SUMMARY
The treatment of bacterial infections suffers from two major problems: spread of multidrug-resistant (MDR) or extensively drug-resistant (XDR) pathogens and lack of development of new antibiotics active against such MDR and XDR bacteria. As a result, physicians have turned to older antibiotics, such as polymyxins, tetracyclines, and aminoglycosides. Lately, due to development of resistance to these agents, fosfomycin has gained attention, as it has remained active against both Gram-positive and Gram-negative MDR and XDR bacteria. New data of higher quality have become available, and several issues were clarified further. In this review, we summarize the available fosfomycin data regarding pharmacokinetic and pharmacodynamic properties, the in vitro activity against susceptible and antibiotic-resistant bacteria, mechanisms of resistance and development of resistance during treatment, synergy and antagonism with other antibiotics, clinical effectiveness, and adverse events. Issues that need to be studied further are also discussed.
INTRODUCTION
The alarmingly increasing antibiotic resistance rates reported among both Gram-positive and Gram-negative pathogens necessitate the implementation of alternative treatment strategies. In view of the rather limited availability of novel antimicrobial agents, the reevaluation of older antibiotic agents seems to be an appealing option. Fosfomycin, an old and rather decommissioned antibiotic, which was previously used mainly as oral (p.o.) treatment for uncomplicated urinary tract infections (UTIs), currently attracts clinicians' interest worldwide. Particularly, the reported activity against pathogens with advanced resistance suggests that this antibiotic may provide a useful option for the treatment of patients with these difficult-to-treat-infections.
Origin and Chemical Structure
Fosfomycin is an old antibiotic agent, discovered in 1969 (1). It is a phosphoenolpyruvate (PEP) analogue that is produced by Streptomyces spp., namely, Streptomyces fradiae (ATCC 21096), S. viridochromogenes (ATCC 21240), and S. wedmorensis (ATCC 21239) (1). It may also be produced synthetically (2).
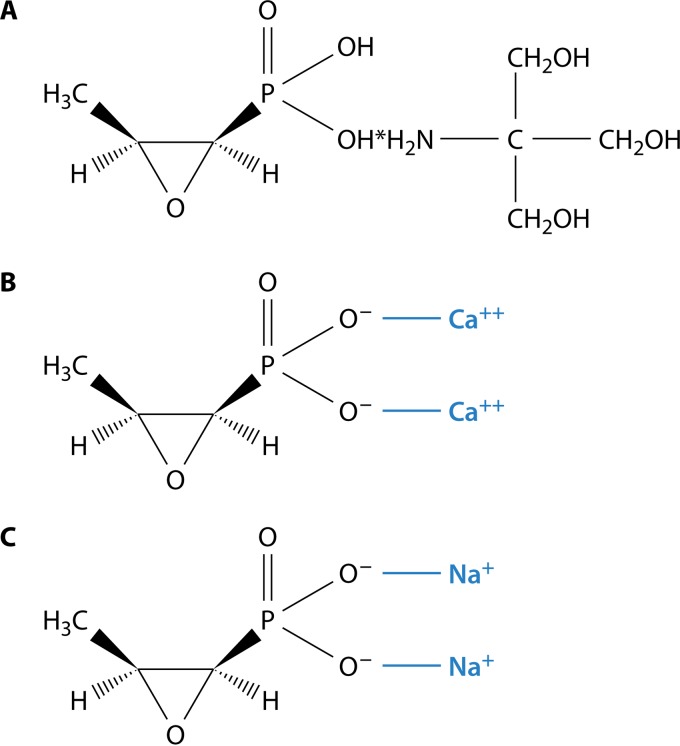
Fosfomycin is a molecule with a low molecular weight (MW) (138) (3). The molecular structure of fosfomycin differs in regard to the available drug formulations. Specifically, fosfomycin is available in two oral formulations, fosfomycin tromethamine (or fosfomycin trometamol) (C3H7O4P · C4H11NO3) Fig. 1A) and fosfomycin calcium (C3H5CaO4P) (Fig. 1B), and one intravenous (i.v.) formulation, fosfomycin disodium (C3H5Na2O4P) (Fig. 1C).
FIG 1.
(A) Molecular structure of fosfomycin trometamol. (B) Molecular structure of fosfomycin calcium. (C) Molecular structure of fosfomycin disodium.
Commercial Formulations
As mentioned above, the commercially available formulations for oral fosfomycin treatment are fosfomycin trometamol and fosfomycin calcium. Fosfomycin trometamol, which is a phosphonic acid derivative of fosfomycin, is available as (1R,2S)-(1,2-epoxypropyl)phosphonic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1) (4). Its commercially available oral formulation consist of a single-dose sachet that contains white granules (4). The MW is 259.2 (4). Fosfomycin calcium salt is the second commercially available formulation for oral fosfomycin treatment. It consists of a white tablet of 500 mg of fosfomycin (titer) (4). The commercially available intravenous fosfomycin formulation consists of 1 to 8 g powder of fosfomycin disodium with succinic acid as sole excipient (https://www.diagnosia.com/de/medikament/infectofos-3-g, http://www.drugs.com/uk/fomicyt-40-mg-ml-powder-for-solution-for-infusion-leaflet.html, http://www.ern.es/wp-content/uploads/2013/01/ENG-FOSFOCINA-INYECTABLE.pdf).
Even though inhaled fosfomycin treatment seems to have potential as an appealing treatment option (5), an aerosolized formulation of fosfomycin that will enable drug delivery directly to the lungs is not commercially available yet. Fosfomycin disodium seems to be the preferred formulation for aerosolized fosfomycin, administered either as a solution for nebulization or as an inhaled dry powder via a metered dose inhaler or dry powder inhaler (6).
Use in Animals
Although fosfomycin has been studied in most of the domestic animals, it is not widely used in veterinary medicine except in countries in Central and South America (7). It is used primarily for the treatment of infectious diseases of broiler chickens and piglets. The drug is eliminated from animal tissues in 2 to 7 days, depending on the testing method, formulation or route of administration, and tissue or animal under study (7). In general, for both pigs and chickens, withdrawal times of 2 and 3 days after intramuscular and p.o. administration, respectively, could be assigned as a precautionary principle for public health (8).
ANTIMICROBIAL PROPERTIES
Mechanism of Action
Fosfomycin is a bactericidal antibiotic agent. It inhibits an enzyme-catalyzed reaction in the first step of the synthesis of the bacterial cell wall (9). Fosfomycin interferes with the first cytoplasmic step of bacterial cell wall biosynthesis, the formation of the peptidoglycan precursor UDP N-acetylmuramic acid (UDP-MurNAc) (10). Specifically, the enzyme UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) is involved in peptidoglycan biosynthesis by catalyzing the transfer of the enolpyruvyl moiety of phosphoenolpyruvate (PEP) to the 3′-hydroxyl group of UDP-N-acetylglucosamine (UNAG) (11). Fosfomycin covalently binds to the thiol group of a cysteine (position 115 in Escherichia coli numbering; target Cys115) in the active site of MurA and consequently inactivates it (11–13). This inhibitory action takes place at an earlier step than the action of β-lactams or glycopeptides.
For the entry inside the bacterium, fosfomycin uses two different uptake pathways (identified at least for E. coli), the l-alpha-glycerophosphate and the hexose-6-phosphate transporter systems (3). The activity of the second uptake system is induced by glucose-6-phosphate (G-6-P) (3). Moreover, the expression of the genes of both the above-mentioned uptake systems requires the presence of cyclic AMP (cAMP), along its receptor protein complex (3). Finally, fosfomycin reduces adherence of bacteria to urinary epithelial cells (14). In a similar manner, fosfomycin suppresses platelet activator factor receptors in respiratory epithelial cells, thus reducing adhesion of Streptococcus pneumoniae and Haemophilus influenzae (15).
Immunomodulating Activity
Fosfomycin exerts immunomodulatory effects by altering lymphocyte, monocyte and neutrophil function. It affects the acute inflammatory cytokine response in vitro and in vivo. It suppresses production of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-1α and increases production of IL-10, while contradictory data have been published regarding IL-6 (15–18). On the other hand, concentrations of TNF-α, IL-1β, and IL-6 expressed as protein and mRNA were almost identical with and without fosfomycin in healthy volunteers (19). Fosfomycin suppresses IL-2 production from T cells (20), the production of leukotriene B4 (LTB4) from neutrophils, and the expression of IL-8 mRNA by LTB4 from monocytes (21). Fosfomycin also exhibits an immunomodulatory effect on B-cell activation (22). Fosfomycin enhances neutrophil phagocytic killing of invading pathogens (23), even in patients on chronic hemodialysis and renal transplantation (24). Fosfomycin resulted in enhanced bactericidal ability of neutrophils compared to other antimicrobials (25). The clinical relevance of the aforementioned actions remains to be elucidated.
Activity in Biofilms
Fosfomycin has the ability to penetrate into biofilms. Several experimental studies (in vitro and biofilm infection models) showed that fosfomycin, alone or in combination with other antibiotics, not only reduced or eradicated clinically significant bacteria from biofilms (26–30) but also resulted in modifications of the biofilm structure. In a rat cellulose-pouch methicillin-resistant Staphylococcus aureus (MRSA) biofilm model, combination therapy with vancomycin and fosfomycin resulted in the disappearance of biofilm-like structures (31). Reductions in the Staphylococcus epidermidis biofilm density were also observed with fosfomycin; the addition of azithromycin in one of the studies had no further effect on the biofilm density or bacterial eradication (32, 33). The combination of prulifloxacin and fosfomycin resulted in destruction and disappearance of P. aeruginosa multilayer biofilms from the surfaces of polyethylene tubes in a urinary tract infection rat model, as seen by scanning electron microscopy (34). Fosfomycin was able to reduce initial and mature E. coli biofilm forms on polystyrene tissue culture plates. Fosfomycin activity was enhanced when it was combined with N-acetylcysteine (35). Finally, fosfomycin was bacteriostatic against vancomycin-resistant enterococci (VRE) in urinary stents biofilms. The MIC90 of VRE strains increased from 64 mg/liter in planktonic cultures to 128 mg/liter in biofilm cultures (36).
Spectrum of Activity
In vitro susceptibility data suggest that fosfomycin is considerably active against both Gram-negative and Gram-positive pathogens. Specifically, fosfomycin is considered active against Enterococcus spp. (including Enterococcus faecalis and E. faecium irrespective of vancomycin resistance), Staphylococcus aureus (irrespective of methicillin resistance), and S. epidermidis (37, 38). Fosfomycin also exhibits considerable activity against Gram-negative pathogens, including Salmonella spp., Shigella spp., E. coli, Klebsiella and Enterobacter spp., Serratia spp., Citrobacter spp., and Proteus mirabilis (37–41). Fosfomycin has been also found to be active against Listeria monocytogenes, Neisseria gonorrhoeae, Aerococcus urinae, and Helicobacter pylori (42–45). Fosfomycin is not active against anaerobes, such as Bacteroides spp., but it is active against Peptococcus spp. and Peptostreptococcus spp. (46, 47). Pseudomonas spp., Acinetobacter spp., Stenotrophomonas maltophilia, Burkholderia cepacia, Staphylococcus capitis, Staphylococcus saprophyticus, and Mycobacterium tuberculosis are intrinsically resistant to fosfomycin (48, 49). Morganella morganii is also resistant to fosfomycin (50).
Intracellular Bactericidal Activity of Fosfomycin
Experiments have shown that some S. aureus strains, in part in the form of the small-colony variant, can resist intracellular killing after phagocytosis from neutrophils or persist inside the host cells, e.g., in the osteoblasts. In this way they can cause relapses of infections (51–53). Fosfomycin was shown to penetrate inside the cells and assist in bacterial clearance in cell line experiments. Compared to other antimicrobials, fosfomycin was more active than glycopeptides and daptomycin but less active than rifampin, ofloxacin, and clindamycin (51–53). Similarly, fosfomycin was able to reduce the intracellular concentration of L. monocytogenes (54). Both positive and negative data have been published regarding the ability of fosfomycin to eliminate intracellular Salmonella enterica serovar Typhimurium (54, 55), while fosfomycin's effectiveness in reducing intracellular E. coli was low (55).
IN VITRO DATA
Susceptibility Testing Methodology
The laboratory methods that have been used for the determination of in vitro susceptibility of Gram-positive and Gram-negative pathogens to fosfomycin include agar (Mueller-Hinton agar) dilution, broth dilution, disk diffusion, and Etest techniques (22, 40, 56–59). Supplementation of agar or broth with G-6-P enhances fosfomycin activity. In this regard, Mueller-Hinton agar or broth supplemented with 25 μg/ml G-6-P is recommended, as it results in maximal enhancement of fosfomycin activity (60). A recent study suggested that regarding P. aeruginosa, which lacks a specific G-6-P transporter, the addition of G-6-P in agar or broth does not affect fosfomycin activity (61). According to the Clinical and Laboratory Standards Institute (CLSI) standard criteria, the approved susceptibility testing methods are disk diffusion and agar dilution for urinary E. coli and E. faecalis isolates, whereas broth microdilution should not be performed (48). On the other hand, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) suggests both agar and broth (http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_5.0_Breakpoint_Table_01.pdf).
In an early study comparing agar dilution, broth microdilution, and disk diffusion for extended-spectrum β-lactamase (ESBL)-producing E. coli and Klebsiella pneumoniae isolates, excellent agreement was observed between the compared methods regarding E. coli, whereas considerable discrepancies were observed for K. pneumoniae (62). In a later study comparing disk diffusion with agar dilution for Gram-negative and Gram-positive isolates, E. coli was found to be uniformly susceptible (63). This finding was consistent regarding K. pneumoniae and Enterobacter cloacae, whereas the prevalence of resistance of P. aeruginosa and S. maltophilia was affected by the choice of MIC (63). On the other hand, particularly for P. aeruginosa, a recent study suggested that broth microdilution is a reliable method, whereas no concordance was observed between agar dilution and disk diffusion/Etest (61). Finally, a recent study evaluating agar dilution, disk diffusion, and Etest for contemporary multidrug-resistant (MDR) Gram-negative pathogens suggested that disk diffusion had poor performance for Acinetobacter baumannii and Enterobacteriaceae and that Etest performed poorly for all tested pathogens (64). The available MICs and zone diameter breakpoints suggested by CLSI and EUCAST for specific bacteria are presented in Table 1.
TABLE 1.
Available fosfomycin MICs and zone diameter breakpoints according to the latest EUCAST and CLSI criteriaa
| Criteriab | Organism(s) and delivery route | MIC (mg/liter) |
Zone diameter (mm) |
||||
|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | ||
| EUCAST | Enterobacteriaceae | ||||||
| Intravenous | ≤32 | >32 | NR | NR | |||
| Oralc | ≤32 | >32 | NR | NR | |||
| Pseudomonas spp. | |||||||
| Intravenousd | |||||||
| Oral | NR | NR | NR | NR | |||
| Staphylococcus spp. | |||||||
| Intravenous | ≤32 | >32 | —e | — | |||
| Oral | NR | NR | NR | NR | |||
| CLSIf | E. colig | ≤64 | 128 | ≥256 | ≥16 | 13–15 | ≤12 |
| E. faecalish | ≤64 | 128 | ≥256 | ≥16 | 13–15 | ≤12 | |
S, susceptible, I, intermediate, R, resistant; NR, not reported.
EUCAST criteria are from version 5.0, 2015 (http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_5.0_Breakpoint_Table_01.pdf); CLSI criteria are from 2015 (48).
For uncomplicated urinary tract infections.
Epidemiological cutoff for wild-type isolates, ≤128 mg/liter.
—, MICs are recommended.
Pseudomonas spp., Acinetobacter spp., B. cepacia complex, S. maltophilia, S. saprophyticus, and S. capitis are considered to have intrinsic resistance, defined as inherent or innate (not acquired) antimicrobial resistance, which is reflected in wild-type antimicrobial patterns of all or almost all representatives of a species. Intrinsic resistance is so common that susceptibility testing is unnecessary.
Testing and reporting only for E. coli urinary isolates.
Testing and reporting only for E. faecalis urinary isolates.
Susceptibility Reports (Gram-Negative and Gram-Positive Isolates)
Early in vitro reports suggested that fosfomycin exhibited considerable activity against Gram-negative and Gram-positive urinary isolates. In this regard, it was considered appropriate therapy for uncomplicated UTIs in many clinical settings worldwide. Specifically, fosfomycin exhibited considerable antimicrobial activity against Gram-negative urinary isolates, including Enterobacteriaceae, as well as Gram-positive urinary isolates, including S. aureus (both methicillin-susceptible S. aureus [MSSA] and MRSA) and E. faecalis (22). Yet, the reported activity of fosfomycin against P. aeruginosa and Acinetobacter baumannii was low (22). Specifically, according to the findings of a review published in 2009 that evaluated 22 studies, fosfomycin exhibited considerable activity against MRSA and penicillin-nonsusceptible pneumococcal isolates (cumulative susceptibility rates, 87.9% [4,240/4,892 isolates] and 87.2% [191/219 isolates], respectively) (65). Activity against vancomycin-resistant enterococci was less promising (cumulative susceptibility rate, 30.3% [183/604 isolates]) and more variable. In a concurrent review of 23 studies, fosfomycin was active against 30.2% (511/1,693 isolates) of MDR P. aeruginosa isolates (66). On the other hand, 3.5% (3/85 isolates) of MDR A. baumannii isolates and none of the 31 MDR Burkholderia species isolates were found to be susceptible to fosfomycin (66). Moreover, fosfomycin was found to be considerably active against MDR Enterobacteriaceae isolates (96.8% [1,604/1,657] of ESBL-producing E. coli isolates and 81.3% [608/748] of ESBL-producing K. pneumoniae isolates) (67).
In Vitro Activity against Contemporary Isolates (Studies Published after 2010)
In the current era, the emergence of MDR and extensively drug-resistant (XDR) pathogens complicated the therapeutic approach to serious infections, such as respiratory tract infections, bacteremia, and surgical infections. In addition, MDR pathogens are now frequently encountered in easy-to-treat infections, such as acute cystitis due to ESBL E. coli isolates. The above factors, as well as the limited options of novel antibiotic agents, necessitated the reevaluation of fosfomycin as a potential therapeutic option for infections caused by contemporary isolates with advanced antimicrobial resistance. Table 2 shows the susceptibility of contemporary bacteria to fosfomycin from the larger studies published from 2010 onwards (36, 56, 57, 61, 68–90).
TABLE 2.
Data on in vitro susceptibility of MDR or XDR bacteria to fosfomycin and relevant antibiotics from the larger studies published from 2010 onwardsa
| Category | First author, yr (reference) | Country, period | Method(s) | Source of infection | Resistance profileb | Organism(s) | No. of isolates | Susceptibility to fosfomycin (%) | Fosfomycin MIC50, MIC90 (mg/liter) |
|---|---|---|---|---|---|---|---|---|---|
| Carbapenem-resistant or carbapenemase-producing Gram-negative bacteria | Jiang, 2015 (75) | China, 2010–2013 | AD | NR | KPC | K. pneumoniae | 278 | 39.2 | 64, >256 |
| Diaz-Aguilar, 2013 (61) | NR | AD, BMD | NR | CR (28.2) | P. aeruginosa | 206 | 80.6 | 64, 256/512d | |
| Tuon, 2013 (88) | Brazil, 2010–2011 | DD | Various | KPC-2 | K. pneumoniae | 311 | 99 | NR | |
| ESBL-producing Enterobacteriaceae | Cho, 2015 (73) | South Korea, 2008–2013 | Microscan | UTI | ESBL | E. coli, K. pneumoniae | 277 | 87.7 (E. coli, 94.9; K. pneumoniae, 61.7) | NR |
| Sultan, 2015 (86) | India | DD | UTI | ESBL, AmpC | Enterobacteriaceae (E. coli, 90%) | 372 | 98.9 (ESBL, 100; AmpC, 95.7) | NR | |
| Asencio, 2014 (69) | Spain, 2010–2012 | Vitek II | Various | ESBL | E. coli | 824 | 95 (ESBL, 82) | NR | |
| K. pneumoniae | 136 | 88 (ESBL, 91) | |||||||
| Khan, 2014 (78) | Pakistan, NR | DD | UTI | ESBL | Enterobacteriaceae | 381 | Total, 84; E. coli, 93; Klebsiella spp., 64; Proteus spp., 50 | NR | |
| Cagan Aktas, 2014 (71) | Turkey, 2011–2012 | DD, Etest | UTI | ESBL (48.4) | E. coli | 244 | 99 (ESBL, 97) | 0.5, 3 | |
| Sorlozano, 2014 (85) | Spain, 2006–2012 | Wider, Microscan | UTI | ESBL (4.4–31.8)c | K. pneumoniae | 3,271 | 40–78 | NR | |
| Villar, 2014 (89) | Argentina, 2012–2013 | DD | UTI | ESBL | E. coli | 374 | 97.6 (ESBL, 98.2) | NR | |
| Villar, 2014 (89) | Argentina, 2012–2013 | DD | UTI | ESBL | K. pneumoniae | 94 | 94.7 | NR | |
| Villar, 2014 (89) | Argentina, 2012–2013 | DD | UTI | ESBL | P. mirabilis | 50 | 72 | NR | |
| Lai, 2014 (79) | China, 2004–2012 | AD | UTI | ESBL (58.1) | E. coli | 908 | 98.4 (ESBL, 93.8) | NR | |
| Karlowsky, 2014 (77) | Canada, 2007–2013 | AD | Various non-UTI | ESBL | E. coli | 254 | 94.9 | 2, 4 | |
| AmpC | 119 | 96.6 | 2, 16 | ||||||
| Morfin-Otero, 2013 (81) | Mexico, 2010–2011 | BMD | NR | ESBL (16) | E. coli | 75 | 96.9 | ≤32, ≤32 | |
| K. pneumoniae | 21 | ≤32, ≤32 | |||||||
| Sahni, 2012 (84) | India, 2009–2010 | DD | UTI | ESBL (47.6) | E. coli | 2,416 | 83 (ESBL, 81) | NR | |
| Araj, 2012 (68) | Lebanon | DD | UTI | ESBL | E. coli | 374 | 86 | NR | |
| K. pneumoniae | 168 | 62 | |||||||
| Briongos-Figuero, 2012 (70) | Spain, 2009 | Vitek II, Etest | UTI | ESBL | E. coli | 372 | 88.7 | NR | |
| Klebsiella spp. | 28 | 46 | |||||||
| Lee, 2012 (80) | South Korea | AD | NR | ESBL | E. coli | 165 | 92.9 | NR | |
| K. pneumoniae | 182 | 95.2 | |||||||
| Hsu, 2010 (74) | Taiwan, 2008–2010 | AD | Various | CRc (43–75), ESBL (42.7) | E. coli | 72 | 99 (ESBL, 96) | 1 and 32 | |
| K. pneumoniae | 167 | 87 (ESBL, 93) | 16, 64 | ||||||
| E. cloacae | 115 | 97 | NR | ||||||
| S. marcescens | 25 | 84 | NR | ||||||
| C. freundii | 20 | 95 | NR | ||||||
| MDR Enterobacteriaceae | Kahlmeter, 2012 (76) | Europe, 2007–2008 | DD | UTI | MDR (18.2) | E. coli | 903 | 98.8 | |
| Falagas, 2010 (57) | Greece, 2007–2009 | Etest | Various | MDR | E. coli | 26 | 100 | 32, 64 | |
| K. pneumoniae | 116 | 90.5 | 1, 4 | ||||||
| MDR Gram-positive bacteria | Champion, 2013 (72) | USA, 2008–2010 | Etest, Vitek II | Cystic fibrosis | MRSA | S. aureus | 277 | 99.6 | |
| Pogue, 2013 (82) | USA, 2008–201 | Microscan, Etest | Various | VRE | E. faecalis | 28 | 96 | 48, 96 | |
| E. faecium | 42 | 76 | |||||||
| Descourouez, 2013 (36) | USA, 2007–2010 | BMD | UTI | VRE | E. faecium | 32 | 100 | 64, 64 | |
| Rebiahi, 2011 (83) | Algeria, 2007–2009 | DD | Surgical wound infections | MRSA | S. aureus | 220 | 94.1 (MRSA, 93.3) | NR | |
| Taj, 2010 (87) | Pakistan, 2009 | DD | Various | MRSA (31.6) | S. aureus | 550 | MSSA, 94.1; MRSA, 68.9 | NR | |
| Yu, 2010 (90) | China, NR | DD | Various | MRSA | S. aureus | 196 | 33.2 | 64, 128 | |
| Falagas, 2010 (56) | Greece, 2008 | DD | Various | Various | Gram positive | 1846 | S. aureus, 99.3; MRSA, 99.2; CoNS, 77.5 | NR | |
| Endimiani, 2010 (93) | USA, 2009 | Etest, AD, DD | NR | KPC | K. pneumoniae | 68 | 62 | 16, 64 |
Abbreviations: AD agar dilution; AMK amikacin; BMD broth microdilution; CR carbapenem resistant; DD disk diffusion; GNM, gentamicin; KPC, Klebsiella pneumoniae carbapenemase; MDR, multidrug resistant; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; NR, not reported; UTI, urinary tract infections; VRE, vancomycin-resistant Enterococcus; XDR, extensively drug resistant.
The number in parentheses is the percentage of pathogens with the specific resistance pattern. If no percentage is provided, all isolates presented the resistance pattern.
Refers to ertapenem-resistant bacteria.
256 with agar dilution and 512 with broth microdilution.
Regarding Gram-positive bacteria, contemporary studies showed that fosfomycin is active against the majority of S. aureus strains (>90%), including MRSA, as well as coagulase-negative staphylococci (CoNS) (36, 56, 61, 72, 75, 82, 83, 87, 88, 90). However, one study reported that only 33.2% of MRSA strains were susceptible to fosfomycin (90). Similarly, fosfomycin activity against enterococci, including VRE strains, varied in the available studies, with some of the studies reporting activity as low as 30% (36, 91, 92). Resistance to fosfomycin did not seem to be associated with vancomycin resistance. In addition, fosfomycin seemed to be less active against E. faecium than against E. faecalis in some series (82, 91). Finally, in the study that provided comparative data, fosfomycin seemed to be less active against coagulase-negative staphylococci and streptococcal strains than against S. aureus (77.5% versus 61.9% versus 99.3%, respectively) (56).
The majority of the recently published studies evaluated the in vitro activity of fosfomycin against ESBL-producing Enterobacteriaceae, particularly E. coli and K. pneumoniae (68–71, 73, 74, 77–81, 84–86, 89). Although studies evaluating the susceptibility of isolates recovered from blood or respiratory specimens have been published, the great majority of these studies focused on urine samples. In general, fosfomycin was more active against E. coli (range, 82% to 100%) than against K. pneumoniae (15% to 100%). Community-acquired strains were in general more susceptible than nosocomial strains. Susceptibility of other Enterobacteriaceae was less frequently reported, but fosfomycin remained active against a significant proportion (72% to 97.5%); P. mirabilis was reported as the least susceptible of them. Finally, fosfomycin was found to be active against 90.5% to 100% of MDR Enterobacteriaceae (57, 76).
Fosfomycin was also evaluated against carbapenem-resistant (CR) or carbapenemase-producing Gram-negative bacteria (57, 76, 93). Most of the data refer to KPC-producing K. pneumoniae strains or, to a lesser extent, to other Enterobacteriaceae. MIC50 and MIC90 values were usually one dilution lower in ESBL-producing K. pneumoniae strains than in CR/KPC-producing K. pneumoniae strains. All CR A. baumannii strains were also resistant to fosfomycin (94), while 80.6% of CR P. aeruginosa strains were reported to be susceptible in one study (61).
The fosfomycin MIC distribution in the available studies was extremely variable and associated with several factors, including species, method used for determination of MIC, underlying fosfomycin resistance mechanisms and coexisting mechanisms conferring resistance to other antibiotics, and geographical region of isolation. Thus, MIC50 and MIC90 values were one dilution lower for ESBL-producing than for CR/KPC-producing K. pneumoniae strains (75, 88, 93). The presence of rmtB genes was also associated with higher MIC values in KPC-producing strains (95). Similarly, the susceptibility of ESBL-producing E. coli strains was slightly lower than that of strains with a nonspecific pattern of resistance. In general, E. coli (including ESBL-producing strains) and S. aureus (including MRSA) displayed a lower MIC distribution in studies published from 2010 onwards. In contrast, enterococci (particularly vancomycin-resistant E. faecium) and K. pneumoniae (especially CR strains) showed higher variation in MIC50 and MIC90 values. The susceptibility of Proteus spp. and Enterobacter spp. was similar to or slightly higher than that of K. pneumoniae, but the available data were limited. Compared with studies published before 2010, no major differences in the susceptibility of Gram-negative bacteria and S. aureus have been reported (65–67, 96). However, the cumulative susceptibility of VRE was found to be 30.3%, considerably lower than the susceptibility in studies after 2010 (65).
MECHANISMS OF RESISTANCE
Inherent Resistance
The mechanism of action and structure of fosfomycin are unique, making cross-resistance uncommon. However, several mechanisms conferring resistance to fosfomycin have been identified (96). Some bacteria are inherently resistant to fosfomycin. First, mutations in murA causing a change from cysteine to aspartate render bacteria (e.g., Chlamydia spp., Mycobacterium tuberculosis, and Vibrio fischeri) resistant to this antimicrobial agent (97) (49, 98). Second, a study reported the identification of a salvage pathway in peptidoglycan synthesis in Pseudomonas putida. Using this pathway, recycling of the peptidoglycan is accomplished instead of its de novo synthesis from UDP-MurNAc, which is the first peptidoglycan precursor (the production of which is catalyzed by MurA) (99). Consequently, the fosfomycin target (MurA) is not involved in peptidoglycan synthesis, resulting in inherent fosfomycin resistance. A similar pathway was recently described for Pseudomonas aeruginosa (10).
Acquired Resistance
In commonly fosfomycin-susceptible bacteria like E. coli, resistance develops when mutations occur in the uptake systems used as means of fosfomycin entry inside the bacteria (100). Mutations in the chromosomal glpT and uhpT genes, which encode fosfomycin transporters, result in blocked or decreased fosfomycin uptake (92, 101). The encoded proteins are glycerol or carbohydrate transporters that are essential for metabolic functions or virulence in E. coli and other bacteria (96). Such mutations were the most common mechanisms of resistance in older series (102). Mutations in cyaA and ptsI genes (which result in lower cAMP levels and downregulation of fosfomycin transporters) have also been described and associated with a decrease in pilus biosynthesis and in the ability to adhere to epithelial cells (96, 103). Mutations in murA result in lower affinity of enolpyruvyl transferase for fosfomycin (104), while overexpression of the enolpyruvyl transferase was also shown to result in fosfomycin resistance (101).
Several fosfomycin-modifying enzymes have been described. FosA (glutathione S-transferase), the first to be described (in 1988), is a metalloenzyme transferred through plasmids in Enterobacteriaceae. It catalyzes the reaction between glutathione and fosfomycin to an inactive adduct (102, 105–107). New subtypes, with similar structure, of the gene have been described (fosA2, fosA3, fosA4, and fosA5) (108–110). Cooccurrence in plasmids with blaCTX-M, blaNDM, blaKPC, blaOXA, blaCMY, blaAmpC, blaTEM, blaSHV, blaSFO-1, gyrA, parC, parE, sul1, sul2, strA, strB, aac(6′)-Ib, aadA5, aphA6, tetA(A), mphA, floR, dfrA7, rmtB, and merA genes has been reported, conferring resistance to β-lactams, quinolones, aminoglycosides, macrolides, sulfonamides, and tetracyclines (75, 80, 111–114). The genes conferring resistance to fosfomycin could be transferred together with genes conferring resistance to other antibiotics in either the same or a conjugate plasmid (114).
FosB is a similar enzyme (its amino acid sequence is 48% identical to that of FosA) that catalyzes the reaction between cysteine and fosfomycin in Gram-positive bacteria (plasmid encoded in Staphylococcus spp. and Enterococcus spp. and chromosomally in Bacillus subtilis) (102, 115–119). FosX is a chromosomal enzyme of Listeria monocytogenes that catalyzes the reaction of fosfomycin with water (120). FosC, found in Pseudomonas syringae, is an enzyme similar to glutathione S-transferase that catalyzes phosphorylation with ATP and inactivation of fosfomycin (121). Finally, kinases that cause fosfomycin degradation through phosphorylation (FomA and FomB, which are structurally and functionally related to FosC) have been identified in Streptomyces spp. and rarely in P. aeruginosa (122).
Heteroresistance
Heteroresistance to fosfomycin has been described for S. pneumoniae. In a recent study, 10 out of 11 tested strains showed heteroresistance to fosfomycin. All heteroresistant strains contained the MurA1 protein. When this was deleted, heteroresistance was abolished. The strain that did not show heteroresistance differed from the other strains by a single amino acid substitution in MurA1 [Ala(364)Thr]. When this gene was introduced into a heteroresistant strain, its heteroresistance phenotype was not changed. Thus, MurA is required for heteroresistance, but it is not the only factor involved (123). Heteroresistance was also described in MDR and non-MDR P. aeruginosa strains (124).
In Vitro and In Vivo Development of Resistance and Spread
Fosfomycin has been associated with rapid development of resistance in vitro, but widespread or increasing resistance in clinical practice has been infrequently reported (96). Several mechanisms can be associated with these observations. Nilsson et al. studied the development of resistance to fosfomycin in E. coli isolates in vitro and the behavior of fosfomycin-resistant isolates recovered from clinical specimens in vitro and in urine. They reported that development of resistance in vitro was highly probable and caused by mutations in ptsI, cyaA, glpT, uhpA/T, and other unspecified genes, while mutations in ptsI and cyaA were not observed in clinical isolates (103). All mutations developing in vitro caused a decrease in the bacterial growth rate of resistant pathogens (in laboratory media or in urine and in the presence or absence of fosfomycin) compared to that of susceptible isolates. Similarly, the growth rate was lower in resistant clinical isolates in the presence of fosfomycin (103).
As described above, in cases of lower urinary tract infections, such mutations may enable bacterial washout and provide a mean for preventing the isolates from establishing in the bladder. Furthermore, it has been postulated that the biological cost of these mutations could be high enough to prohibit the growth of the mutants in the intestines or outside the host (125). However, slower growth was not observed in fosfomycin-resistant clinical isolates in the absence of fosfomycin (103), suggesting that in real-case scenarios other compensatory mutations might ameliorate the biologic cost and enable persistence of resistant bacteria. The ability of fosfomycin to decrease adhesion of E. coli in the bladder wall and the high concentrations achieved in urine may further prevent bacterial establishment, at least in the urinary tract, even for isolates with a high fosfomycin MIC. Finally, the mutations described above can be found in chromosomes, but the emergence of resistant loci in plasmids (FosA and FosB) could potentially provide a better means for the spread of resistance mechanisms than the chromosomal ones (126). Several recently published studies have shown dissemination of fosfomycin-resistant strains (mainly due to the presence of FosA) in patients as well environmental reservoirs in livestock and animals (75, 113, 127, 128).
Fosfomycin administration was not as widespread as that for other antibiotics, e.g., β-lactams or fluoroquinolones. Thus, studies that did not account for fosfomycin consumption did not show major differences in fosfomycin resistance with time (96). However, in a study that evaluated 17,602 urinary tract infections due to E. coli during a 5-year period (2003 to 2008), a 50% increase in fosfomycin use resulted in an increase of fosfomycin-resistant, extended-spectrum β-lactamase (ESBL)-producing E. coli strains from 2.2% at the beginning of the study to 21.7% at the end (P < 0.0001) (129). A similar increase in fosfomycin resistance was reported among all isolates (from 1.6% in 2003 to 3.8% in 2008; P < 0.0001). A significant increase in fosfomycin resistance against uropathogens was reported in a second Spanish study during a 7-year period (2006 to 2012) (85). On the other hand, the limited available data from 5 randomized controlled trials (RCTs) included in a meta-analysis showed that resistance did not develop after a single-dose treatment for cystitis (130). These RCTs were conducted in Europe and the United States and included a total of 739 patients (nonpregnant women; children, 3%) (131–135). One disadvantage of this analysis was that 4 of these RCTs were published more than 15 years ago (1987 to 1998).
Older studies reported development of resistance during treatment in between 0% and 6.7% of all cases; development of resistance was more pronounced among P. aeruginosa strains (7 to 20%) (96). Recent in vitro experiments in 59 MDR and non-MDR P. aeruginosa strains confirmed the propensity of P. aeruginosa to develop resistance to fosfomycin (124). Although 61% of the studied strains were considered fosfomycin susceptible at the beginning of the study (MIC ≤ 64 mg/liter), they were replaced by fosfomycin-resistant colonies even when the inoculum was low. We should acknowledge that heteroresistance was detected at baseline in all tested isolates (124). Development of resistance to fosfomycin during treatment along with an increase in β-lactam MICs was reported in 3 isolates in a Greek hospital. The resistant bacteria were considered mutants of the pretreatment ones (136).
SYNERGY AND ANTAGONISM
Table 3 shows the synergy of fosfomycin in combination with other antibiotics against clinically relevant bacteria.
TABLE 3.
Synergistic activity of fosfomycin in combination with other antibiotics against several clinically relevant bacteria
| Organism(s) | Antibiotics with activity in combination with fosfomycin (reference[s]) |
||
|---|---|---|---|
| Synergy | Indifference | Antagonism | |
| MSSA | Linezolid (174), ciprofloxacin (24), ceftriaxone (137), ciprofloxacin (137), rifampin (137) | Vancomycin (137), ceftriaxone (137), gentamicin (137) | |
| MRSA | Cefamandole (138, 139, 268), cefazolin (138, 139, 268), vancomycin (29, 137, 229), rifampin (29, 90, 142, 147), carbapenems (13, 152), cefmetazole (13), cefoperazone-sulbactam (13), linezolid (161), quinupistin-dalfopristin (162), fusidic acid (90), minocycline (163), tigecycline (29), daptomycin (29, 165) | Aminoglycosides (268), fusidic acid (268), trimethoprim (268), vancomycin (137) | Rifampin (139) |
| Glycopeptide-intermediate S. aureus | Imipenem (166), vancomycin (166), linezolid (166) | ||
| CoNS | Ciprofloxacin (137), imipenem (137), rifampin (137) | Vancomycin (137) | |
| MR S. epidermidis | Vancomycin (167) | ||
| Enterococcus | Cefotaxime (137), daptomycin (137),a imipenem (137)a | ||
| VRE | Daptomycin (36), teicoplanin (92), amoxicillin (36), linezolid (36, 92),b ampicillin (92),b vancomycin (92),a tigecycline (92),a rifampin (92)a | Nitrofurantoin (92), minocycline (92) | Ampicillin (92) |
| Streptococcus spp. | Penicillin (137),a cefminox (137),a cefotaxime (137)a | Vancomycin (137), imipenem (137), ceftriaxone (137), cefepime (137) | |
| E. coli | Gentamicin (137)a | ||
| ESBL-producing E. coli | Carbapenems (84),a aztreonam (153),colistin (84),b aminoglycosides (28, 84),a tigecycline (28, 84),a colistin (28) | ||
| ESBL-producing K. pneumoniae | Carbapenems (84), colistin (84),b netilmicin (84),a tigecycline (84)a | ||
| K. pneumoniae | Gentamicin (137)a | ||
| MDR K. pneumoniae | Carbapenem (152), aztreonam (153) | ||
| CR K. pneumoniae | Carbapenems (84, 164, 179), colistin (84, 164, 179),a tigecycline (84),a netilmicin (84)a | Gentamicin (179) | Colistin (156), (OXA-48-producing strain) |
| NDM-1-producing Enterobacteriaceae | Colistin (158), tigecycline (158) | ||
| Salmonella | Amilacin (137),a cefepime (137) | ||
| P. aeruginosa | Aztreonam (137),a levofloxacin (137),a ciprofloxacin (137),a cefepime (137),a gentamicin (137),a piperacillin (137),a ceftazidime (137),a imipenem (137)a | Imipenem (137), ceftazidime (137), ciprofloxacin (137), gentamicin (137) | |
| CR P. aeruginosa | Colistin (149),a carbapenems (149, 150),a aminoglycosides (27, 40), piperacillin-tazobactam (40), ceftazidime (40), cefepime (40), ciprofloxacin (40) | ||
| MDR P. aeruginosa | Carbapenems (84), colistin (189),b tigecycline (84),b netilmicin (84)b | Carbapenems (152), aminoglycosides (168) | |
| Acinetobacter | Amikacin (137)a | Imipenem (137), ceftazidime (137), ciprofloxacin (137) | |
| OXA-23-producing Acinetobacter | Colistin (59, 89),a,b sulbactam (89) | ||
| Pan-drug-resistant Acinetobacter | Polymyxin B (90),b minocycline (90)b | ||
| N. gonorrhoeae | Ceftriaxone (44) | Cefixime (159, 253), ceftriaxone (159, 253), azithromycin (253), colistin (253), ertapenem (253), gentamicin (253), minocycline (253), oxifloxacin (253) | |
Synergy was observed in ≤50% of tested strains.
Synergy was observed in <20% of tested strains.
Older Studies
Fosfomycin's unique mechanism of action provides a mean for possible synergy with other antibiotics. Older studies evaluating synergy of fosfomycin with other antibiotics against Gram-positive and Gram-negative bacteria were summarized in a review published in 2009 (137). The fractional inhibitory concentration index (FICI) and the efficacy time index (ETI) were used to define synergy. Time-kill experiments, checkerboards, broth microdilution, and agar dilution were used. Fosfomycin was synergistic with cefamandole, cefazolin, and methicillin for MRSA strains (138–140); however, data were discouraging for antibiotics more likely to be used for MRSA treatment, i.e., aminoglycosides, fusidic acid, and trimethoprim (140). Conflicting data were reported for vancomycin (139–141) and rifampin (139, 142). Other studies also showed synergy with ciprofloxacin and linezolid for MSSA strains (143, 144). Synergy against some strains of Streptococcus spp. was observed between fosfomycin and penicillin, cefminox, and cefotaxime but not vancomycin, imipenem, ceftriaxone, and cefepime (137). Regarding Enterococcus spp., synergy was observed with cefotaxime and for some strains with daptomycin and imipenem (137).
Fewer data were available for Gram-negative bacteria. Regarding P. aeruginosa, synergy was observed for most of the strains with aztreonam, cefepime, and levofloxacin, while conflicting or partially encouraging data were reported for imipenem, ceftazidime, ciprofloxacin, and aminoglycosides (137). One study showed synergy with ceftazidime, imipenem, and ciprofloxacin against only 1 of the 34 MDR A. baumannii strains and for 38% of strains with amikacin (145). Synergy was also observed between fosfomycin and gentamicin in some E. coli, K. pneumoniae, and S. marcescens strains (145).
Newer Studies
During the last few years, more data have become available regarding the potential synergistic activity between fosfomycin and other antibiotics against contemporary strains, for which fewer treatment options are available. The fractional inhibitory concentration index (FICI), reduction in colonies, and the efficacy time index (ETI) were used to define synergy. Time-kill experiments, checkerboards, broth microdilution, agar dilution, and Etest were used.
Nonfermenting Gram-negative bacteria.
Two studies evaluated the potential synergistic activity between fosfomycin and colistin against OXA-23-producing A. baumannii; they reported synergy against 50% of the strains in one study (checkerboards were used) and 12.5% of strains in the other (checkerboards and time-kill assays were used) (146, 147). One of these studies reported synergy against 75% of strains when fosfomycin was combined with sulbactam (146). Discouraging results were reported when fosfomycin was combined with polymyxin B or minocycline for pan-drug-resistant A. baumannii strains (synergy was observed in 16% and 12% of strains using checkerboards and FICI, respectively) (148).
More promising data have been reported for CR P. aeruginosa strains; three studies reported synergy between fosfomycin and colistin (22%) or carbapenems (up to 40%) against clinical isolates using checkerboards and time-kill assays (40, 149, 150). Using checkerboards/FICI, aminoglycosides, piperacillin-tazobactam, ceftazidime, cefepime, and ciprofloxacin were also synergistic with fosfomycin against CR P. aeruginosa strains (151). In one more study, in which the resistance profile of P. aeruginosa was not reported, synergy (using FICI) between fosfomycin and aminoglycosides was observed for 60% to 80% of tested strains, with amikacin and isepamicin demonstrating the higher synergy rates (27). Against MDR P. aeruginosa, synergy against 50% to 70% of isolates (the Etest was used) was reported with carbapenems (mainly doripenem), while synergy with colistin, tigecycline, or netilmicin was reported for <15% of strains (59). However, we should note that other published studies reported no synergy against P. aeruginosa between fosfomycin and aminoglycosides or carbapenems using checkerboards and time-kill assays (6, 152).
Enterobacteriaceae.
The available studies showed that synergy between fosfomycin and other antibiotics against K. pneumoniae depends on the underlying enzymes conferring resistance. The fosfomycin-doripenem, fosfomycin-aztreonam, and fosfomycin-aztreonam-amdinocillin combinations were highly effective in reducing bacterial populations of drug-resistant K. pneumoniae using checkerboards and time-kill assays (152, 153). In ESBL-producing K. pneumoniae strains, synergy with carbapenems (43% to 78%, with imipenem showing the highest rate), colistin (7%), netilmicin (43%), and tigecycline (21%) was reported (59). Against CR K. pneumoniae without specification of the exact mechanism of resistance, synergy with carbapenems (70%), colistin (36%), tigecycline (30%), and netilmicin (42%) was reported (59). The Etest was used in that study. Against KPC-2-producing K. pneumoniae strains, synergy with meropenem primarily (65%) and colistin secondarily (12%) was reported using time-kill assays, while combination with gentamicin resulted in indifference (154). Similarly, the fosfomycin-colistin and fosfomycin-colistin-meropenem combinations showed synergy against 2 VIM- and 2 NDM-producing K. pneumoniae strains (155). However, antagonism between colistin and fosfomycin against OXA-48-producing K. pneumoniae isolates was reported (checkerboards were used) (156). Regarding E. coli, the data refer to ESBL-producing strains; synergy was reported with carbapenems (by checkerboards, time-kill assays, and Etest), aztreonam (by checkerboards and time-kill assays), colistin (by time-kill assay and Etest), netilmicin (by Etest), and tigecycline (by time-kill assays and Etest) (28, 59, 152, 153, 157). In addition, synergy was reported with cefoxitin (by time-kill assays) at concentrations equal to the MIC of the isolate but not at higher concentrations (157). Finally, synergy was reported with colistin, but not with tigecycline, against NDM-1-producing Enterobacteriaceae using checkerboards (158).
Studies on Neisseria gonorrhoeae showed no synergy between several antibiotics (cefixime, ceftriaxone, azithromycin, colistin, ertapenem, gentamicin, minocycline, oxifloxacin, rifampin, and spectinomycin) and fosfomycin when the agar dilution or Etest method was used (159, 160). However, synergy was observed with ceftriaxone in a time-kill study (44).
Gram-positive bacteria.
Several studies have evaluated the synergistic activity of fosfomycin with various antibiotics against S. aureus, especially MRSA. All these studies reported high synergy rates in vitro among clinical isolates (by time-kill assays and checkerboards): with doripenem (against 95% of isolates) (152), linezolid (98%) (161), quinupristin-dalfopristin (100%) (162), fusidic acid (88%) (90), and minocycline (87%) (163). Lower synergy was reported with rifampin (50%) (164). It is also noteworthy that antagonism was not reported for any of the above combinations. Similarly, in vivo biofilm models showed synergy between fosfomycin and vancomycin or daptomycin against MRSA strains (31, 165). Another study showed that the fosfomycin-rifampin combination was the most successful in reducing MRSA bacterial colonies (time-kill assay); other combinations tested in this study, in order of decreasing efficacy, were fosfomycin-daptomycin, fosfomycin-vancomycin, and fosfomycin-tigecycline (29). In a peritonitis model against a glycopeptide-intermediate S. aureus isolate, the combination of fosfomycin-imipenem was more effective than the combination of fosfomycin with vancomycin or linezolid (166). The effectiveness of these combinations was confirmed histologically (by disappearance of biofilm-like structures, marked decrease in necrosis, and formation of granular tissue) in the aforementioned studies. Finally, synergy between fosfomycin and vancomycin against methicillin-resistant S. epidermidis in vitro (by checkerboards) was not reported (167).
Two studies evaluated the potential synergistic activity between fosfomycin and other antibiotics against VRE clinical isolates. In general, synergy was observed in vitro with daptomycin, teicoplanin, and amoxicillin (by time-kill assays) (36, 91). Synergy was observed with linezolid or ampicillin against few vancomycin-resistant E. faecalis strains, while no synergy was reported with nitrofurantoin or minocycline (36, 91). Similar synergy between fosfomycin and vancomycin, tigecycline, or rifampin against both E. faecium and E. faecalis was reported (20% to 33%) (91). In biofilm models, synergy was observed against most E. faecalis strains with teicoplanin (44%), tigecycline (56%), or rifampin (100%), but these combinations were less successful against E. faecium (10%, 10%, and 40%, respectively). No synergy was observed in biofilm models with linezolid and ampicillin (91). In addition, antagonism between fosfomycin and ampicillin was reported for 2 VRE isolates.
Although some of these studies provide promising data for the selection of specific antibiotic combinations in real clinical scenarios in the future, it is evident that not all isolates would be susceptible to these combinations. For example, the same antibiotic combination (most notable and clinically relevant, fosfomycin with colistin, carbapenems, or aminoglycosides) resulted in variable synergy, or even antagonism, against CR K. pneumoniae isolates. It is probable that other coexisting mechanisms conferring resistance, including efflux pumps and modified antibiotic targets, and transferred together with ESBL genes in the same or conjugated plasmids contribute to these phenotypes. Enhanced antibiotic uptake (168) or downregulation of vital genes for bacterial growth (169), as shown for tobramycin in the presence of mucin and under anaerobic conditions in patients with cystic fibrosis, may also contribute to the synergistic activity. Future studies should compare the outcomes for patients infected by bacteria which were susceptible to antibiotic combinations in vitro to those for patients infected by bacteria that remained resistant.
PHARMACOKINETICS AND PHARMACODYNAMICS
Oral Fosfomycin
The oral bioavailability of fosfomycin trometamol ranges between 34 and 58% (38, 122). Absorption occurs in the small intestine, and evidence suggests that coadministration of fosfomycin trometamol with food may reduce absorption of the drug (37% fasting versus 30% with food) (4, 170). The maximum concentration in serum (Cmax) was also higher under fasting conditions (12.1 ± 0.6 mg/liter and 7.8 ± 1.6 mg/liter, respectively), but urinary recovery rates were similar (58% versus 52%) (170). Age does not seem to affect absorption (38). Metoclopramide increases gastrointestinal motility and results in lower absorption and lower serum concentrations. The rate and extent of absorption of fosfomycin trometamol were approximately 6 times greater than those of fosfomycin calcium during the first 2 h postdose and approximately 3 to 4 times greater during the 12-h postdose period (4). In a study comparing the pharmacokinetic (PK) properties of fosfomycin trometamol and fosfomycin calcium, mean peak serum concentrations following a single 2-g dose of fosfomycin trometamol were found to be 2- to 4-fold higher than those obtained after a single 3-g dose of fosfomycin calcium (171). The reason for this observation is that fosfomycin calcium is hydrolyzed and thus inactivated by gastric acid (172–174).
The mean serum elimination half-life (t1/2) of fosfomycin trometamol is estimated at 5.7 h (38). The t1/2 was relatively prolonged in elderly patients (38). The area under the concentration-time curve (AUC) is 145 to 228 mg · h/liter (38) Conflicting data regarding the apparent volume of distribution has been published (40 to 136 liters) (94). The degree of binding of the fosfomycin molecule with proteins is negligible (174). Fosfomycin is excreted nonmetabolized in the urine, through glomerular filtration (175). Depending on age, fasting, and renal function, 11 to 60% of the drug can be found in the urine within 24 h from administration (122). Specifically, older age, administration with a meal, and deteriorating renal function result in slower elimination through the kidneys (122).
Following a single 3-g dose of fosfomycin trometamol, peak urine concentrations are reached within 4 h (38). High urine as well as bladder tissue concentrations (>128 mg/liter) are retained for 1 to 2 days, which is sufficient to eliminate the majority of common uropathogens (38, 176) However, the activity of fosfomycin at concentrations equal to the MIC is impaired against a variety of pathogens when the urine pH is below 6, resulting in bacterial regrowth (177). Contemporary published evidence suggests that following a single 3-g dose of oral fosfomycin trometamol, sufficient intraprostatic concentrations in uninflamed prostatic tissue are achieved (178).
No contraindications exist for the administration of fosfomycin with other medications. Unless the benefits outweigh the risks, typhoid (live attenuated) and BCG vaccines should be withheld in patients receiving fosfomycin, as with other antimicrobials, as the coadministration may lower vaccine effectiveness due to pharmacodynamic (PD) antagonism (http://reference.medscape.com/drug/formulary/monurol-fosfomycin-342560#3). Fosfomycin may increase the levels or effect of digoxin; patients should be monitored closely when digoxin and fosfomycin are coadministered. A low risk for contraceptive failure exists when fosfomycin is coadministered with conjugated estrogens. Minor or insignificant interactions may result in lower absorption of vitamin B complex, metoclopramide, and balsalazide (http://reference.medscape.com/drug/formulary/monurol-fosfomycin-342560#3). Finally, fosfomycin trometamol should not be coadministered with probenecid, which decreases renal clearance and excretion of fosfomycin (4).
Parenteral Fosfomycin Disodium
In vivo studies suggest that following a 15-mg/kg intravenous dose of fosfomycin disodium in piglets, the AUC from 0 to 12 h (AUC0–12) was 120.00 ± 23.12 μg · h/ml, whereas the volume of distribution was 273.00 ± 40.70 ml/kg; plasma clearance was 131.50 ± 30.07 ml/kg/h, and the t1/2 was 1.54 ± 0.40 h (179). In the same study, following intramuscularly administered fosfomycin disodium, the AUC0–12 and bioavailability were 99.00 ± 0.70 μg · h/ml and 85.5% ± 9.90%, respectively (179). Another in vivo study evaluating a 20-mg/kg/day dose of intravenous/intramuscular fosfomycin disodium in cattle suggested that effective fosfomycin plasma concentrations for susceptible pathogens could be achieved up to 8 h after intravenous administration and approximately 10 h after intramuscular administration (180).
Following intravenous administration, variable peak, mean, and trough fosfomycin levels have been reported in humans. In general, peak concentrations were high (up to 606 mg/liter) (174). Nonrenal elimination of intravenous fosfomycin is negligible, with 93 to 99% excreted unchanged in the urine (22, 175, 181). With regard to fosfomycin's tissue penetration following intravenous administration in patients or healthy volunteers, a review suggested that intravenously administered fosfomycin has greater penetration into subcutaneous and muscle tissue, followed by lung and bone tissue (174). Substantial concentrations following intravenous doses were also achieved in cerebrospinal fluid (CSF), soft tissues, and bone tissues, whereas data regarding the distribution of fosfomycin into intra-abdominal sites were scarce.
Skin, soft tissue, and abscesses.
Data from healthy volunteers and patients showed that fosfomycin achieves high concentrations in skin and soft tissues. In healthy volunteers, administration of a single 8-g dose of fosfomycin resulted in AUC0–8 ratios between the interstitial fluid of muscles and adipose tissue over that of serum of 0.53 and 0.71, respectively (182). In intensive care unit (ICU) patients with soft tissue infections, the AUC0–4 ratio for muscle over plasma was 0.71 (183). Similar findings were reported for diabetic foot infections with osteomyelitis (184). Fosfomycin also exhibited similar penetration into subcutaneous tissue regardless of the presence of inflammation (185). However, fosfomycin levels in the abscess fluid were highly variable. It seems that fosfomycin penetration into abscesses depends on morphological characteristics (e.g., the permeability of the outer wall or the vascularity of the surrounding tissues) beyond plasma concentrations or the individual ratios of abscess surface area to volume (186, 187).
An advantage of fosfomycin in the case of abscesses could be the increased bactericidal activity against both Gram-positive and Gram-negative bacteria (188). The MICs for fosfomycin were lower under anaerobic conditions. The culture media and the strains tested significantly affected the degree of change in MICs. The growth-inhibitory diameter in the paper disc assay increased in parallel with the decrease in the redox potential of the agar medium. As the increase of the activity of fosfomycin in anaerobic cultures was not associated with the change of medium pH or the change of mobility of the drug in agar, it was assumed that the uptake of fosfomycin through the cell membrane increases under anaerobic conditions (188).
Lower respiratory tract.
An intravenously administered fosfomycin dose of 2 g was reported to achieve substantial concentrations of 12 to 16 mg/liter in healthy lung tissue, approximately half of that achieved in serum. The concentration in tumor cells was half of that in healthy tissue (189). In patients with tracheostomy, fosfomycin concentrations (7 ± 7.14 mg/liter) in bronchial secretions 2 h after the end of a 4-h infusion were 13% of those in serum (190). Intravenous fosfomycin seems also to exhibit good penetration into infected lung tissue; the ratio of the AUC0–∞ for lung to the AUC0–∞ for plasma was 0.63 in a study evaluating the ability of a single 4-g intravenous dose of fosfomycin to penetrate lung tissue of septic patients. In that study, fosfomycin's mean Cmax and AUC0–∞ were higher in healthy than in infected lungs (131.6 ± 110.6 mg/liter versus 107.5 ± 60.2 mg/liter and 367.6 ± 111.9 mg · h/liter versus 315.1 ± 151.2 mg · h/liter, respectively) (191). Finally, fosfomycin achieves adequate concentrations in pleural fluid (due to both infectious and noninfectious etiology) for at least 12 h following the end of infusion. However, the presence of pachypleuritis may impede penetration in pleural effusion (192).
CNS and CSF.
Fosfomycin crosses the blood-brain barrier, and meningeal inflammation increases its concentration in the CSF (193). However, an in vitro study showed that the antibacterial activity of fosfomycin against S. aureus was lower in CSF than in Mueller-Hinton broth, suggesting that fosfomycin may not be sufficient for isolates with higher MICs (194). In a rabbit model for pneumococcal meningitis, it was also suggested that fosfomycin concentrations in CSF should be at least 8 times higher than the MIC for the isolate in order to obtain adequate bacterial killing (195). Therefore, fosfomycin may not be considered adequate as monotherapy for patients with meningitis (195). In patients with CSF drainage, a single 5-g or 10-g dose resulted in CSF levels 9.2% and 13.8%, respectively, of those in plasma. When given at a dose of 5 g every 8 h, its CSF levels were 30 mg/liter or more after the second day of treatment and tripled in cases of inflammation (196). In a small study enrolling 6 ICU patients with ventriculostomy-associated ventriculitis, fosfomycin's AUC at steady state in CSF was 27% of that in plasma (197). Finally, data from 2 neurosurgical patients without central nervous system (CNS) infection showed that fosfomycin could achieve clinically relevant levels in the brain parenchyma (198).
Bone.
Fosfomycin penetrates in both cortical and cancellous bone, and penetration correlates with plasma levels and the presence of inflammation. In patients undergoing hip replacement, a 4-h infusion of fosfomycin (4 g) resulted in a slightly higher concentration in cancellous than in cortical bone as measured 1 h and 3 h after the end of infusion (199). Much higher concentrations in the interstitial bone fluid were reported for patients receiving intravenous fosfomycin for chronic osteomyelitis or diabetic foot infections with osteomyelitis (184, 200).
Intra-abdominal sites.
Although fosfomycin is eliminated almost entirely through the kidneys, its concentrations in the bile and gallbladder were high in patients undergoing cholecystectomy, especially soon after administration; its levels decreased gradually over time (201). In a study of 4 patients, fosfomycin achieved concentrations higher than the MIC for the causative bacteria in purulent ascitic fluid as well as in the inflamed appendix (202).
Heart valves and biofilms.
In patients undergoing open heart surgery for valvulopathies, prophylactic administration of intravenous fosfomycin (5 g) resulted in variably high valve concentrations (27 to 77 mg/liter) depending on the degree of valvular degeneration. The levels were maintained for at least 60 min (203). Data regarding penetration of fosfomycin into biofilms have not been published, but several studies have evaluated the effectiveness of fosfomycin alone or in combination in experimental biofilm models (see below).
Concentration- or time-dependent action.
It is not fully elucidated whether bacterial killing with fosfomycin is time or concentration dependent. It seems that this depends on the microorganism under study. Thus, it seems that for P. aeruginosa and S. aureus, fosfomycin demonstrates time- or non-concentration-dependent killing (6, 168). Recently, a study suggested that fosfomycin demonstrates both time- and concentration-dependent activity against S. aureus (13). On the other hand, concentration-dependent killing was demonstrated against Enterococcus faecium, E. coli, and P. mirabilis (36, 204).
Clinical Significance of PK and PD Aspects in Specific Patient Groups
Elderly individuals.
Comparative pharmacokinetic evidence with regard to elderly and younger individuals suggested that the serum AUC0–∞ for both fosfomycin tromethamine and fosfomycin calcium was significantly increased in elderly compared to younger individuals (171). On the other hand, fosfomycin trometamol and fosfomycin calcium clearance was significantly decreased in elderly compared to younger individuals (171). Dose adjustment for both oral fosfomycin formulations is not recommended for elderly individuals with endogenous creatinine clearances of >50 ml/min per 1.73 m2 (171). However, in elderly patients with impaired renal function (mean creatinine clearance of 40 ml/min), the fosfomycin urinary concentration was higher than that in healthy adults (205).
Children and neonates.
In children 3 to 15 years old, the elimination half-life of fosfomycin is similar to or slightly lower than that in adults with normal renal function (206). However, the half-life is prolonged in both full- and preterm neonates due to their larger volume of distribution and lower glomerular filtration rate (207). High mean serum and urine concentrations are achieved; in addition, 58% to 78% of the dose is excreted in the urine (206). Early published evidence regarding the pharmacokinetic aspects of intravenous fosfomycin in children suggests a dose response in blood and urine concentrations of the drug, given at 25 mg/kg and 50 mg/kg either through intravenous injection or through a 1-h intravenous infusion (206). However, a recent study that focused on the pharmacokinetic and dosing aspects of fosfomycin treatment in children and neonates suggested that fosfomycin exhibits a time-dependent bactericidal activity (207).
Pregnancy and lactation.
Fosfomycin trometamol has been assigned to pregnancy category B (i.e., animal reproduction studies have failed to demonstrate a risk to the fetus, and there are no adequate and well-controlled studies in pregnant women) (4); thus, it should be used during pregnancy only if clearly indicated (174). Fosfomycin is reported to cross the placental barrier through simple diffusion but does not affect the placental transport of other nutrients (208). Teratogenic effects have not been reported with fosfomycin doses of ≤1,000 mg/kg/day (corresponding to 1.4 and 9 times the human dose) in pregnant rats, whereas when doses of ≥1,000 mg/kg/day were administered to pregnant rabbits, fetotoxicities, concomitantly with maternal toxicity, were observed (4). Currently, there are no available data on excretion of fosfomycin in human milk. However, due to the low molecular weight of the drug, excretion is expected (4).
Critically ill patients.
Pharmacokinetic data on intravenous administration of an 8-g dose of fosfomycin in critically ill patients with sepsis suggest that the drug exhibits a “tissue pharmacokinetic profile,” with median fosfomycin concentrations in the interstitium and plasma exceeding MICs for Streptococcus pyogenes, S. aureus, and Pseudomonas aeruginosa for a period of 4 h (183). On the other hand, a more recent review suggested that the alterations in volume of distribution and creatinine clearance that are observed during critical illness may result in a need for fosfomycin loading doses and/or dose adjustments in order to avoid toxicity, as well as inadequate treatment (209).
Patients with renal function impairment.
Since nonrenal clearance of fosfomycin disodium is negligible and fosfomycin trometamol is eliminated primarily in the urine, impairment of renal function was expected to affect the pharmacokinetic aspects of fosfomycin. Indeed, early data suggested that following a single dose of 3 g fosfomycin trometamol, the Cmax and AUC were significantly higher in uremic patients with various degrees of renal insufficiency than in healthy controls (210). Early evidence also suggested that following injection of 1 g fosfomycin disodium, serum levels and time of elimination were related to the degree of renal insufficiency (211). Fosfomycin is also actively eliminated through the hemodialyzer (211–213). However, adjustment of the fosfomycin dose was not deemed necessary in critically ill patients under continuous venovenous hemofiltration (214). In addition, in a recent study focusing on the pharmacokinetic aspects of intravenous and intraperitoneal fosfomycin, in patients on automated peritoneal dialysis without peritonitis, fosfomycin exhibited good systemic exposure after intraperitoneal administration but limited peritoneal fluid penetration following intravenous administration (215).
DOSING GUIDELINES
Oral Fosfomycin
According to published bacteriological and clinical evidence, the recommended dose for oral fosfomycin trometamol treatment regarding uncomplicated urinary tract infections (cystitis) is a 3-g single dose (130, 216). Regarding complicated urinary tract infections (complicated cystitis), a higher-than-approved dose (a single oral dose of 3 g fosfomycin trometamol every 2 to 3 days for a total of 3 doses) (Table 4) is recommended by several authors (22). Evidence suggests that adjustment of the oral 3-g dose of fosfomycin trometamol is not necessary in vulnerable subpopulations, including pregnant women, elderly individuals, and patient with impaired renal/liver function (4, 22). However, regarding pediatric patients, lower oral dosages 1 to 2 g of fosfomycin trometamol have been reported in relevant studies (22, 132, 217, 218).
TABLE 4.
Studies with clinical outcomes after fosfomycin administration for urinary tract infections published from 2010 onwardsa
| First author, yr of publication (reference) | Study place, yr | Design | Patients, n | Infection | Bacteria | Fosfomycin treatment | Comparator | Mortality | Clinical cure | Microbiological cure |
|---|---|---|---|---|---|---|---|---|---|---|
| Ceran, 2010 (224) | Turkey, NA | RCT | Female adults, 260 | Lower uUTI | E. coli, Enterobacter spp. | p.o. trometamol, 3-g single dose | Ciprofloxacin at 500 mg q12h for 5 days | NA | 64/77 (83%) vs 53/65 (81%) | 64/77 (83%) vs 51/65 (78%) |
| Usta, 2011 (226) | Turkey, 2007–2008 | RCT | Pregnant females, 90 | Lower uUTI | E. coli, Enterobacter spp., K. pneumoniae | p.o. trometamol, 3-g single dose | Amoxicillin-clavulanate at 625 mg q12h or cefuroxime at 500 mg q12h | NA | 22/28 (79%) vs 46/56 (82%) | 23/28 (82%) vs 48/56 (86%) |
| Palou, 2013 (225) | Spain, NR | SB RCT | Postmenopausal females, 118 | Lower uUTI | E. coli (76.8%), K. pneumoniae (7.3%), P. mirabilis (4.9%), Enterococus spp. (3.7%) | p.o. trometamol, 3 g, 2 doses | Ciprofloxacin at 250mg q12h for 3 days | NA | 32/37 (86.5%) vs 32/39 (82.1%) | 23/37 (62.2%) vs 23/39 (59%) |
| Matsumoto, 2011 (227) | Japan, 2008 | Prospective | Community infections, 40 | Lower uUTI | E. coli (79.5%) | p.o. calcium, 1 g q8h for 2 days | NA | NR | 34/40 (85%) | 30/40 (75%) |
| Senol, 2010 (229) | Turkey, 2005–2006 | Prospective | Community infections, 47 | Lower cUTI | ESBL-producing E. coli | p.o. trometamol, 3 g every other day for 3 doses | Carbapenems | NA | 21/27 (77.8%) vs 19/20 (95%) | 16/27 (59.3%) vs 16/20 (80%) |
| Neuner, 2012 (230) | USA, 2006–2010 | Retrospective | 41 | UTI | CR K. pneumoniae (13), P. aeruginosa (8), ESBL producers (7), VRE (7), E. coli (5) | p.o. in combination with other antibiotics in 27% of patients | NA | 4/41 (10%) | NA | 24/41 (59%); CR K. pneumoniae, 46%; P. aeruginosa, 38%; ESBL producers, 71%; VRE, 71% |
| Reid, 2013 (231) | USA, 2010–2011 | Retrospective | Kidney transplantation, 14 | UTI | ESBL-producing E. coli (7), KPC-producing K. pneumoniae (5), P. aeruginosa (2) | p.o. trometamol, 3 g, median of 3 doses | NA | NR | NA | 4/13 (31%); recurrence, 7/13 (54%); persistence, 3/13 (21%) |
| Qiao, 2013 (228) | China, 2011 | Prospective | Community infections, 356 | Lower UTI | NR | p.o. trometamol, 3 g for 3 doses | NA | NR | uUTI, 179/189 (94.7%); recurrent UTI, 61/79 (77.2%); cUTI, 42/67 (62.7%) | uUTI, 83/85 (97.7%); recurrent UTI, 34/36 (94.4%); cUTI, 26/31 (83.9%) |
| Wu, 2014 (232) | Taiwan, 2003–2013 | Prospective | Children with vesicoureteral reflux disease, 6 | Recurrent UTI | Enterobacteriaceae, P. aeruginosa | 100–200 mg/kg/day for 7–10 days plus amikacin at 15 mg/kg/day for 5 days or ceftazidime at 100–150 mg/kg/day for 7–10 days | NA | NR | 1/6 (16.7%) | 1/6 (16.7%) |
Abbreviations: CR carbapenem resistant; cUTI, complicated urinary tract infection; ESBL extended spectrum β-lactamase; KPC, Klebsiella pneumoniae carbapenemase; MDR multidrug resistant; NA, not available; NR, not reported; p.o., per os; q8h, every 8 h; RCT randomized controlled trial; SB, single blind; uUTI, uncomplicated urinary tract infections.
Parenteral Fosfomycin
The dose regimens for intravenous fosfomycin range with regard to the severity of the disease. Specifically, daily intravenous fosfomycin dosages in patients with normal renal function (creatinine clearance of ≥80 ml/min) range from a 12- to 16-g total daily dose, administered as 2 to 4 divided doses (22, 207, 219–221). In most cases, fosfomycin is administered intravenously as a dose of 8 g of fosfomycin disodium twice daily (every 12 h) (3). However, higher daily doses (up to 24 g) have been given to patients with CNS or other severe infections (220). Intravenous fosfomycin is administered as a slow infusion after dilution in 100 ml of normal saline.
With regard to patients with impaired renal function, currently it is not clear if dose adjustment is required for an estimated creatinine clearance of 40 to 80 ml/min. For patients with estimated creatinine clearances of 40, 30, 20, and 10 ml/min, a reduction to 70%, 60%, 40%, and 20% of the daily recommended dose, respectively, is proposed. In patients undergoing intermittent dialysis (every 48 h), 2 g after each session is recommended (https://www.medicines.org.uk/emc/medicine/28971). There are no data for dose reduction in patients with hepatic impairment or for elderly patients without renal impairment.
With regard to children and neonates, the dose of intravenous fosfomycin is adjusted according to body weight and age. Specifically, according to the instructions provided in the package insert of the intravenous fosfomycin formulation, the recommended doses are: 100 mg/kg in 2 divided doses for premature babies, 200 mg/kg in 3 divided doses in neonates, 200 to 300 mg/kg in 3 divided doses for infants up to 1 year (and up to 10 kg), and 200 to 400 mg/kg in 3 to 4 divided doses for children 1 to 12 years (http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con309596.pdf) (222). Published data regarding administration of fosfomycin through other parenteral routes (mainly intramuscularly) are scarce.
CLINICAL DATA
Urinary Tract Infections
The majority of the available clinical data regarding fosfomycin's effectiveness refer to treatment or prevention of lower UTIs, primarily cystitis. Current guidelines recommend fosfomycin for the treatment of female patients with uncomplicated cystitis. However, it was also stated that according to FDA data, 1 dose of fosfomycin may be associated with lower effectiveness than other short-course regimens (223). In contrast, pooled data from 27 RCTs on pregnant (16 trials) and nonpregnant (5 trials) females, males and nonpregnant females (3 trials), and children (3 trials) with cystitis or other lower UTIs did not support the FDA data (130). Most RCTs were open label and with a low mean Jadad score (≤2). A single 3-g dose of fosfomycin was administered in these RCTs with cystitis patients. Comparator antibiotics included quinolones, β-lactams, aminoglycosides, nitrofurantoin, and sulfonamides. Clinical and microbiological cure, relapses, and reinfections were similar for fosfomycin and comparators. Pregnancy, gender, age, double blinding, and duration of administration of comparators did not affect clinical and microbiological success (130). Three additional RCTs evaluating the effectiveness and safety of fosfomycin for lower UTIs in females have been published since then (Table 4). All of them reported that fosfomycin trometamol was as affective as comparator antibiotics, regardless of the patients' hormonal or pregnancy status (224–226).
Besides RCTs, work on several cohorts studying the effectiveness of fosfomycin for the treatment of lower UTIs has been published (Table 4). These studies confirmed the effectiveness of fosfomycin for the treatment of patients with UTIs due to isolates susceptible to fosfomycin and several other antibiotics (227, 228) but also showed that fosfomycin monotherapy may not suffice for the treatment of recurrent UTIs or UTIs due to MDR bacteria in patients with significant comorbidity. In a small observational study of patients with complicated lower UTI due to ESBL-producing E. coli, oral fosfomycin trometamol was compared with carbapenem treatment. Clinical and microbiological success with fosfomycin and carbapenems was not significantly different (77.8% versus 95% and 59.3% versus 80%, respectively; P > 0.05) (229). In addition, a discordance between in vitro susceptibility to fosfomycin and microbiological effectiveness was observed in patients with P. aeruginosa (75% versus 38%) and CR K. pneumoniae UTIs (92% versus 46%) (230). Furthermore, in a small case series of kidney transplantation patients and children with vesicoureteral reflux disease, a high rate of recurrent infections was reported, mainly due to different or more susceptible bacteria than those for which fosfomycin was initially prescribed (231, 232). More robust data from well-designed and adequately powered studies should become available in order to reach safer conclusions. Finally, few case reports support fosfomycin use, alone or in combination with other antibiotics, for the treatment of patients with acute prostatitis (233–235).
Non-Urinary Tract Infections
The effectiveness of fosfomycin for the treatment of patients with Gram-negative or Gram-positive non-urinary tract infections has been evaluated in several studies since its discovery. In a comprehensive review of the older studies (until 2008), fosfomycin was effective in 84% of patients (81.1% cures; 1,302/1,604) (2). In the studies included in that review, fosfomycin was prescribed for various infections (pneumonia and other respiratory infections, osteomyelitis or septic arthritis, meningitis or encephalitis, ear, nose, and throat infections, obstetric and gynecological infections, septicemia or endocarditis, peritonitis, cervical lymphadenitis, eye infections, diabetic foot infections, and typhoid fever) due to several bacteria (most prevalently S. aureus, S. epidermidis, P. aeruginosa, E. coli, K. pneumoniae, and Enterobacter spp.) and in variable doses (1 to 24 g per day in 3 or 4 divided doses, when provided). Fosfomycin was administered primarily in combination with other antibiotics. In several cases it was administered when treatment failure with other antibiotics was documented. The duration of treatment was up to 2 months, depending on the infection under study (2).
An even older review that included studies performed in Japan soon after the discovery of fosfomycin reported that oral fosfomycin (per os 2 to 3 g/day for adults or 100 to 130 mg/kg for infants and children in most cases) was effective in 76% (912/1,200) of patients, while the parenteral form (i.v. 2 to 4 g/day for adults or 100 to 250 mg/kg for infants and children) was effective for 68% (340/500) (236). Fosfomycin in combination with other antibiotics was also found to be effective against MDR P. aeruginosa (90.9%; 30/33) and S. Typhimurium infections (66, 67). Finally, preliminary data suggest that fosfomycin is active against H. pylori and could be used as salvage therapy in patients not responding to first-line regimens (42, 237).
The major drawbacks of the aforementioned studies were the lack of randomization and the heterogeneity of patients under study, indications, and dosing of fosfomycin. In addition, several of them were conducted years or even decades ago, and their findings may not apply to the resistance profiles of contemporary isolates or the complexity and severity of diseases and infections that patients face nowadays. Data from RCTs are still not available, but RCTs evaluating the comparative efficacy of fosfomycin and meropenem for bacteremic UTIs and fosfomycin in addition to daptomycin for MRSA bacteremia are under way (238, 239). Similarly, fosfomycin is being evaluated in combination with other antibiotics for MDR or XDR infections (NCT01297894, NCT02142751, and NCT00871104).
Table 5 summarizes the characteristics and outcomes of contemporary studies regarding the effectiveness of fosfomycin for the treatment of infections due to MDR bacteria (219, 220, 240–246). Fosfomycin was prescribed for several indications in variable doses and always in combination with other antibiotics. When provided, all-cause mortality ranged from 18.2% to 40.8%, values similar to those reported for combinations of colistin, tigecycline, and aminoglycosides (247–250). Importantly, 3 of the studies provided data regarding development of resistance during treatment; in 2 studies, resistant bacteria were not isolated, while 1 reported that 3 isolates developed resistance (220, 243, 246). Of note, the initial MIC of these isolates was at the highest within susceptible values (32 to 64 mg/liter; according to EUCAST, an MIC of 64 mg/liter denotes resistance). Infections due to such isolates have been associated with higher mortality (251, 252).
TABLE 5.
Studies with clinical outcomes after fosfomycin administration for non-urinary tract infections published from 2010 onwardsa
| First author, yr of publication (reference) | Study place, yr | Design | Patients, n | Infections (n) | Bacteria | Fosfomycin | Comparator | Mortality | Clinical cure | Microbiological cure |
|---|---|---|---|---|---|---|---|---|---|---|
| Michalopoulos, 2010 (220) | Greece, 2008 | Prospective | Adults, 11 | ICU infections | CR K. pneumoniae | i.v. 4 g q6h plus other antibiotics | NA | 2/11 (18.2%) | NR | NR |
| Apisarnthanarak, 2010 (219) | Thailand, 2009–2010 | Retrospective | Adults, 8 | HAP/VAP | CR P. aeruginosa | i.v. 2 g q8h plus i.v. doripenem (1 g q8h, extended infusion) | NA | 2/8 (25%) | 6/8 (75%) | 6/7 (86%) |
| Florent, 2011 (243) | France, 2005–2010 | Retrospective arm, prospective arm | Adults, 72 | BJI (33), CNS infections (11), EaS infections (9), UTI (9), BSI (5), SSTI (4), pneumonia (1) | Enterobacteriaceae (24, including 5 ESBL- and 4 AmpC-producing strains), P. aeruginosa (13, including 5 MDR strains), staphylococci (12, including 6 MRSA strains); overall, MDR, 28% | i.v. 4 g q8h plus other antibiotics | NA | NR | 63/72 (87%) | NR |
| Kusachi, 2011 (244) | Japan, NA | NA | Adults, 114 | Intra-abdominal abscess | NA | i.v. added on previously failing antibiotic | NA | NR | 91/104 (87.5%) | NA |
| Dihn, 2012 (242) | France, 2007 | Prospective | Adults and children, 116 | Lung infections (33), BJI (32), UTI (16), BSI (9), IAI, endocarditis, CNS infections (7) | P. aeruginosa (43), Enterobacteriaceae (29), MRCNS (23), MRSA (15), Streptococcus spp (6), MDR (83), ESBL (49) | i.v. 4 g q6h–q8h plus other antibiotics | NA | 30/116 (25.9%) | 77/99 (77%) | 66/83 (79.5%) |
| Apisarnthanarak, 2012 (240) | Thailand, 2007–2011 | Retrospective | 49 | HAP/VAP | CR P. aeruginosa | i.v. for ≥2 days plus doripenem (1 g q8h) or colistin (5 mg/kg/day in 2 divided doses) | NA | 20/49 (40.8%) | 29/49 (59.2%) | 33/49 (67.3%) |
| Navarro-San Francisco, 2013 (245) | Spain, 2010–2012 | Prospective | 5 | Bacteremia | OXA-48-producing K. pneumoniae | i.v. plus either tigecycline or colistin | Combinations of tigecycline, colistin, carbapenems, aminoglycosides | 2/5 (40%) vs 24/35 (68.6%) | NR | NR |
| Pontikis, 2014 (246) | Greece 2010–2012 | Prospective | ICU, 66 | Primary BSI, VAP, CR-BSI, IAI | KPC-producing K. pneumoniae (41), P. aeruginosa (17) | i.v. 16–24 g in divided doses plus other antibiotics | NA | 18/48 (37.5%) | 26/48 (54.2%) | 27/48 (56.3%) |
| Del Rio, 2014 (241) | Spain, 2001–2010 | Prospective | 16 | BSI (75% endocarditis) | MRSA | i.v. 2 g q6h plus imipenem (1 g q6h) | NA | 5/16 (31%) | NR | NR |
Abbreviations: BJI, bone and joint infections; BSI, bloodstream infections; CNS, central nervous system; CR, carbapenem resistant; CR-BSI, catheter-related bloodstream infections; EaS, ear and sinus infections; ESBL, extended-spectrum β-lactamase; HAP, hospital-acquired pneumonia; IAI, intra-abdominal infections, i.v., intravenous; KPC, Klebsiella pneumoniae carbapenemase; MDR, multidrug resistant; MRCNS, methicillin-resistant coagulase-negative staphylococci; MRSA, methicillin-resistant S. aureus; NA, not available; SSTI, skin and soft tissue infections; UTI, urinary tract infections; VAP, ventilator-associated pneumonia.
Prophylaxis
Increasing antimicrobial resistance, especially among quinolones, increased the interest in alternative prophylactic regimens for urologic procedures. In a recently published review regarding the effectiveness of fosfomycin in preventing UTI after endourological interventions or surgical procedures, the authors concluded that one or two doses of fosfomycin trometamol could be an effective alternative. The conclusion was limited by the small number of available patients for every indication (253). In an RCT not included in that review, fosfomycin (3 g by mouth every 48 h for two doses) was compared with ciprofloxacin (500 mg by mouth every 12 h for 5 days) for prevention of prostatitis after transrectal prostate biopsy; 671 patients were enrolled. Overall, complications were equally distributed between the compared antibiotics (22.6% versus 27.6%; P = 0.17). Bacteriuria was more frequently reported in patients receiving fosfomycin (8.6% versus 4.2%; P = 0.02), but resistance was more frequently reported after ciprofloxacin treatment (41.9% versus 69.2%; P = 0.0004). Both treatments were well tolerated (254).
In another, nonrandomized trial, fosfomycin prophylaxis after prostate biopsy was associated with fewer UTIs (5/104; 4.8%) than both levofloxacin (12/110; 10.9%) and ciprofloxacin (53/406; 13.1%), but microbiological failure was not significantly different (255). It seems that the administration of oral fosfomycin (3 g) 1 to 4 h prior to prostate biopsy results in concentrations in the prostate that are adequate to prevent infections due to bacteria with an MIC of <4 mg/liter (256).
Oral fosfomycin was also studied for the prevention of subsequent infections in 152 patients with recurrent UTIs. Fosfomycin at 3 g every week was compared to prulifloxacin at 600 mg every week, for a total of 12 doses. At the end of 3 months, recurrent UTI occurred in 50% of patients receiving fosfomycin and 63% of patients in the prulifloxacin arm; 9 months later, the corresponding figures were 68% and 73%, respectively (257).
Fosfomycin was evaluated in combination with metronidazole for prophylaxis in colorectal surgery in several RCTs published more than 2 decades ago, (125, 258) as well as for prophylaxis in upper gastrointestinal tract and hepatobiliary procedures (259). Although fosfomycin was as effective as comparators in these RCTs, it is not included in the recommended regimens in relevant guidelines (260–262). Finally, in an RCT, fosfomycin was compared with cefuroxime for knee arthroplasty. None of the fosfomycin-treated patients had an infection 6 months after surgery, compared to 1 patient presenting with superficial wound infection in the cefuroxime group (263).
Inhaled Preparations
The effectiveness of inhaled fosfomycin in combination with tobramycin for the treatment of patients with cystic fibrosis and chronic P. aeruginosa infection was evaluated in a double-blind, randomized, placebo-controlled trial. Patients were initially treated with inhaled aztreonam for 28 days (5). Two different dosage schemes (80/20 mg or 160/40 mg) were studied against placebo for another 28 days. Patients receiving both schemes of fosfomycin and tobramycin combinations showed significantly less decline in values for median forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) at the end of treatment and the time of randomization than patients who received the placebo (5). Changes in P. aeruginosa density in sputum between the end of treatment and the time of randomization were not significantly different between patients receiving the 160/40 mg fosfomycin-tobramycin combination and the placebo group (mean, 0.39 log10 CFU/g versus 0.67 log10 CFU/g; P = 0.48), but it was different between the group receiving the 80/20 mg fosfomycin-tobramycin combination and the placebo group (mean, −0.37 log10 CFU/g versus 0.67 log10 CFU/g; P = 0.01). The need for more antipseudomonal antibiotics, hospitalizations, and missed days at work or school during the study were not significantly different between the antibiotic combination groups at either dose and the placebo group (5). The efficacy of the amikacin-fosfomycin combination in mechanically ventilated patients for prophylaxis in colonized patients or treatment in patients with pneumonia is under evaluation in a clinical trial (NCT01969799).
ADVERSE EVENTS
Fosfomycin is contraindicated when known hypersensitivity exists, but it is generally considered safe (4). Mild and self-limited gastrointestinal disturbances, such as diarrhea (including from Clostridium difficile), nausea, abdominal pain, and dyspepsia, are the most common adverse events following oral administration. Headaches, dizziness, infections of the upper respiratory tract, vaginitis, and bacterial or fungal superinfections have been also reported. Transient laboratory alterations concern all blood series (neutropenia, eosinophilia, anemia, and low platelet count) and increases in liver enzymes and bilirubin but not renal function (22). In fact, studies in rats have shown that fosfomycin protects against aminoglycoside nephrotoxicity by inhibiting aminoglycoside-induced histamine release following mast cell destruction (265). Similar findings regarding vancomycin, amphotericin B, and cisplatin nephrotoxicity have been published (266–268). Clinical data confirming these observations are not available. In a meta-analysis of RCTs comparing oral fosfomycin with other antibiotics for the treatment of patients with lower UTIs, no significant difference was observed in the development of adverse events or withdrawals from the studies. However, fosfomycin was associated with fewer adverse events among pregnant women (130).
Sodium overload and hypokalemia are listed among the potential adverse events after i.v. administration. Every gram of i.v. fosfomycin contains 0.32 g of sodium (222). In addition, fosfomycin is thought to increase potassium urinary excretion in the distal part of the renal tubules. In a French study, hypokalemia was reported in 26% of patients (19/72) (243). The authors reported that while potassium was administered in all patients, hypokalemia was found only when fosfomycin was administered in 30- to 60-min infusions, while it did not occur when the period of administration was extended to 4 h. Other adverse events reported in that study were infusion site reactions (4%), heart failure and hypertension due to sodium overload (6%), and alanine aminotransferase increase (1%) (243). Thus, potassium supplements should be administered and its levels monitored regularly in patients receiving fosfomycin. Caution is also required in patients with heart failure.
Finally, fosfomycin was not mutagenic or genotoxic in the Ames test in cultured human cells (lymphocytes), in Chinese hamster cells, and the in vivo mouse micronucleus assay. Fosfomycin did not affect fertility or reproductive performance in male and female rats (4).
CONCLUDING REMARKS
Fosfomycin has been used since its discovery mainly for the treatment of outpatients with UTIs. It has a unique mechanism of action that makes cross-resistance uncommon and allows for synergy with other antibiotics. In addition, it has a broad spectrum of activity and is still active against several of the contemporary problematic antibiotic-resistant bacteria. It penetrates adequately, and its levels are maintained in human tissues. Thus, interest in its effectiveness against MDR or XDR nosocomial infections, when limited treatment options are available, has been reawakened. There is also interest in its potential synergistic activity with glycopeptides, rifampin, or daptomycin against MRSA infections as well as in monotherapy against infections with ESBL-producing Enterobacteriaceae. Although the current microbiological data favor its use, there are few clinical data regarding both effectiveness and safety. The currently available data derive from case series or small cohorts, in which fosfomycin was administered mainly in combination with other antibiotics. In addition, it may prove to be useful for the treatment of other infections, such as H. pylori infection, when first-line antibiotic regimens fail. Also, there is a lack of data comparing the trometamol and calcium salt oral preparations. Finally, its safety profile should be better studied in order to avoid serious adverse events such as hypokalemia and congestive heart failure.
There is insufficient evidence for widely accepted breakpoints for all bacteria besides Enterobacteriaceae, Staphylococcus spp., and E. faecalis. Thus, most studies extrapolate these breakpoints to other bacteria, such as P. aeruginosa, which is classified by the CLSI as inherently resistant to fosfomycin but for which several studies reported variable susceptibility rates. Accordingly, discordance between susceptibility to fosfomycin and effectiveness of fosfomycin monotherapy against UTIs due to P. aeruginosa was reported. In the same time, synergy and higher effectiveness was reported when combination treatment with other antibiotics was employed. In addition, there is a discrepancy between EUCAST and CLSI criteria for susceptibility, making interpretation and comparison of results from different studies difficult. Concerns over the potential development of resistance should prompt clinicians to use it judiciously in order to prevent the development of resistance inside hospitals and to prevent the dissemination of resistant strains from outpatients to inpatients.
In an era of antibiotic resistance and limited new treatment options, interest in fosfomycin is expected to culminate in the next decade. Several issues regarding effectiveness, safety, and resistance need to be addressed, namely, the susceptibility breakpoints, the appropriate dose and duration of administration for both oral and intravenous formulations, the effectiveness of oral fosfomycin for the treatment of complicated UTIs or non-UTIs, the everlasting question of the effectiveness of monotherapy and combination regimens (including existing [e.g., polymyxins, aminoglycosides, and glycopeptides] or forthcoming [e.g., combinations of β-lactams and new β-lactamase inhibitors, new aminoglycosides, or polypeptide antibiotics] antibiotics), and the concerns over increased probability of development of resistance during treatment. Finally, the intravenous formulation is not available in several countries, including the United States. In a recent case report, the oral formulation was used successfully for a bacteremic MDR infection (269).
Biographies

Matthew E. Falagas, M.D., M.Sc., D.Sc., is founder and director of the Alfa Institute of Biomedical Sciences (AIBS), Athens, Greece, Adjunct Associate Professor of Medicine at Tufts University School of Medicine, Boston, MA, and Director of the Department of Internal Medicine-Infectious Diseases, Iaso General Hospital, Iaso Group, Athens, Greece. His research interests include antimicrobial resistance and nosocomial infections. Prof. Falagas is the author of more than 650 peer-reviewed original papers, reviews, and editorials that received more than 30,000 citations. His current Hirsch index (h-index) is 87.

Evridiki Vouloumanou, born in 1984, studied medicine at the Medical School of the National and Kapodistrian University of Athens, Greece, during the period 2001 to 2007. She has been working as a research fellow in the Alfa Institute of Biomedical Sciences (AIBS) in Athens, Greece, since 2008, with a particular interest in pediatric infection diseases and antimicrobial agents. She currently holds a position as a pediatric resident in a tertiary care hospital in Athens, Greece.

George Samonis is a medical oncologist and infectious diseases specialist, Professor of Medicine, at the University of Crete, Greece. He studied at the University of Athens, Greece, and holds a doctoral degree from the same university. He has served at the M. D. Anderson Cancer Center in Houston, TX, USA, as a fellow and the University of Crete as Head of the Department of Hematology and Head of the Department of Internal Medicine of the University Hospital of Heraklion. His research resulted in 260 articles that have attracted ∼8,100 citations; he is coinventor of two antifungal formulations. He has participated in 300 congresses and has given 60 lectures at scientific institutions. Dr. Samonis has been on the editorial boards of three medical journals and is reviewer for several others. He is member of 15 medical societies and has been the President of the Hellenic Society of Medical Mycology. His main interest is infections in immunocompromised hosts, with emphasis on fungal infections.

Konstantinos Z. Vardakas was born in Kozani, Greece. He obtained his medical degree from the National University of Athens School of Medicine in 2002. He obtained his Ph.D. in prophylaxis against fungal infections in special populations from the University of Crete School of Medicine. He completed his training in internal medicine in 2010. He is currently senior researcher in infectious diseases at the Alfa Institute of Biomedical Sciences and internist at Iaso General Hospital in Athens, Greece. He is interested in the treatment of patients with multidrug-resistant Gram-negative and Gram-positive infections and in prophylaxis against fungal and microbial infections in special populations.
REFERENCES
- 1.Hendlin D, Stapley EO, Jackson M, Wallick H, Miller AK, Wolf FJ, Miller TW, Chaiet L, Kahan FM, Foltz EL, Woodruff HB, Mata JM, Hernandez S, Mochales S. 1969. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science 166:122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- 2.Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. 2008. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis 46:1069–1077. doi: 10.1086/527442. [DOI] [PubMed] [Google Scholar]
- 3.Frimodt-Moller N. 2010. Fosfomycin, p 935–944. In Grayson ML. (ed), Kucers' The use of antibiotics, 6th ed Edward Arnold Ltd, London, United Kingdom. [Google Scholar]
- 4.Paladin Labs. 2007. Monurol package insert. Paladin Labs, Quebec, Canada. [Google Scholar]
- 5.Trapnell BC, McColley SA, Kissner DG, Rolfe MW, Rosen JM, McKevitt M, Moorehead L, Montgomery AB, Geller DE. 2012. Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with pseudomonas airway infection. Am J Respir Crit Care Med 185:171–178. doi: 10.1164/rccm.201105-0924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLeod DL, Barker LM, Sutherland JL, Moss SC, Gurgel JL, Kenney TF, Burns JL, Baker WR. 2009. Antibacterial activities of a fosfomycin/tobramycin combination: a novel inhaled antibiotic for bronchiectasis. J Antimicrob Chemother 64:829–836. doi: 10.1093/jac/dkp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez DS, Tapia MO, Soraci AL. 2014. Fosfomycin: uses and potentialities in veterinary medicine. Open Vet J 4:26–43. [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez D, Soraci A, Tapia MO. 2013. Tissue disposition and withdrawal time of fosfomycin in swines after oral and intramuscular administration. J Anim Prod Adv 3:107–119. doi: 10.5455/japa.20130407054809. [DOI] [Google Scholar]
- 9.Skarzynski T, Mistry A, Wonacott A, Hutchinson SE, Kelly VA, Duncan K. 1996. Structure of UDP-N-acetylglucosamine enolpyruvyl transferase, an enzyme essential for the synthesis of bacterial peptidoglycan, complexed with substrate UDP-N-acetylglucosamine and the drug fosfomycin. Structure 4:1465–1474. doi: 10.1016/S0969-2126(96)00153-0. [DOI] [PubMed] [Google Scholar]
- 10.Borisova M, Gisin J, Mayer C. 2014. Blocking peptidoglycan recycling in Pseudomonas aeruginosa attenuates intrinsic resistance to fosfomycin. Microb Drug Resist 20:231–237. doi: 10.1089/mdr.2014.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eschenburg S, Priestman M, Schonbrunn E. 2005. Evidence that the fosfomycin target Cys115 in UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) is essential for product release. J Biol Chem 280:3757–3763. doi: 10.1074/jbc.M411325200. [DOI] [PubMed] [Google Scholar]
- 12.Kahan FM, Kahan JS, Cassidy PJ, Kropp H. 1974. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci 235:364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- 13.Petek M, Baebler S, Kuzman D, Rotter A, Podlesek Z, Gruden K, Ravnikar M, Urleb U. 2010. Revealing fosfomycin primary effect on Staphylococcus aureus transcriptome: modulation of cell envelope biosynthesis and phosphoenolpyruvate induced starvation. BMC Microbiol 10:159. doi: 10.1186/1471-2180-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlone NA, Borsotto M, Cuffini AM, Savoia D. 1987. Effect of fosfomycin trometamol on bacterial adhesion in comparison with other chemotherapeutic agents. Eur Urol 13(Suppl 1):S86–S91. [DOI] [PubMed] [Google Scholar]
- 15.Yokota S, Okabayashi T, Yoto Y, Hori T, Tsutsumi H, Fujii N. 2010. Fosfomycin suppresses RS-virus-induced Streptococcus pneumoniae and Haemophilus influenzae adhesion to respiratory epithelial cells via the platelet-activating factor receptor. FEMS Microbiol Lett 310:84–90. doi: 10.1111/j.1574-6968.2010.02049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto T, Tateda K, Miyazaki S, Furuya N, Ohno A, Ishii Y, Hirakata Y, Yamaguchi K. 1999. Fosfomycin alters lipopolysaccharide-induced inflammatory cytokine production in mice. Antimicrob Agents Chemother 43:697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morikawa K, Watabe H, Araake M, Morikawa S. 1996. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother 40:1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morikawa K, Zhang J, Nonaka M, Morikawa S. 2002. Modulatory effect of macrolide antibiotics on the Th1- and Th2-type cytokine production. Int J Antimicrob Agents 19:53–59. doi: 10.1016/S0924-8579(01)00457-5. [DOI] [PubMed] [Google Scholar]
- 19.Sauermann R, Marsik C, Steiner I, Seir K, Cvitko T, Zeitlinger M, Wagner O, Joukhadar C. 2007. Immunomodulatory effects of fosfomycin in experimental human endotoxemia. Antimicrob Agents Chemother 51:1879–1881. doi: 10.1128/AAC.00914-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morikawa K, Oseko F, Morikawa S, Sawada M. 1993. Immunosuppressive activity of fosfomycin on human T-lymphocyte function in vitro. Antimicrob Agents Chemother 37:2684–2687. doi: 10.1128/AAC.37.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honda J, Okubo Y, Kusaba M, Kumagai M, Saruwatari N, Oizumi K. 1998. Fosfomycin (FOM: 1 R-2S-epoxypropylphosphonic acid) suppress the production of IL-8 from monocytes via the suppression of neutrophil function. Immunopharmacology 39:149–155. doi: 10.1016/S0162-3109(98)00003-4. [DOI] [PubMed] [Google Scholar]
- 22.Michalopoulos AS, Livaditis IG, Gougoutas V. 2011. The revival of fosfomycin. Int J Infect Dis 15:e732–739. doi: 10.1016/j.ijid.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Perez Fernandez P, Herrera I, Martinez P, Gomez-Lus ML, Prieto J. 1995. Enhancement of the susceptibility of Staphylococcus aureus to phagocytosis after treatment with fosfomycin compared with other antimicrobial agents. Chemotherapy 41:45–49. doi: 10.1159/000239323. [DOI] [PubMed] [Google Scholar]
- 24.Tullio V, Cuffini AM, Banche G, Mandras N, Allizond V, Roana J, Giacchino F, Bonello F, Ungheri D, Carlone NA. 2008. Role of fosfomycin tromethamine in modulating non-specific defence mechanisms in chronic uremic patients towards ESBL-producing Escherichia coli. Int J Immunopathol Pharmacol 21:153–160. [DOI] [PubMed] [Google Scholar]
- 25.Krause R, Patruta S, Daxbock F, Fladerer P, Wenisch C. 2001. The effect of fosfomycin on neutrophil function. J Antimicrob Chemother 47:141–146. doi: 10.1093/jac/47.2.141. [DOI] [PubMed] [Google Scholar]
- 26.Anderson GG, Kenney TF, Macleod DL, Henig NR, O'Toole GA. 2013. Eradication of Pseudomonas aeruginosa biofilms on cultured airway cells by a fosfomycin/tobramycin antibiotic combination. Pathog Dis 67:39–45. doi: 10.1111/2049-632X.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai Y, Fan Y, Wang R, An MM, Liang BB. 2009. Synergistic effects of aminoglycosides and fosfomycin on Pseudomonas aeruginosa in vitro and biofilm infections in a rat model. J Antimicrob Chemother 64:563–566. doi: 10.1093/jac/dkp224. [DOI] [PubMed] [Google Scholar]
- 28.Corvec S, Furustrand Tafin U, Betrisey B, Borens O, Trampuz A. 2013. Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-beta-lactamase-producing Escherichia coli in a foreign-body infection model. Antimicrob Agents Chemother 57:1421–1427. doi: 10.1128/AAC.01718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mihailescu R, Furustrand Tafin U, Corvec S, Oliva A, Betrisey B, Borens O, Trampuz A. 2014. High activity of fosfomycin and rifampin against methicillin-resistant staphylococcus aureus biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother 58:2547–2553. doi: 10.1128/AAC.02420-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliva A, Furustrand Tafin U, Maiolo EM, Jeddari S, Betrisey B, Trampuz A. 2014. Activities of fosfomycin and rifampin on planktonic and adherent Enterococcus faecalis strains in an experimental foreign-body infection model. Antimicrob Agents Chemother 58:1284–1293. doi: 10.1128/AAC.02583-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J, Mao NF, Wang L, Zhang HB, Chen Q, Liu H, Tang X, Jin T, Zhu CT, Li FB, Sun LH, Xu XM, Xu YQ. 2014. Efficacy of combined vancomycin and fosfomycin against methicillin-resistant Staphylococcus aureus in biofilms in vivo. PLoS One 9:e113133. doi: 10.1371/journal.pone.0113133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajdu S, Lassnigg A, Graninger W, Hirschl AM, Presterl E. 2009. Effects of vancomycin, daptomycin, fosfomycin, tigecycline, and ceftriaxone on Staphylococcus epidermidis biofilms. J Orthop Res 27:1361–1365. doi: 10.1002/jor.20902. [DOI] [PubMed] [Google Scholar]
- 33.Presterl E, Hajdu S, Lassnigg AM, Hirschl AM, Holinka J, Graninger W. 2009. Effects of azithromycin in combination with vancomycin, daptomycin, fosfomycin, tigecycline, and ceftriaxone on Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 53:3205–3210. doi: 10.1128/AAC.01628-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikuniya T, Kato Y, Ida T, Maebashi K, Monden K, Kariyama R, Kumon H. 2007. Treatment of Pseudomonas aeruginosa biofilms with a combination of fluoroquinolones and fosfomycin in a rat urinary tract infection model. J Infect Chemother 13:285–290. doi: 10.1007/s10156-007-0534-7. [DOI] [PubMed] [Google Scholar]
- 35.Marchese A, Bozzolasco M, Gualco L, Debbia EA, Schito GC, Schito AM. 2003. Effect of fosfomycin alone and in combination with N-acetylcysteine on E. coli biofilms. Int J Antimicrob Agents 22(Suppl 2):S95–S100. [DOI] [PubMed] [Google Scholar]
- 36.Descourouez JL, Jorgenson MR, Wergin JE, Rose WE. 2013. Fosfomycin synergy in vitro with amoxicillin, daptomycin, and linezolid against vancomycin-resistant Enterococcus faecium from renal transplant patients with infected urinary stents. Antimicrob Agents Chemother 57:1518–1520. doi: 10.1128/AAC.02099-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barry AL, Brown SD. 1995. Antibacterial spectrum of fosfomycin trometamol. J Antimicrob Chemother 35:228–230. doi: 10.1093/jac/35.1.228. [DOI] [PubMed] [Google Scholar]
- 38.Patel SS, Balfour JA, Bryson HM. 1997. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 53:637–656. [DOI] [PubMed] [Google Scholar]
- 39.Fukuyama M, Furuhata K, Oonaka K, Hara T, Sunakawa K. 2000. Antibacterial activity of fosfomycin against the causative bacteria isolated from bacterial enteritis. Jpn J Antibiot 53:522–531. [PubMed] [Google Scholar]
- 40.Samonis G, Maraki S, Rafailidis PI, Kapaskelis A, Kastoris AC, Falagas ME. 2010. Antimicrobial susceptibility of Gram-negative nonurinary bacteria to fosfomycin and other antimicrobials. Future Microbiol 5:961–970. doi: 10.2217/fmb.10.47. [DOI] [PubMed] [Google Scholar]
- 41.Stock I, Wiedemann B. 1999. Natural antibiotic susceptibility of Escherichia coli, Shigella, E vulneris, and E hermannii strains. Diagn Microbiol Infect Dis 33:187–199. doi: 10.1016/S0732-8893(98)00146-1. [DOI] [PubMed] [Google Scholar]
- 42.Barahona-Garrido J, Quinonez NF, Cerda-Contreras E, Maria Sarti H, Tellez-Avila FI. 2013. Fosfomycin-containing second-line treatment for Helicobacter pylori infection. Am J Gastroenterol 108:858–859. doi: 10.1038/ajg.2013.48. [DOI] [PubMed] [Google Scholar]
- 43.Hirzel C, Guilarte YN, Hirzberger L, Furrer H, Marschall J, Endimiani A. 2015. In vitro susceptibility of Aerococcus urinae isolates to antibiotics used for uncomplicated urinary tract infection. J Infect 71:395–397. doi: 10.1016/j.jinf.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Hauser C, Hirzberger L, Unemo M, Furrer H, Endimiani A. 2015. In vitro activity of fosfomycin alone and in combination with ceftriaxone or azithromycin against clinical Neisseria gonorrhoeae isolates. Antimicrob Agents Chemother 59:1605–1611. doi: 10.1128/AAC.04536-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lepe JA, Torres MJ, Smani Y, Parra-Millan R, Pachon J, Vazquez-Barba I, Aznar J. 2014. In vitro and intracellular activities of fosfomycin against clinical strains of Listeria monocytogenes. Int J Antimicrob Agents 43:135–139. doi: 10.1016/j.ijantimicag.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Altes Gutierrez A, Rodriguez Noriega A. 1977. In vitro sensitivity of anaerobic bacteria to fosfomycin. Chemotherapy 23(Suppl 1):S51–S57. [DOI] [PubMed] [Google Scholar]
- 47.Piriz S, Cuenca R, Valle J, Vadillo S. 1992. Susceptibilities of anaerobic bacteria isolated from animals with ovine foot rot to 28 antimicrobial agents. Antimicrob Agents Chemother 36:198–201. doi: 10.1128/AAC.36.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.CLSI. 2015. Performance standards for antimicrobial susceptibility testing. Document M100-S25; twenty-fifth informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 49.De Smet KA, Kempsell KE, Gallagher A, Duncan K, Young DB. 1999. Alteration of a single amino acid residue reverses fosfomycin resistance of recombinant MurA from Mycobacterium tuberculosis. Microbiology 145:3177–3184. doi: 10.1099/00221287-145-11-3177. [DOI] [PubMed] [Google Scholar]
- 50.Stock I, Wiedemann B. 1998. Identification and natural antibiotic susceptibility of Morganella morganii. Diagn Microbiol Infect Dis 30:153–165. doi: 10.1016/S0732-8893(97)00243-5. [DOI] [PubMed] [Google Scholar]
- 51.Guggenbichler JP, Bonatti H, Rottensteiner F. 1989. Resistance of staphylococci to intracellular killing by macrophages—a new pathophysiologic concept of acute hematogenous osteomyelitis in childhood and its therapeutic consequences. Padiatr Padol 24:21–32. [PubMed] [Google Scholar]
- 52.Trautmann M, Meincke C, Vogt K, Ruhnke M, Lajous-Petter AM. 1992. Intracellular bactericidal activity of fosfomycin against staphylococci: a comparison with other antibiotics. Infection 20:350–354. doi: 10.1007/BF01710683. [DOI] [PubMed] [Google Scholar]
- 53.Valour F, Trouillet-Assant S, Riffard N, Tasse J, Flammier S, Rasigade JP, Chidiac C, Vandenesch F, Ferry T, Laurent F. 2015. Antimicrobial activity against intraosteoblastic Staphylococcus aureus. Antimicrob Agents Chemother 59:2029–2036. doi: 10.1128/AAC.04359-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okada N, Nishio M, Danbara H. 2003. Intracellular activity of fosfomycin against two distinct enteropathogenic bacteria, Salmonella enterica and Listeria monocytogenes, alive inside host cells. Chemotherapy 49:49–55. doi: 10.1159/000069787. [DOI] [PubMed] [Google Scholar]
- 55.Kihlstrom E, Andaker L. 1985. Inability of gentamicin and fosfomycin to eliminate intracellular Enterobacteriaceae. J Antimicrob Chemother 15:723–728. doi: 10.1093/jac/15.6.723. [DOI] [PubMed] [Google Scholar]
- 56.Falagas ME, Maraki S, Karageorgopoulos DE, Kastoris AC, Kapaskelis A, Samonis G. 2010. Antimicrobial susceptibility of Gram-positive non-urinary isolates to fosfomycin. Int J Antimicrob Agents 35:497–499. doi: 10.1016/j.ijantimicag.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 57.Falagas ME, Maraki S, Karageorgopoulos DE, Kastoris AC, Mavromanolakis E, Samonis G. 2010. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents 35:240–243. doi: 10.1016/j.ijantimicag.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 58.Maraki S, Samonis G, Rafailidis PI, Vouloumanou EK, Mavromanolakis E, Falagas ME. 2009. Susceptibility of urinary tract bacteria to fosfomycin. Antimicrob Agents Chemother 53:4508–4510. doi: 10.1128/AAC.00721-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samonis G, Maraki S, Karageorgopoulos DE, Vouloumanou EK, Falagas ME. 2012. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur J Clin Microbiol Infect Dis 31:695–701. doi: 10.1007/s10096-011-1360-5. [DOI] [PubMed] [Google Scholar]
- 60.Barry AL, Fuchs PC. 1991. In vitro susceptibility testing procedures for fosfomycin tromethamine. Antimicrob Agents Chemother 35:1235–1238. doi: 10.1128/AAC.35.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diez-Aguilar M, Morosini MI, del Campo R, Garcia-Castillo M, Zamora J, Canton R. 2013. In vitro activity of fosfomycin against a collection of clinical Pseudomonas aeruginosa isolates from 16 Spanish hospitals: establishing the validity of standard broth microdilution as susceptibility testing method. Antimicrob Agents Chemother 57:5701–5703. doi: 10.1128/AAC.00589-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Cueto M, Lopez L, Hernandez JR, Morillo C, Pascual A. 2006. In vitro activity of fosfomycin against extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: comparison of susceptibility testing procedures. Antimicrob Agents Chemother 50:368–370. doi: 10.1128/AAC.50.1.368-370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu CL, Liu CY, Huang YT, Liao CH, Teng LJ, Turnidge JD, Hsueh PR. 2011. Antimicrobial susceptibilities of commonly encountered bacterial isolates to fosfomycin determined by agar dilution and disk diffusion methods. Antimicrob Agents Chemother 55:4295–4301. doi: 10.1128/AAC.00349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perdigao-Neto LV, Oliveira MS, Rizek CF, Carrilho CM, Costa SF, Levin AS. 2014. Susceptibility of multiresistant gram-negative bacteria to fosfomycin and performance of different susceptibility testing methods. Antimicrob Agents Chemother 58:1763–1767. doi: 10.1128/AAC.02048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Falagas ME, Roussos N, Gkegkes ID, Rafailidis PI, Karageorgopoulos DE. 2009. Fosfomycin for the treatment of infections caused by Gram-positive cocci with advanced antimicrobial drug resistance: a review of microbiological, animal and clinical studies. Expert Opin Invest Drugs 18:921–944. doi: 10.1517/13543780902967624. [DOI] [PubMed] [Google Scholar]
- 66.Falagas ME, Kastoris AC, Karageorgopoulos DE, Rafailidis PI. 2009. Fosfomycin for the treatment of infections caused by multidrug-resistant non-fermenting Gram-negative bacilli: a systematic review of microbiological, animal and clinical studies. Int J Antimicrob Agents 34:111–120. doi: 10.1016/j.ijantimicag.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 67.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. 2010. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis 10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 68.Araj GF, Jaber FA. 2012. In vitro activity of fosfomycin and other antimicrobials against uropathogenic Escherichia coli and Klebsiella pneumoniae at a tertiary care center in Lebanon. J Med Liban 60:142–147. [PubMed] [Google Scholar]
- 69.Asencio MA, Huertas M, Carranza R, Franco M, Castellanos J, Barbera JR, Conde Mdel C, Tenias JM. 2014. Trend in the susceptibility of the most frequent bacterial pathogens isolated at Hospital General La Mancha Centro over 2010-2012 period. Rev Esp Quimioter 27:261–268. [PubMed] [Google Scholar]
- 70.Briongos-Figuero LS, Gomez-Traveso T, Bachiller-Luque P, Dominguez-Gil Gonzalez M, Gomez-Nieto A, Palacios-Martin T, Gonzalez-Sagrado M, Duenas-Laita A, Perez-Castrillon JL. 2012. Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended-spectrum beta-lactamase (ESBL)-producing enterobacteria. Int J Clin Pract 66:891–896. doi: 10.1111/j.1742-1241.2012.02991.x. [DOI] [PubMed] [Google Scholar]
- 71.Cagan Aktas S, Gencer S, Batirel A, Haciseyitoglu D, Ozer S. 2014. Fosfomycin susceptibility of urinary Escherichia coli isolates producing extended-spectrum beta-lactamase according to CLSI and EUCAST recommendations. Mikrobiyol Bul 48:545–555. doi: 10.5578/mb.8327. [DOI] [PubMed] [Google Scholar]
- 72.Champion EA, Miller MB, Popowitch EB, Hobbs MM, Saiman L, Muhlebach MS. 2014. Antimicrobial susceptibility and molecular typing of MRSA in cystic fibrosis. Pediatr Pulmonol 49:230–237. doi: 10.1002/ppul.22815. [DOI] [PubMed] [Google Scholar]
- 73.Cho YH, Jung SI, Chung HS, Yu HS, Hwang EC, Kim SO, Kang TW, Kwon DD, Park K. 2015. Antimicrobial susceptibilities of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in health care-associated urinary tract infection: focus on susceptibility to fosfomycin. Int Urol Nephrol 47:1059–1066. doi: 10.1007/s11255-015-1018-9. [DOI] [PubMed] [Google Scholar]
- 74.Hsu MS, Liao CH, Liu CY, Yang CJ, Huang YT, Hsueh PR. 2011. In vitro susceptibilities of clinical isolates of ertapenem-non-susceptible Enterobacteriaceae to nemonoxacin, tigecycline, fosfomycin and other antimicrobial agents. Int J Antimicrob Agents 37:276–278. doi: 10.1016/j.ijantimicag.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 75.Jiang Y, Shen P, Wei Z, Liu L, He F, Shi K, Wang Y, Wang H, Yu Y. 2015. Dissemination of a clone carrying a fosA3-harbouring plasmid mediates high fosfomycin resistance rate of KPC-producing Klebsiella pneumoniae in China. Int J Antimicrob Agents 45:66–70. doi: 10.1016/j.ijantimicag.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 76.Kahlmeter G, Poulsen HO. 2012. Antimicrobial susceptibility of Escherichia coli from community-acquired urinary tract infections in Europe: the ECO.SENS study revisited. Int J Antimicrob Agents 39:45–51. doi: 10.1016/j.ijantimicag.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 77.Karlowsky JA, Denisuik AJ, Lagace-Wiens PR, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. 2014. In vitro activity of fosfomycin against Escherichia coli isolated from patients with urinary tract infections in Canada as part of the CANWARD surveillance study. Antimicrob Agents Chemother 58:1252–1256. doi: 10.1128/AAC.02399-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khan IU, Mirza IA, Ikram A, Ali S, Hussain A, Ghafoor T. 2014. In vitro activity of fosfomycin tromethamine against extended spectrum beta-lactamase producing urinary tract bacteria. J Coll Physicians Surg Pak 24:914–917. [PubMed] [Google Scholar]
- 79.Lai B, Zheng B, Li Y, Zhu S, Tong Z. 2014. In vitro susceptibility of Escherichia coli strains isolated from urine samples obtained in mainland China to fosfomycin trometamol and other antibiotics: a 9-year surveillance study (2004-2012). BMC Infect Dis 14:66. doi: 10.1186/1471-2334-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee SY, Park YJ, Yu JK, Jung S, Kim Y, Jeong SH, Arakawa Y. 2012. Prevalence of acquired fosfomycin resistance among extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae clinical isolates in Korea and IS26-composite transposon surrounding fosA3. J Antimicrob Chemother 67:2843–2847. doi: 10.1093/jac/dks319. [DOI] [PubMed] [Google Scholar]
- 81.Morfin-Otero R, Mendoza-Olazaran S, Silva-Sanchez J, Rodriguez-Noriega E, Laca-Diaz J, Tinoco-Carrillo P, Petersen L, Lopez P, Reyna-Flores F, Alcantar-Curiel D, Garza-Ramos U, Garza-Gonzalez E. 2013. Characterization of Enterobacteriaceae isolates obtained from a tertiary care hospital in Mexico, which produces extended-spectrum beta-lactamase. Microb Drug Resist 19:378–383. doi: 10.1089/mdr.2012.0263. [DOI] [PubMed] [Google Scholar]
- 82.Pogue JM, Marchaim D, Abreu-Lanfranco O, Sunkara B, Mynatt RP, Zhao JJ, Bheemreddy S, Hayakawa K, Martin ET, Dhar S, Kaye KS, Lephart PR. 2013. Fosfomycin activity versus carbapenem-resistant Enterobacteriaceae and vancomycin-resistant Enterococcus, Detroit, 2008-10. J Antibiot (Tokyo) 66:625–627. doi: 10.1038/ja.2013.56. [DOI] [PubMed] [Google Scholar]
- 83.Rebiahi SA, Abdelouahid DE, Rahmoun M, Abdelali S, Azzaoui H. 2011. Emergence of vancomycin-resistant Staphylococcus aureus identified in the Tlemcen university hospital (North-West Algeria). Med Mal Infect 41:646–651. doi: 10.1016/j.medmal.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 84.Sahni RD, Balaji V, Varghese R, John J, Tansarli GS, Falagas ME. 2013. Evaluation of fosfomycin activity against uropathogens in a fosfomycin-naive population in South India: a prospective study. Future Microbiol 8:675–680. doi: 10.2217/fmb.13.31. [DOI] [PubMed] [Google Scholar]
- 85.Sorlozano A, Jimenez-Pacheco A, de Dios Luna Del Castillo J, Sampedro A, Martinez-Brocal A, Miranda-Casas C, Navarro-Mari JM, Gutierrez-Fernandez J. 2014. Evolution of the resistance to antibiotics of bacteria involved in urinary tract infections: a 7-year surveillance study. Am J Infect Control 42:1033–1038. doi: 10.1016/j.ajic.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 86.Sultan A, Rizvi M, Khan F, Sami H, Shukla I, Khan HM. 2015. Increasing antimicrobial resistance among uropathogens: is fosfomycin the answer? Urol Ann 7:26–30. doi: 10.4103/0974-7796.148585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taj Y, Abdullah FE, Kazmi SU. 2010. Current pattern of antibiotic resistance in Staphylococcus aureus clinical isolates and the emergence of vancomycin resistance. J Coll Physicians Surg Pak 20:728–732. [PubMed] [Google Scholar]
- 88.Tuon FF, Rocha JL, Formighieri MS, Sfair S, Bertoldi MB, Palmeiro JK, Dalla Costa LM. 2013. Fosfomycin susceptibility of isolates with blaKPC-2 from Brazil. J Infect 67:247–249. doi: 10.1016/j.jinf.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 89.Villar HE, Jugo MB, Macan A, Visser M, Hidalgo M, Maccallini GC. 2014. Frequency and antibiotic susceptibility patterns of urinary pathogens in male outpatients in Argentina. J Infect Dev Ctries 8:699–704. doi: 10.3855/jidc.3766. [DOI] [PubMed] [Google Scholar]
- 90.Yu XH, Song XJ, Cai Y, Liang BB, Lin DF, Wang R. 2010. In vitro activity of two old antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus. J Antibiot (Tokyo) 63:657–659. doi: 10.1038/ja.2010.105. [DOI] [PubMed] [Google Scholar]
- 91.Tang HJ, Chen CC, Zhang CC, Su BA, Li CM, Weng TC, Chiang SR, Ko WC, Chuang YC. 2013. In vitro efficacy of fosfomycin-based combinations against clinical vancomycin-resistant Enterococcus isolates. Diagn Microbiol Infect Dis 77:254–257. doi: 10.1016/j.diagmicrobio.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 92.Takahata S, Ida T, Hiraishi T, Sakakibara S, Maebashi K, Terada S, Muratani T, Matsumoto T, Nakahama C, Tomono K. 2010. Molecular mechanisms of fosfomycin resistance in clinical isolates of Escherichia coli. Int J Antimicrob Agents 35:333–337. doi: 10.1016/j.ijantimicag.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 93.Endimiani A, Patel G, Hujer KM, Swaminathan M, Perez F, Rice LB, Jacobs MR, Bonomo RA. 2010. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 54:526–529. doi: 10.1128/AAC.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rizek C, Ferraz JR, van der Heijden IM, Giudice M, Mostachio AK, Paez J, Carrilho C, Levin AS, Costa SF. 2015. In vitro activity of potential old and new drugs against multidrug-resistant gram-negatives. J Infect Chemother 21:114–117. doi: 10.1016/j.jiac.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 95.Li JJ, Sheng ZK, Deng M, Bi S, Hu FS, Miao HF, Ji ZK, Sheng JF, Li LJ. 2012. Epidemic of Klebsiella pneumoniae ST11 clone coproducing KPC-2 and 16S rRNA methylase RmtB in a Chinese university hospital. BMC Infect Dis 12:373. doi: 10.1186/1471-2334-12-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karageorgopoulos DE, Wang R, Yu XH, Falagas ME. 2012. Fosfomycin: evaluation of the published evidence on the emergence of antimicrobial resistance in Gram-negative pathogens. J Antimicrob Chemother 67:255–268. doi: 10.1093/jac/dkr466. [DOI] [PubMed] [Google Scholar]
- 97.McCoy AJ, Sandlin RC, Maurelli AT. 2003. In vitro and in vivo functional activity of Chlamydia MurA, a UDP-N-acetylglucosamine enolpyruvyl transferase involved in peptidoglycan synthesis and fosfomycin resistance. J Bacteriol 185:1218–1228. doi: 10.1128/JB.185.4.1218-1228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar S, Parvathi A, Hernandez RL, Cadle KM, Varela MF. 2009. Identification of a novel UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) from Vibrio fischeri that confers high fosfomycin resistance in Escherichia coli. Arch Microbiol 191:425–429. doi: 10.1007/s00203-009-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gisin J, Schneider A, Nagele B, Borisova M, Mayer C. 2013. A cell wall recycling shortcut that bypasses peptidoglycan de novo biosynthesis. Nat Chem Biol 9:491–493. doi: 10.1038/nchembio.1289. [DOI] [PubMed] [Google Scholar]
- 100.Tsuruoka T, Miyata A, Yamada Y. 1978. Two kinds of mutants defective in multiple carbohydrate utilization isolated from in vitro fosfomycin-resistant strains of Escherichia coli K–12. J Antibiot (Tokyo) 31:192–201. doi: 10.7164/antibiotics.31.192. [DOI] [PubMed] [Google Scholar]
- 101.Horii T, Kimura T, Sato K, Shibayama K, Ohta M. 1999. Emergence of fosfomycin-resistant isolates of Shiga-like toxin-producing Escherichia coli O26. Antimicrob Agents Chemother 43:789–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arca P, Reguera G, Hardisson C. 1997. Plasmid-encoded fosfomycin resistance in bacteria isolated from the urinary tract in a multicentre survey. J Antimicrob Chemother 40:393–399. doi: 10.1093/jac/40.3.393. [DOI] [PubMed] [Google Scholar]
- 103.Nilsson AI, Berg OG, Aspevall O, Kahlmeter G, Andersson DI. 2003. Biological costs and mechanisms of fosfomycin resistance in Escherichia coli. Antimicrob Agents Chemother 47:2850–2858. doi: 10.1128/AAC.47.9.2850-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Venkateswaran PS, Wu HC. 1972. Isolation and characterization of a phosphonomycin-resistant mutant of Escherichia coli K-12. J Bacteriol 110:935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arca P, Hardisson C, Suarez JE. 1990. Purification of a glutathione S-transferase that mediates fosfomycin resistance in bacteria. Antimicrob Agents Chemother 34:844–848. doi: 10.1128/AAC.34.5.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arca P, Rico M, Brana AF, Villar CJ, Hardisson C, Suarez JE. 1988. Formation of an adduct between fosfomycin and glutathione: a new mechanism of antibiotic resistance in bacteria. Antimicrob Agents Chemother 32:1552–1556. doi: 10.1128/AAC.32.10.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bernat BA, Laughlin LT, Armstrong RN. 1997. Fosfomycin resistance protein (FosA) is a manganese metalloglutathione transferase related to glyoxalase I and the extradiol dioxygenases. Biochemistry 36:3050–3055. doi: 10.1021/bi963172a. [DOI] [PubMed] [Google Scholar]
- 108.Ma Y, Xu X, Guo Q, Wang P, Wang W, Wang M. 2015. Characterization of fosA5, a new plasmid-mediated fosfomycin resistance gene in Escherichia coli. Lett Appl Microbiol 60:259–264. doi: 10.1111/lam.12366. [DOI] [PubMed] [Google Scholar]
- 109.Wachino J, Yamane K, Suzuki S, Kimura K, Arakawa Y. 2010. Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob Agents Chemother 54:3061–3064. doi: 10.1128/AAC.01834-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu H, Miao V, Kwong W, Xia R, Davies J. 2011. Identification of a novel fosfomycin resistance gene (fosA2) in Enterobacter cloacae from the Salmon River, Canada. Lett Appl Microbiol 52:427–429. doi: 10.1111/j.1472-765X.2011.03016.x. [DOI] [PubMed] [Google Scholar]
- 111.Qin S, Fu Y, Zhang Q, Qi H, Wen JG, Xu H, Xu L, Zeng L, Tian H, Rong L, Li Y, Shan L, Yu Y, Feng X, Liu HM. 2014. High incidence and endemic spread of NDM-1-positive Enterobacteriaceae in Henan Province, China. Antimicrob Agents Chemother 58:4275–4282. doi: 10.1128/AAC.02813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tseng SP, Wang SF, Kuo CY, Huang JW, Hung WC, Ke GM, Lu PL. 2015. Characterization of fosfomycin resistant extended-spectrum beta-lactamase-producing Escherichia coli isolates from human and pig in Taiwan. PLoS One 10:e0135864. doi: 10.1371/journal.pone.0135864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Villa L, Guerra B, Schmoger S, Fischer J, Helmuth R, Zong Z, Garcia-Fernandez A, Carattoli A. 2015. IncA/C plasmid carrying blaNDM-1, blaCMY-16, and fosA3 in a Salmonella enterica serovar Corvallis strain isolated from a migratory wild bird in Germany. Antimicrob Agents Chemother 59:6597–6600. doi: 10.1128/AAC.00944-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao JY, Zhu YQ, Li YN, Mu XD, You LP, Xu C, Qin P, Ma JL. 2015. Coexistence of SFO-1 and NDM-1 beta-lactamase genes and fosfomycin resistance gene fosA3 in an Escherichia coli clinical isolate. FEMS Microbiol Lett 362:1–7. doi: 10.1093/femsle/fnu018.. [DOI] [PubMed] [Google Scholar]
- 115.Qu TT, Shi KR, Ji JS, Yang Q, Du XX, Wei ZQ, Yu YS. 2014. Fosfomycin resistance among vancomycin-resistant enterococci owing to transfer of a plasmid harbouring the fosB gene. Int J Antimicrob Agents 43:361–365. doi: 10.1016/j.ijantimicag.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 116.Xu X, Chen C, Lin D, Guo Q, Hu F, Zhu D, Li G, Wang M. 2013. The fosfomycin resistance gene fosB3 is located on a transferable, extrachromosomal circular intermediate in clinical Enterococcus faecium isolates. PLoS One 8:e78106. doi: 10.1371/journal.pone.0078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cao M, Bernat BA, Wang Z, Armstrong RN, Helmann JD. 2001. FosB, a cysteine-dependent fosfomycin resistance protein under the control of sigma(W), an extracytoplasmic-function sigma factor in Bacillus subtilis. J Bacteriol 183:2380–2383. doi: 10.1128/JB.183.7.2380-2383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Etienne J, Gerbaud G, Fleurette J, Courvalin P. 1991. Characterization of staphylococcal plasmids hybridizing with the fosfomycin resistance gene fosB. FEMS Microbiol Lett 68:119–122. [DOI] [PubMed] [Google Scholar]
- 119.Zilhao R, Courvalin P. 1990. Nucleotide sequence of the fosB gene conferring fosfomycin resistance in Staphylococcus epidermidis. FEMS Microbiol Lett 56:267–272. [DOI] [PubMed] [Google Scholar]
- 120.Fillgrove KL, Pakhomova S, Schaab MR, Newcomer ME, Armstrong RN. 2007. Structure and mechanism of the genomically encoded fosfomycin resistance protein, FosX, from Listeria monocytogenes. Biochemistry 46:8110–8120. doi: 10.1021/bi700625p. [DOI] [PubMed] [Google Scholar]
- 121.Garcia P, Arca P, Evaristo Suarez J. 1995. Product of fosC, a gene from Pseudomonas syringae, mediates fosfomycin resistance by using ATP as cosubstrate. Antimicrob Agents Chemother 39:1569–1573. doi: 10.1128/AAC.39.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duez JM, Mousson C, Siebor E, Péchinot A, Freysz M, Sixt N, Bador J, Neuwirth C. 2011. Fosfomycin and its application in the treatment of multidrug-resistant Enterobacteriaceae infections. Clin Med Rev Ther 3:123–142. [Google Scholar]
- 123.Engel H, Gutierrez-Fernandez J, Fluckiger C, Martinez-Ripoll M, Muhlemann K, Hermoso JA, Hilty M, Hathaway LJ. 2013. Heteroresistance to fosfomycin is predominant in Streptococcus pneumoniae and depends on the murA1 gene. Antimicrob Agents Chemother 57:2801–2808. doi: 10.1128/AAC.00223-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walsh CC, McIntosh MP, Peleg AY, Kirkpatrick CM, Bergen PJ. 2015. In vitro pharmacodynamics of fosfomycin against clinical isolates of Pseudomonas aeruginosa. J Antimicrob Chemother 70:3042–3050. doi: 10.1093/jac/dkv221. [DOI] [PubMed] [Google Scholar]
- 125.Olsson-Liljequist B, Burman LG. 1993. Introducing fosfomycin for surgical prophylaxis—emergence of resistance in aerobic faecal gram-negative bacteria of in-patients, but not among strains causing infection after elective colorectal procedures. Scand J Infect Dis 25:725–733. doi: 10.3109/00365549309008570. [DOI] [PubMed] [Google Scholar]
- 126.Giske CG. 2015. Contemporary resistance trends and mechanisms for the old antibiotics colistin, temocillin, fosfomycin, mecillinam and nitrofurantoin. Clin Microbiol Infect 21:899–905. doi: 10.1016/j.cmi.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 127.Ho PL, Chan J, Lo WU, Law PY, Li Z, Lai EL, Chow KH. 2013. Dissemination of plasmid-mediated fosfomycin resistance fosA3 among multidrug-resistant Escherichia coli from livestock and other animals. J Appl Microbiol 114:695–702. doi: 10.1111/jam.12099. [DOI] [PubMed] [Google Scholar]
- 128.Li Y, Zheng B, Zhu S, Xue F, Liu J. 2015. Antimicrobial susceptibility and molecular mechanisms of fosfomycin resistance in clinical Escherichia coli isolates in mainland China. PLoS One 10:e0135269. doi: 10.1371/journal.pone.0135269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oteo J, Orden B, Bautista V, Cuevas O, Arroyo M, Martinez-Ruiz R, Perez-Vazquez M, Alcaraz M, Garcia-Cobos S, Campos J. 2009. CTX-M-15-producing urinary Escherichia coli O25b-ST131-phylogroup B2 has acquired resistance to fosfomycin. J Antimicrob Chemother 64:712–717. doi: 10.1093/jac/dkp288. [DOI] [PubMed] [Google Scholar]
- 130.Falagas ME, Vouloumanou EK, Togias AG, Karadima M, Kapaskelis AM, Rafailidis PI, Athanasiou S. 2010. Fosfomycin versus other antibiotics for the treatment of cystitis: a meta-analysis of randomized controlled trials. J Antimicrob Chemother 65:1862–1877. doi: 10.1093/jac/dkq237. [DOI] [PubMed] [Google Scholar]
- 131.Boerema JB, Willems FT. 1990. Fosfomycin trometamol in a single dose versus norfloxacin for seven days in the treatment of uncomplicated urinary infections in general practice. Infection 18(Suppl 2):S80–S88. doi: 10.1007/BF01643433. [DOI] [PubMed] [Google Scholar]
- 132.Careddu P, Borzani M, Scotti L, Varotto F, Garlaschi L, Fontana P. 1987. Treatment of lower urinary tract infections in children: single dose fosfomycin trometamol versus pipemidic acid. Chemioterapia 6:290–294. [PubMed] [Google Scholar]
- 133.Gupta K, Hooton TM, Stamm WE. 2005. Isolation of fluoroquinolone-resistant rectal Escherichia coli after treatment of acute uncomplicated cystitis. J Antimicrob Chemother 56:243–246. doi: 10.1093/jac/dki169. [DOI] [PubMed] [Google Scholar]
- 134.Minassian MA, Lewis DA, Chattopadhyay D, Bovill B, Duckworth GJ, Williams JD. 1998. A comparison between single-dose fosfomycin trometamol (Monuril) and a 5-day course of trimethoprim in the treatment of uncomplicated lower urinary tract infection in women. Int J Antimicrob Agents 10:39–47. doi: 10.1016/S0924-8579(98)00021-1. [DOI] [PubMed] [Google Scholar]
- 135.Naber KG, Thyroff-Friesinger U. 1990. Fosfomycin trometamol versus ofloxacin/co-trimoxazole as single dose therapy of acute uncomplicated urinary tract infection in females: a multicentre study. Infection 18(Suppl 2):S70–S76. doi: 10.1007/BF01643431. [DOI] [PubMed] [Google Scholar]
- 136.Karageorgopoulos DE, Miriagou V, Tzouvelekis LS, Spyridopoulou K, Daikos GL. 2012. Emergence of resistance to fosfomycin used as adjunct therapy in KPC Klebsiella pneumoniae bacteraemia: report of three cases. J Antimicrob Chemother 67:2777–2779. doi: 10.1093/jac/dks270. [DOI] [PubMed] [Google Scholar]
- 137.Kastoris AC, Rafailidis PI, Vouloumanou EK, Gkegkes ID, Falagas ME. 2010. Synergy of fosfomycin with other antibiotics for Gram-positive and Gram-negative bacteria. Eur J Clin Pharmacol 66:359–368. doi: 10.1007/s00228-010-0794-5. [DOI] [PubMed] [Google Scholar]
- 138.Chang SC, Hsieh WC, Luh KT, Ho SW. 1989. Effects of antibiotic combinations on methicillin-resistant Staphylococcus aureus in vitro. Taiwan Yi Xue Hui Za Zhi 88:488–492. [PubMed] [Google Scholar]
- 139.Fosse T, David MF, Duluc F, Darmusey D, Tamalet C, Toga B. 1984. In vitro study of the cefamandole-fosfomycin combination against methicillin-resistant staphylococci. Pathol Biol (Paris) 32:528–531. [PubMed] [Google Scholar]
- 140.Utsui Y, Ohya S, Magaribuchi T, Tajima M, Yokota T. 1986. Antibacterial activity of cefmetazole alone and in combination with fosfomycin against methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother 30:917–922. doi: 10.1128/AAC.30.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakazawa H, Kikuchi Y, Honda T, Isago T, Nozaki M. 2003. Enhancement of antimicrobial effects of various antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) by combination with fosfomycin. J Infect Chemother 9:304–309. doi: 10.1007/s10156-003-0266-2. [DOI] [PubMed] [Google Scholar]
- 142.Hamilton-Miller JM. 1992. In vitro activity of fosfomycin against ‘problem’ gram-positive cocci. Microbios 71:95–103. [PubMed] [Google Scholar]
- 143.Sahuquillo Arce JM, Colombo Gainza E, Gil Brusola A, Ortiz Estevez R, Canton E, Gobernado M. 2006. In vitro activity of linezolid in combination with doxycycline, fosfomycin, levofloxacin, rifampicin and vancomycin against methicillin-susceptible Staphylococcus aureus. Rev Esp Quimioter 19:252–257. [PubMed] [Google Scholar]
- 144.Ullmann U. 1987. Synergism between ciprofloxacin and fosfomycin in vitro. Infection 15:264. [DOI] [PubMed] [Google Scholar]
- 145.Martinez-Martinez L, Rodriguez G, Pascual A, Suarez AI, Perea EJ. 1996. In-vitro activity of antimicrobial agent combinations against multiresistant Acinetobacter baumannii. J Antimicrob Chemother 38:1107–1108. doi: 10.1093/jac/38.6.1107. [DOI] [PubMed] [Google Scholar]
- 146.Santimaleeworagun W, Wongpoowarak P, Chayakul P, Pattharachayakul S, Tansakul P, Garey KW. 2011. In vitro activity of colistin or sulbactam in combination with fosfomycin or imipenem against clinical isolates of carbapenem-resistant Acinetobacter baumannii producing OXA-23 carbapenemases. Southeast Asian J Trop Med Public Health 42:890–900. [PubMed] [Google Scholar]
- 147.Wei W, Yang H, Liu Y, Ye Y, Li J. 15 May 2015. In vitro synergy of colistin combinations against extensively drug-resistant Acinetobacter baumannii producing OXA-23 carbapenemase. J Chemother doi: 10.1179/1973947815Y.0000000030. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Y, Chen F, Sun E, Ma R, Qu C, Ma L. 2013. antibacterial activity of combinations of fosfomycin, minocycline and polymyxin B on pan-drug-resistant. Exp Ther Med 5:1737–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Di X, Wang R, Liu B, Zhang X, Ni W, Wang J, Liang B, Cai Y, Liu Y. 2015. In vitro activity of fosfomycin in combination with colistin against clinical isolates of carbapenem-resistant Pseudomas aeruginosa. J Antibiot (Tokyo) 68:551–555. doi: 10.1038/ja.2015.27. [DOI] [PubMed] [Google Scholar]
- 150.Kunakonvichaya B, Thirapanmethee K, Khuntayaporn P, Montakantikul P, Chomnawang MT. 2015. Synergistic effects of fosfomycin and carbapenems against carbapenem-resistant Pseudomonas aeruginosa clinical isolates. Int J Antimicrob Agents 45:556–557. doi: 10.1016/j.ijantimicag.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 151.Santos DA, Nascimento MM, Vitali LH, Martinez R. 2013. In vitro activity of antimicrobial combinations against multidrug-resistant Pseudomonas aeruginosa. Rev Soc Bras Med Trop 46:299–303. doi: 10.1590/0037-8682-0012-2013. [DOI] [PubMed] [Google Scholar]
- 152.Lingscheid T, Tobudic S, Poeppl W, Mitteregger D, Burgmann H. 2013. In vitro activity of doripenem plus fosfomycin against drug-resistant clinical blood isolates. Pharmacology 91:214–218. doi: 10.1159/000348572. [DOI] [PubMed] [Google Scholar]
- 153.Hickman RA, Hughes D, Cars T, Malmberg C, Cars O. 2014. Cell-wall-inhibiting antibiotic combinations with activity against multidrug-resistant Klebsiella pneumoniae and Escherichia coli. Clin Microbiol Infect 20:O267–273. doi: 10.1111/1469-0691.12374. [DOI] [PubMed] [Google Scholar]
- 154.Souli M, Galani I, Boukovalas S, Gourgoulis MG, Chryssouli Z, Kanellakopoulou K, Panagea T, Giamarellou H. 2011. In vitro interactions of antimicrobial combinations with fosfomycin against KPC-2-producing Klebsiella pneumoniae and protection of resistance development. Antimicrob Agents Chemother 55:2395–2397. doi: 10.1128/AAC.01086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tangden T, Hickman RA, Forsberg P, Lagerback P, Giske CG, Cars O. 2014. Evaluation of double- and triple-antibiotic combinations for VIM- and NDM-producing Klebsiella pneumoniae by in vitro time-kill experiments. Antimicrob Agents Chemother 58:1757–1762. doi: 10.1128/AAC.00741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Evren E, Azap OK, Colakoglu S, Arslan H. 2013. In vitro activity of fosfomycin in combination with imipenem, meropenem, colistin and tigecycline against OXA 48-positive Klebsiella pneumoniae strains. Diagn Microbiol Infect Dis 76:335–338. doi: 10.1016/j.diagmicrobio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 157.Lefort A, Chau F, Lepeule R, Dubee V, Kitzis MD, Dion S, Fantin B. 2014. Activity of fosfomycin alone or combined with cefoxitin in vitro and in vivo in a murine model of urinary tract infection due to Escherichia coli harbouring CTX-M-15-type extended-spectrum beta-lactamase. Int J Antimicrob Agents 43:366–369. doi: 10.1016/j.ijantimicag.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 158.Bercot B, Poirel L, Dortet L, Nordmann P. 2011. In vitro evaluation of antibiotic synergy for NDM-1-producing Enterobacteriaceae. J Antimicrob Chemother 66:2295–2297. doi: 10.1093/jac/dkr296. [DOI] [PubMed] [Google Scholar]
- 159.Barbee LA, Soge OO, Holmes KK, Golden MR. 2014. In vitro synergy testing of novel antimicrobial combination therapies against Neisseria gonorrhoeae. J Antimicrob Chemother 69:1572–1578. doi: 10.1093/jac/dkt540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wind CM, de Vries HJ, van Dam AP. 2015. Determination of in vitro synergy for dual antimicrobial therapy against resistant Neisseria gonorrhoeae using Etest and agar dilution. Int J Antimicrob Agents 45:305–308. doi: 10.1016/j.ijantimicag.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 161.Xu-hong Y, Falagas ME, Dong W, Karageorgopoulos DE, De-feng L, Rui W. 2014. In vitro activity of fosfomycin in combination with linezolid against clinical isolates of methicillin-resistant Staphylococcus aureus. J Antibiot (Tokyo) 67:369–371. doi: 10.1038/ja.2014.5. [DOI] [PubMed] [Google Scholar]
- 162.Duez JM, Adochitei A, Pechinot A, Siebor E, Sixt N, Neuwirth C. 2008. In vitro combinations of five intravenous antibiotics with dalfopristin-quinupristin against Staphylococcus aureus in a 3-dimensional model. J Chemother 20:684–689. doi: 10.1179/joc.2008.20.6.684. [DOI] [PubMed] [Google Scholar]
- 163.Sun C, Falagas ME, Wang R, Karageorgopoulos DE, Yu X, Liu Y, Cai Y, Liang B, Song X, Liu Z. 2011. In vitro activity of minocycline combined with fosfomycin against clinical isolates of methicillin-resistant Staphylococcus aureus. J Antibiot (Tokyo) 64:559–562. doi: 10.1038/ja.2011.52. [DOI] [PubMed] [Google Scholar]
- 164.Tang HJ, Chen CC, Cheng KC, Wu KY, Lin YC, Zhang CC, Weng TC, Yu WL, Chiu YH, Toh HS, Chiang SR, Su BA, Ko WC, Chuang YC. 2013. In vitro efficacies and resistance profiles of rifampin-based combination regimens for biofilm-embedded methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:5717–5720. doi: 10.1128/AAC.01236-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lingscheid T, Poeppl W, Bernitzky D, Veletzky L, Kussmann M, Plasenzotti R, Burgmann H. 2015. Daptomycin plus fosfomycin, a synergistic combination in experimental implant-associated osteomyelitis due to methicillin-resistant Staphylococcus aureus in rats. Antimicrob Agents Chemother 59:859–863. doi: 10.1128/AAC.04246-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pachon-Ibanez ME, Ribes S, Dominguez MA, Fernandez R, Tubau F, Ariza J, Gudiol F, Cabellos C. 2011. Efficacy of fosfomycin and its combination with linezolid, vancomycin and imipenem in an experimental peritonitis model caused by a Staphylococcus aureus strain with reduced susceptibility to vancomycin. Eur J Clin Microbiol Infect Dis 30:89–95. doi: 10.1007/s10096-010-1058-0. [DOI] [PubMed] [Google Scholar]
- 167.Liu LG, Zhu YL, Hu LF, Cheng J, Ye Y, Li JB. 2013. Comparative study of the mutant prevention concentrations of vancomycin alone and in combination with levofloxacin, rifampicin and fosfomycin against methicillin-resistant Staphylococcus epidermidis. J Antibiot (Tokyo) 66:709–712. doi: 10.1038/ja.2013.87. [DOI] [PubMed] [Google Scholar]
- 168.MacLeod DL, Velayudhan J, Kenney TF, Therrien JH, Sutherland JL, Barker LM, Baker WR. 2012. Fosfomycin enhances the active transport of tobramycin in Pseudomonas aeruginosa. Antimicrob Agents Chemother 56:1529–1538. doi: 10.1128/AAC.05958-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McCaughey G, Gilpin DF, Schneiders T, Hoffman LR, McKevitt M, Elborn JS, Tunney MM. 2013. Fosfomycin and tobramycin in combination downregulate nitrate reductase genes narG and narH, resulting in increased activity against Pseudomonas aeruginosa under anaerobic conditions. Antimicrob Agents Chemother 57:5406–5414. doi: 10.1128/AAC.00750-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Borgia M, Longo A, Lodola E. 1989. Relative bioavailability of fosfomycin and of trometamol after administration of single dose by oral route of fosfomycin trometamol in fasting conditions and after a meal. Int J Clin Pharmacol Ther Toxicol 27:411–417. [PubMed] [Google Scholar]
- 171.Borsa F, Leroy A, Fillastre JP, Godin M, Moulin B. 1988. Comparative pharmacokinetics of tromethamine fosfomycin and calcium fosfomycin in young and elderly adults. Antimicrob Agents Chemother 32:938–941. doi: 10.1128/AAC.32.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bergan T. 1990. Degree of absorption, pharmacokinetics of fosfomycin trometamol and duration of urinary antibacterial activity. Infection 18(Suppl 2):S65–S69. doi: 10.1007/BF01643430. [DOI] [PubMed] [Google Scholar]
- 173.Goto M, Sugiyama M, Nakajima S, Yamashina H. 1981. Fosfomycin kinetics after intravenous and oral administration to human volunteers. Antimicrob Agents Chemother 20:393–397. doi: 10.1128/AAC.20.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. 2009. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents 34:506–515. doi: 10.1016/j.ijantimicag.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 175.Bergan T, Thorsteinsson SB, Albini E. 1993. Pharmacokinetic profile of fosfomycin trometamol. Chemotherapy 39:297–301. doi: 10.1159/000239140. [DOI] [PubMed] [Google Scholar]
- 176.Scaglione F, Cicchetti F, Demartini G, Arcidiacono M. 1994. Fosfomycin distribution in the lower urinary tract after administration of fosfomycin trometamol salt. Int J Clin Pharmacol Res 14:107–109. [PubMed] [Google Scholar]
- 177.Burian A, Erdogan Z, Jandrisits C, Zeitlinger M. 2012. Impact of pH on activity of trimethoprim, fosfomycin, amikacin, colistin and ertapenem in human urine. Pharmacology 90:281–287. doi: 10.1159/000342423. [DOI] [PubMed] [Google Scholar]
- 178.Gardiner BJ, Mahony AA, Ellis AG, Lawrentschuk N, Bolton DM, Zeglinski PT, Frauman AG, Grayson ML. 2014. Is fosfomycin a potential treatment alternative for multidrug-resistant gram-negative prostatitis? Clin Infect Dis 58:e101–e105. doi: 10.1093/cid/cit704. [DOI] [PubMed] [Google Scholar]
- 179.Soraci AL, Perez DS, Martinez G, Dieguez S, Tapia MO, Amanto F, Harkes R, Romano O. 2011. Disodium-fosfomycin pharmacokinetics and bioavailability in post weaning piglets. Res Vet Sci 90:498–502. doi: 10.1016/j.rvsc.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 180.Sumano LH, Ocampo CL, Gutierrez OL. 2007. Intravenous and intramuscular pharmacokinetics of a single-daily dose of disodium-fosfomycin in cattle, administered for 3 days. J Vet Pharmacol Ther 30:49–54. doi: 10.1111/j.1365-2885.2007.00812.x. [DOI] [PubMed] [Google Scholar]
- 181.Kawabata N, Shiraha Y, Doi S, Umemura K, Yaginuma K. 1978. A study on serum level and urinary excretion of fosfomycin-Na in man with special reference to pharmacokinetic analysis. Jpn J Antibiot 31:549–560. [PubMed] [Google Scholar]
- 182.Frossard M, Joukhadar C, Erovic BM, Dittrich P, Mrass PE, Van Houte M, Burgmann H, Georgopoulos A, Muller M. 2000. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob Agents Chemother 44:2728–2732. doi: 10.1128/AAC.44.10.2728-2732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Joukhadar C, Klein N, Dittrich P, Zeitlinger M, Geppert A, Skhirtladze K, Frossard M, Heinz G, Muller M. 2003. Target site penetration of fosfomycin in critically ill patients. J Antimicrob Chemother 51:1247–1252. doi: 10.1093/jac/dkg187. [DOI] [PubMed] [Google Scholar]
- 184.Schintler MV, Traunmuller F, Metzler J, Kreuzwirt G, Spendel S, Mauric O, Popovic M, Scharnagl E, Joukhadar C. 2009. High fosfomycin concentrations in bone and peripheral soft tissue in diabetic patients presenting with bacterial foot infection. J Antimicrob Chemother 64:574–578. doi: 10.1093/jac/dkp230. [DOI] [PubMed] [Google Scholar]
- 185.Legat FJ, Maier A, Dittrich P, Zenahlik P, Kern T, Nuhsbaumer S, Frossard M, Salmhofer W, Kerl H, Muller M. 2003. Penetration of fosfomycin into inflammatory lesions in patients with cellulitis or diabetic foot syndrome. Antimicrob Agents Chemother 47:371–374. doi: 10.1128/AAC.47.1.371-374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Petsch M, Mayer-Helm BX, Sauermann R, Joukhadar C, Kenndler E. 2005. Determination of fosfomycin in pus by capillary zone electrophoresis. J Chromatogr A 1081:55–59. doi: 10.1016/j.chroma.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 187.Sauermann R, Karch R, Langenberger H, Kettenbach J, Mayer-Helm B, Petsch M, Wagner C, Sautner T, Gattringer R, Karanikas G, Joukhadar C. 2005. Antibiotic abscess penetration: fosfomycin levels measured in pus and simulated concentration-time profiles. Antimicrob Agents Chemother 49:4448–4454. doi: 10.1128/AAC.49.11.4448-4454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Inouye S, Watanabe T, Tsuruoka T, Kitasato I. 1989. An increase in the antimicrobial activity in vitro of fosfomycin under anaerobic conditions. J Antimicrob Chemother 24:657–666. doi: 10.1093/jac/24.5.657. [DOI] [PubMed] [Google Scholar]
- 189.Farago E, Kiss IJ, Nabradi Z. 1980. Serum and lung tissue levels of fosfomycin in humans. Int J Clin Pharmacol Ther Toxicol 18:554–558. [PubMed] [Google Scholar]
- 190.Berthelot G, Bergogne-Berezin E, Kafe H, Daumal M, Gillon JC. 1983. Penetration of fosfomycin into bronchial secretions. Pathol Biol (Paris) 31:519–521. [PubMed] [Google Scholar]
- 191.Matzi V, Lindenmann J, Porubsky C, Kugler SA, Maier A, Dittrich P, Smolle-Juttner FM, Joukhadar C. 2010. Extracellular concentrations of fosfomycin in lung tissue of septic patients. J Antimicrob Chemother 65:995–998. doi: 10.1093/jac/dkq070. [DOI] [PubMed] [Google Scholar]
- 192.Lastra CF, Marino EL, Barrueco M, Gervos MS, Gil AD. 1984. Disposition of phosphomycin in patients with pleural effusion. Antimicrob Agents Chemother 25:458–462. doi: 10.1128/AAC.25.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Drobnic L, Quiles M, Rodriguez A. 1977. A study of the levels of fosfomycin in the cerebrospinal fluid in adult meningitis. Chemotherapy 23(Suppl 1):S180–S188. [DOI] [PubMed] [Google Scholar]
- 194.Sauermann R, Schwameis R, Fille M, Ligios ML, Zeitlinger M. 2009. Cerebrospinal fluid impairs antimicrobial activity of fosfomycin in vitro. J Antimicrob Chemother 64:821–823. doi: 10.1093/jac/dkp261. [DOI] [PubMed] [Google Scholar]
- 195.Nau R, Zysk G, Reinert RR, Mergeryan H, Eiffert H, Prange HW. 1995. Activity of fosfomycin in a rabbit model of experimental pneumococcal meningitis. J Antimicrob Chemother 36:997–1004. doi: 10.1093/jac/36.6.997. [DOI] [PubMed] [Google Scholar]
- 196.Kuhnen E, Pfeifer G, Frenkel C. 1987. Penetration of fosfomycin into cerebrospinal fluid across non-inflamed and inflamed meninges. Infection 15:422–424. doi: 10.1007/BF01647220. [DOI] [PubMed] [Google Scholar]
- 197.Pfausler B, Spiss H, Dittrich P, Zeitlinger M, Schmutzhard E, Joukhadar C. 2004. Concentrations of fosfomycin in the cerebrospinal fluid of neurointensive care patients with ventriculostomy-associated ventriculitis. J Antimicrob Chemother 53:848–852. doi: 10.1093/jac/dkh158. [DOI] [PubMed] [Google Scholar]
- 198.Brunner M, Reinprecht A, Illievich U, Spiss CK, Dittrich P, van Houte M, Muller M. 2002. Penetration of fosfomycin into the parenchyma of human brain: a case study in three patients. Br J Clin Pharmacol 54:548–550. doi: 10.1046/j.1365-2125.2002.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sirot J, Lopitaux R, Dumont C, Rampon S, Cluzel R. 1983. Diffusion of fosfomycin into bone tissue in man. Pathol Biol (Paris) 31:522–524. [PubMed] [Google Scholar]
- 200.Meissner A, Haag R, Rahmanzadeh R. 1989. Adjuvant fosfomycin medication in chronic osteomyelitis. Infection 17:146–151. doi: 10.1007/BF01644014. [DOI] [PubMed] [Google Scholar]
- 201.Muller O, Ruckert U, Walter W, Haag R, Sauer W. 1982. Fosfomycin concentrations in serum and bile. Infection 10:18–20. doi: 10.1007/BF01640831. [DOI] [PubMed] [Google Scholar]
- 202.Nakamura T, Hashimoto I, Sawada Y, Mikami J, Bekki E. 1985. Clinical studies on fosfomycin sodium following intravenous administration (tissue concentration and clinical efficacy). Jpn J Antibiot 38:2057–2067. [PubMed] [Google Scholar]
- 203.Hirt SW, Alken A, Muller H, Haverich A, Vomel W. 1990. Perioperative preventive antibiotic treatment with fosfomycin in heart surgery: serum kinetics in extracorporeal circulation and determination of concentration in heart valve tissue. Z Kardiol 79:615–620. [PubMed] [Google Scholar]
- 204.Mazzei T, Cassetta MI, Fallani S, Arrigucci S, Novelli A. 2006. Pharmacokinetic and pharmacodynamic aspects of antimicrobial agents for the treatment of uncomplicated urinary tract infections. Int J Antimicrob Agents 28(Suppl 1):S35–S41. [DOI] [PubMed] [Google Scholar]
- 205.Janknegt R, Hooymans PM, Fabius GT, Nohlmans-Paulssen MK, Machielsen C, Boogaard-van den Born J, Rang J, Smits CA, Willems-Thissen ME, Krommenhoek A. 1994. Urinary concentrations of fosfomycin after a single 3 g dose of fosfomycin to elderly nursing-home patients. Pharm World Sci 16:149–153. doi: 10.1007/BF01877485. [DOI] [PubMed] [Google Scholar]
- 206.Iwai N, Nakamura H, Miyazu M, Watanabe Y. 1991. A study of the absorption and excretion of fosfomycin sodium in children. Jpn J Antibiot 44:345–356. [PubMed] [Google Scholar]
- 207.Traunmuller F, Popovic M, Konz KH, Vavken P, Leithner A, Joukhadar C. 2011. A reappraisal of current dosing strategies for intravenous fosfomycin in children and neonates. Clin Pharmacokinet 50:493–503. doi: 10.2165/11592670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 208.Iioka H, Moriyama I, Kyuma M, Tsuji Y, Ichijo M. 1986. The transport mechanism of antibiotics using microvillous membrane vesicles (placental transport of fosfomycin). Nihon Sanka Fujinka Gakkai Zasshi 38:1702–1706. [PubMed] [Google Scholar]
- 209.Parker S, Lipman J, Koulenti D, Dimopoulos G, Roberts JA. 2013. What is the relevance of fosfomycin pharmacokinetics in the treatment of serious infections in critically ill patients? A systematic review. Int J Antimicrob Agents 42:289–293. doi: 10.1016/j.ijantimicag.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 210.Fillastre JP, Leroy A, Josse S, Moulin B. 1988. Pharmacokinetics of trometamol-fosfomycin in patients with renal insufficiency. Pathol Biol (Paris) 36:728–730. [PubMed] [Google Scholar]
- 211.Gobernado M, Garcia J, Santos M, Panadero J, Diosdado N. 1977. Renal insufficiency and fosfomycin. Chemotherapy 23(Suppl 1):S200–S203. [DOI] [PubMed] [Google Scholar]
- 212.Bouchet JL, Quentin C, Albin H, Vincon G, Guillon J, Martin-Dupont P. 1985. Pharmacokinetics of fosfomycin in hemodialyzed patients. Clin Nephrol 23:218–221. [PubMed] [Google Scholar]
- 213.Fernandez Lastra C, Marino EL, Dominguez-Gil A, Tabernero JM, Grande Villoria J. 1984. Pharmacokinetics of phosphomycin during haemofiltration. Br J Clin Pharmacol 17:477–480. doi: 10.1111/j.1365-2125.1984.tb02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gattringer R, Meyer B, Heinz G, Guttmann C, Zeitlinger M, Joukhadar C, Dittrich P, Thalhammer F. 2006. Single-dose pharmacokinetics of fosfomycin during continuous venovenous haemofiltration. J Antimicrob Chemother 58:367–371. doi: 10.1093/jac/dkl251. [DOI] [PubMed] [Google Scholar]
- 215.Tobudic S, Matzneller P, Stoiser B, Wenisch JM, Zeitlinger M, Vychytil A, Jaeger W, Boehmdorfer M, Reznicek G, Burgmann H. 2012. Pharmacokinetics of intraperitoneal and intravenous fosfomycin in automated peritoneal dialysis patients without peritonitis. Antimicrob Agents Chemother 56:3992–3995. doi: 10.1128/AAC.00126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Keating GM. 2013. Fosfomycin trometamol: a review of its use as a single-dose oral treatment for patients with acute lower urinary tract infections and pregnant women with asymptomatic bacteriuria. Drugs 73:1951–1966. doi: 10.1007/s40265-013-0143-y. [DOI] [PubMed] [Google Scholar]
- 217.Principi N, Corda R, Bassetti D, Varese LA, Peratoner L. 1990. Fosfomycin trometamol versus netilmicin in children's lower urinary tract infections. Chemotherapy 36(Suppl 1):S41–S45. [DOI] [PubMed] [Google Scholar]
- 218.Varese LA. 1987. Trometamol salt of fosfomycin versus netilmicin: randomized multicenter study in children's lower urinary tract infections. Eur Urol 13(Suppl 1):S119–S121. [DOI] [PubMed] [Google Scholar]
- 219.Apisarnthanarak A, Mundy LM. 2010. Use of high-dose 4-hour infusion of doripenem, in combination with fosfomycin, for treatment of carbapenem-resistant Pseudomonas aeruginosa pneumonia. Clin Infect Dis 51:1352–1354. doi: 10.1086/657249. [DOI] [PubMed] [Google Scholar]
- 220.Michalopoulos A, Virtzili S, Rafailidis P, Chalevelakis G, Damala M, Falagas ME. 2010. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin Microbiol Infect 16:184–186. doi: 10.1111/j.1469-0691.2009.02921.x. [DOI] [PubMed] [Google Scholar]
- 221.Miro JM, Entenza JM, Del Rio A, Velasco M, Castaneda X, Garcia de la Maria C, Giddey M, Armero Y, Pericas JM, Cervera C, Mestres CA, Almela M, Falces C, Marco F, Moreillon P, Moreno A. 2012. High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 56:4511–4515. doi: 10.1128/AAC.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Infectopharm Arzneimittel und Consilium GmbH. 2015. Fomicyt package insert. Infectopharm, Heppenheim, Germany. [Google Scholar]
- 223.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 224.Ceran N, Mert D, Kocdogan FY, Erdem I, Adalati R, Ozyurek S, Goktas P. 2010. A randomized comparative study of single-dose fosfomycin and 5-day ciprofloxacin in female patients with uncomplicated lower urinary tract infections. J Infect Chemother 16:424–430. doi: 10.1007/s10156-010-0079-z. [DOI] [PubMed] [Google Scholar]
- 225.Palou J, Angulo JC, Ramon de Fata F, Garcia-Tello A, Gonzalez-Enguita C, Boada A, Sanz M. 2013. Randomized comparative study for the assessment of a new therapeutic schedule of fosfomycin trometamol in postmenopausal women with uncomplicated lower urinary tract infection. Actas Urol Esp 37:147–155. doi: 10.1016/j.acuro.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 226.Usta TA, Dogan O, Ates U, Yucel B, Onar Z, Kaya E. 2011. Comparison of single-dose and multiple-dose antibiotics for lower urinary tract infection in pregnancy. Int J Gynaecol Obstet 114:229–233. doi: 10.1016/j.ijgo.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 227.Matsumoto T, Muratani T, Nakahama C, Tomono K. 2011. Clinical effects of 2 days of treatment by fosfomycin calcium for acute uncomplicated cystitis in women. J Infect Chemother 17:80–86. doi: 10.1007/s10156-010-0092-2. [DOI] [PubMed] [Google Scholar]
- 228.Qiao LD, Zheng B, Chen S, Yang Y, Zhang K, Guo HF, Yang B, Niu YJ, Wang Y, Shi BK, Yang WM, Zhao XK, Gao XF, Chen M. 2013. Evaluation of three-dose fosfomycin tromethamine in the treatment of patients with urinary tract infections: an uncontrolled, open-label, multicentre study. BMJ Open 3:e004157. doi: 10.1136/bmjopen-2013-004157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Senol S, Tasbakan M, Pullukcu H, Sipahi OR, Sipahi H, Yamazhan T, Arda B, Ulusoy S. 2010. Carbapenem versus fosfomycin tromethanol in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related complicated lower urinary tract infection. J Chemother 22:355–357. doi: 10.1179/joc.2010.22.5.355. [DOI] [PubMed] [Google Scholar]
- 230.Neuner EA, Sekeres J, Hall GS, van Duin D. 2012. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 56:5744–5748. doi: 10.1128/AAC.00402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Reid GE, Grim SA, Layden JE, Akkina S, Tang I, Campara M, Clark NM. 2013. The use of fosfomycin to treat urinary tract infections in kidney transplant recipients. Transplantation 96:e12–14. doi: 10.1097/TP.0b013e318298dd26. [DOI] [PubMed] [Google Scholar]
- 232.Wu TH, Huang FL, Fu LS, Chou CM, Chien YL, Huang CM, Lin CF, Chen PY. 21 November 2014. Treatment of recurrent complicated urinary tract infections in children with vesicoureteral reflux. J Microbiol Immunol Infect doi: 10.1016/j.jmii.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 233.Cunha BA, Gran A, Raza M. 2015. Persistent extended-spectrum beta-lactamase-positive Escherichia coli chronic prostatitis successfully treated with a combination of fosfomycin and doxycycline. Int J Antimicrob Agents 45:427–429. doi: 10.1016/j.ijantimicag.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 234.Dubos M, Barraud O, Fedou AL, Fredon F, Laurent F, Brakbi Y, Cypierre A, Francois B. 2014. Prostatic abscesses and severe sepsis due to methicillin-susceptible Staphylococcus aureus producing Panton-Valentine leukocidin. BMC Infect Dis 14:466. doi: 10.1186/1471-2334-14-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Grayson ML, Macesic N, Trevillyan J, Ellis AG, Zeglinski PT, Hewitt NH, Gardiner BJ, Frauman AG. 2015. Fosfomycin for treatment of prostatitis: new tricks for old dogs. Clin Infect Dis 61:1141–1143. doi: 10.1093/cid/civ436. [DOI] [PubMed] [Google Scholar]
- 236.Fujii R. 1977. Fosfomycin in the treatment of bacterial infections: summary of clinical trials in Japan. Chemotherapy 23(Suppl 1):S234–S246. [DOI] [PubMed] [Google Scholar]
- 237.Boyanova L, Davidkov L, Gergova G, Kandilarov N, Evstatiev I, Panteleeva E, Mitov I. 2014. Helicobacter pylori susceptibility to fosfomycin, rifampin, and 5 usual antibiotics for H. pylori eradication. Diagn Microbiol Infect Dis 79:358–361. doi: 10.1016/j.diagmicrobio.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 238.Rosso-Fernandez C, Sojo-Dorado J, Barriga A, Lavin-Alconero L, Palacios Z, Lopez-Hernandez I, Merino V, Camean M, Pascual A, Rodriguez-Bano J. 2015. Fosfomycin versus meropenem in bacteraemic urinary tract infections caused by extended-spectrum beta-lactamase-producing Escherichia coli (FOREST): study protocol for an investigator-driven randomised controlled trial. BMJ Open 5:e007363. doi: 10.1136/bmjopen-2014-007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Shaw E, Miro JM, Puig-Asensio M, Pigrau C, Barcenilla F, Murillas J, Garcia-Pardo G, Espejo E, Padilla B, Garcia-Reyne A, Pasquau J, Rodriguez-Bano J, Lopez-Contreras J, Montero M, de la Calle C, Pintado V, Calbo E, Gasch O, Montejo M, Salavert M, Garcia-Pais MJ, Carratala J, Pujol M. 2015. Daptomycin plus fosfomycin versus daptomycin monotherapy in treating MRSA: protocol of a multicentre, randomised, phase III trial. BMJ Open 5:e006723. doi: 10.1136/bmjopen-2014-006723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Apisarnthanarak A, Mundy LM. 2012. Carbapenem-resistant Pseudomonas aeruginosa pneumonia with intermediate minimum inhibitory concentrations to doripenem: combination therapy with high-dose, 4-h infusion of doripenem plus fosfomycin versus intravenous colistin plus fosfomycin. Int J Antimicrob Agents 39:271–272. doi: 10.1016/j.ijantimicag.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 241.del Rio A, Gasch O, Moreno A, Pena C, Cuquet J, Soy D, Mestres CA, Suarez C, Pare JC, Tubau F, Garcia de la Maria C, Marco F, Carratala J, Gatell JM, Gudiol F, Miro JM. 2014. Efficacy and safety of fosfomycin plus imipenem as rescue therapy for complicated bacteremia and endocarditis due to methicillin-resistant Staphylococcus aureus: a multicenter clinical trial. Clin Infect Dis 59:1105–1112. doi: 10.1093/cid/ciu580. [DOI] [PubMed] [Google Scholar]
- 242.Dinh A, Salomon J, Bru JP, Bernard L. 2012. Fosfomycin: efficacy against infections caused by multidrug-resistant bacteria. Scand J Infect Dis 44:182–189. doi: 10.3109/00365548.2011.616221. [DOI] [PubMed] [Google Scholar]
- 243.Florent A, Chichmanian RM, Cua E, Pulcini C. 2011. Adverse events associated with intravenous fosfomycin. Int J Antimicrob Agents 37:82–83. doi: 10.1016/j.ijantimicag.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 244.Kusachi S, Nagao J, Saida Y, Watanabe M, Okamoto Y, Asai K, Nakamura Y, Enomoto T, Arima Y, Kiribayashi T, Watanabe R, Saito T, Uramatsu M, Sato J. 2011. Antibiotic time-lag combination therapy with fosfomycin for postoperative intra-abdominal abscesses. J Infect Chemother 17:91–96. doi: 10.1007/s10156-010-0167-0. [DOI] [PubMed] [Google Scholar]
- 245.Navarro-San Francisco C, Mora-Rillo M, Romero-Gomez MP, Moreno-Ramos F, Rico-Nieto A, Ruiz-Carrascoso G, Gomez-Gil R, Arribas-Lopez JR, Mingorance J, Pano-Pardo JR. 2013. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect 19:E72-79. doi: 10.1111/1469-0691.12091. [DOI] [PubMed] [Google Scholar]
- 246.Pontikis K, Karaiskos I, Bastani S, Dimopoulos G, Kalogirou M, Katsiari M, Oikonomou A, Poulakou G, Roilides E, Giamarellou H. 2014. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int J Antimicrob Agents 43:52–59. doi: 10.1016/j.ijantimicag.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 247.Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, Kapaskelis A, Nikita D, Michalopoulos A. 2010. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents 35:194–199. doi: 10.1016/j.ijantimicag.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 248.Falagas ME, Rafailidis PI, Matthaiou DK, Virtzili S, Nikita D, Michalopoulos A. 2008. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: characteristics and outcome in a series of 28 patients. Int J Antimicrob Agents 32:450–454. doi: 10.1016/j.ijantimicag.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 249.Falagas ME, Vardakas KZ, Tsiveriotis KP, Triarides NA, Tansarli GS. 2014. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents 44:1–7. doi: 10.1016/j.ijantimicag.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 250.Vardakas KZ, Matthaiou DK, Falagas ME, Antypa E, Koteli A, Antoniadou E. 2015. Tigecycline for carbapenem-resistant Klebsiella pneumoniae infections in the intensive care unit. Infect Dis (Lond) 47:755–757. [DOI] [PubMed] [Google Scholar]
- 251.Falagas ME, Tansarli GS, Rafailidis PI, Kapaskelis A, Vardakas KZ. 2012. Impact of antibiotic MIC on infection outcome in patients with susceptible Gram-negative bacteria: a systematic review and meta-analysis. Antimicrob Agents Chemother 56:4214–4222. doi: 10.1128/AAC.00663-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mavros MN, Tansarli GS, Vardakas KZ, Rafailidis PI, Karageorgopoulos DE, Falagas ME. 2012. Impact of vancomycin minimum inhibitory concentration on clinical outcomes of patients with vancomycin-susceptible Staphylococcus aureus infections: a meta-analysis and meta-regression. Int J Antimicrob Agents 40:496–509. doi: 10.1016/j.ijantimicag.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 253.Wagenlehner FM, Thomas PM, Naber KG. 2014. Fosfomycin trometamol (3,000 mg) in perioperative antibiotic prophylaxis of healthcare-associated infections after endourological interventions: a narrative review. Urol Int 92:125–130. doi: 10.1159/000355103. [DOI] [PubMed] [Google Scholar]
- 254.Lista F, Redondo C, Meilan E, Garcia-Tello A, Ramon de Fata F, Angulo JC. 2014. Efficacy and safety of fosfomycin-trometamol in the prophylaxis for transrectal prostate biopsy. Prospective randomized comparison with ciprofloxacin. Actas Urol Esp 38:391–396. [DOI] [PubMed] [Google Scholar]
- 255.Ongun S, Aslan G, Avkan-Oguz V. 2012. The effectiveness of single-dose fosfomycin as antimicrobial prophylaxis for patients undergoing transrectal ultrasound-guided biopsy of the prostate. Urol Int 89:439–444. doi: 10.1159/000342370. [DOI] [PubMed] [Google Scholar]
- 256.Rhodes NJ, Gardiner BJ, Neely MN, Grayson ML, Ellis AG, Lawrentschuk N, Frauman AG, Maxwell KM, Zembower TR, Scheetz MH. 2015. Optimal timing of oral fosfomycin administration for pre-prostate biopsy prophylaxis. J Antimicrob Chemother 70:2068–2073. doi: 10.1093/jac/dkv067. [DOI] [PubMed] [Google Scholar]
- 257.Costantini E, Zucchi A, Salvini E, Cicalese A, Li Marzi V, Filocamo MT, Bini V, Lazzeri M. 2014. Prulifloxacin vs fosfomycin for prophylaxis in female patients with recurrent UTIs: a non-inferiority trial. Int Urogynecol J 25:1173–1178. [DOI] [PubMed] [Google Scholar]
- 258.Andaker L, Burman LG, Eklund A, Graffner H, Hansson J, Hellberg R, Hojer H, Ljungqvist U, Kjellgren K, Kling PA, et al. 1992. Fosfomycin/metronidazole compared with doxycycline/metronidazole for the prophylaxis of infection after elective colorectal surgery. A randomised double-blind multicentre trial in 517 patients. Eur J Surg 158:181–185. [PubMed] [Google Scholar]
- 259.Shinagawa N, Mizuno I, Fukui T, Takeyama H, Yasuda A, Matsumoto K, Ueda N, Mouri N, Nagasaki T, Yokoyama T, Shinbara K, Isaka M, Kurisu Y, Akagi S, Tagawa K, Kano M, Niitani N, Watatani Y. 2006. Prophylactic effect of fosfomycin on postoperative infection in gastroenterological surgery. Jpn J Antibiot 59:417–427. [PubMed] [Google Scholar]
- 260.Alfonsi P, Slim K, Chauvin M, Mariani P, Faucheron JL, Fletcher D. 2014. French guidelines for enhanced recovery after elective colorectal surgery. J Visc Surg 151:65–79. doi: 10.1016/j.jviscsurg.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 261.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA. 2013. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 14:73–156. doi: 10.1089/sur.2013.9999. [DOI] [PubMed] [Google Scholar]
- 262.Zerey M, Hawver LM, Awad Z, Stefanidis D, Richardson W, Fanelli RD. 2013. SAGES evidence-based guidelines for the laparoscopic resection of curable colon and rectal cancer. Surg Endosc 27:1–10. doi: 10.1007/s00464-012-2592-x. [DOI] [PubMed] [Google Scholar]
- 263.Chareancholvanich K, Udomkiat P, Waikakul S. 2012. A randomized control trial between fosfomycin and cefuroxime as the antibiotic prophylaxis in knee arthroplasty. J Med Assoc Thai 95(Suppl 9):S6–13. [PubMed] [Google Scholar]
- 264.Reference deleted. [Google Scholar]
- 265.Bendirdjian JP, Morin JP, Foucher B, Fillastre JP. 1978. The effect of fosfomycin on the respiration of rat kidney motochondria. Minerva Med 69:4079–4086. [PubMed] [Google Scholar]
- 266.Kreft B, de Wit C, Marre R, Sack K. 1991. Experimental studies on the nephrotoxicity of amphotericin B in rats. J Antimicrob Chemother 28:271–281. doi: 10.1093/jac/28.2.271. [DOI] [PubMed] [Google Scholar]
- 267.Nakamura T, Hashimoto Y, Kokuryo T, Inui KI. 1998. Effects of fosfomycin and imipenem/cilastatin on nephrotoxicity and renal excretion of vancomycin in rats. Pharm Res 15:734–738. doi: 10.1023/A:1011971019868. [DOI] [PubMed] [Google Scholar]
- 268.Umeki S, Watanabe M, Yagi S, Soejima R. 1988. Supplemental fosfomycin and/or steroids that reduce cisplatin-induced nephrotoxicity. Am J Med Sci 295:6–10. doi: 10.1097/00000441-198801000-00003. [DOI] [PubMed] [Google Scholar]
- 269.Kyle JM, Stollings JL, White KD, Noto MJ, Wheeler AP. 2015. Fosfomycin for multidrug treatment of Klebsiella pneumoniae carbapenemase bacteremia. Ann Pharmacother 49:366–367. doi: 10.1177/1060028014564395. [DOI] [PubMed] [Google Scholar]