Abstract
Duchenne muscular dystrophy (DMD) is a degenerative muscle disease caused by genetic mutations that lead to the disruption of dystrophin in muscle fibers. There is no curative treatment for this devastating disease. Clustered regularly interspaced short palindromic repeat/Cas9 (CRISPR/Cas9) has emerged as a powerful tool for genetic manipulation and potential therapy. Here we demonstrate that CRIPSR-mediated genome editing efficiently excised a 23-kb genomic region on the X-chromosome covering the mutant exon 23 in a mouse model of DMD, and restored dystrophin expression and the dystrophin-glycoprotein complex at the sarcolemma of skeletal muscles in live mdx mice. Electroporation-mediated transfection of the Cas9/gRNA constructs in the skeletal muscles of mdx mice normalized the calcium sparks in response to osmotic shock. Adenovirus-mediated transduction of Cas9/gRNA greatly reduced the Evans blue dye uptake of skeletal muscles at rest and after downhill treadmill running. This study provides proof evidence for permanent gene correction in DMD.
Introduction
Muscular dystrophies are a heterogeneous group of inherited disorders characterized by progressive muscle weakness and muscle wasting.1,2 Duchenne muscular dystrophy (DMD) is the most common form caused by mutations in the DMD gene,3 leading to the loss of dystrophin protein in striated muscle. This fatal muscle disease affects approximately 1 in 3,500 male births.4 Although significant progress has been made in the last two decades to understand the biology and pathogenesis of this devastating disease, no effective treatment is currently available. Disruption of dystrophin expression results in the collapse of the dystrophin-glycoprotein complex at the sarcolemma,5,6 and renders the skeletal muscle prone to contraction-induced injury.1 Previous work has shown that deletion of a large portion of the dystrophin protein in the central region did not appear to affect the function of dystrophin protein,7 thus providing a promising therapy by skipping the mutant exon while preserving the reading frame. This has been extensively studied using the exon skipping technology,8,9,10 which works at the transcription level by interfering with the splicing mechanism.
RNA-guided, nuclease-mediated genome editing, based on type II CRISPR (clustered regularly interspaced short palindromic repeat)/Cas (CRISPR-associated) systems has been recently introduced as a promising genome editing tool.11,12 Unlike other gene therapy methods, this system can effectively correct the primary genetic defect without retaining the original dysfunctional copy of the gene.13,14,15 A recent study16 showed that CRISPR/Cas9-mediated genome editing11,12 could be used in one-cell mouse embryos to correct the dystrophin gene mutation in the germ line of mdx mice, a model for DMD.17 In addition, two other studies demonstrated that gene correction could also be achieved with the use of CRISPR in cultured human DMD patient-derived cells.18,19 In this study, we investigate the feasibility of CRISPR/Cas9-mediated genome editing as a novel therapeutic tool to correct the genetic defect for the first time in postnatal mdx mice.
Results
CRISPR-mediated gene editing restores dystrophin reading frame in vitro
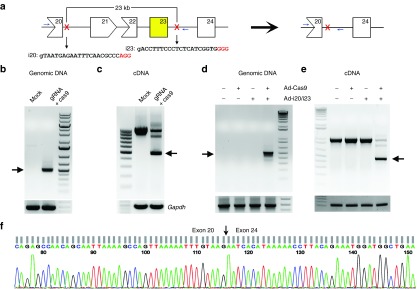
The mdx mouse carries a point mutation in exon 23, resulting in the formation of a premature stop codon and the disruption of dystrophin expression. We hypothesized that in-frame deletion of the genomic DNA covering exon 23 would restore functional dystrophin expression in mdx mice. We initially attempted to delete exon 23 (213 bp) alone, but no specific gRNA target in intron 22 could be identified. Therefore, we expanded our search for gRNA targets within intron 20 so that exon 21 (181 bp), 22 (146 bp), and 23 could be deleted altogether from the genomic sequence (Figure 1a). Two gRNA target sites were chosen from intron 20 and 23 (Figure 1a). A pair of primers specific for intron 20 and 23 beyond the gRNA target sites (Figure 1a and Supplementary Table S1) were used to genotype the cells for genomic editing. Cotransfection of the two gRNA with cas9 plasmids (Supplementary Table S1) into mouse C2C12 cells resulted in the detection of a small polymerase chain reaction (PCR) product of 510 bp as predicted (Figure 1b), indicating successful CRISPR-mediated genome editing. No PCR product could be amplified from mock-transfected C2C12 cells due to the large size of the region (~23 kb). We also performed reverse transcription-polymerase chain reaction (RT-PCR) to determine whether the deletion could lead to the expression of a truncated dystrophin transcript. As shown in Figure 1c, a smaller band (475 bp) together with the WT band (1,075 bp) could be readily amplified from the transfected cells using a primer pair annealed to exon 20 and 26, respectively (Supplementary Table S1). We then examined whether these reagents could also work in primary myoblasts isolated from mdx mice. To this end, adenoviral vectors expressing EGFP-2A-cas9 and the gRNAs were generated. Three days after transduction of mdx myoblasts with the adenoviruses, genomic DNA and RNA were extracted from the cells to examine the gene-editing activity and the dystrophin transcript expression. A 510-bp PCR product was amplified from the genomic DNA of the cells coinfected with cas9 and gRNA viruses, but not from the control cells (Figure 1d). Moreover, a smaller transcript of 475 bp was detected in the gRNA/cas9-coinfected cells (Figure 1e). DNA sequencing confirmed that the smaller transcripts from both C2C12 cells and mdx cells treated with gRNA/cas9 were formed due to successful deletion of exons 21–23 (Figure 1f).
Figure 1.
CRISPR-mediated deletion of a large region in mouse Dmd gene in vitro. (a) Diagram showing the genomic locus of mouse X-chromosome and the gRNA targeting sites. The mutant exon 23 is highlighted in yellow. (b) Polymerase chain reaction (PCR) analysis of genomic DNA extracted from C2C12 cells treated with or without gRNA and Cas9 constructs. (c) RT-PCR analysis of the dystrophin transcript expression in C2C12 cells. (d) PCR analysis of genomic DNA extracted from mdx myoblasts transduced with or without gRNA (Ad-i20/i23) and Cas9 expressing adenovirus. (e) RT-PCR analysis of the dystrophin transcript expression in mdx myoblasts as treated in (d). (f) DNA sequencing analysis of the smaller RT-PCR product (475 bp) in (c) and (e). Arrows indicate the expected bands after gene editing. All data are representative of a minimum of three experiments.
CRISPR-mediated dystrophin rescue normalizes calcium sparks in mdx muscle fibers
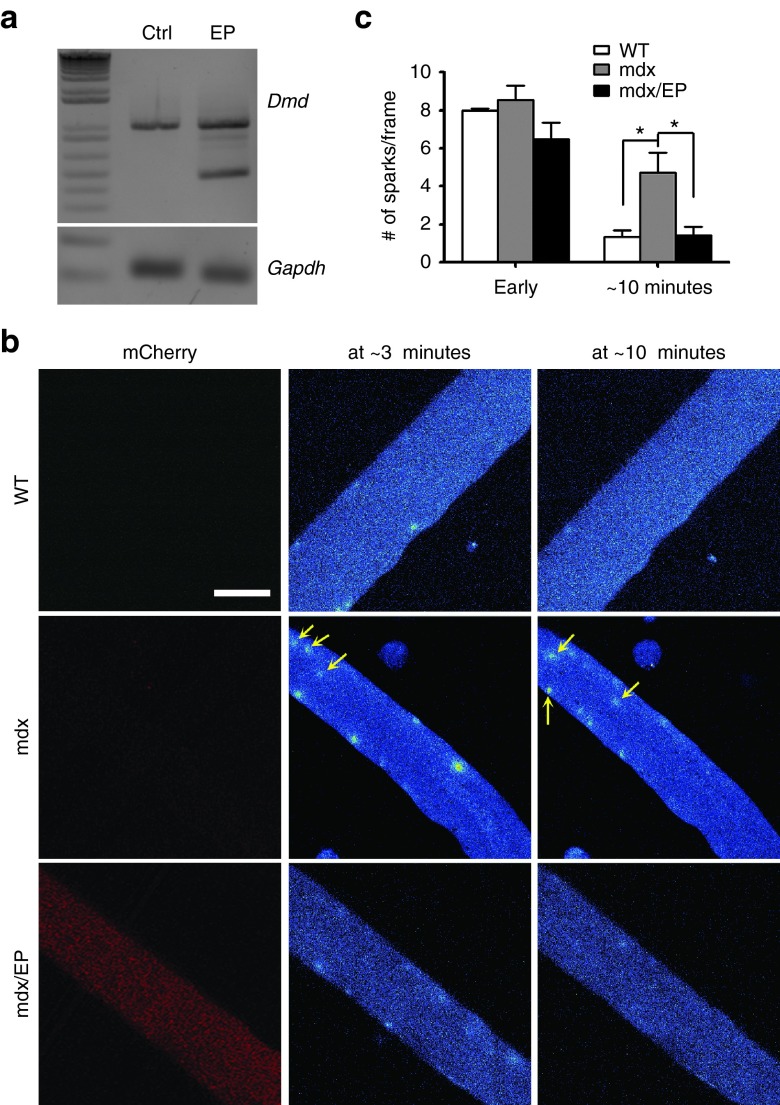
To test whether CRISPR-mediated editing of DMD restores dystrophin expression and function in mdx muscle fibers, we electroporated the cas9/gRNA plasmids into the flexor digitorum longus muscles of adult mdx mice.20,21,22 Male mdx mice at the age of 2 months were electroporated with a combination of the gRNAs and mCherry-2A-Cas9 plasmids and muscles were analyzed 10 days later. Male WT controls and mdx mice were used as controls. Muscle fibers with red fluorescence were widely observed in transfected muscles (data not shown). By RT-PCR analysis, truncated dystrophin transcript can be detected in the treated muscles but not in the control muscles (Figure 2a).
Figure 2.
Osmotic shock-induced calcium sparks in mdx muscle fibers treated with or without mCherry-2A-Cas9/gRNA. (a) RT-PCR analysis of the total RNA extracted from the control flexor digitorum longus (FDB) muscles (Ctrl) or those electroporated with mCherry-2A-Cas9/gRNA plasmids (EP). (b) Osmotic shock-induced calcium sparks at ~3 minutes and ~10 minutes in isolated FDB muscle fibers from mdx mice electroporated with (left) or without (right) mCherry-2A-Cas9/gRNA plasmids. Arrows point to the calcium sparks located at the center of the muscle fibers. Scale bar: 50 µm. (c) Statistical analysis of calcium sparks in mdx muscle fibers electroporated with (male mdx/EP, n = 3) or without (male mdx, n = 4) mCherry-2A-Cas9/gRNA plasmids immediately (Early) or 10 minutes after osmotic shock and male WT controls (n = 4). *P < 0.05.
It has previously been reported that subtle membrane deformation by osmotic shock evokes uncontrolled calcium sparks in mdx muscle fibers but not in controls.23 To examine whether CRISPR-mediated rescue of dystrophin expression could correct the functional defect in mdx skeletal muscle, we studied the calcium sparks in the enzymatically isolated mdx muscle fibers induced by osmotic shock. As shown previously,23 osmotic shock induced an uncontrolled calcium spark response within 25 minutes of recording in nontransfected mdx muscle fibers (Figure 2b,c and Supplementary Figure S1, Supplementary Movie S1), however, the calcium sparks in the transfected mdx muscle fibers (shown by red fluorescence) faded out within 15 minutes similar to the wild-type muscle fibers (Figure 2b,c and Supplementary Figure S1, Supplementary Movie S2) as previously reported.23 Moreover, it is noted that the calcium sparks spread into the center of the mdx muscle fibers (see Figure 2b, middle panel). Such center-localized calcium sparks were spared in the CRISPR-treated mdx muscle fibers. These results suggest that CRISPR-mediated deletion of exons 21–23 restores the expression of dystrophin in mdx muscle fibers and normalizes the osmotic shock-induced Ca2+ sparks in these cells.
CRISPR-mediated gene editing restores dystrophin and dystrophin-glycoprotein complex expression at the sarcolemma of mdx muscles
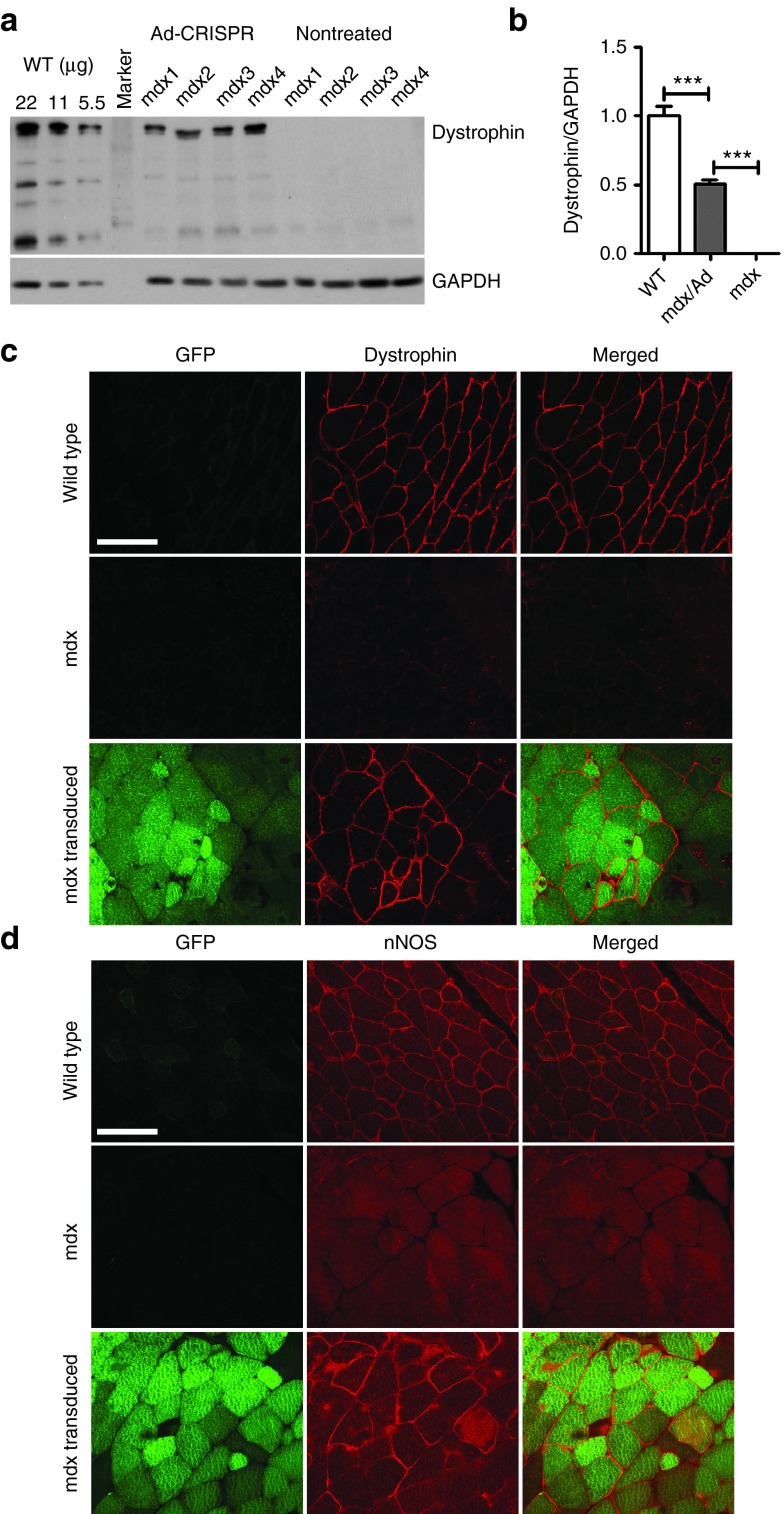
To further investigate whether CRISPR-mediated gene editing could restore dystrophin and its associated protein complex, we injected the adenoviral vectors carrying GFP-2A-cas9 and gRNAs into the gastrocnemius (GA) muscles of newborn pups. Green fluorescent protein (GFP) signals were readily detectable indicating that the adenovirus transduction was successful (Supplementary Figure S2). Dystrophin protein could be detected by western blotting analysis in the mdx muscles transduced with cas9/gRNA-expressing adenoviral particles (Figure 3a). Quantitative analysis showed that dystrophin expression in the mdx muscles transduced with the adenoviral vectors was restored to about 50% of that in WT muscles (P < 0.01) (Figure 3b). We also performed immunofluorescence staining of the muscle sections to study the localization of dystrophin and its associated proteins. Three weeks after adenovirus transduction, dystrophin expression was restored in the muscle fibers that were positive for GFP (Figure 3c). In GFP-negative or nontreated mdx muscle fibers, no dystrophin-positive clusters were observed (Figure 3c). Immunofluorescence staining also demonstrated that neuronal nitric oxide synthase (nNOS), α-sarcoglycan, β-dystroglycan, which are normally located to the sarcolemma in healthy muscles via interaction with dystrophin-glycoprotein complex,24,25 were also restored at the sarcolemma of GFP-positive muscle fibers (Figure 3d, Supplementary Figure S3). These data suggest that the entire dystrophin-glycoprotein complex were restored at the sarcolemma by gene editing. Consistent with the western blotting and immunofluorescence staining results, RT-PCR analysis showed that the smaller dystrophin transcript with the exons 21–23 deleted was expressed in the gene edited muscles (Supplementary Figure S4).
Figure 3.
Restoration of dystrophin and its associated proteins at the sarcolemma of mdx muscles by genome-editing. (a) Western blotting of gastrocnemius muscle homogenates from wild-type (WT), mdx, and mdx with adenoviral vectors carrying GFP-2A-cas9 and gRNA using anti-dystrophin and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies. 22, 11, and 5.5 µg total proteins from the WT muscles were loaded per lane. (b) Quantitative analysis of western blotting in WT, mdx, and mdx injected with adenoviral vectors. (c,d) Confocal immunofluorescence images of dystrophin (c, red) and neuronal nitric oxide synthase (nNOS) (d, red) in muscle cryosections treated with or without EGFP-2A-cas9/gRNA adenovirus. The images are representative of five experiments. Scale bar: 100 µm. ***P < 0.001.
CRISPR-mediated gene editing protects mdx muscles at rest or stress conditions
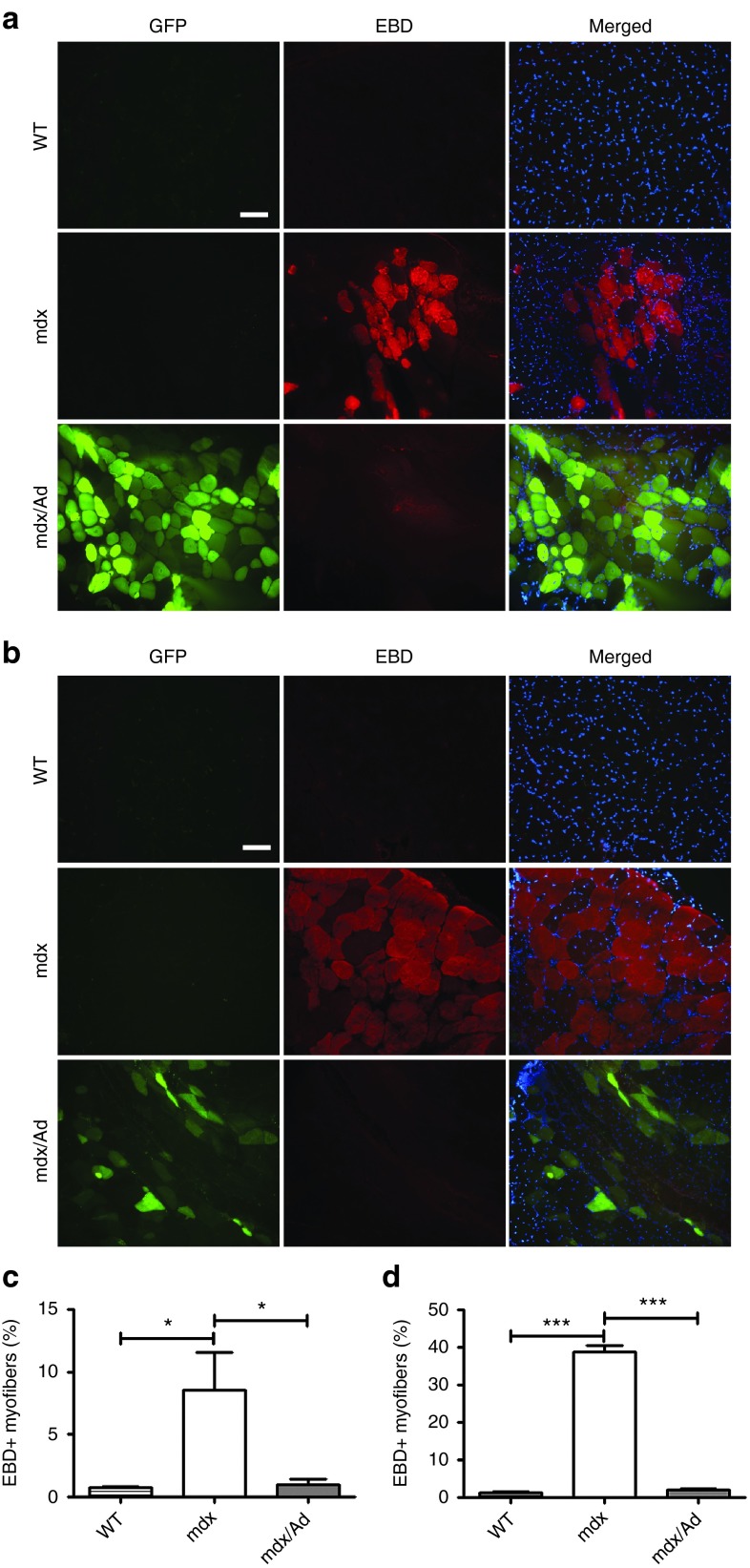
Loss of dystrophin and its interacting complex results in fragile membrane integrity.2 Evans blue dye (EBD) is a reliable in vivo marker of myofiber damage.26 To determine whether the rescue of dystrophin expression could functionally maintain the sarcolemmal integrity of mdx muscles, we assessed the EBD uptake in skeletal muscles of adult male mdx mice injected with or without cas9/gRNA adenovirus. Skeletal muscles of mdx mice at rest showed typical clusters of EBD-positive fibers indicating a compromised sarcolemma integrity (Figure 4a). Interestingly, the EBD uptake in the gene-edited muscles was greatly reduced (Figure 4a,b). Downhill treadmill running further increased the percentage of EBD-positive fibers from mdx mice (Figure 4c,d), but EBD uptake was dramatically inhibited in the gene-edited muscles of mdx mice (Figure 4c,d and Supplementary Figure S5). These results suggest that CRISPR-mediated correction of dystrophin expression also functionally protects the skeletal muscle from injury.
Figure 4.
Functional rescue of dystrophin in mdx mice by CRISPR-mediated gene surgery. (a,b) Evans blue dye (EBD) uptake in the gastrocnemius muscles from male WT (n = 4), mdx (n = 4) and mdx (n = 4) mice injected with cas9/gRNA adenovirus at rest (a) or after downhill treadmill running exercise (b). The images are representative of four experiments. Red: EBD, green: GFP, blue: 4′,6-diamidino-2-phenylindole (DAPI). Scale bar: 100 µm. (c,d) Statistical analysis of EBD-positive muscle fiber percentage in WT, mdx and mdx/Ad preparations at rest (c) and after downhill treadmill running exercise (d). *P < 0.05; ***P < 0.001.
Discussion
Although the genetic cause of DMD has been identified for over three decades,27 and several gene and cell therapies have been developed to deliver a functional copy of DMD or dystrophin-like protein to the diseased tissue, no curative treatment exists.28 In this study, we developed a novel therapeutic strategy based on the CRISPR gene-editing platform to restore dystrophin reading frame in living mdx mice by creating in-frame deletion of the genomic DNA covering exon 23. Our data demonstrated that CRISPR-mediated gene editing efficiently excises a 23-kb region from the Dmd allele and restores the expression of dystrophin and its associated proteins in mdx muscle fibers. Moreover, the restored dystrophin expression functionally corrects muscle membrane defect and normalizes intracellular calcium signaling.
Previous studies by other investigators have demonstrated that the CRISPR technology could be used to correct dystrophin mutations in cultured human DMD patient cells17,18 or one-cell embryos derived from mdx mice,16 but none have taken this approach of restoring dystrophin expression and function to postnatal animals. By injecting Cas9, gRNA and a homologous single-stranded oligodeoxynucleotide (ssODN) template into mouse zygotes, Olson's laboratory demonstrated that up to 9% of live pups carry the corrected dystrophin gene,16 which is consistent with generally lower efficiency through homologous recombination versus nonhomologous recombination end joining. CRISPR-mediated correction of the dystrophin gene was also reported in patient-derived induced pluripotent stem cells by Hotta's group19 and myoblasts by Gersbach's group,18 respectively. Although correcting genetic defects in cultured patient cells holds great potential for translational application, it requires cell transplantation, which poses another big hurdle for success. Even with the immunodeficient mice, dystrophin expression was only scarcely detected in the transplanted tissue.18 As compared to these previous reports, our work took advantage of the highly efficient nonhomologous recombination end joining DNA repair following CRISPR-mediated double-strand break formation to remove mutant exons and demonstrated that dystrophin protein could be restored to about 50% of that in WT skeletal muscle directly in live postnatal mdx mice. Moreover, the restored dystrophin expression normalizes the functional defects in mdx skeletal muscle. These results provide the first evidence that CRISPR-mediated gene correction of disease-causing mutations can be achieved in the muscle tissues of postnatal animals.
Traditional gene therapy delivers a functional copy of the defective gene in the cDNA format. For example, it has been tested to deliver a mini-dystrophin cDNA into animal models of DMD as treatment. The CRISPR-based gene therapy as developed in this study has several advantages over the traditional gene replacement therapy or exon skipping therapy. First, the CRISPR-based therapy works at the genomic level and thus have a long-term effectiveness in restoring the defective gene. Second, it does not require repeated treatment as seen in the exon skipping therapy or traditional gene therapy. This would significantly reduce the cost and complications associated with repeating treatments. Third, it retains most if not all regulatory elements for controlling proper expression of the target gene, whereas the traditional gene replacement therapy lacks. Our study provided the first proof-of-principle evidence that the CRISPR-based gene-editing approach can be applied to restore dystrophin expression in live mdx mice, suggesting that the CRISPR-based gene therapy may be a viable option to treat DMD and other genetic disorders. However, further studies are required to evaluate the durability and safety profile of the CRISPR-based gene therapy approaches via systemic delivery in animal models before they can be used in clinical trials. Specifically, targeted deep sequencing analysis can be used to improve the sensitivity of rare gene editing events, and the safety profile of the in vivo CRISPR-based gene-editing therapy should be rigorously examined for the duration of the animal's life span.
Taken together, we show for the first time that CRISPR-mediated gene editing functionally restores dystrophin expression in live mdx mice. This approach holds promises not only for muscular dystrophy but also for genetic diseases in general.
Materials and Methods
Mice. Mice (C57BL/10ScSn and C57BL/10ScSn-Dmdmdx/J) were maintained at The Ohio State University Laboratory Animal Resources in accordance with animal use guidelines. All animal studies were authorized by the Animal Care, Use, and Review Committee of The Ohio State University.
Construction of Cas9 and gRNA plasmids. All plasmids for CRISPR/Cas9 vector system were constructed as summarized in Supplementary Table S1.
Generation of EGFP-2A-cas9, i20-gRNA, and i23-gRNA adenoviruses. EGFP-2A-cas9, i20-gRNA, and i23-gRNA cassettes were subcloned into pShuttle-CMV vector (Clontech, Mountain View, CA) and recombinant adenovirus genomic DNA were generated using the AdEasy-1 Adenovirus system (Agilent Technologies, La Jolla, CA) according to the manufacturer's instructions. The adenoviral particles were packaged and amplified in AD293 cells and purified by cesium chloride gradient ultracentrifugation followed by dialysis in storage buffer (10 mmol/l Tris-HCl pH 8.0, 2 mmol/l MgCl2, 4% sucrose). The titers of the adenovirus preparations were quantified by measuring the OD260.29
Cell culture and transfection. C2C12 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and electroporated with Neon Transfection System (Invitrogen, Carlsbad, CA). Briefly, 1 × 105 cells were electroporated with 0.25 µg cas9 and 0.125 µg i20-gRNA and 0.125 µg i23-gRNA plasmids. The electroporation conditions were 1,650 V, 10 ms, 3 pulses. Primary mdx myoblasts were isolated from the hind-limb skeletal muscles of mdx mice of 6 weeks old by digestion with collagenase type IA (Sigma-Aldrich, St Louis, MO), and cultured in DMEM/F-12 supplemented with 20% FBS. mdx myoblasts at 50–60% confluence were infected with EGFP-2A-cas9, i20-gRNA, and i23-gRNA adenoviruses at 100 multiplicity of infection. After 48 hours, C2C12 and mdx myoblasts were collected for the following genomic DNA analysis. Electroporated C2C12 cells were cultured in differentiation medium. mdx myotubes were harvested to analyze the dystrophin expression by RT-PCR after 3 days in differentiation medium.
Adenovirus transduction in vivo. To transduce skeletal muscle in vivo, the quadriceps and gastrocnemius muscles of mdx pups (day 1–3) were injected with ~2.5 × 1010 each viral particles. Adult mdx mice were treated similarly with ~6 × 1010 each viral particles. Tissues were collected for the genomic DNA, RNA, western blotting, and immunofluorescence staining experiments.
Extraction of DNA and RNA, and PCR analysis. Total genomic DNA from muscle tissues, C2C12 and myoblast cells were isolated and precipitated by isopropanol. Total RNA was extracted from muscle tissues or cultured cells by using Trizol reagent (Life Technologies, Carlsbad, CA). Total RNA was pretreated with an RNase-free DNase and 5 μg of treated RNA was used as template for first-strand cDNA synthesis by using RevertAid RT Reverse Transcription Kit (Life Technologies). Aliquots of the RT product were used for RT-PCR analysis of dystrophin expression. Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was used as a reference gene for PCR analysis. The primers used for mouse dystrophin genomic DNA were: 5′-GGCCAAAGCAAACTCTGGTA and 5′-TTTAATCCCACGTCATGCAA. The primers used for mouse dystrophin mRNA were: 5′-GGCTAGAGTATCAAACCAACATCAT and 5′-TGGAGGCTTACGGTTTTATCC. The primers used for Gapdh were: 5′-GGAGTTGCTGTTGAAGTC and 5′-ACCTGCCAAGTATGATGA.
Western blotting. Gastrocnemius muscles from mdx mice treated with or without cas9/gRNA AD virus injection were lysed with cold radioimmunoprecipitation assay buffer buffer supplemented with protease inhibitors and extracted protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (BioRad, Hercules, CA, 4–20%) and transferred onto polyvinylidene fluoride membranes (0.45 μm). The rabbit polyclonal anti-dystrophin (E2660, 1:500, Spring Bioscience, Pleasanton, CA) and mouse monoclonal anti-GAPDH (MAB374, 1:2,500, Millipore, Billerica, MA) antibodies were used for immunoblotting analysis. Horseradish peroxidase-conjugated rabbit anti-mouse (1:3,000) and goat anti-rabbit secondary antibodies (1:3,000) were obtained from Millipore (Billerica, CA). The membranes were developed using enhanced chemiluminescence western blotting substrate (Pierce Biotechnology, Rockford, IL) and exposed to film (Kodak, Rochester).
Immunofluorescence staining and confocal imaging. Quadriceps and gastrocnemius muscles were collected from the mdx mice injected with cas9/gRNA-expressing adenovirus or phosphate-buffered saline (PBS). Ten micrometer frozen sections were fixed with 4% paraformaldehyde for 15 minutes at room temperature. The samples were then washed twice with PBS and incubated with blocking solution (10% horse serum) for 1 hour before overnight incubation at 4 °C with primary antibodies. Primary antibodies against dystrophin (ab15277, 1:200, Abcam, San Francisco, CA), nNOS (H-299, 1:200, Santa Cruz Biotechnology, Dallas, TX), α-sarcoglycan (A-SARC-L-CE, 1:100, Leica, Buffalo Grove, IL), and β-dystroglycan (B-DG-CE, 1:100, Leica) were used. The slides were then extensively washed with PBS and incubated with secondary antibodies (Alexa Fluor 555 goat anti-mouse IgG, 1:200, Invitrogen or Alexa Fluor 594 goat anti-rabbit IgG 1:200, Invitrogen, Carlsbad, CA) for 1h at room temperature. Finally, the glass slides were mounted using VECTASHIELD Mounting Medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Then the slides were imaged with an inverted confocal microscope (Zeiss 780, Germany).
EBD uptake. Sterilized EBD/PBS (1% w/v; Sigma-Aldrich) was administered at 100 µl per 10 g body weight through intraperitoneal injection of mdx mice ~24 hours before tissue collection. Muscle sections from EBD-injected animals were incubated in ice-cold acetone at 20 °C for 10 minutes, washed 3 × 10 minutes with PBS, and mounted with Vectashield mounting medium (Vector Laboratories). EBD-positive muscle fibers were counted independently by two investigators on 10-µm cryosections of dye-injected mice. All sections were examined and photographed under a Nikon Ti-E fluorescence microscope (Nikon, Melville, NY).
Calcium spark recording. Flexor digitorum longus muscles electroporated with 40 µg mCherry-2A-cas9/gRNA plasmids were dissected under a fluorescence microscope for mCherry fluorescence as previously described.22 Briefly, mice were deeply anesthetized using 2.5% isoflurane in O2 (1 l/minute). Using a dissection microscope, 10 µl of hyaluronidase solution (2 mg/ml hyaluronidase in sterile Tyrode) was injected under the footpads of one foot of the mouse subcutaneously toward the base of the toes. After 90 minutes, procedure was repeated and then 40 µg mCherry-2A-cas9/gRNA plasmids were injected. After 10–15 minutes, mice were anesthetized for the third time and one gold-plated acupuncture needle was placed under the skin at the heel of the foot and a second one was placed at the base of the toes. Electrodes were oriented parallel to each other and perpendicular to the long axis of the foot. The head of the needles (electrodes) were connected to the electrical stimulator using micro-clip connectors. Muscles were electroplated by applying 20 pulses, 20 ms in duration/each at 1Hz. The voltage was adjusted to 100 V/cm. Freshly isolated flexor digitorum longus muscle fibers were loaded with the calcium indicator Fluo-4-AM (10 µmol/l) for 60 minutes at room temperature.30 Fibers that were selected for analysis were confirmed to have intact sarcolemmal membranes and regular striation patterns by phase-contrast microscopy. Measurements of calcium sparks were performed using a BioRad (Hercules, CA) Radiance-2100 confocal microscope equipped with an argon laser (488 nm) and a 40×, 1.3 NA oil-immersion objective. Serial x–y images of muscle fibers were acquired at 3.08 seconds per frame. Digital Ca2+ image analysis was performed using ImageJ software (NIH) and customer-devised routines.
Statistical analysis. Data are expressed as mean ± standard error of the mean. Statistical differences were determined by unpaired Student's t-test for two groups and one-way analysis of variance with Bonferroni's post-tests for multiple group comparisons using Prism 5.02 (Graphpad). A P value less than 0.05 was considered to be significant.
SUPPLEMENTARY MATERIAL Figure S1. Representative plots of calcium sparks per frame over the 25-minute recording period in isolated FDB muscle fibers of WT, mdx and mdx electroporated with CRISPR plasmids (mdx/EP). Figure S2. Fluorescence images of GFP fluorescence in the GA muscles of newborn pups two weeks after intramuscular injection with or without cas9/gRNA adenovirus. Figure S3. Confocal immunofluorescence images of α-sarcoglycan and β-dystroglycan in muscle cryosections treated with or without EGFP-2A-cas9/gRNA adenovirus. Figure S4. RT-PCR analysis of the total RNA extracted from the gastrocnemius muscles injected with EGFP-2A-Cas9/gRNA adenovirus (mdx/Ad). Figure S5. EBD fluorescence micrograph of the entire cross section of both gastrocnemius muscles (the left side was injected with cas9/gRNA adenovirus and the contralateral side was used as control) from the same mdx mouse after downhill treadmill running exercise Movie S1. Osmotic shock-induced calcium sparks in non-transfected mdx muscle fibers. Movie S2. Osmotic shock-induced calcium sparks in Cas9/gRNA-transfected mdx muscle fibers. Table S1. List of the plasmids used in this study.
Acknowledgments
R.H. is supported by US National Institutes of Health grants R01-HL116546 and R01- AR064241. J.M. is supported by RO1-AG028614 and RO1-AR061385. M.E.R. is a recipient of the NIH T32 postdoctoral fellowship (T32HL0980391). The authors declare no competing financial interests.
Supplementary Material
Osmotic shock-induced calcium sparks in non-transfected mdx muscle fibers.
Osmotic shock-induced calcium sparks in Cas9/gRNA-transfected mdx muscle fibers.
References
- Cohn, RD and Campbell, KP (2000). Molecular basis of muscular dystrophies. Muscle Nerve 23: 1456–1471. [DOI] [PubMed] [Google Scholar]
- Wallace, GQ and McNally, EM (2009). Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol 71: 37–57. [DOI] [PubMed] [Google Scholar]
- Koenig, M, Hoffman, EP, Bertelson, CJ, Monaco, AP, Feener, C and Kunkel, LM (1987). Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50: 509–517. [DOI] [PubMed] [Google Scholar]
- Emery, AE (1991). Population frequencies of inherited neuromuscular diseases–a world survey. Neuromuscul Disord 1: 19–29. [DOI] [PubMed] [Google Scholar]
- Campbell, KP (1995). Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell 80: 675–679. [DOI] [PubMed] [Google Scholar]
- Blake, DJ, Weir, A, Newey, SE and Davies, KE (2002). Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82: 291–329. [DOI] [PubMed] [Google Scholar]
- England, SB, Nicholson, LV, Johnson, MA, Forrest, SM, Love, DR, Zubrzycka-Gaarn, EE et al. (1990). Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature 343: 180–182. [DOI] [PubMed] [Google Scholar]
- Goyenvalle, A, Griffith, G, Babbs, A, El Andaloussi, S, Ezzat, K, Avril, A et al. (2015). Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat Med 21: 270–275. [DOI] [PubMed] [Google Scholar]
- Aartsma-Rus, A and van Ommen, GJ (2007). Antisense-mediated exon skipping: a versatile tool with therapeutic and research applications. RNA 13: 1609–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aartsma-Rus, A, Fokkema, I, Verschuuren, J, Ginjaar, I, van Deutekom, J, van Ommen, GJ et al. (2009). Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum Mutat 30: 293–299. [DOI] [PubMed] [Google Scholar]
- Mali, P, Yang, L, Esvelt, KM, Aach, J, Guell, M, DiCarlo, JE et al. (2013). RNA-guided human genome engineering via Cas9. Science 339: 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong, L, Ran, FA, Cox, D, Lin, S, Barretto, R, Habib, N et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H, Xue, W, Chen, S, Bogorad, RL, Benedetti, E, Grompe, M et al. (2014). Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol 32: 551–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y, Liang, D, Wang, Y, Bai, M, Tang, W, Bao, S et al. (2013). Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell 13: 659–662. [DOI] [PubMed] [Google Scholar]
- Schwank, G, Koo, BK, Sasselli, V, Dekkers, JF, Heo, I, Demircan, T et al. (2013). Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13: 653–658. [DOI] [PubMed] [Google Scholar]
- Long, C, McAnally, JR, Shelton, JM, Mireault, AA, Bassel-Duby, R and Olson, EN (2014). Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 345: 1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfield, G, Siller, WG, Wight, PA and Moore, KJ (1984). X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81: 1189–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousterout, DG, Kabadi, AM, Thakore, PI, Majoros, WH, Reddy, TE and Gersbach, CA (2015). Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat Commun 6: 6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, HL, Fujimoto, N, Sasakawa, N, Shirai, S, Ohkame, T, Sakuma, T et al. (2015). Precise correction of the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Reports 4: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara, H and Miyazaki, J (1998). Gene transfer into muscle by electroporation in vivo. Nat Biotechnol 16: 867–870. [DOI] [PubMed] [Google Scholar]
- McMahon, JM, Signori, E, Wells, KE, Fazio, VM and Wells, DJ (2001). Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase – increased expression with reduced muscle damage. Gene Ther 8: 1264–1270. [DOI] [PubMed] [Google Scholar]
- Tjondrokoesoemo, A, Park, KH, Ferrante, C, Komazaki, S, Lesniak, S, Brotto, M et al. (2011). Disrupted membrane structure and intracellular Ca2+ signaling in adult skeletal muscle with acute knockdown of Bin1. PLoS One 6: e25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X, Weisleder, N, Collet, C, Zhou, J, Chu, Y, Hirata, Y et al. (2005). Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat Cell Biol 7: 525–530. [DOI] [PubMed] [Google Scholar]
- Chang, WJ, Iannaccone, ST, Lau, KS, Masters, BS, McCabe, TJ, McMillan, K et al. (1996). Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci USA 93: 9142–9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie, RH, Barresi, R and Campbell, KP (2002). Loss of sarcolemma nNOS in sarcoglycan-deficient muscle. FASEB J 16: 1786–1791. [DOI] [PubMed] [Google Scholar]
- Hamer, PW, McGeachie, JM, Davies, MJ and Grounds, MD (2002). Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat 200(Pt 1): 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, EP, Brown, RH Jr and Kunkel, LM (1987). Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51: 919–928. [DOI] [PubMed] [Google Scholar]
- van Deutekom, JC and van Ommen, GJ (2003). Advances in Duchenne muscular dystrophy gene therapy. Nat Rev Genet 4: 774–783. [DOI] [PubMed] [Google Scholar]
- Sweeney, JA and Hennessey, JP Jr (2002). Evaluation of accuracy and precision of adenovirus absorptivity at 260 nm under conditions of complete DNA disruption. Virology 295: 284–288. [DOI] [PubMed] [Google Scholar]
- Han, R, Grounds, MD and Bakker, AJ (2006). Measurement of sub-membrane [Ca2+] in adult myofibers and cytosolic [Ca2+] in myotubes from normal and mdx mice using the Ca2+ indicator FFP-18. Cell Calcium 40: 299–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Osmotic shock-induced calcium sparks in non-transfected mdx muscle fibers.
Osmotic shock-induced calcium sparks in Cas9/gRNA-transfected mdx muscle fibers.