Abstract
Novel object and location recognition tasks harness the rat’s natural tendency to explore novelty (Berlyne, 1950) to study incidental learning. The present study examined the ontogenetic profile of these two tasks and retention of spatial learning between postnatal day (PD) 17 and 31. Experiment 1 showed that rats ages PD17, 21, and 26 recognize novel objects, but only PD21 and PD26 rats recognize a novel location of a familiar object. These results suggest that novel object recognition develops before PD17, while object location recognition emerges between PD17 and PD21. Experiment 2 studied the ontogenetic profile of object location memory retention in PD21, 26, and 31 rats. PD26 and PD31 rats retained the object location memory for both 10-min and 24-hr delays. PD21 rats failed to retain the object location memory for the 24-hr delay, suggesting differential development of short- versus long-term memory in the ontogeny of object location memory.
Keywords: hippocampus, perirhinal cortex, short-term retention, long-term retention, incidental learning, Long-Evans
INTRODUCTION
Novel object recognition (OR) has become a widely used paradigm in behavioral neuroscience (Ainge & Langston, 2012; Barker, Bird, Alexander, & Warburton, 2007; Barker & Warburton, 2011; Bermudez-Rattoni, Okuda, Roozendaal, & McGaugh, 2005; Dix & Aggleton, 1999; Ennaceur & Delacour, 1988; Kruger, Brockmann, Salamon, Ittrich, & Hanganu-Opatz, 2012; Mumby, Gaskin, Glenn, Schramek, & Lehmann, 2002; Norman & Eacott, 2005; Reger, Hovda, & Giza, 2009; Warburton, Barker, & Brown, 2013; Winters, Saksida, & Bussey, 2008). The task harnesses the natural tendency of rats to explore novel objects (Berlyne, 1950), in order to study the underlying neural mechanisms of perceptual and memory processes involved in object recognition. In the OR task, the rat explores two identical objects during the study phase, is removed for a delay, and returned to the chamber where one object is the same as the study phase (familiar) and one object has been replaced (novel). The rat expresses its recognition of the familiar object by preferentially exploring the novel object. Using this basic principle, other tasks have been developed to examine object location recognition (OL), object-in-context recognition, and many more.
The object location recognition (OL) task has recently been of interest in our lab (Jablonski, Schreiber, Westbrook, Brennan, & Stanton, 2013). The OL task is similar to the OR task, except the novel manipulation is the movement of one familiar object to a different location within the chamber. Following the logic of the OR task, the rat expresses its recognition of the object locations in the OL task by preferentially exploring the familiar object in the novel location. The OL task involves learning about spatial information in the absence of any reinforcement. This type of learning, called incidental spatial learning, is studied in our lab using another paradigm called the context preexposure facilitation effect, or CPFE (Jablonski, Schiffino, & Stanton, 2012; Schiffino, Murawski, Rosen, & Stanton, 2011). This type of learning is useful for studying memory functions that depend on temporal cortical structures (perirhinal, postrhinal, and entorhinal cortices) and their interactions with the hippocampal formation (Rudy, 2009; see General Discussion section).
Both the novel object and object location recognition tasks have been studied ontogenetically (Ainge & Langston, 2012; Jablonski et al., 2013; Kruger et al., 2012; Reger et al., 2009). Novel object recognition has been shown in rats as young as PD15-17 (Kruger et al., 2012). On the other hand, the recent literature on the ontogeny of spatial variants of object recognition tasks has been mixed. Kruger et al. (2012) demonstrated OL performance in rats as young as PD16, while Ainge & Langston (2012) showed that PD24 rats could not perform an object-in-place task that also elicits exploration of a familiar object in a novel location. We have obtained OL performance in PD21 and 26 rats (Jablonski et al., 2013) but the present report is the first to determine whether preweanling rats can perform the OL (or OR) tasks using our procedures.
Other mixed results have been obtained with the object location recognition task after a 24 hr retention interval in adult rats (Barker & Warburton, 2011; Larkin et al., 2008; Ozawa, Kumeji, Yamada, & Ichitani, 2012; Ozawa, Yamada, & Ichitani, 2011). Some studies have shown that adult rats can remember object location for up to 24 hr (Barker & Warburton, 2011; Larkin et al., 2008), while other studies have shown that adult rats cannot remember object location at 24 hr (Ozawa et al., 2011, 2012). To our knowledge, the present study is the first to examine long-term retention of object location performance in developing rats.
The present study extends the novel object recognition literature by examining the ontogenetic emergence of OL task performance after a short delay in PD17, 21, and 26 rats (Exps. 1A–C); and of long-term retention (up to 24 hr) in PD21, 26 and 31 rats (Exps. 2A and B). The ages chosen are lab precedents which are used because they span the period from preweanling to juvenile to adolescence in the rat and are transitional for many kinds of learning and memory tasks (e.g., Stanton, 2000).
EXPERIMENT 1A: ONTOGENY OF OBJECT AND SPATIAL MEMORY
Experiment 1 extended the developmental literature and recent research in our lab (Jablonski et al., 2013) on novel object and object location recognition in developing rats by examining factors relevant to task performance. These factors included age (Exp. 1A), weaning (Exp. 1B), and object study time (Exp. 1C).
Experiment 1A sought to examine the ontogenetic profile of performance on the OR and OL tasks in PD17, 21, or 26 rats. The data from PD21 and 26 rats were taken from our previous report (Jablonski et al., 2013). They are analyzed together with PD17 rats that were trained and tested with identical procedures and re-reported here to provide a broader age comparison. Although data from the previous report were collected at a different time, the stability of our data across time and across experiments justifies their inclusion in this report (see Data Collection and Analysis section, for further details). PD17 and 21 rats remained in their litters during testing whereas PD26 rats were weaned on PD21 and were housed individually during testing. At each age, animals of both sexes were tested on either the OR or OL tasks.
Materials and Methods
Subjects
The animal colony and maintenance are the same as described in our previous report (Jablonski et al., 2013). Subjects were Long-Evans rats bred at the University of Delaware, Office of Laboratory Animal Medicine (OLAM). Females were time-bred and housed in clear polypropylene cages when pregnant. The cages were 45 cm × 24 cm × 21 cm in dimensions containing standard bedding and allowing ad lib food and water access for the dam. During the light cycle (12:12), the cages were checked for births. The day on which a newborn litter was found was designated postnatal day (PD) 0, indicating the offspring’s birth-date. Litters were moved from the breeding facility to the laboratory colony rooms on PD2. On PD3, litters were culled to eight pups (usually four males and four females) and were paw-marked by a subcutaneous injection of nontoxic black ink for identification purposes.
A total of 104 (51 M; 53 F) Long-Evans rats from 22 litters were the subjects. Subjects were assigned to one of three age groups—PD17, PD21, or PD26—and one of two tasks—OR or OL. Instances in which same-sex littermates contributed data to the same experimental group (Task, Age, Sex) were averaged in order to analyze the data as a single observation statistically. Sixteen instances of same-litter sampling occurred (OR Task, PD17, F, n = 1, M, n = 2; OR Task, PD21, F, n = 1, M, n = 1; OR Task, PD26, F, n =2, M, n = 2; OL Task, PD17, F, =1, M, =1; OL Task, PD21, F, n = 2; OL Task, PD26, F, n = 2, M, n = 1). Rats in the PD17 and PD21 age groups were kept as litters with their dam throughout the study. Rats in the PD26 age group were weaned on PD21 and housed by sex with littermates in clear polypropylene cages (45 cm × 24 cm ×17 cm) with ad lib access to food and water. Two days prior to the beginning of habituation (PD23), weaned rats were individually housed in white polypro-pylene cages (24 × 18 × 13 cm) with ad lib access to food and water, where they stayed for the length of the study. Rats in the PD17 and PD21 group were transported individually to and from the behavioral testing room in the same type of cages in which the PD26 group was individually housed.
Apparatus
The apparatus has been described by Jablonski et al. (2013). We used two identical circular chambers made of white polyester resin panels—78.7 cm in diameter, 48.9 cm walls—raised 26.7 cm off the floor, with spatial cues (black “X” and striped circle) placed at the top of the walls out of reach of the rats. In addition to these proximal cues, the well-lit room allowed the sight of more distal cues of the room surrounding the arena. A tripod-mounted camera permitted digital recording of data (see Data Analysis section). The objects—a fake green apple (7 cm × 7 cm at the widest point), a glass jar filled with blue stones (8.9 cm × 7.6 cm at the widest point), and a white hook with a flat bottom (7.6 cm × 8.9 cm at the widest point) —and their placement in the test arena were also the same as described previously (Jablonski et al., 2013).
Procedure
The procedure was exactly as described previously (Jablonski et al., 2013).
Habituation
Rats received three habituation sessions to the arena devoid of objects. Before each habituation session, the rats were handled for 3 min in the laboratory animal housing room. Rats were then weighed and carted to the behavioral testing room in their home cage, where they waited on the cart while the arenas were cleaned with 70% ethanol solution. Each habituation (and testing) session began by placing the rat in the center of the arena facing the north position. The rat was left in the empty arena for 10 min before being placed back in its home cage and carted back to the animal housing room. Habituation sessions 1 and 2 took place the morning (~0800–1200 hr) and approximately 5 hr later in the afternoon (~1300–1700 hr), respectively, of PD16 for the PD17 age group, PD20 for the PD21 age group, and PD25 for the PD26 age group. The final habituation session (Session 3) occurred the following morning.
Testing
The testing session (Session 4) occurred the afternoon of PD17, PD21, or PD26. Rats were weighed in the animal housing room, before being carted to the behavioral testing room in their home cages. Rats remained on the cart in the hallway of the behavioral testing room, while the arenas and objects were cleaned with 70% ethanol solution. Rats were tested on either the Novel Object Recognition (OR) task or the Object Location Recognition (OL) task. Arena configuration (see Fig. 1 in Jablonski et al., 2013), arena number, task order, object identity (OR), and object movement (OL) were counterbalanced across the sex and age group variables (Jablonski et al., 2013).
Novel Object Recognition (OR) task
Two of the same objects (fake apple or glass jar) were securely placed in the arena (the handle of the glass jar always pointed to the east wall). For the study phase, the rat was placed in the center of the arena facing the north position. The rat was removed from the arena containing the objects after 5 min and put back in its home cage. The rat remained undisturbed on the cart in the lab hallway for a 5-min delay period. During this delay, one of the objects was replaced with the opposite object for the test phase. For example, if the arena contained two copies of the fake apple during the study phase, one would be replaced so that it contained one fake apple (familiar object) and one glass jar (novel object) during the test phase (Fig. 1A). The arena and objects were cleaned with 70% ethanol solution. At the end of the 5-min delay period, the rat was returned to the arena for a test phase lasting 3 min. At the end of the test phase, the rat was returned to its home cage and carted back to the animal housing room. Exploration of each object in the study and test phases was measured. Exploration was defined as actively sniffing, pawing at, or whisking with its snout directed toward the object (see Data Analysis section).
FIGURE 1.
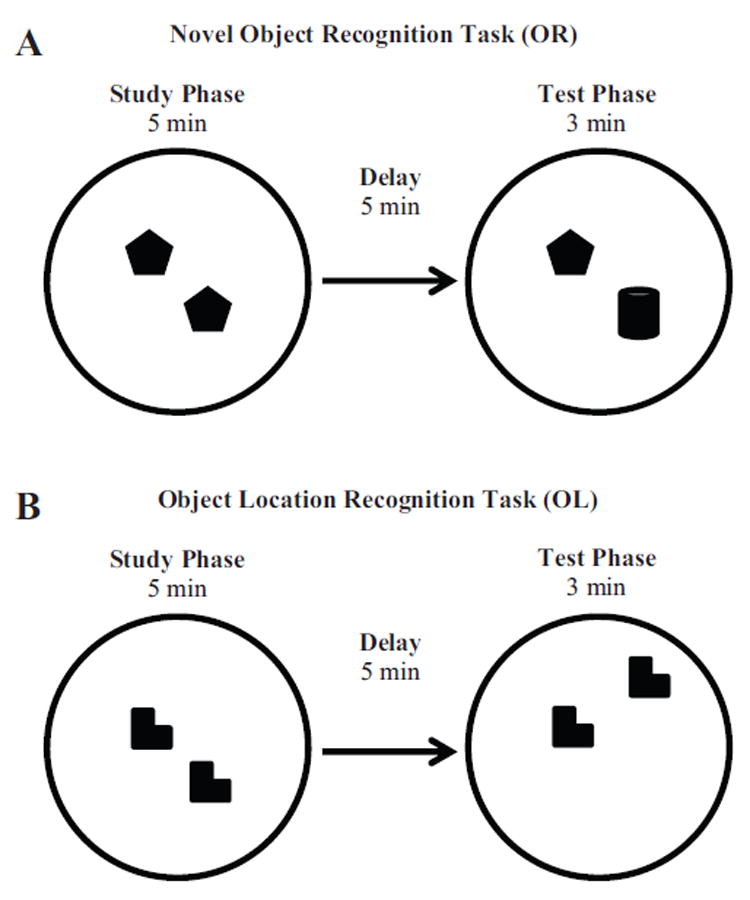
A: A schematic diagram of the novel object recognition (OR) task. The objects here are shown in Configuration 1. In the sample phase, the rat is placed in a chamber with two identical objects (fake apple) for 5 min. Following the sample phase, the rat is removed from the arena for a 5-min delay. When the rat is returned to the chamber for the test phase, one object from the sample phase has been replaced with a novel object (glass jar). B: A schematic diagram of the object location recognition (OL) task. Objects are shown in Configuration 1. The procedure is the same as that for the OR task except the test phase. In the test phase, one of the familiar objects is moved to a novel location in the arena.
Object Location Recognition (OL) Task
Previous studies in our lab have shown that the hook produces the best results for this task (Jablonski et al., 2013); thus, this object was exclusively used for the OL task in the present study. Two copies were securely placed in the arena so that the hook pointed toward the east wall. The procedure for the OL task was the same as that previously described for the OR task, except that the objects in the test phase were both familiar but one was moved to a novel location in the arena (Fig. 1B). For example, for Configuration 1, the study phase consisted of one hook in position 1 and one hook in position 4. For the test phase, the hook in position 4 was moved to position 2n (novel location), while the hook in position 1 remained in that position (familiar location). The distance between the familiar location and novel location was conserved across the study and test phase.
Data Collection and Analysis
As described previously (Jablonski et al., 2013), all habituation and testing sessions were recorded using a video camcorder (Panasonic USA, Model #SDR-H85P). The digital recordings were transferred to a personal computer and scored later by an observer blind to the experimental conditions. The observer analyzed the test phase using a timing program (NILA Lab, Temple University), where the rat’s exploration time of each object could be tracked by clicking on the respective left and right timer buttons. A second blind observer analyzed a subset of the data in order to calculate inter-observer reliability. Correlation analysis revealed high agreement (median r =.881; range =.451–.992; all ps <.01) and t-tests revealed no significant difference between observers (median p =.746; range =.078–.999).
Study phase exploration times were analyzed between age groups to determine any differences in total exploration across ages and were analyzed by ANOVA (using STATISTICA 12 software). When appropriate, post-hoc Newman-Keuls analyses were conducted. To quantify object preference during the test phase, an exploration ratio was converted from the raw exploration times corresponding to each object in the test phase. Exploration ratios were calculated using the equation, tnovel/(tnovel + tfamiliar), where the time spent exploring the novel object is divided by the total time spent exploring both objects during the test phase (Mumby et al., 2002). Following conventions in the literature (Dix & Aggleton, 1999), one-sample t-tests were used to compare exploration ratios of each age group for each task to an exploration ratio indicating no preference (.5). ANOVAs were not performed because they do not include this no-preference comparison condition and therefore do not test the experimental hypothesis. In addition, ANOVAs are often insufficiently powered, relative to one-sample t-tests, to detect group × task interactions involving the small effect sizes that are typical of object recognition tasks (Kruger et al., 2012; Matagne, Budden, Ojeda, & Raber, 2013; Mumby et al., 2002; Reger et al., 2009).
To address a potential concern that PD21 and 26 data from our previous report should not be analyzed together with PD17 data because of cross-experiment variability, we examined how stable and replicable our findings are across studies by performing t-tests of animals run under the same conditions in different experiments. For PD21 and 26 rats, we compared previous data used here in Exp. 1A with their age and group (nonrestricted) counterparts in Exp. 1C as well as in Exp. 2B (10-min retention). The mean (±SE) for the PD26 rats in Exps. 1A, 1C and 2B, respectively were .66 (±.03), .60 (±.04), and .65 (±.04). None of these differences were statistically significant (all ps >.31). For PD21 rats, these respective means were .69 (±.03), .65 (±.06), and .64 (±.04), and also failed to differ statistically (all ps >.40). More importantly, the presence of novelty preference assessed by one-sample t-test is significant in all cases.
Results and Discussion
Results
After accounting for same-litter sampling, data from five animals met the criteria of a statistical outlier and were excluded from the analyses (OR Task, PD17, M, n = 1; OR Task, PD21, F, n = 1; OL Task, PD21, F, n = 1; OR Task, PD26, F, n = 1; OL Task, PD26, M, n = 1). Outliers were defined as a mean total exploration ratio that exceeded ±2 standard deviations from the group mean in an individual session. Data from the remaining 81 subjects were used in the analyses (OR Task, PD17, n = 9; OR Task, PD21, n = 14; OR Task, PD26, n = 17; OL Task, PD17, n = 10; OL Task, PD21, n = 13; OL Task, PD26, n = 17).
Study Phase
Baseline exploration increased with age (Fig. 2). Comparison of the baseline exploration levels in the study phase between Age and Sex were conducted using a × (age) ×2 (task) ×2 (sex) factorial ANOVA. The ANOVA showed a main effect of Age, such that the PD17 total exploration during the study phase was significantly less than that of PD21 and PD26 [F(2,75) =36.52, p <.0001]. No other main effects or interactions were found (all Fs <1.15). New-man-Keuls post-hoc analysis showed that the PD17 group total exploration time (11.87 ± 2.24) during the study phase differed significantly from that of the PD21 group (44.63 ± 2.99; p <.001) and the PD26 group (51.90 ± 3.51; p <.001). However, the total exploration time did not differ between the PD21 and PD26 groups (p >.12).
FIGURE 2.
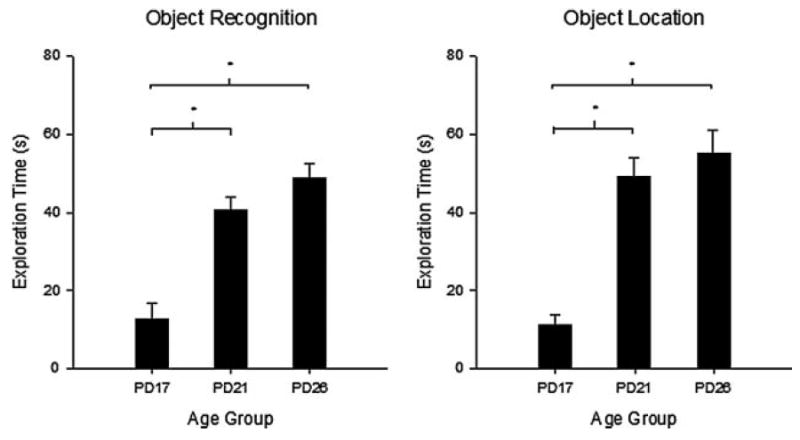
Total exploration time (s) for each age group during the study phase of Experiment 1A. Mean exploration times are shown with error bars representing SEM. (*p <.01). Rats in the PD17 age group explored objects for significantly less time than rats in either the PD21 or PD26 groups, which did not differ.
Test Phase
The results of novelty preference appear in Figure 3. Rats performed the OR task at all ages, but performance on the OL task emerged between PD17 and PD21. One-sample t-tests of exploration ratios compared to chance performance (.5) revealed high novel object preference, regardless of age (PD17: .652, p <.01; PD21: .752, p <.001; PD26: .724, p <.001). The PD21 and PD26 groups showed significant object location novelty preference as well (PD21: .621, p <.009; PD26: .599, p <.05). The PD17 group did not show a significant object location novelty preference (.519; p >.83).
FIGURE 3.
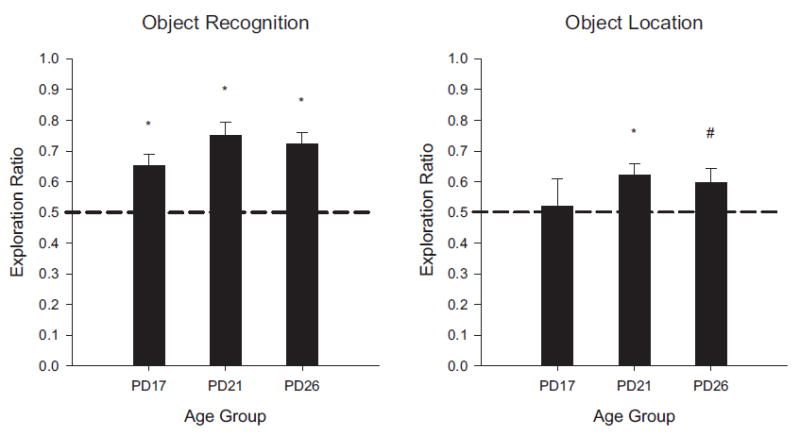
Results of Experiment 1A shown as novelty exploration ratios by age group in the object recognition task (left panel) and the object location recognition task (right panel). Exploration ratios were calculated as (time exploring novel)/(total exploration time of both objects). Rats in each condition exhibited significant novelty preference, except for the PD17 rats in the OL task which did not significantly differ from chance performance (.5). (#p <.05; *p <.01)
Discussion
PD17, 21, and 26 rats displayed significant novelty preference in the novel object recognition task. However, only the PD21 and 26 rats exhibited significant novelty preference in the object location recognition task, suggesting that object location recognition emerges between PD17 and PD21.
EXPERIMENT 1B: WEANING EFFECT ON ONTOGENY OF OBJECT AND SPATIAL MEMORY
The previous experiment showed that PD17, 21, and 26 rats displayed novel object recognition, while only PD21 and 26 rats showed object location recognition after the short retention interval. In Experiment 1A, the PD21 rats were not weaned prior to the experiment, while the PD26 rats were weaned normally on PD21. Although performance did not differ between PD21 and 26 rats, it is possible that the confound with weaning over this age range obscured potential age differences that would be evident if the weaning variable was held constant. In our lab, weaning usually occurs on PD21. If we were to wean the PD21 group, they would be weaned early (PD20) because the first day of the experimental protocol begins on PD20. Early weaning is not the best way to examine age difference obscured by weaning because of the dangers of weaning rats too early and also the stress associated with weaning would coincide with the experimental testing. Therefore, Experiment 1B sought to examine any effects of weaning on the OR and OL tasks by studying PD26 rats that were not weaned and remained with the dam for the duration of the experiment. Their data were compared with weaned PD26 rats, to examine the effect of weaning at this age, and with non-weaned PD21 rats, to examine age effects without the confounding effect of weaning.
Materials and Methods
Subjects
Subjects were 16 (8 M; 8 F) Long-Evans rats from 4 litters. The animal colony and maintenance were the same as in Experiment 1A, with the exception of weaning. The rats were not weaned at PD21, but rather stayed with the dam throughout the experiment (PD25-PD26). Only one instance of same-litter sampling occurred (OR Task, M, n = 1) and data were averaged to yield a single score as described in Experiment 1A.
Apparatus and Procedure
The apparatus and procedure were the same as in Experiment 1A.
Data collection and Analysis
Data collection and analysis were the same as in Experiment 1A. A one-sample t-test was used to compare exploration ratios of the test phase against that of chance performance (.5). An independent samples t-test was used to compare exploration ratios of the non-weaned PD26 rats to that of the weaned PD26 rats and non-weaned PD21 rats from Experiment 1A.
Results and Discussion
Results
After accounting for same-litter sampling, data from two animals met the criteria as a statistical outlier as described in Experiment 1A and were excluded from the analyses (OR Task, F, n = 1; OL Task, F, n = 1). Data from the remaining 13 subjects were used in the analyses (OR Task, n = 6; OL Task, n = 7).
Study Phase
Comparison of the baseline exploration levels in the study phase between Task and Sex were conducted using a mixed factorial ANOVA. The ANOVA showed no significant main effects or interactions (all Fs < 1.7), confirming that there was no statistical difference in total exploration levels during the study phase between weaned PD26 rats (51.90 ± 3.51 s) and non-weaned PD26 rats (54.43 ± 6.55 s). The ANOVA between the non-weaned PD21 rats (44.63 ± 2.99 s) and non-weaned PD26 rats (54.43 ± 6.55 s) revealed no significant main effects or interactions (all Fs <2.1), showing that there was no statistical difference between the different aged non-weaned groups in object exploration levels during the study phase.
Test Phase
The novelty preference results appear in Figure 4. Non-weaned PD26 rats performed both the OR and OL tasks, consistent with that of the weaned PD26 rats from Experiment 1A, suggesting that weaning does not affect task performance. One-sample t-tests were conducted on exploration ratios compared to chance performance (.5). In the OR task, the non-weaned PD26 rats explore the novel object significantly more than the familiar object (.748 ±.071, p <.02). In addition, the non-weaned PD26 rats exhibit significant object location novelty preference in the OL task (.621 ±.039, p <.03). An independent-samples t-test was conducted on exploration ratios between weaned (Experiment 1A) and non-weaned PD26 rats for each task, revealing no significant difference between groups in either task (OR Task, Weaned PD26, Non-weaned PD26; p >.74; OL Task, Weaned PD26, Non-weaned PD26; p >.77). Another independent-samples t-test was conducted on exploration ratios between non-weaned PD21 rats (Experiment 1A) and non-weaned PD26 rats for each task, showing no significant differences (OR Task, Non-weaned PD21, Non-weaned PD26; p >.95; OL Task, Non-weaned PD21, Non-weaned PD26; p >.99).
FIGURE 4.
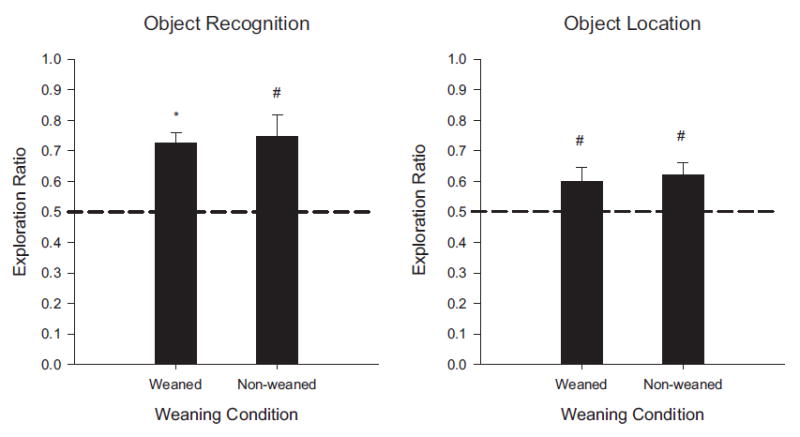
Results of Experiment 1B shown as novelty exploration ratios by weaning group in the OR task (left panel) and OL task (right panel). The data for the weaned PD26 group is the same as that reported in Experiment 1A. Exploration ratios were calculated as (time exploring novel)/(total exploration time of both objects). Rats in each condition exhibited significant novelty preference. (#p <.05; *p <.01).
Discussion
Experiment 1B showed that failure to wean rats at PD21 neither enhanced nor impaired subsequent performance of novel object or location recognition on PD26, compared to weaned PD26 rats and non-weaned PD21 rats from Experiment 1A. Previous studies have shown impairments as well as enhancements in other tests of spatial memory in adult rats subjected to isolation during the third postnatal week (Frisone, Frye, & Zimmerberg, 2002; Sandstrom, 2005; Sandstrom & Hart, 2005). Weaning stress could be reduced relative to isolation stress since weaned rats are housed with same-sex littermates. Traditionally, weaning occurs during the third postnatal week around PD21. The null effect of weaning in rats tested at PD26 suggests that the stress of weaning neither impairs nor improves recognition memory as assessed with the OR and OL tasks used here.
EXPERIMENT 1C: RESTRICTED STUDY PHASE
In Experiment 1A, rats in the PD17 age group explored the objects in the study phase significantly less than the rats in the older age groups (PD21 and PD26). Although, the PD17 rats were able to display a preference for the novel object in the OR task, the reduced object study time may have prevented these young rats from showing a preference for the object in a novel location in the OL task. As a result, the age differences in Experiment 1A may reflect the longer object study times on the OL task shown by the older age groups. Experiment 1C sought to address this problem with the age comparison by manipulating the object exploration time during the study phase of the older rats (PD21 and PD26) so it was comparable to PD17 rats in object exploration. This was achieved by restricting the exploration period to 1 min (Group Restricted). Performance of these rats was compared with groups given the standard 5-min exploration used previously in Exp. 1A (Group Non-restricted). If object study time, rather than age, accounted for differences in OL performance in Exp. 1A, then the restricted groups in the present study should not be able to perform the OL task.
Materials and Methods
Subjects
Subjects were 64 (35 M; 29 F) Long-Evans rats sampled from 13 litters obtained, housed, and reared as described previously. Subjects were assigned to either PD21 or PD26 age group and either Restricted or Non-restricted group. The weaning procedure for the age groups was the same as described in Experiment 1A. One animal failed to meet the exploration criteria (greater than 1 s exploration) in the study phase (Restricted, PD21, F, n = 1), and another failed to meet the exploration criteria in the test phase (Non-restricted, PD26, F, n = 1). Both animals were excluded from the analyses. Instances of same-litter sampling were averaged as previously described. There were two instances of same-litter sampling (Non-restricted, PD26, M, n = 2).
Apparatus, Design, and Procedure
The apparatus and procedure were the same as described in Experiment 1A. An Age (PD21, 26) × Study Time (Restricted, Non-restricted) × Sex between-groups factorial design was used. Study time was manipulated by reducing the duration of the study phase to 1 min in the Restricted group. Pilot studies determined that this manipulation produced durations of exploration in PD21-26 rats that were comparable to PD17 rats given a 5-min study phase (Exp. 1A). Performance of the Restricted group was compared with rats allowed 5 min of exploration (Non-restricted) in order to directly examine the effect of the exploration time factor.
Data Collection and Analysis
Data collection and analysis was the same as Experiment 1A.
Results and Discussion
Results
Data from six animals met the criteria for a statistical outlier as previously described and were excluded from the analyses (Non-restricted, PD21, M, n = 1, F, n = 1; Restricted, PD21, M, n =1; Non-restricted, PD26, M, n = 1, F, n = 1; Restricted, PD26, M, n = 1, F, n = 1). Data from the remaining 54 subjects were used in the analyses (Non-restricted, PD21, n = 14; Restricted, PD21, n = 13; Non-restricted, PD26, n = 12; Restricted, PD26, n = 15).
Study Phase
A mixed factorial ANOVA was conducted to compare baseline exploration levels between Sex, Age, and Study Time. The ANOVA revealed only a main effect of Study Time [F(1,46) = 52.718, p <.001], such that the Restricted group (16.00 ± 1.26 s) explored significantly less than the Non-restricted group (56.63 ± 5.58 s). A mixed factorial ANOVA comparing the PD17 group from Exp. 1A and the restricted PD21 and PD26 groups from the present experiment revealed a main effect of Age group [F(2,33) =3.812, p <.04], such that the PD26 group spent significantly more time exploring than both the PD17 and PD21 groups, which did not differ (Fig. 5). This confirms that the manipulation effectively reduced study time in PD21 rats, comparable to that of PD17 rats in Exp. 1A.
FIGURE 5.
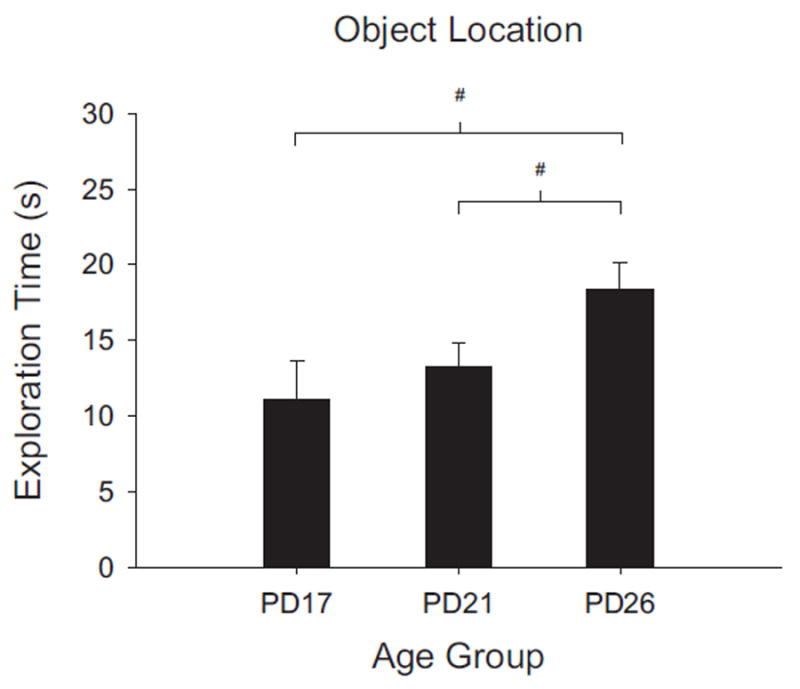
Total exploration time (s) for each age group during the study phase of Experiment 1C. The PD17 group is the same as that reported from Experiment 1A. The PD21 and PD26 groups shown are only from the Restricted group in Exp. 1C. Mean exploration times are shown with error bars representing SEM. (#p <.05). Rats in the Restricted PD26 age group explored objects for significantly more time than rats in either the PD17 or Restricted PD21 groups, which did not differ. The restricted time manipulation made the study phase exploration times of the PD21 rats comparable to that of the PD17 rats from Experiment 1A.
Test Phase
One-sample t-tests were conducted to compare exploration ratios to chance performance (.5). PD21 rats display a preference for the familiar object in the novel location in the Restricted group (.60 ± 04) and in the Non-restricted group (.65 ±.06). Both groups, Restricted (.60 ±.04) and Non-restricted (.60 ±.04), of PD26 rats also show the same preference. The exploration ratios of the Restricted PD21 and PD26 groups as well as the PD17 group from Exp. 1A are shown in Figure 6.
FIGURE 6.
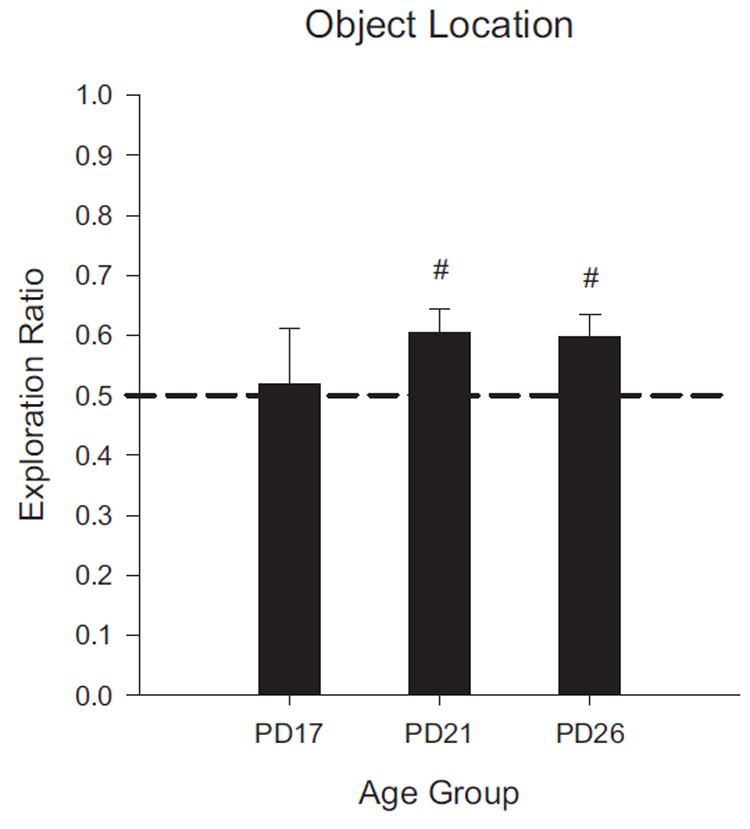
Results of Experiment 1C shown as novelty exploration ratios by age group. The PD17 group is the same as that reported from Exp. 1A. The PD21 and PD26 groups shown are only from the Restricted group in Exp. 1C. Exploration ratios were calculated as (time exploring novel)/(total exploration time of both objects). Rats exhibited significant preference for the displaced object only at PD21 and 26 ages. (#p <.05).
Discussion
Experiment 1C showed that shortening the length of the study phase reduces the overall exploration times in the study phase, regardless of age, but only the manipulation in PD21 rats reduced the study phase exploration times to a comparable level to the PD17 rats from Exp. 1A. In addition, the reduction in exploration time has no effect on performance of the OL task. Thus, the differential OL performance between PD17 and PD21 rats in Exp. 1A cannot be accounted for by study time exploration alone.
EXPERIMENT 2A: RETENTION OF SPATIAL MEMORY
Experiment 1 described the ontogeny of novel object and location recognition after a short retention interval of 5 min. Experiment 2 sought to examine any ontogenetic differences in short-term versus long-term memory in the object location recognition task by varying the length of the retention interval between the study and test phases. This was accomplished in two experiments, the first (Exp. 2A) examining a range of retention intervals in PD31 rats and the second (Exp. 2B) examining performance at the longest retention interval in PD21 and 26 rats.
Experiment 2A explored the effect of longer retention intervals—10 min, 1 hr, 4 hr, and 24 hr—on the OL task in PD31 rats, an age at which our lab has previously shown that rats exhibit novelty preference after a short delay of 5 min (Jablonski et al., 2013).
Materials and Methods
Subjects
Subjects were 88 (49 M; 39 F) Long-Evans rats derived from 16 litters. Subjects were assigned to one of four retention interval groups—10 min, 1 hr, 4 hr, or 24 hr—or the control group (Test Only). Same-litter sampling was analyzed as previously described in Experiment 1A. Data from same-sex littermates were averaged together in five instances: (10 min, M, n = 2; 1 hr, M, n = 1; 4 hr, M, n = 1; 24 hr, M, n = 1). The animal colony and maintenance was the same as Experiment 1A. Subjects were individually housed two days prior to habituation. All rats began habituation on PD30.
Apparatus and Procedure
Only the novel location recognition (OL) task was studied in Experiment 2A. The apparatus and procedure were the same as in the previous experiments, except for the length of the delay period between the study phase and the test phase. The delay length was either 10 min, 1 hr, 4 hr, or 24 hr. Rats in each delay length group were carted back to the colony room for the duration of the retention interval, with the exception of the 10-min group. The 10-min group remained on the cart in the hallway of the behavioral testing room similar to the 5-min delay groups in the two previous experiments. In addition, another group (Test Only) was included to control for memory. For this group, the OL task was modified so that there were no objects present during the study phase, but the rest of the procedure and test phase remained the same. Arena configuration, arena number, and object movement were counterbalanced with sex and delay length variables.
Data Collection and Analysis
Data collection and analysis were the same as in Experiment 1.
Results and Discussion
Results
After accounting for same-litter sampling, data from seven animals met the criteria as a statistical outlier as described in Experiment 1A and were excluded from the analyses (10 min, F, n = 1; 1 hr, F, n = 2; 4 hr, F, n = 2, M, n = 1; 24 hr, M, n = 1). Data from the remaining 76 subjects were used in the analyses (Test Only, n = 9; 10 min, n = 17; 1 hr, n = 17; 4 hr, n = 16; 24 hr, n = 17).
Study Phase
Comparison of the baseline exploration levels in the study phase between Sex and Retention Interval were conducted using a mixed factorial ANOVA. The ANOVA showed no significant main effects or interactions (all Fs < 1.3), showing that there was no statistical difference in exploration levels between the 10 min (68.96 ± 5.80 s), 1 hr (66.99 ± 4.29 s), 4 hr (55.87 ± 6.01 s), and 24 hr (56.56 ± 7.27 s) retention intervals to account for differences in novelty exploration.
Test Phase
The novelty preference results appear in Figure 7. PD31 rats remembered the locations of the objects after all four retention intervals, including up to 24 hr. In addition, the Test Only control group did not show significant preference for the object in the displaced location. One-sample t-tests of exploration ratios compared to chance performance (.5) were conducted for each delay length group. PD31 rats showed an exploration preference for the object in the novel location after all four retention intervals (10 min: p <.02; 1 hr: p <.001; 4 hr: p <.01; 24 hr: p <.01). The Test Only control group did not show significant novelty preference (p <.75).
FIGURE 7.
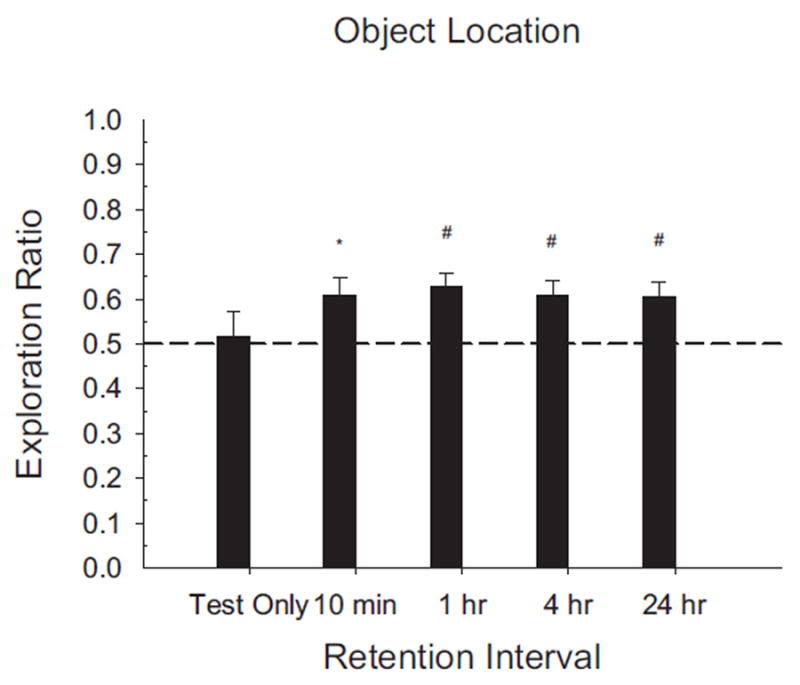
Results of Experiment 2A shown as novelty exploration ratios by retention interval condition. Exploration ratios were calculated as (time exploring novel)/(total exploration time of both objects). Rats exhibited significant preference for the displaced object after all four retention interval lengths. Rats that had not previously studied the objects (Test only) showed no object preference. (#p <.05; *p <.01).
Discussion
The lack of preference in the Test Only control group suggests that memory is indeed used to perform the OL Task. PD31 rats remember the location of objects after various retention interval lengths of up to 24 hr. These results indicate that by PD31, the spatial memory system of rats has developed sufficiently to support both short and long-term retention of object location information.
EXPERIMENT 2B: ONTOGENY OF SPATIAL MEMORY RETENTION
Experiment 2A showed that PD31 rats displayed a preference for the displaced object in the OL task after up to 24 hr. In addition, PD21 and 26 rats exhibited object location novelty preference after a short (5-min) retention interval in Experiment 1. The present experiment (Experiment 2B) examined the effect of retention interval ontogenetically at these two younger age groups. In order to extend the finding of OL performance after a 5-min delay at these ages, the retention intervals used in the present experiment were 10 min and 24 hr. Both age groups were predicted to exhibit object location recognition after the 10 min delay, with the key question being whether object location novelty preference after a 24-hr retention interval would be evident in these age groups. This is the first systematic developmental study of the effect of short versus long-term retention of spatial memory using the OL task.
Materials and Methods
Subjects
Sixty-two (33 M; 29 F) Long-Evans rats sampled from 10 litters were the subjects. The animal colony and maintenance was the same as the previous experiments. Subjects were assigned to one of two age groups—PD21 or PD26—and one of two retention intervals—10 min or 24 hr. Rats in the PD26 age group were weaned normally (on PD21). Rats in the PD21 age group were not weaned and remained with the dam for the duration of the experiment. Data from one animal was excluded due to experimenter error during the test phase (24 hr, PD21, M, n = 1). One animal failed to meet the exploration criteria (total exploration of the objects greater than 1 s) for the test phase and was excluded from the analyses (10 min, PD21, F, n = 1). Analysis of same-litter sampling instances was the same as previously described in Experiment 1A. Two instances of same-litter sampling occurred (10 min, PD21, M, n = 1; 24 hr, PD21, M, n = 1).
Apparatus and Procedure
Only the object location recognition (OL) task was conducted in Experiment 2B. The apparatus and procedure were the same as Experiment 2A, except only the 10 min and 24 hr delay lengths were used. Arena configuration, arena number, and object movement were counterbalanced with sex, delay length, and age group variables.
Data Collection and Analysis
Data collection and analysis were the same as described in the previous experiments.
Results and Discussion
Results
Data from four animals were excluded from the analyses for meeting statistical outlier criteria as described in Experiment 1A (10 min, PD21, M, n = 1; 10 min, PD26, M, n = 1, F, n = 1; 24 hr, PD26, F, n = 1). Data from the remaining 54 subjects were used in the analyses (10 min, PD21, n = 13; 10 min, PD26, n = 13; 24 hr, PD21, n = 13; 24 hr, PD26, n = 15).
Study Phase
Comparison of the baseline exploration levels in the study phase between Age and Sex were conducted using a mixed factorial ANOVA. The ANOVA showed no significant main effects or interactions (all Fs <3.7), showing that there was no statistical difference in exploration levels between PD21 rats (56.85 ± 4.10 s) and PD26 rats (59.51 ± 5.73 s) to account for differences in novelty exploration.
Test Phase
The test phase results appear in Figure 8. Rats in the PD26 age group performed the OL task after both retention intervals, while those in the PD21 group only showed an object location novelty preference after the shorter delay (10 min). One-sample t-tests showed that both age groups, PD21 and PD26, explored the object in the novel location more during the test phase after a delay of 10 min (p < .01). After a 24 hr delay, only the PD26 group showed an exploration preference toward the object in the novel location (p < .05).
FIGURE 8.
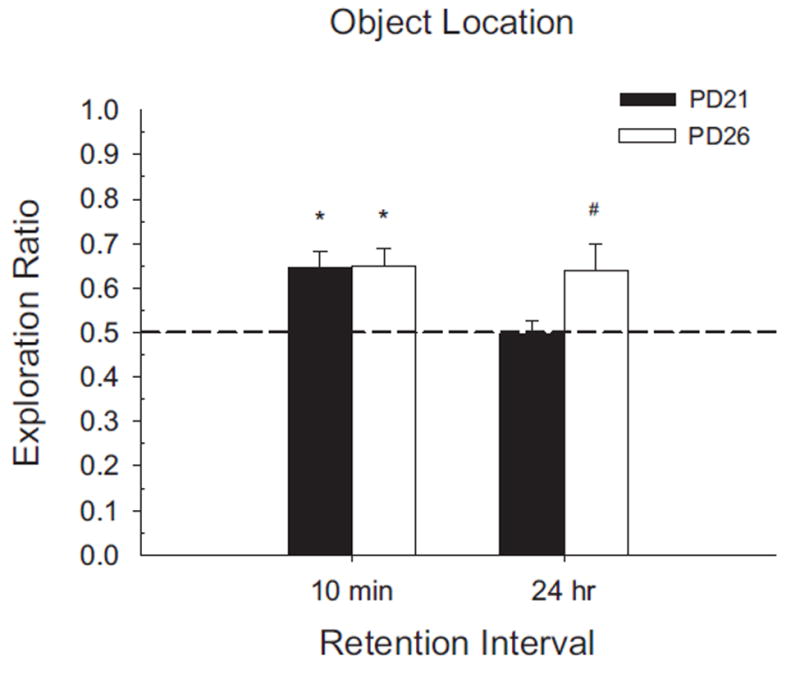
Results of Experiment 2B shown as novelty exploration ratios by retention interval and age groups. Exploration ratios were calculated as (time exploring novel)/(total exploration time of both objects). PD26 rats exhibited significant preference for the displaced object after both the short and long retention intervals. PD21 rats showed significant preference after the 10-min delay, but failed to differ significantly from chance performance (.5) after a 24-hr retention interval. (#p <.05; *p <.01)
Discussion
Experiment 2B was the first study to examine retention effects of spatial memory ontogenetically in the object location recognition task. In this experiment, PD26 rats showed object location recognition after both a short (10 min) and long (24 hr) retention interval. PD21 rats, on the other hand, only displayed a novelty preference after the short retention interval. Thus, acquisition of OL emerges ontogenetically by PD21; however, long-term retention of OL develops further between PD21 and PD26.
GENERAL DISCUSSION
The results of the present study provide insights into the ontogenetic development of novel object and location recognition. Experiment 1A showed that PD17, 21, and 26 rats display novel object recognition; however, only PD21 and 26 rats show object location recognition. Experiment 1B showed that weaning did not influence performance of PD26 rats on either task. Experiment 1C revealed that age differences in OL performance in Experiment 1A are not related to age differences in object exploration time during the study phase. Experiment 2A examined the effect of increasing retention interval on object location recognition, showing that PD31 rats remember object location after up to 24 hr. Experiment 2B extended these results by showing 24-hr retention of object location recognition in PD26 rats but not PD21 rats. Performance effects cannot account for these findings because PD21 rats performed the object location recognition task after the 10 min interval. The results of the present study help to further characterize the development of spatial memory in rats, by showing that short-term spatial memory of object location emerges between PD17 and PD21 followed by increasing development of 24-hr retention after PD21.
The results of Experiment 1A indicate that the ontogenetic emergence of novel object recognition occurs by PD17, while object location recognition emerges between PD17 and PD21. The novel object recognition results agree with those of Kruger et al. (2012), in which PD14-17 rats exhibited novel object recognition after a 10-min delay. In contrast, the object location recognition results in the present study do not agree with Kruger et al. (2012). Our failure to observe OL on PD17 contrasts with their finding that PD15-17 rats can perform this task. Our finding that PD21-26 rats can perform the OL task contrasts with Ainge and Langston’s (2012) report that PD24 rats do not exhibit spatial novelty recognition on an object-in-place task, which may be a more difficult task than the object location task. Although additional studies are needed to identify the many experimental factors, such as task parameters or strain differences, which could be responsible for discrepant findings in this literature, the preponderance of evidence suggests differential ontogeny of novel object and location recognition. This is consistent with neurobiological studies in adult rats indicating these two types of memory are mediated by different regions of the brain (Barker et al., 2007; for review, Dere, Huston, & De Souza Silva, 2007).
In Experiment 1A there was a significant difference in study phase total exploration time of the objects by age, such that the PD17 group spent significantly less time exploring the objects compared to the PD21 and PD26 groups (which did not differ from each other). The decreased exploration times did not prevent PD17 rats from showing a preference for a novel object, but they did not prefer an object in a novel location. Experiment 1C addressed the role of differential exploration time across ages in the OL task by reducing the study phase exploration time of the older rats to make it comparable to that of the PD17 rats. Despite the reduction in exploration times, PD21 and PD26 rats still displayed a preference for the object in the novel location. A related question is whether increasing exploration time in PD17 rats would improve their OL performance. While we have not examined this ourselves, the literature suggests that increasing time in the arena might not increase object exploration time in pre-juvenile rats (Kruger et al., 2012). In any case, Experiment 1C shows that OL performance changes with age under conditions in which exploration times across age are similar.
Experiment 2A showed that performance of the OL task requires memory of studied objects, and PD31 rats remembered object location for as long as 24 hr. This is the first study to examine this question in developing rats. However, previous studies have reported mixed results in adult rats, with some studies showing object location memory after 24 hr (Barker & Warburton, 2011; Larkin et al., 2008) and others not (Ozawa et al., 2011, 2012). While there are many experimental variables, such as strain differences or task parameters, which could contribute to these conflicting findings, our study supports previous ones showing 24-hr retention in the OL task.
Experiment 2B replicates and extends Experiment 1A by showing that PD21 and PD26 rats show object location recognition after both 5-min (Experiment 1A) and 10-min retention intervals. In addition, Experiment 2B showed that 24-hr retention of OL observed on PD31 (Experiment 2A) is also present in PD26 but not PD21 rats. Sometime between 10 min and 24 hr after encoding the object location memory, PD21 rats either forget or are unable to retrieve the information that is required for task performance after 24 hr. Further studies are needed to determine the time-course over which this information is forgotten in PD21 rats. The impairment of the PD21 group after 24 hr suggests that acquisition and retention of spatial memory processes subserving OL performance are dissociable during ontogeny. Long-term retention of most forms of learning in the rat shows protracted ontogenetic development relative to short-term retention (Rudy & Morledge, 1994; Schweitzer & Green, 1982). This phenomenon of infantile amnesia has been recognized for many years (Campbell & Spear, 1972), but understanding of its neurobiological basis remains poorly understood. The present findings show that the object novelty preparation in general, and the OR and OL tasks in particular, may provide a useful approach to addressing these questions.
The present study did not examine the neural basis of developmental differences in acquisition and retention of OL performance; however, it is instructive to consider the neural substrates of these tasks and ask how normal development of these substrates may contribute to the findings of the present study. Visual information is dissociated into object and spatial pathways in the brain (Bussey, Saksida, & Murray, 2005; Deshmukh, Johnson, & Knierim, 2012; Forwood, Winters, & Bussey, 2005; Haxby et al., 1991; Murray, Bussey, & Saksida, 2007). The perirhinal cortex is involved in the object, or “what,” pathway used in object recognition memory in adult rats (Barker et al., 2007; Bussey, Duck, Muir, & Aggleton, 2000; Deshmukh et al., 2012; Mumby & Pinel, 1994; Mumby et al., 2002; Winters & Bussey, 2005; for review, Brown, Barker, Aggleton, & Warburton, 2012), but not tasks that involve spatial memory (Barker et al., 2007; Norman & Eacott, 2005; Winters, Forwood, Cowell, Saksida, & Bussey, 2004). The postrhinal cortex (POR), medial entorhinal cortex (MEC), presubiculum, and parasubiculum constitute the spatial, or “where,” pathway, which is supported by physiological studies of grid and head-direction cells (Hafting, Fyhn, Molden, Moser, & Moser, 2005; Taube, Muller, & Ranck, 1990).
The “where” and the “what” pathways ultimately terminate in the hippocampal formation, where the information is combined to form one conjunctive representation. The parahippocampal-hippocampal system, which supports conjunctive representations (Rudy, 2009), is important for incidental spatial memory but not object recognition (Barker & Warburton, 2011; Mumby et al., 2002).
The differential ontogeny of the object recognition task and the object location task in the present study could reflect differences in development of the two pathways for visual information. Since the time course of the neuroanatomical development of these two pathways is similar (Bayer & Altman, 1978; Fricke & Cowan, 1977; Canto & Witter, 2012a; Canto & Witter, 2012b; Furtak, Moyer, & Brown, 2007), their differential ontogeny may rise from a difference in the functional development of these pathways, which is supported by neurophysiological studies (Canto & Witter, 2012b; Langston et al., 2010; Wills, Cacuci, Burgess, & O’Keefe, 2010). In particular, place cells in the hippocampus and grid cells in the MEC (Langston et al., 2010; Wills et al., 2010) show protracted functional development that parallels the ontogenetic profiles of task performance reported here.
The present study further characterizes the development of spatial memory through behavioral paradigms of incidental learning. The age of emergence of spatial memory used in the object location recognition task coincides with that of other behavioral paradigms examining hippocampal-dependent learning and memory (Dumas and Rudy, 2010; Schiffino et al., 2011; for review, Stanton, 2000), further suggesting that hippo-campal system functioning comes “on-line” between PD17 and PD21. Further studies should investigate more directly the functional development of grid cells in the entorhinal cortex, as well as place cells in the hippocampus, and behavior using object location recognition to determine the changes in neural development that give rise to spatial memory represented in the parahippocampal-hippocampal system.
References
- Ainge JA, Langston RF. Ontogeny of neural circuits underlying spatial memory in the rat. Frontiers in Neural Circuits. 2012;6:1–10. doi: 10.3389/fncir.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GRI, Bird F, Alexander V, Warburton EC. Recognition memory for objects, places, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and the perirhinal cortex. Journal of Neuroscience. 2007;27(11):2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC. When is the hippocampus involved in recognition memory. Journal of Neuroscience. 2011;31(29):10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Hippocampal development in the rat: Cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation. Journal of Comparative Neurology. 1978;158(1):55–79. doi: 10.1002/cne.901580105. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. Novelty and curiosity as determinants of exploratory behavior. British Journal of Psychology. 1950;41:68. [Google Scholar]
- Bermudez-Rattoni F, Okuda S, Roozendaal B, McGaugh JL. Insular cortex is involved in consolidation of object recognition memory. Learning & Memory. 2005;12(5):447–49. doi: 10.1101/lm.97605. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Duck J, Muir JL, Aggleton JP. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behavourial Brain Research. 2000;111(1-2):187–202. doi: 10.1016/s0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. The perceptual-mnemonic/feature conjunction model of peri-rhinal cortex function. Quarterly Journal of Experimental Psychology B. 2005;58(3-4):269–282. doi: 10.1080/02724990544000004. [DOI] [PubMed] [Google Scholar]
- Brown MW, Barker GRI, Aggleton JP, Warburton EC. What pharmacological interventions indicate concerning the role of the perirhinal cortex in recognition memory. Neuropsychologia. 2012;50:3122–3140. doi: 10.1016/j.neuropsychologia.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BA, Spear NE. Ontogeny of memory. Psychological Review. 1972;79(3):215–236. doi: 10.1037/h0032690. [DOI] [PubMed] [Google Scholar]
- Canto CB, Witter MP. Cellular properties of principal neurons in the rat entorhinal cortex. II. The lateral entorhinal cortex. Hippocampus. 2012a;22:1256–1276. doi: 10.1002/hipo.20997. [DOI] [PubMed] [Google Scholar]
- Canto CB, Witter MP. Cellular properties of principal neurons in the rat entorhinal cortex. II. The medial entorhinal cortex. Hippocampus. 2012b;22:1277–1299. doi: 10.1002/hipo.20993. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neuroscience and Biobehavioral Reviews. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Johnson JL, Knierim JJ. Perirhinal cortex represents nonspatial, but not spatial, information in rats foraging in the presence of objects: Comparison with lateral entorhinal cortex. Hippocampus. 2012;22:2045–2058. doi: 10.1002/hipo.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behavioural Brain Research. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Dumas TC, Rudy JW. Development of the hippocampal memory system: Creating networks and modifiable synapses. New York, NY: Oxford University Press; 2010. [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats 1: Behavioral data. Behavourial Brain Research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15(3):347–355. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Fricke R, Cowan WM. An autoradiographic study of the development of the entorhinal and commissural afferents to the dentate gyrus of the rat. Journal of Comparative Neurology. 1977;173(2):231–250. doi: 10.1002/cne.901730203. [DOI] [PubMed] [Google Scholar]
- Frisone DF, Frye CA, Zimmerberg B. Social isolation stress during the third week of life has age-dependent effects on spatial learning in rats. Behavioural Brain Research. 2002;128(2):153–160. doi: 10.1016/s0166-4328(01)00315-1. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Moyer JR, Brown TH. Morphology and ontogeny of rat perirhinal cortical neurons. Journal of Comparative Neurology. 2007;505:493–510. doi: 10.1002/cne.21516. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, Rapoport SI. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(5):1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Schreiber WB, Westbrook SR, Brennan LE, Stanton ME. Determinants of novel object and location recognition during development. Behavioural Brain Research. 2013;256:140–150. doi: 10.1016/j.bbr.2013.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Schiffino FL, Stanton ME. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Developmental Psychobiology. 2012;54:714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger HS, Brockmann MD, Salamon J, Ittrich H, Hanganu-Opatz IL. Neonatal hippocampal lesion alters the functional maturation of the prefrontal cortex and the early cognitive development in pre-juvenile rats. Neurobiology of Learning and Memory. 2012;97:470–481. doi: 10.1016/j.nlm.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, Moser MB. Development of the spatial representation system in the rat. Science. 2010;328:1576–1580. doi: 10.1126/science.1188210. [DOI] [PubMed] [Google Scholar]
- Larkin AE, Fahey B, Gobbo O, Callaghan CK, Cahill E, O’Mara SM, Kelly AM. Blockade of NMDA receptors pre-training, but not post-training, impairs object displacement learning in the rat. Brain Research. 2008;1199:126–132. doi: 10.1016/j.brainres.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Matagne V, Budden S, Ojeda SR, Raber J. Correcting deregulated Fxyd1 expression ameliorates a behavioral impairment in a mouse model of Rett syndrome. Brain Research. 2013;1496:104–114. doi: 10.1016/j.brainres.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learning and Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Pinel JP. Rhinal cortex lesions and object recognition in rats. Behavioral Neuroscience. 1994;108(1):11–18. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: A new view of medial temporal lobe function in primates and rodents. Annual Review of Neuroscience. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behavioral Neuroscience. 2005;119(2):557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Kumeji M, Yamada K, Ichitani Y. D-Cycloserine enhances spatial memory in spontaneous place recognition in rats. Neuroscience Letters. 2012;509:13–16. doi: 10.1016/j.neulet.2011.12.031. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Yamada K, Ichitani Y. Long-term object location memory in rats: Effects of sample phase and delay length in spontaneous place recognition test. Neuroscience Letters. 2011;497:37–41. doi: 10.1016/j.neulet.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Reger ML, Hovda DA, Giza CG. Ontogeny of rat recognition memory measured by the novel object recognition task. Developmental Psychobiology. 2009;51:672–678. doi: 10.1002/dev.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learning and Memory. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: Implications for consolidation, infantile amnesia, and hippocampal system function. Behavioral Neuroscience. 1994;108(2):227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ. Sex differences in the long-term effect of preweanling isolation stress on memory retention. Hormones and Behavior. 2005;47(5):556–562. doi: 10.1016/j.yhbeh.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Hart SR. Isolation stress during the third postnatal week alters radial arm maze performance and corticosterone levels in adulthood. Behavioural Brain Research. 2005;156(2):289–296. doi: 10.1016/j.bbr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiology of Learning and Memory. 2011;95:190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer L, Green L. Acquisition and extended retention of a conditioned taste aversion in preweanling rats. Journal of Comparative and Physiological Psychology. 1982;96(5):791–806. doi: 10.1037/h0077916. [DOI] [PubMed] [Google Scholar]
- Stanton ME. Multiple memory systems, development, and conditioning. Behavioural Brain Research. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. Journal of Neuroscience. 1990;10(2):436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Barker GR, Brown MW. Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology. 2013;74:41–47. doi: 10.1016/j.neuropharm.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Cacuci F, Burgess N, O’Keefe J. Development of hippocampal cognitive map in prewean-ling rats. Science. 2010;328:1573–1576. doi: 10.1126/science.1188224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. Journal of Neuroscience. 2005;25(1):52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippo-campal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. Journal of Neuroscience. 2004;24(26):5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BW, Saksida LM, Bussey TJ. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neuroscience & Biobehavioral Reviews. 2008;32(5):1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]


