Abstract
The current study investigated whether motivational dysfunction in Parkinson’s patients is related to a deficit in preparing for motivated behavior. Based on previous studies, it was hypothesized that PD patients would show reduced preparation for action specifically when faced with threat (of loss) and that reduced action preparation would relate to self-report of apathy symptoms. The study measured an electrocortical correlate of preparation for action (CNV amplitude) in PD patients and healthy controls, as well as defensive and appetitive activation during emotional perception (LPP amplitude). The sample included 18 non-demented PD patients (tested on dopaminergic medications) and 15 healthy controls who responded as quickly as possible to cues signaling threat of loss or reward, in which the speed of the response determined the outcome. Results indicated that, whereas PD patients showed similar enhanced action preparation with the addition of incentives to controls, PD patients showed generally reduced action preparation, evidenced by reduced CNV amplitude overall. Results suggest that PD patients may have behavioral issues due to globally impaired action preparation but that this deficit is not emotion-specific, and movement preparation may be aided by incentive in PD patients.
Keywords: CNV, Parkinson’s, LPP, Motivation, Incentive, Motor preparation
1. Introduction
Parkinson’s disease (PD) is a degenerative disease of the central nervous system, typically recognized by cardinal motor symptoms of tremor, rigidity, bradykinesia, and postural instability. In addition to motor symptoms, PD patients experience a number of cognitive and emotional changes, including high rates of depression, anxiety, and apathy (Schneider et al., 2008; Pedersen et al., 2009), even when compared to other disabled medical populations (Pluck and Brown, 2002). Although research has made significant strides in understanding the pathophysiology underlying motor symptoms in PD (e.g. Delong, 1990), emotional and motivational changes are less well understood and remain some of the most debilitating symptoms of the disease (Weintraub et al., 2004; Visser et al., 2008). To better understand the nature of motivational and emotional dysfunction in Parkinson’s disease, the current study investigated physiological correlates of these constructs in the laboratory, from preparation for motivated behavior to emotional perception.
According to the defense cascade model of emotion (Lang et al., 1997), emotion can be considered an action disposition, characterized by a physiological cascade where, as threat becomes more imminent, physiological reactivity shifts from enhanced orienting to preparation for action, in order to avoid a threat or capture a reward (Lang et al., 1997). Theoretically, affective pathology could be related to deficits at any level of the response cascade, from preparation for action or goal-directed behavior to oriented perceptual intake. Löw et al. (2008) developed an experimental paradigm to simulate the defense cascade in the laboratory, which measures physiological correlates of preparation for imminent action and emotional perception (Löw et al., 2008). In the current study, this paradigm was adapted for use in exploring motivational and emotional processes in Parkinson patients.
A number of theorists have proposed that disordered motivation in PD or “apathy” (Marin, 1991) may be due to a deficit in preparing for and initiating goal-directed behavior (Levy and Dubois, 2006). Apathy is defined as a primary deficit in motivation associated with reduced goal-directed behaviors (Marin, 1991) and is highly prevalent in frontal-subcortical diseases such as Parkinson’s. In addition, data suggest that apathy may be related to Parkinson’s disease pathology itself, as Zahodne et al. (2012) found that apathy follows a similar trajectory as motor symptom progression in Parkinson’s. Stuss and Alexander (2000) suggested that apathy results from a variety of deficits in the central nervous system, including affective (flattening of emotional responsiveness), behavioral (reduced initiation of spontaneous behavior), and executive dysfunction (difficulty planning/executing), processes that are interrelated and frequent concomitants of Parkinson’s disease. The hypothesis examined in the current study is that motivational dysfunction in PD is a deficit primarily in preparation for motivated behavior.
Previous studies have reported that PD patients show reduced potentiation of the startle reflex compared to healthy control participants when viewing aversive pictures (Bowers et al., 2006; Miller et al., 2009; Zahodne, 2011), and at least one of these studies also found that attenuated startle reflex is positively related to apathy symptoms (Bowers et al., unpublished data). On the other hand, whereas Parkinson patients demonstrate normal sympathetic arousal when viewing affective pictures, reduced exploration via voluntary eye movements is found (Dietz et al., 2011), prompting the hypothesis that PD patients may be impaired at the level of initiating motivated behavior, rather than showing deficits in affective arousal when confronted with emotionally engaging cues. This hypothesis is consistent with findings of reduced startle potentiation in PD, as the startle eye-blink is a somato-motor reflex that reflects readiness for defensive behavior (Lang et al., 1997; Carlsen et al., 2004).
When electrocortical responses in PD patients are measured during affective picture viewing, PD patients demonstrate reduced cortical response specifically to aversive (not pleasant or neutral pictures; Dietz et al., 2013) as evidenced by reduction of the late positive potential or “LPP”, a positive-going event-related potential that is maximal 400–700 ms over centro-parietal sensors (e.g., Cuthbert et al., 1995; Schupp et al., 2000). Similar to the startle reflex data, reduced LPP to unpleasant pictures is also associated with higher self-reported apathy in Parkinson patients. Findings of reduced cortical response and reduced startle potentiation during unpleasant picture viewing in Parkinson patients suggest that the defensive motivational system—the brain’s innate physiological response to threatening stimuli (Bradley et al., 2001)—may be specifically impaired in Parkinson’s disease. Based on these findings, we hypothesized that motivational dysfunction in PD would be evidenced by diminished preparation for defensive behavior, and that the deficit is related to self-report of apathy symptoms.
On the other hand, some data support a hypothesis of generally reduced preparation for action in PD that is not specific to defensive motivation. For example, several studies have found that PD patients show a smaller contingent negative variation (CNV) prior to a simple motor response than controls (Tsuda, 1982; Amabile et al., 1986; Oishi et al., 1995; Verleger et al., 1999; Pulvermüller et al., 1996). The CNV is a slow event-related potential that is largest in the anticipatory period just prior to a motor response (Simons et al., 1979). The CNV is also modulated by affective arousal or the addition of an incentive tied to the behavioral response (Simons et al., 1979; Chwilla and Brunia, 1991; Kotani et al., 2001, 2003; Masaki et al., 2006; Ohgami et al., 2004, 2006). Although PD patients, compared to controls, have shown reduced CNV amplitude in a variety of simple motor tasks (e.g. go/no-go task, Pulvermüller et al., 1996), the question investigated here is whether the CNV will be differentially modulated by incentive.
Thus, the current study measured electrocortical activity in PD patients and controls using a task adapted from Löw et al. (2008) in which ERPs to salient cues are measured during preparation for action. On each trial, specific cues are presented that signal the possibility of either loss or reward which the participant can either avoid (loss) or gain (reward) based on the speed of their response to an imperative stimulus, and the amplitude of the CNV leading up to the motor response is measured. It is hypothesized that Parkinson’s patients will show blunted activation of the defense system, evidenced by smaller CNV in PD patients compared to controls during threat (of loss) condition. An alternative hypothesis is that PD patients will show reduced preparation for action across all conditions, regardless of incentive, which would indicate that the deficit in preparation for action is broad and not specific to motivated behavior.
A secondary aim of the current study was to replicate findings of reduced LPP amplitude during unpleasant picture viewing in PD patients (Dietz et al., 2013). To do so, unpleasant, pleasant, or neutral pictures were presented on pseudorandomized trials, separate from preparation for action trials, and the amplitude of the late positive potential was measured and correlated with self-report of apathy symptoms. A deficit in defensive motivational activation would be evidenced by reduced LPP amplitude during unpleasant picture viewing compared to controls (based on Dietz et al., 2013) that varies with self-reported apathy.
2. Material and methods
2.1. Participants
A total of 18 non-demented patients with idiopathic Parkinson’s disease (PD) and 15 healthy age-matched controls participated in the study. The Parkinson patients were recruited through the clinics of the University of Florida’s Center for Movement Disorders and Neurorestoration (CMDNR); the control participants were spouses of patients or recruited from the community.
To be included in the Parkinson group, participants: a) had a clinical diagnosis of idiopathic PD (Hughes et al., 1992) based on the presence of at least two of three cardinal motor signs of PD (i.e., bradykinesia, resting tremor, rigidity) and a positive response to dopaminergic therapy based on improved motor subscore of the Unified Parkinson’s Disease Rating Scale-Third Edition (UPDRS-III; Fahn et al., 1987) and b) were on stable regimen of Parkinson medication (6 months) prior to participating in this study. Patients were tested “on” their dopaminergic medications in order to accurately reflect their day to day functional state, especially given that emotion and motivation dysfunction persist in Parkinson’s patients despite dopaminergic therapy (possibly due to the fact that motor and limbic functions have different optimal dopaminergic dosages; Cools, 2006). Indices for gauging disease severity were obtained as part of normal clinical care and included in correlational analyses only if within six months of the testing session. Specific measures included the motor scale of the UPDRS-III and staging from the Hoehn–Yahr scale.
Specific exclusion criteria employed during recruitment for the Parkinson group included (a) evidence of secondary or atypical parkinsonism, b) brain surgery including deep brain stimulation, pallidotomy, thalamotomy, or fetal cell transplants, c) evidence of dopamine dysregulation disorder; or d) dopamine-related dyskinesias that would affect EMG or ERP recordings, e) neurologic disturbance (other than Parkinson disease for the PD group) or chronic medical illness (i.e., HIV, metastatic cancer, etc.). Other exclusion criteria, assessed at the time of the testing session, included: a) significant cognitive disturbance based on MMSE <25; b) current or past history of major psychiatric disturbance based on the self-report (e.g., schizophrenia, bipolar, anxiety disorders, major depressive disorder (anxiety and depressive disorders were screened out only for patients whose disorders preexisted the Parkinson diagnosis, as we intended to include patients with a range of affective symptoms due to PD in the current study for the purposes of correlational analyses). Axis I disorders were screened with a structured clinical interview. Participants who were taking anti-depressant medications were included, since many of the PD patients seen at the UF Center for Movement Disorders and Neurorestoration are prescribed anti-depressants even in the absence of a diagnosis of major depressive disorder for sub-clinical depressive symptoms and/or mood maintenance.
2.2. Screening evaluation (Parkinson patients and control group)
All participants were fully informed of the nature of the study and written informed consent was obtained according to University of Florida and federal guidelines. All participants received neurocognitive, psychiatric, and mood screening to rule out dementia and other significant psychopathology, mood disturbance, or other factors (i.e., medical history) that would interfere with their participation in this study. This screening evaluation occurred on the same day as the acquisition of physiological data and included the MMSE and structured Axis I disorders interview. Additional baseline measures of mood, anxiety, and motivation were collected including the Beck Depression Inventory-II (BDI-II) (Beck et al., 1996; considered a valid and reliable measure of depression in PD with an optimal cut-off of 14, Visser et al., 2006), the Apathy Scale (Starkstein et al., 1992; recommended for use in PD, see Leentjens et al., 2008), the State/Trait Anxiety Inventory (STAI; Spielberger et al., 1999), the Snaith–Hamilton Pleasure Scale (SHAPS; Snaith et al., 1995; suggested for use in PD, Leentjens et al., 2008), Temporal Experience of Pleasure Scale (TEPS, Gard et al., 2006) and the Behavioral Inhibition and Behavioral Activation Scales (BIS/BAS; Carver and White, 1994). The TEPS provides indices of anticipatory and consummatory anhedonia (lower scores indicate greater anhedonia), whereas the BIS/BAS provides scales of one’s tendency towards approach/avoidance behavior (BIS/BAS) (higher scores indicate higher aversion to punishment and higher pleasure seeking, respectively). These exploratory measures have not been specifically validated in a PD population; however the TEPS is strongly correlated with the SHAPS (Gard et al., 2006) which has been suggested for use in PD.
2.3. Participant characteristics
Table 1 lists demographic characteristics of PD patients and healthy controls, as well as scores on mood and emotion measures. The groups did not differ in age or education. PD patients scored significantly higher on the depression, apathy, and anxiety inventories. Additionally, over half of the PD patients had scores that fell in the clinical range for clinically significant apathy in Parkinson’s disease, using the recommended cut-off of 14 (Starkstein et al., 1992). On the other hand, only three PD patients fell at or above the recommended cut-off of 14 on the BDI-II for depression. Parkinson patients and controls did not differ on the experimental questionnaires related to anhedonia and behavioral activation/inhibition questionnaires (TEPS, SHPS, BIS/BAS).
Table 1.
Demographic characteristics and self-report of emotional symptoms in Parkinson patients (N = 18) and controls (N = 15).
| Parkinson (N = 18) | Range | Control (N = 15) | Range | df | t | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Gender (M/F) | 14/4 | 11/6 | |||||||
| Antidepressant (Y/N) | 8/10 | 0/15 | |||||||
| Age | n = 18 | 66 (7.99) | 51–82 | n = 15 | 70 (6.94) | 52–77 | 31 | 1.33 | |
| Education | n = 18 | 17.17(3.55) | 12–25 | n = 15 | 17.00 (2.70) | 12–24 | 31 | .15 | |
| BDIa | n = 18 | 7.82 (4.85) | 0–17 | n = 15 | 2.60 (2.44) | 0–9 | 31 | 3.77 | p < .01 |
| Apathy scalea | n = 17 | 13.12 (6.07) | 2–22 | n = 15 | 8.47 (4.34) | 2–16 | 30 | 2.46 | p < .05 |
| STAI trait | n = 16 | 32.81 (8.38) | 20–40 | n = 15 | 27.60 (5.41) | 20–38 | 25.82 | 2.07 | p < .05 |
| TEPS anticipatory pleasure | n = 17 | 46.06 (7.15) | 35–59 | n = 15 | 47.33 (7.03) | 37–58 | 30 | .51 | |
| TEPS consummatory pleasure | n = 17 | 37.59 (5.62) | 29–48 | n = 15 | 36.47 (6.22) | 27–44 | 30 | .04 | |
| Snaith Hamilton pleasure scalea | n = 17 | 21.41 (4.05) | 15–30 | n = 15 | 20.47 (4.29) | 14–29 | 30 | .64 | |
| BIS/BAS totala | n = 17 | 65.41 (8.08) | 46–77 | n = 15 | 68.00 (8.17) | 52–80 | 30 | .90 | |
| UPDRS motor “on”b | n = 16 | 26 (9.55) | 11–41 | – | – | – | – | – | |
| UPDRS motor “off”b | n = 17 | 34 (10.39) | 16–55 | – | – | – | – | – | |
| Disease duration (years)/severity (HY stagec) | n = 18 | 8.39 (4.57) | 2–17 | – | – | – | – | – |
Scores ≥14 are considered clinically significant scores for Parkinson’s patients on the BDI-II and the Apathy Scale (Leentjens et al., 2000; Starkstein et al., 1992). On the SHAPS and TEPS, lower scores indicate greater anhedonia (Snaith et al., 1995; Gard et al., 2006). On the BIS, higher scores indicate anticipatory anxiety and sensitivity to punishment, on the BAS, higher scores indicate higher pleasure seeking tendency (Carver and White, 1994.)
Of the UPDRS motor “on” scores, 9 patients had UPDRS motor scores obtained “on” medications within the last 6 months; these were the only patients included in regression analyses. Only 3 patients had UPDRS motor testing “off” medications in the last six months; thus, this data was not used in analyses.
Hoehn Yahr stages for patients were as follows (N = 15): Stage 1.5 (N = 1), Stage 2 (N = 10), Stage 2.5 (N = 1), Stage 3 (N = 1) and Stage 4 (N = 2). HY “on” stages were recorded within the last 1.5 years.
2.4. Experimental procedure
Experimental testing took place at the UF Center for the Study of Emotion and Attention (CSEA) at the University of Florida. On the testing day, written informed consent was obtained from participants. Patients were tested while “on” their anti-parkinsonian medication. Patients were evaluated with the MMSE and structured clinical interview and then completed psychological measures and the psychophysiology paradigm, all of which took place over the course of one testing session. Participants were debriefed and paid at the conclusion of the experimental session.
2.5. Stimuli
Fig. 1 (top) illustrates the three different conditions for preparation for action trials. On these trials, one of three visual cues was presented for three seconds that consisted of: 1) a picture of a fistful of money, which signaled the possibility of obtaining a reward (money), 2) a fistful of burning money, which signaled the possibility of losing money, and 3) an empty palm, which signaled no outcome. On each trial, one of these three cues was presented for 3 s and signaled the upcoming presentation of an imperative (reaction time) stimulus, to which the participant was instructed to respond by making a button press as quickly as possible. A cue showing a fistful of money indicated that a fast response would earn money (between $.05–$1.50); a fistful of burning money indicated that a fast response would avoid a loss; an empty palm indicated that no money could be gained or lost. Reward and loss amounts were varied on each trial in order to enhance participant engagement in the experimental task, as feedback uncertainty (lack of predictability) yields enhanced cortical correlates of engagement (Catena et al., 2012). A 1000 Hz auditory tone was presented concurrent with the preparatory cue to accentuate the announcement of the preparatory period (Wynn et al., 2010). Following the 3-second preparatory interval, the auditory signal ended and the screen changed to red, signaling that the participant should press a button on a hand-held response pad as quickly as possible. Each trial was followed by an intertrial interval that averaged three seconds (2–5 s).
Fig. 1.

Top: Illustration of threat of loss, reward, and neutral preparation for action trials. Bottom: Schematic of experimental design. On a preparation for action trial, the warning cue was presented for 3 s, followed by a red screen which indicated that the participant should press the space bar as fast as possible. The participant then received feedback about whether money was won, lost, or neither, based on whether the reaction time on that trial was faster than an individual’s 90th percentile reaction time cut-off. Affective and neutral pictures were pseudo-randomized with preparation for action trials. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
For perception trials, the picture stimuli were selected from the International Affective Picture System (Lang et al., 2008) and consisted of 36 unpleasant, 36 neutral, and 36 pleasant pictures. During picture viewing, each picture was presented for three seconds, followed by a variable 2–5 second ITI.
Fig. 1 (bottom) illustrates the experimental sequence. In total, the task consisted of 108 preparation for action trials, including 36 reward trials, 36 neutral trials, and 36 loss trials, pseudorandomized with 108 picture viewing trials, consisting of 36 pleasant pictures, 36 neutral pictures, and 36 unpleasant pictures, for a total of 216 trials.
2.6. Baseline reaction time testing
To determine the reaction time used to determine the speed of “successful” trials, a baseline procedure measured the individual’s reaction times for 20 neutral (no-win/no-loss) preparatory trials prior to beginning the experimental sequence. Based on these 20 trials, the criterion RT for subsequent win/loss trials was set at the individual’s 90th percentile, meaning that a participant’s reaction time had to be in the top 10% of fastest baseline responses to gain a reward or escape loss.
2.7. Data recording
Electroencephalogram (EEG) was recorded at 250 Hz with an EGI 129 sensor net. A vertex reference was used and the signal was filtered on-line (0.01–48 Hz). The data were filtered offline at 20 Hz, corrected for artifacts (Junghöfer et al., 2000) and re-referenced to the average reference. Eye movements were corrected using BioSig, an open source software library for biomedical signal processing (Schlogl et al., 2007). Bad channels were later interpolated using spherical splines, a method of data interpolation that uses the signal obtained from sensors located within close spatial distances to estimate data from a missing sensor.
On average, across all participants, 16% of trials were rejected due to artifact, with a maximum of 38% of trials rejected for a single participant. Each ERP waveform was averaged for a minimum of 20 trials. There was no difference in the number of rejected trials between groups (Mean patient group = 30, SD = 18, i.e. 14% of 216 trials, Mean control group = 40, SD = 22, i.e. 19% of 216 trials; t = 1.52; p = .14). A 200 ms baseline correction was applied to all ERP waveforms, and ERPs during the preparatory cue were extracted from −200 to 3000 ms post cue onset and the CNV was calculated as the average voltage in the last second of the cue presentation (i.e., 2000–3000 s after cue onset). Reaction time for each of the response trials was recorded. ERPs during picture viewing were extracted from −200 to 1000 ms post picture onset and the LPP was calculated from the average voltage from 500 to 1000 ms after picture onset.
2.8. Data analysis
Based on visual inspection (see Fig. 2A), the CNV was measured over sensors in a centro-parietal cluster.2 Group differences in preparation for motivated behavior were tested in a 2 (Group: Parkinson vs. Controls) × 3 (Motivational Context: reward, threat, neutral) repeated measures MANOVA using SPSS statistical software (IBM, version 22, Armonk, New York). For all analyses, MANOVA (Wilks lambda) statistics are reported when sphericity was violated (see O’Brien and Kaiser, 1985); univariate ANOVA statistics are reported when sphericity assumptions were not violated.
Fig. 2.
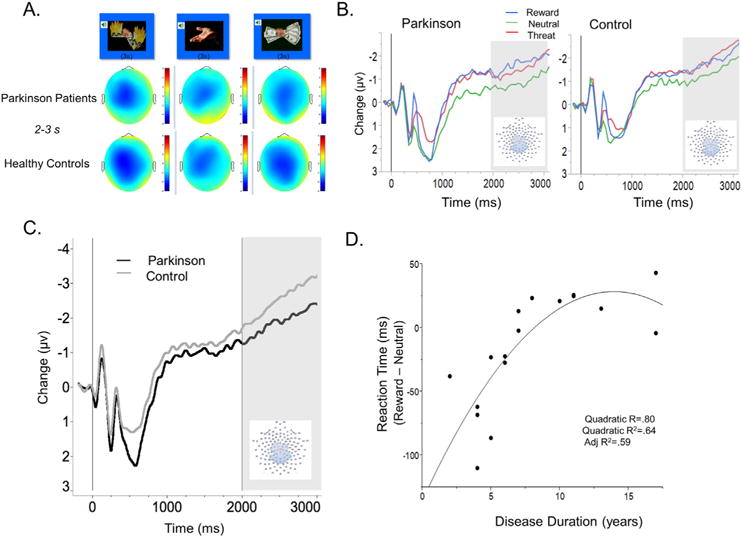
A: Topographies showing average changes in voltage at the scalp for Parkinson patients (top) and healthy controls (bottom) during the CNV time window, from 2000 to 3000 ms after cue onset during preparation for action trials. B: Event-related potentials during the presentation of reward, threat, and neutral incentive motivation cues, prior to the motor response for a centroparietal sensor group. Grayed background indicates time window for ERP analysis. Effect of incentive motivation on CNV amplitude was significant in both Parkinson and Control groups. C: The contingent negative variation, measured at a centro-parietal sensor group, during the last second prior to the motor response. Grayed background indicates time window for ERP analysis. PD patients showed reduced late CNV amplitude compared to controls. D: Relationship between disease duration and reduction in reaction time during reward (compared to neutral) trials. Note that reaction times have been back-transformed from the log transformation applied to raw reaction time data for analyses, and are thus presented in milliseconds.
Based on visual inspection of the topographies (see Fig. 3A), the LPP was measured over a group of central-posterior sensors3 where the topographies for both groups showed the greatest overlap in terms of maximal LPP amplitude. The LPP amplitude was scored as the average amplitude from 500 to 1000 ms after picture and submitted to a 2 (Group: Parkinson vs. Control) × 3 (Hedonic Content: pleasant, neutral, unpleasant) between-within ANOVA.
Fig. 3.
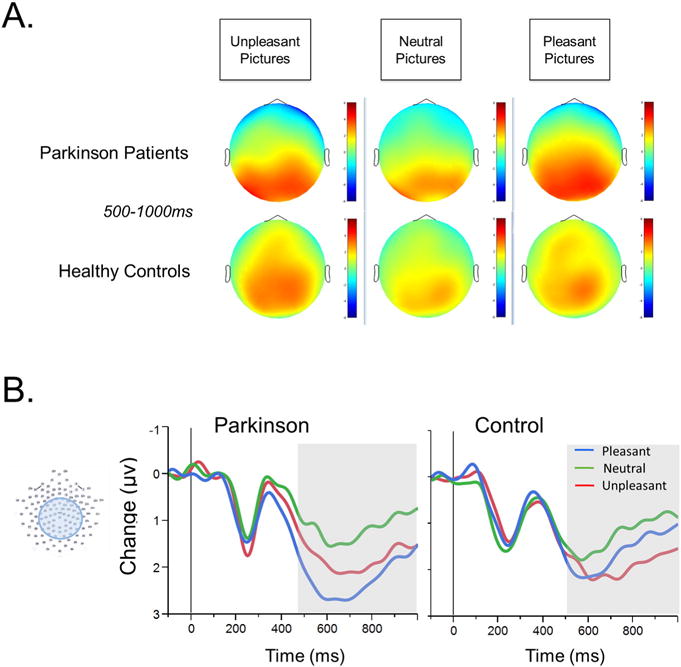
A: Topographies showing average changes in voltage for Parkinson patients (top) and healthy controls (bottom) during unpleasant, neutral, and pleasant picture viewing in the LPP time window, from defined as 500–1000 ms following picture onset, during. B: Event-related potentials during the presentation of pleasant, unpleasant, and neutral pictures, at central-posterior sensor group. Grayed background indicates time window for ERP analysis. PD patients showed enhanced LPP during pleasant compared to unpleasant picture viewing, whereas controls do not.
For reaction time, a log10 transformation was applied to correct for skewness and the data was analyzed in a 2 (Group: Parkinson vs. Controls) × 3 (Motivational Context: reward, threat, neutral) between-within MANOVA.
To examine the relationship between self-reported symptoms of emotion and motivational dysfunction, differences were computed for each dependent measure (CNV, RT, LPP) in motivated (CNV, RT: reward, threat; LPP: pleasant, unpleasant) and non-motivated (neutral) contexts. In some cases, follow-up linear and quadratic regression analyses were conducted to further analyze significant relationships. Regression analyses were scrutinized for outliers in terms of standardized residuals.
3. Results
3.1. Preparation for action: contingent negative variation (CNV)
Table 2 presents means and standard deviations for CNV amplitudes in PD and control groups. Fig. 2A illustrates scalp topographies for the time window occurring one second prior to the imperative response stimulus for Parkinson patients (top) and for control participants (bottom) during preparation for action (threat of loss, neutral, and reward). A significant main effect of incentive motivation was found for CNV amplitude and Fig. 2B illustrates the significant effect of motivational context, F(2,30) = 5.79, p < .01, , for both Parkinson’s and control participants. Both groups showed a larger CNV when preparing to respond to signals of potential reward or loss, compared to neutral cues, t’s(30) = 3.25 and 2.99, p < .05. The predicted Group by Motivational Context interaction was not significant, however F(2,30) = .18, p = .85, . Rather, both PD patients and control participants showed a significant effect of incentive on the CNV amplitude.
Table 2.
Means and (standard deviations) for physiological and behavioral responses measured in Parkinson patients and controls during emotional picture viewing and preparation for action.
| Threat | Neutral | Reward | |
|---|---|---|---|
| CNV | |||
| Control | −3.17 (1.46) | −2.51 (1.22) | −3.30 (1.66) |
| Parkinson | −1.83 (1.31) | −1.25 (.99) | −1.62 (1.30) |
| Reaction time | |||
| Control | 194.98 (1.34) | 204.17 (1.29) | 194.98 (1.32) |
| Parkinson | 269.15 (1.51) | 302.00 (1.45) | 281.84 (1.48) |
| Success rate of RT | |||
| Control | .77 (.26) | .72 (.25) | .77 (.26) |
| Parkinson | .58 (.28) | .52 (.29) | .57 (.28) |
| Unpleasant | Neutral | Pleasant | |
|
| |||
| LPP | |||
| Control | 1.90 (1.23) | 1.22 (.91) | 1.62 (1.13) |
| Parkinson | 1.83 (1.05) | 1.16 (.94) | 2.27 (1.11) |
As illustrated in Fig. 2C, a main effect of Group indicated that PD patients showed an overall smaller CNV in the last second prior to the imperative response window, compared to control participants, regardless of the motivational context (loss, reward, neutral), suggesting a general reduction in action preparation in Parkinson’s patients, F(1,31) = 8.29, p < .01, . On the other hand, PD patients and controls did not differ in amplitude in earlier CNV time windows (1000–2000 ms), F(1,31 = .53, p = .47), suggesting the late CNV difference is not simply an inter-individual group difference.
There was no relationship between CNV modulation and apathy in the PD patient group, N = 17, r = .05, p = .84 for threat-related CNV modulation, or motor symptom severity, N = 9, r = .28, p = .47, or disease duration, N = 18, r = −.09, p = .73.
3.2. Reaction time
Table 2 presents reaction time data and success rate. Motivational context (reward, threat) did not significantly affect reaction time, F(2, 62) = 2.1, p = .13, , possibly due to the simplicity of the motor task (simple button press), and the interaction of Group and Incentive Motivation was not significant. A one-tailed test indicated only that PD patients tended to be somewhat slower overall, t(31) = 3.41, one-tailed p = .04, , with a lower probability of success (56% success rate for PD patients, compared to 75% success rate for controls vs. Mann–Whitney U(31) = 2.31, p < .05). This difference in success rate occurred in spite of reaction time cut-offs that were based on each individuals’ own baseline reaction times.
There were no significant relationships between reaction time speed during threat of loss trials and self-report of apathy or indices of disease severity in patients. On the other hand, within the PD group, there was a significant relationship between reaction time speed during reward trials and disease duration. As illustrated in Fig. 2D, as disease duration increased, the facilitation in RT for reward, compared to neutral, preparation trials decreased. Although the linear relationship between disease duration and RT modulation for reward trials was significant, r = .70, p < .001, the quadratic trend explained significant variance above and beyond the linear trend, r = .80, r2 = .64, adjusted r2 = .59, Δr p < .01. Fig. 2D illustrates that the facilitative effect of reward incentive on reaction time diminished, reaching an asymptote of zero (no difference in RT’s to reward vs. neutral), as disease duration increased.
3.3. Picture viewing: late positive potential (LPP)
Table 2 lists means and standard deviations for LPP amplitude during picture viewing; Fig. 3B illustrates ERPs for patients and controls. There was a main effect of Hedonic Content, F(2,62) = 19.29, p < .01, , such that the LPP was larger when participants viewed unpleasant, t(32) = 4.56, p < .001, or pleasant, t(32) = 5.77, p < .001, compared to neutral, pictures. The main effect was qualified by a significant Group × Hedonic Content interaction, F(2,62) = 4.77, p < .05, . Although PD patients showed larger LPP amplitude when viewing unpleasant, t(17) = 3.34 p < .01, and pleasant, t(17) = 6.12 p < .001, compared to neutral, pictures, the LPP was significantly larger when PD patients viewed pleasant, compared to unpleasant, pictures, t(17) = 3.21, p < .05. Healthy controls showed no difference in LPP amplitude when viewing unpleasant and pleasant pictures, t(14) = .50, p = .35, and only showed reliable LPP modulation during unpleasant relative to neutral picture viewing, t(14) = 3.10, p < .04. The difference between LPP amplitude during pleasant and neutral picture viewing was not statistically significant in the healthy control group, t(14) = 2.03, p = .15.
The predicted relationship between LPP modulation during unpleasant picture viewing and apathy within the patient group was not significant, N = 17, r = −.30, p = .12, and there was no significant relationship with either motor symptom severity, N = 9, r = −.43, p = .25, or disease duration, N = 17, r = .16, p = .54, nor were there relationships between LPP amplitude during pleasant picture viewing and self-report or disease-related variables.
4. Discussion
This study investigated the hypothesis that PD patients show blunted preparation specifically for defensive actions. This hypothesis was not supported. Rather, PD patients demonstrated a significant effect of incentive motivation on CNV amplitude, in which CNV amplitude was enhanced during both threat of loss and reward trials, relative to a neutral context, which was similar to that found for control participants. The main difference between PD patients and controls was overall reduced CNV amplitude during preparation for action—regardless of incentive.
These findings suggest that, although impaired motor processes may play a role in the lack of spontaneous, goal-directed behavior in Parkinson’s disease, this does not necessarily reflect reduced arousal or emotional responsiveness. Because PD patients demonstrated an effect of incentive on preparation for action similar to controls, the augmenting effect of incentive or arousal on movement preparation could perhaps be leveraged in Parkinson’s disease therapies for movement initiation. Relatedly, one study has reported that both pleasant and unpleasant emotional states positively impact the initiation of forward gait in Parkinson’s disease (Naugle et al., 2011).
PD patients did not differ from controls in earlier time windows of the CNV (e.g. 1–2 s after cue onset), suggesting the difference in amplitude during preparation for action does not reflect an overall between group difference in ERP amplitude that could reflect a number of irrelevant variables (e.g. head size, hydration of sensors, thickness of skull/scalp, etc.). Instead, patients and controls only differed in CNV amplitude during the last second prior to the button press response, when preparing to make a motor movement of any type. The timing of this modulation is consistent with modulation of the “late” CNV component (Simons et al., 1979), which is thought to specifically reflect motor response preparation (Simons et al., 1979). The generally reduced amplitude of the late CNV component (regardless of incentive) just prior to the motor response is consistent with other studies that have found reduced movement-related potentials (MRPs) in Parkinson’s disease (Cunnington et al., 1997; Deecke et al., 1977; Shibasaki et al., 1978; Simpson and Khuraibet, 1987; Dick et al., 1989; Jahanshahi et al., 1995).
Movement-related potentials are thought to reflect pre-motor activity in the supplementary motor area (SMA) (Cunnington et al., 1997; Tanji, 1985; Alexander and Crutcher, 1990). Consistent with this, PET studies have found that PD patients show reduced SMA activity prior to a motor response (Playford et al., 1992). Thus, the reduced motor preparation exhibited by PD patients in the current study may be due to diminished SMA activation prior to the execution of a movement, which could potentially be augmented by the addition of an incentive or increased arousal. Neurophysiologically, this might be accomplished via the amplification of compromised basal ganglia signals to the frontal lobes by input from limbic structures and/or brainstem connections (Takakusaki et al., 2004). Therapeutically, use of incentive based behavioral therapies or stimulant pharmacologic treatment could be used in PD patients to increase behavioral activation. For example, there is evidence that methylphenidate improves motor (and possibly cognitive) function in Parkinson’s disease (Auriel et al., 2009), and a case study showed that the use of methylphenidate also improved symptoms of apathy in a patient with Parkinson’s disease (Fahn and Chatterjee, 2002). Pramipexole has also been shown to improve depression and motivational symptoms in PD (Leentjens et al., 2009).
PD patients showed overall slower reaction times and were less successful on action trials than control participants, even though patients were tested on medication and the reaction time criteria were tailored to their own personal 90th percentile reaction time (thus taking into account an individual’s baseline speed). It is possible that the lower overall success rate in PD patients is related to motor timing difficulties in PD. Studies that use the “synchronization-continuation paradigm”, in which patients tap their fingers at a certain pace, either concurrently with the onset of a tone or independently without the aid of a cue (e.g., Jones et al., 2011; Avanzino et al., 2013) find poorer accuracy for PD patients specifically during the “continuation,” self-initiated portion of the timed tapping task, with performance decreasing at longer tapping intervals (i.e. greater than 500 ms). PD patients in the current study similarly may have had more difficulty consistently timing the motor response (tapping the response button) following the relatively long three second preparatory interval. A deficit in motor timing and reduced late CNV amplitude in the current study together suggest reduced or aberrant action preparation in PD.
Additionally, there was a significant relationship between reaction time during reward trials and disease duration, such that the facilitative effect of reward incentive on reaction time diminished as disease duration increased. This is consistent with increasing damage to the mesolimbic dopaminergic pathway as the disease progresses (Ito et al., 2002), which likely affects relevant reward circuitry that provides amplification to basal ganglia output, giving rise to facilitated reaction time during reward trials (Takakusaki et al., 2004). As the disease progresses over time, reward related behavior might be preferentially affected in Parkinson’s disease.
With respect to emotional perception, the current study elaborated on findings of aberrant LPP modulation in PD patients reported by Dietz et al. (2013). Results were consistent with those reported by Dietz et al. (2013), such that PD patients showed disparate LPP amplitudes during unpleasant compared to pleasant picture viewing. However, in the current data set, PD patients were most different from controls in their LPP amplitudes during pleasant picture viewing. Healthy controls did not show a reliable difference in LPP amplitude during pleasant compared to neutral viewing, whereas PD patients showed the largest LPP amplitudes during pleasant picture viewing, compared to all other picture contents. These results suggest that differences in LPP modulation between Parkinson’s and healthy controls may reflect exaggerated response to pleasant, as opposed to reduced response to unpleasant pictures in the PD group.
4.1. Limitations
Because PD patients were less successful than controls in the reaction time task, differences in CNV amplitude in the PD patients might be interpreted as overall decreased engagement in the task, less effort, etc. An engagement deficit, however, would expect overall attenuation of CNV amplitude, rather than specifically the amplitude of the late CNV component, in the last second prior to the response window. Another point to consider is that patients were tested “on” their dopaminergic and antidepressant medications, in which motivational symptoms typically persist despite medical treatment. Nonetheless, interpretations regarding specific dopaminergic influences on incentive-based motor preparation in Parkinson’s patients are not conclusive when patients are medicated. Finally, as always, correlation data should be interpreted with caution, given limited sample size.
4.2. Summary and conclusions
PD patients showed a general deficit in preparation for action using CNV amplitude prior to response mobilization, but showed the same effect of incentive (both reward and threat of loss) on CNV enhancement as did healthy controls. Taken together, the data suggest that behavioral deficits in PD may be related to globally impaired preparation for action that is not emotion-specific, and that movement preparation in PD may be aided by the addition of an incentive or by pharmacologically-induced arousal. Future directions include the development of incentive-based behavioral therapies (similar to token economy approaches for behavior modification) or randomized controlled trials investigating stimulant medications in the treatment of motivational disorders in Parkinson’s disease.
Supplementary Material
Acknowledgments
This work was instrumentally supported by an NRSA awarded to the first author (JR) by the NIH National Institute of Neurological Disorders and Stroke (NINDS) (F31 NS 073331-01), as well as the UF INFORM database and NPF center of excellence.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ijpsycho.2015.11.014.
Footnotes
Sensors included in CNV analysis: 7,13,30,31,36,37,52,53,54,55,60,61,62,67,72,77,78, 79,80,85,86,87,92,105,106,129.
Sensors included in LPP analysis: 5,6,7,12,13,20,29,30,31,35,37,41,42,47,51,52,53, 54,55,59,60,61,62,66,67,71,72,76,77,78,79,80,84,85,86,87,91,92,93,96,97,98,103,104, 105,106,110,111,112,118,129.
References
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. doi: 10.1152/jn.1990.64.1.133. [DOI] [PubMed] [Google Scholar]
- Amabile G, Fattapposta F, Pozzessere G, Albani G, Sanarelli L, Anrea Rizzo P, Morocutti M. Parkinson disease: electrophysiological (CNV) analysis related to pharmacological treatment. Electroencephalogr Clin Neurophysiol. 1986;64(6):521–524. doi: 10.1016/0013-4694(86)90189-6. [DOI] [PubMed] [Google Scholar]
- Auriel E, Hausdorff JM, Giladi N. Methylphenidate for the treatment of Parkinson disease and other neurological disorders. Clin Neuropharmacol. 2009;32(2):75–81. doi: 10.1097/WNF.0B013E318170576C. [DOI] [PubMed] [Google Scholar]
- Avanzino L, Pelosin E, Martino D, Abbruzzese G. Motor timing deficits in sequential movements in Parkinson disease are related to action planning: a motor imagery study. PLoS One. 2013;28(9):e75454. doi: 10.1371/journal.pone.0075454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer R, Brown G. The Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bowers D, Miller K, Mikos A, Kirsch-Darrow L, Springer U, Fernandez H, et al. Startling facts about emotion in Parkinson’s disease: blunted reactivity to aversive stimuli. Brain J Neurol. 2006;129(12):3356–3365. doi: 10.1093/brain/awl301. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276. [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis J, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Mot Behav. 2004;36(3):253–264. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67(2):319–333. [Google Scholar]
- Catena A, Perales JC, Megías A, Cándido A, Jara E, Maldonado A. The brain network of expectancy and uncertainty processing. PLoS ONE. 2012:e40252. doi: 10.1371/journal.pone.0040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwilla DJ, Brunia CH. Event-related potentials to different feedback stimuli. Psychophysiology. 1991;28:123–132. doi: 10.1111/j.1469-8986.1991.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function—implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Johnson KA, Bradshaw JL. Movement-related potentials in Parkinson’s disease. Motor imagery and movement preparation. Brain. 1997;120(8):1339–1353. doi: 10.1093/brain/120.8.1339. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp H, McManis M, Hillman C, Bradley MM, Lang PJ. Cortical slow waves: emotional perception and processing. Psychophysiology. 1995;32:S26. [Google Scholar]
- Deecke L, Englitz HG, Kornhuber HH, Schmitt G. Cerebral potentials preceding voluntary movement in patients with bilateral or unilateral Parkinson akinesia. In: Desmedt JE, editor. Attention, Voluntary Contraction and Event-related Cerebral Potentials. Progress in clinical neurophysiology. 1977. pp. 151–163. [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Dick JPR, Rothwell JC, Day BL, Cantello R, Buruma O, Gioux M, et al. The Bereitschaftspotential is abnormal in Parkinson’s disease. Brain. 1989;112:233–244. doi: 10.1093/brain/112.1.233. [DOI] [PubMed] [Google Scholar]
- Dietz J, Bradley MM, Okun MS, Bowers D. Emotion and ocular responses in Parkinson’s disease. Neuropsychologia. 2011;49(12):3247–3253. doi: 10.1016/j.neuropsychologia.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz J, Bradley MM, Jones JJ, Okun MS, Perlstein WM, Bowers DD. The late positive potential, emotion and apathy in Parkinson’s disease. Neuropsychologia. 2013;51(5):960–966. doi: 10.1016/j.neuropsychologia.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Chatterjee A. Methylphenidate treats apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2002;14(4):461–462. doi: 10.1176/jnp.14.4.461. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, Committee M. o. t. U. D . Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, Clane DB, editors. Recent Developments in Parkinson’s disease. Macmillan Health Care Information; Florham Park: 1987. pp. 153–163. [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Pers. 2006;40:1086–1102. [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease. A clinico-pathological study of 100 cases. JNNP. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Nagano-Saito A, Kato T, Arahata Y, Nakamura A, Kawasumi Y, Brooks DJ. Striatal and extrastriatal dysfunction in Parkinson’s disease with dementia: a 6-[18F] fluoro-l-dopa PET study. Brain. 2002;125(6):1358–1365. doi: 10.1093/brain/awf134. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins I, Brown RG, Marsden C, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements: I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal Parkinson’s disease subjects. Brain J Neurol. 1995;118(4):913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jones CG, Claassen DO, Yu M, Spies JR, Malone T, Dirnberger G, Kubovy M. Modeling accuracy and variability of motor timing in treated and untreated Parkinson’s disease and healthy controls. Front Integr Neurosci. 2011;5:1–13. doi: 10.3389/fnint.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–532. [PubMed] [Google Scholar]
- Kotani Y, Hiraku S, Suda K, Aihara Y. Effect of positive and negative emotion on stimulus-preceding negativity prior to feedback stimuli. Psychophysiology. 2001;38:873–878. doi: 10.1111/1469-8986.3860873. [DOI] [PubMed] [Google Scholar]
- Kotani Y, Kishida S, Hiraku S, Suda K, Ishii M, Aihara Y. Effects of information and reward on stimulus-preceding negativity prior to feedback stimuli. Psychophysiology. 2003;40:818–826. doi: 10.1111/1469-8986.00082. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: affect, activation, and action. Attention and orienting: Sensory and motivational processes. 1997:97–135. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): affective ratings of pictures and instruction manual Technical Report A-8. University of Florida; Gainesville, FL: 2008. [Google Scholar]
- Leentjens AF, Verhey FR, Luijckx GJ, Troost J. The validity of the Beck Depression Inventory as a screening and diagnostic instrument for depression in patients with Parkinson’s disease. Mov Disord. 2000;15(6):1221–1224. doi: 10.1002/1531-8257(200011)15:6<1221::aid-mds1024>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE, Goetz CG. Apathy and anhedonia rating scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2008;23(14):2004–2014. doi: 10.1002/mds.22229. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Koester J, Fruh B, Shephard DTS, Barone P, Houben JJ. The effect of pramipexole on mood and motivational symptoms in Parkinson’s disease: a meta-analysis of placebo-controlled studies. Clin Ther. 2009;31(1):89–98. doi: 10.1016/j.clinthera.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Löw A, Lang P, Smith J, Bradley M. Both predator and prey: emotional arousal in threat and reward. Psychol Sci. 2008;19(9):865–873. doi: 10.1111/j.1467-9280.2008.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- Masaki H, Takeuchi S, Gehring WJ, Takasawa N, Yamazaki K. Affective-motivational influences on feedback-related ERPs in a gambling task. Brain Res. 2006;1105:110–121. doi: 10.1016/j.brainres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Miller KM, Okun MS, Marsiske M, Fennell EB, Bowers D. Startle reflex hyporeactivity in Parkinson’s disease: an emotion-specific or arousal-modulated deficit? Neuropsychologia. 2009;47:1917–1927. doi: 10.1016/j.neuropsychologia.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugle KM, Hass CJ, Joyner J, Coombes SA, Janelle CM. Emotional state affects the initiation of forward gait. Emotion. 2011;11(2):267. doi: 10.1037/a0022577. [DOI] [PubMed] [Google Scholar]
- O’Brien RG, Kaiser MK. MANOVA method for analyzing repeated measures designs: an extensive primer. Psychol Bull. 1985;97(2):316. [PubMed] [Google Scholar]
- Ohgami Y, Kotani Y, Hiraku S, Aihara Y, Ishii M. Effects of Reward and Stimulus modality on stimulus-preceding negativity. Psychophysiology. 2004;41:729–738. doi: 10.1111/j.1469-8986.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- Ohgami Y, Kotani Y, Tsukamoto T, Omura K, Inoue Y, Aihara Y, et al. Effects of monetary reward and punishment on stimulus-preceding negativity. Psychophysiology. 2006;43:227–236. doi: 10.1111/j.1469-8986.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- Oishi M, Mochizuki Y, Du C, Takasu T. Contingent negative variation and movement-related cortical potentials in parkinsonism. Electroencephalogr Clin Neurophysiol. 1995;95:346–349. doi: 10.1016/0013-4694(95)00084-c. [DOI] [PubMed] [Google Scholar]
- Pedersen KF, Larsen JP, Alves G, Aarsland D. Prevalence and clinical correlates of apathy in Parkinson’s disease: a community-based study. Parkinsonism Relat Disord. 2009;15(4):295–299. doi: 10.1016/j.parkreldis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RSJ, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Pluck GC, Brown RG. Apathy in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2002;73(6):636–642. doi: 10.1136/jnnp.73.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F, Lutzenberger W, Müller V, Mohr B, Dichgans J, Birbaumer N. P3 and contingent negative variation in Parkinson’s disease. Electroencephalogr Clin Neurophysiol. 1996;98:456–467. doi: 10.1016/0013-4694(96)95537-6. [DOI] [PubMed] [Google Scholar]
- Schlogl A, Keinrath C, Zimmermann D, Scherer R, Leeb R, Pfurtscheller G. A fully automated correction method of EOG artifacts in EEG recordings. Clin Neurophysiol. 2007;118:98–104. doi: 10.1016/j.clinph.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Schneider F, Althaus A, Backes V, Dodel R. Psychiatric symptoms in Parkinson’s disease. Eur Arch Psychiatry Clin Neurosci. 2008;258(S):55–59. doi: 10.1007/s00406-008-5012-4. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37(2):257–261. [PubMed] [Google Scholar]
- Shibasaki H, Shima F, Kuroiwa Y. Clinical studies of the movement-related cortical potential (MP) and the relationship between the dentatorubrothalamic pathway and readiness potential (RP) J Neurol. 1978;219:15–25. doi: 10.1007/BF00313365. [DOI] [PubMed] [Google Scholar]
- Simons RF, Öhman A, Lang PJ. Anticipation and response set: cortical, cardiac, and electrodermal correlates. Psychophysiology. 1979;16(3):222–233. doi: 10.1111/j.1469-8986.1979.tb02982.x. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Khuraibet AJ. Readiness potential of cortical area 6 preceding self-paced movement in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1987;50:1184–1191. doi: 10.1136/jnnp.50.9.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith–Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Sydeman SJ, Owen AE, Marsh BJ. Measuring anxiety and anger with the state-trait anxiety inventory (STAI) and the state-trait anger expression inventory (STAXI) the use of Psychological Testing for Treatment Planning and Outcomes Assessment, 2. Lawrence Erlbaum Associates Publishers; Mahwah, NJ: 1999. pp. 993–1021. [Google Scholar]
- Starkstein S, Mayberg H, Preziosi T, Andrezejewski P. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1992;4(2):134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Disorders of frontal lobe functioning. Semin Neurol. 2000;20(4):427–437. doi: 10.1055/s-2000-13175. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia–brainstem pathways in the control of motor behaviors. Neurosci Res. 2004;50(2):137–151. doi: 10.1016/j.neures.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Tanji J. Comparison of neuronal activities in the monkey supplementary and precentral motor areas. Behav Brain Res. 1985;18:137–142. doi: 10.1016/0166-4328(85)90069-5. [DOI] [PubMed] [Google Scholar]
- Tsuda T. Contingent negative variation in parkinsonism. Tokushima J Exp Med. 1982;29:87–94. [PubMed] [Google Scholar]
- Verleger R, Wascher E, Arolt V, Daase C, Strohm A, Kompf D. Slow EEG potentials (contingent negative variation and post-imperative negative variation) in schizophrenia: their association to the present state and to Parkinsonian medication effects. Clin Neurophysiol. 1999;110:1175–1192. doi: 10.1016/s1388-2457(99)00023-1. [DOI] [PubMed] [Google Scholar]
- Visser M, Leentjens AF, Marinus J, Stiggelbout AM, van Hilten JJ. Reliability and validity of the Beck depression inventory in patients with Parkinson’s disease. Mov Disord. 2006;21(5):668–672. doi: 10.1002/mds.20792. [DOI] [PubMed] [Google Scholar]
- Visser M, van Rooden SM, Verbaan D, Marinus J, Stiggelbout AM, van Hilten JJ. A comprehensive model of health-related quality of life in Parkinson’s disease. J Neurol. 2008;255(10):1580–1587. doi: 10.1007/s00415-008-0994-4. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson’s disease. J Am Geriatr Soc. 2004;52(5):784–788. doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Horan WP, Kring AM, Simons RF, Green MF. Impaired anticipatory event-related potentials in schizophrenia. Int J Psychophysiol. 2010;77(2):141–149. doi: 10.1016/j.ijpsycho.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB. Doctoral Dissertation submitted at the University of Florida. 2011. Components of Depression in Parkinson disease: Relation to Psycho-physiology and Affective Chronometry. [Google Scholar]
- Zahodne LB, Mariske M, Okun MS, Rodriguez RL, Malaty I, Bowers D. Mood and motor trajectories in Parkinson’s disease: multivariate latent growth curve modeling. Neuropsychology. 2012;26(1):71–80. doi: 10.1037/a0025119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


