Abstract
Background
Geraniol is an acyclic monoterpene alcohol, which exhibits good prospect as a gasoline alternative. Geraniol is naturally encountered in plants at low concentrations and an attractive target for microbial engineering. Geraniol has been heterologously produced in Escherichia coli, but the low titer hinders its industrial applications. Moreover, bioconversion of geraniol by E. coli remains largely unknown.
Results
Recombinant overexpression of Ocimum basilicum geraniol synthase, Abies grandis geranyl diphosphate synthase, and a heterotic mevalonate pathway in E. coli BL21 (DE3) enabled the production of up to 68.6 ± 3 mg/L geraniol in shake flasks. Initial fed-batch fermentation only increased geraniol production to 78.8 mg/L. To further improve the production yield, the fermentation conditions were optimized. Firstly, 81.4 % of volatile geraniol was lost during the first 5 h of fermentation in a solvent-free system. Hence, isopropyl myristate was added to the culture medium to form an aqueous-organic two-phase culture system, which effectively prevented volatilization of geraniol. Secondly, most of geraniol was eventually biotransformed into geranyl acetate by E. coli, thus decreasing geraniol production. For the first time, we revealed the role of acetylesterase (Aes, EC 3.1.1.6) from E. coli in hydrolyzing geranyl acetate to geraniol, and production of geraniol was successfully increased to 2.0 g/L under controlled fermentation conditions.
Conclusions
An efficient geraniol production platform was established by overexpressing several key pathway proteins in engineered E. coli strain combined with a controlled fermentation system. About 2.0 g/L geraniol was obtained using our controllable aqueous-organic two-phase fermentation system, which is the highest yield to date. In addition, the interconversion between geraniol and geranyl acetate by E. coli was first elucidated. This study provided a new and promising strategy for geraniol biosynthesis, which laid a basis for large-scale industrial application.
Keywords: Geraniol, Geranyl acetate, Fed-batch fermentation, Acetylesterase, Engineered Escherichia coli
Background
Monoterpene geraniol (trans-isomer of 3,7-dimethyl-2, 6-octadiene-1-ol), which is emitted from flowers, has been widely applied in perfume, pharmaceutical, and other industries [1–3]. As a gasoline alternative, geraniol is superior to ethanol due to low hygroscopicity, high energy content, and relatively low volatility [4, 5]. Geraniol is derived from geranyl diphosphate (GPP) which is synthesized from either the mevalonate (MVA) pathway or the methylerythritol phosphate pathway in plants [6, 7]. However, geraniol has low economic value because it can only be extracted naturally from plants at very low concentrations. Large amounts of value-added products can be generated through the metabolic engineering of microbial hosts [8–10]. Unlike plants, microorganisms usually do not carry a specific GPP synthase (GPPS) and cannot make monoterpenes with the exception of a few winemaking Saccharomyces cerevisiae strains which manage to do so (5 mg/L monoterpenes) [11–13]. In recent years, geraniol has been successfully heterologously produced in Escherichia coli and S. cerevisiae. Mutations in farnesyl diphosphate synthase (FPPS) allow GPP release for monoterpene biosynthesis in recombinant microorganisms harboring monoterpene synthases [11, 14, 15]. A recent study demonstrated that GPP accumulation in yeast bearing mutated FPPS enabled geraniol formation in the absence of a heterologous geraniol synthase probably through endogenous dephosphorylation [11, 14]. Geraniol can also be generated even in the absence of specific GPPS or mutated FPPS in E. coli by simply overexpressing an Ocimum basilicum geraniol synthase (GES), although the GPP release mechanism remains unclear [16]. By co-overexpression of a FPPS mutant and GES in S. cerevisiae, 5 mg/L geraniol was obtained after 7 days of culture [11]. Production of geraniol was further increased to 36.04 mg/L in S. cerevisiae harboring both regulator gene MAF1 and GES after 48 h of culture by overexpressing key rate-limiting enzymes of the MVA pathway [17]. So far, maximum geraniol (182.5 mg/L) has been produced by geraniol dehydrogenase mutant E. coli with the whole MVA pathway and GES after 48 h of culture [5]. However, the titer is still too low for industrial applications.
In addition, geraniol usually undergoes biotransformation to other terpenoids in aromatic plants, which influences the quality of distilled essential oils [18, 19]. The conversion of geraniol to trans-citral in Cymbopogon flexuosus leaves is catalyzed by NADP+-dependent geraniol dehydrogenase [20]. Similarly, some wine yeasts can modify the free terpenoid contents, although they only have limited capability to produce monoterpenoids [21–23]. S. cerevisiae is able to convert geraniol into citronellol under the catalysis of enzyme OYE2 [24], and ATF1 alcohol acetyltransferase is involved in the acetylation of geraniol during S. cerevisiae fermentation [24]. Unlike the extensive studies on yeast, the bioconversion of geraniol in E. coli has seldom been referred and only until recently, geraniol has been dehydrogenized and isomerized into other geranoids (nerol, neral, and geranial) in E. coli by enzyme YjgB [5].
Thereby motivated, we created an effective geraniol-biosynthesizing strain and developed a new high-performance fermentation strategy to increase geraniol production. In addition, we observed the interconversion between geraniol and geranyl acetate in E. coli. The mechanism by which geranyl acetate was hydrolyzed into geraniol was thus investigated and controlled to further increase geraniol production.
Results and discussion
Regulated biosynthesis of geraniol from glucose in E. coli
A highly efficient strain LWG6 was constructed to produce geraniol from glucose in E. coli, comprising a heterotic MVA pathway from Enterococcus faecalis and S. cerevisiae, GPP synthase GPPS2 from Abies grandis, and codon-optimized GES from O. basilicum. This biosynthetic pathway (Fig. 1) was adapted from a previous study with genes from different origins [5]. It has previously been reported that GPP was efficiently synthesized by this heterotic MVA pathway and GPP synthase from A. grandis [25, 26]. After 48 h of shake-flask culture (OD600 = 2), 68.6 ± 3 mg/L geraniol was obtained from glucose by LWG6, while the control strain LWG10 without GES failed to produce geraniol. The geraniol production efficiency of strain LWG6 (34.3 mg/L/OD600) doubled that of strain GEOLW (about 16 mg/L/OD600) after 48 h of culture in flask [5], which followed a similar geraniol synthesis pathway to that of wild type E. coli MG 1655. Accordingly, LWG6 was a promising strain for geraniol synthesis.
Fig. 1.
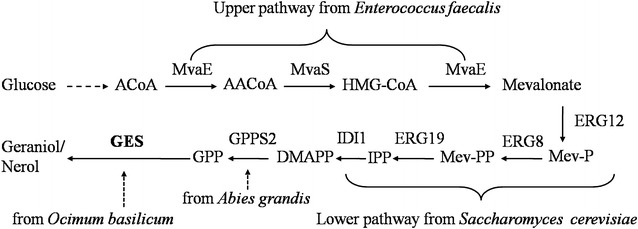
Production of geraniol via the MVA pathways used in this study. Enzymes involved in this pathway: MvaE, E. faecalis acetyl-CoA acetyltransferase/HMG-CoA reductase; MvaS, E. faecalis HMG-CoA synthase; ERG12, S. cerevisiae mevalonate kinase; ERG8, S. cerevisiae phosphomevalonate kinase; ERG19, S. cerevisiae mevalonate pyrophosphate decarboxylase; IDI1, S. cerevisiae IPP isomerase; GPPS2, A. grandis geranyl diphosphate synthase; GES, O. basilicum synthase was optimized to the preferred codon usage of E. coli. Pathway intermediates: A-CoA, acetyl-CoA; AA-CoA, acetoacetyl-CoA; HMG-CoA, hydroxymethylglutaryl-CoA; Mev-P, mevalonate 5-phosphate; Mev-PP, mevalonate pyrophosphate. IPP isopentenyl pyrophosphate, DMAPP dimethylallyl pyrophosphate, GPP geranyl diphosphate
Geranyl acetate formation in E. coli under fed-batch fermentation condition
The fed-batch fermentation was carried out with LWG6 based on the results obtained above in shake flask. Geraniol accumulation was monitored over the course of fermentation (Fig. 2). The highest concentration of geraniol was just 78.8 mg/L after being induced by isopropyl β-d-thiogalactoside (IPTG) for 5 h. The low titer then plummeted to 12.9 mg/L after 24 h, which may be ascribed to the volatilization of geraniol during fermentation and the accumulated toxicity may further prevent its synthesis by engineered E. coli [27, 28]. To prove the volatility of geraniol during fermentation, an authentic geraniol standard was fed to the culture of E. coli BL21 (DE3) in a 5 L fermentor. As shown in Fig. 3a, 81.4 % of fed geraniol is lost during the first 5 h of fermentation, probably through volatilization. In order to prevent volatilization, isopropyl myristate that also can reduce monoterpene toxicity was added [27], forming an aqueous-organic two-phase culture system. With this system, the amount of fed geraniol was kept stable during 20 h of culture (Fig. 3b), which was conducive to geraniol fermentation.
Fig. 2.
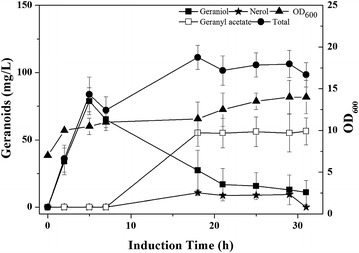
Fed-batch fermentation by LWG6 in a solvent-free system. Accumulation of total geranoids (filled circle) including geraniol (filled square), nerol (circle), and geranyl acetate (square). Induction was carried out when OD600 (filled triangle) reached about 10 using 0.5 mM IPTG. Results are the mean of three replicates with error bars representing the standard deviation
Fig. 3.
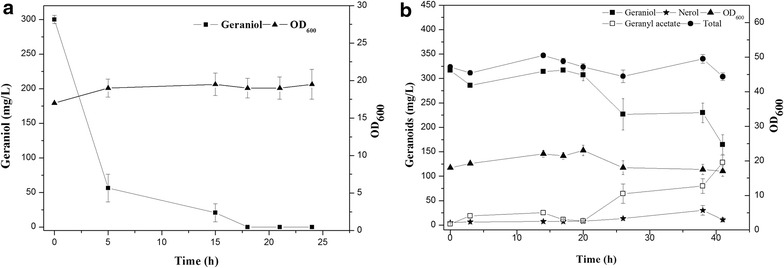
Geranyl acetate feeding experiments by E. coli BL21 (DE3). a Feeding experiments in a solvent-free system. b Feeding experiments in aqueous-organic two-phase culture system. Isopropyl myristate was added when OD600 (filled triangle) reached about 20 according to one over ten of the fermentation medium volume. Accumulation of total geranoids (filled circle) including geraniol (filled square), nerol (circle), and geranyl acetate (square). Results are the mean of three replicates with error bars representing the standard deviation
Moreover, the dehydrogenation and isomerization of geraniol into other geranoids (nerol, neral, and geranial) may also be responsible for the loss of geraniol [5]. Only 10.8 mg/L nerol was detected at 18 h, which descended to zero at the end of fermentation (Fig. 2). Instead of nerol, neral, and geranial, a new compound appeared at 18 h of culture, which was identified as geranyl acetate by GC-MS analysis. At the end of fermentation, geranyl acetate accounted for 83.7 % of total geranoids.
The formation of geranyl acetate was analyzed. Firstly, cat gene in plasmid pSTV28 encoding chloramphenicol acetyltransferase (CAT) is known to be responsible for chloramphenicol resistance, which exhibits non-specific esterification activity toward esterification of geraniol into geranyl acetate [5]. Plasmid pACYDuet-1 used in our study also harbors CAT, forming geranyl acetate. Secondly, in a previous study, acetyltransferase ATF1 (EC 2.3.1.84) from S. cerevisiae mainly contributed to geranyl acetate synthesis [24]. It is highly possible that similar functional enzymes of o-acetyltransferase (EC 2.3.1.9) also exist in E. coli and cause the esterification of geraniol therein [29]. This postulation was supported by the fed experiment shown in Fig. 3b. Geraniol decreased after 20 h of culture along with the accumulation of geranyl acetate. After 40 h of culture, more than 40 % of fed geraniol was converted into geranyl acetate (128.3 mg/L) by E. coli BL21 (DE3). Nerol was also detected but the total production was lower than 8 %, suggesting that geraniol was not lost mainly through dehydrogenation in E. coli BL21 (DE3) under such fed-batch conditions. Geranial and neral were not found and the amounts of total geranoids (geraniol, nerol, and geranyl acetate) were relatively stable during fermentation.
Conversion of geranyl acetate to geraniol by AES from E. coli
Simple gene knockout may not effectively prevent geranyl acetate synthesis, so geraniol production can feasibly be augmented by converting geranyl acetate to geraniol in engineered E. coli. Acetylesterase GAE (EC 3.1.1.6) from Cymbopogon martinii is involved in the transformation of geranyl acetate into geraniol [18]. Acetylesterase (Aes, EC 3.1.1.6) also exists in E. coli, although its effect on geranyl acetate is still not clear [30]. In our study, Aes was overexpressed in E. coli BL21 (DE3), the activity of which was indicated by incubating geranyl acetate under defined conditions and monitoring the amount of produced geraniol with GC-MS. Since about 75 % of geranyl acetate was converted into geraniol after 2 h of incubation, E. coli was capable of geranyl acetate hydrolyzation. No geraniol was produced in the control experiment using boiled and denatured enzyme.
Geraniol production under fed-batch fermentation with biotransformation of geranyl acetate to geraniol
Considering the effect of Aes, geraniol production can feasibly be increased through biotransformation of geranyl acetate to geraniol during fermentation. It can be allowed by overexpression of Aes in LWG6 which, however, further burdens the cell metabolism system, since eight heterologous genes have already been designed to be overexpressed. Moreover, the above transformation can be realized by regulating fermentation. In the absence of glucose, E. coli cells reuse acetate [31], thereby facilitating the formation of geraniol catalyzed by Aes. Geraniol feeding experiment was used to identify whether geranyl acetate, which was esterified from geraniol, can reproduce geraniol by this glucose starvation strategy. The control strain LWG10 was used in feeding experiment to ensure similar conversion of geraniol to geranyl acetate. As shown in Fig. 4a, fed geraniol is lost quickly by LWG10 and about 86 % of geraniol is converted to geranyl acetate at 21 h. From 21 to 28 h, the geraniol amount remained stable, suggesting the reaction between geraniol and geranyl acetate reached equilibrium. Then glucose supply was stopped at 28 h, and geraniol production rose from 33 to 160 mg/L at 39 h when residual glucose was exhausted, probably because the reuse of acetate disturbed the reaction balance and moved the reaction toward geraniol formation under the catalysis of Aes. For comparison, glucose was continuously added and most of geraniol was quickly converted to geranyl acetate that was kept at a low concentration thereafter (Fig. 4b).
Fig. 4.
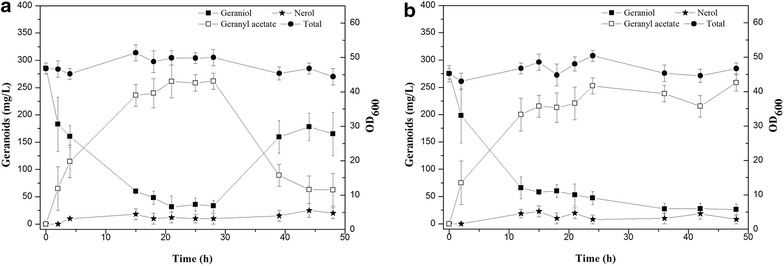
Geranyl acetate feeding experiments by LWG10. a Glucose addition was stopped after 28 h of culture. b The residual glucose was maintained below 5 g/L by a feeding solution containing 800 g/L glucose at appropriate rates. Isopropyl myristate was added when OD600 reached about 20 according to one over ten of the fermentation medium volume. Accumulation of total geranoids (filled circle) including geraniol (filled square), nerol (circle), and geranyl acetate (square). Results are the mean of three replicates with error bars representing the standard deviation
Thus, a new fermentation strategy was established based on the above results. First, isopropyl myristate was added to form an aqueous-organic two-phase culture system. Second, glucose starvation was employed to convert geranyl acetate into geraniol. Therefore, this new fermentation condition was used with LWG6 (Fig. 5). At the beginning, both geraniol and geranyl acetate increased quickly and OD600 value of the bacterial culture increased from 20 to 32 rapidly. The titers of geraniol and geranyl acetate reached 1.04 and 1.01 g/L at 24 h of culture, respectively. During the next 24 h, geranyl acetate increased to 1.43 g/L with decreasing geraniol, suggesting that the strain no longer synthesized geraniol that was converted to geranyl acetate. Then, glucose supply was stopped at 48 h and the culture was continued under the glucose starvation condition. As expected, geranyl acetate was converted to geraniol after 56 h of culture while OD600 value of the bacterial culture began to decline slightly. At the end of fermentation, the concentration of geraniol reached maximum (2.0 g/L), and the yield (from glucose to geraniol) was 14 % which is approximately 11-fold that reported before [5]. Geranyl acetate production was reduced to 0.16 g/L at 68 h. Geraniol or neral was not detected while nerol was kept at a very low concentration during fermentation (0.05 g/L at 52 h). About 1.27 g/L (88.8 %) geranyl acetate was successfully converted to geraniol during the later stage of fermentation by glucose starvation.
Fig. 5.
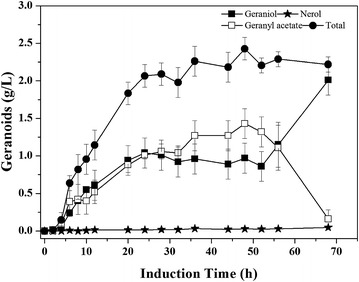
Geraniol production under fed-batch fermentation with biotransformation of geranyl acetate to geraniol. Accumulation of total geranoids (filled circle) including geraniol (filled square), nerol (circle), and geranyl acetate (square). Induction was carried out when OD600 reached about 20 using 0.5 mM IPTG. Isopropyl myristate was added 4 h after IPTG induction according to one over ten of the fermentation medium volume, and glucose addition was stopped after 48 h of culture. Results are the mean of three replicates with error bars representing the standard deviation
Although higher concentration of geraniol was obtained in this study, geraniol production needs to be elevated at least 3 to 4 times in the future to meet the requirements of industrialization. Of all possible improvement strategies, engineering of the host can be realized by employing a chromosome integration technique to decrease the cell growth burden that results from overexpression of heterologous genes. Another approach is optimization of fermentation conditions such as induction time, inoculum age, and organic solvent for geraniol production.
Conclusions
In summary, an efficient strain LWG6 was constructed and an isopropyl myristate-overlaid two-phase fed-batch fermentation system was established to biosynthesize geraniol from glucose. For the first time, the interconversion between geraniol and geranyl acetate by E. coli was observed, and Aes from E. coli was involved in the hydrolysis of geranyl acetate. Geraniol production reached up to 2.0 g/L with biotransformation of 88.8 % geranyl acetate to geraniol under controlled fermentation condition, which is the highest from glucose hitherto. This study provided a new strategy for geraniol biosynthesis.
Methods
Medium and culture conditions
Luria broth (LB) medium (10 g/L tryptone, 10 g/L NaCl, and 5 g/L yeast extract) was used for gene cloning and shake-flask fermentation. For geraniol production, recombinant strains were cultured in shake-flask or fed-batch fermentation with the medium containing 20 g/L glucose, 9.8 g/L K2HPO4, 5 g/L beef extract, 0.3 g/L ferric ammonium citrate, 2.1 g/L citric acid monohydrate, and 0.06 g/L MgSO4 as well as 1 ml of trace element solution which included 0.37 g/L (NH4)6Mo7O24·4H2O, 0.29 g/L ZnSO4·7H2O, 2.47 g/L H3BO4, 0.25 g/L CuSO4·5H2O, and 1.58 g/L MnCl2·4H2O. Appropriate antibiotics were added to the culture medium according to selectable marker gene of each plasmid listed in Table 1 at the following concentrations: ampicillin (Amp, 100 mg/ml), kanamycin (Kan, 50 mg/ml), and chloramphenicol (Cm, 34 mg/ml).
Table 1.
Strains and plasmids used in this study
| Name | Relevant characteristics | References |
|---|---|---|
| primersa | ||
| GES-rbs-F | GGAAGATCTAGGAGGTAAAAAATATGTCTTGCGCTCGTATCACCG | This study |
| GES-R | CCGCTCGAGTTACTGGGTGAAGAACAGAGCG | This study |
| Aes-F-NcoI | CCCATGGCTATGAAGCCGGAAAACAAACT | This study |
| Aes-R-EcoRI | GGAATTCCTAAAGCTGAGCGGTAAAGAACTG | This study |
| Strains | ||
| BL21(DE3) | F− omp T, hsdSB (r−Bm−B), gal, dcm me131, λ(DE3) | Invitrogen |
| LWG6 | E.coli BL21(DE3)/pLWG6, pYJM14 | This study |
| LWG10 | E.coli BL21(DE3)/p YJM26, pYJM14 | This study |
| LWG11 | E.coli BL21(DE3)/p LWG11 | This study |
| Plasmids | ||
| pET-30a | F1 (pBR322), Kan | Novagen |
| pGH/GES | pGH carrying GES from O. basilicum | This study |
| pLWG 6 | pACYCDuet-1 carrying mvaE and mvaS from E. faecalis, GPPS2 from A. grandis,GES from O. basilicum, Cm | This study |
| pLWG11 | pET-30a carrying aes from E. coli, Kan | This study |
| pYJM26 | pACYCDuet-1 carrying mvaE and mvaS from E. faecalis, GPPS2 from A. grandis, Cm | [27] |
| pYJM14 | pTrcHis2B carrying ERG12, ERG8, ERG19 and IDI1 from S.cerevisiae, Amp | [27] |
a Restriction sites are underlined
Strains and plasmids
All strains and plasmids used in this study are listed in Table 1. The nucleotide sequences of GES gene from O. basilicum (sweet basil) (GenBank No. AY362553.1) were analyzed (http://www.genscript.com/cgi-bin/tools/rare_codon_analysis) and optimized to the preferred codon of E. coli (http://www.jcat.de/) online. The codon-optimized GES gene was synthesized by Genray Company with plasmid pGH as vector pGH/GES. GES gene was PCR-amplified from plasmid DNA of pGH/GES with primer set GES-rbs-F/GES-R. The PCR products were digested with BglII/XhoI, respectively, and introduced into the corresponding sites of pYJM26 to create pLWG 6. Plasmids pLWG 6 and pYJM14 were co-expressed in E. coli BL21 (DE3) to form strain LWG6. Aes was PCR-amplified from genomic DNA of BL21 (DE3) with primer set Aes-F-NcoI/Aes-R-EcoRI. The PCR product digested with NcoI and EcoRI was cloned into pET30a cut with the same restriction enzymes, creating pLWG11.
Enzyme extraction and assay
LWG11 was cultured in LB broth and induced by IPTG at a final concentration of 0.1 mM when OD600 of the bacterial culture reached 0.6–0.8. After being incubated at 30 °C for 4–6 h, the cells were harvested by centrifugation at 6000 g for 5 min, washed with distilled water, and then resuspended in 0.5 M Tris-HCl (pH 8). All the extraction procedures were carried out at 4 °C. The cells were broken by sonic treatment for 10 min at 0 °C (3 s pulse on, 3 s pulse off, 40 W, Sonics VCX130, China). The Aes activity was determined by GC-MS monitoring of geraniol produced by the hydrolysis of geranyl acetate. The assay system consisted of 0.05 M Tris-Cl, pH 8.0, 5 mM MgSO4, 1 mM DTE, 2 mM geranyl acetate, and the enzyme extract (about 0.4 mg protein) in a total volume of 0.5 ml. The reaction mixture was incubated at 30 °C in a sealed capped tube for 2 h. A blank control with boiled enzyme was also run simultaneously.
Shake-flask cultures
A single colony of LWG6 was grown in LB broth overnight at 37 °C. The culture was used to inoculate the same medium (1:100 dilution) and grown at 37 °C until an OD600 of 0.6–0.8 was reached. IPTG was added to a final concentration of 0.1 mM, and the culture was further incubated at 30 °C for 48 h. The samples were added with the same volume of ethyl acetate, vortexed briefly, and centrifuged to separate the phases, and the organic phase was analyzed by GC-MS. LWG10 strain was used as control. The experiment was performed in triplicate.
Fed-batch fermentation for geraniol biosynthesis from glucose
LWG6 strain was grown overnight at 37 °C in 100 ml of LB medium and inoculated to a 5-L fermentor (BIOSTAT B plus MO5L, Sartorius, Germany) containing 2 L of fermentation medium. The temperature was maintained at 37 °C, and pH was maintained at 7.0 by automatically adding ammonia. Antifoam 204 was used to prohibit foam development. The stirring speed was first set at 400 rpm to maintain a 20 % saturation of dissolved oxygen. The expression of plasmid-born exogenous gene(s) for geraniol production was induced with 0.5 mM IPTG at 30 °C. During the course of fermentation, residual glucose was measured using a glucose analyzer (SBA-40D, China) and maintained below 5 g/L by a feeding solution containing 800 g/L glucose at appropriate rates. The samples were added with the same volume of ethyl acetate, vortexed briefly, and centrifuged to separate the phases, and the organic phase was analyzed.
To prevent violation, isopropyl myristate was added 4 h after IPTG induction according to one over ten of the fermentation medium volume. Glucose was stopped feeding after 48 h of culture. The samples were collected on time, and the organic phase was separated by centrifugation at 13,000 rpm for 10 min, then added with ten volumes of ethyl acetate and analyzed by GC-MS.
Geranyl acetate feeding experiments
About 300 mg per liter medium of geraniol was fed to the cultures of E. coli BL21 (DE3) or LWG10 (OD600 at 20) to investigate the fate of geraniol during fermentation. Other fermentation conditions were the same as above.
Geraniol characterization by GC-MS
Putative geraniol products were identified by GC-MS. A HP-INNOWAX capillary column (30 m × 0.25 mm; 0.25-μm-film thickness; Agilent Technologies) was used. The oven temperature was initially held at 80 °C for 1 min and sequentially increased at the rate of 10 °C/min to 180 and 30 °C/min to 250 °C. Peak identification was based on a relative retention time and total ion mass spectral comparison with an external standard (Sigma-Aldrich, USA). The peak areas were converted into concentrations in comparison with standard curves plotted with a set of known concentrations of standards.
Authors’ contributions
HL and WL developed the idea for the study. WL and MX designed the research, did the literature review, and prepared the manuscript. YC and JG helped to revise the manuscript. XX, RZ, TC, and XXL did the lab work, plasmid construction, strain cultivation, fed-batch fermentation, and product detection. WL and YC drafted the initial manuscript together and all authors contributed in analyzing the data and writing the draft. All authors read and approved the final manuscript.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21376255), Key Program of the Chinese Academy of Sciences (KGZD-EW-606-1-3), and Taishan Scholars Climbing Program of Shandong (tspd20150210).
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- Aes
acetylesterase
- MVA
mevalonate
- GPP
geranyl diphosphate
- GPPS
geranyl diphosphate synthase
- FPPS
farnesyl diphosphate synthase
- GES
Ocimum basilicum geraniol synthase
- Amp
ampicillin
- Kan
kanamycin
- Cm
chloramphenicol
- IPTG
isopropyl β-d-thiogalactoside
Contributor Information
Wei Liu, Email: liuwei@qibebt.ac.cn.
Xin Xu, Email: xuxin@qibebt.ac.cn.
Rubing Zhang, Email: zhangrb@qibebt.ac.cn.
Tao Cheng, Email: chengtao@qibebt.ac.cn.
Yujin Cao, Email: caoyj@qibebt.ac.cn.
Xiaoxiao Li, Email: 1154803919@qq.com.
Jiantao Guo, Email: jguo4@unl.edu.
Huizhou Liu, Email: liuhuizhou@qibebt.ac.cn.
Mo Xian, Email: xianmo1@qibebt.ac.cn.
References
- 1.Chen W, Viljoen AM. Geraniol—a review of a commercially important fragrance material. S Afr J Bot. 2010;76(4):643–651. doi: 10.1016/j.sajb.2010.05.008. [DOI] [Google Scholar]
- 2.Lapczynski A, Bhatia SP, Foxenberg RJ, Letizia CS, Api AM. Fragrance material review on geraniol. Food Chem Toxicol. 2008;46(Suppl 11):S160–S170. doi: 10.1016/j.fct.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 3.Polo M, Crespo R. Bravo Md. Geraniol and simvastatin show a synergistic effect on a human hepatocarcinoma cell line. Cell Biochem Funct. 2011;29(6):452–458. doi: 10.1002/cbf.1772. [DOI] [PubMed] [Google Scholar]
- 4.Peralta-Yahya PP, Keasling JD. Advanced biofuel production in microbes. Biotechnol J. 2010;5(2):147–162. doi: 10.1002/biot.200900220. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Wang C, Yoon S-H, Jang H-J, Choi E-S, Kim S-W. Engineering Escherichia coli for selective geraniol production with minimized endogenous dehydrogenation. J Biotechnol. 2014;169:42–50. doi: 10.1016/j.jbiotec.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 7.Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep. 1999;16(5):565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- 8.Chang MC, Keasling JD. Production of isoprenoid pharmaceuticals by engineered microbes. Nat Chem Biol. 2006;2(12):674–681. doi: 10.1038/nchembio836. [DOI] [PubMed] [Google Scholar]
- 9.Clomburg JM, Gonzalez R. Biofuel production in Escherichia coli: the role of metabolic engineering and synthetic biology. Appl Microbiol Biotechnol. 2010;86(2):419–434. doi: 10.1007/s00253-010-2446-1. [DOI] [PubMed] [Google Scholar]
- 10.Leonard E, Lim K-H, Saw P-N, Koffas MA. Engineering central metabolic pathways for high-level flavonoid production in Escherichia coli. Appl Environ Microbiol. 2007;73(12):3877–3886. doi: 10.1128/AEM.00200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer MJ, Meyer S, Claudel P, Bergdoll M, Karst F. Metabolic engineering of monoterpene synthesis in yeast. Biotechnol Bioeng. 2011;108(8):1883–1892. doi: 10.1002/bit.23129. [DOI] [PubMed] [Google Scholar]
- 12.Carrau FM, Medina K, Boido E, Farina L, Gaggero C, Dellacassa E, et al. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol Lett. 2005;243(1):107–115. doi: 10.1016/j.femsle.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 13.Zea L, Moreno J, Ortega JM, Medina M. Content of free terpenic compounds in cells and musts during vinification with three Saccharomyces cerevisiae races. J Agric Food Chem. 1995;43(4):1110–1114. doi: 10.1021/jf00052a046. [DOI] [Google Scholar]
- 14.Oswald M, Fischer M, Dirninger N, Karst F. Monoterpenoid biosynthesis in Saccharomyces cerevisiae. FEMS Yeast Res. 2007;7(3):413–421. doi: 10.1111/j.1567-1364.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard L, Karst F. Characterization of a lysine-to-glutamic acid mutation in a conservative sequence of farnesyl diphosphate synthase from Saccharomyces cerevisiae. Gene. 1993;125(2):185–189. doi: 10.1016/0378-1119(93)90326-X. [DOI] [PubMed] [Google Scholar]
- 16.Fischer MJ, Meyer S, Claudel P, Perrin M, Ginglinger JF, Gertz C, et al. Specificity of Ocimum basilicum geraniol synthase modified by its expression in different heterologous systems. J Biotechnol. 2013;163(1):24–29. doi: 10.1016/j.jbiotec.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Zhang W, Du G, Chen J, Zhou J. Over production of geraniol by enhanced precursor supply in Saccharomyces cerevisiae. J Biotechnol. 2013;168(4):446–451. doi: 10.1016/j.jbiotec.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Dubey VS, Luthra R. Biotransformation of geranyl acetate to geraniol during palmarosa (Cymbopogon martinii, Roxb. wats. var. motia) inflorescence development. Phytochemistry. 2001;57(5):675–680. doi: 10.1016/S0031-9422(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 19.Cormier F, Ambid C. Extractive bioconversion of geraniol by a Vitis vinifera cell suspension employing a two-phase system. Plant Cell Rep. 1987;6(6):427–430. doi: 10.1007/BF00272774. [DOI] [PubMed] [Google Scholar]
- 20.Sangwan RS, Singh-Sangwan N, Luthra R. Metabolism of acyclic monoterpenes: partial purification and properties of geraniol dehydrogenase from lemongrass (Cymbopogon flexuosus Stapf.) leaves. J Plant Physiol. 1993;142(2):129–134. doi: 10.1016/S0176-1617(11)80952-1. [DOI] [Google Scholar]
- 21.Fia G, Giovani G, Rosi I. Study of β-glucosidase production by wine-related yeasts during alcoholic fermentation. A new rapid fluorimetric method to determine enzymatic activity. J Appl Microbiol. 2005;99(3):509–517. doi: 10.1111/j.1365-2672.2005.02657.x. [DOI] [PubMed] [Google Scholar]
- 22.Arifin AA, Don MM, Uzir MH. Baker’s yeast mediated biotransformation of geraniol into citronellol using a continuous-closed-gas-loop bioreactor (CCGLB) system. Biochem Eng J. 2011;56(3):219–224. doi: 10.1016/j.bej.2011.07.001. [DOI] [Google Scholar]
- 23.Gamero A, Manzanares P, Querol A, Belloch C. Monoterpene alcohols release and bioconversion by Saccharomyces species and hybrids. Int J Food Microbiol. 2011;145(1):92–97. doi: 10.1016/j.ijfoodmicro.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 24.Steyer D, Erny C, Claudel P, Riveill G, Karst F, Legras J-L. Genetic analysis of geraniol metabolism during fermentation. Food Microbiol. 2013;33(2):228–234. doi: 10.1016/j.fm.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Xian M, Su S, Zhao G, Nie Q, Jiang XL, Zheng YN, Liu W. Enhancing production of bio-Isoprene using hybrid MVA pathway and isoprene synthase in E. coli. PLoS One. 2012;7(4):e33509. doi: 10.1371/journal.pone.0033509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Nie Q, Ren M, Feng H, Jiang X, Zheng Y, et al. Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol Biofuels. 2013;6(1):60. doi: 10.1186/1754-6834-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan TC, Turner CD, Krömer JO, Nielsen LK. Alleviating monoterpene toxicity using a two-phase extractive fermentation for the bioproduction of jet fuel mixtures in Saccharomyces cerevisiae. Biotechnol Bioeng. 2012;109(10):2513–2522. doi: 10.1002/bit.24536. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Yoon SH, Shah AA, Chung YR, Kim JY, Choi ES, et al. Farnesol production from Escherichia coli by harnessing the exogenous mevalonate pathway. Biotechnol Bioeng. 2010;107(3):421–429. doi: 10.1002/bit.22831. [DOI] [PubMed] [Google Scholar]
- 29.Kursula P, Sikkilä H, Fukao T, Kondo N, Wierenga RK. High resolution crystal structures of human cytosolic thiolase (CT): a comparison of the active sites of human CT, bacterial thiolase, and bacterial KAS I. J Mol Biol. 2005;347(1):189–201. doi: 10.1016/j.jmb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Farias T, Mandrich L, Rossi M, Manco G. Biochemical and thermostability features of acetyl esterase aes from Escherichia coli. Protein Pept Lett. 2007;14(2):165–169. doi: 10.2174/092986607779816113. [DOI] [PubMed] [Google Scholar]
- 31.Prieto MA, Galán B, Torres B, Ferrández A, Fernández C, Miñambres B, et al. Aromatic metabolism versus carbon availability: the regulatory network that controls catabolism of less-preferred carbon sources in Escherichia coli. FEMS Microbiol Rev. 2004;28:503–518. doi: 10.1016/j.femsre.2004.04.004. [DOI] [PubMed] [Google Scholar]


