Abstract
Aim:
In the continuing search for safe and efficient antidiabetic drug, marine algae become important source which provide several compounds of immense therapeutic potential. Alpha-amylase, alpha-glucosidase inhibitors, and antioxidant compounds are known to manage diabetes and have received much attention recently. In the present study, four green algae (Chaetomorpha aerea, Enteromorpha intestinalis, Chlorodesmis, and Cladophora rupestris) were chosen to evaluate alpha-amylase, alpha-glucosidase inhibitory, and antioxidant activity in vitro.
Materials and Methods:
The phytochemical constituents of all the extracts were qualitatively determined. Antidiabetic activity was evaluated by inhibitory potential of extracts against alpha-amylase and alpha-glucosidase by spectrophotometric assays. Antioxidant activity was determined by 2,2-diphenyl-1-picrylhydrazyl, hydrogen peroxide (H2O2), and nitric oxide scavenging assay. Gas chromatography-mass spectrometry (GC-MS) analysis was carried out to determine the major compound responsible for its antidiabetic action.
Results:
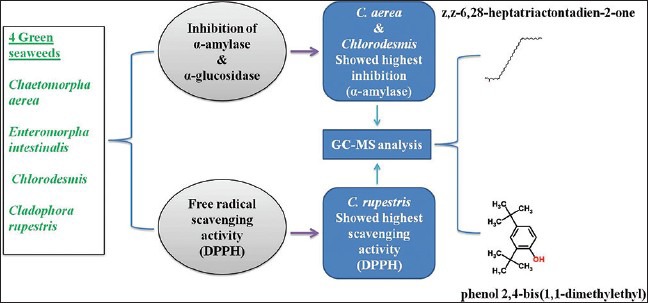
Among the various extracts screened, chloroform extract of C. aerea (IC50 − 408.9 μg/ml) and methanol extract of Chlorodesmis (IC50 − 147.6 μg/ml) showed effective inhibition against alpha-amylase. The extracts were also evaluated for alpha-glucosidase inhibition, and no observed activity was found. Methanol extract of C. rupestris showed notable free radical scavenging activity (IC50 – 666.3 μg/ml), followed by H2O2 (34%) and nitric oxide (49%). Further, chemical profiling by GC-MS revealed the presence of major bioactive compounds. Phenol, 2,4-bis (1,1-dimethylethyl) and z, z-6,28-heptatriactontadien-2-one were predominantly found in the methanol extract of C. rupestris and chloroform extract of C. aerea.
Conclusion:
Our results demonstrate that the selected algae exhibit notable alpha-amylase inhibition and antioxidant activity. Therefore, characterization of active compounds and its in vivo assays will be noteworthy.
SUMMARY
Four green algae were chosen to evaluate alpha-amylase, alpha-glucosidase inhibitory, and antioxidant activity in vitro
C. aerea and Chlorodesmis showed significant inhibition against alpha-amylase, and C. rupestris showed notable free radical scavenging activity
No observed activity was found against alpha-glucosidase
GC-MS analysis of the active extracts reveals the presence of major compounds which gives an insight on the antidiabetic and antioxidant activity of these algae.
Abbreviations used: DPPH: 2,2-diphenyl-1-picrylhydrazyl, BHT: Butylated hydroxytoluene, GC-MS: Gas chromatography-mass spectrometry.
Keywords: 2,2-diphenyl-1-picrylhydrazyl; alpha-amylase; alpha-glucosidase; Chaetomorpha aerea; Chlorodesmis; Cladophora rupestris
INTRODUCTION
Diabetes mellitus is a major endocrine disorder characterized by chronic hyperglycemia. It mainly affects the humans due to defects in insulin secretion or resistance.[1] Pancreatic alpha-amylase and alpha-glucosidase are key enzymes in the small intestine. These enzymes play a major role in the digestion of starch yielding glucose and maltose, leads to increased postprandial glucose levels.[2] Hence, reducing the starch digestion rate by inhibition of enzymes such as alpha-amylase and alpha-glucosidase is the best way for the management of diabetes.[3] Oxidative stress induced by free radical is also one of the causative factors for diabetes. Antioxidant compounds play a major role in scavenging free radicals and control of oxidative stress related diseases such as diabetes.[4]
Recently, marine algae and their bioproducts are getting greater attention toward the treatment of diabetes.[5] There is a great interest in screening marine algae for therapeutic drugs, as their ability to produce secondary metabolites has been extensively documented. Marine algae contain considerable amounts of polyphenols, which are effective antioxidants and have particular biological activities.[5] However, only a few studies have investigated the biological potential of Indian marine algae. In this study, four seaweeds (Chaetomorpha aerea, Enteromorpha intestinalis, Chlorodesmis, and Cladophora rupestris) belongs to the class of Chlorophyta were sequentially extracted using various solvents and screened for in vitro alpha-amylase, alpha-glucosidase, and various free radical scavenging assays (2,2-diphenyl-1-picrylhydrazyl [DPPH], hydrogen peroxide (H2O2), and nitric oxide). Preliminary phytochemical and gas chromatography-mass spectrometry (GC-MS) analyses were carried out to determine the probable inhibitory constituent.
MATERIALS AND METHODS
Algal samples
Fresh algae were collected from Parangipettai (Latitude 11°30’14”N; Longitude 79°45’8” E), Cuddalore (C. aerea, E. Intestinalis, and Chlorodesmis) and Kovalam (C. rupestris) (Latitude 12°79’25”N; Longitude 80°25’30"E) Chennai, Tamil Nadu, India during September 2012. The collected algae were cleaned, and holdfasts were removed, and they were shade dried, powdered, and stored under −20°C until extraction. The algal samples were identified and authenticated by Dr. P. Kaladharan, Principle Scientist and Scientist In Charge, Calicut Regional Centre of Central Marine Fisheries Research Institute.
Preparation of algal extracts
The dried algal samples (25 g) were milled and extracted using 250 ml of solvents such as chloroform, ethanol, and methanol for 24 h by using Soxhlet apparatus. Each filtrate was concentrated to dryness under reduced pressure using rotary evaporator (Super Fit, Rotavap, model, PBU-6, India). The samples were lyophilized by using freeze dryer (Lark, Penguin Classic Plus, India) and stored in a refrigerator at 2–8°C for use in subsequent experiments.
Preliminary phytochemical screening
Preliminary phytochemical screenings were carried out, as per the standard protocols of Harborne.[6]
In vitro alpha-amylase inhibition study
The alpha-amylase inhibitory activity was determined as described by Jayasri et al.[7] with minor modifications. Briefly, 250 μl of algal extracts with varying concentrations (125–500 μg/ml) and 250 μl of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M sodium chloride) containing alpha-amylase (porcine pancreatic alpha-amylase Sigma, St. Louis, USA) solution (0.5 mg/ml) were incubated for 10 min at 25°C. After preincubation, 250 μl of 1% starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M sodium chloride) was added to each tube at 5 s intervals. The reaction mixtures were then incubated at 25°C for 10 min. The reaction was stopped with 500 μl dinitrosalicylic acid (Sigma, St. Louis, USA) color reagent. The tubes were then incubated in a boiling water bath for 5 min and cooled to room temperature. The reaction mixture was then diluted by adding 5 ml of distilled water, and absorbance was measured at 540 nm in the ultraviolet (UV)-visible spectrophotometer (Systronics AU-2700, India). The experiments were performed in triplicates, and the alpha-amylase inhibitory activity was calculated as percentage inhibition, using the formula.
% Inhibition =([Abscontrol - Abssamples]/ Abscontrol)×100
In vitro alpha-glucosidase inhibition study
The alpha-glucosidase inhibitory activity was determined as described by Jayasri et al.[7] with slight modifications. Briefly, 50 μl of algal extracts with varying concentrations (250–1000 μg/ml) and 100 μl of 0.1 M phosphate buffer (pH-6.9) containing alpha-glucosidase (SRL, India) solution (1.0 U/ml) were incubated in 96 well plates at 25°C for 10 min. After preincubation, 50 μl of 5 mm p-nitrophenyl alpha-D-glucopyranoside (Sigma, St. Louis, USA) in 0.1 M phosphate buffer (pH-6.9) was added to each well at 5 s intervals. The reaction mixture was then incubated at 25°C for 5 min. After incubation, absorbance readings were recorded at 405 nm using a plate reader (Bio-TEK, USA) and compared to the control which contained 50 μl of buffer solution in place of algal extract. The experiments were performed in triplicates, and alpha-glucosidase inhibitory activity was calculated as percentage inhibition. The percentage of enzyme inhibition was calculated as follows.
% Inhibition =([Abscontrol - Abssamples]/ Abscontrol)×100
Free radical scavenging activity (2,2-diphenyl-1-picrylhydrazyl)
Free radical scavenging activity was determined according to the method of Mensor et al.[8] Briefly, 500 μl of 0.3 mM alcoholic solution of DPPH (Himedia, India) was added to 2.5 ml of test samples at varying concentrations (250–1000 μg/ml). The samples were incubated in dark for 30 min, and absorbance was measured at 518 nm using UV-visible spectrophotometer (Systronics AU-2700, India). Synthetic antioxidant butylated hydroxytoluene (BHT) were used as positive control. The experiments were performed in triplicates, and scavenging activity was expressed as percentage inhibition, using the following formula.
% Scavenging =([Abscontrol - Abssamples]/ Abscontrol)×100
Hydrogen peroxide radical scavenging activity
H2O2 radical scavenging activity was determined according to the method of Ruch et al.[9] A solution of H2O2 (40 mM) was prepared in phosphate buffer (50 mM, pH 7.4). Briefly, 1 ml of test samples of varying concentrations (250–1000 μg/ml) were added to the H2O2 solution and incubated for 10 min. Absorbance was measured at 230 nm against blank solution containing phosphate buffer without H2O2. Synthetic antioxidant ascorbic acid was used as positive control. The experiments were performed in triplicates, and scavenging activity was expressed as percentage scavenging, using the following formula.
% Scavenging =([Abscontrol - Abssamples]/ Abscontrol)×100
Nitric oxide scavenging activity
The nitric oxide scavenging activity was determined according to the method of Marcocci et al.[10] Briefly, 2 ml of the test extracts with varying concentrations (250–1000 μg/ml) were incubated with 0.5 ml of sodium nitroprusside (5 mM) for 2 h at 27°C. Aliquot 1 ml of the incubated solution and mixed with 0.6 ml of Griess reagent (1.0 mL sulfanilic acid reagent [0.33%] in 20% glacial acetic acid at room temperature for 5 min with 1 ml of naphthyl ethylenediamine dichloride [0.1%]). The absorbance was measured immediately at 550 nm, and synthetic antioxidant BHT was used as positive control. The experiments were performed in triplicates, and scavenging activity was expressed as percentage scavenging, using the following formula.
% Scavenging =([Abscontrol - Abssamples]/ Abscontrol)×100
Gas chromatography-mass spectrometry analysis
GC-MS (Perkin Elmer, Clarus 680 GC coupled to a Clarus 600 MS) analysis was performed for the detection of major compounds present in the methanol extracts of C. rupestris and chloroform extracts of C. aerea. Column used in GC-MS was Elite-5MS (30.0 m, 0.25 mm ID, 250 μm df). Carrier gas used was helium at a constant flow rate of 1 ml/min. Electron Ionization mode with electron energy set at 70 eV was used. About 1 ml of extract diluted with methanol was injected to GC-MS, and the compounds were identified based on the molecular structure, molecular mass, and calculated fragment ratio of resolved spectra with that of mass spectra available from the library. Spectral data were interpreted using the database of National Institute Standard and Technology (NIST).
Statistical analysis
All the data reported were expressed as mean ± standard error of the mean. Statistical analysis was performed using two-way ANOVA. The values were considered to be significantly different when the P < 0.0001 compared to the baseline values. Graph-Pad Prism, version 5 (GraphPad software, Inc.) was used for statistical analysis.
RESULTS AND DISCUSSION
Seaweeds and their organic extracts pose wide array of bioactive compounds with potential health benefits.[11] Marine seaweeds that can reduce postprandial hyperglycemia by inhibiting enzymes such as alpha-amylase and alpha-glucosidase is found to be an effective strategy for the management of diabetes.[12] In this study, four seaweeds (C. aerea, E. intestinalis, Chlorodesmis, and C. rupestris) collected from the southern coast of India were screened for alpha-amylase, alpha-glucosidase inhibitory activity, and antioxidant potential in vitro. Among the four seaweeds, C. aerea, E. Intestinalis, and C. rupestris were previously reported for its antibacterial and antioxidant activity.[13,14] However, none of the seaweeds have been screened for alpha-amylase and alpha-glucosidase inhibitory activity to elucidate the hypoglycemic activity in vitro. Therefore, the present study validates alpha-amylase and alpha-glucosidase inhibitory activity in vitro.
Initially, the extracts were screened for the presence of various phytocompounds [Table 1]. The phytochemical analysis indicates the presence of phenols, flavonoids, alkaloids, lipids, glycosides, and tannins. The extracts were then assayed for alpha-amylase inhibitory activity. The results obtained were showed in Figures 1 and 2. Among the four seaweeds, Chlorodesmis methanol extract (72%) and C. aerea chloroform extract (86%) exhibited highest inhibition against alpha-amylase at a concentration of 500 μg/ml. The IC50 values were found to be 147.6 and 408.9 μg/ml, respectively. Moreover, methanol extract of E. Intestinalis (59%) and C. rupestris (14%) showed moderate to low alpha-amylase inhibitory activity. Previously, these green seaweeds (C. aerea and C. rupestris) were reported for its antibacterial activity[14,15] and, to the best of our knowledge, this is the first time report on alpha-amylase inhibitory activity of these four seaweeds. Our results also demonstrate that these extracts did not influence the activity of alpha-glucosidase enzyme. This study confers that the phytocompounds present in these green algal extracts may not be responsible for significant alpha-glucosidase inhibition.
Table 1.
Phytochemical screening of various extracts of the four seaweeds
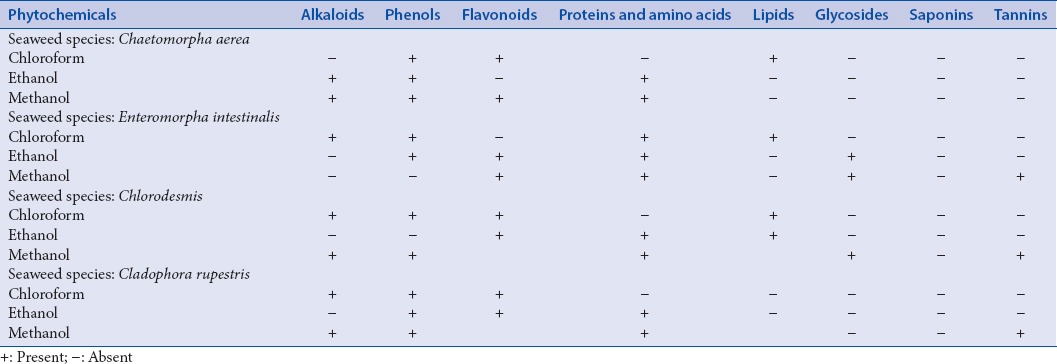
Figure 1.
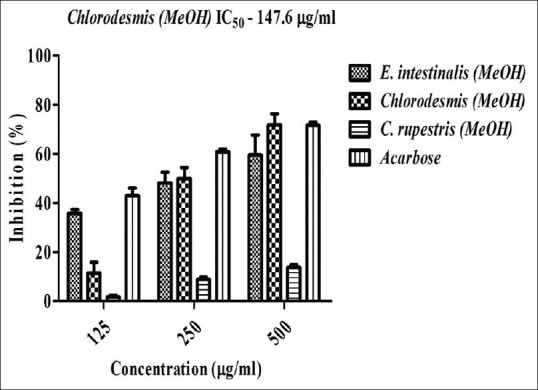
Effect of various seaweed extracts on alpha-amylase inhibition in vitro. Acarbose is used as positive control and absorbance was measured at 540 nm. Values are means ± standard deviation (n = 3), ***P < 0.0001 considered as significant
Figure 2.
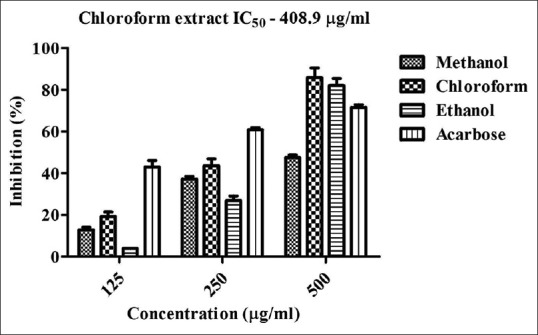
In vitro alpha-amylase inhibitory activity of Chaetomorpha aerea. Acarbose is used as positive control and absorbance was measured at 540 nm. Values are means ± standard deviation (n = 3), ***P < 0.0001 considered as significant
Further, seaweeds were assayed for free radical scavenging activity and/or antioxidant potential of the selected extracts. The extracts were screened for three different free radical scavenging activity (DPPH, H2O2, and nitric oxide) [Figures 3, 4a and b]. The results indicate that methanol extract of C. rupestris showed the highest scavenging activity against DPPH (72%), H2O2 (34%), and nitric oxide (49%). Among them, C. rupestris showed highest scavenging activity against DPPH with an IC50 value of 666.3 μg/ml. Methanol extract of C. aerea also showed moderate to low activity against DPPH (32%), H2O2 (16%), and nitric oxide (24%). Methanol extracts of Chlorodesmis (20%) and E. Intestinalis (5%) showed very less scavenging ability against DPPH and no activity was found against H2O2 and nitric oxide at a concentration of 1000 μg/ml. Earlier studies report that Cladophora glomerata, and E. Intestinalis, collected from Konya, Turkey possess notable free radical scavenging activity. Due to high phenolic content, C. glomerata methanolic and aqueous extracts exhibit highest scavenging activity as compared to E. Intestinalis.[13] In accordance with previous studies, our result confers that Cladophora species exhibit potent antioxidant and/or free radical scavenging activity in vitro.
Figure 3.
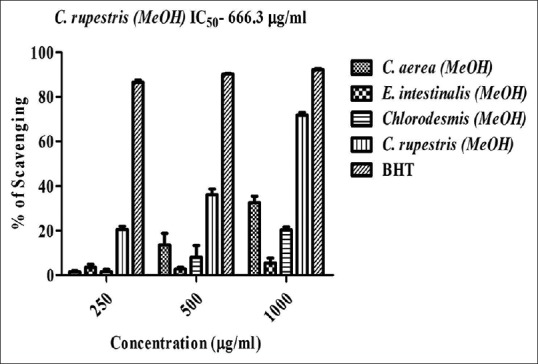
2,2-diphenyl-1-picrylhydrazyl radical scavenging activity of seaweed extracts. Butylated hydroxytoluene is used as positive control and absorbance was measured at 517 nm. Values are means ± standard deviation (n = 3), ***P < 0.0001 considered as significant
Figure 4.
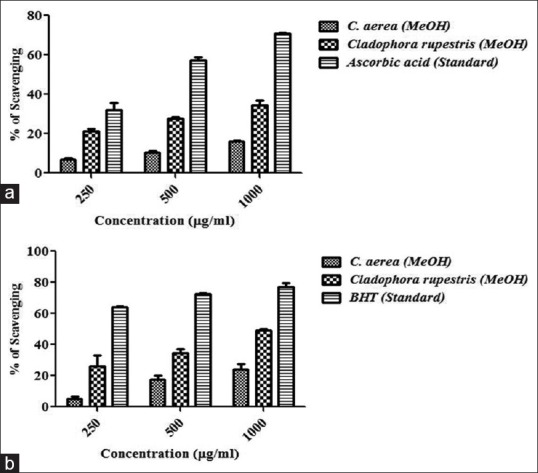
(a) Hydrogen peroxide radical scavenging activity of seaweed extracts. Ascorbic acid is used as standard and absorbance was measured at 230 nm. (b) Nitric oxide scavenging activity of seaweed extracts. Butylated hydroxytoluene is used as positive control and absorbance was measured at 550 nm. Values are means ± standard deviation (n = 3), ***P < 0.0001 considered as significant
Chemical profiling by GC-MS, based on the retention time (RT) and molecular mass, revealed the presence major phytoconstituents. The methanol extract of C. rupestris and chloroform extract of C. aerea which showed the highest antioxidant and alpha-amylase inhibitory activity were analyzed by GC-MS and compared with the RT and mass spectra available in the data library of NIST. The chromatograms obtained were showed in Figure 5. Methanol extract of C. rupestris showed the presence of phenol 2,4-bis (1,1-dimethylethyl) and z,z-6,28-heptatriactontadien-2-one. This is early stated that the highest scavenging activity is proportional to its phenolic content.[16] Phenol 2,4-bis (1,1-dimethylethyl) present in the methanol extract of C. rupestris has previously isolated from a herb Plumbago zeylanica and reported for its antioxidant activity.[17] z,z-6,28-heptatriactontadien-2-one is another major compound identified in both methanol extract of C. rupestris and chloroform extract of C. aerea. However, no known antidiabetic compound was identified by GC-MS.
Figure 5.
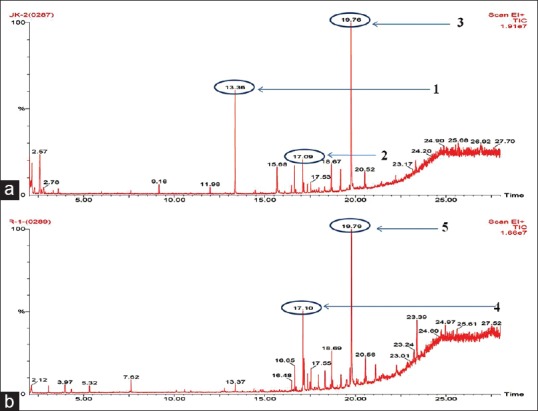
(a) Gas chromatogram of methanol extract of Cladophora rupestris (1) phenol, 2,4-bis (1,1-dimethylethyl), (2) z, z-6,28-heptatriactontadien-2-one, (3) z, z-6,28-heptatriactontadien-2-one. (b) Gas chromatogram of chloroform extract of Chaetomorpha aerea, (4) z, z-6,28-heptatriactontadien-2-one, (5) z, z-6,28-heptatriactontadien-2-one
CONCLUSION
Hence, it is anticipated that the tested algae produces a novel antidiabetic compound and further advancement of sophisticated spectroscopic techniques are required for the identification. The isolation and characterization of active compound will only give a conclusive data about the antidiabetic and antioxidant activity of these respective seaweeds and are underway.
Financial support and sponsorship
Department of Biotechnology, Government of India for their financial support (Grant number – No. BT/Bio-CARe/03/347/2010-11) and VIT University for providing all necessary facilities.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

P. S. Unnikrishnan
Unnikrishnan P. S. is a doctoral student at School of Biosciences and Technology, VIT University, Vellore. His doctoral research mainly focuses on the evaluation of antidiabetic compounds from marine seaweeds collected from Southern coast of India. He has experience in characterization of compounds using various chromatographical and spectroscopical techniques and studying its molecular mechanism of pure compound in in-vitro and in-vivo models.

K. Suthindhiran
Suthinidhiran. K. is an Assistant Professor (Senior) at School of Biosciences and Technology, VIT University, Vellore. His research mainly focuses on the diversity and exploration of marine bacteria (Magnetotactic bacteria, Actinobacteria) and marine algae (red, brown, and green algae). He has experience in identifying new sources of marine bioproducts, providing a sustainable source of supply and optimizing recovery and production of bioproducts.

M. A. Jayasri
Jayasri M. A. is an Assistant Professor (Senior) at School of Biosciences and Technology, VIT University, Vellore. Her research mainly focuses on the chemistry and molecular mechanisms of natural products for the management of diabetes. She has projects funded by Department of Biotechnology (Government of India) and has collaborations with international institutions.
Acknowledgment
The authors wish to thank Department of Biotechnology, Government of India for their financial support (Grant number – No. BT/Bio-CARe/03/347/2010-11) and VIT University for providing all necessary facilities.
REFERENCES
- 1.Nickavar B, Yousefian N. Evaluation of α-amylase inhibitory activities of selected antidiabetic medicinal plants. J Verbr Lebensm. 2011;6:191–5. [Google Scholar]
- 2.Eichler HG, Korn A, Gasic S, Pirson W, Businger J. The effect of a new specific alpha-amylase inhibitor on post-prandial glucose and insulin excursions in normal subjects and Type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1984;26:278–81. doi: 10.1007/BF00283650. [DOI] [PubMed] [Google Scholar]
- 3.Sudha P, Zinjarde SS, Bhargava SY, Kumar AR. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement Altern Med. 2011;11:5. doi: 10.1186/1472-6882-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu XJ, Hansen C. Antioxidant capacity, phenolic content, and polysaccharide content of Lentinus edodes grown in whey permeate-based submerged culture. J Food Sci. 2008;73:M1–8. doi: 10.1111/j.1750-3841.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 5.Lordan S, Smyth TJ, Soler-Vila A, Stanton C, Ross RP. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013;141:2170–6. doi: 10.1016/j.foodchem.2013.04.123. [DOI] [PubMed] [Google Scholar]
- 6.Harborne JB. Chapman and Hall, London: Springer Science and Business Media; 1998. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 7.Jayasri MA, Radha A, Mathew TL. a-amylase and α-glucosidase inhibitory activity of Costus pictus D. Don in the management of diabetes. J Herb Med Toxicol. 2009;3:91–4. [Google Scholar]
- 8.Mensor LL, Menezes FS, Leitão GG, Reis AS, dos Santos TC, Coube CS, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–30. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 9.Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–8. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 10.Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994;201:748–55. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 11.Rindi F, Soler-Vila A, Guiry MD. Taxonomy of marine macroalgae used as sources of bioactive compounds. In: Hayes M, editor. Marine Bioactive Compounds: Sources, Characterization and Applications. New York: Springer; 2012. pp. 1–53. [Google Scholar]
- 12.Etxeberria U, de la Garza AL, Campión J, Martínez JA, Milagro FI. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin Ther Targets. 2012;16:269–97. doi: 10.1517/14728222.2012.664134. [DOI] [PubMed] [Google Scholar]
- 13.Akköz C, Arslan D, Ünver A, Özcan MM, Yilmaz B. Chemical composition, total phenolic and mineral contents of Enteromorpha intestinalis (L.) Kütz and Cladophora glomerata (L.) Kütz seaweeds. J Food Biochem. 2011;35:513–23. [Google Scholar]
- 14.Pierre G, Sopena V, Juin C, Mastouri A, Graber M, Maugard T. Antibacterial activity of a sulfated galactan extracted from the Marine alga Chaetomorpha aerea against Staphylococcus aureus. Biotechnol Bioprocess Eng. 2011;16:937–45. [Google Scholar]
- 15.Stabili L, Acquaviva MI, Biandolino F, Cavallo RA, De Pascali SA, Fanizzi FP, et al. Biotechnological potential of the seaweed Cladophora rupestris (Chlorophyta, Cladophorales) lipidic extract. N Biotechnol. 2014;31:436–44. doi: 10.1016/j.nbt.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Wang T, Jonsdottir R, Olafsdottir G. Total phenolic compounds, radical scavenging and metal chelation of extracts from icelandic seaweeds. Food Chem. 2009;116:240–8. [Google Scholar]
- 17.Ajayi GO, Olagunju JA, Ademuyiwa O, Martins OC. Gas chromatography-mass spectrometry analysis and phytochemical screening of ethanolic root extract of Plumbago zeylanica, Linn. J Med Plants Res. 2011;5:1756–61. [Google Scholar]


