Abstract
Background:
Tulsi, Banyan, and Jamun are popular Indian medicinal plants with notable hypoglycemic potentials. Now the work reports chemo-profiling of the three species with in-vitro screening approach for natural enzyme inhibitors (NEIs) against enzymes pathogenic for type 2 diabetes. Further along with the chemometrics optimized extraction process technology, phyto-synergistic studies of the composite polyherbal blends have also been reported.
Objective:
Chemometrically optimized extraction procedures, ratios of polyherbal composites to achieve phyto-synergistic actions, and in-vitro screening of NEIs amongst leaves of Tulsi, Banyan, and Jamun.
Materials and Methods:
The extraction process parameters of the leaves of three plant species (Ficus benghalensis, Syzigium cumini and Ocimum sanctum) were optimized by rotatable central composite design of chemometrics so as to get maximal yield of bio-actives. Phyto-blends of three species were prepared so as to achieve synergistic antidiabetic and antioxidant potentials and the ratios were optimized by chemometrics. Next, for in vitro screening of natural enzyme inhibitors the individual leaf extracts as well as composite blends were subjected to assay procedures to see their inhibitory potentials against the enzymes pathogenic in type 2 diabetes. The antioxidant potentials were also estimated by DPPH radical scavenging, ABTS, FRAP and Dot Blot assay.
Results:
Considering response surface methodology studies and from the solutions obtained using desirability function, it was found that hydro-ethanolic or methanolic solvent ratio of 52.46 ± 1.6 and at a temperature of 20.17 ± 0.6 gave an optimum yield of polyphenols with minimal chlorophyll leaching. The species also showed the presence of glycosides, alkaloids, and saponins. Composites in the ratios of 1:1:1 and 1:1:2 gave synergistic effects in terms of polyphenol yield and anti-oxidant potentials. All composites (1:1:1, 1:2:1, 2:1:1, 1:1:2) showed synergistic anti-oxidant actions. Inhibitory activities against the targeted enzymes expressed in terms of IC50 values have shown that hydro-ethanolic extracts in all cases whether individual species or composites in varying ratios gave higher IC50 values thus showing greater effectivity.
Conclusion:
Current research provides the state-of-the-art of search of NEIs amongst three species by in-vitro assays which can be further utilized for bioactivity-guided isolations of such enzyme inhibitors. Further, it reports the optimized phyto-blend ratios so as to achieve synergistic anti-oxidative actions.
SUMMARY
The current research work focuses on the optimization of the extraction process parameters and the ratios of phyto-synergistic blends of the leaves of three common medicinal plants viz. banyan, jamun and tulsi by chemometrics. Qualitative and quantitative chemo profiling of the extracts were done by different phytochemical tests and UV spectrophotometric methods. Enzymes like alpha amylase, alpha glucosidase, aldose reductase, dipeptidyl peptidase 4, angiotensin converting enzymes are found to be pathogenic in type 2 diabetes. In vitro screening of natural enzyme inhibitors amongst individual extracts and composite blends were carried out by different assay procedures and the potency expressed in terms of IC50 values. Antioxidant potentials were estimated by DPPH radical scavenging, ABTS, FRAP and Dot Blot assay. Hydroalcoholic solvent (50:50) gave maximal yield of bio-actives with minimal chlorophyll leaching. Hydroethanolic extract of tulsi showed maximal antioxidant effect. Though all composites showed synergism, maximal effects were shown by the composite (1:1:2) in terms of polyphenol yield, antioxidant effect and inhibitory actions against the targeted enzymes.
Abbreviations used: DPP4- dipeptidyl peptidase 4; AR- aldose reductase; ACE- angiotensin converting enzyme; PPAR-γ- peroxisome proliferator activated receptor-γ; NEIs- natural enzyme inhibitors; BE- binding energy; GLP-1- Glucagon like peptide -1; ROS- Reactive oxygen species; CAT- catalase; GSH-Px- glutathione per-oxidase; SOD- superoxide dismutase; pNPG- para-nitro phenyl-α-D-gluco-pyranoside solution; DPPH- 1,1-diphenyl-2-picrylhydrazyl; RSM- Response surface methodology; CCD- central composite design; DMSO- dimethyl sulfoxide; HHL- hippuryl-L-histidyl-L-leucine; GPN-Tos- Gly-Pro p-nitroanilide toluenesulfonate salt; ESC- experimental scavenging capacity; TSC- theoretical scavenging capacity; FRAP- Ferric Reducing Assay Procedure; ABTS- 2, 2’- azinobis (3-ethylbenzothiazoline-6 – sulfonic acid.
Keywords: Anti-oxidant, chemometrics, hypoglycemic, natural enzyme inhibitors, phyto-synergistic, polyherbal
INTRODUCTION
Enzymes are essential for maintaining different biochemical life processes such as metabolism, cell cycling, and signal transduction, etc., But, hyper or hypo activity of enzymes are the underlying causes of diseases like diabetes, Alzheimer's disease, Myasthenia gravis, and Parkinson's disease, etc., as evidenced by etiopathogenesis of diseases at the molecular level. It is anticipated that enzyme inhibitors can serve as important therapeutic bullets for these diseases.[1,2,3] Diabetes mellitus (DM) is one of the major killers of the health of mankind after AIDS, cancer, and cerebrovascular diseases and have shown a steady growth in the number of diabetics to 366 million by 2030 as reported by International Diabetes Federation.[1,2,3] The figure in Indian scenario is about 40.9 million, which is expected to grow to 60.9 million by 2025.[1,2,3] Despite tremendous strides in modern medicines, availability of insulin therapy and synthetic hypoglycemics and their failure to restore normoglycemia without adverse effects has made a resurgence of interest in phytotherapy.[4,5] Pathophysiology of type 2 DM have shown that enzymes like α-amylase, α-glucosidase, dipeptidyl peptidase-4 (DPP-4), aldose reductase (AR), angiotensin converting enzyme (ACE), and peroxisome proliferator-activated receptor-γ (PPAR-γ) contribute significantly to the pathogenesis of the disease. Phyto-molecules serving as natural enzyme inhibitors (NEIs) can serve as successful therapeutic targets in the control of this chronic disease.[4,5,6,7,8,9,10,11,12,13,14,15] Inhibition of carbohydrate hydrolyzing enzymes like α-amylase and α-glucosidase helps to reduce postprandial hyperglycemia. Inhibition of other enzymes like AR, DPP-4, ACE, and PPAR-γ also presents an effective strategy to combat type 2 DM naturally.[4,5,6,7,8,9,10,11,12,13,14,15]
AR, a member of the aldo-keto reductases superfamily, is the first and rate-limiting enzyme in the polyol pathway that reduces glucose to sorbitol, utilizing nicotinamide adenine dinucleotide phosphate (reduced form of NADPH) as a cofactor. In type 2 DM, there is excess sorbitol formation due to increased availability of glucose and sorbitol gets accumulated in insulin- sensitive tissues like lens, nerves, retina leading to cataract, retinopathy, and neuropathy. AR-inhibitors prevent the conversion of glucose to sorbitol and are capable of controlling diabetic complications.[7,8,9,10,11] Limited literature data and molecular docking analysis in terms of binding energy have shown that natural bio-molecules such as quercetin, quercitrin, myricitrin, coumarins, monoterpenes, and stilbenes are potent AR inhibitors.[12]
Enzyme ACE is associated with hypertension, a long-term complication of diabetes. ACE activates angiotensin-I into a potent vasoconstrictor called angiotensin-II. Angiotensin-II influences aldosterone release which increases blood pressure by promoting sodium retention in distal tubules. Bio-molecules such as flavonoids, flavonols, anthocyanins, and triterpenes are potent ACE inhibitors further evidenced by molecular docking studies.[13,14,15] Glucagon-like peptide-1 (GLP-1) is a remarkable anti-diabetic gut hormone with combinatorial actions of stimulating insulin secretion, inhibiting glucagon secretion, increasing beta cell mass, reducing the rate of gastric emptying, and inducing satiety. DPP-4 rapidly deactivates GLP-1. Phyto-molecules, mostly triterpenoids, steroids, and phenolic constituents with DPP-4 inhibitory activity, help to increase the levels of endogenous active GLP-1 and act as an important therapeutic compound against type 2 DM, the fact being further supported by molecular docking studies.[16,17,18]
Reactive oxygen species (ROS) like singlet oxygen or various radicals is detrimental for anabolic activities and ROS-induced oxidative damages to cellular tissues leading to diseases like cancer, chorionic villous sampling, nephropathy, and aging. Attenuation in ROS level may be due to increased production or diminished depletion of enzymes like catalase, glutathione peroxidase, and superoxide dismutase. Natural anti-oxidants which scavenge free radicals can provide a synergistic action to the overall anti-diabetic potential of any plant.[19,20,21]
The current research work focuses on the chemo-profiling and explorations of NEIs by in-vitro methods amongst the leaves of three common Indian medicinal plants viz. Ficus benghalensis (FB, Family: Moraceae) or Banyan tree, Syzygium cumini (SC, Family: Myrtaceae) or Jamun, and Ocimum sanctum (OS, Family: Lamiaceae) or Tulsi. They are available throughout India and their anti-diabetic potentials are documented in several in vivo animal trials.[21,22,23,24,25,26,27] However, novelty of this work lies on the in-vitro screening of NEIs amongst the leaves of the three species; optimization of the extraction process parameters by chemometrics (central composite design [CCD] and mixed design approaches) so as get maximal yield of bio-actives and also the ratios of polyherbal composites so as to achieve phyto-synergistic anti-oxidant effects. In this context, the work is novel to the best of our knowledge.
MATERIALS AND METHODS
Plant materials
Fresh leaves of FB (voucher specimen: IITKGP/HB/2014/J1), SC (voucher specimen: IITKGP/HB/2014/J2), and OS (voucher specimen: IITKGP/HB/2014/J3) were collected from natural and man-made forest areas of IIT Kharagpur and adjoining areas like Balarampur, Gopali, and Prembazar and authenticated by Dr. Shanta AK, Biotechnologist, Nirmala College of Pharmacy, Guntur, India.
Reagents
Yeast α-glucosidase, bovine serum albumin, sodium azide, para-nitro phenyl-α-D-gluco-pyranoside solution (pNPG), ACE (from rabbit lung, 3.5 units/mg of protein), starch azure, porcine pancreatic amylase, tris-HCL buffer, hippuryl-L-histidyl-L-leucine (HHL), and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were obtained from Sigma Chemicals, USA. Other chemicals like diagnostic reagents, surfactants, polyphosphate, dextran sulfate, etc., were purchased from Merck Co., India. Acarbose (Acar) was a kind gift sample from Zota Pharmaceuticals Pvt., Ltd., Chennai, India. All chemicals and reagents used for the experimentation were all of analytical grade and were purchased either from Merck (India) and Sigma-Aldrich.
Instruments
Electric grinder (Bajaj GX 11); centrifuge (Remi, R-8C Lab Centrifuge); ultraviolet (UV) spectrophotometer (Thermo Scientific).
Software
Experimental design, data analysis, and generation of surface plots were performed by using Design Expert Trial version 7.0. (Design Expert Software, Stat Ease, Inc).
Experimental methodology
Collection of plant leaves
Good, fresh, disease-free mature leaves of FB (Vata or Banyan), OS (Tulsi), and SC (Jamun) were collected from the natural and man-made forests of IIT Kharagpur and adjoining areas like Prembazar, Gopali, and Balarampur, etc. Collected leaves were washed thoroughly under running tap water and spreaded on large plastic trays under direct sunlight for 10–15 days. Thoroughly dried leaves were grinded in an electrical grinder (Bajaj GX 11) and stored in airtight amber colored plastic containers with proper labeling until use.
Proximate analysis
Leaf powders of each species (FB, SC, OS) were exhaustively processed for various parameters of proximate analysis (carbohydrates, fats, crude protein, moisture, dry matter, crude fiber, nitrogen free extract, and ash) according to the Association of Official Analytical Chemists methods (1990) and other standard literatures.[28]
Optimization of extraction conditions and phyto-blend ratios by response surface methodology
An experimental design methodology should be such that it is economical for extracting the maximum amount of complex information, a significant reduction in experimentation time with simultaneous saving of material and personnel cost. Response surface methodology (RSM) using desirability function may be a good statistical analysis tool to justify the above statement. The most popular RSM design is the CCD. In this study, for extraction of individual species there are two independent variables viz. solvent ratio (volume of ethanol or methanol) and temperature and the response is the yield of polyphenols. For optimization of ratios of herbal composites, mixed design approach of RSM was adopted and the ratios showing a maximum yield of polyphenols was considered as the optimized one.[29,30,31]
Preparation of plant extracts
Extraction procedure was a combination of dynamic maceration for 6 h in magnetic stirrer (Remi, 2MLH) followed by a static maceration of 18 h. The same sequence was followed for three days. Solvents viz. methanol, ethanol, 1-butanol, chloroform, ethyl acetate, acetone, n-hexane, and diethyl ether were selected from the eluotropic series.[32,33,34,35,36,37] However, as evidenced by literature, a huge amount of chlorophyll got extracted with solvents like acetone, ethyl acetate, diethyl ether, and chloroform. Presence of chlorophyll in extracts is a serious problem during HPLC operations since chlorophyll sticks to HPLC columns which cannot be removed by any solvent wash, and in future is sure to compromise with the functionality of HPLC column. There is an option to remove chlorophylls by sephadex columns, but our objective was to optimize a suitable solvent ratio which will facilitate maximal elution of bio-actives of interest (here, total phenolics) without extraction of chlorophyll.[32,33,34,35,36,37]
Lab experimentations and desirability functions of RSM showed hydro-ethanolic and hydro-methanolic extracts in 50:50 solvent ratio gave an optimal yield of polyphenols. Aqueous extractions were carried out in all cases to find the water soluble components. Polyherbal composites or phyto-blend were prepared by intermixing of three different species (SC: FB: OS) in the ratios (1:1:1; 1:2:1; 2:1:1; and 1:1:2) optimized by mixed design approach by RSM with solvent systems mentioned above.[32,33,34,35,36,37]
Estimation of chlorophyll content in the extracts by ultraviolet method
The chlorophyll contents in the extracts were determined according to the methods given by Mackinney, 1941; Arnon, 1949. Definitively weighed leaf samples were put separately into 100% acetone, 96% alcohol and, 95% diethyl ether (50 mL for each gm) and then subjected to homogenization at 1500 rpm for 3 min. The homogenate was filtered and centrifuged at 3000 rpm for 10 min. The supernatant was separated, and absorbances were read at 663 nm for chlorophyll a and 645 nm for chlorophyll b and the amount of these pigments were calculated.[38,39]
Preliminary phytochemical and quantitative evaluations of extracts
Phytochemical analysis of the major bioactive compounds of interest of the three different species (FB, SC, and OS) was performed using the methods of Harborne (1984), Trease, and Evans (1989) and other literature methods.[40,41,42,43,44,45,46,47,48]
Total polyphenol content of the extracts was determined using Folin-Ciocalteu (FC) reagent and recording the absorbance at 760 nm after 30 min of incubation in dark as per the literatures methods of Othman et al., 2007 and Modnicki and Balcerek 2009.[40,41,42] Total flavonoid content of the extracts was determined by method of Djeridane et al., 2006 which is based on the formation of a complex of flavonoid-aluminum showing absorbance at 430 nm and the flavonoid concentration expressed in terms of quercetin equivalent (QE) per gram of the extract.[43] Flavonol content of the extracts was determined according to Abdel-Hameed, 2009 and its absorbance recorded at 440 nm and expressed in terms of QE per gram of the extract.[44] Tannins were estimated according to the protocol of Hagerman and Butler, 1978 which is based on the obtention of a colored complex Fe2+ - phenol whose absorbance was measured spectrophotometrically at 510 nm and expressed as tannic acid equivalent per gram of the extract.[45]
Estimations of alkaloid, saponins, steroids, and glycosides
Alkaloid
The total alkaloid content was determined by the UV-spectrophotometric method according to Ganapaty et al., 2013 which is based on the reaction between alkaloid and bromocresol green and the absorbance measured at 470 nm.[46]
Glycosides
Glycoside content was determined spectrophotometrically as per the method given by Rizwan A et al., 2011 and absorbance recorded at 515 nm and calculated as aloe-emodin/Rhein.[47]
Saponins
Saponin estimations were carried out spectrophotometrically according to the method given by Devanaboyina et al., 2013 and absorbance recorded at 544 nm against reagent blank. Diosgenin was used as the standard material.[48]
Steroids
Steroids were determined with 1 mL of the methanolic extracts taken in 10 mL of the volumetric flask; sulfuric acid (4 N, 2 mL), and FeCl3 (0.5% w/v, 2 mL) were added followed by potassium hexacyanoferrate solution (III) (0.5% w/v, 0.5 mL). After heating the mixture in a water bath at 70 ± 20°C for 30 min with occasional shaking and further diluted up to mark with distilled water. The absorbance was measured at 780 nm against reagent blank.[48]
In-vitro assay procedures
After phytochemical investigations, α-amylase, α-glucosidase, AR, ACE, DPP-4 in-vitro inhibitory assays, and anti-oxidant assays like DPPH free radical scavenging activity, total anti-oxidant activity in the ferric reducing assay procedure (FRAP) assay, 2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay, rapid DPPH radical scavenging by dot-blot assay of the individual extracts, and that of the composites (SC: FB: OS in different ratios) were carried out following standard methods with slight modifications.[46,47,48,49,50,51,52,53,54,55,56,57]
α-Amylase inhibitory assay
The study was carried out following standard literature methodologies with slight modifications.[49,50] Briefly, 2 mg of starch azure was suspended in a tube containing 0.2 mL of 0.5 M tris-phosphate buffer (pH 6.9) containing 0.01 M calcium chloride as the substrate. After boiling the tube for 5 min, it was preincubated for 5 min at 37°C. Different concentrations (10, 20, 40, 60, 80, and 100 μg/mL) of the extracts of FB, SC, and OS and the composites were prepared by dissolving in 1 mL of 0.1% dimethyl sulfoxide. Then 0.2 mL of the extract of particular concentrations was put in the tube containing the substrate solution. Next, 0.1 mL of porcine pancreatic amylase in tris-HCL buffer (2 units/mL) was added to the tube containing extracts and substrate, at 37°C. After 10 min, the reaction was stopped by adding 0.5 mL of 50% acetic acid in each tube, and the reaction mixture was centrifuged (Eppendorf-5804R) at 3000 rpm for 5 min at 4°C. The absorbance of the resulting supernatant was measured at 595 nm. Acar in the concentration range of 1.25, 2.5, 5, and 10 μg/mL in distilled water was used to create the calibration curve. The assay was performed in triplicate. The concentration of the extracts of three species (FB, SC, and OS) and composites required inhibiting 50% of a amylase activity under the assay conditions is referred to as IC50 values. Absorbance was calculated using the formula:
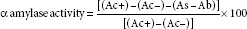
α-Glucosidase inhibitory assay
The assay procedure was developed as described by Basak et al., 2010 and Subramanian et al., 2008, with slight modifications.[49,50] An aqueous ethanolic extract of the three species (FB, SC, OS, and composites) was used for the study. The yeast α-glucosidase enzyme solution was prepared by dissolving at a concentration of 0.1U/mL in 100 mM phosphate buffer, pH 7.0, containing bovine serum albumin, and sodium azide which was used as enzyme source. This enzyme solution was added to the aqueous ethanolic extracts of FB, SC, and OS in increasing concentrations (1, 1.5, 2, 2.5, 3, and 3.5 μL mL−1). The reaction was initiated by adding 0.20 mL of the pNPG solution; 2 mM pNPG in 50 mM sodium phosphate buffer (pH 6.9) which acted as the substrate. The reaction was terminated by adding 1 mL 0.1 M Na2HPO4. The test tubes were cooled under tap water, and α-glucosidase inhibitory activity was determined at 405 nm by measuring the quantity of p-nitrophenol released from pNPG. The assay was performed in triplicate for each extract and the data presented as mean ± standard deviation. The concentration of the extracts (FB, SC, OS, and composites) required inhibiting 50% of α-glucosidase activity under experimental conditions is defined as the IC50 value. Acar was dissolved in distilled water to prepare a series of dilutions (1.25, 2.5,5, and 10 mg/ml) and was used as the positive control. The percent inhibition was calculated according to the formula:
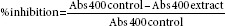
IC50 values were determined from the plots of percent inhibition versus log inhibitor concentration and were calculated by nonlinear regression analysis from the mean inhibitory values.
Aldose reductase inhibitory assay
The assay was carried out following reported literature methods and the experimental protocol was approved by Institutional Ethical Committee.[8,9,10,11] About 2–3 months, old healthy adult Wistar albino rats weighing about 150–200 g were acclimatized to laboratory conditions (12 h light and 12 h dark cycle, 25 ± 5°C, and 30–60% relative humidity) with free access to pelleted food and water ad libitum. Immediately after sacrifice, eye lenses were removed, washed with saline, and the fresh weight of a lens measured. Next, a 10% homogenate was prepared from the rat lens in 0.1 M phosphate-buffered saline (PBS) at pH 7.4, centrifuged at × 5000 g for 10 min in the cold, and the supernatant was collected. The protein content of the supernatant was determined by literature methods.[8,9,10,11]
For the determination of the AR inhibitory activity, 0.7 mL of phosphate buffer (0.067 M), 0.1 mL of NADPH (25 × 10−5 M), 0.1 mL of DL-glyceraldehyde (substrate, 5 × 10−4 M), and 0.1 mL of lens supernatant were mixed in the sample cuvette. Absorbance was taken against a reference cuvette containing all other components except the substrate and DL-glyceraldehyde. The final pH of the reaction mixture was adjusted to pH 6.2. On adding substrate to the solution mixture, the enzymatic reaction starts and absorbance (OD) was recorded at 340 nm for 3 min at 30 s intervals. AR activity was calculated and expressed as OD/min/mg protein. Next, a stock solution was prepared by dissolving the extracts (FB, SC, OS, and composites) in PBS, and different concentrations were prepared; reaction was initiated by the addition of 0.1 mL DL-glyceraldehyde and the reaction rate measured as mentioned above. Quercetin, a known AR inhibitor was used as the positive control.
Angiotensin converting enzyme inhibitory assay
The assay method was based on the liberation of hippuric acid from HHL catalyzed by the ACE. The assay procedure was carried as described by Chaudhary et al., 2013, and other methods with slight modifications.[13,14,51] Briefly, 50 μL of sample solutions (extracts of FB, SC, OS, and composites) in the concentration range of 0.1–2.5 mg/mL were preincubated with 50 μL of ACE (25 mU/mL) at 37°C for 10 min. Next, 150 μL of substrate solution (8.3 mM HHL in 50 mM sodium borate buffer containing 0.5 M NaCl at pH 8.3) was added and incubated for 30 min at 37°C. The reaction was terminated by addition of 250 μL of 1.0 M HCL. To the resulting solution, 0.5 mL of ethyl acetate was added and centrifuged (Eppendorf-5804R) for 15 min. Then, 0.2 mL of the upper layer was transferred to a test tube, evaporated under room temperature in vacuum, the liberated hippuric acid was dissolved in 1 mL distilled water, and the absorbance was measured at 228 nm. Experiments were performed in triplicates. Captopril was used as standard (3.5 μg/mL) in the assay. The percentage of inhibition (ACEI) was calculated using the formula:
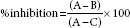
Where A is the OD at 228 nm with ACE but without inhibitor; B is the OD in the presence of both ACE and inhibitor; and C is the OD without ACE and inhibitor.
Dipeptidyl peptidase-4 inhibitory assay
The assay was carried out following reported literature methods using Gly-Pro p-nitroanilide toluenesulfonate salt as the substrate.[52,53] Briefly, 0.5 mL of the assay mixture contained 40 mM K-Na-phosphate buffer, pH 7.5, an enzyme sample. The reaction was initiated by adding a substrate to a concentration of 0.24 mM and stopped by adding 0.2 M acetic buffer at pH 5.5. The differential absorption at 390 nm was recorded against an identical mixture without the enzyme, and the amount of p-nitroaniline depleted was evaluated from its extinction coefficient at the wavelength of 9.9 mM/cm−1.
Evaluation of anti-oxidant activity
1,1-diphenyl-2-picrylhydrazyl free radical scavenging activity
The anti-oxidant activity of three extracts (FB, SC, OS, and composites) was determined on the basis of the scavenging effect on the stable DPPH free radical activity.[19,54,55,56] Briefly, 0.2 mM DPPH solution was prepared by dissolving 0.08 g of DPPH in methanol in a 100 mL standard flask and volume made up to mark with methanol. Next 1.5 mL of 0.2 mM DPPH solution and 1.5 mL of sample solutions in different concentrations were mixed. In another series, 1.5 mL of different concentrations of sample solutions were mixed with 1.5 mL of methanol. All solutions were kept for 30 min at room temperature and allowed to react. Absorbance was read at 517 nm. Calculations were done basing on the equation:
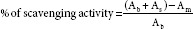
Where Ab = absorbance of 1.5 mL DPPH + 1.5 mL methanol; Am = absorbance of 1.5 mL DPPH + 1.5 mL sample solution; and As = absorbance of 1.5 mL sample solution + 1.5 mL methanol.
Plotting was done on percent inhibition versus concentration, and the concentration of sample required for 50% inhibition is regarded as the IC50 value for each of the test samples.
Selection of the ratio of anti-oxidants to achieve synergism
The synergistic effect (SE) was calculated by comparing the scavenging capacities of the composites in variant ratios to the arithmetic sum of the scavenging capacities of the individual extracts.
The experimental scavenging capacity (ESC) of the composites (in different ratios) was calculated using the equations:
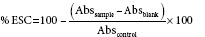
Here, Abs is recorded at 518 nm. The blank consisted of 1 mL methanol + 2.5 mL of extract, the control solution consisted of 1 mL of 0.3 mM DPPH + 2.5 mL methanol, and the sample solution consisted of 1 mL of 0.3 mM DPPH + 2.5 mL of sample extract.
The theoretical scavenging capacity (TSC) is the sum of the scavenging capacities of each extract, calculated using the individual scavenging capacity in the following equation:
%TSC = 100 – ([100 − ESCA1/100] + [100 − ESCA2/100]) where ESCA1, ESCA2 represent the percentage ESC of the individual anti-oxidants.
The SE of the combination of anti-oxidants is based on the ratio of the ESCs and TSCs, calculated using the following equation (Fuhrman et al., 2000): SE = ESC/TSC, where SE value >1 (SE >1) will exhibit synergism.[57,58,59]
Total anti-oxidant activity (ferric reducing assay procedure assay)
Total anti-oxidant activity was determined by the FRAP assay as per the standard literature procedure.[54,55] The procedure is based on the reduction of ferric to ferrous form in the presence of anti-oxidants in the test samples (plant extracts). Plant extracts (200 μL) were allowed to react with FRAP solution (2900–3000 μL) for 30 min in the dark. Absorbance of the colored product formed (ferrous tripyridyltriazine complex) was recorded at 595 nm. Results were expressed in μM equivalent to FeSO4 by extrapolation from the calibration curve.
2, 2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) assay
The assay was carried out according to standard literature with slight modifications.[20,24,56] The main principle is based on the ability of test samples to scavenge 2,2’- azino-bis (ethylbenzthiazoline-6-sulphonic acid) (ABTS+) radical cation. The ABTS + solution was diluted with ethanol to get an absorbance of 0.700 ± 0.05 at 734 nm for measurements. The anti-oxidative activities of the extracts were calculated by determining the decrease in absorbance at different concentrations by using the equation:
E = ([Ac – At]/Ac) × 100 where At and Ac are the respective absorbance of tested samples and ABTS + expressed as μmol.
Rapid screening of anti-oxidant activity by dot-blot assay
Rapid screening of anti-oxidant by dot-blot assay with DPPH staining was carried out as per the literature methodology. An aliquot of each dilution of different extracts (FB, SC, and OS) were loaded on a 10 cm × 20 cm silica gel TLC plate (Merck) and allowed to dry. Drops of each sample were loaded in order of decreasing concentrations along the column. The staining of silica plate was done by dipping the plate in 0.4 mM DPPH solution in methanol for 2–3 s. The intensity of the yellow color depends upon the amount and nature of radical scavenger present in the sample.[60]
RESULTS
The results of the proximate analysis of three leaves species have shown that moisture content of FB, SC, and OS to be 10.75, 54.75, and 70.1, respectively. Carbohydrate content of FB, SC, and OS was found to be 24.5, 14, 1.5, respectively, protein contents are FB - 2.1, SC - 0.99, and OS - 0.45; fat contents are FB - 4.6, SC - 0.23, and OS - 0.23; the ash values of the three species were found to be 10.92, 12.35, and 12.19 for FB, SC, and OS, respectively. Loss on drying was found to be 15.7 for FB, 17.1 for SC, and 16.21 for OS.
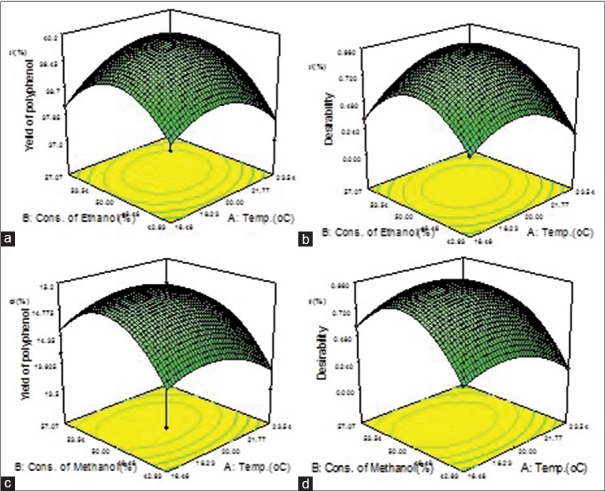
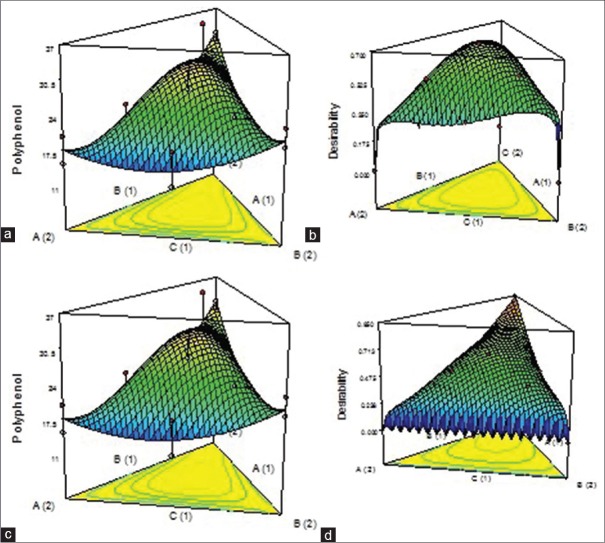
RSM generated three-dimensional surface plots for individual and composite extracts were presented in Figures 1 and 2.
Figure 1.
(a) Three-dimensional surface plot for an ethanolic extract of Tulsi. (b) Three-dimensional surface plot of desirability for an ethanolic extract of Tulsi. (c) Three-dimensional surface plot for a methanolic extract of Tulsi. (d) Three-dimensional surface plot of desirability for methanolic extract of Tulsi
Figure 2.
(a) Three-dimensional surface plot for aqueous composite 1:1:1. (b) Three-dimensional surface plot of desirability for aqueous composite 1:1:1. (c) Three-dimensional surface plot for aqueous composite 1:1:2. (d) Three-dimensional surface plot of desirability for aqueous composite 1:1:2
The FB extracts were light brown in color with methanolic extract showing a highest percent yield of 18.4, extracts of Jamun were dark blackish brown and its ethanolic extract showed highest percent yield of 23.6, and the extracts of Tulsi were light brown with its aqueous extract showing the maximum percent yield of 17.6.
The chlorophyll content with 100% acetone was found to be 4.01 ± 0.02 and 1.96 ± 0.01 for chlorophyll a and b, respectively. The values with 95% diethyl ether were 3.54 ± 0.03 and 1.01 ± 0.02 and with 96% methanol it is 0.96 ± 0.04 and 0.11 ± 0.34 for chlorophyll a and b, respectively.
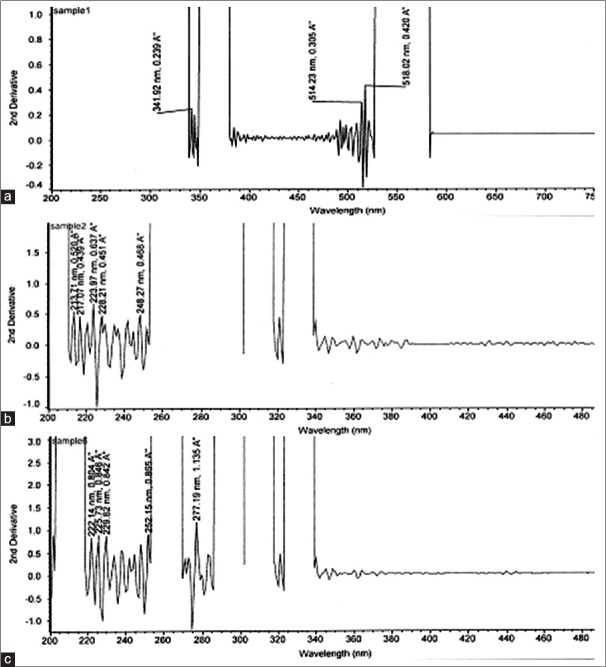
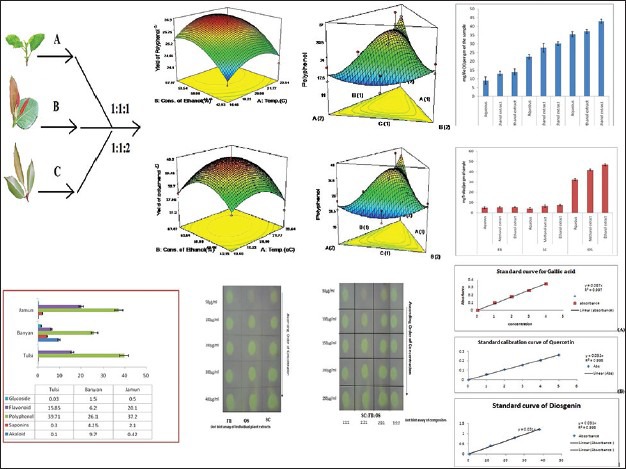
Preliminary phytochemical tests showed the presence of bio-actives like alkaloids, flavonoids, saponins, steroids, glycosides, phenolics, tannins, tri-terpenoids, and the quantitative yield of bio-actives present in the individual species and their composites have been presented in Table 1. Comparative histograms of the various bio-actives have been presented in the graphical abstract shows the presence of glycoside, flavonoid, polyphenol, saponin, and alkaloid. The UV scanned graphs of the FB, SC, and OS showing the presence of polyphenols and flavonoids have been provided in Figure 3. The IC50 values for the different assays have been presented in Tables 2 and 3.
Table 1.
Quantitative data of the major chemical constituents in the extracts
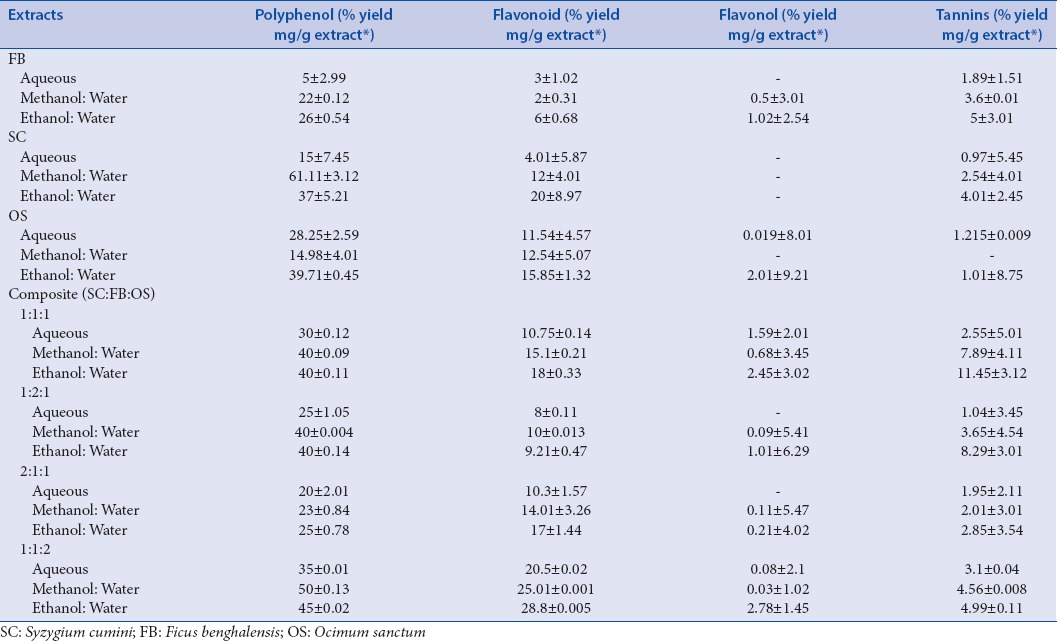
Figure 3.
(a) Ultraviolet scanned graphs showing the presence of polyphenols in Ficus. (b) Ultraviolet scanned graphs showing the presence of flavonoids in Tulsi. (c) Ultraviolet scanned graphs showing the presence of flavonoids in Jamun
Table 2.
IC50 values of the extracts and composites in different enzyme inhibitory assays
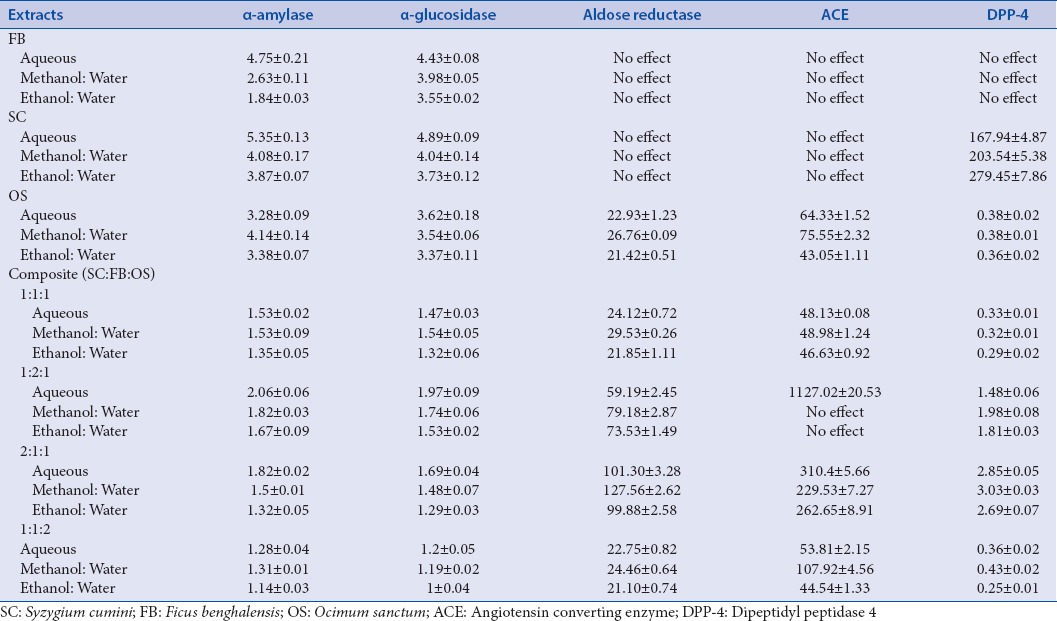
Table 3.
IC50 values of DPPH radical scavenging of extracts
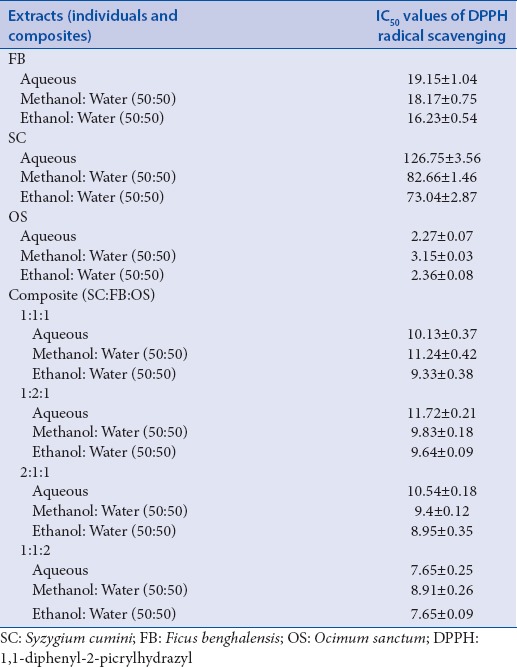
The SE was calculated by comparing the scavenging capacities of the composites in variant ratios to the arithmetic sum of the scavenging capacities of the individual extracts. Basing on this concept, the %TSC value was found to be 41.7% and the %ESC of the individual extracts of OS, SC, and FB was found to be 26.7, 13.5, and 8.2, respectively. For composites viz. 1:1:1, 1:1:2, 2:1:1, and 1:2:1%ESC values were found to be 56.2, 49.1, 42.9, and 42.1, respectively. In contrast to individual extracts, all composites showed SE. SE values for 1:1:1, 1:1:2, 2:1:1, and 1:2:1 were found to be 1.35, 1.18, 1.02, and 1.00, respectively. All values >1 exhibited synergism.
The total anti-oxidant activity of the three species carried out by FRAP and ABTS assay have been presented in the form of comparative histogram (graphical abstract) which shows that OS has highest anti-oxidant potentials and the hydro-ethanolic extracts of all species showed maximum scavenging capacities. All composites (1:1:1, 1:1:2, 2:1:1, and 1:2:1) exhibited synergism (SE >1), the maximum activity shown by the composite ratio 1:1:2 followed by 1:1:1. The TLC plates for rapid DPPH radical scavenging by dot-blot assay (graphical abstract) for both individual species and composites clearly depicts the difference in scavenging capacities of the extracts along with the variance in concentrations.
DISCUSSIONS
Proximate analysis was done, and the purpose of such analysis was to see the results in the climatic and environmental conditions of IIT Kharagpur.
Considering the RSM, the process order fits to quadratic design model and prediction for desirability function of ethanolic and methanolic extract of FB are 0.92 and 1.0, respectively. Predictions for the desirability of ethanolic and methanolic extract of OS are 0.86 and 0.84, respectively, and for ethanolic and methanolic extract of SC are 0.94 and 0.92, respectively. From the solutions obtained using desirability function, it was found that a hydroalcoholic solvent ratio of 52.46 ± 1.6 and at a temperature of 20.17 ± 0.6 gave an optimum yield of polyphenols. However, based on practical experimentations on the yield of polyphenols, hydro-ethanolic and hydro-methanolic solutions in the ratio of 50:50 were used for the purpose of extractions. In case of composites, SEs in terms of polyphenol yield was observed with ratios of 1:1:1 and 1:1:2. Hence, these two are the optimized ratios.
As regards, percent yield of the optimized process technology under the optimized process parameters gave satisfactory yield in all cases.
The chlorophyll estimations carried out showed maximum chlorophyll content with acetone and diethyl ether and minimum with methanol. Hydroalcoholic solutions (50:50) gave best yields of bio-actives with minimal leaching of chlorophylls.
Solvent selection is a crucial parameter in any extraction procedure so as to achieve a maximal yield of any bio-actives. Methanol, ethanol, or acetone and their mixtures in water are so far the most commonly used solvents in the extraction of phenolic compounds from the plant materials.[34,35,36,37] The extractability of the phenolic compounds depends on the type of the solvent, nature of the material to be extracted, chemical structure of phenolic compounds, temperature, extraction time, solid-liquid ratio, extraction method employed, and the possible presence of interfering substances.[34,35,36,37] In contrast to aqueous extracts, hydro-ethanolic solutions as extraction solvents gave higher mass fractions of volatile oil contents. Coming to the chemistry point of view, it is seen that alcohol-water mixture offers the advantage of modulating the polarity of alcohol solvents, and the solubility of polyphenols largely depends on the hydroxyl groups, the molecular size, and length of hydrocarbon.[34,35,36,37] Solvent mixtures are ideal and selective for extraction of great number of bioactive compounds. When water alone is used as the solvent, only the water soluble bioactive components get extracted but there are many other components which may be soluble in the organic counterparts. Another fact to be considered is that there is a balance between polarity and polyphenolics extraction yield.[34,35,36,37] Polar solvents such as water, ethanol, methanol, hydroalcohol, and acetone were used for extraction of polyphenols. Solvents like ethyl acetate being less polar gives low extraction yield and those with higher polarity like ethanol and methanol, or their hydroalcoholic solutions will give higher extraction yields.[34,35,36,37]
The quantitative yield of polyphenols and flavonoids follows a descending order from hydro-ethanolic extracts, hydro-methanolic extracts, and aqueous extracts. However, there is an appreciable increase in polyphenol and flavonoid content amongst the composites of the three species blended in different ratios [Table 1], and the hydro-ethanolic extract of the composite (1:1:2) showed highest polyphenol and flavonoid content.
For in-vitro explorations of NEIs amongst the leaves of three plant species, it was found that hydro-ethanolic extracts in all cases gave higher IC50 values, thus, showing greater effectivity. Composites, whether aqueous extracts, hydro-methanolic, or hydro-ethanolic showed synergistic inhibitory actions; the appreciable one being 1:1:2 followed by 1:1:1.
Prior research suggested a strong positive correlation (R2 = 0.99) between phenolic content and anti-oxidative potential.[61] Polyphenols can prevent damage from ROS through radical scavenging or prevent the generation of these species by iron chelation.[13,61,62,63,64] Polyphenols also bind and inhibit the enzymes α-amylase and α-glucosidase.[13,61,62,63,64] Polyphenols have also shown to facilitate insulin response and attenuate secretion of glucose-dependent insulinotropic polypeptide and GLP-1. Other suggested mechanisms for the hypoglycemic actions of polyphenols were down regulation of the expression of liver glucokinase, upregulation of phosphoenolpyruvate carboxykinase (PEPCK), induction of the AMP-activated protein kinase pathway, and enhancing peripheral glucose utilization by stimulating glucose transporter subtype 4, etc.[62,63,64]
Flavonoids inhibit the ACE enzyme by generating chelate complexes within the active center of ACE.[13] Flavonoids were found to attenuate hepatic gluconeogenesis by decreasing the activity of glucose-6-phosphate and PEPCK, subsequently improving glycemic control.[13] Our research data are in accordance with this phenomenon. A strong correlation was found between polyphenol (R2 = 0.81–0.92) and flavonoid contents (R2 = 0.57–0.99) with the anti-oxidative and enzyme inhibitory potentials of the extracts.
CONCLUSIONS
NEIs can serve as an important therapeutic tool against type 2 DM. The current research aims to provide the state-of-the-art of search of NEIs amongst three species of Indian medicinal plants by in-vitro assays which can be further utilized for bioactivity-guided isolations of such enzyme inhibitors. Further development of polyherbal composites and achieving phyto-synergistic actions was another goal of the work. Our research results showed the multi-dimensional hypoglycemic potential of the three species of Indian medicinal plants for future exploitations in phytotherapy of type 2 DM. However, extensive pharmacology and toxicological studies in animal and human models are further warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Dr. Analava Mitra
Dr. Analava Mitra, MBBS, PhD is currently the Associate Professor of School of Medical Science and Technology, IIT Kharagpur. He has 34 yrs of experience in clinical practice, teaching and research with 150 publications, 4 Indian patents, 1 book and four book chapters. He has guided several PhD scholars and is the editorial board member, reviewer of different national and international journals as well as member of various professional bodies. His major areas of research interest include herbal medicine, clinical trials, drug encapsulations, cognitive science etc.

Dr. Prakash Katakam
Dr. Prakash Katakam, M. Pharm, PhD has 17 years of experience in Teaching and research both in academics and industries. He is currently the Faculty of Pharmacy, at University of Zawia, Libya. His research areas include Novel Drug Delivery Systems, Synthetic Chemistry, Analytical Chemistry, Biomaterials, Bioanalytical method development and Natural Products Chemistry. He has 91 publications, 15 conference papers, 5 Indian Patents and is the reviewer of several journals and member of various professional bodies. He has guided several masters and PhD scholars both in India and abroad. He is the consultant at K.N. Biosciences and Nishka Labs Hyderabad, India.
Acknowledgments
The fund for carrying out the current research has been provided by WBDST sponsored DTE project (Sanction Order No: 904(SANC)/ST/P/S and T/9G-14/2011) at IIT Kharagpur. The sponsor is deeply acknowledged.
REFERENCES
- 1.Mitra A, Dewanjee D, Dey B. Mechanistic studies of lifestyle interventions in type 2 diabetes. World J Diabetes. 2012;3:201–7. doi: 10.4239/wjd.v3.i12.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dey B, Mitra A. Chemo-profiling of Eucalyptus and study of its hypoglycemic potential. World J Diabetes. 2013;4:170–6. doi: 10.4239/wjd.v4.i5.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dey B, Mitra A, Katakam P, Singla RK. Exploration of natural enzyme inhibitors with hypoglycemic potentials amongst Eucalyptus Spp. by in vitro assays. World J Diabetes. 2014;5:209–18. doi: 10.4239/wjd.v5.i2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ata A, Naz S, Elias EM. Naturally occurring enzyme inhibitors and their pharmaceutical applications. Pure Appl Chem. 2011;83:1741–9. [Google Scholar]
- 5.Kumar S, Kumar V, Rana M, Kumar D. Enzyme inhibitors from plants: An alternate approach to treat diabetes. Pharmacogn Commun. 2012;2:18–33. [Google Scholar]
- 6.Bachhawat JA, Shihabudeen MS, Thirumurugan K. Screening of fifteen Indian Ayurvedic plants for alpha-glucosidase inhibitory activity and enzyme kinetics. Int J Pharm Pharm Sci. 2011;3:267–74. [Google Scholar]
- 7.Lamba HS, Bhargava CS, Thakur M, Bhargava S. a-glucosidase and aldose reductase inhibitory activity in vitro and anti-diabetic activity in vivo of Tribulus terrestris L.(Dunal) Int J Pharm Pharm Sci. 2011;3:270–2. [Google Scholar]
- 8.Patel D, Kumar R, Kumar M, Sairam K, Hemalatha S. Evaluation of in vitro aldose reductase inhibitory potential of different fraction of Hybanthus enneaspermus Linn F. Muell. Asian Pac J Trop Biomed. 2012;2:134–9. doi: 10.1016/S2221-1691(11)60207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman A, Guerrero RO. Inhibition of aldose reductase by herbs extracts and natural substances and their role in prevention of cataracts. Rev Cubana Plant Med. 2005;10:1–7. [Google Scholar]
- 10.Vyshali P, Saraswati KJ, Sanakal R, Kaliwal BB. Inhibition of aldose activity by essential phytochemicals of Cymbopogon citratus (DC.) Stapf. Int J Biom Bioinform. 2011;5:257–67. [Google Scholar]
- 11.Halder N, Joshi S, Gupta SK. Lens aldose reductase inhibiting potential of some indigenous plants. J Ethnopharmacol. 2003;86:113–6. doi: 10.1016/s0378-8741(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 12.Madeswaran A, Muthuswamy UM, Kuppusamy AK, Sivasanmugam T, Varadharajan SD, Puliyath J. In-silico docking studies of aldose reductase inhibitory activity of commercially available flavonoids. Bangladesh J Pharmacol. 2012;7:266–71. [Google Scholar]
- 13.Guerrero L, Castillo J, Quiñones M, Garcia-Vallvé S, Arola L, Pujadas G, et al. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS One. 2012;7:e49493. doi: 10.1371/journal.pone.0049493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balasuriya BW, Rupasinghe HP. Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Funct Foods Health Dis. 2011;1:172–88. [Google Scholar]
- 15.Priya V, Jananie RK, Vijayalakshmi K. Molecular docking analysis of compounds present in Trigonella foenum graceum with angiotensin converting enzyme in-silico analysis. J Chem Pharm Res. 2011;3:129–39. [Google Scholar]
- 16.Bellé LP, Bitencourt PE, Abdalla FH, Bona KS, Peres A, Maders LD, et al. Aqueous seed extract of Syzygium cumini inhibits the dipeptidyl peptidase IV and adenosine deaminase activities, but it does not change the CD26 expression in lymphocytes in vitro . J Physiol Biochem. 2013;69:119–24. doi: 10.1007/s13105-012-0195-6. [DOI] [PubMed] [Google Scholar]
- 17.Goto T, Takahashi N, Hirai S, Kawada T. Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR Res 2010. 2010 doi: 10.1155/2010/483958. 483958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng Y, Lu ZM, Huang W, Xu HY, Shi JS, Xu ZH. Bioassay-guided isolation of DPP-4 inhibitory fractions from extracts of submerged cultured of Inonotus obliquus. Molecules. 2013;18:1150–61. doi: 10.3390/molecules18011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakila BS, Manjula A, Latha MT, Ravi TK. De novo drug design and synthesis of certain indole derivatives and screening for their xanthine oxidase inhibitory activity. Int J PharmTech Res. 2010;2:2128–38. [Google Scholar]
- 20.Shalini D, Sudha G. Anti-oxidative activity of various tea extracts Camellia sinensis. J Biosci Res. 2010;1:271–8. [Google Scholar]
- 21.Zhang LL, Yi ML. Antioxidant tannins from Syzygium cumini fruit. Afr J Biotechnol. 2009;8:2301–9. [Google Scholar]
- 22.Arif T, Sharma B, Gahlaut A, Kumar V, Dabur R. Anti-diabetic agents from medicinal plants: A review. Chem Biol Lett. 2014;1:1–13. [Google Scholar]
- 23.Yadav HN. Effect of concentration of Ocimum sactum Linn (Tulsi) leaves extract on the a amylase, a glucosidase activity and microorganism growth. Int J Eng Sci Res Technol. 2013;2:312–15. [Google Scholar]
- 24.Freitas TC, Pereira CA, Pereira LL. Syzygium sp (Myrtaceae) extracts: Inhibition of alpha amylase. Eur J Med Plants. 2014;4:116–25. [Google Scholar]
- 25.Ruan ZP, Zhang LL, Lin YM. Evaluation of the anti-oxidant activity of Syzygium cumini leaves. Molecules. 2008;13:2545–56. doi: 10.3390/molecules13102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sirisha N, Reenivasulu M, Sangeeta K, Madhusudhana C. Anti-oxidant properties of Ficus Species – A review. Int J PharmTech Res. 2010;2:2174–82. [Google Scholar]
- 27.Singh AK, Jatwa R, Jashi J. Cytoprotective and dipeptidyl peptidase IV (Dpp-IV/Cd26) inhibitory roles of Ocimum sanctum and Momordica charantia extract. Asian J Pharm Clin Res. 2014;7:115–20. [Google Scholar]
- 28.Akande IS, Samuel TA, Agbazue U, Olowolagba BL. Comparative proximate analysis of ethanolic and water extracts of Cymbopgon citrtus, Lemongrass and four tea brands. Plant Sci Res. 2011;3:29–35. [Google Scholar]
- 29.Zhang X, Lu X, Li S, Zhong M, Shi X, Luo G. Investigation of 2,4.dichlorophenoxyacetic acid adsorption onto MIEX resin: Optimization using response surface methodology. J Taiwan Inst Chem Eng. 2014;45:1835–41. [Google Scholar]
- 30.Demirel M, Kayan B. Application of response surface methodology and central composite design for the optimization of textile dye degradation by wet air oxidation. Int J Ind Chem. 2012;3:1–10. [Google Scholar]
- 31.Cukor G, Jurkovi Z, Sekulic MS. Rotatable central composite design of experiments versus Taguchi method in the optimization of turning. Metabk. 2011;50:17–20. [Google Scholar]
- 32.Turkmen N, Velioglu YS, Sari F, Polat G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules. 2007;12:484–96. doi: 10.3390/12030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franco D, Sineiro J, Rubilar M, Sanchez M, Jerez M, Pinelo M, et al. Polyphenols from plant materials: Extraction and anti-oxidant power. Electron J Environ Agri Food Chem. 2008;7:3210–6. [Google Scholar]
- 34.Tatiya AU, Tapadiya GG, Kotecha S, Surana SJ. Effect of solvents on total phenolics, anti-oxidant and antimicrobial properties of Bridelia retusa Spreng stem bark. Indian J Nat Prod Resour. 2011;2:442–7. [Google Scholar]
- 35.Mohammedi Z. Impact of solvent extraction type on total polyphenols content and biological activity from Tamarix aphylla (L.) Karst. Int J Pharm Biosci. 2011;2:609–15. [Google Scholar]
- 36.Khedher O, Moussaoui Y, Salem RB. Solvent effects on phenolic contents and anti-oxidant activities of the Echinops spinosus and the Limoniastrum monopetalum. Res J Pharm Biol Chem Sci. 2014;5:66–76. [Google Scholar]
- 37.Dent M, Uzelac VD, Penic M, Bosiljkov T, Levaj B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol Biotechnol. 2013;51:84–91. [Google Scholar]
- 38.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;140:315–22. [Google Scholar]
- 40.Othman A, Ismail A, Abdul GN, Adenan I. Anti-oxidant capacity and phenolic content of cocoa beans. Food Chem. 2007;100:1523–30. [Google Scholar]
- 41.Modnicki D, Balcerek M. Estimation of total phenol contents in Ocimum basilicum L., Origanum vulgare L., Thymus vulgaris L., commercial samples. Herba Pol. 2009;55:35–42. [Google Scholar]
- 42.Atanassova M, Georgieva S, Ivancheva K. Total phenolic and total flavonoid contents, antio-xidant capacity and biological contaminants in medicinal herbs. J Univ Chem Technol Metall. 2011;46:81–8. [Google Scholar]
- 43.Djeridane K, Yousfi M, Nadjemi D, Boutassouna D, Stocker P, Vidal N. Anti-oxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–60. [Google Scholar]
- 44.Abdel-Hameed el-SS. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2009;114:1271–7. [Google Scholar]
- 45.Hagerman AE, Butler LG. Protein precipitation method for the quantitative determination of tannin. J Agric Food Chem. 1978;26:809–12. [Google Scholar]
- 46.Ganapaty S, Ramaiah M, Yasaswini K, Nuthakki VK, Dibbanti HK. Quantitative phytochemical estimation and evaluation of hepatoprotective activity of methanolic extract of Dendrobium ovatum (L.) Kraenzl whole plant against CCl4 induced hepatotoxicity. J Pharmacog Phytochem. 2013;2:113–8. [Google Scholar]
- 47.Rizwan A, Kushagra N, Tekeshwar K, Singh M, Dewangan D. Phytochemical estimations of anthraquinones from Cassia species. Int J Res Ayurveda Pharm. 2011;2:1320–3. [Google Scholar]
- 48.Devanaboyina N, Ramalakshmi N, Sudeepthi BS, Hemachakradhar K, Raju NP. Preliminary phytochemical screening, quantitative estimation and evaluation of antimicrobial activity of Alstonia macrophylla stem bark. Int J Sci Invent Today. 2013;2:31–9. [Google Scholar]
- 49.Subramanian R, Asmawi MZ, Sadikun A. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim Pol. 2008;55:391–8. [PubMed] [Google Scholar]
- 50.Basak SS, Candan F. Chemical composition and in vitro anti-oxidant and antidiabetic activities of Eucalyptus camaldulensis Dehnh, essential oil. J Iran Chem Soc. 2010;7:216–26. [Google Scholar]
- 51.Chaudhary SK, Mukherjee PK, Maiti N, De AK, Bhadra S, Saha BP. Evaluation of angiotensin converting enzyme inhibition and anti-oxidant activity of Piper longum L. Indian J Tradit Knowl. 2013;12:478–82. [Google Scholar]
- 52.Mardanyan S, Sharoyan S, Antonyan A, Zarkanyan N. Dipeptidyl peptidase IV and adenosine deaminase inhibition by Armenian plants and anti-diabetic drugs. Int J Diabetes Metab. 2011;19:69–74. [Google Scholar]
- 53.Sharoyan S, Antonyan A, Mardanyan S, Lupidi G, Cristalli G. Influence of dipeptidyl peptidase IV on enzymatic properties of adenosine deaminase. Acta Biochim Pol. 2006;53:539–46. [PubMed] [Google Scholar]
- 54.Patel G, Gauni B, Mehta K, Patel BN. Comparative Study of anti-oxidant capacity of raw powder and waste black tea by FRAP assay. Res J Recent Sci. 2014;3:42–44. [Google Scholar]
- 55.Durre S, Muhammad AR, Sana B, Gulashan B. Ferric reducing anti-oxidant power of essential oils extracted from Eucalyptus and Curcuma species. Asian Pac J Trop Biomed. 2012;2:1633–6. [Google Scholar]
- 56.Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 57.Andreja RH, Majda H, Zi EK, Davorin B. Comparison of anti-oxidative and synergistic effects of Rosemary extract with a tocopherol, ascorbyl palmitate and citric acid in sun flower oil. Food Chem. 2000;71:229–33. [Google Scholar]
- 58.Mensor LL, Menezes FS, Leitão GG, Reis AS, dos Santos TC, Coube CS, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–30. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 59.Liu D, Shi J, Ibarra AC, Kakuda Y, Xue SJ. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. LWT. 2008;41:1344–9. [Google Scholar]
- 60.Guillemin N, Meunier B, Jurie C, Cassar-Malek I, Hocquette JF, Leveziel H, et al. Validation of a Dot-Blot quantitative technique for large scale analysis of beef tenderness biomarkers. J Physiol Pharmacol. 2009;60(Suppl 3):91–7. [PubMed] [Google Scholar]
- 61.Perron NR, Brumaghim JL. A review of the anti-oxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 62.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J Diabetes Metab Disord. 2013;12:43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004;4:18. doi: 10.1186/1471-2210-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diabetes Metab J. 2013;37:173–5. doi: 10.4093/dmj.2013.37.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]