Abstract
Introduction:
Inflammation is a well-known physiological response to protect the body against infection and restore tissue injury. Nevertheless, the chronic inflammation can trigger various inflammatory associated diseases/disorder. Moringa oleifera is a widely grown plant in most tropical countries and it has been recognized traditionally for several medicinal benefits.
Objectives:
The objective of this study was to investigate the anti-inflammatory properties of M. oleifera extract on lipopolysaccharide (LPS) - stimulated macrophages.
Materials and Methods:
The anti-inflammatory effect of M. oleifera hydroethanolic bioactive leaves extracts was evaluated by assessing the inhibition of nitric oxide (NO) production during Griess reaction and the expression of pro-inflammatory mediators in macrophages.
Results:
Interestingly, we found that M. oleifera hydroethanolic bioactive leaves extract significantly inhibited the secretion of NO production and other inflammatory markers such as prostaglandin E2, tumor necrosis factor alpha, interleukin (IL)-6, and IL-1β. Meanwhile, the bioactive extract has induced the production of IL-10 in a dose-dependent manner. In addition, M. oleifera hydroethanolic bioactive leaves extract effectively suppressed the protein expression of inflammatory markers inducible NO synthase, cyclooxygenase-2, and nuclear factor kappa-light-chain-enhancer of activated B-cells p65 in LPS-induced RAW264.7 macrophages in a dose-dependent manner.
Conclusion:
These findings support the traditional use of M. oleifera plant as an effective treatment for inflammation associated diseases/disorders.
SUMMARY
Hydroethanolic extracts of Moringa oleifera effectively inhibit the NO production in LPS induced inflammatory model.
M. oleifera crude extracts successfully modulate the production of pro-inflammatory mediators in LPS stimulated macrophages.
M. oleifera extracts suppressed the expression of inflammatory mediators in LPS stimulated macrophages.
Keywords: Anti-inflammatory, lipopolysaccharide, Moringa oleifera, pro-inflammatory, RAW264.7 murine macrophage
INTRODUCTION
Inflammation is a protective mechanism of organisms to defense against harmful stimuli.[1,2] It involves various molecular pathways with a wide variety of physiological processes.[3] However, up-regulated inflammation can lead to many diseases such as cancer,[4] asthma, allergic rhinitis, atopic dermatitis, and rheumatoid arthritis.[1] In the United States, inflammatory diseases were reported with the high prevalence among their population.[5] Around 46.4 million individuals were diagnosed with arthritis; 1.3 million adults and 294,000 children were reported for rheumatoid arthritis and juvenile arthritis, respectively.[6] Inflammation process of cellular dysfunction can be induced through microbial stimulus; lipopolysaccharide (LPS) is a common prototypical endotoxin can directly activate macrophages. Monitoring evidence have been recommended that activated macrophage produce a higher amount of inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and other inflammatory mediators.[1] Macrophages produced by bone marrow cells are directed to different body organs and tissues such as lung and liver are closely connected with tissue homeostasis; moreover, it performed crucial role within the natural and acquired immune system. The macrophage is the foremost cell in the inflammatory process and exerts two opposite impacts, stimulatory and inhibitory effects on inflammation process, which include phagocytic effect based on the nature and intense of the stimulus. Macrophages, derived from blood (monocytes), are actively involved in tissue inflammation site against noxious insult; they are capable to perform a wide array of function. Inflammatory macrophages have the ability to kill through apoptosis, clearance of proliferating resident stromal, infiltrating leukocytes, and parenchymal cells.[7]
The World Health Organization has estimated that 80% of the population in the developing countries use traditional medicine for their primary health care needs and the majority of this therapy requires the use of herbal extract and their active components.[8] Almost all modern drugs are produced originally through traditional herbal resources. These have progressed to develop the conventional medicines that use both isolated natural compounds and synthetic drugs. Herbal supplements are popular and their formulations have led to the several novel generation of phytomedicines that are more effective than before.[9] Development of efficient nonsteroidal anti-inflammatory drugs (NSAIDs) with minimal or no gastrointestinal (GI) side effects is an area of interest in drug discovery industry. The use of most commonly recommended drug from analgesics such as aspirin currently have been restricted because of their potential side effects such as severe gastric disorders. Lesser gastrointestinal side effects seen with Cyclooxygenase -2 inhibitors are resolved at a slow phase.[10,11,12] Furthermore, an enhancement of cardiovascular and cerebrovascular events, specifically in patients with an increased risk of thrombosis has been evaluated in the chronic use of some COX-2-specific inhibitors. Anti-inflammatory drugs which include “biologicals” such as anti-cytokine therapies show a significant decrease in host defense toward the infection.[3] As a result of the steroidal and NSAID side effects, the researchers started to focus on natural compounds as a keen source of alternative drugs. Numerous herbal medicines pave the way for leading novel therapeutic compounds against inflammation, despite they are inexpensive, safe, highly tolerated, and convenient for numerous patients.[9,13]
Moringa oleifera Lam (syn. Moringa pterygosperma known as “The Miracle Tree,” “Horseradish-tree,” or “Ben oil tree”) is well-known and the most commonly spread species of Moringaceace family. It offers a remarkable range of medicinal application due to its possession of numerous nutritional/therapeutic candidates. M. oleifera grows through the diverse part of the world Asia, Africa, India, Pakistan, Cambodia, Philippines, and America.[14] Different parts of the plant extracts and derived compounds have been reported with various medicinal properties. The root extract has been demonstrated to have anti-fertility and anti-inflammatory agents. Moreover, the seed possesses anti-inflammatory impact, anti-hypertensive effects, and ability to decrease lipid peroxidation. Moringa Leaves extract have been studied for its antioxidant effect through various in vitro and in vivo approaches and also it has various pharmacological properties such as hypolipidemic, anti-atherosclerotic,[15] wound healing,[16] hepatoprotective,[17,18,19] antimicrobial, antinociceptive, and radioprotective properties.[20] These extracts have eliminated the neuropathic pain[21,22] and protection against oxidative stress in an in vivo model of Alzheimer's disease.[23] The pods, leaves, and flowers of M. oleifera commonly have been consumed as a vegetable in the south of Asia. Since, the whole plant provides nutrition and medicinal value against diabetes, cancer, rheumatoid arthritis, and other ailments; M. oleifera is also called as “miracle tree.”[24] The reason behind the enormous pharmacological activities of M. oleifera might possibly due to various secondary plant metabolites present in M. oleifera such as flavonoids, phenolics, vitamins, carotenoids, minerals, sterols, amino acids, alkaloids, and glycosides.[8] M. oleifera leaves contains phenolic acids such as quinic acid and chlorogenic acid that exhibit high antioxidant activities[25,26] and recently β-sitosterol, was isolated from Moringa leaves fraction[27] and it has reported to reduce the intestinal uptake of dietary cholesterol effects.[28] M. oleifera seeds contains antitumor and anti-hypertensive effects of O-ethyl-4-(a-L-rhamnosyloxy) benzyl carbamate and its seven derivatives such as niazirin, niazimicin, and β-sitosterol.[29] Therefore, the purpose of this study was to screen three different hydroethanolic solvent gradients of M. oleifera bioactive leaves extract for cytotoxicity and NO production. Furthermore, the anti-inflammatory activity of M. oleifera hydroethanolic bioactive leaves extract in LPS-stimulated RAW264.7 murine macrophage cells was evaluated by quantifying the expression of various inflammatory markers in comparison with dexamethasone, a known anti-inflammatory compound.
MATERIALS AND METHODS
Chemicals and reagents
The murine macrophage RAW264.7 cell line (ATCC, TIB-71) was examined as an in vitro model to investigate the anti-inflammatory properties of M. oleifera hydroethanolic extract. Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS), penicillin, streptomycin for cell culture were obtained from Nacalai (Kyoto, Japan). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), LPSs isolated from Escherichia coli 0111:B4 were purchased from Sigma–Aldrich Co., (St. Louis, MO, USA). Prostaglandin E2 (PGE2) parameter assay kit, TNF-α, IL-6, IL-1β, and IL-10 mouse ELISA kits were purchased from R and D Systems (Minneapolis, MN, USA). The bicinchoninic acid (BCA) protein assay kit was obtained from Pierce (Thermo scientific, USA). Anti-β-actin–horseradish peroxidase (HRP) and secondary polyclonal antibody-conjugated HRP, were purchased from Santa Cruz (CA, USA). Primary antibodies against inducible nitric oxide synthase (iNOS), COX-2, nuclear factor kappa-light-chain-enhancer of activated B-cells p65 (NFκB-p65), were purchased from Abcam (Cambridge, MA, USA).
Preparation of plant extract
Fresh and mature M. oleifera leaves were collected from Garden-2, Universiti Putra Malaysia, Serdang, Selangor, Malaysia, washed and dried at room temperature for 18 h. Then, the leaves were oven dried at 42°C for 2 consecutive days and grinded them with electronic laboratory blender. Three different hydroethanolic solvent gradients were prepared (ethanol: Distilled water, 50:50 [50%], 70:30 [70%], and 90:10 [90%]), and they were macerated for 3 days at room temperature. The soaked material was stirred every day and filtered through filter paper. Then, the extract obtained from maceration process was concentrated under vacuum on a rotary evaporator at 40°C. The crude extract was freeze-dried, weighed, and stored at −80°C for future use. For each experiment prior to the treatment of cell culture, M. oleifera hydroethanolic extract was dissolved in DMEM and sonicated in an ultrasonic bath (Mettler Electronic Corporation, Anaheim, USA) at 25°C.
Cell culture
Murine RAW264.7 macrophage cell line was purchased from ATCC (VA, USA) and cultured in DMEM supplemented with 10% FBS, 1% streptomycin/penicillin at 37°C in a 5% CO2 incubator. Dexamethasone, which is commonly known as a classic glucocorticosteroid drug and frequently used in clinical practice, was selected as a positive control with a concentration of 0.5 μg/ml.[30] RAW264.7 macrophage cells stimulated by LPS (1 μg/ml) without any other intervention component were considered as a negative control[31] while cells incubated by medium were considered as normal control.
3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay for cell viability
The effect of M. oleifera hydroethanolic bioactive leaves extract on cell viability was measured by the mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT) to violet formazan crystals (nonwater-soluble) inside the cell. The cells were cultured in 96-well plates at a density of 105 cells/ml (100 μL/well) and incubated for 24 h. Cells were treated with three different hydroethanolic gradients (50%, 70%, and 90%) with different concentrations through serial dilution (1000–15.62 μg/ml) and this was followed by 24 h incubation. Then, 20 μl MTT solution at 37°C was added (5 mg/ml in phosphate-buffered saline) with 4 h incubation. The optical density of formazan in solution was measured by using microplate reader at 570 nm wavelength.
Determination of nitric oxide
The effects of M. oleifera hydroethanolic bioactive leaves extract on NO production in RAW264.7 cells were determined based on Griess reaction. In brief, RAW264.7 cells were cultured in 24-well plates at a density of 105/ml and incubated for 24 h followed by 1-h pretreatment with M. oleifera hydroethanolic (50%, 70%, and 90%) extract and dexamethasone and co-incubated with LPS for 24 h. Griess reagent (0.1% N-[1-naphthyl] ethylenediamine-HCl and 1% sulfanilamide and 5% H3PO4) was added to the supernatant and after 10 min incubation reading by ELISA microplate reader at 540 nm wavelength.
Determination of inflammatory mediator's by ELISA
Cells (1 × 106 cells/ml) were plated into 6-well culture plate and treated with M. oleifera 90% hydroethanolic bioactive leaves extract or dexamethasone, then stimulated for 24 h with LPS. In brief, capture antibodies for TNF-α, IL-6, IL1-β, and IL-10 were coated in 96-well plates by overnight incubation. After the incubation time and washing immune complex of capture antibody, samples/standard, and detection antibody. Finally, plates were detected by reacting with streptavidin horseradish – HRP – tetramethylbenzidine detection system. Reactions were stopped by addition of 2M H2SO4, and absorbance was read at 450 nm by a microplate reader. In addition, PGE2 production was determined using an ELISA kit (with coated capture antibody) according to the manufacturer's instructions.
Western blot analysis
The cells were cultured in 6-well plates and incubated with M. oleifera 90% hydroethanol bioactive leaves extract in the presence and the absence of LPS for 24 h. After washing with ice-cold phosphate buffer saline (PBS), the cells were lysed in a cell lysis buffer containing the protease and phosphatase inhibitor cocktail. Cells lysed dilution was centrifuged at 14,000 rpm for 25 min to discard cell debris. The protein concentration was measured by using BCA assay according to the manufacture's instruction. Equal amounts of total cellular proteins (30 μg) were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (GE Healthcare, USA). Each membrane was incubated with blocking solution (5% nonfat skim milk) in PBS containing 0.5% Tween 20 (PBST, pH 7.4) at room temperature for 1-h. This was followed by an overnight incubation at 4°C with the appropriate primary antibodies iNOS, COX-2, NFκB-p65 (1:1000 dilution), the membranes were washed with PBS containing Tween 20 for 1-h, then incubated with a 1:2000 dilution of HRP-conjugated secondary antibodies for 1-h at room temperature. The membranes were washed again with PBST for 1-h then the immune reacted proteins were detected using a Chemiluminescence System (ChemiDoc, BioRad, USA). The bands obtained were quantitated and analyzed using ImageJ Software (BioTechniques, New York, NY, USA).
Statistical analysis
The results reported are summarized from three independent experiments and expressed as the mean ± standard deviation (SD). Data were presented as means ± SD and the P < 0.05 and P < 0.001 were considered as statistically significant. Significant differences were examined using an analysis of variance with SPSS 20.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
Effect of Moringa oleifera on cell viability
Three different hydroethanolic gradients of M. oleifera bioactive leaves extract were examined for cytotoxicity study with MTT assay. The 90% hydroethanolic extract showed the highest cell viability and more than 85% of the cells were viable at 125 μg/ml and 250 μg/ml concentrations (*P < 0.05) [Figure 1]. Accordingly, (90:10) 90% M. oleifera hydroethanolic bioactive leaves extract at 250, 125 μg/ml concentrations was selected for future anti-inflammatory experiments.
Figure 1.
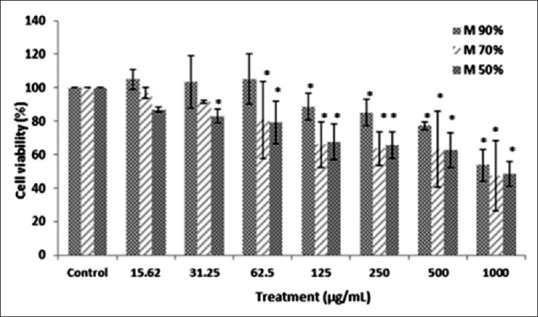
The effect of three different hydroethanolic gradients of Moringa oleifera leaves extract (90%, 70%, and 50%) on the viability of RAW 264.7 cells. Cytotoxicity was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reduction assay after 24 h treatment with Moringa oleifera extract (15.62–1000) µg/ml. Values are presented as the mean of three independent experiments in triplicate, and data are shown as mean ± standard deviation. Statistical analysis using one-way analysis of variance with Tukey's post-hoc test (*P < 0.05) as compared with the untreated control group
Inhibition of nitric oxide production by Moringa oleifera in lipopolysaccharide-stimulated RAW264.7 cells
RAW264.7 murine macrophage cells were shown to produce NO through stimulation with LPS. Significant inhibition of NO production was observed after treatment with three different hydroethanolic gradients of M. oleifera bioactive leaves extract. In addition, NO production was significantly suppressed after treatment with dexamethasone (*P < 0.001) [Figure 2].
Figure 2.
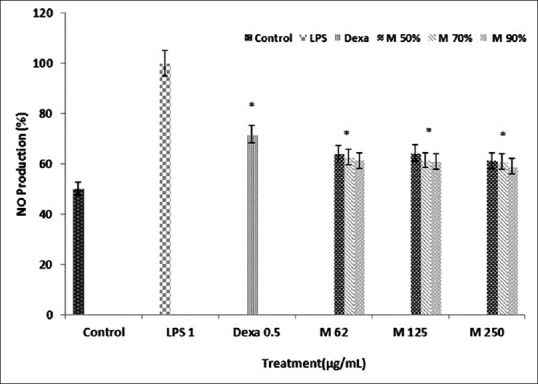
Effects of three different hydroethanolic gradients of Moringa oleifera bioactive leaves extract (90%, 70%, and 50%) on lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage. Cells were treated with different concentration of Moringa oleifera (62 µg/ml, 125 µg/ml, and 250 µg/ml) in the presence of 1 µg/ml lipopolysaccharide for 24 h. Dexamethasone (0.5 µg/ml) was used as a positive control. Control values were obtained in the absence of lipopolysaccharide or Moringa oleifera. Three independent assays were performed in triplicate, and data are shown the mean ± standard deviation statistical analysis using one-way analysis of variance with Tukey's post-hoc test. (*P < 0.001) indicates significant differences from the lipopolysaccharide treated group and nontreated group
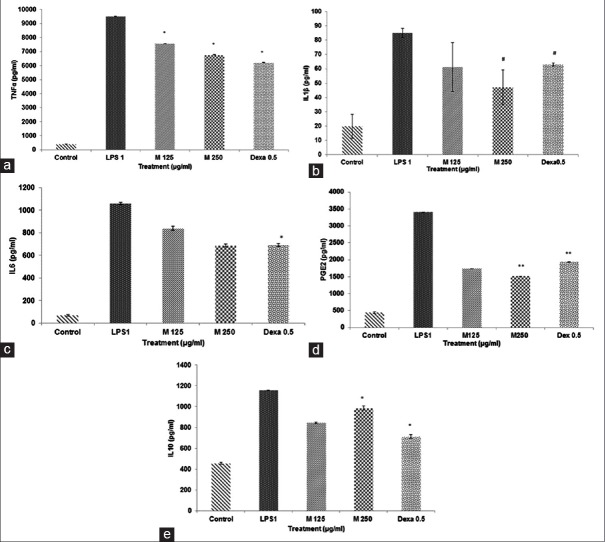
The effect of Moringa oleifera on lipopolysaccharide-induced inflammatory cytokines and prostaglandin E2 production
The cytokine production levels of TNF-α, IL-1β, IL-6, and PGE2 in the cell-free culture supernatants were significantly enhanced in respond to LPS induction [Figure 3a–d], which has proven the successful establishment of in vitro inflammatory model. The 90% M. oleifera hydroethanolic bioactive leaves extract significantly suppressed the production level of TNF-α, IL-1β, IL-6, and PGE2 in a concentration-dependent manner (*P < 0.001) (**P < 0.001) (#P < 0.05) [Figure 3a–d] and meantime, it has increased the production of IL-10 in a dose-dependent manner (*P < 0.001) [Figure 3e]. Meanwhile, dexamethasone has also exhibited the significant inhibition in TNF-α, IL-1β, IL-6, PGE2 production, and enhancement in IL-10 level (*P < 0.001) (**P < 0.001) (#P < 0.05) [Figure 3a–e].
Figure 3.
(a-e) Effect of Moringa oleifera 90% hydroethanolic bioactive leaves extract on the production of tumor necrosis factor alpha, interleukin-1β, interleukin-6, prostaglandin E2, and interleukin-10 cytokines in lipopolysaccharide-stimulated RAW 264.7 cells. Cells have been exposed with lipopolysaccharide (1 µg/ml) alone or lipopolysaccharide plus different concentrations of Moringa oleifera (125 µg/ml, 250 µg/ml) and dexamethasone for 24 h. Three independent assays were performed in triplicate, and the data are shown the mean ± standard deviation statistical analysis using one-way analysis of variance with Tukey's post-hoc test. It shows a significant difference with lipopolysaccharide treated and untreated groups (*P < 0.001). It exhibits significant difference with lipopolysaccharide treated group (**P < 0.001). It shows a significant difference with lipopolysaccharide treated group (#P < 0.05)
Effect of Moringa oleifera on expression of inflammatory markers inducible nitric oxide synthase, cyclooxygenase-2, and nuclear factor kappa-light-chain-enhancer of activated B-cells p65 on lipopolysaccharide-induced macrophages
Western blot analysis was performed to evaluate the inhibitory effects of M. oleifera on inflammatory mediators in LPS stimulated macrophages. RAW264.7 cells were treated with 90% M. oleifera hydroethanolic bioactive leaves extract in the presence and the absence of LPS for 24 h. The protein expression of iNOS and COX-2 was clearly enhanced through stimulation with LPS, and M. oleifera hydroethanolic bioactive leaves extract considerably suppressed iNOS and COX-2 pro-inflammatory mediators, and NFκB-p65 protein expression in a dose-dependent fashion [Figure 4a]. In addition, to understand whether M. oleifera hydroethanolic bioactive leaves extract suppressed NF-κB pathway activity, we measured the protein expression of NFκB-p65 and it exhibited significant inhibition effect through LPS stimulated RAW264.7 cells. In the quantitative determination, β-actin expression level as an internal standard was comparable between each target protein. The density ratio of iNOS, COX-2, and NFκB-p65 was significantly decreased after treatment with M. oleifera through LPS-induced RAW264.7 cells (*P < 0.05) [Figure 4b].
Figure 4.
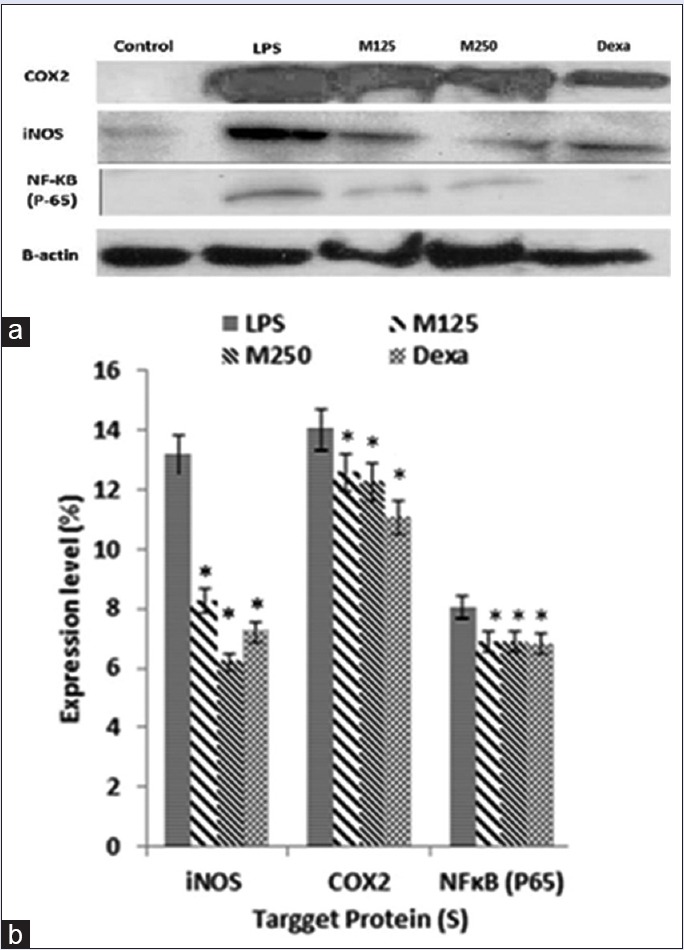
(a) Effect of Moringa oleifera hydroethanolic bioactive leaves extract on lipopolysaccharide-induced inducible nitric oxide synthase, cyclooxygenase-2, and nuclear factor kappa-light-chain-enhancer of activated B-cells p65 expression in RAW264.7 cells. Cells were stimulated with lipopolysaccharide (1 µg/ml) in the presence of Moringa oleifera hydroethanolic leaves extract (125 µg/ml, 250 µg/ml) and dexamethasone (0.5 µg/ml) for 24 h at 37°C. Cell lysates were extracted, and protein expression levels were analyzed by Western blot. β-actin was used as the loading control. (b) The level of each protein was measured and normalized to β-actin. The values are reported as the mean ± standard deviation percentage of three independent experiments. Statistical analysis using one-way analysis of variance with Tukey's post-hoc test. It shows a significant difference with lipopolysaccharide treated group (*P < 0.05)
DISCUSSION
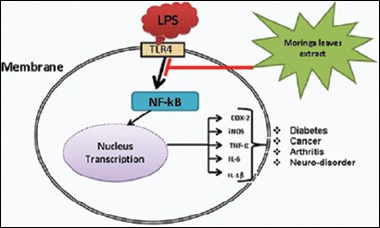
Macrophages perform a crucial function in host defenses toward noxious substances and the inflammation process.[2,3,4,5,6,7] It has been well-established that LPS, a part of Gram-negative bacteria membranes is identified by toll-like receptor 4 (TLR4) on the cell membrane of the macrophages. It activates the release of pro-inflammatory mediators through TLR4-NFκB signaling pathways, which mediate host harmful injury.[31] Overproduction of pro-inflammatory cytokines by activated macrophages has been concerned in the pathophysiology of numerous chronic inflammatory illnesses. Therefore, LPS-stimulated macrophages are used as a model to investigate on inflammation and the mechanisms action of anti-inflammatory agents.[2,7] Recently, it has been notable that more natural products are becoming utilized as a treatment to reduce several acute and chronic diseases.[32,33]
In the field of natural products drug discovery, plant extractions process with the appropriate solvent system is very essential to identify and isolate the pharmacological compounds from medical plants. A previous investigation by Fakurazi et al.,[34] has been reported that hepatoprotective properties of 80% hydroethanolic M. oleifera leaves extract in hepatotoxicin induced animal model. However, in the present study, we have evaluated three different hydroethanolic gradients of M. oleifera leaves extracts based on cell specific toxicity, anti-oxidant, and anti-inflammatory properties.[35] Several anti-inflammatory drugs have recently been exhibited to carry a free radical scavenging mechanism as part of their pharmacological activities.[36] Oxidative stress-induced activation of NFκB has been identified in several chronic inflammatory diseases exhibiting a crucial link among modulation of NFκB molecules and antioxidant properties.[37] Furthermore, it could be suggested that antioxidant potential of M. oleifera leaves might be one of the cellular mechanisms of its anti-inflammatory potential in LPS stimulated macrophages.[38,39,40]
Cell viability assay was performed for three different M. oliefera hydroethanolic leaves gradients 90% hydroethanolic bioactive leaves extract revealed the highest cell viability as compared with 70% and 50% concentration. It can be due to the availability of more nutrition and some certain compounds for cells. This result was in agreement with our previous study by Karthivashan et al.,[35] which introduced 90% hydroethanolic solvent as the best solvent. It could possibly digest leaves tissue and may release antioxidant and nutritionally important active compounds of M. oliefera leaves. Consistent with the previous findings by Araújo et al.,[41] indicated that M. oleifera aqueous seeds extract also exhibited higher cell viability on peritoneal macrophages cells. Previous investigations have shown that, the maximum duration of release of inflammatory mediators from LPS stimulated macrophages was 24 h with 1 μg/ml concentration and this LPS concentration is nontoxic to macrophages.[42,43,44,45]
NO is an essential mediator and regulator of inflammatory responses. In the inflammatory processes overproduction of NO reacts with superoxide causes cytotoxicity and tissue damage in several organisms. NO-induced oxidative stress is associated with several illnesses for instance atherosclerosis, septic shock, diabetes, Alzheimer's disease, and Parkinson's disease. NO inhibitor compounds have been reported as an effective treatment for such diseases.[46] In animal tissues synthases enzyme (NOS) oxidase L-arginine to L-citrulline and produce NO supplements. Three isoforms of NOS which include NOS I or (nNOS) - the neuronal form, NOS II or iNOS, contained in numerous cell types following inflammatory stimulation (e.g., macrophages), and NOS III (or eNOS) - constitutive enzyme mainly reveal in the endothelium. The three isoforms have the same molecular construction and involve numerous cofactors.[47] Recent study by Lee, et al. has demonstrated that licochalcone E which is isolated and characterized from the roots of Glycyrrhiza inflata, revealed potent anti-inflammatory effect by suppressing NO production and protein expression of iNOS in a dose-dependent fashion.[48] In this study, the effect of three different hydroethanolic M. oliefera leaves gradients (50:50 [50%], 70:30 [70%], and 90:10 [90%]) were found to significantly inhibit LPS-induced NO production. Moreover, the inhibitory effect of positive control (dexamethasone) was similar to M. oleifera leaves extract. In addition, protein expression of iNOS was inhibited after treatment with M. oleifera hydroethanolic bioactive leaves extract confirming the suppressive effect of M. oleifera on NO production. These findings might conclude that M. oleifera leaves acts as an effective anti-inflammatory agent against various ailments.
The mechanism of action of numerous anti-inflammatory drugs is through PG synthesis suppression which usually mediated by COX. Between two isoforms of COX (COX-1 and COX-2), COX-1 has been recommended to produce a physiological amount of PGs for typical platelet, kidney, and stomach function. COX-2 is a mediator with high activity at inflammatory sites in animals and human patients with the inflammatory disorder.[49] PGE2 is one of the most effective inflammatory mediators which acts in all inflammatory processes leading to typical signs of inflammation such as pain, swelling, and redness.[50] PGE2 has been altered from arachidonic acid through the COX-2 catalytic activity. COX-2 also might be affected specifically at its enzymatic activity via NO and iNOS.[49] A recent study by Kim et al., has exhibited that composition of essential oil from fingered citron (Citrus medica L. var. sarcodactylis) caused dose-dependent suppression of LPS-stimulated PGE2 production and inhibited COX-2 protein expression.[2] In this study M. oleifera hydroethanolic bioactive leaves extracts significantly reduced PGE2 production and COX-2 protein expression. It may suggest that the anti-inflammatory effect of M. oleifera hydroethanolic bioactive leaves extracts could possibly be due to its suppressive effect on PGE2 production via blocking COX-2 protein expression. The cross-talk among the iNOS and COX-2 pathways has been identified through several researchers and few researches have concluded that NO seems to reduce COX-2 expression.[51] Targeting iNOS and COX-2 has been known as a useful strategy to prevent acute and chronic inflammatory diseases. These inflammatory mediators regulate the inflammation process by producing NO and PGE2, respectively.[52] As a result active compounds with dual suppression effects on iNOS and COX-2 expression would have remarkable potential on healing the acute and chronic inflammation.
Pro-inflammatory cytokines, which includes TNF-α and IL-6 are classified as main inflammatory mediators which are produced by monocyte and macrophages in the inflammatory procedure.[15] TNF-α is a key mediator in inflammatory responses, as well as the initiation of apoptosis. It might stimulate the development or expression of IL-6, IL-1β, PGE2, collagenase, and adhesion molecules eliciting a variety of physiological functions such as septic shock, inflammation and cytotoxicity.[46] In addition, it represents a function on the developmental pathogenesis of numerous inflammation-associated chronic illnesses such as cancer, obesity, and cardiovascular diseases. TNF-α and IL-6 are mainly produced by macrophages in response to LPS through NFκB activation.[15] The IL-1 family plays a crucial role in both host defense and inflammation. To date, up to 11 members of this family have been recognized. IL-1α and IL-1β both are synthesized as precursor molecules by several different cell types and both are pro-inflammatory cytokines. IL-1β is a main mediator of inflammation which performs a basic role in tissue injury restore along with protection toward microbial pathogens. In these conditions, systemic (i.e. bone marrow) and/or local (i.e., endothelial) reactions to this cytokine are responsible for beneficial impacts, which include cellular infiltration and neutrophil mobilization, respectively. However, an extra amount of this cytokine may possibly have deleterious impacts on a diversity of cells and tissue.[53] IL-6, which has been originally defined as a B-cell differentiation factor is regarded as a multifunctional cytokine which regulates immune responses, hematopoiesis, acute phase response, and inflammation. Increased IL-6 amount in many cases is correlated with several diseases which include rheumatoid arthritis, systemic-onset juvenile chronic arthritis, osteoporosis, psoriasis, polyclonal plasmacytosis, malignant plasmacytoma, Crohn's disease, and encephalomyelitis. Therefore, inhibitors of IL-6 could possibly be beneficial in treating of inflammatory autoimmune illnesses.[54] A recent study has been published on anti-inflammatory properties of plumbagin, derived from the plants of the Plumbaginaceae family which exhibited suppressing effect on the production of TNF-α, IL-1β, and IL-6 from LPS-stimulated RAW264.7 cells in a dose-dependent fashion.[55] In the present study, M. oleifera hydroethanolic bioactive leaves extract exhibited significant inhibition in LPS-induced TNF-α, IL-1 β, and IL-6 production in a dose-dependent manner. These results recommended that M. oleifera leaves may possess anti-inflammatory characteristic and help to reduce some inflammatory associated disorders. We believe that these anti-inflammatory properties of M. oleifera bioactive leaves extracts might be related to the presence of various active compounds which was identified by us through liquid chromatography-mass spectrometry (MS)/MS.[35]
IL-10 is one of the pleiotropic cytokines, which regulated the function of numerous adaptive immune-related cells. Although, IL-10 is strongly known as an immunosuppressive and anti-inflammatory cytokine, it performs other crucial roles such as the ability to activate B-cells, T-cells, natural killer cells, and mast cells.[56] A study by Zhao, et al. has reported that Corilagin (beta-1-O-galloyl-3,6-(R)-hexa hydroxyl diphenoyl-D-glucose) a member of the tannin family significantly enhanced IL-10 production in a dose-dependent manner through LPS stimulated RAW264.7 cells.[30] In the current study, M. oleifera hydroethanolic bioactive leaves extract revealed significant induction in IL-10 amount as an anti-inflammatory cytokine in a dose-dependent fashion which was in agreement with anti-inflammatory properties of M. oleifera bioactive extract.
NFκB characteristics as a hetero- or homo-dimer, which is often classify in five NFκB subunits NFκB1 (p50 and its precursor p105), NFκB2 (p52 and its precursor p100), RelA (p65), RelB, and c-Rel. Among all, p50:p65 is the most studied heterodimers, which are activated through the classical pathway and basically stimulated gene expression. The transcription factor NFκB is often a crucial regulator of numerous cellular functions such as cell survival and inflammation. Interestingly, for this kind of functions the role of p50:p65 dimer is most highlighted. Moreover, cell survival and inflammation stimulation are related to the p50:p65 dimer function.[57] In resting cells NFκB is situated in the cytoplasm as a nonactive complex bound to I-κBa, as an inhibitory protein. When cells become activated through inflammatory stimuli the inhibitory protein is phosphorylated and consequently degraded then, dissociated to provide free NFκB. As a result, free NFκB will translocate into the nucleus where it connected to κB binding sites in the promoter part of target genes. Basically, transcription of pro-inflammatory markers increases through this binding connection. Therefore, suppression of NFκB signaling pathway production might describe the potent activity of M. oleifera bioactive leaves extract as an inhibitor of inflammatory cytokines and mediators. Basically, NFκB factor p65 after stimulation with LPS translocate from the cytoplasm to the nucleus.[31] In the current study, we found that the translocation of NFκB factor p65 was significantly inhibited by M. oleifera bioactive leaves extract in a dose-dependent fashion. It could be recommended that the anti-inflammatory impact of M. oleifera bioactive leaves extract is through NFκB pathway inhibition.
CONCLUSION
Although a few immunotherapy studies on M. oleifera have been reported in the past the anti-inflammatory activity of M. oleifera hydroethanolic bioactive leaves extracts was first explored. The current study demonstrated that M. oleifera hydroethanolic bioactive leaves extract exhibited a remarkable anti-inflammatory effect on LPS induced inflammation in macrophages. Bioactive extract of M. oleifera effectively suppressed iNOS and COX-2 protein expression and also the production of NO and PGE2 stimulated by LPS. Moreover, it decreased the pro-inflammatory cytokines production (TNF-α, IL-1β, and IL-6) induced by LPS in macrophages, and the level of IL-10 was increased through suppression of signaling cascades leading to the activation of NFκB-p65. In addition, this study suggested that the anti-inflammatory activity from bioactive compounds contain in M. oleifera hydroethanolic bioactive leaves extract can promote effective treatment to manage the inflammatory disorder.
Financial support and sponsorship
This work was supported by a research grant from the Ministry of Science, Technology and Innovation of Malaysia under E-science Project No 02-01-04-SF1144 and the Ministry of Agriculture Malaysia under science fund (agriculture section) No. 05-01-04-SF1148.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Sharida Fakurazi
Dr. Sharida Fakurazi, is an Associate Professor at Pharmacology unit, Department of Human Anatomy at Faculty of Medicine and Health Science, Universiti Putra Malaysia (UPM), Malaysia and also holds the post of Head of the Laboratory of Vaccines and Immunotherapeutics, Institute of Bioscience at the same university. She has co-authored over 70 publications in internationally reputed peer-reviewed journals and holds 4 Malaysia patents. The current research interests mainly Drug research on bioactive phyto-constituents against drug induced hepatotoxicity; synthesis, characterize and development of nano-drugs against inflammation, hepato and neuro-toxicity.

Palanisamy Arulselvan
Dr. Palanisamy Arulselvan received his Doctorate in the field of Biochemistry from the University of Madras, India and he was trained as a post-doctoral researcher at Academia Sinica, Taiwan. Currently, he is continuing his scientific research career as a Research Fellow at Institute of Bioscience, Universiti Putra Malaysia, Malaysia. He has published over 50 papers in internationally reputed journals, refereed proceedings and book chapter. His current research focuses on natural products based drug discovery; nano-drug delivery system and role of inflammatory signaling targets in diabetic wound healing.
REFERENCES
- 1.Huo M, Cui X, Xue J, Chi G, Gao R, Deng X, et al. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J Surg Res. 2013;180:e47–54. doi: 10.1016/j.jss.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 2.Kim KN, Ko YJ, Yang HM, Ham YM, Roh SW, Jeon YJ, et al. Anti-inflammatory effect of essential oil and its constituents from fingered citron (Citrus medica L. var. sarcodactylis) through blocking JNK, ERK and NF-κB signaling pathways in LPS-activated RAW 2647 cells. Food Chem Toxicol. 2013;57:126–31. doi: 10.1016/j.fct.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA. Anti-inflammatory agents: Present and future. Cell. 2010;140:935–50. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajput S, Wilber A. Roles of inflammation in cancer initiation, progression, and metastasis. Front Biosci (Schol Ed) 2010;2:176–83. doi: 10.2741/s55. [DOI] [PubMed] [Google Scholar]
- 5.Chu X, Ci X, Wei M, Yang X, Cao Q, Guan M, et al. Licochalcone a inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo . J Agric Food Chem. 2012;60:3947–54. doi: 10.1021/jf2051587. [DOI] [PubMed] [Google Scholar]
- 6.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 7.Naik SR, Wala SM. Inflammation, allergy and asthma, complex immune origin diseases: Mechanisms and therapeutic agents. Recent Pat Inflamm Allergy Drug Discov. 2013;7:62–95. [PubMed] [Google Scholar]
- 8.Alhakmani F, Kumar S, Khan SA. Estimation of total phenolic content, in-vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed. 2013;3:623–7. doi: 10.1016/S2221-1691(13)60126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agnihotri S, Wakode S, Agnihotri A. An overview on anti-inflammatory properties and chemo-profiles of plants used in traditional medicine. Indian J Nat Prod Resour. 2010;1:150–67. [Google Scholar]
- 10.Dutt V, Dutt R, Kumar S, Dhar V. Evaluation of analgesic activity of Solanum platanifolium sims of fruits. Indian Drugs Bombay. 2007;44:405. [Google Scholar]
- 11.Chhabria M, Doda S, Jani M. Synthesis and anti-inflammatory activity of some novel 2-Phenyl-3-Methylthio-3-(substituted) arylamino acrylamide. Indian Drugs Bombay. 2005;42:604. [Google Scholar]
- 12.Park EK, Ryu MH, Kim YH, Lee YA, Lee SH, Woo DH, et al. Anti-inflammatory effects of an ethanolic extract from Clematis mandshurica Rupr. J Ethnopharmacol. 2006;108:142–7. doi: 10.1016/j.jep.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Wei WC, Lin SY, Chen YJ, Wen CC, Huang CY, Palanisamy A, et al. Topical application of marine briarane-type diterpenes effectively inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and dermatitis in murine skin. J Biomed Sci. 2011;18:94. doi: 10.1186/1423-0127-18-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luqman S, Srivastava S, Kumar R, Maurya AK, Chanda D. Experimental assessment of Moringa oleifera Leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/519084. 519084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muangnoi C, Chingsuwanrote P, Praengamthanachoti P, Svasti S, Tuntipopipat S. Moringa oleifera pod inhibits inflammatory mediator production by lipopolysaccharide-stimulated RAW 264.7 murine macrophage cell lines. Inflammation. 2012;35:445–55. doi: 10.1007/s10753-011-9334-4. [DOI] [PubMed] [Google Scholar]
- 16.Muhammad AA, Pauzi NA, Arulselvan P, Abas F, Fakurazi S. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. Biomed Res Int 2013. 2013 doi: 10.1155/2013/974580. 974580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fakurazi S, Sharifudin SA, Arulselvan P. Moringa oleifera hydroethanolic extracts effectively alleviate acetaminophen-induced hepatotoxicity in experimental rats through their anti-oxidant nature. Molecules. 2012;17:8334–50. doi: 10.3390/molecules17078334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar E, Harsha K, Shaik S, Rao NN, Giribabu N. Evaluation of in vitro antioxidant activity and in vivo hepatoprotective activity of Moringa oleifera seeds extract against ethanol induced liver damage in wistar rats. Evaluation. 2013;3:10–5. [Google Scholar]
- 19.Ujah O, Ujah I, Johnson J, Ekam V, Udenze E. Hepatoprotective property of ethanolic leaf extract of Moringa oleifera on carbon tetrachloride (CCl4) induced hepatotoxicity. J Nat Prod Plant Resour. 2013;3:15–22. [Google Scholar]
- 20.Bhattacharya A, Naik MR, Agrawal D, Sahu PK, Kumar S, Mishra SS. CNS Depressant and muscle relaxant effect of ethanolic leaf extract of Moringa oleifera on albino rats. Int J PharmTech Res. 2014;6:1441–9. [Google Scholar]
- 21.Khongrum J, Wattanathorn J, Muchimapura S, Thukhum-Mee W, Thipkaew C, Wannanon P, et al. Moringa oleifera Leaves extract attenuates neuropathic pain induced by chronic constriction injury. Am J Appl Sci. 2012;9:1182–7. [Google Scholar]
- 22.Kirisattayakul W, Wattanathorn J, Tong-Un T, Muchimapura S, Wannanon P. Moringa oleifera Lam mitigates oxidative damage and brain infarct volume in focal cerebral ischemia. Am J Appl Sci. 2012;9:1457–63. [Google Scholar]
- 23.Hannan MA, Kang JY, Mohibbullah M, Hong YK, Lee H. Moringa oleifera with promising neuronal survival and neurite outgrowth promoting potentials. J Ethnopharmacol. 2014;152:142–50. doi: 10.1016/j.jep.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 24.Chumark P, Khunawat P, Sanvarinda Y, Phornchirasilp S, Morales NP, Phivthong-Ngam L, et al. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J Ethnopharmacol. 2008;116:439–46. doi: 10.1016/j.jep.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Amaglo NK, Bennett RN, Lo Curto RB, Rosa EA, Lo Turco V, Giuffrida A, et al. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L. grown in Ghana. Food Chem. 2010;122:1047–54. [Google Scholar]
- 26.Mbikay M. Therapeutic potential of Moringa oleifera Leaves in chronic hyperglycemia and dyslipidemia: A review. Front Pharmacol. 2012;3:24. doi: 10.3389/fphar.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain PG, Patil SD, Haswani NG, Girase MV, Surana SJ. Hypolipidemic activity of Moringa oleifera Lam. Moringaceae, on high fat diet induced hyperlipidemia in albino rats. Rev Bras Farmacognosia. 2010;20:969–73. [Google Scholar]
- 28.Lin X, Racette SB, Lefevre M, Spearie CA, Most M, Ma L, et al. The effects of phytosterols present in natural food matrices on cholesterol metabolism and LDL-cholesterol: A controlled feeding trial. Eur J Clin Nutr. 2010;64:1481–7. doi: 10.1038/ejcn.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preedy VR, Watson RR, Patel VB. London, Burlington, San Diego: Academic Press; 2011. Nuts and Seeds in Health and Disease Prevention. [Google Scholar]
- 30.Zhao L, Zhang SL, Tao JY, Pang R, Jin F, Guo YJ, et al. Preliminary exploration on anti-inflammatory mechanism of Corilagin (beta-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-D-glucose) in vitro . Int Immunopharmacol. 2008;8:1059–64. doi: 10.1016/j.intimp.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Xiong H, Liu L. Effects of taraxasterol on inflammatory responses in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol. 2012;141:206–11. doi: 10.1016/j.jep.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Sellamuthu PS, Arulselvan P, Kamalraj S, Fakurazi S, Kandasamy M. Protective nature of mangiferin on oxidative stress and antioxidant status in tissues of streptozotocin-induced diabetic rats. ISRN Pharmacol 2013. 2013 doi: 10.1155/2013/750109. 750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YT, Wen CC, Chen YH, Huang WC, Huang LT, Lin WC, et al. Dietary uptake of Wedelia chinensis extract attenuates dextran sulfate sodium-induced colitis in mice. PLoS One. 2013;8:e64152. doi: 10.1371/journal.pone.0064152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fakurazi S, Hairuszah I, Nanthini U. Moringa oleifera Lam prevents acetaminophen induced liver injury through restoration of glutathione level. Food Chem Toxicol. 2008;46:2611–5. doi: 10.1016/j.fct.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Karthivashan G, Tangestani Fard M, Arulselvan P, Abas F, Fakurazi S. Identification of bioactive candidate compounds responsible for oxidative challenge from hydro-ethanolic extract of Moringa oleifera leaves. J Food Sci. 2013;78:C1368–75. doi: 10.1111/1750-3841.12233. [DOI] [PubMed] [Google Scholar]
- 36.Mehta JP, Parmar PH, Vadia SH, Patel MK, Tripathi CB. In vitro antioxidant and in vivo anti-inflammatory activities of aerial parts of Cassia species. Arabian J Chem. 2013 doi: 10.1016/jarabjc201306010. [Google Scholar]
- 37.Siriwatanametanon N, Fiebich BL, Efferth T, Prieto JM, Heinrich M. Traditionally used Thai medicinal plants: In vitro anti-inflammatory, anticancer and antioxidant activities. J Ethnopharmacol. 2010;130:196–207. doi: 10.1016/j.jep.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 38.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–85. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 40.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araújo LC, Aguiar JS, Napoleão TH, Mota FV, Barros AL, Moura MC, et al. Evaluation of cytotoxic and anti-inflammatory activities of extracts and lectins from Moringa oleifera seeds. PLoS One. 2013;9(8):e81973. doi: 10.1371/journal.pone.0081973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong H, Cheng Y, Zhang X, Zhang X. Effects of taraxasterol on iNOS and COX-2 expression in LPS-induced RAW 264.7 macrophages. J Ethnopharmacol. 2014;155:753–7. doi: 10.1016/j.jep.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Yuan F, Chen J, Sun PP, Guan S, Xu J. Wedelolactone inhibits LPS-induced pro-inflammation via NF-kappaB pathway in RAW 264.7 cells. J Biomed Sci. 2013;20:84. doi: 10.1186/1423-0127-20-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soromou LW, Zhang Z, Li R, Chen N, Guo W, Huo M, et al. Regulation of inflammatory cytokines in lipopolysaccharide-stimulated RAW 264.7 murine macrophage by 7-O-methyl-naringenin. Molecules. 2012;17:3574–85. doi: 10.3390/molecules17033574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae DS, Kim YH, Pan CH, Nho CW, Samdan J, Yansan J, et al. Protopine reduces the inflammatory activity of lipopolysaccharide-stimulated murine macrophages. BMB Rep. 2012;45:108–13. doi: 10.5483/BMBRep.2012.45.2.108. [DOI] [PubMed] [Google Scholar]
- 46.Li C, Wang MH. Anti-inflammatory effect of the water fraction from hawthorn fruit on LPS-stimulated RAW 264.7 cells. Nutr Res Pract. 2011;5:101–6. doi: 10.4162/nrp.2011.5.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–87. [PubMed] [Google Scholar]
- 48.Lee HN, Cho HJ, Lim do Y, Kang YH, Lee KW, Park JH. Mechanisms by which licochalcone e exhibits potent anti-inflammatory properties: Studies with phorbol ester-treated mouse skin and lipopolysaccharide-stimulated murine macrophages. Int J Mol Sci. 2013;14:10926–43. doi: 10.3390/ijms140610926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang QS, Cui YL, Dong TJ, Zhang XF, Lin KM. Ethanol extract from a Chinese herbal formula, “Zuojin Pill”, inhibit the expression of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 mouse macrophages. J Ethnopharmacol. 2012;141:377–85. doi: 10.1016/j.jep.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 50.Legler DF, Bruckner M, Uetz-von Allmen E, Krause P. Prostaglandin E2 at new glance: Novel insights in functional diversity offer therapeutic chances. Int J Biochem Cell Biol. 2010;42:198–201. doi: 10.1016/j.biocel.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Nahar PP, Driscoll MV, Li L, Slitt AL, Seeram NP. Phenolic mediated anti-inflammatory properties of a maple syrup extract in RAW 264.7 murine macrophages. J Funct Foods. 2014;6:126–36. [Google Scholar]
- 52.Jeong JB, Hong SC, Jeong HJ, Koo JS. Anti-inflammatory effect of 2-methoxy-4-vinylphenol via the suppression of NF-kB and MAPK activation, and acetylation of histone H3. Arch Pharm Res. 2011;34:2109–16. doi: 10.1007/s12272-011-1214-9. [DOI] [PubMed] [Google Scholar]
- 53.Allantaz F, Chaussabel D, Banchereau J, Pascual V. Microarray-based identification of novel biomarkers in IL-1-mediated diseases. Curr Opin Immunol. 2007;19:623–32. doi: 10.1016/j.coi.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonelli A, Ferri C, Ferrari SM, Ghiri E, Goglia F, Pampana A, et al. Serum levels of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor alpha in mixed cryoglobulinemia. Arthritis Rheum. 2009;60:3841–7. doi: 10.1002/art.25003. [DOI] [PubMed] [Google Scholar]
- 55.Wang T, Wu F, Jin Z, Zhai Z, Wang Y, Tu B, et al. Plumbagin inhibits LPS-induced inflammation through the inactivation of the nuclear factor-kappa B and mitogen activated protein kinase signaling pathways in RAW 264.7 cells. Food Chem Toxicol. 2014;64:177–83. doi: 10.1016/j.fct.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 56.Yoon SB, Lee YJ, Park SK, Kim HC, Bae H, Kim HM, et al. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J Ethnopharmacol. 2009;125:286–90. doi: 10.1016/j.jep.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 57.Pereira SG, Oakley F. Nuclear factor-κB1: Regulation and function. Int J Biochem Cell Biol. 2008;40:1425–30. doi: 10.1016/j.biocel.2007.05.004. [DOI] [PubMed] [Google Scholar]