Abstract
Background:
Plant species from genus Carduus are widely distributed in the world and represented in Bulgaria by 14 species. Previous investigations on this genus demonstrated a strong antioxidant potential of extract from some Bulgarian Carduus species.
Objective:
The present study investigates the phenolic profile and the antioxidant potential of different extracts obtained from four endemic Compositae herbs, growing wild in Bulgaria: Carduus armatus Boiss and Heldr., Carduus candicans Waldst. et Kit ssp. globifer (Velen.) Kazmi., Carduus rhodopaeus Velen. and Carduus thracicus (Velen.) Hayek.
Materials and Methods:
Antioxidant capacity of the obtained extracts was estimated with 2,2-diphenyl-1-picrylhydrazyl, 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid), and ferric reducing antioxidant power and copper reduction antioxidant assays. Phenolic profile was estimated by high performance liquid chromatography.
Results:
Eleven phenolic acids and eight flavonoids were quantified in the inflorescences. Sinapic (2760.72 ± 15.68 μg/g dry weight [dw]), chlorogenic (2564.50 ± 19.73 μg/g dw) and ferulic acids (1648.71 ± 19.57 μg/g dw), as well as luteolin (2345.45 ± 18.61 μg/g dw) and apigenin (1332.75 ± 12.05 μg/g dw) were found to be the predominant compounds. The above contents are the highest values found in C. candicans ssp. globifer. The highest established antioxidant activity (AOA) was in favor of the ethanolic extracts, and the extract of C. rhodopaeus affirmed with the highest AOA among the investigated plant species.
Conclusion:
All identified phenolic compounds were reported for the 1st time in the studied endemic Carduus species, as well as their antioxidant capacities. The present study revealed that these plant species could be used as sources of antioxidants with potential medicinal properties.
SUMMARY
Phenolic acids and flavonoid profiles of four endemic compositae herbs, growing wild in Bulgaria: Carduus armatus, Carduus candicans ssp. globifer, Carduus rhodopaeus and Carduus thracicus, were quantified for the first time by high performance liquid chromatography (HPLC). Eleven phenolic acids and eight flavonoids were determined in the inflorescences. Sinapic, chlorogenic and ferulic acids, as well as luteolin and apigenin were found to be the predominant compounds. The highest values of established phenolic compounds were found in C. candicans ssp. globifer. The studied plant extracts of Carduus species possessed antioxidant activity in favor of C. rhodopaeus and results confirmed 70 % ethanol as more appropriate solvent. The present study revealed that these plant species could be used as sources of antioxidants with potential medicinal properties.
Keywords: Carduus species, high-performance liquid chromatography, in vitro antioxidant activity, phenolic profile
INTRODUCTION
Polyphenols are secondary metabolites of plants and are generally involved in defense against ultraviolet radiation or aggression by pathogens.[1] In food, polyphenols may contribute to the bitterness, astringency, color, flavor, odor, and oxidative stability. Polyphenols and other food phenolics are currently subjects of increasing scientific interest because of their possible beneficial effects on human health. Toward the end of 20th century, epidemiological studies and associated meta-analyses strongly suggested that long-term consumption of diets rich in plant polyphenols offered some protection against development of cancers, cardiovascular diseases, diabetes, osteoporosis, and neurodegenerative diseases.[2,3] Plant species from genus Carduus belongs to family Asteraceae. It is widely distributed in Europe and represented in Bulgaria by 14 species.[4,5] Some species of this plant genus are well-known herbs and have been used in phytotherapy as diuretic, cardiotonic, liver tonic, and antihemoroidal remedy.[6]
The flavonoids are more exhaustive studied phenolic compounds (present as different aglycons and glycosides attached) in different Carduus species,[7,8,9,10] but the profile of phenolic acids is less studied for this plant genus. First data for the presence of phenolic acids in genus Carduus was showed by Liu et al.,[11] for Carduus acanthoides and Slavov et al.,[12] for Carduus thoermeri.
As a result of the conducted phytochemical screening of Bulgarian Carduus L. species in terms of main phenolic compounds content, flavonoids, and total phenols were revealed as dominant constituents, followed by phenolic acids and anthocyanins.[13] Furthermore, the screening of Carduus species in terms of antioxidant activity (AOA) revealed that the species C. thoermeri and Carduus candicans ssp. globifer are more potent and could be evaluated as sources of antioxidants.[14,15]
The aim of the present investigation was to establish the phenolic profile (flavonoids and phenolic acids), to examine the total phenolic content, and to evaluate the related total antioxidant potential in four endemic Carduus species, which showed good quantitative values according our previous screening studies. Thus, this research will enrich the scanty available information on these plant species and outlines the promising potential practical application.
MATERIALS AND METHODS
Plant material
The plant materials (inflorescences) were collected from natural habitats from Bulgaria, during the 2011–2013 vegetative seasons [Table 1]. The collected plant materials were air-dried in darkness at room temperature. Species identification was carried out at the Department of Botany of University of Plovdiv “Paisij Hilendareski,” according to Tutin et al.,[4] and Delipavlov and Cheshmedzhiev.[5] Voucher specimens of the different species were deposited in the herbarium at the Agriculture University of Plovdiv, Bulgaria (Herbarium SOA).
Table 1.
Collection locality, floristic region, altitude, and voucher specimen number of the studied Carduus species

Preparation of plant extracts
Dried plant material of all four Carduus species was grounded and 0.5 g of the accurately weighed sample was refluxed exhaustively triple with 70% (v/v) methanol at 70°C (raw material to solvent 1:20) for 30 min. The extracts were combined and made up to 30 ml with methanol in a volumetric flask.
High-performance liquid chromatography analysis
The high performance liquid chromatography (HPLC) analysis of phenolic acids and flavonoids were performed by using Waters 1525 Binary Pump HPLC systems (Waters, Milford, MA, USA), equipped with Waters 2484 dual Absorbance Detector (Waters, Milford, MA, USA) and Supelco Discovery HS C18 column (5 μm, 25 cm × 4.6 mm), operated under control of Breeze 3.30 software. Detailed conditions of HPLC analyses are reported previously.[16] Concentration of each individual compound was calculated based on the external standard method and was converted to microgram compound per gram dry weight (dw).
Total polyphenol content determination
The total polyphenol content (TPC) was analyzed using the Folin–Ciocalteu method[17] with some modifications.[18] Each sample (1 ml) was mixed with 5 ml of Folin–Ciocalteu phenol reagent and 4 ml of 7.5% Na2CO3. The mixture was then vortexed well and left for 5 min at 50°C. After incubation, the absorbance was measured at 765 nm. The TPC in the extracts was expressed as milligram gallic acid equivalent (GAE) per gram dw.
2,2-diphenyl-1-picrylhydrazyl free-radical scavenging activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was determined following the described method[19] which was slightly modified.[18] Freshly prepared 4 × 10−4 M methanolic solution of DPPH was mixed with the samples in a ratio of 2:0.5 (v/v). The light absorption was measured at 517 nm. The DPPH radical scavenging activity was presented as a function of the concentration of Trolox - Trolox equivalent antioxidant capacity (TEAC) and was defined as the concentration of Trolox having equivalent AOA expressed as μM/Trolox equivalent (TE) gram dw.
2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical cation decolorization assay
The scavenging activity of the extract against radical cation 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS·+) was estimated according to Re et al.,[20] Briefly, ABTS was dissolved to a 7 mM concentration. ABTS radical cation (ABTS·+) was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate (final concentration) and allowing the mixture to stand in the dark at room temperature for 12–16 h before use. Afterward, the ABTS·+ solution was diluted with ethanol to an absorbance of 0.7 ± 0.02 at 734 nm and equilibrated at 30°C. After the addition of 1.0 ml of diluted ABTS·+ solution to 10 ml of samples, the absorbance reading was taken at 30°C after 6 min. The results were expressed as TEAC value (μM/TE gram dw).
Ferric reducing antioxidant power assay
The ferric reducing antioxidant power (FRAP) assay was carried out according to the procedure of Benzie and Strain[21] with some changes.[18] The FRAP reagent was prepared fresh daily and was warmed to 37°C prior to use. One-hundred and fifty microliters of plant extracts were allowed to react with 2850 μl of the FRAP reagent for 4 min at 37°C, and the absorbance was recorded at 593 nm. The results were expressed as μM/TE gram dw.
Copper reduction antioxidant assay
Copper reduction antioxidant (CUPRAC) assay was performed according to the method of Ak and Gülçin.[22] To a test tube were added 1 ml of CuCl2 solution (1.0 × 10−2 M), 1 ml of neocuproine methanolic solution (7.5 × 10−3 M), and 1 ml NH4 Ac buffer solution (pH 7.0), and mixed with 0.1 ml of herbal extract (sample) followed by 1 ml of water were added (total volume = 4.1 ml) and mixed well. Absorbance against a reagent blank was measured at 450 nm after 30 min. Trolox was used as a standard and total antioxidant capacity of extracts was expressed as μM/TE gram dw.
Statistical analysis
The presented results are average from two independent experiments carried out in triplicates. The results were expressed as mean ± standard deviation.
RESULTS AND DISCUSSION
Flavonoid and phenolic acid profile
Flavonoids as a main class of phenolic compounds demonstrate a wide range of biochemical and pharmacological effects.[23,24]
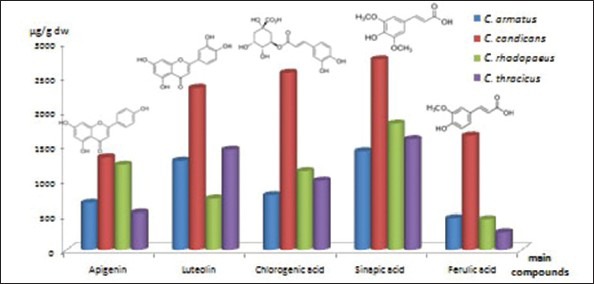
In the present study, the methanol extract obtained from flower heads of the species was subjected to HPLC analysis for the presence of flavonoids. The analysis showed eight flavonoids and flavonoid glycosides in studied Carduus species. Among all established flavonoid, aglycones luteolin and apigenin were found to be with the highest content [Table 2].
Table 2.
Content of flavonoids (aglycons and glycosides) in inflorescences of Carduus species, (μg/g dw)
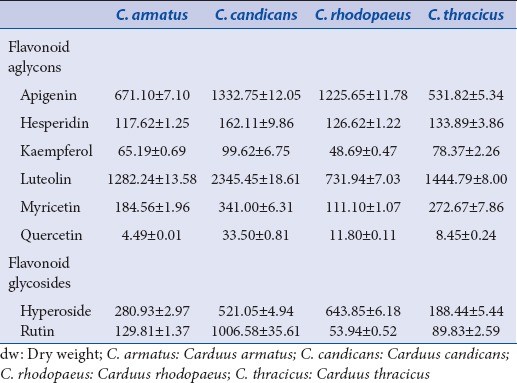
Luteolin was the predominant compound in C. candicans (2345.45 ± 18.61 μg/g dw), followed by Carduus thracicus (1444.79 ± 8.00 μg/g dw) and Carduus armatus (1282.24 ± 13.58 μg/g dw) extracts, respectively. Luteolin expresses antihypertensive,[25] anti-inflammatory,[26] and anticancer activity.[27] Apigenin was evaluated in similar concentrations in C. candicans and Carduus rhodopaeus extracts (1332.75 ± 12.05 and 1225.65 ± 11.78 μg/g dw, respectively), as well as in C. armatus and C. thracicus extracts (671.10 ± 7.10 and 531.82 ± 5.34 μg/g dw, respectively). As antioxidant apigenin significantly induced glutathione transferase it is a protectant against cardiotoxic agents.[28]
The glycosides hyperoside and rutin were detected to be present in all four species, as the highest concentration of rutin was established in C. candicans extract - 1006.58 ± 35.61 μg/g dw and of hyperoside in C. rhodopaeus extract - 643.85 ± 6.18 μg/g dw [Table 2].
In brief, all identified flavonoids were reported for the 1st time in the investigated Carduus species. However, in comparison with the study established by Slavov et al.,[12] for the profile of C. thoermeri, the concentrations of luteolin and myricetin were higher in the present study of endemic Carduus species (especially in C. candicans - 4–5 time higher, respectively).
Eleven phenolic acids were identified in the investigated extracts, except C. candicans extract, where they were 10 [Table 3]. Sinapic, ferulic, and chlorogenic acids were the main phenolic acids identified in the investigated extracts. The presence of caffeic acid, p-coumaric acid, 2-hydroxybenzoic acid, vanillic acid, syringic acid, gallic acid, cinnamic acid, and 3,4-dihydroxybenzoic acid was also established.
Table 3.
Content of phenolic acids in inflorescences of Carduus species, (μg/g dw)
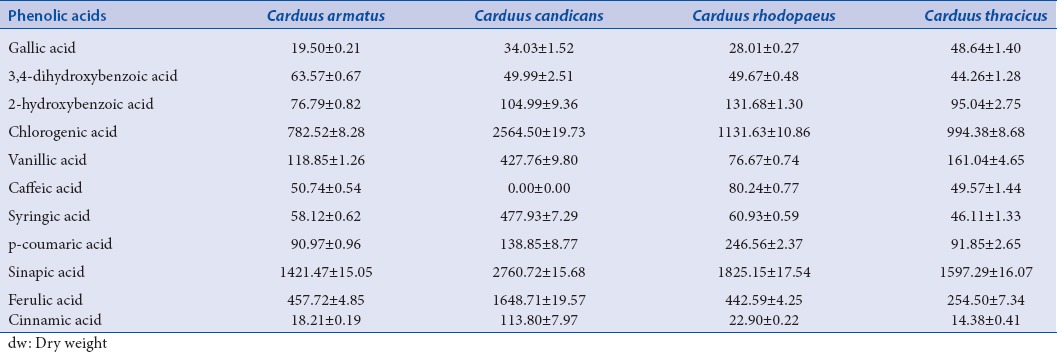
In C. candicans extract, sinapic, chlorogenic, and ferulic acids were with the highest concentrations (2760.72 ± 15.68, 2564.50 ± 19.73 and 1648.71 ± 19.57 μg/g dw, respectively), followed by C. rhodopaeus, C. thracicus, and C. armatus [Table 3]. However, relatively high concentrations of syringic acid (477.93 ± 7.29 –g/g dw) and vanillic acid (427.76 ± 9.80 μg/g dw) in C. candicans, as well of p-coumaric acid (246.56 ± 2.37 μg/g dw) in C. rhodopaeus were established. Comparatively, Slavov et al.,[12] reported sinapic, ferulic, and chlorogenic acids as predominant acids in C. thoermeri, as well.
Ferulic and sinapic acids as natural phenolic compounds are known for free-radical scavenger and reactive toward free-radicals.[29,30] Other studies suggest that ferulic acid may have antitumor activity.[31,32] Chlorogenic acid possess other pharmacological activities such as antihypertensive effect,[33] improved lipid and glucose metabolism,[34] protection of dopaminergic neurons in neuroinflammatory conditions,[35] and also antitumor activity.[31] Briefly, in the present investigation, the above-mentioned phenolic acids were established and quantified for the 1st time for the studied species.
Free-radical scavenging activity
Nowadays, the current focus of researchers is toward natural antioxidants, especially polyphenolics with plant origin. More than one extraction system is recommendable for the detailed assessment of the antioxidant properties of plants; therefore, we investigated two types of solvent extraction.
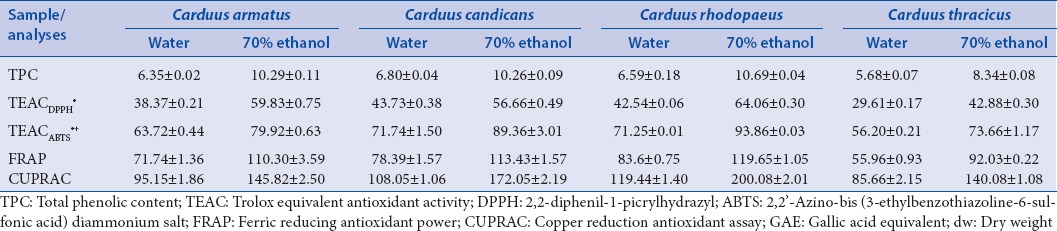
Since polyphenols contribute significantly to the overall antioxidant capacity, it was reasonable for us to determine their total amount in the investigated extracts of C. armatus, C. candicans, C. rhodopaeus, and C. thracicus. The established total phenolic compounds concentrations by Folin-Ciocalteu's method varied widely and are shown in Table 4. By comparing the used solvents, better results were established by 70% ethanol extracts. However, the best result among the investigated samples was found for the C. rhodopaeus extracts 10.69 ± 0.04 mg GAE/g dw. It has to be noted that the other investigated species (excl. C. thracicus) showed quite similar results in prevalence of the 70% ethanol as the extraction solvent.
Table 4.
Total phenol content (mg GAE/g dw) and in vitro antioxidant activity (μM/TE g dw) in inflorescences of Carduus species
The observed differences could be explained by the different polarity of the polyphenol compounds present in the investigated plant species. These findings are in agreement with the previously reported by Kratchanova et al.,[36] significant influence of the extraction solvent over the efficiency of the polyphenol content in the extract.
It is known that phenolics have various functions in plants. The role of polyphenols in preventing many chronic diseases including cancer, cardiovascular diseases, and diabetes has been well-documented.[37] Therefore, the presence of those compounds in the studied extract suggests their important role in the plant and might contribute toward its probable AOA.
In order to investigate the AOA, experiments with two stable radicals DPPH· and ABTS·+ were conducted. In addition, the FRAP and copper reduction (CUPRAC) assays were also performed. The results were expressed as TEAC-values [Table 4]. TEACDPPH· values were in range of 29.61 ± 0.17–64.06 ± 0.30 μM/TE g dw and the TEACABTS·+ values were in range from 56.20 ± 0.21 to 93.86 ± 0.03 μM/TE g dw, respectively. Higher TEAC value indicates that a sample has a stronger antioxidant potential. Therefore, based on the results it can be conducted in agreement with the total polyphenolic content that the best effectiveness was achieved by extracting C. rhodopaeus with 70% ethanol.
In addition, the same tendency was observed for the FRAP values of the investigated extracts of Carduus species as the results were in range from 55.96 ± 0.93 to 119.65 ± 1.05 μM/TE g dw, respectively. Using the CUPRAC assay the highest values were found to be 200.08 ± 2.01 μM/TE g dw [Table 4] by the C. rhodopaeus 70% ethanol extract, corresponding to the results mentioned already in this research. Thus, the ethanol extracts of C. rhodopaeus definitely affirmed with the highest AOA among the investigated plant species in relation to the estimated polyphenolic content.
The results of all conducted antioxidant capacity assays showed that the investigated extracts possessed AOA, which for the 70% ethanol extracts was higher than the capacity of the water ones. This confirmed the results obtained from the TPC assay. Therefore, it can be concluded that the 70% ethanol is more efficient as a solvent in order to obtain extracts with higher content of biologically active substances in terms of AOA. This is in agreement with the study reported by Sultana et al.,[38] for better results achieved with aqueous organic solvent.
A slight difference among the results of the applied assays was observed. Interestingly, the highest antioxidant values were measured by the CUPRAC assay. This could be explained by the unique mechanism and the unequal sensitivity of each method applied. The authors, therefore, strongly suggested that, when analyzing the AOA of samples, it is better to use at least two methods due to the differences between the test systems.[39]
CONCLUSIONS
In the present study, the phenolic acids and flavonoid profiles of C. armatus, C. candicans ssp. globifer, Carduus rhodopaeus, and C. thracicus from Bulgaria were investigated and quantified for the 1st time. The main compounds established were sinapic, chlorogenic, and ferulic acids, and the flavonoids luteolin, apigenin, and rutin as well using HPLC-methods. Among all investigated species, C. candicans ssp. globifer was with the highest concentration of identified phenolic compounds. The studied Carduus species extracts revealed AOA with in favor of Bulgarian endemic C. rhodopaeus. The detailed investigation confirmed 70% ethanol as a more appropriate solvent according to all conducted assays.
The present study revealed that the investigated Carduus species could be used as sources of bioactive compounds with potential medicinal properties, in particular, the AOA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Ivanka Dimitrova-Dyulgerova
Dr. Ivanka Dimitrova-Dyulgerova, Assoc. Prof., Department of Botany, Faculty of Biology, Plovdiv University “Paisii Hilendarski”, Plovdiv, Bulgaria Scientific area: Botany, phytoindication, medicinal plants and herbs.

Iliya Zhelev
Iliya Zhelev, Assist. Prof., Department of Pharmaceutical Technologies, Faculty of Pharmacy, Medicinal University of Varna, Varna, Bulgaria Scientific area: Pharmacognosy.

Dasha Mihaylova
Dr. Dasha Mihaylova, Assoc. Prof., Department of Biotechnology, University of food Technologies, Plovdiv, Bulgaria Scientific area: Phytochemistry of medicinal plants and herbs, bioactive substances.
Acknowledgments
The authors gratefully acknowledge the Department of Industrial Microbiology, Laboratory of Applied Biotechnologies, The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences for their technical help in HPLC analyses.
REFERENCES
- 1.Beckman CH. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants. Physiol Mol Plant Pathol. 2000;57:101–10. [Google Scholar]
- 2.Graf BA, Milbury PE, Blumberg JB. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J Med Food. 2005;8:281–90. doi: 10.1089/jmf.2005.8.281. [DOI] [PubMed] [Google Scholar]
- 3.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S–25. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 4.Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, et al., editors. Flora Europaea. Plantaginaceae to Compositae (and Rubiaceae) Vol. 4. Cambridge: Cambridge University Press; 1976. [Google Scholar]
- 5.Delipavlov D, Cheshmedzhiev I. Key to the plants in Bulgaria. Plovdiv (Bulgaria): Academic Publishing House of Agricultural University; 2003. [Google Scholar]
- 6.Petkov V. Modern Phytotherapy. Sofia (Bulgaria): State Publishing House “Medicina i Fizkultura”;; 1982. [Google Scholar]
- 7.Bain JF, Desrochers AM. Flavonoids of Carduus nutans and Carduus acanthoides. Biochem Syst Ecol. 1988;16:265–8. [Google Scholar]
- 8.Jordon-Thaden IE, Louda SM. Chemistry of Cirsium and Carduus: A role in ecological risk assessment for biological control of weeds. Biochem Syst Ecol. 2003;31:1353–96. [Google Scholar]
- 9.Terentjev SV, Krasnov EA. Coumarins and flavonoids of above-ground part of Carduus crispus L. and Carduus nutans L. Rastitelnye Resursy. 2003;39:55–64. [Google Scholar]
- 10.Kozyra M, Głowniak K, Boguszewska M. The analysis of flavonoids in the flowering herbs of Carduus acanthoides L. Curr Issues Pharm Med Sci. 2013;26:10–5. [Google Scholar]
- 11.Liu SK, Que S, Cheng W, Zhang QY, Liang H. Chemical constituents from whole plants of Carduus acanthoides. Zhongguo Zhong Yao Za Zhi. 2013;38:2334–7. [PubMed] [Google Scholar]
- 12.Slavov I, Mihaylova D, Dimitrova-Dyulgerova I. Phenolic acids, flavonoids profile and antioxidant activity of Carduus thoermeri Weinm. extract. Oxid Commun. 2014;37:247–53. [Google Scholar]
- 13.Zhelev I, Dimitrova-Dyulgerova I, Belkinova D, Mladenov R. Content of phenolic compound in the genus Carduus L. from Bulgaria. Ecologia Balkanica. 2013;5:13–21. [Google Scholar]
- 14.Zheleva-Dimitrova D, Zhelev I, Dimitrova-Dyulgerova I. Antioxidant activity of some Carduus species growing in Bulgaria. Free Radic Antioxid. 2011;1:14–9. [Google Scholar]
- 15.Mihaylova D, Georgieva L, Zhelev I, Dimitrova-Dyulgerova I. Influence of the extraction conditions over the antioxidant activity of Carduus thoermeri. Bulg J Agric Sci. 2013;19:61–4. [Google Scholar]
- 16.Marchev A, Georgiev V, Ivanov I, Badjakov I, Pavlov A. Two-phase temporary immersion system for Agrobacterium rhizogenes genetic transformation of sage (Salvia tomentosa Mill.) Biotechnol Lett. 2011;33:1873–8. doi: 10.1007/s10529-011-0625-5. [DOI] [PubMed] [Google Scholar]
- 17.Kujala TS, Loponen JM, Klika KD, Pihlaja K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J Agric Food Chem. 2000;48:5338–42. doi: 10.1021/jf000523q. [DOI] [PubMed] [Google Scholar]
- 18.Mihaylova D, Lante A, Krastanov A. Total phenolic content, antioxidant and antimicrobial activity of Haberlea rhodopensis extracts obtained by pressurized liquid extraction. Acta Aliment Hung. 2014 [Google Scholar]
- 19.Brand-Williams W, Cuvelier M, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995;28:25–30. [Google Scholar]
- 20.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 21.Benzie IF, Strain JJ. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 22.Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Cooks N, Samman S. Flavonoids – Chemistry, metabolism, cardioprotective effects and dietary sources. J Nutr Biochem. 1996;7:66–76. [Google Scholar]
- 24.Agrawal A. Pharmacological activities of flavonoids: A review. Int J Pharm Sci Nanotechnol. 2011;4:1394–98. [Google Scholar]
- 25.Shen C, Kuan Y, Lee S, Yang M, Kuo H, Chiu Y. The protective effect of luteolin on cytotoxicity and genotoxicity of bisphenol-A-glycidyldimethacrylate in macrophages involved in DNA damage and caspases activation. Life Sci J. 2013;10:3137–42. [Google Scholar]
- 26.Odontuya G, Hoult JR, Houghton PJ. Structure-activity relationship for antiinflammatory effect of luteolin and its derived glycosides. Phytother Res. 2005;19:782–6. doi: 10.1002/ptr.1723. [DOI] [PubMed] [Google Scholar]
- 27.Byun S, Lee KW, Jung SK, Lee EJ, Hwang MK, Lim SH, et al. Luteolin inhibits protein kinase C (epsilon) and c-Src activities and UVB-induced skin cancer. Cancer Res. 2010;70:2415–23. doi: 10.1158/0008-5472.CAN-09-4093. [DOI] [PubMed] [Google Scholar]
- 28.Breinholt V, Lauridsen ST, Dragsted LO. Differential effects of dietary flavonoids on drug metabolizing and antioxidant enzymes in female rat. Xenobiotica. 1999;29:1227–40. doi: 10.1080/004982599237903. [DOI] [PubMed] [Google Scholar]
- 29.Yoon BH, Jung JW, Lee JJ, Cho YW, Jang CG, Jin C, et al. Anxiolytic-like effects of sinapic acid in mice. Life Sci. 2007;81:234–40. doi: 10.1016/j.lfs.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Valentão P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, Bastos ML. Antioxidant activity of Centaurium erythraea infusion evidenced by its superoxide radical scavenging and xanthine oxidase inhibitory activity. J Agric Food Chem. 2001;49:3476–9. doi: 10.1021/jf001145s. [DOI] [PubMed] [Google Scholar]
- 31.Huang MT, Smart RC, Wong CQ, Conney AH. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48:5941–6. [PubMed] [Google Scholar]
- 32.Li W, Li N, Tang Y, Li B, Liu L, Zhang X, Fu H, Duan JA. Biological activity evaluation and structure – activity relationship analysis of ferulic acid and caffeic acid derivatives for anticancer. Bioorg Med Chem Lett. 2012;22:6085–8. doi: 10.1016/j.bmcl.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y, Wang J, Ballevre O, Luo H, Zhang W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens Res. 2012;35:370–4. doi: 10.1038/hr.2011.195. [DOI] [PubMed] [Google Scholar]
- 34.Ong KW, Hsu A, Tan BK. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem Pharmacol. 2013;85:1341–51. doi: 10.1016/j.bcp.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Shen C, Kuan Y, Lee S, Yang M, Kuo H, Chiu Y. The protective effect of luteolin on cytotoxicity and genotoxicity of bisphenol-A-glycidyldimethacrylate in macrophages involved in DNA damage and caspases activation. Life Sci J. 2013;10:3137–42. [Google Scholar]
- 36.Kratchanova M, Denev P, Ciz M, Lojek A, Mihailov A. Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems. Acta Biochim Pol. 2010;57:229–34. [PubMed] [Google Scholar]
- 37.Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: Antioxidants and beyond. Am J Clin Nutr. 2005;81:215S–17. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 38.Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–80. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlesier K, Harwat M, Böhm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Radic Res. 2002;36:177–87. doi: 10.1080/10715760290006411. [DOI] [PubMed] [Google Scholar]


