Abstract
Objective:
Xanthii fructus (Compositae) is a traditional herbal medicine used for treating headache, toothache, pruritus, empyema, and rhinitis. In this study of the quality control of X. fructus, we performed simultaneous analysis of nine marker compounds: Protocatechuic acid (1), chlorogenic acid (2), caffeic acid (3), 4,5-dicaffeoylquinic acid (4), ferulic acid (5), 3,5-dicaffeoylquinic acid (6), 1,3-dicaffeoylquinic acid (7), 1,4-dicaffeoylquinic acid (8), and 4,5-dicaffeoylquinic acid (9).
Materials and Methods:
Nine components were separated using reversed-phase SunFire™ C18 analytical column and analyzed using high-performance liquid chromatography. We examined the biological effects of the nine marker compounds by determining their anti-inflammatory activities in the murine macrophage cell line RAW 264.7.
Results:
Among the nine marker compounds, eight significantly inhibited lipopolysaccharide (LPS)-stimulated tumor necrosis factor-alpha (TNF-α) production. 1, 3, 5 had significant inhibitory effects on LPS-induced prostaglandin E2 (PGE2) production in RAW 264.7 cells. None of the tested marker compounds had a significant effect on interleukin-6 production in LPS-treated RAW 264.7 cells. Our data demonstrated that each marker compound from X. fructus exerts anti-inflammatory activity by targeting different inflammation-related pathways such as the TNF-α or PGE2 pathway.
Conclusion:
Further experiments using in vitro and in vivo models are needed to identify the mechanisms responsible for the anti-inflammatory properties of each marker compound.
SUMMARY
Simultaneous analysis of nine phenylpropanoids in the Xanthii fructus was established using HPLC-PDA system.
1,4-dicaffeoylquinic acid significantly inhibited LPS-stimulated TNF-a production.
Protocatechuic acid, caffeic acid and ferulic acid had significant inhibitory effects on LPS-induced PGE2 production in RAW 264.7 cells.

Keywords: Anti-inflammatory effect, herbal medicine, high-performance liquid chromatography, RAW 264.7, Xanthii fructus
INTRODUCTION
Xanthii fructus (Compositae) is derived from the fruit of Xanthium strumarium L., which is widely distributed in Asian countries such as Korea, Japan, and China.[1] Some investigations showed that X. fructus has a variety of pharmacological properties regarding antioxidant, anti-cancer, and analgesic effects.[2,3] Phytochemical studies of X. fructus have identified carboxyatractyloside, xanthanol, alkaloids, thiazinediones, and phenylpropanoids.[2,3,4] Among these compounds, we simultaneously examined nine phenylpropanoid marker compounds for quality control of X. fructus extracts using high-performance liquid chromatography (HPLC).
Inflammation is a complex immuno- and biological-reaction against various harmful stimuli.[5] Activated macrophages by inflammatory stimulator lead to produce pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6.[6] Since pro-inflammatory cytokines have been considered as the attractive target molecule(s) for treating inflammatory diseases,[7] it is important to examine the effects of the drug on their production. Prostaglandin E2 (PGE2) also plays a crucial role in the regulation of inflammatory response.[8] PGE2 is synthesized by cyclooxygenase (COX)-2 that is mediated by inflammatory stimuli.
In recent years, nonsteroidal anti-inflammatory drugs are most popularly used for treating inflammatory disorders. However, several adverse effects such as gastrointestinal problems and damage of central nervous system can limit therapeutic effects.[9] To overcome these problems, recent research has been focused on the anti-inflammatory action of phytochemical (s).[10,11,12,13] In our study, we investigated anti-inflammatory effects of nine marker compounds from X. fructus. Inflammatory reaction in macrophages was induced by stimulating with lipopolysaccharide (LPS) and analyzed production of major inflammation markers such as TNF-α, IL-6, and PGE2.
MATERIALS AND METHODS
Plant materials
The X. fructus used in this study was purchased from HMAX (Jecheon, Korea) in October 2008. The botanical origin of this sample was taxonomically confirmed by Professor Je-Hyun Lee, Dongguk University, Gyeongju, Republic of Korea. A voucher specimen (2008-ST-25) has been deposited at the Herbal Medicine Formulation Research Group, Korea Institute of Oriental Medicine.
Chemicals and reagents
Chlorogenic acid and caffeic acid were purchased from Acros Organics (Pittsburgh, PA, USA). 4,5-Dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 1,3-dicaffeoylquinic acid, and 1,5-dicaffeoylquinic acid were obtained from Chengdu Biopurify Phytochemicals Ltd., (Chengdu, China). Protocatechuic acid, ferulic acid, and 1,4-dicaffeoylquinic acid were obtained from ChromaDex (Irvine, CA, USA), Wako Chemicals (Osaka, Japan), and ChemFaces (Wuhan, China), respectively. The purity of these reference standards was >98.0% by HPLC analysis. The chemical structures of these components are shown in Figure 1. The HPLC-grade solvents methanol, acetonitrile, and water were obtained from JT Baker (Phillipsburg, NJ, USA). Glacial acetic acid (analytical grade) was purchased from Merck KGaA (Darmstadt, Germany).
Figure 1.

Chemical structures of nine marker compounds in Xanthii fructus
Preparations of seventy percentage ethanol extract
Dried X. fructus (200 g) was extracted three times with 70% (v/v) ethanol (2 L) by sonication for 60 min. The extracted solution was filtered through filter paper, evaporated at 40°C using a Büchi R-210 rotary evaporator (Flawil, Switzerland) under vacuum to dryness and freeze-dried. The yield of the freeze-dried 70% ethanol extract obtained was 5.86% (11.72 g).
Quantitative analysis of the marker components in Xanthii fructus
The sample was analyzed using a Shimadzu Prominence LC-20A series HPLC (Shimadzu Co., Kyoto, Japan) comprising a solvent delivery unit (LC-20AT), online degasser (DGU-20A3), column oven (CTO-20A), auto sample injector (SIL-20AC), and photodiode array detector (PDA) (SPD-M20A). Data were collected and processed using LC solution software (Version 1.24, Shimadzu Co., Kyoto, Japan). The stationary phase for the separation of the nine components used a reversed-phase SunFire™ C18 analytical column (Waters, Milford, MA, USA; 150 mm × 4.6 mm and 5 μm particle size). The mobile phase comprised 1.0% aqueous acetic acid (eluent A) and 1.0% acetic acid in acetonitrile (eluent B). The gradient flow of the two-solvent system was as follows: 5–5% B (3 min), 5–40% B (15 min), 40% B (20 min), and 40–5% B (25 min). The flow rate was 1.0 mL/min, the column temperature was maintained at 40°C, and the injection volume was 10 μL. The detection wavelength of quantification was set over the range of 190–400 nm and was recorded at 260 and 325 nm.
Cell culture
The murine macrophage cell line RAW 264.7 was obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were cultured in Dulbecco's minimal essential medium (DMEM; Gibco BRL, Carlsbad, CA) supplemented with 5.5% fetal bovine serum (Gibco BRL), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37°C.
Cytotoxicity assay
To test the cytotoxicity of the nine markers, RAW 264.7 cells were incubated for 24 h in the presence or absence of each marker compound from X. fructus. Cell counting kit-8 (CCK-8) solution (Dojindo, Kumamoto, Japan) was added, and the cells were incubated for 4 h. After the incubation, the absorbance was read at 450 nm in a Microplate Reader (Benchmark Plus, Bio-Rad, MN, USA). Cell viability was calculated as the percentage of viable cells in the X. fructus-treated group versus untreated controls.
Measurement of tumor necrosis factor-alpha, interleukin-6, and prostaglandin E2 production
RAW 264.7 cells were plated at a density of 2.4 × 104 cells on 24 well and incubated for 24 h. Cells were treated with various concentration of each marker compound from X. fructus for 4 h prior to LPS (1 μg/mL) stimulation. After an additional incubation for 20 h, the concentrations of TNF-α (BD Biosciences, Mountain View, CA, USA), IL-6 (BD Biosciences), and PGE2 (Cayman Chemical Co., Ann Arbor, MI, USA) in the supernatants were measured by enzyme-linked immunosorbent assays (ELISAs) according to manufacturers’ instructions.
Statistical analysis
All values are expressed as the mean ± standard error of the mean of three independent samples of each marker compound from X. fructus. One-way analysis of variance was used to identify significant differences between the treatment groups. Dunnett's test was used for multiple group comparisons. Differences were considered significant at P < 0.05.
RESULTS AND DISCUSSION
Quantitative determination of nine compounds in Xanthii fructus
An optimized HPLC method was applied for the simultaneous analysis of nine phenylpropanoids in the X. fructus extract. Five components were eluted within 20 min using the two mobile phases. Each compound in the chromatogram was identified by comparing the retention time and UV spectra compared with those of the standards [Figure 2]. The retention times of protocatechuic acid, chlorogenic acid, caffeic acid, 1,4-dicaffeoylquinic acid, 1,3-dicaffeoylquinic acid, ferulic acid, 1,5-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid were 6.51, 11.45, 11.80, 12.16, 13.22, 14.67, 15.63, 15.89, and 16.55 min, respectively. Representative HPLC chromatograms of standards and the extracts are shown in Figure 2. The coefficients of determination (r2) of the calibration curves for the nine analytes were ≥0.9996, and the calibration curves had good linearity within the tested concentration range.
Figure 2.
High-performance liquid chromatography chromatograms of the standard mixture (a) and Xanthii fructus extract (b) at ultraviolet detection of 260 nm (I) and 325 nm (II). Protocatechuic acid (1), chlorogenic acid (2), caffeic acid (3), 1,4-dicaffeoylquinic acid (4), 1,3-dicaffeoylquinic acid (5), ferulic acid (6), 1,5-dicaffeoylquinic acid (7), 3,5-dicaffeoylquinic acid (8), and 4,5-dicaffeoylquinic acid (9)
As determined by the HPLC–PDA method, the amounts of the nine phenylpropanoids, protocatechuic acid, chlorogenic acid, caffeic acid, 1,4-dicaffeoylquinic acid, 1,3-dicaffeoylquinic acid, ferulic acid, 1,5-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid in X. fructus extract ranged from 0 to 33.56 mg/g [Table 1]. Among these components, chlorogenic acid and 1,5-dicaffeoylquinic acid were detected as the main constituents (33.56 mg/g and 10.12 mg/g) of X. fructus. Protocatechuic acid and ferulic acid, which have been isolated and analyzed as bioactive compounds from X. fructus,[2,14,15] were not detected in this sample.
Table 1.
The concentrations of nine marker compounds in the Xanthii fructus extract (n=3)

Cytotoxic effects of marker compounds from Xanthii fructus in RAW 264.7 cells
The cytotoxicity of the nine marker compounds from X. fructus was evaluated in RAW 264.7 cells using the CCK-8 assay. Cells were treated with various concentrations (0, 6.25, 12.5, 25, 50, or 100 μM) of each compound for 24 h. As shown in Figure 3, none of the tested marker compounds had a cytotoxic effect at a concentration up to 50 μM, although they caused a marked reduction in the viability of RAW 264.7 cells. Thus, nontoxic concentrations of ≤50 μM were used for subsequent in vitro assays.
Figure 3.
Cytotoxicity of nine marker compounds from Xanthii fructus in RAW 264.7 cells. Cells were seeded into 96-well plates and treated with nine marker compounds from Xanthii fructus for 24 h. Cell viability was assessed using a cell counting kit-8 assay. (a) Protocatechuic acid, (b) chlorogenic acid, (c) caffeic acid, (d) 1,4-dicaffeoylquinic acid, (e) 1,3-dicaffeoylquinic acid, (f) ferulic acid, (g) 1,5-dicaffeoylquinic acid, (h) 3,5-dicaffeoylquinic acid, and (i) 4,5-dicaffeoylquinic acid
Inhibitory effects of the marker compounds from Xanthii fructus on tumor necrosis factor-alpha and interleukin-6 production by RAW 264.7 cells
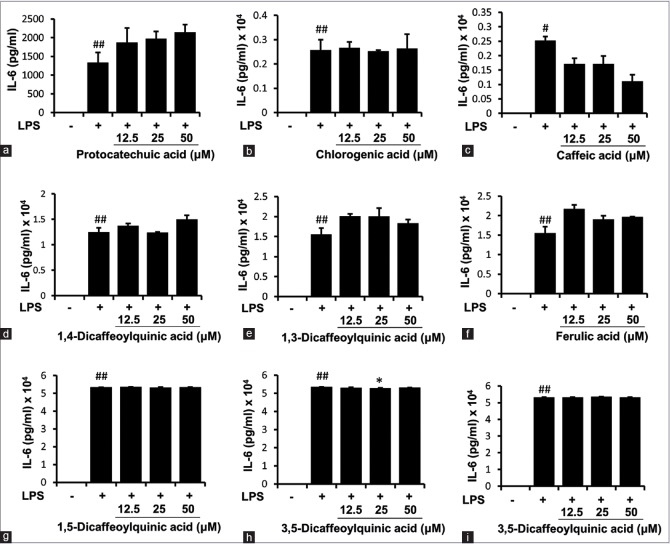
To determine the biological activities of the nine marker compounds from X. fructus, we used an in vitro experimental model of inflammation using the murine macrophage cell line RAW 264.7. We chose these cells because macrophages are major immune cells involved in the initiation, maintenance, and resolution of the inflammatory process.[16,17] Macrophages are activated by various stimuli such as bacterial LPS released during inflammatory reactions, and secreted proinflammatory cytokines such as TNF-α[18] and IL-6.[19] Thus, we examined whether the marker compounds from X. fructus could affect the production of proinflammatory cytokines in RAW 264.7 cells. ELISAs for mouse TNF-α and IL-6 were used to measure the concentrations in the culture supernatant from LPS-treated RAW 264.7 cells incubated with or without each of the marker compounds. Among the nine marker compounds tested, only 1,4-dicaffeoylquinic acid [Figure 4d] had a significant inhibitory effect on TNF-α production by the cells [Figure 4]. None of the marker compounds had an effect on IL-6 production [Figure 5].
Figure 4.

Effect of nine marker compounds from Xanthii fructus on lipopolysaccharide-stimulated tumor necrosis factor-alpha production in RAW 264.7 cells. Each bar represents the mean of three independent experiments (#P < 0.05 and ##P < 0.01 versus untreated control; **P < 0.01 versus lipopolysaccharide-treated cells). (a) Protocatechuic acid, (b) chlorogenic acid, (c) caffeic acid, (d) 1,4-dicaffeoylquinic acid, (e) 1,3-dicaffeoylquinic acid, (f) ferulic acid, (g) 1,5-dicaffeoylquinic acid, (h) 3,5-dicaffeoylquinic acid, and (i) 4,5-dicaffeoylquinic acid
Figure 5.
Effect of nine marker compounds from Xanthii fructus on lipopolysaccharide-stimulated interleukin-6 production in RAW 264.7 cells. Each bar represents the mean of three independent experiments (#P < 0.05 and ##P < 0.01 versus untreated control; **P < 0.01 versus lipopolysaccharide-treated cells). (a) Protocatechuic acid, (b) chlorogenic acid, (c) caffeic acid, (d) 1,4-dicaffeoylquinic acid, (e) 1,3-dicaffeoylquinic acid, (f) ferulic acid, (g) 1,5-dicaffeoylquinic acid, (h) 3,5-dicaffeoylquinic acid, and (i) 4,5-dicaffeoylquinic acid
Effects of marker compounds from Xanthii fructus on prostaglandin E2 level in RAW 264.7 cells
We also examined effects of nine marker compounds from X. fructus on the production of PGE2 because PGE2 is the major product of COX-2, a pro-inflammatory mediator.[20] As shown in Figure 6, LPS stimulation significantly increased level of PGE2 whereas indomethacin, a positive control, significantly reduced LPS-mediated PGE2 production in RAW 264.7 cells. Among nine marker compounds from X. fructus, protocatechuic acid [Figure 6a] and caffeic acid [Figure 6c] had significant inhibitory effects on PGE2 production ranged from 12.5 to 50 μM. Ferulic acid [Figure 6f] revealed a significant inhibition of PGE2 production at 50 μM, but not lower concentration.
Figure 6.
Effect of nine marker compounds from Xanthii fructus on lipopolysaccharide-stimulated prostaglandin E2 production in RAW 264.7 cells. Indomethacin was used as a positive control. Each bar represents the mean of three independent experiments (#P < 0.05 and ##P < 0.01 versus untreated control; **P < 0.01 versus lipopolysaccharide-treated cells). (a) Protocatechuic acid, (b) chlorogenic acid, (c) caffeic acid, (d) 1,4-dicaffeoylquinic acid, (e) 1,3-dicaffeoylquinic acid, (f) ferulic acid, (g) 1,5-dicaffeoylquinic acid, (h) 3,5-dicaffeoylquinic acid, and (i) 4,5-dicaffeoylquinic acid
CONCLUSION
We successfully developed a method to simultaneously identify the nine marker compounds and this method will help to improve the quality control of X. fructus extract. In addition, nine marker compounds from X. fructus had inhibitory effects on inflammatory responses by targeting different proinflammatory factors, even though some of these compounds have similar chemical structures. However, we found no relationships between the amount detected by HPLC analysis and the in vitro anti-inflammatory activities of each of the compounds. Further experiments are needed to identify the major bioactive compound (s) and the molecular mechanisms responsible for the anti-inflammatory effects of each compound.
Financial support and sponsorship
This research was supported by a grant (No. K15251) from the Korea Institute of Oriental Medicine.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Sae-Rom Yoo
Sae-rom, Yoo is a researcher at the K-herb Research Center, Korea Institute of Oriental Medicine (KIOM), Daejeon, Republic of Korea.

Soo-Jin Jeong
Soo-Jin, Jeong is a principal researcher at the KM Convergence Research Division in KIOM. Their research interest is in the area of metabolic diseases such as obesity, skin diseases, inflammatory diseases, and cancer especially using natural products.

Chang-Seob Seo
Chang-Seob, Seo is a principal researcher at K-herb Research Center, Korea Institute of Oriental Medicine. He is an expert in natural product. His expertise involves natural products, separation, purification, identification, and quantitation of bioactive components in complex matrix of herbs.

Na-Ri Lee
Na-Ri, Lee is a researcher at K-herb Research Center, Korea Institute of Oriental Medicine. Her major is biotechnology. She is interested in separation, purification, and in vitro assay from the natural products.

Hyeun-Kyoo Shin
Hyeun-Kyoo, Shin, Korean medicine doctor (KMD), is a principal researcher at K-herb Research Center, Korea Institute of Oriental Medicine. Her major is Korean medicine. He is interested in the area of traditional herbal medicines.
REFERENCES
- 1.Bae KH. The medicinal plants of Korea. Seoul: Kyo-Hak Publishing Co. Ltd; 2000. p. 514. [Google Scholar]
- 2.Han T, Li HL, Hu Y, Zhang QY, Huang BK, Zheng HC, et al. Phenolic acids in Fructus Xanthii and determination of contents of total phenolic acids in different species and populations of Xanthium in China. Zhong Xi Yi Jie He Xue Bao. 2006;4:194–8. doi: 10.3736/jcim20060217. [DOI] [PubMed] [Google Scholar]
- 3.Lee YM, Kang DG, Kim MG, Choi DH, Lee HS. Isolation of antioxidants from the seeds of Xanthium strumarium. Korean J Orient Physiol Pathol. 2004;18:792–6. [Google Scholar]
- 4.Ma YT, Huang MC, Hsu FL, Chang HF. Thiazinedione from Xanthium strumarium. Phytochemistry. 1998;48:1083–5. [Google Scholar]
- 5.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147:227–35. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottomley MJ, Webb NJ, Watson CJ, Holt PJ, Freemont AJ, Brenchley PE. Peripheral blood mononuclear cells from patients with rheumatoid arthritis spontaneously secrete vascular endothelial growth factor (VEGF): Specific up-regulation by tumour necrosis factor-alpha (TNF-αlpha) in synovial fluid. Clin Exp Immunol. 1999;117:171–6. doi: 10.1046/j.1365-2249.1999.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–67. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang YJ, Mbonye UR, DeLong CJ, Wada M, Smith WL. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog Lipid Res. 2007;46:108–25. doi: 10.1016/j.plipres.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev. 2002;4:CD002296. doi: 10.1002/14651858.CD002296. [DOI] [PubMed] [Google Scholar]
- 10.Das U, Manna K, Sinha M, Datta S, Das DK, Chakraborty A, et al. Role of ferulic acid in the amelioration of ionizing radiation induced inflammation: A murine model. PLoS One. 2014;9:e97599. doi: 10.1371/journal.pone.0097599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim KM, Bae S, Koo JE, Kim ES, Bae ON, Lee JY. Suppression of skin inflammation in keratinocytes and acute/chronic disease models by caffeic acid phenethyl ester. Arch Dermatol Res. 2015;307:219–27. doi: 10.1007/s00403-014-1529-8. [DOI] [PubMed] [Google Scholar]
- 12.Ruifeng G, Yunhe F, Zhengkai W, Ershun Z, Yimeng L, Minjun Y, et al. Chlorogenic acid attenuates lipopolysaccharide-induced mice mastitis by suppressing TLR4-mediated NF-κB signaling pathway. Eur J Pharmacol. 2014;729:54–8. doi: 10.1016/j.ejphar.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhou J, Fu S, Wang C, Zhou B. Preventive effects of protocatechuic acid on LPS-induced inflammatory response in human gingival fibroblasts via activating PPAR-γ. Inflammation. 2015;38:1080–4. doi: 10.1007/s10753-014-0073-1. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Z, Wang L, Chen B, Li F, Wang M. Chemical constituents from Fructus xanthii. Chin J Appl Environ Biol. 2011;17:350–2. [Google Scholar]
- 15.Yang L, Su ZJ, Xu SJ, Wu JX, Chen LL, Zhou RL, et al. Simultaneous determination of 4 phenolic acids in cangerzi by ultra-performance liquid chromatography. Yao Xue Xue Bao. 2010;45:1537–40. [PubMed] [Google Scholar]
- 16.Ahmed JS, Mehlhorn H. Review: The cellular basis of the immunity to and immunopathogenesis of tropical theileriosis. Parasitol Res. 1999;85:539–49. doi: 10.1007/s004360050593. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–6. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 18.Tracey KJ, Cerami A. Tumor necrosis factor: A pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 19.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 20.Crofford LJ, Wilder RL, Ristimäki AP, Sano H, Remmers EF, Epps HR, et al. Cyclooxygenase-1 and-2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. J Clin Invest. 1994;93:1095–101. doi: 10.1172/JCI117060. [DOI] [PMC free article] [PubMed] [Google Scholar]