Abstract
Context:
Our survey was performed near Iquitos (Peruvian Amazon) and its surroundings and leads us to consider Mestizo ethnomedical practices. The plant species reported here are traditionally used for ailments related to microbial infections. Inhabitants of various ethnic origins were interviewed, and 52 selected plants extracts were evaluated for their antimicrobial properties against a panel of 36 sensitive and multi-resistant bacteria or yeast. The study aimed at providing information on antimicrobial plant extract activities and the ethnomedical context of Mestizo riverine populations from Loreto (Peru).
Material and Method:
The minimum inhibitory concentrations (MICs) of the plant crude extracts were carried out using the agar dilution method and ranged between 0.075 and 5.0 mg/ml.
Results:
Of the 40 plants analyzed, 9 species showed MIC ≤0.3 mg/ml (Anacardium occidentale, Couroupita guianensis, Croton lechleri, Davilla rugosa, Erythrina amazonica, Jacaranda copaia subsp. Spectabilis, Oenocarpus bataua, Peperomia macrostachya, and Phyllanthus urinaria) for one or several of the 36 microorganisms and only 6 drug extracts were inactive. Among the 40 plants, 13 were evaluated for the first time for an antibacterial activity.
Conclusion:
This evaluation of the antimicrobial activity of 40 plants using an approved standard methodology allowed comparing those activities against various microbes to establish antimicrobial spectra of standardized plant extracts, and give support to the traditional use of these plants. It may also help discovering new chemical classes of antimicrobial agents that could serve against multi-resistant bacteria.
SUMMARY
This study leads us to consider Mestizo ethnomedical practices near Iquitos (Peruvian Amazon) and its surroundings. The plant species reported here are traditionally used for ailments related to microbial infections. 52 selected plants extracts were evaluated for their antimicrobial properties against a panel of 36 sensitive and multi resistant bacteria or yeast. The study aimed at providing information on antimicrobial plant extract activities and the ethnomedical context of Mestizo riverine populations from Loreto.
Keywords: Antimicrobial activity, Iquitos, Loreto, medicinal plant, traditional use
INTRODUCTION
Resistance to antibiotics in numerous pathogens has become a real public health problem, it concerns specific pathogens causing diseases such as tuberculosis or gonorrhea but also opportunistic pathogens involved in nosocomial infections.[1,2,3,4,5] Recently, the WHO expressed urgent need for the development of new antibacterial compounds.[6,7,8] But the number of new drugs in development is low, raising the question for alternative research.
As plants have evolved since hundreds of millions of years in the presence of bacteria and develop rarely bacterial diseases (even in warm and damp conditions, which are favorable for bacterial development), this provides a rational basis for the search for vegetal antibacterial compounds. In addition, the long standing empirical use of herbal medicine against infectious ailments is consistent with the previous observation. Our study took place in the Northern region of Peru called Loreto (one of the Peruvian biggest Amazonian province) with a high level of both biodiversity and traditional knowledge. This knowledge has been transmitted and has circulated through constant interactions and exchanges between Indigenous groups and Mestizo populations, in particular during the Rubber Boom era, leading to a large repository of medicinal practices of various origins.
Our ethnographic survey was performed in Iquitos and its surroundings (Tamshi Yacu) and leads us to consider Mestizo ethnomedical practices as part of a larger ethnomedical system including neighboring Amerindian knowledge and practices. On the basis of traditional medicinal reports gathered during our ethnopharmacological survey, 40 different plants used in the Amazonian district of Peru were selected, and their antimicrobial activity was assessed. The minimal inhibitory concentration (MIC) was determined in vitro against 36 microbial strains belonging to 21 different species.
MATERIALS AND METHODS
Ethnomedical context
An intensive ethnographic survey was necessary to understand the relevance and the variable selective criteria of traditional herbal medicines. A “participant-observation” methodology was adopted. Many interviews were performed in the Belén Market in Iquitos, where Mestizo local people commercialized most of the plant species studied, for medical use. The medicinal plants market of Belén is located on the shore of the Itaya River. It started in the late sixties with two women: María de Marreros and María de Yap. In the late nineties, the majority of the “puestos” (around 30 in total) were under the responsibility of women engaged in the commercialization of medicinal plants to urban population and mainly to local shamans called “curanderos”, “vegetalistas” or “medicos” in local Spanish. The local shamans were identified as belonging to different indigenous origins: Mainly Cocama (A. Curico Murayari), Lowland Napo Quechua or Lamista Quechua and Shipibo from the Ucayali. The majority of the 140 commercialized plant species do not come from cultivated areas but from more distant primary forest areas. The three main zones of extraction are the River Napo, the River Nanay, and the rural surroundings of the main road stretching from Iquitos to Nauta.[9,10]
Although Mestizo shamanism and plant therapy have been mentioned as playing an important role in the medical system of Iquitos and its rural surroundings, an extensive documentation on medicinal plants use linked with ethnomedical practices has never been fully undertaken.[11,12,13] Why such a lack of interest for disclosing an ethnomedical overview of Mestizo traditional therapy? Mestizo culture has for a long time appeared as less “traditional” than other indigenous systems, such as the neighboring Shipibo groups, where more extensive ethnopharmacological studies were performed.[14,15,16] However, the Mestizo riverine population dwelling the area of study have been, since the early colonial period until nowadays, engaged in constant communication and exchanges with Indigenous population: There are the Shipibo from the Ucayali River (Panoan linguistic family), the Quechua Napu Runa from the Napo and Tigre River, the Quechua Lamista from the Huallaga, or the Cocama from the Amazon and Marañon River. The interactions between Mestizo and Amerindian groups did not restrict to the exchanges of manufactured goods during the Rubber Boom era (1875-1914) and after, but both population have influenced each other from the perspective of medical knowledge and more specifically plant therapy.[17,18]
In this ethnopharmacological investigation, a close attention was paid to the practices of plant therapy among the Mestizo population. Speaking of mestizo pharmacopoeia without giving a short insight on their conception of the therapeutic process and their representations of illness could be missing the chore of ethnomedical practices in the Peruvian Amazon. For Mestizo riverine populations, most severe diseases etiologies such as strong skin infections, malaria, leishmaniasis, or other physical disorders are often classified in the category of sorcery: “brujeria” in local Spanish. In these contexts, traditional ritual specialists are called to diagnose the spiritual causes of physical disorders, thanks to their dreams and visionary power. Most of the Mestizo ritual specialists refer to themselves as “curanderos” or “vegetalistas” – specialized in the use of medicinal plants.
Ethnobotanical survey
The ethnobotanical study was conducted during June-August 2011 and 2013. Claims of effective therapy for the treatment of cough, diarrhea, dysentery, piles, skin diseases, conjunctivitis, chronic bronchitis, tuberculosis, genito-urinary diseases, and sore throat by traditional herbalists have prompted our interest in this scientific investigation. Information presented here was compiled through general conversations, informal interviews, and rainforest walks with healers, midwives, and local people with knowledge in self-medication using plants.
Plant material
The plants were collected (in forest or in the market), identified, and deposited in the herbarium of the Universidad Nacional de la Amazonia Peruana (UNAP, Iquitos) by the botanist Juan Celidonio Ruiz Macedo, then a second collection was made in higher quantities in the surroundings of Iquitos (voucher specimen, Table 1) for biological essay. In few cases, the collected part of the plant did not match to the drug traditionally used, due to the collection opportunities.
Table 1.
Alphabetic list and traditional anti-infectious uses of the species investigated

The plant samples were dried, finely grounded with a hammer mill, and extracted overnight with methanol at room temperature, with gentle shaking (methanol provide a more complete extraction, including less polar compounds, and still more representative of the traditional preparations as aqueous or ethanolic extracts). The extracts were filtered through filter paper, dried under reduced pressure at 40°C, and weighed. Thus, crude extracts were dissolved in methanol to a final concentration of 10 mg/ml.
Minimal inhibitory concentration determination
Selected microorganisms
In the setting of malnutrition, HIV, aging, organ transplantation, other immunodeficiency etiologies, and opportunistic infections are common causes of morbidity and mortality. That is the reason why we selected various bacteria, some of which display resistance to antibiotics or can be involved in opportunistic or nosocomial infections or in diseases cited by the informants. The various microorganisms (Gram-positive, Gram-negative, yeast) were all able to grow aerobically in Mueller Hinton Agar (MHA) media.
The investigations use standardized methodology with internationally recognized protocols (CLSI, 2006), American Type Culture Collection strains (reference strains from the ATCC), and drug multi-resistant clinical strains (that reflect what is encountered today in hospital from the CHRU Lille, France).[19] For each strain, we added the registration number in the CFPL collection, followed by the mentions of antimicrobial resistance or the type of enzymes implicated in resistance. Species identification (determined by mass spectrometry) and antibiogram of each bacteria (cultivated or cryopreserved) are evaluated 3 times in the year.
Bacteria could be divided into several groups
The first three groups include enterobacteria (Gram-negative Bacilli)
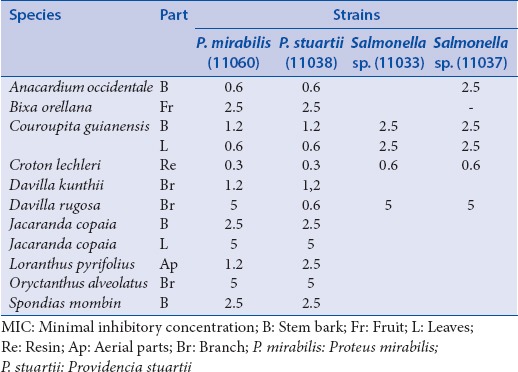
The first of those includes lactose-positive, VP negative enterobacteria (which are usually poorly resistant and pathogenic): Escherichia coli (8137; 8138: Penicillin resistance; 8157 penicillin and fluoroquinolone resistances; ATCC 25922: NA) and Citrobacter freundii (11041; 11042: Cephalosporin resistance; 11043: TEM 3) [Table 2]. The second group of enterobacteria includes lactose-positive, VP positive enterobacteria with frequent implication in nosocomial infections and high antibiotic resistance: Klebsiella pneumoniae (11016; 11017: Penicillin resistance), Enterobacter cloacae (11050; 11051: Cephalosporin resistance; 11053: NDM-1), Enterobacter aerogenes (9004: BLSE) and Serratia marcescens (11056; 11057: Cephalosporin resistance) [Table 3]. The third group includes lactose-negative and more pathogenic enterobacteria: Proteus mirabilis (11060), Providencia stuartii (11038), Salmonella spp. (11033; 11037: CMY 2: Penicillin resistance) [Table 4].
Table 2.
MIC concentrations (mg/ml) for the bacteria of the first group of enterobacteria
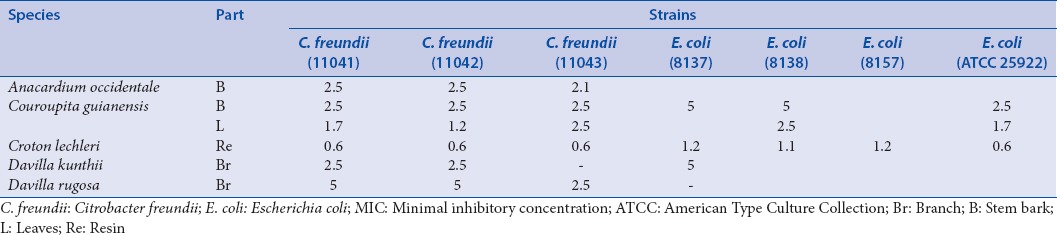
Table 3.
MIC concentrations (mg/ml) for the bacteria of the second group of enterobacteria
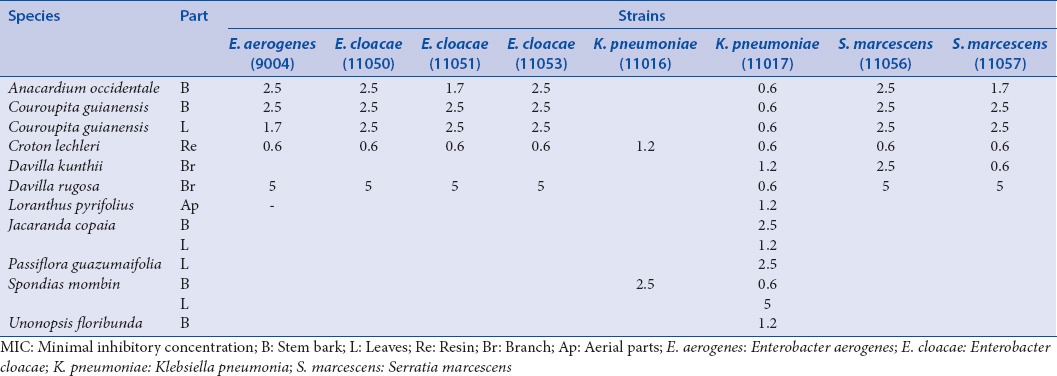
Table 4.
MIC concentrations (mg/ml) for the bacteria of the third group of enterobacteria
The fourth group includes other Gram-negative bacteria also found with a high frequency in nosocomial infections: Pseudomonas aeruginosa (8131; ATCC 27583), Acinetobacter baumannii (9010: VEB-1; 9011: Multi-resistant), Stenotrophomonas maltophilia [Table 5].
Table 5.
MIC concentrations (mg/mL) for the bacteria of the fourth group (nonenterobacteria Gram-negative)
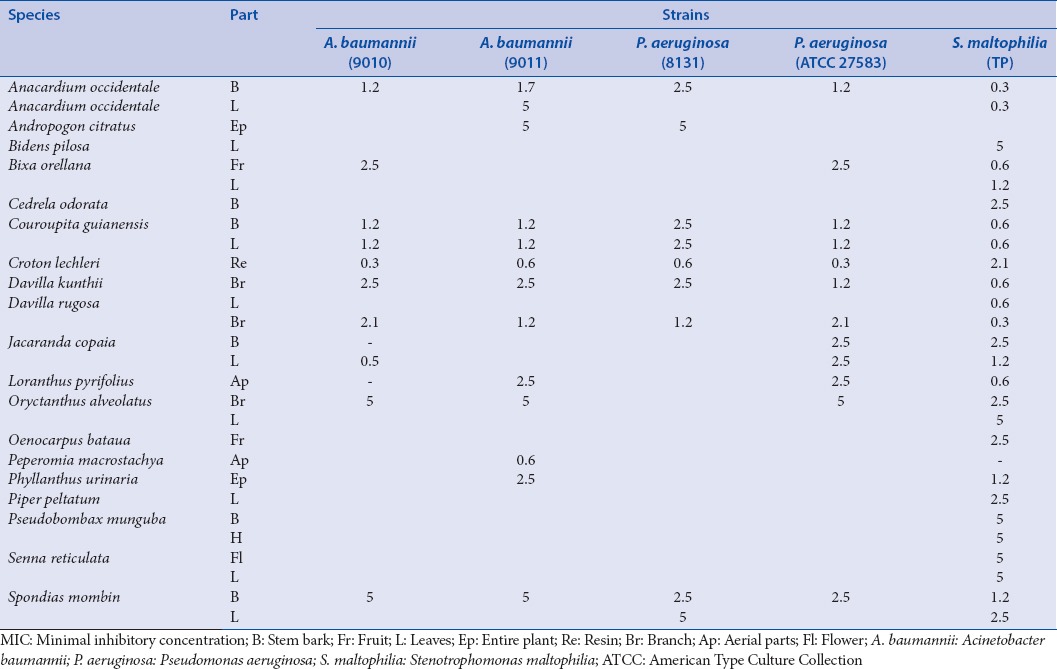
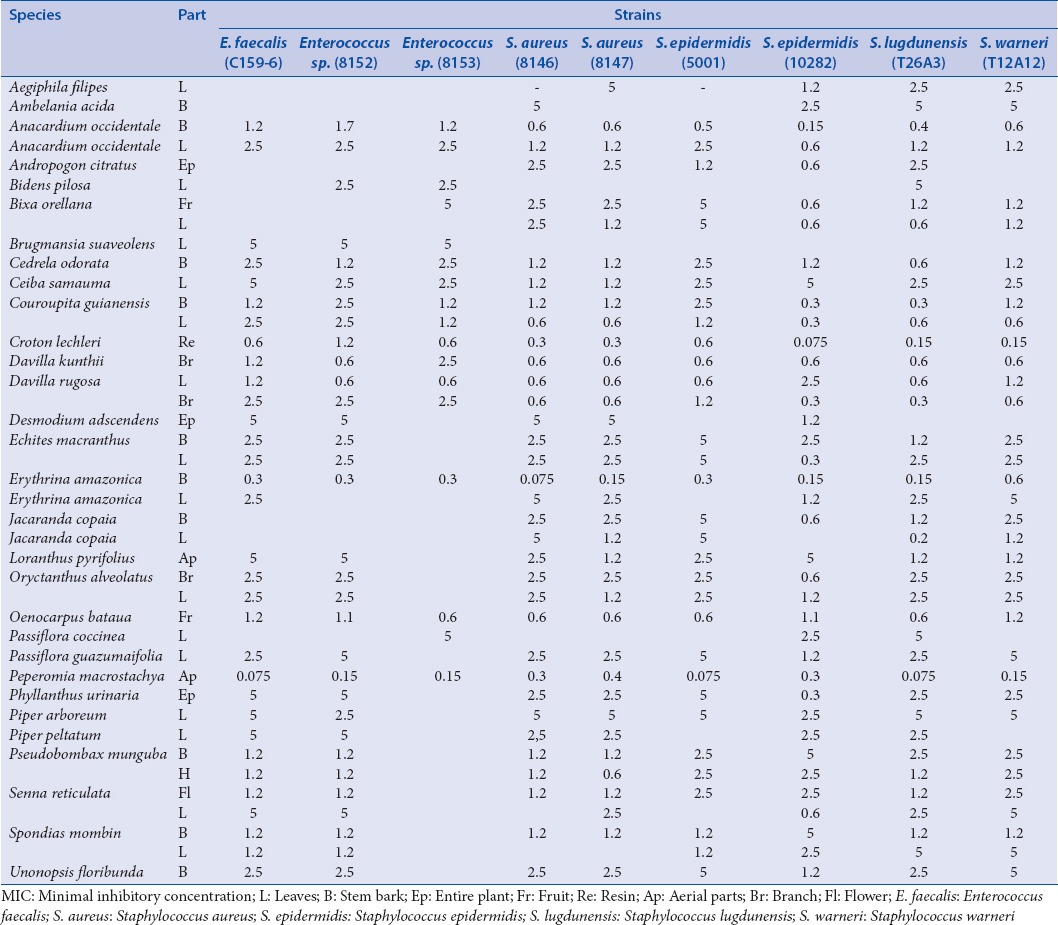
The fifth group includes Gram-positive cocci (that can be involved in external infections): Staphylococcus aureus (8146: Meticillin- and kanamycin-resistant; 8147), Staphylococcus epidermidis (5001, 10282), Staphylococcus lugdunensis (T26A3), Staphylococcus warneri (T12A12), Enterococcus spp. (8152 aminoglycoside-resistant; 8153: erythromycin- and clindamycin-resistant), and Enterococcus faecalis (C159-6 vancomycin-susceptible) [Table 6].
Table 6.
MIC concentrations (mg/mL) for the bacteria of the fifth group (Gram-positive cocci)
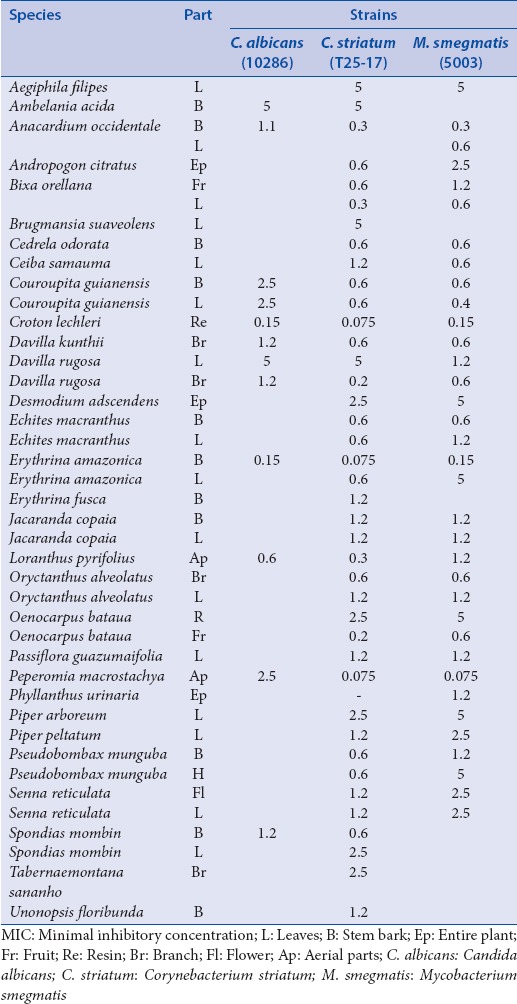
The last group contains other miscellaneous microorganisms: Mycobacterium smegmatis (5003), Corynebacterium striatum (T25-17), Candida albicans (10286) [Table 7].
Table 7.
MIC concentrations (mg/mL) for the bacteria of the sixth group comprising miscellaneous strains
Antimicrobial tests
MIC determinations of the plant crude extracts were carried out using the agar dilution method stipulated by the CLSI agar dilution methods (CLSI2006).
The plant extracts and the antimicrobial drugs were evaluated, the MIC of the extracts was determined for 35 bacterial strains and 1 yeast by diluting the extracts in MHA media. The inhibitory concentrations ranged between 0.075 and 5.0 mg/ml in seven dilutions (5.0, 2.5, 1.2, 0.6, 0.3, 0.15, and 0.075 mg/ml), 0.075 mg was considered as the lower concentration for a preliminary screening and MIC >5.0 mg/ml were considered as nonactive against tested strains.[20] Petri dishes (controls and extracts), were inoculated with strains (104 CFU, obtained by dilution in brain heart) using a Steer's replicator and were incubated at 37°C for 24 h. MIC was defined as the lowest concentration of extract without bacterial growth after incubation. Control antibiotics were evaluated, and specific anti-biograms were available for each strain (see additional file). Moreover, if we rely on the fact that antibiotics do not have a vegetal origin, we cannot expect that plant extracts could have the same efficiency as that of pure antibiotics compounds. For each group of bacteria, only plants showing an activity are mentioned. The extracts that exhibited MIC ≤0.3 mg/ml were tested in triplicate at lower concentrations (down to 0.075 mg/ml, and standard deviation are done for values: 4.2 ± 1.7; 2.1 ± 0.8; 1.7 ± 0.8; 1.1 ± 0.2; 0.5 ± 0.1; 0.4 ± 0.1, and <0.0 for other values).
Thin-layer chromatography bioautography
Plates (Silica gel 60 Xtra Sil G/UV254) were developed with ethyl acetate:methanol:water (16:2:1). Tannins were revealed with FeCl3 aqueous solution. Other developed thin-layer chromatography (TLC) plates were dried and overlaid by nutrient agar seeded with an overnight culture of S. epidermidis (5001). The plate was incubated for 24 h at 37°C and then sprayed with a solution of p-iodonitrotetrazolium violet (1-h later, clear zones appears corresponding to bacterial inhibition).
RESULTS
Ethnobotanical study
Bark or roots are also often prepared as hydro-alcoholic maceration and need special diet and cannot be mixed with other plants or food.[21] Some common names, such as Sapo huasca, Amor seco, Masisa, Suelda con suelda, and Cordoncillo, can refer to different species [Table 1]; this may be ascribed to a loss in traditional medical knowledge in the district of Iquitos. For few species (Oryctanthus alveolatus, Pseudobombax munguba, Tabernaemontana sananho), the plant organ tested do not exactly match with the part used (cause of collection opportunities).
Biological screening methodology and activities
As the authors well known the difficulties to ascribe any specific identified “bacteria” to a so-called “traditional use” they decided to test plant extracts on a large panel of 36 bacteria or yeast, in order to be more representative of the infectious pathogens enhanced in the described disease. The agar dilution method (macrodilution) with methanolic extract enabled to test the antibiotic activity of all our plants without problem of solubility, to obtain standardized numeric value (MIC) for each of the 36 microorganisms.
Only six plant extracts were active against the first group of enterobacteria [Table 2] with the lowest MIC values for Croton lechleri on all strains. As concerns E. coli, only four extracts have an activity against at least one of the four strain species.
Twenty-one plants show activities against the second group of enterobacteria, especially against the Enterobacter cloacae strain 11053 [Table 3]. We also observed the global activity of the C. lechleri extract and the multi-resistance of K. pneumonia (inhibited by only two extracts).
Concerning the last group of enterobacteria [Table 4], we also observed the highest antibacterial activity for C. lechleri and the highest level of resistance for the Salmonella spp. strains.
Usually, Plant extracts were more active against the fourth group of bacteria (nonenterobacteria Gram-negative) that are involved in nosocomial infections and often highly resistant to antibiotics. We can underline a high sensitivity of the S. maltophilia strain that is emerging pathogen in hospital with life-threatening infections [Table 5]. Best antibacterial activities were obtained for C. lechleri, Anacardium occidentale, Couroupita guianensis, Davilla rugosa, and Spondias mombin extracts.
A majority of plant extracts showed good activity against Gram-positive cocci especially against the genera Enterococcus and Staphylococcus, which validates the use against skin infections frequently stated in the survey [Table 6]. Peperomia macrostachya shows an unexpected activity with low MIC concentrations for both Enterococcus and S. aureus, even for the meticillin-resistant (8146) strain whereas it has never been studied before for its biological or chemical properties.
The sixth group also contains the nonpathogenic species M. smegmatis, which is close in structure to the highly pathogenic Mycobacterium tuberculosis [Table 7]. An activity on this strain is a good argument for further investigation, as drug resistance is on the rise and primarily in HIV-infected patients, is associated with a significantly higher mortality rate and short survival period, which makes the search for new anti-tuberculosis molecules of great interest.
Of the 40 plants analyzed (52 methanolic extracts), 9 species showed MIC ≤0.3 mg/ml (A. occidentale, C. guianensis, C. lechleri, D. rugosa, Erythrina amazonica, Jacaranda copaia subsp. Spectabilis, Oenocarpus bataua, P. macrostachya, Phyllanthus urinaria) for one or several of the 36 microorganisms and only 7 extracts were inactive (Bidens pilosa, Jatropha gossypiifolia, Laportea aestuans, Petiveria alliacea, Phlebodium decumanum, Piper tuberculatum, Tripogandra serrulata).
Examination by bio-autography of the Davilla kunthii and D. rugosa (chemically unknown species) revealed an antibacterial activity due to polar compounds. Revelation with FeCl3 aqueous solution of the TLC permitted to identify hydrolysable tannins as responsible of the antibacterial activity.
Among the 40 plants, 13 of them were evaluated for the first time for an antibacterial activity (Aegiphila filipes, Ceiba samauma, Echites machrantus, O. bataua, O. alveolatus, Passiflora coccinea, Passiflora guazumaefolium, P. macrostachya, P. decumanum, P. munguba, Senna bacillaris, T. sananho, T. serrulata). The more sensitive microorganisms were Gram-positive: C. striatum, P. aeruginosa, S. epidermidis, S. lugdunensis, S. warneri.
DISCUSSION/CONCLUSION
This is the first time that those plants were tested on so many different bacteria in a same standardized experiment with a MIC value. It permitted a comparative evaluation of the plant activities in absolutely equal conditions of assessment on microbial species. Strains of the same species (with very different anti-biograms) displayed only marginal differences (K. pneumoniae resistant strains were more sensible to plant extracts than the nonresistant strain) which is in favor of very different thus readily complementary-modes of action of such extracts in comparison with antibiotics (similar plant extract activities are observed for resistant and sensitive strains from C. Freundi, E. coli, E. cloacae: Gram-negative; S epidermidis, S. aureus: Gram-positive).
Bibliographic and experimental studies of the more active species (MIC ≤0.3 mg/ml) permitted to describe their potential active compounds:
A. occidentale: Tannins, alkaloids, saponins, alkyl phenols, and flavonoids from leaves and bark may be responsible of bactericidal activity.[22]
C guianensis: Benzyl benzoate (from leaves against Gram-positive bacteria) and saponins, flavonoïds, and tanins from leaves and bark may be active compounds.[23,24,25]
E. amazónica: Phytochemical reports have never been published for this species but alkaloids and isoflavonoids are suspected to be responsible of antibacterial activity in other Erythrina species.[26]
C. lechleri: Diterpenoids (antibacterial compounds) but steroids, procyanidins, and alkaloids are considered to be the most active constituents of the sap.[27]
D. rugosa: Experimental data as bio-autography of this chemically unknown species revealed an antibacterial activity due to hydrolysable tannins.
J. copaia: Benzoquinole (jacaranone) was isolated from the leaves and exhibited anti-infectious activities.[28]
O. bataua: Anthocyanins, condensed tannins, stilbenes, and phenolic acids are suspected to be the biological active compounds of the fruit.[29]
P. urinaria: Sesquiterpene as phyllanthocin is known to have antibacterial activity in this genus.[30]
P. macrostachya: Essential oil (with bisabolol, caryophyllene oxide, myristicin, and limonene) from aerial parts may be responisble of the in vitro bactericidal activity.[31]
Some of the plants assayed were quite well-studied (C. lechleri, C. guianensis) (Jones, Al-Dhabi) but their activity had never been evaluated on all those bacteria (or yeast) and permitted to confirm our method and results. Other plants have specific scientific interest for further chemical investigations because they have never been tested on bacteria and their antimicrobial compounds still yet unknown (A. filipes, C samauma, E. machrantus, O. bataua, O. alveolatus, P. guazumaifolia, P. macrostachya, P. munguba, T. sananho).
Regarding this ethnopharmacological study allowed to compare the antimicrobial activities of various plant species, to establish the antimicrobial spectra of standardized plant extracts, and give support to the traditional use of these plants.[32,33,34,35,36,37,38] Further examination by bio-autography is ongoing in order to determine new active constituents. When observing the antimicrobial versatility of C. lechleri on enterobacteria, it should be kept in mind that proanthocyanidins in this resin also display anti-secretoty activity, as has been well established and put into use with the recently marketed crofelemer (Fulyzaq®), in perfect consistency with the traditional uses of numerous tannin-contain plants.[8] This reminds us of the up-to-now unavoidable narrow scope and insufficiencies of screening studies, but it also warns us against too straightforward analysis of numeric values and harsh discarding of traditional remedies, as these assays may only partly highlight the potential usefulness of herbal medicines.
Minimum inhibitory concentration (MIC) of active plants (mg/mL)
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Vincent Roumy
Vincent Roumy, Assistant professor in Pharmacognosy, Institut Charles Viollette, Université Lille.

Andréa-Luz Gutierrez- Choquevilca
Andréa-Luz Gutierrez-Choquevilca, Assistant professor anthropology Ecole pratique des hautes études (EPHE), Laboratoire d'anthropologie sociale, Paris.

Jean Pierre Lopez Mesia
Jean Pierre Lopez Mesia, Student in medicine and phytochemistry, Universidad Nacional de la Amazonía Peruana, Iquitos.

Lastenia Ruiz
Lastenia Ruiz, Professor in Phytochemistry, Universidad Nacional de la Amazonía Peruana, Iquitos.

Juan Celidonio Ruiz Macedo
Juan Celidonio Ruiz Macedo, botanist, Universidad Nacional de la Amazonía Peruana, Iquitos.

Amin Abedini
Amin Abedini, PhD Pharmacognosy, Institut Charles Viollette, Université Lille.

Ameni Landoulsi
Ameni Landoulsi, Student in Pharmacognosy Institut Charles Viollette, Université Lille.

Jennifer Samaillie
Jennifer Samaillie, Technical assistant, Pharmacognosy, Institut Charles Viollette, Université Lille.

Thierry Hennebelle
Thierry Hennebelle, Professor in Pharmacognosy, Institut Charles Viollette, Université Lille.

Céline Rivière
Céline Rivière, Assistant professor in Pharmacognosy, Institut Charles Viollette, Université Lille.

Christel Neut
Christel Neut, Assistant professor in microbiology, Laboratoire de Bactériologie, Faculté des Sciences Pharmaceutiques et Biologiques, Université Lille.
Acknowledgments
The authors gratefully acknowledge: Séverine Mahieux (from the laboratory of bacteriology, Lille 2), the ARPIA Peru association (Association pour la Recherche sur les Pharmacopées indigenes d’Amazonie), the EDF foundation (Electricité De France), the Nord Pas de Calais department (SISA: Solidarité Ici Solidarité Ailleurs), as well as Clara Mori, Corina Güivinsinti and other Peruvian people who were willing to share with us their knowledge about medicinal plants. The authors declare that they have no conflict of interest.
REFERENCES
- 1.Arias CA, Murray BE. The rise of the Enterococcus: Beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266–78. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: The epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–7. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 3.Gould IM, David MZ, Esposito S, Garau J, Lina G, Mazzei T, et al. New insights into meticillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int J Antimicrob Agents. 2012;39:96–104. doi: 10.1016/j.ijantimicag.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Gyssens IC. Antibiotic policy. Int J Antimicrob Agents. 2011;38(Suppl):11–20. doi: 10.1016/j.ijantimicag.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Unemo M, Shafer WM. Antibiotic resistance in Neisseria gonorrhoeae: Origin, evolution, and lessons learned for the future. Ann N Y Acad Sci. 2011;1230:E19–28. doi: 10.1111/j.1749-6632.2011.06215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zumla A, Hafner R, Lienhardt C, Hoelscher M, Nunn A. Advancing the development of tuberculosis therapy. Nat Rev Drug Discov. 2012;11:171–2. doi: 10.1038/nrd3694. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan W, Laing R. Geneva, Switzerland: World Health Organization; 2004. Priority Medicines for Europe and the World: A Public Health Approach to Innovation. [Google Scholar]
- 8.Macarthur RD, Hawkins TN, Brown SJ, Lamarca A, Clay PG, Barrett AC, et al. Efficacy and safety of crofelemer for noninfectious diarrhea in HIV-seropositive individuals (ADVENT trial): A randomized, double-blind, placebo-controlled, two-stage study. HIV Clin Trials. 2013;14:261–73. doi: 10.1310/hct1406-261. [DOI] [PubMed] [Google Scholar]
- 9.Dobkin de Rios M. The vidente phenomenon in third world traditional healing: An Amazonian example. Med Anthropol. 1984;8:60–70. doi: 10.1080/01459740.1984.9965889. [DOI] [PubMed] [Google Scholar]
- 10.Gow P, Thomas N, Humphrey C. Ann Arbor, Mishigan: The University of Michigan Press; 1994. River People: Shamanism and History in Western Amazonia. In Shamanism, History and the State. [Google Scholar]
- 11.Galy S, Rengifo E, Hay YO. Factores de la organización del mercado de las plantas medicinales en Iquitos – Amazonía Peruana. Folia Amaz. 2000;11:139–58. [Google Scholar]
- 12.Gutierrez-Choquevilca AL. Sisyawaytii tarawaytii. Sifflements serpentins et autres voix d’esprits dans le chamanisme quechua du haut Pastaza (Amazonie péruvienne) J Soc Am. 2011;97:179–222. [Google Scholar]
- 13.Luna LE. The concept of plants as teachers among four mestizo shamans of Iquitos, northeastern Peru. J Ethnopharmacol. 1984;11:135–56. doi: 10.1016/0378-8741(84)90036-9. [DOI] [PubMed] [Google Scholar]
- 14.Luna LE. Stockholm: Almqvist and Wiksell International; 1986. Vegetalismo shamanism among the mestizo population of the Peruvian Amazon. [Google Scholar]
- 15.Jauregui X, Clavo ZM, Jovel EM, Pardo-de-Santayana M. “Plantas con madre”: Plants that teach and guide in the shamanic initiation process in the East-Central Peruvian Amazon. J Ethnopharmacol. 2011;134:739–52. doi: 10.1016/j.jep.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Tournon J, Reategui U. Enfermedad y medicina entre los Shipibo-Conibo del Alto Ucayali. Amaz Peru. 1988;8:83–97. [Google Scholar]
- 17.Alexiades M. United States: University of New York; 1999. Ethnobotany of the Ese Eja: Plants, Health, and Change in an Amazonian Society. Thesis. [Google Scholar]
- 18.Gutierrez-Choquevilca AL. Imaginaire acoustique et apprentissage d’une ontologie animiste. Le cas des Quechua d’Amazonie péruvienne, Ateliers d’Anthropologie du LESC. 2010:34. [Google Scholar]
- 19.Vol. 2. Wayne, New Jersey, PA: CLSI; 2006. Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard M7-A7. [Google Scholar]
- 20.Das K, Tiwari RK, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J Med Plants Res. 2010;4:104–11. [Google Scholar]
- 21.Sanz-Biset J, Cañigueral S. Plant use in the medicinal practices known as “strict diets” in Chazuta valley (Peruvian Amazon) J Ethnopharmacol. 2011;137:271–88. doi: 10.1016/j.jep.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Ayepola OO, Ishola RO. Evaluation of Antimicrobial Activity of Anacardium occidentale (Linn.) J Med Dent Sci. 2009;3:1–3. [Google Scholar]
- 23.Al-Dhabi NA, Balachandran C, Raj MK, Duraipandiyan V, Muthukumar C, Ignacimuthu S, et al. Antimicrobial, antimycobacterial and antibiofilm properties of Couroupita guianensis Aubl. fruit extract. BMC Complement Altern Med. 2012;12:242. doi: 10.1186/1472-6882-12-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinheiro MM, Fernandes SB, Fingolo CE, Boylan F, Fernandes PD. Anti-inflammatory activity of ethanol extract and fractions from Couroupita guianensis Aublet leaves. J Ethnopharmacol. 2013;146:324–30. doi: 10.1016/j.jep.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 25.Sivakumar T, Shankar T, Baskar PV, Geetha G. Efficacy of Couroupita guianensis against selected human pathogens. Adv Biol Res. 2012;6:59–63. [Google Scholar]
- 26.Tanaka H, Sato M, Oh-Uchi T, Yamaguchi R, Etoh H, Shimizu H, et al. Anti-bacterial properties of a new isoflavonoid from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus. Phytomedicine. 2004;11:331–7. doi: 10.1078/0944711041495137. [DOI] [PubMed] [Google Scholar]
- 27.Jones K. Review of sangre de drago (Croton lechleri) – A South American tree sap in the treatment of diarrhea, inflammation, insect bites, viral infections, and wounds: Traditional uses to clinical research. J Altern Complement Med. 2003;9:877–96. doi: 10.1089/107555303771952235. [DOI] [PubMed] [Google Scholar]
- 28.Valadeau C, Pabon A, Deharo E, Albán-Castillo J, Estevez Y, Lores FA, et al. Medicinal plants from the Yanesha (Peru): Evaluation of the leishmanicidal and antimalarial activity of selected extracts. J Ethnopharmacol. 2009;123:413–22. doi: 10.1016/j.jep.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 29.Rezaire A, Robinson JC, Bereau D, Verbaere A, Sommerer N, Khan MK, et al. Amazonian palm Oenocarpus bataua (“patawa”): Chemical and biological antioxidant activity – phytochemical composition. Food Chem. 2014;149:62–70. doi: 10.1016/j.foodchem.2013.10.077. [DOI] [PubMed] [Google Scholar]
- 30.Macrae WD, Hudson JB, Towers GH. Studies on the pharmacological activity of Amazonian euphorbiaceae. J Ethnopharmacol. 1988;22:143–72. doi: 10.1016/0378-8741(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 31.de Lira PN, da Silva JK, Andrade EH, Sousa PJ, Silva NN, Maia JG. Essential oil composition of three Peperomia species from the Amazon, Brazil. Nat Prod Commun. 2009;4:427–30. [PubMed] [Google Scholar]
- 32.Arévalo Valera G. El ayahuasca y el curandero Shipibo-Conibo del Ucayali (Perú) Am Indíg. 1986;46:147–61. [Google Scholar]
- 33.Vasquez R. Useful Plants of the Amazonian Peru. Beltsville: Field Work USDA National Agricultural Library; 1990. [Google Scholar]
- 34.Duke JA, Vasquez R. Amazonian Ethnobotanical Dictionary. Boca Raton: CRC Press; 1994. [Google Scholar]
- 35.Jovel EM, Cabanillas J, Towers GH. An ethnobotanical study of the traditional medicine of the Mestizo people of Suni Miraño, Loreto, Peru. Ethnopharmacol. 1996;53:149–56. doi: 10.1016/0378-8741(96)01437-7. [DOI] [PubMed] [Google Scholar]
- 36.Mejia K, Rengifo E. Plantas Medicinales de Uso Popular en la Amazonia Peruana. 2nd ed. Lima: Tarea Asociación Grafica Educativa; 2000. [Google Scholar]
- 37.Rutter RA. Catalogo de Plantas Útiles de la Amazonia Peruana. Pucallpa: Instituto Linguistico de Verano; 1990. [Google Scholar]
- 38.Vilaça A. Making kin out of others in Amazonia. J R Anthropol Inst. 2002;8:347–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Minimum inhibitory concentration (MIC) of active plants (mg/mL)


