Abstract
Background
In children, integration of HIV in MNCH services has been shown to incr. ease uptake of early infant diagnosis. This article examines bottlenecks and opportunities for scaling up integrated pediatric HIV services in Nepal.
Methods
This is a descriptive study using both mixed qualitative and quantitative methods, conducted in January 2015 in 19 facilities in five regions of Nepal most affected by HIV epidemic. The qualitative methods comprised in-depth structured interviews with key informants (leadership of The National Center for AIDS and STD Control and National Public Health Laboratory, district management teams, medical officers in charge of health facilities and HIV clinics, frontline staff at antenatal care and HIV clinics and laboratory). The quantitative methods were used to abstract data of HIV-infected pregnant women seen between January and December 2014, HIV-exposed infants aged less than 12 months, and HIV infected children aged less than 15 years who were initiated HIV treatment from 2010 to 2014. Structured tools were used to collect data which were analysed using IBM SPSS.
Results
Of the 19 facilities assessed, 18(98%), 18(98%), 14(75%), and 11(58%) provided prevention of mother-to-child transmission (PMTCT), Expanded Program on Immunization (EPI), pediatric ART and nutrition rehabilitation services, respectively. However, only 1(5%) facility collected onsite dried blood spots (DBS) for PCR HIV testing and 6(32%) facilities provided counselling and referral for DBS. In 2014, of the 121 HIV-exposed infants recorded, only 21(17%) received PCR test. The median turnaround time of the PCR test results was 54 days. Of the 21 records with PCR test, 11(52.5%) were from PMTCT clinics, 7(33%) from Nutritional rehabilitation clinics, and 3(14.5%) from pediatric outpatient clinic.
Conversely, 934 children were initiated ART between 2010 and 2014, of which 5% were infants and 29% aged between 1 and 5 years. 298(32%) had comorbidities of which 64% had malnutrition. A total of 534(57%) had tuberculosis (TB) status assessed of which 58(11%) had active TB. Infants had lowest retention (63%), high mortality (17.4%), and loss to follow-up (10.9%).
Conclusion
Few facilities collect DBS and few children receive PCR tests with limited linkage to ART. This has led to late ART initiation, comorbidities, including TB coinfections and poor outcomes. The results indicate that there are opportunities for improving HIV case finding among HIV-exposed infants in PMTCT, EPI, TB, and nutrition services if provider initiated testing and counselling at the point of service delivery is institutionalized in these settings.
Keywords: dried blood spots, nutrition, pediatric ART, prevention of mother-to-child transmission
INTRODUCTION
There has been 2.3 (1.9–2.7) million new HIV infections globally, showing a 33% decline in the number of new infections from 3.4 (3.1–3.7) million in 2001 [1]. Some of the greatest reductions seen in new infections have been among newborn, declining from 530 000 in 2000 to 260 000 in 2013, as a result of national and global efforts invested in prevention of mother-to-child transmission (PMTCT) programs [2].
Nepal had a HIV prevalence rate 0.2% (0.2–0.3%) among adults of 15–49 years of age; with 1900 (1500–2500) of children 0–14 years of age living with HIV in 2013. According to Nepal National Center for AIDS and STD Control (NCASC), Nepal's HIV epidemic is largely concentrated among most at risk populations and some migrants. HIV prevalence rates among intravenous drug users (IDUs) in 2011 in Kathmandu was 6.3% and there were between 24 649 and 28 359 female sex workers in Nepal with an estimated HIV prevalence rates of 1.7%. HIV infection rate among street-based sex workers in the Kathmandu Valley is 4.2%. Nationally, clients of female sex workers accounted for 4.4% of total estimated HIV infections [3]. In addition, high number of sex workers who migrated or were trafficked to Mumbai, India to work, increase HIV prevalence rates in the sex workers’ network in Nepal, [3]. Children born from these groups of populations were the most at high risk of contracting HIV mainly during perinatal period.
Recently, the NCASC developed a new National HIV/AIDS Strategy 2011–2016, which highlights prevention, including prevention PMTCT as a key strategic direction. National Public Health Laboratory (NPHL) has been equipped to perform early infant diagnosis (EID); and the linkages among health facilities and referral system put in place. However, the coverage of pediatric HIV care remains unacceptably low [4–6]. Limited follow-up with mother–child pairs up to 18 months postpartum and EID, were still critical gaps in the current pediatric HIV programs [7]. Other missed opportunities in these settings included the systemic screening, prevention, and treatment of tuberculosis (TB) and viral hepatitis.
The study aim is to describe the delivery of pediatric HIV care and treatment program setup with regard to the comprehensiveness of EID services for HIV-exposed and ART services for HIV-infected children. The clinical profile and retention in care, and HIV managers’ and healthcare providers’ perspectives toward pediatric HIV program have also been investigated.
METHODS
Population
This was a retrospective review of routinely recorded data for EID and pediatric ART program, retrieved at selected health facilities in Nepal between January and February 2015. No children neither women/mothers were interviewed.
The selection of sites was based on the regions that are most affected by HIV epidemic according to the National surveillance report of the country. The criteria for selection included the following: having introduced HIV services, geographical locations (urban/rural), level (tertiary to community), ownership (public/private), and having high patients’ load (more than 10 cases of HIV infection in 2014). Thus, 19 Health facilities were selected and are shown in Table 1.
Table 1.
Health facilities assessed
| Region | Health facility | Geographic location |
| Eastern | 1. Mechi Zonal Hospital, Bhadrapur | Urban |
| 2. Kosi Zonal hospital, Biratnagar | Urban | |
| 3. BPKIHS, Dharan | Urban | |
| 4. Itahari PHC | Rural | |
| 5. Sonapur subhealth post | Rural | |
| Central | 1. Sukra Raj Tropical and Infectious Disease Hospital, Kathmandu | Urban |
| 2. Kanti Children's Hospital, Kathmandu | Urban | |
| 3. TU Teaching Hospital, Kathmandu | Urban | |
| 4. Bharatpur Hospital, Chitwan | Urban | |
| 5. Khaireni PHC, Chitwan | Rural | |
| Western | 1. Western Regional Hospital, Pokhara | Urban |
| Mid-western | 1. Bheri Zonal Hospital, Nepalgunj | Urban |
| 2. Mid-western Regional Hospital, Surkhet | Urban | |
| 3. Mehal Kuna PHC, Surkhet | Rural | |
| Far-west | 1. Mahakali Zonal Hospital, Mahendranagar | Urban |
| 2. Sub-Regional Hospital, Dadeldhura | Urban | |
| 3. Doti Hospital, Doti | Rural | |
| 4. Seti Zonal Hospital, Dhangadi | Urban |
The abstraction of data was done from HIV clinics, antenatal care clinics and maternity wards. Interviews were conducted with Key informants (KI) from all these 19 health facilities. Only laboratories with complete and up-to-date data for HIV infection up to the month preceeding the study were considered. Thus, only nine laboratory registers were analysed. Among these nine laboratories one was private, which was supported by Family Health International (FHI).
Study design
This was a descriptive cross-sectional study. We collected data between January and February, 2015 for quantitative analysis and conducted interviews for qualitative study of the pediatric program. The interview was conducted with the leadership of NCASC and NPHL at the central level, district management teams, medical officers in charge of health facilities and HIV clinics, frontline staff at antenatal care and HIV clinics and laboratory. The assessment used questions that have been utilized in similar efforts in other countries in similar efforts. Thus, it was not necessary to pilot testing them. The interview lasted approximately 15–20 min. It covered the following four sets of questions that were designed to identify downstream, midstream, and upstream of pediatric HIV care and treatment, among which included: aspects related to the policy framework, national guidelines, strategy on HIV/AIDS, and service delivery for prevention mother-to-child transmission of HIV (PMTCT), HIV-exposed, and infected children. The elements in policy framework that were looked at included the coordination and decentralization of pediatric HIV services, data-informed planning and budgeting plan, organizational and delivery model of HIV testing services for pregnant women and HIV-exposed children, community engagement and participation, supply chain management, overarching services design, EID and antiretroviral treatment (ART). Questions for KI at national level also looked at the legal environment such monitoring, and task shifting/sharing for HIV treatment and care for children. For the district health management, the questions were related to the supervision and local coordination of interventions. The questions related to operations of activities and service provisions were asked to the health facility in-charge and frontline staff (physicians, nurses, and counselors) at HIV clinics.
The collection of medical recorded data was conducted following the PMTCT cascade between January to December 2014 for all HIV-exposed children aged between 1 and 12 months; and HIV-infected children below 15 years who were treated from 2010 to 2014 at selected sites. Registers at HIV clinics, antenatal care, and maternity wards were the primary source of these data. Registers for PMTCT, HIV-exposed children and pediatric HIV care from visited facilities were used to estimate the coverage of services and median value of HIV rapid test for pregnant women and children above 12 months of age, EID, cotrimoxazole prophylaxis (CPT), clinical profile, regimen of ART, CD4 trend, lost to follow up (LTFU), and outcomes. Data identifying women and children in this study were not collected.
Data analysis
Data were recorded in excel sheet and imported into IBM Statistical Package for the Social Sciences 23 software for analysis. We used descriptive statistics to assess our results. Quantitative indicators were measured for initiation of antiretroviral (ARV) prophylaxis for the newborn; EID, initiation of CPT and ART for children. The retention in care was measured by the rates of continuation of follow-ups. The additional variables included the clinical profile, WHO clinical staging, the HIV treatment regimens, and rates of TB/HIV coinfections. Transfer-outs and stopped treatment were excluded as outcomes from the analysis.
For qualitative analysis, we transcribed each open question of the interview and enter it into Quality System Regulation (QSR) Nvivo10. This software allowed us to utilize the data for the creation of concepts, categories, and themes, as opposed to relying on predetermined analysis items. Once the codebook was developed, two of our collaborators independently coded the interviews. Thus, QSR Nvivo10 supported the data analysis and identified trends in the data. The analysis itself was an inductive process, which allowed themes to emerge from the data. The themes were coded into categories which were continuously refined throughout the analysis. Finally, relationships between categories were created and inferences were made. The analysis and its process were discussed among the team members.
Ethical considerations
Personal identifying data were all removed to ensure confidentiality and privacy of patient information and cleared by relevant authorities. The National Health Research Council has approved the study protocol for ethical clearance.
RESULTS
Profile of health facilities and key informants
A total of 67 staff (KI) at different levels of HIV program management and implementation from all five regions and the 19 selected health facilities were interviewed. Fifty seven percentage were men and 43% women; and 53% of them have been working for more than 10 years. There were 41% healthcare providers, 28% district health management staff, 28% hospital managers, and 3% national HIV management staff. 52% respondents were aged between 41 and 50; and 27% were 31–40 years old. The mean of age was (mean ± SD = 45 ± 6; minimum = 25; maximum = 60 years).
Quantitative data
Knowledge of policy, guidelines, and organization of HIV programs
Just over three out of 10 KI knew Nepal HIV policy, guidelines, Annual plan to scale up EID, Annual plan to scale up Initiation of HIV treatment in children, Annual plan to scale up retention of HIV-infected children task shifting policy for pediatric HIV treatment and care.
Availability of pediatric HIV care at health facilities
Out of 19 facilities surveyed, only 31% offered counseling and referrals for EID services to either to Kathmandu or to project run by FHI360. Stock-out for HIV kits and cotrimoxazole for the last 3 months have been reported, respectively in 3% and 18% of health facilities, respectively. Prescriptions for HIV treatment were dispensed by lay counsellors and nurses in 16% and 84% health facilities, respectively. Eighty percentage and 20% of pediatric ART were initiated by pediatricians and medical officers, respectively. Only one percentage of facilities had trained nurses on the collection of DBS and 10% of them had trained laboratory staff on DBS collection. Nurses at HIV clinics were dispensing ARV treatments in 84% of facilities and the remaining 16% were done by lay counselors.
Sixty percentage of facilities had nutritional rehabilitation clinics and 95% of these 19 facilities were offering EPI services to the population. Only 10% of them had trained staff for the collection of DBS and none of them were nurses. Pediatricians and medical officers prescribed ART for children in respectively 80 and 20% of health facilities. Eighteen and 3% of health facilities had stock-out for the last 3 months for cotrimoxazole and HIV testing kits, respectively. Five percentage of health facilities did not assist HIV-infected pregnant women to deliver onsite preferring to refer them to other facilities. No reason was provided to why this is done so (Fig. 1).
FIGURE 1.
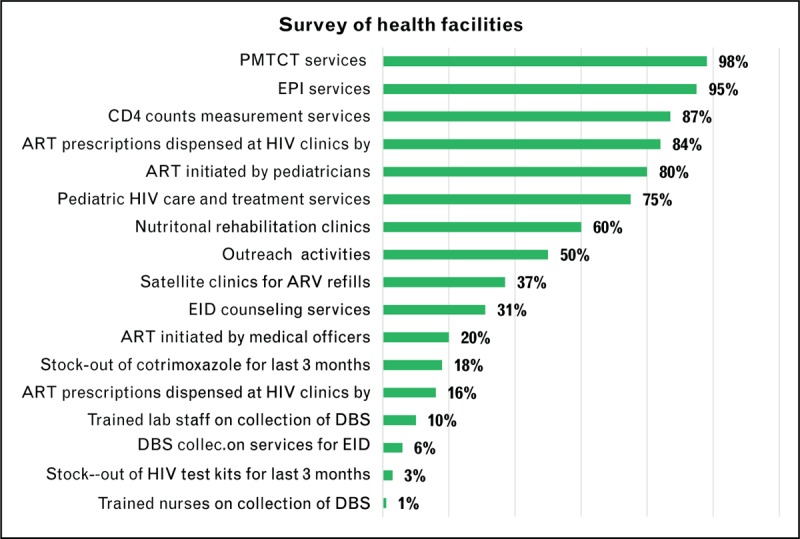
Survey of HIV related services in selected 19 health facilities.
Early infant diagnosis tests
Only 17% of health facilities offered EID services. The prescription of HIV DNA/PCR tests varied between health facilities. Facilities in Mid-West, Western and Central regions prescribed EID tests more than Eastern region. Most of EID tests were prescribed from Far-West and Central regions, except for BPKIHS in Dharan. These all tests were done through FHI 360 program. Only 27% (21/78) of HIV-exposed infants were tested for HIV using DNA/PCR technology as seen in Fig. 2. Approximately, 43% (9/21) of infants had the first PCR test between 11 and 16 weeks, and the remaining 29% (7/21) children were tested between 17 and 20 weeks of age. The mean overall turnaround time for PCR tests was 54 days. Of the 21 records with PCR test results abstracted, 52.5% (11/21) were from PMTCT settings, 33% (7/21) from Nutritional rehabilitation clinics, and 14.5% (3/21) from pediatric outpatient clinic. There were no DBS from EPI clinics despite their high coverage in the country. The HIV prevalence rates among the 21 infants varied from 6 to 20 weeks. These HIV prevalence rates were 5% at 6–10 weeks, 7% at 11–19 weeks, and 6% at 17–20 weeks of age.
FIGURE 2.
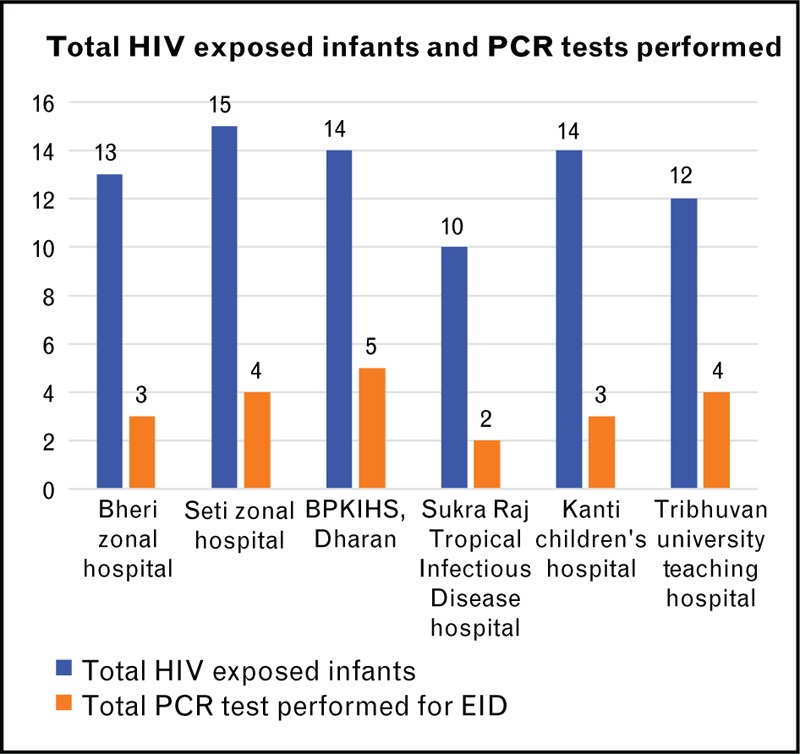
Early infant diagnosis for HIV.
Cotrimoxazole prophylaxis
About 78% of infants received cotrimoxazole prophylaxis treatment (CPT) at 6 weeks of age, this proportion increased to 88% at 10 weeks, at 95% by the age of 12 weeks, and all infants were on CPT by the age of 16 weeks of age. Twenty two percentage (16/78) infants did not receive CPT at 6 weeks of age because it was out of stock. Cotrimoxazole was dispensed by ART clinic nurses and/or counsellors.
Services provided to HIV-infected children
Just 934 children have treated with ART between 2010 and 2014. Malnutrition (64%), gingivitis (63%), respiratory infections (45%), and muco-cutaneous diseases (34%) were the most common clinical presentations. Twenty two (13.1%) of children were asymptomatic.
About ART regimen pattern for children, the most commonly used ART regimen was AZT + 3TC + NVP with 57% among all records for children aged between 1 and 4 years, followed by d4T + 3TC + NVP in 29%, d4T + 3TC + EFV in 9% of children, and TDF + 3TC + LPV/r in 5% of children. The majorities of children on ART were aged between 5 and 15 years, and represented 66% of the overall data abstracted from all 19 health facilities.
ART outcomes
The outcomes of ART were well documented in 836 records out of a total of 934 data after 12 months of initiation of ART. Of 836 children on ART, 65% of children were aged between 5 and 15 years and 95% among them had cumulative death rates of 5.5%. Infants (<1 year) were the most vulnerable and represented 4.9% of the cohort with the lowest ART initiation (63%) and the highest death rates of 17.4%, most probably because of low EID uptake. The children aged between 1 and 4 years were the second least enrolled children on ART in comparison to other age groups. LTFU levels were at 11% for infants and between 7 and 9% for the remaining of the age groups. There has been no difference between urban and urban facilities. The registers do not have data related to the outcomes of those who were lost to follow-up, as seen in Table 2.
Table 2.
Summary of ART outcomes
| Age group | Cohort | On ART | Death | LTFU |
| <1 year | 46 (4.9%) | 29 (63%) | 8 (17.4%) | 5 (10.9%) |
| 1–4 years | 275 (29.4%) | 199 (72.4%) | 9 (3.3%) | 19 (6.9%) |
| 5–9 years | 311 (33.3%) | 298 (95.8%) | 11 (3.5%) | 27 (8.7%) |
| 10–15 years | 302 (32.3%) | 300 (99.3%) | 7 (2.3%) | 22 (7.3%) |
| Total | 934 (100%) | 836 (9.5%) | 34 (3.6%) | 73 (7.8%) |
ART adherence and retention in care
This variable was poorly provided in all records for children. We have used refills of prescriptions as proxy to adherence. Considering this approach, there were 356 records that provided adequate information on refills. Out of these 356 records, only 15% (53/356) records did not show regular collection of refills.
Qualitative analysis
Bottlenecks and causes
The key bottlenecks and related causes on the above findings are summarized in Table 3. This includes enabling environment, supply, and demand of services. Twenty four percent of pregnant women have delivered without knowing their HIV status.
Table 3.
Key bottlenecks and related causes
| Area of intervention | Domain of intervention | Key bottlenecks | Causes |
| Enabling environment | Policy | HIV testing services are still fragmented | Collection of blood samples are done by only laboratory staff |
| Inconsistence of policy on gratuity of health services for pregnant women and children | Payment of services in one region and gratuity in another one | ||
| Service delivery system | Task shifting is not well articulated in the policy | Unclear messages | |
| Initiation of ART is done in all health facilities | Not all clinicians are trained on pediatric HIV treatment | ||
| Human resource not trained on collection of dried blood spots for early infant diagnosis | No available data on trainings conducted and current coverage of trained staff across health facilities |
DISCUSSION
This study describes the implementation of pediatric HIV care and treatment program in some regions of Nepal, with emphasis on the HIV program staff's perspectives and pediatric HIV care cascades. There are eleven main findings. First, there is no clear understanding of policies on EID services and task shifting. Study observed that few HIV program staff were still not sure whether EID services can be provided in the all country or not and that task shifting can help to increase DBS collection and initiation of ART. This situation can be improved with staff orientation and dissemination of all national documents, training, and closed supervision as found by previous investigators [8,9].
Second, the lack of money to pay for other blood tests, husband permission before testing, stigma [10], and out-stock of testing kits were the main reasons for pregnant women giving birth without knowing their HIV status. Because, HIV tests were performed with blood drawn for other tests which are charged for at laboratory, if women did not pay for these other blood tests, HIV tests will also be deferred until such time the payment is made.
Third, the study showed that the prescription of HIV DNA/PCR tests varied between health facilities, with Mid-West, Western, and Central regions prescribing more than Eastern region. Very few HIV-exposed infants were offered EID services because of low-skilled staff, operational challenges, organizational model, and socio-economic situations of parents [11–13]. The mean overall turnaround time for all PCR tests was 54 days. This long turnaround time was mainly due to the lack of money for parents to bring the children for blood collection facilities, at FHI360 site or NLHP in Kathmandu, the only sites where DBS could be collected. Most of DBS were from PMTCT, Nutritional rehabilitation clinics, and pediatric outpatient clinic; and no DBS from EPI clinics despite their high coverage in the country. The study underlined the need of integrating EID with other MNCH services, as shown in other studies [14].
Forth, the supply of commodities was a serious challenge in some settings. Twenty two percentage of HIV-exposed infants did not receive CPT at 6 weeks of age because it was out of stock, as found. by many investigators [15–18].
Fifth, HIV-infected children were treated very late and limited numbers of them were screening for TB and viral hepatitis. Like previous investigators, we found a high prevalence rate of TB/HIV coinfections [19–20] and the prevalence rates of viral hepatitis coinfections were also high in children living with HIV [21–23].
Sixth, the study observed a low rate of retention in care and a very high LTFU rate; as shown by previous studies [24–30]. Caregivers and healthcare providers’ behaviors and attitudes impact on the retention in care. These characteristics included low socio-economic status and level of household support and issues such as lack of food, time constraints to bring the child to the health facilities, perceptions that the child is healthy in the absence of symptoms, stigma, religious beliefs, and male partner support [31].
Seventh, the knowledge of professionals in charge of HIV programs on policies, strategies, guidelines, and priority of pediatric HIV program is critical to delivery services [32]. This was weak in this study. This situation can be improved with training and closed supervision [33]. PMTCT has shown to be cost-effective intervention that leads to the uptake of EID and pediatric HIV care and treatment. However, the identification of pregnant women living with HIV and follow-up of pair mother–child remain a challenge in many settings [34–37] like in this study.
Eighth, this study found that the long-standing health-systems issues were the most common reason for the long turnaround time, including cost of transportation for the collection of DBS and referral of children to the laboratory instead of blood samples at the two sites of collection for DBS in all country, which was due to limited skilled staff and service accessibility, and community-level factors (particularly stigma, fear of disclosure and lack of partner support) [38,39], and weak integration [40].
Ninth, study observed that the delay in initiation CPT was delayed because of unavailability of cotrimoxazole at HIV clinics rather than failure for prescriptions.
Tenth, study observed that 42% of HIV infected children were at WHO stage II; 38% at stage III; 17% at stage IV, and the remaining 3% at stage I [41]. Sixty six percentage of children were 5–14 years old, 29% were 1–4 years old; and only 5% of children were less than 12 months old. The study observed that TB screening were conducted only in 11% of HIV-infected children.
Eleventh, adherence to ART and retention in care are still a big challenge as shown by previous study [42]. We observed that adherence to ART was poorly provided in all records for children. Using prescription refills as proxy, 15% records did not show regular adherence; and this can be improved by engaging with parents, strengthening community support, and usage of mHealth technologies, as evidenced by others [43–44].
LIMITATIONS OF THE STUDY
This study did not look at quality of care. Although the quality of care can be assessed using the quality of pediatric ART services using 2010 WHO guidelines, this approach has limitation on its own. For example, the timing that laps between the diagnosis and the initiation of ART does not provide the quality of services per se. Thus, assessment of quality should look at processes that have been applied to meet a set of criteria which ensure that the services have achieved the acceptable level as set by guidelines and standards. Unfortunately, there were no data in any register at health facilities that recorded quality events, quality materials, and quality metrics which can be linked to the measurement of quality of services. This is an area that needs more attention.
Nepal has adapted all recommended registers from WHO. These registers include all variables and interventions related to good pediatric HIV care and treatment; and are available at all selected facilities. However, these tools are not filled in regularly with complete data. Only one facility was moving to electronic system.
The fact that the government wished that the assessment be limited to the health facilities that had big number of people living with HIV, could limit the real picture of overall regions, because data from these facilities excluded data from other health facilities in the study. However, as the excluded data represented not more 10% than of the total number of PLHIV seen during this period of the study, we estimated that the impact of this bias was minimal.
CONCLUSION
Few facilities collect DBS and few children receive PCR tests with limited linkage to ART. This has led to late ART initiation, comorbidities, including TB coinfections, and poor outcomes. The results indicate that there are opportunities for improving HIV case finding among HIV-exposed infants in PMTCT, EPI, TB, and nutrition services if provider initiated testing and counselling at the point of service delivery are institutionalized in these settings (Tables 4 and 5).
Table 4.
Key bottlenecks and related causes for enabling environment and supply of services (continue)
| Area of intervention | Domain of intervention | Key bottlenecks | Causes |
| Coordination | Regular supervision is limited | Irregular support from the supervisors | |
| HIV program is vertical within health facilities | Weak integration of HIV with other health services program | ||
| Management and planning | District annual plans fall short on addressing the bottlenecks | No bottlenecks analysis conducted to inform planning | |
| Budget/financing | District budget and HIV budget are not known | Lack of detailed budget for many activities under pediatric HIV and | |
| Ensuring quality | Not much is done to ensure quality | No quality improvement plan | |
| Supply factors | Accessibility of early infant diagnosis services | early infant diagnosis is limited to few sites | No decentralization of services |
| Skilled staff to perform specific tasks (dried blood spots…) | Only laboratory staff are allowed to collect blood samples | Limited trained staff | |
| Operations system in place to transfer specimen to lab and reception of results | Sending parents and children to another city and town just for collection blood samples | Poor system for collection and transfer of blood samples to the lab | |
| Ensuring quality of services through quality improvement efforts | No priority on quality improvement planning | No quality improvement framework activities |
Table 5.
Key bottlenecks and related causes for demand of services
| Area of intervention | Domain of intervention | Key bottlenecks | Causes |
| Demand factors | Access to early infant diagnosis | Limited sites provide these services | Initiation of ART only done at hospitals despite that PHCs having medical officers onsite who can be trained |
| Poverty for population who are affected by HIV | Limited uptake of services | High cost of transportation and living conditions to travel to early infant diagnosis collection sites and ART clinics | |
| Stigma and discrimination | Cultural and religious beliefs | Some hospitals refer HIV-infected pregnant women even for normal delivery because of HIV status |
Acknowledgements
Special thanks to Ronawk Khan, UNICEF, Nepal for her support.
Financial support and sponsorship
UNICEF Nepal has provided financial support to conduct this study.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Global Report UNAIDS. www.unaids.org/en/resources/campaigns/globalreport 2013. [Accessed 10 January 2014]. [Google Scholar]
- 2.UNAIDS. Global Plan for the elimination of mother-to-child transmission of HIV and keeping their mothers alive 2011. www.unaids.org/documents [Accessed 3 December 2013]. [Google Scholar]
- 3.UNAIDS. Report on the Global AIDS Epidemic 2013. http://www.unaids.org/globalreport/default.htm [Accessed 24 January 2014] [Google Scholar]
- 4.UNAIDS UNAIDS. Report on the Global AIDS Epidemic 2014. http://www.unaids.org/globalreport/default.htm [Accessed 24 January 2014] [Google Scholar]
- 5.Chetty T, S. Knight, J. Giddy, et al. A retrospective study of Human Immunodeficiency Virus transmission, mortality and loss to follow-up among infants in the first 18 months of life in a prevention of mother-to-child transmission programme in an urban hospital in KwaZulu-Natal, South Africa. BMC Pediatr 2012; 12:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan Phelpsa B, Saeed Ahmedb, Anouk Amzelc, et al. Linkage, initiation and retention of children in the antiretroviral therapy cascade: an overview. AIDS 2013; 27:S207–S213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran DA, Shakeshaft A, Ngo AD, et al. Structural barriers to timely initiation of antiretro-viral treatment in Vietnam: findings from six outpatient clinics. PLoS One 2012; 7:e51289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ditekemena J, Koole O, Engmann C, et al. Determinants of male involvement in maternal and child health services in sub-Saharan Africa: a review. Reprod Health 2012; 9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mipando LN, Nkanaunena K, Seyama L, Kumwenda N. Barriers to HIV testing in prevention of mother to child transmission services in Blantyre. Glob Health Action 2013; 6:22780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theilgaard ZP, Katzenstein TL, Chiduo MG, et al. Addressing the fear consequences of stigmatization-a necessary step towards making HAART accesibles to women in Tanzania: a qualitative study. AIDS Res Ther 2011; 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creek TL, Sherman G, Nkengasong J, et al. Infant human immunodeficiency virus diagnosis in resource-limited settings: issues, technologies and country experiences. Am J Obstet Gynecol 2007; 197 (Suppl 3):S64–71. [DOI] [PubMed] [Google Scholar]
- 12.Dube Q, Dow A, Chirambo C, et al. Implementing early infant diagnosis of HIV infection at the primary care level: experience and challenges in Malawi. Bull World Health Organ 2012; 90:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciaranello L, Park J, Ramirez L, et al. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med 2011; 9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rollins N, Little K, Mzolo S, et al. Surveillance of motherto-child transmission prevention programmes at immunization clinics: the case for universal screening. AIDS 2007; 21:1341–1347. [DOI] [PubMed] [Google Scholar]
- 15.Moodley D, Reddy L, Mahungo W, Masha R. Factors associated with coverage of cotrimoxazole prophylaxis in HIV-exposed children in South Africa. PLoS One 2013; 8:e63273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Date AA, Vitoria M, Granich R, et al. Implementation of co-trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull World Health Organ 2010; 88:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zachariah R, Harries AD, Luo C, et al. Scaling-up co-trimoxazole prophylaxis in HIV-exposed and HIV-infected children in high HIV-prevalence countries. Lancet Infect Dis 2007; 7:686–693. [DOI] [PubMed] [Google Scholar]
- 18.Ryan M, Griffin S, Chitah B, et al. The cost-effectiveness of cotrimoxazole prophylaxis in HIV-infected children in Zambia. AIDS 2008; 30: 22:749–757. [DOI] [PubMed] [Google Scholar]
- 19.Elenga N, Kouakoussui KA, Bonard D, et al. Diagnosed tuberculosis during the follow-up of a cohort of human immunodeficiency virus-infected children in Abidjan, Cote d’Ivoire: ANRS 1278 study. Pediatr Infect Dis J 2005; 24:1077–1082. [DOI] [PubMed] [Google Scholar]
- 20.Kouakoussui A, Fassinou P, Anaky MF, et al. Respiratory manifestations in HIV-infected children pre and post-HAART in Abidjan, the Ivory Coast. Paediatr Respir Rev 2004; 5:311–315. [DOI] [PubMed] [Google Scholar]
- 21.Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis 2009; 48:108–114. [DOI] [PubMed] [Google Scholar]
- 22.Cotton MF, Schaaf HS, Lottering G, et al. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis 2008; 12:225–227. [PubMed] [Google Scholar]
- 23.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the prechemotherapy era. Int J Tuberc Lung Dis 2004; 8:392–402. [PubMed] [Google Scholar]
- 24.Braitstein P, Songok J, Vreeman R, et al. Outcomes of HIV-positive and HIV exposed children lot to follow up from large HIV treatment program in western Kenya. J Acquir Immune Defic Syndr 2011; 57:e40–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chetty T, S. Knight, J. Giddy, et al. A retrospective study of human immunodeficiency virus transmission, mortality and loss to follow-up among infants in the first 18 months of life in a prevention of mother-to-child transmission programme in an urban hospital in KwaZulu-Natal, South Africa. BMC Pediatr 2012; 12:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phelpsa BR, Ahmedb S, Amzelc A, Diallo MO. Linkage, initiation and retention of children in the antiretroviral therapy cascade: an overview. AIDS 2013; 27:S207–S213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Posse M, Meheus F, van Asten H, et al. Barriers to access to antiretroviral treatment in developing countries: a review. Trop Med Int Health 2008; 13:904–913. [DOI] [PubMed] [Google Scholar]
- 28.Tran DA, Shakeshaft A, Ngo AD, et al. Structural barriers to timely initiation of antiretro-viral treatment in Vietnam: findings from six outpatient clinics. PLoS One 2012; 7:e51289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MH, Ahmed S, Buck WC, et al. The Tingathe programme: a pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV (PMTCT) cascade of services in Malawi. J Int AIDS Soc 2012; 15 (Suppl 2):17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scanlon ML, Vreeman RC. Current strategies for improving access and adherence to antiretroviral therapies in resource-limited settings. HIV AIDS 2013; 5:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wachira J, Middlestadt SE, Vreeman R, Braitstein P. Factors underlying taking a child to HIV care: implications for reducing loss to follow-up among HIV-infected and -exposed children. SAHARA J 2012; 9:20–29. [DOI] [PubMed] [Google Scholar]
- 32.Bogart LM, Chetty S, Giddy J, et al. Barriers to care among people living with HIV in South Africa: contrasts between patient and healthcare provider perspectives. AIDS Care 2013; 25:843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwapong GD, Boateng D, Agyei-Baffour P, Addy EA. Health service barriers to HIV testing and counseling among pregnant women attending Antenatal Clinic; a cross-sectional study. BMC Health Serv Res 2014; 14:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tudor Car L, Van Velthoven M, Brusamento S, et al. Integrating prevention of mother-to-child HIV transmission programs to improve uptake: a systematic review. PLoS One 2012; 7:e35268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malaju MT, Alene GD. Assessment of utilization of provider-initiated HIV testing and counseling as an intervention for prevention of mother to child transmission of HIV and associated factors among pregnant women in Gondar town, North West Ethiopia. BMC Public Health 2012; 12:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki1 Y, Ali M, Sathiarany V, et al. Prevalence and barriers to HIV testing among mothers at a tertiary care hospital in Phnom Penh, Cambodia. Barriers to HIV testing in Phnom Penh, Cambodia. BMC Public Health 2010; 10:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gourlay A, Birdthistle I, Mburu G, et al. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc 2013; 16:18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bharat S. A systematic review of HIV/AIDS-related stigma and discrimination in India: current understanding and future needs. SAHARA J 2011; 8:138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamla D, Mbori-Ngachab D, Newman M. Evidence from the field: missed opportunities for identifying and linking HIV-infected children for early initiation of ART. AIDS 2013; 27 (Suppl 2):S139–S146. [DOI] [PubMed] [Google Scholar]
- 40.Ramalho LC, Gonçalves EM, De Carvalho WR, et al. Abnormalities in body composition and nutritional status in HIV-infected children and adolescents on antiretroviral therapy. Int J STD AIDS 2011; 22:453–456. [DOI] [PubMed] [Google Scholar]
- 41.Scanlon ML, Vreeman RC. Current strategies for improving access and adherence to antiretroviral therapies in resource-limited settings. HIV AIDS 2013; 5:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bain-Brickley D, Butler LM, Kennedy GE, Rutherford GW. Interventions to improve adherence to antiretroviral therapy in children with HIV infection. Cochrane Database Syst Rev 2011; 12:CD009513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wachira J, Middlestadt SE, Vreeman R, Braitstein P. Factors underlying taking a child to HIV care: implications for reducing loss to follow-up among HIV-infected and -exposed children. SAHARA J 2012; 9:20–29. [DOI] [PubMed] [Google Scholar]
- 44.Scanlon ML, Vreeman RC. Current strategies for improving access and adherence to antiretroviral therapies in resource-limited settings. HIV AIDS 2013; 5:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


