Abstract
Background
Avian pathogenic Escherichia coli strains cause extraintestinal diseases in birds, leading to substantial economic losses to the poultry industry worldwide. Bacteria that invade cells can overcome the host humoral immune response, resulting in a higher pathogenicity potential. Invasins are members of a large family of outer membrane proteins that allow pathogen invasion into host cells by interacting with specific receptors on the cell surface.
Results
An in silico analysis of the genome of a septicemic APEC strain (SEPT362) demonstrated the presence of a putative invasin homologous to the ychO gene from E. coli str. K-12 substr. MG1655. In vitro and in vivo assays comparing a mutant strain carrying a null mutation of this gene, a complemented strain, and its counterpart wild-type strain showed that ychO plays a role in the pathogenicity of APEC strain SEPT362. In vitro assays demonstrated that the mutant strain exhibited significant decreases in bacterial adhesiveness and invasiveness in chicken cells and biofilm formation. In vivo assay indicated a decrease in pathogenicity of the mutant strain. Moreover, transcriptome analysis demonstrated that the ychO deletion affected the expression of 426 genes. Among the altered genes, 93.66 % were downregulated in the mutant, including membrane proteins and metabolism genes.
Conclusion
The results led us to propose that gene ychO contributes to the pathogenicity of APEC strain SEPT362 influencing, in a pleiotropic manner, many biological characteristics, such as adhesion and invasion of in vitro cultured cells, biofilm formation and motility, which could be due to the possible membrane location of this protein. All of these results suggest that the absence of gene ychO would influence the virulence of the APEC strain herein studied.
Keywords: Escherichia coli, APEC, ychO, Virulence, Pathogenicity
Background
Escherichia coli strains that cause diseases outside the intestine are known as extraintestinal pathogenic E. coli (ExPEC). These strains include human uropathogenic E. coli (UPEC), neonatal meningitis E. coli (NMEC) and avian pathogenic E. coli (APEC) [1–3]. APEC are frequently associated with extraintestinal infections in poultry, leading to respiratory or systemic diseases, which are responsible for large economic losses to the poultry industry worldwide [4, 5]. Colibacillosis is a general term used to describe the large number of existing infections, including septicemia, cellulitis, omphalitis, peritonitis, respiratory tract infections, the egg yolk disease and swollen head syndrome. Colisepticemia, the most severe systemic disease, is characterized by pericarditis, perihepatitis and airsacculitis, and it leads to multiple organ failure and death [6, 7]. In APEC, although several virulence factors such as adhesins, secretion and iron uptake systems, increased serum survival and cytotoxic proteins, have already been identified [1, 6, 8], many others could exist and participate in the pathogenicity process. These currently unknown virulence factors could play major roles in pathogenicity and could be significant for the development of measures for controlling the infectious processes.
Adherence to and invasion of host cells are important steps in the pathogenesis of many bacteria [9]. Bacterial adherence is mediated by adhesins, which recognize receptors on the cell surface. Bacteria that invade host cells possess an important advantage in pathogenicity, overcoming the humoral immune response [10]. A large family of bacterial outer membrane proteins facilitates the entry of the pathogen into host cells by allowing tight adherence to and invasion of the cells. This family of proteins interacts with receptors displayed on the cell surface, triggering signaling cascades to rearrange the host cell cytoskeleton and induce the uptake of bacteria [11, 12]. The first two members of this family (intimin and invasin), although acting differently to promote the invasion of host cells, show significant sequence similarity, especially in the amino terminal region [13, 14]. The first invasin (inv) to be described is produced by Yersinia pseudotuberculosis and Y. enterocolitica [14], and it mediates bacterial entry into eukaryotic cells by high-affinity binding to members of the β1 integrin family [12, 15], which are heterodimeric integral membrane proteins that mediate communication between the extracellular environment and the cytoskeleton [16]. The intimins, implicated in attaching and effacing lesions, are produced by enterohemorrhagic (EHEC) and enteropathogenic E. coli (EPEC) [13, 17]. In contrast to invasin, the receptor for intimin binding is Tir (Translocated Intimin Receptor), a protein that is secreted into the host cell membrane by the bacterium itself [18]. The intimins and invasins have similar domain structures: an N-terminal signal sequence, a conserved β-barrel domain, and a C-terminal passenger domain (the transported part of the protein) [19]. The β-barrel structure is necessary for the passenger domain, which mediates interactions with host cells, to cross the outer membrane [20].
The in silico analysis of recently sequenced genomes of some APEC strains [21–23] enabled the identification of possible new virulence genes that could lead to a better understanding of the infectious process. The in silico analysis of the sequenced genome of the APEC strain SEPT362 [22, 24] identified a putative invasin gene, which is homologous to the not yet described ychO gene from E. coli str. K-12 substr. MG1655 (98 % of identity) and is present in 120 sequenced E. coli strains (NCBI). The role of this protein in pathogenicity or biological function of the APEC strains was not previously established, and considering the importance of intimin/invasin-like proteins in other Gram-negative pathogens, this work aimed to test the hypothesis that ychO might contribute to the pathogenesis of APEC strain SEPT362. In this work we showed that the ychO gene is highly expressed in the lungs and spleen during in vivo infection assays by strain SEPT362, what suggests the importance of this gene in in vivo colonization of the host. A mutant strain for gene ychO was constructed for in vivo and in vitro comparative analysis with the complemented strain and its counterpart wild-type strain. In this study, we demonstrated, for the first time, that the gene ychO is expressed in vitro and in vivo and is involved in the bacterial capacity for adhesion to and invasion of cultivated cells in vitro, motility and biofilm formation and also influences the pathogenicity and the expression of many other genes. These properties are important for pathogenicity in vivo, and our results suggest an important role for ychO in the pathogenesis of strain SEPT362.
Results and discussion
In silico characterization of the putative invasin gene ychO
The APEC strain SEPT362 was isolated from the liver of a laying hen presenting clinical signs of septicemia [24]. A survey of the genome of strain SEPT362 [GenBank: AOGL00000000.1] revealed a putative invasin homologous to the ychO gene from E. coli str. K-12 substr. MG1655 (98 % identity). To initiate this work, the re-annotation of this gene using RAST [25] was first performed, which showed a signal peptide sequence of 141 bp that was not present in the GI:449323183 protein annotated in the SEPT362 genome. This peptide was considered to be part of the gene because it is known that this N-terminal signal sequence is an important domain of a typical invasin, predicted to mediate the translocation of the protein from the bacterial cytoplasm through the inner membrane [26]. The ychO product alignment also showed that different E. coli pathotypes possess a peptide signal on this protein, reinforcing the decision to retain this sequence as part of the gene for the mutant construction. This protein contains an N-terminal signal sequence, a conserved β-domain that forms a transmembrane β-barrel structure, and a C-terminal passenger domain that could be exported to the outside of the cell. These domains together are the main characteristics of the intimin/invasin superfamily. The intimin/invasin-like proteins normally form a longer structure, after the passenger domain, composed of repeated bacterial immunoglobulin-like domains (BID). In some cases, C-type lectin-like domains are present at the C-terminus [27]. These structures were not found in the YchO protein.
ychO expression in vitro and in vivo
In this study, first, ychO expression was verified in the APEC strain SEPT362 (Fig. 1). The results showed that this gene is expressed not only in culture conditions but is highly expressed in the lungs and spleen of chicks 24 and 48 h after inoculation, which suggests the importance of this gene in in vivo colonization of the host. With these results, a mutant strain of the ychO gene and a complemented derivative strain containing the wild-type gene on a plasmid were constructed for comparative analysis with the wild-type APEC strain SEPT362. The mutant and complemented strains were verified by quantitative Real Time-PCR (qRT-PCR). The high expression and high standard deviation of ychO expression in complemented strain is related to the number of plasmid copies present in this strain, which has an average of 15 copies per cell [28]. A growth curve was constructed for the three strains, using LB and DMEM media. No differences were found between strains in both growing conditions (data not shown).
Fig. 1.
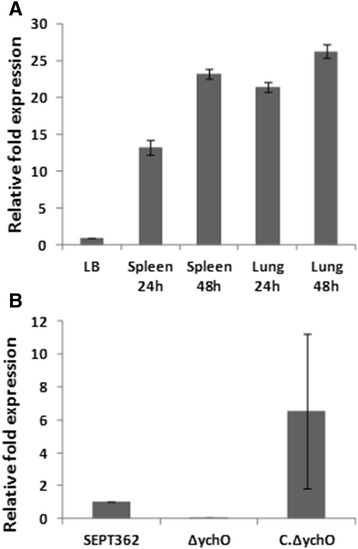
Relative fold expression levels of ychO verified using quantitative Real Time-PCR (qRT-PCR). (a) Expression of ychO in SEPT362 in the spleen and lungs after 24 and 48 h post infection compared to expression in vitro using LB media. (b) In vitro analysis of ychO expression in the SEPT362, ΔychO and complemented strains grown in DMEM medium
The influence of the ychO gene on SEPT362 cell adherence
Invasins are a class of proteins that allow bacteria to penetrate cells, normally by tight adherence to and invasion of eukaryotic cells [11, 12]. Adherence and invasion are important steps for bacterial pathogenesis and contribute to the colonization, persistence and dissemination of a pathogen in the host organism [9, 10, 29, 30]. Therefore, understanding the role of a possible invasin might help to elucidate the mechanisms of invasion and pathogenicity of host cells.
The capacity of the strains to adhere to chicken embryonic fibroblasts cell line CEF was assessed to understand the possible influence of ychO gene on this phenotype. Because type 1 fimbriae binds to D-mannose residues and is important for APEC adherence to chicken cells [31–33], adhesion assays were also performed in the presence of alpha-D-mannopyranoside (D-mannose analog), a potent non metabolized FimH antagonist. The number of bacteria that adhered to pre-fixed CEF cells was significantly lower for the ychO mutant than for the wild-type strain in the presence and absence of methyl-alpha-D-mannopyranoside (Fig. 2a). The decrease in the presence of a D-mannose analog shows that the disorder was not due to a disturb in type 1 fimbriae expression. The complemented strain restored the bacterial adhesion. These data suggest that ychO is involved in SEPT362 adherence. To investigate whether the change in adhesion ability was due to a disturbance in the expression of other genes possibly related to the adhesion capacity of this strain, different bacterial adhesin genes (adhesin fimH [34, 35], E. coli common pilus ecp [36], long polar fimbria subunit A lpfA [37], curlin fimbriae csgA [38], autotransporters aatA [39, 40] and aatB [41]), that were identified by in silico analysis to exist in the genome of strain SEPT362, were studied by qRT-PCR (Fig. 2b). The expression levels of these genes were not significantly changed indicating that none of these genes are influenced by the lack of ychO, and the decreased adherence of the mutant strain is not due to a decreased expression of those genes. Thus, even without the BIDs and the C-type lectin-like domains [42, 43], the YchO probably acts as an adhesin in APEC strain SEPT362.
Fig. 2.
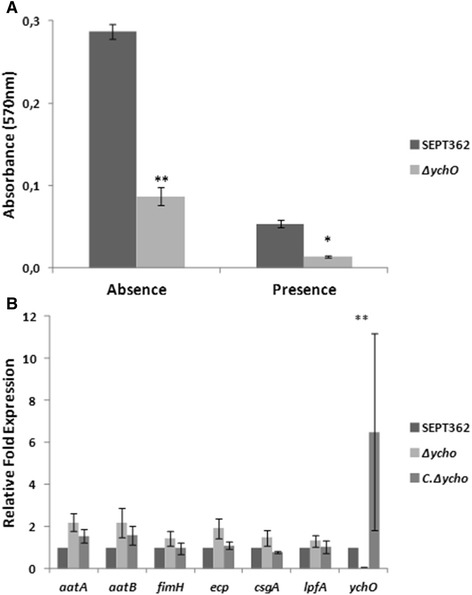
Bacterial adhesion assay. (a) Comparison of adhesion between the strains. Quantification of bacteria adhered to the chicken embryo fibroblast cell line (CEF) in the absence or presence of methyl-alpha-D-mannopyranoside. (b) Relative fold expression of genes related to adhesion in SEPT362 verified using quantitative Real Time-PCR (qRT-PCR). Statistical significance was determined by Tukey’s test in comparison with SEPT362 (**, p < 0.01; *, p < 0.05)
Gene ychO contributes to the invasion of chicken embryonic fibroblast cells
We assessed the ability of SEPT362 to invade the chicken embryonic fibroblast cell line CEC-32 and the potential contribution of the ychO gene to this phenotype. This cell line of avian origin was chosen because it resembles the environment bacteria would find in vivo. SEPT362 was able to invade CEC-32 cells efficiently, and the ychO gene played an important role in this phenotype. The invasion assay was performed with two sets of cells: one set was lysed immediately after incubation with 50 μg ml−1 of gentamycin and the other was lysed after 1.5 h of incubation with 5 μg ml−1 of gentamicyn. The number of viable bacteria inside cells after the invasion assay was significantly lower in strain ∆ychO than in the wild-type strain, whereas the complemented strain restored this capacity (Fig. 3). The decreased invasiveness was significant, but was not completely abolished. This results suggest that other genes reported to be related to invasion, such as fliC (flagellin), motA (flagellar motor protein), bamB (outer membrane protein biogenesis), ompA (outer membrane protein A), ibeB (invasion protein), among others [44–46] that are present in this strain could also contribute to this process. After 1.5 hour of invasion, the number of surviving viable mutants was decreased while the wild-type and complemented strains retained their viability. These data suggest that this protein plays a role not only in invasion, but also in bacterial survival in this cell type.
Fig. 3.
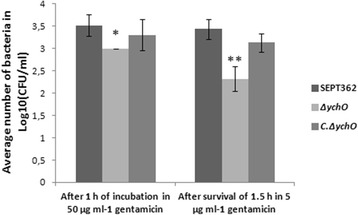
Invasion assay in CEC-32. Comparison of strains after 1 h of incubation in 50 μg ml−1 gentamicin and survival after 1.5 h in 5 μg ml−1 gentamicin. Statistical significance was determined by Tukey’s test in comparison with the count of the strain SEPT362 (**, p < 0.01, *, p < 0.05)
Biofilm formation is influenced by ychO
Biofilms are multicellular communities within a self-produced extracellular matrix attached to a surface [47, 48]. The extracellular matrix helps resist environmental changes and avoid host defenses. Given the capacity of strain SEPT362 to form biofilms on abiotic surfaces [49], we assessed this ability in the mutant strain using the crystal violet biofilm test on polystyrene (Fig. 4). Although the growth curves of strains ∆ychO and the C.∆ychO did not show any difference when compared with the wild-type strain (data not shown), strain ∆ychO formed 50 % less biofilm than the wild-type strain after 24 h, indicating that ychO influences biofilm formation. The complemented strain formed 35 % more biofilm than the wild-type strain, which is explained by the overexpression of the gene in its plasmidial structure and suggests the importance of this protein in biofilm formation. These results corroborate a previous report in which a similar putative invasin protein of Edwardsiella tarda was found to be essential for biofilm formation [50], even though this protein contains a long structure of repeated BID, whereas ychO does not.
Fig. 4.
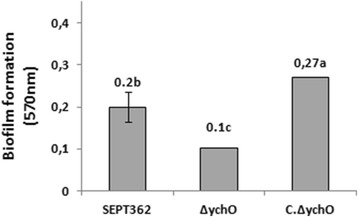
Biofilm formation. Cells were grown for 24 h in polystyrene plates with DMEM. Averages followed by different letters are significantly different (Tukey, p < 0.05)
The lack of ychO affects the virulence of strain SEPT362
In vivo assays were used to evaluate the pathogenicity of strain SEPT362 and its derivatives. For this purpose, separated groups of one-day-old chicks (n = 20 on each group) were infected with the mutant, its complemented derivative and the wild-type strain (Fig. 5). The percent survival of chicks infected with the wild-type was 70 % on the first day, 30 % on the third day, and 10 % at the end of the experiment. For strain ∆ychO, the percent survival was 65 % on the first day, 60 % on the third day, and 40 % at the end of the seventh day. No mortality was observed for the negative control infected with 109 CFU ml−1 of E. coli DH10β (data not shown). Although there were no significant differences among the strains considering the seven days of the experiment (Log-rank test, p > 0.05), a significant change in the survival profile of the mutant strain compared to the wild-type was observed, after the second day (Log-rank test, p > 0.05). The complemented strain presented a profile similar to that of the wild-type strain. The lack of ychO gene decreases the virulence of strain SEPT362 after 48 h in the host, which indicates YchO has a role in survival of the strain in the host. Although this gene is also found in non pathogenic E. coli strains, all of these results suggest that the absence of gene ychO influences the virulence of the APEC strain herein studied in a direct way.
Fig. 5.
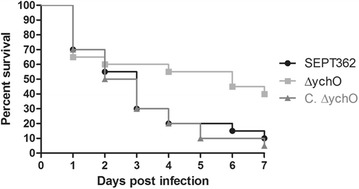
Survival assay. One-day-old broiler chicks were infected with 109 CFU ml−1 bacteria (n = 20). There was no statistical significance determined by the Log-rank (Mantel-Cox) test compared with the SEPT362 strain (p > 0.05)
It is known that APEC pathogenesis is controlled by a number of virulence factors, including adhesins (F1-, P-, AC/I-, and F17-fimbriae, curli fimbriae, and afimbrial adhesins), iron acquisition systems (aerobactin and yersiniabactin), hemolysins and the temperature-sensitive hemagglutinin Tsh, antibactericidal factors (outer membrane protein A, a protein for increased serum survival, lipopolysaccharide, K1-capsule, and colicin production), and toxins (heat stable toxin, cyto-/verotoxin, flagella toxin, and vacuolating autotransporter toxin) [4, 51–59]. Many of these virulence factors (fimH, tshH, fliC, icmF, iucC, yoeB, among others) have been found in strain SEPT362 by in silico search and in vivo analysis [22, 24, 49], which most likely makes the pathogenicity of this strain multifactorial. Thus, ychO activity is one of several factors that could contribute to SEPT362 pathogenicity, and the full virulence observed in this strain would result from the sum of all the virulence factors present in it.
The lack of ychO affected the expression several genes in SEPT362 strain
The results presented so far showed that ychO gene has a pleiotropic effect on several biological characteristics, including those related to invasin like proteins, such as biofilm formation, adhesion to and invasion of in vitro cultured cells. To further investigate the pleiotropic effect of gene ychO to all of these biological characteristics we used the transcriptome sequencing (RNAseq) analysis to compare the transcription profiles of the mutant strain and its wild-type strain. We found that 426 genes were affected upon ychO deletion, which were classified into 14 broad categories (Fig. 6a) using Ecocyc and Uniprot. Most of affected genes are classified in metabolism (23.17 %), hypothetical (20.42 %) and membrane transport (17.61 %). Among the altered genes, 93.66 % were downregulated in the mutant (Fig. 6b). The RNAseq was validated by comparison of the expression levels of the genes csgA, lpfA, fimH, fliC, flhD, motA, flgE, ecp and ychO by qRT-PCR technique. The results were similar for both techniques (data not shown).
Fig. 6.
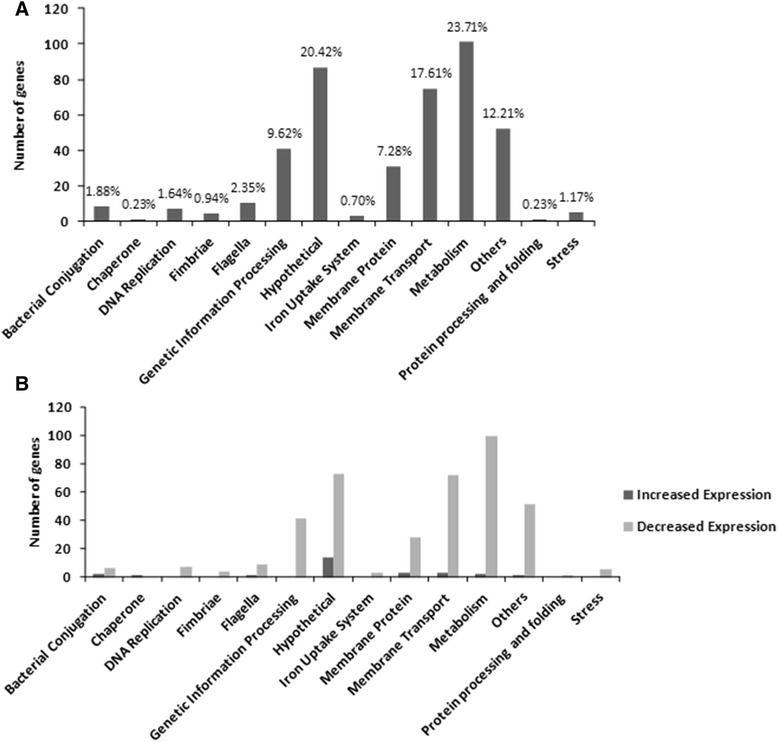
Classification of the genes affected in ychO mutant, based on RNAseq analyses. (a) Each gene is represented once, and is classified in the most relevant category. Percentage indicates the total number of affected factors in each category, relative to the total number of affected genes. (b) Upregulated and Downregulated genes classified in the most relevant category
The high expression of ychO in strain SEPT362 in lungs and spleens during infection and the possible membrane location of protein YchO suggest that the lack of this protein could generate a breakdown of the bacterial membrane, deregulating other membrane-located proteins and membrane transport proteins, triggering a pleiotropic effect, deregulating genes related to metabolism and thus several biological characteristics.
Conclusions
The adhesion, invasion and biofilm formation results presented in this paper together with the presence of the three basic structures of the protein (peptide signal, β-Barrel and passenger domain), allow us to suggest that gene Ycho protein plays roles as an adhesin/invasion in this specific strain. Despite its short passenger protein structure, without the BID, the protein is important for the virulence of APEC strain SEPT362. With the lack of ychO gene, there was a decrease in mortality in chicks. These results, together with the alteration of the expression of 426 genes, led us to propose that ychO has a pleiotropic action in APEC strain SEPT362 probably due to an imbalance effect on membrane structure what leads to the altered expression of membrane-related and metabolic genes.
Methods
Ethics statement
This study was conducted according to the animal welfare guidelines of the World Organization for Animal Health [60], and approved by the “Ethics Committee on Animal Use-CEUA Unicamp” (Protocol number 2669–1) according to the Brazilian Legislation N° 11794. The chickens were reared in boxes placed in warmed room, according to thermal comfort conditions required by chickens, fed with pathogen-free food and free access to water. After infection, animals were monitored every 6–8 hours. After the experiments, the birds that did not die from colibacillosis caused by inoculated bacteria were euthanized by cervical dislocation and then dissected with aseptic surgical techniques. The birds were sacrificed as a measure for preventing spreading of the disease. All efforts were made to minimize suffering.
Bacterial strains and growth conditions
The APEC strain SEPT362 (OR:H10) was isolated from the liver of a laying hen presenting clinical signs of septicemia, and it belongs to the bacterial collection of the Bacterial Molecular Biology Laboratory of the Institute of Biology of the State University of Campinas (LBMB) [24, 49]. The genome of SEPT362 has been sequenced [22]. All strains and plasmids used in this study are listed in Table 1. Strains were grown aerobically in Dulbecco’s Modified Eagle’s Medium (DMEM - Nutricell) or Luria-Bertani medium (LB). Antibiotics were added in both media at the following concentrations: 100 μg ml−1 ampicillin (Amp), 30 μg ml−1 chloramphenicol (Cm), 25 μg ml−1 tetracycline (Tet) and 50 μg ml−1 kanamycin (Km). Molecular biology techniques were performed as previously described [28].
Table 1.
Strains and plasmids used in this work
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| SEPT362 | APEC strain isolated from a septicemic laying hen | [24] |
| SEPT362Cm | SEPT362 with plasmid pKD46Cm | This study |
| ∆ychO | ychO mutant | This study |
| C.∆ychO | ychO mutant complemented with plasmid pC.∆ychO | This study |
| pACYC184 | cloning vector | New England Biolabs |
| pKD4 | pANTS derivative plasmid containing FRT-flanked kanamycin resistance | [63] |
| pKD3 | pANTS derivative plasmid containing FRT-flanked chloramphenicol resistance | [63] |
| pKD46 | λ Red recombinase expression plasmid | [63] |
| pKD46Cm | Plasmid pKD46 with a chloramphenicol cassette insertion | This study |
| pC.∆ychO | Plasmid pACYC184 with ychO | This study |
In silico characterization of the invasin ychO
The NCBI Prokaryotic Genomes Automatic Annotation Pipeline (PGAAP) was employed for gene annotation (http://www.ncbi.nlm.nih.gov/genome/annotation_prok/) of APEC strain SEPT362 [22]. A survey of this genome demonstrated the existence of a gene (ychO) described as a putative invasin homologous to the not yet described gene ychO from E. coli str. K-12 substr. MG1655 and present in contig24 from base 40482 to base 41735. This gene was re-annotated by RAST (Rapid Annotation using Subsystem Technology) (http://rast.nmpdr.org/rast.cgi) [25] to confirm the gene size and composition. A topological prediction was obtained by the I–Tasser server [61].
RNA extraction
Cultures were grown overnight in LB medium at 37 °C, diluted 1:100 in DMEM and grown at 150 rpm to an OD600 of 0.5. RNA was extracted by using the RNAeasy Mini Kit (QIAGEN) according to the manufacturer’s protocol.
In vivo analysis of ychO gene expression
The expression of the ychO gene in the lungs and spleen of infected chicks was verified using qRT-PCR. For this purpose, bacterial cultures were grown overnight in LB medium at 37 °C, washed and resuspended in 0.1 mL of sterile phosphate buffered saline (PBS) at a density of 109 CFU ml−1 and injected into the right thoracic air sac of ten one-day-old chicks (Cobb line). At 24 or 48 h post infection, surviving chicks were euthanized, and the lungs and spleens were removed, processed for RNA extraction using the RNAeasy Mini Kit (QIAGEN) and assayed by qRT-PCR.
Quantitative real time PCR assay
The qRT-PCR assay was performed in a one-step reaction using the ABI StepOne Plus Real-Time PCR (Applied Biosystems), in triplicate (technical replicates) using samples from three independent experiments (biological replicates). Each reaction contained 6 μl of 2X SYBR Green Reaction Mix with Rox, 0.25 μl of SuperScript III RT/Platinum Taq Mix (Invitrogen), 100 nM of each primer, 1 ng μl−1 RNA and sufficient DEPC water for a final volume of 12 μl. The rpoA gene was used as the endogenous control. Data collection was performed using the ABI StepOneTM Real-Time PCR software v.2.1 (Applied Biosystems). Data were normalized to levels of rpoA and analyzed using the comparative critical threshold (CT) method [62]. Error bars represent the standard deviations (SD) of the CT values.
Construction of the ychO mutant and the complemented strain
The putative invasin gene ychO of strain SEPT362 (1,254 bp) was deleted together with the sequence of its signal peptide (141 bp upstream of the ychO gene). The mutant strain ∆ychO was constructed using the λ Red system [63], with modifications. Briefly, a pair of primers, flanked by 50 nucleotide extensions homologous to the adjacent regions of the target gene, was designed to amplify the kanamycin cassette from the plasmid pKD4. One microgram of purified PCR product was electroporated into strain SEPT362 containing the λ Red recombinase plasmid, pKD46Cm (modified by the insertion of the chloramphenicol cassette amplified from plasmid pKD3). Transformed bacterial cells were plated and grown at 37 °C on LB agar containing kanamycin. Deletion of the ychO gene was confirmed by PCR (data not shown) using external primers for the gene. To complement the mutant strain, a DNA fragment covering the ychO coding region plus its putative upstream promoter was amplified and cloned into the BamHI and SalI sites of plasmid pACYC184. The plasmid pC.∆ychO was transformed into the ∆ychO strain, generating the complemented strain C.∆ychO. All oligonucleotides used in this work are listed in Table 2.
Table 2.
Oligonucleotides used for mutant and complement strains construction in this work
| Primer | Sequence |
|---|---|
| Amplification of the Cm cassette | |
| Forward | GAATTTTTTCGCTATAGTGTAGGCTGGAGCTGCTTC |
| Reverse | GAATTTTTTCGCTATACATATGAATATCCTCCTTAG |
| Mutagenesis | |
| Forward | TATTCTTTAGGGCTATGGTTTTTCATTTTTTACCGGAAGTTACCGACGTTGTGTAGGCTGGAGCTGCTTC |
| Reverse | AGTCTCGCGTGGAAGCTGCGGTATGGGTGCATCAGGAGCGCATTTTCTGACATATGAATATCCTCCTTAG |
| Mutagenesis confirmation | |
| Forward | TACCGGAAGTTACCGACGTT |
| Reverse | ATCAGGAGCGCATTTTCTGA |
| Genetic Complementation | |
| Forward | GCTATAGTCGACGCGAGAAAATACGACAAAAG |
| Reverse | GTAAGGATCCCACATGCTGAAGAAAATGAA |
Growth curves
Cultures grown overnight in LB medium were adjusted to the same density based on OD600, diluted 1:100 and cultivated in DMEM and LB medium at 37 °C with agitation at 150 r.p.m. The OD600 was measured every 0.5 h until the bacteria reached the stationary phase.
In vivo pathogenicity experiments
Chick infections were performed as described by de Pace et al. [24] with minor modifications. Cultures of wild-type, mutant and complemented strains were grown overnight in LB medium at 37 °C, washed and resuspended in 0.1 mL sterilized PBS at a density of 1010 CFU ml−1 and injected into the right thoracic air sac of each one-day-old broiler chick. A group of 20 chicks was used for each bacterial culture. The Escherichia coli str. K-12 substr. DH10β was used as a negative control. The groups were observed for seven days post infection, and death was recorded every 24 h.
Bacterial adhesion to Chicken Embryo Fibroblast (CEF) cells
Strains were evaluated for CEF cell adherence in the presence and absence of the D-mannose analog, methyl-alpha-D-mannopyranoside (Sigma cat. n° M6882, Saint Louis, MO, USA), a potent FimH antagonist [64]. E. coli adherence to CEF cells was detected by the reduction of the tetrazolium dye MTT to formazan as previously described [65] and by determining the numbers of live bacteria adhering to the surface of pre-fixed cells. CEF cells were cultured in a 96-well plate in DMEM medium supplemented with 10 % fetal bovine serum (FBS) until they reached confluence. The cells were subsequently inoculated with bacteria (OD600 ≈ 1.0) at a multiplicity of infection (M.O.I.) of 10:1. The strains were grown with or without 1 % of the mannose analog methyl-alpha-D-mannopyranoside. The E. coli HB101 was used as a negative control. The infection time was 1 h, and the multiplication time was 3 h in LB broth. After the multiplication time, cells were washed six times with sterile PBS, and MTT solution [2 mg ml−1 (Sigma, cat. n° M2003)] was added for another 2 h of incubation at 37 °C for MTT reduction to formazan. Following the reduction time, the supernatant solution was removed, 100 μl of isopropyl alcohol:hydrochloric acid (24:1) was added, and the absorbance was measured at 570 nm.
CEC-32 invasion assay
The chicken embryonic fibroblast cell line CEC-32 [66] was used as a non-phagocytic cell to test APEC-host cell interactions. Fibroblasts were cultivated for 48 h in 24-well culture-plates at 7.5 x 104 cells cm−2 in DMEM medium (Nutricell) with 10 % FBS at 37 °C in 5 % CO2. Cultures were washed and infected with bacteria at an MOI of 150:1 and incubated for 1 h at 37 °C in 5 % CO2. E. coli HB101 was used as a negative control. The cells were then washed 3 times with PBS and incubated in DMEM with 10 % FBS and 50 μg ml−1 of gentamicin for 1 h at 37 °C in 5 % CO2. One set of cells was lysed and plated to count the bacteria. The other set of cultured cells was incubated for 1.5 h in DMEM (Nutricell) with 10 % of fetal calf serum containing 5 μg ml−1 of gentamicin before the lysis step. For the lysis step, the wells were washed 3 times with PBS and incubated with 1 ml of 1 % Triton X-100 for 5 minutes. The suspensions were diluted (serial dilutions of 1:10) and plated on LB agar, and the CFU number per ml was determined by counting the colonies for each dilution.
Biofilm formation
Biofilm formation was analyzed using crystal violet staining as previously described by Christensen et al. [67]. Overnight cultures of SEPT362, ∆ychO and C.∆ychO were inoculated in triplicate into DMEM at a 1:100 dilution in 24-well cell culture plates (polystyrene) to a final volume of 1 ml. The plates were incubated at 37 °C in a 5 % CO2 atmosphere for 24 h. The wells were then washed 3 times with 1x PBS, pH 7.4. The cells were fixed with 1 ml of 75 % ethanol, washed 3 more times with 1x PBS and stained with 0.5 % crystal violet for 5 min. After washing 4 times with 1x PBS, pH 7.4, the crystal violet was solubilized by adding 1 ml of 95 % ethanol to each well for 2 minutes. The absorbance of this solution was determined in a spectrophotometer at 570 nm.
Global gene expression analyses by transcriptome sequencing (RNAseq)
The mRNA transcriptome was isolated using the Ribominus™ Transcriptome Isolation Kit (Yeast and Bacteria) (Invitrogen - Life Technologies) according to the manufacturer’s protocol. The transcriptome sequencing (RNAseq) was performed using the platform HISEQ2000 (ILLUMINA). Samples containing 2 mg of mRNA were used for preparation of cDNA libraries specific sizes that were fragmented enzymatically repaired and linked to adapters discerned in the samples bioinformatics analyzes. After preparation, the libraries were deposited in one slide (flowcell) containing 8 channels (lanes) via the robotic instrument cBot (Illumina). The flowcell was then placed in HiSeq2000 where the sequencing occurred. At the end of the race, bioinformatics analyses were performed for gene expression comparison. The reads were aligned with the Open Read Frames (ORFs) and non-coding transcripts from SEPT362 strain. The alignment was performed by using the Bowtie program [68], allowing 2 mismatches. The transcript expression calculation was based on the number of aligned reads, according to Mortazavi et al. (2008) [69]. Only reads with one alignment against the ORFs were considered. Differently expressed ORFs were indentified based on the number of reads with one alignment each. Statistic analyses were performed using R De-Seq [70]. According to the program authors, ORFs less expressed in each of the analyses were excluded from the statistics tests. The validation was performed by qRT-PCR for genes csgA, lpfA, fimH, fliC, flhD, motA, flgE, ecp and ychO.
Statistical analysis
All in vitro assays were performed in triplicate. The results of adhesion, invasion, biofilm formation and qRT-PCR assays were compared using a Tukey test. Statistical analyses were performed with ASSISTAT Version 7.6 Beta (2012). RNAseq statistic analyses were performed using R De-Seq. For the chick infection assays, the Log-rank (Mantel-Cox) test was performed using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com. Differences were considered significant at a P value of <0.05.
Acknowledgements
The authors are thankful to Globo Aves for donating the one-day-old chicks, to Prof. Dr. Fabiana Horn (Federal University of Rio Grande do Sul) for providing the avian macrophage cells and to Prof. Dr. Clarisse Weis Arns for providing the chicken embryo fibroblast cells. This work was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (2012/04391-1 and 2012/09655-7). WDS has a scientific fellowship from CNPq.
We thank the staff of the Life Sciences Core Facility (LaCTAD) from State University of Campinas (UNICAMP), for the Transcriptome and Bioinformatics analysis.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LP participated in the design of the study, carried out the invasion, macrophage, biofilm, motility and RNAseq assays, performed the statistical analysis, and drafted the manuscript. JBP and LP carried out the construction of mutant and complemented strain and the in vivo pathogenicity experiments. TCGR carried out the in silico characterization. JLL carried out the quantitative Real-Time-PCR analysis. RAC and GN carried out the adhesion assays. WDS conceived the study, participated in its design and coordination, was responsible for all the necessary funds and helped out to draft the manuscript. All authors red and approved the final manuscript.
Contributor Information
Livia Pilatti, Email: lipilatti@gmail.com.
Jacqueline Boldrin de Paiva, Email: jackboldrin@hotmail.com.
Thaís Cabrera Galvão Rojas, Email: thaiscgrojas@gmail.com.
Janaína Luisa Leite, Email: jana.garbim@gmail.com.
Rogério Arcuri Conceição, Email: roarcuri@yahoo.com.br.
Gerson Nakazato, Email: gersonakazato@yahoo.com.br.
Wanderley Dias da Silveira, Email: wds@unicamp.br.
References
- 1.Ewers C, Li G, Wilking H, Kieβling S, Alt K, Antáo E-M, Laturnus C, Diehl I, Glodde S, Homeier T, Böhnke U, Steinrück H, Philipp H-C, Wieler LH. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Int J Med Microbiol. 2007;297:163–176. doi: 10.1016/j.ijmm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Johnson JR, Russo T a. Extraintestinal pathogenic Escherichia coli: “The other bad E coli”. J Lab Clin Med. 2002;139:155–162. doi: 10.1067/mlc.2002.121550. [DOI] [PubMed] [Google Scholar]
- 3.Ron EZ. Host specificity of septicemic Escherichia coli: human and avian pathogens. Curr Opin Microbiol. 2006;9:28–32. doi: 10.1016/j.mib.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Dho-Moulin M, Fairbrother JM. Avian pathogenic Escherichia coli (APEC) Vet Res. 1999;30:299–316. [PubMed] [Google Scholar]
- 5.Barnes HJ, Vaillancourt JP, Gross WB. Colibacillosis. Dis Poult. 2003;11:631–652. [Google Scholar]
- 6.La Ragione RM, Woodward MJ. Virulence factors of Escherichia coli serotypes associated with avian colisepticaemia. Res Vet Sci. 2002;73:27–35. doi: 10.1016/S0034-5288(02)00075-9. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Siek KE, Giddings CW, Doetkott C, Johnson TJ, Nolan LK. Characterizing the APEC pathotype. Vet Res. 2005;36:241–256. doi: 10.1051/vetres:2004057. [DOI] [PubMed] [Google Scholar]
- 8.Boyer F, Fichant G, Berthod J, Vandenbrouck Y, Attree I. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics. 2009;10:104. doi: 10.1186/1471-2164-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer DH, Mintz KP, Fives-Taylor PM. Models of invasion of enteric and periodontal pathogens into epithelial cells: a comparative analysis. Crit Rev Oral Biol Med. 1997;8:389–409. doi: 10.1177/10454411970080040301. [DOI] [PubMed] [Google Scholar]
- 10.Niemann HH, Schubert W-D, Heinz DW. Adhesins and invasins of pathogenic bacteria: a structural view. Microbes Infect. 2004;6:101–112. doi: 10.1016/j.micinf.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Saltman LH, Lu Y, Zaharias EM, Isberg RR. A region of the Yersinia pseudotuberculosis invasin protein that contributes to high affinity binding to integrin receptors. J Biol Chem. 1996;271:23438–23444. doi: 10.1074/jbc.271.38.23438. [DOI] [PubMed] [Google Scholar]
- 12.Isberg RR, Leong JM. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–71. doi: 10.1016/0092-8674(90)90099-Z. [DOI] [PubMed] [Google Scholar]
- 13.Jerse a E, Yu J, Tall BD, Kaper JB. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990;87:7839–43. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isberg RR, Voorhis DL, Falkow S. Identification of invasin: A protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 15.Grassl G a, Bohn E, Müller Y, Bühler OT, Autenrieth IB. Interaction of Yersinia enterocolitica with epithelial cells: invasin beyond invasion. Int J Med Microbiol. 2003;293:41–54. doi: 10.1078/1438-4221-00243. [DOI] [PubMed] [Google Scholar]
- 16.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-S. [DOI] [PubMed] [Google Scholar]
- 17.Frankel G, Candy DC, Everest P, Dougan G. Characterization of the C-terminal domains of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect Immun. 1994;62:1835–42. doi: 10.1128/iai.62.5.1835-1842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol. 1998;30:911–21. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 19.Fairman JW, Dautin N, Wojtowicz D, Liu W, Noinaj N, Barnard TJ, Udho E, Przytycka TM, Cherezov V, Buchanan SK. Crystal structures of the outer membrane domain of intimin and invasin from Enterohemorrhagic E. coli and Enteropathogenic Y. pseudotuberculosis. Structure. 2012;20:1233–1243. doi: 10.1016/j.str.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touzé T, Hayward RD, Eswaran J, Leong JM, Koronakis V. Self-association of EPEC intimin mediated by the β-barrel-containing anchor domain: a role in clustering of the Tir receptor. Mol Microbiol. 2004;51:73–87. doi: 10.1046/j.1365-2958.2003.03830.x. [DOI] [PubMed] [Google Scholar]
- 21.Rojas TCG, Parizzi LP, Tiba MR, Chen L, Pereira GAG, Sangal V, Yang J, Yu J, Dias da Silveira W. Draft genome of a brazilian avian-pathogenic Escherichia coli strain and in silico characterization of virulence-related genes. J Bacteriol. 2012;194:3023. doi: 10.1128/JB.00394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojas TCG, Maluta RP, Parizzi LP, Koenigkan LV, Yang J, Yu J, et al. Genome sequences of avian pathogenic Escherichia coli strains isolated from brazilian commercial poultry. Genome Announc. 2013;1. [DOI] [PMC free article] [PubMed]
- 23.Mangiamele P, Nicholson B, Wannemuehler Y, Seemann T, Logue CM, Li G, Tivendale KA, Nolan LK. Complete genome sequence of the avian pathogenic Escherichia coli. Genome Announc. 2013;1:2–3. doi: 10.1128/genomeA.00026-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Pace F, Nakazato G, Pacheco A, Boldrin de Paiva J, Sperandio V, Dias da Silveira W. The type VI secretion system plays a role in type 1 fimbria expression and pathogenesis of an avian pathogenic Escherichia coli strain. Infect Immun. 2010;78:4990–4998. doi: 10.1128/IAI.00531-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards R a, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek R a, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai JC, Yen M-R, Castillo R, Leyton DL, Henderson IR, Saier MH. The bacterial intimins and invasins: a large and novel family of secreted proteins. PLoS One. 2010;5:e14403. doi: 10.1371/journal.pone.0014403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamburger ZA, Brown MS, Isberg RR, Bjorkman PJ. Crystal structure of invasin: a bacterial integrin-binding protein. Science (80- ) 1999;286:291–295. doi: 10.1126/science.286.5438.291. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. 2nd edition. Volume 3. New York: Cold Spring Harbor Laboratory; 1989.
- 29.Antão E-M, Wieler LH, Ewers C. Adhesive threads of extraintestinal pathogenic Escherichia coli. Gut Pathog. 2009;1:22. doi: 10.1186/1757-4749-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finlay BB, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 31.Bahrani-Mougeot FK, Buckles EL, Lockatell CV, Hebel JR, Johnson DE, Tang CM, Donnenberg MS. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol. 2002;45:1079–1093. doi: 10.1046/j.1365-2958.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- 32.Boudeau J, Barnich N, Darfeuille-Michaud A. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn’s disease is involved in bacterial invasion of intestinal epithelial cells. Mol Microbiol. 2001;39:1272–1284. doi: 10.1111/j.1365-2958.2001.02315.x. [DOI] [PubMed] [Google Scholar]
- 33.Khan NA, Kim Y, Shin S, Kim KS. FimH-mediated Escherichia coli K1 invasion of human brain microvascular endothelial cells. Cell Microbiol. 2007;9:169–178. doi: 10.1111/j.1462-5822.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- 34.Marc D, Arné P, Brée A, Dho-Moulin M. Colonization ability and pathogenic properties of a fim- mutant of an avian strain of Escherichia coli. Res Microbiol. 1998;149:473–485. doi: 10.1016/S0923-2508(98)80002-8. [DOI] [PubMed] [Google Scholar]
- 35.Marc D, Arne P, Brée A, Schouler C, Dho-Moulin M. Increased tracheal colonization in chickens without impairing pathogenic properties of avian pathogenic Escherichia coli MT78 with a fimH deletion. Avian Dis. 2000;44:343–55. doi: 10.2307/1592549. [DOI] [PubMed] [Google Scholar]
- 36.Rendón MA, Saldaña Z, Erdem AL, Monteiro-Neto V, Vázquez A, Kaper JB, Puente JL, Girón J a. Commensal and pathogenic Escherichia coli use a common pilus adherence factor for epithelial cell colonization. Proc Natl Acad Sci U S A. 2007;104:10637–42. doi: 10.1073/pnas.0704104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farfan MJ, Cantero L, Vidal R, Botkin DJ, Torres AG. Long polar fimbriae of enterohemorrhagic Escherichia coli O157:H7 bind to extracellular matrix proteins. Infect Immun. 2011;79:3744–50. doi: 10.1128/IAI.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uhlich G a, Gunther NW, Bayles DO, Mosier D a. The CsgA and Lpp proteins of an Escherichia coli O157:H7 strain affect HEp-2 cell invasion, motility, and biofilm formation. Infect Immun. 2009;77:1543–52. doi: 10.1128/IAI.00949-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li G, Feng Y, Kariyawasam S, Tivendale KA, Wannemuehler Y, Zhou F, Logue CM, Miller CL, Nolan LK. AatA is a novel autotransporter and virulence factor of avian pathogenic Escherichia coli. Infect Immun. 2010;78:898–906. doi: 10.1128/IAI.00513-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Xia Y, Dai J, Shi Z, Kou Y, Li H, Bao Y, Lu C. Novel roles for autotransporter adhesin AatA of avian pathogenic Escherichia coli: colonization during infection and cell aggregation. FEMS Immunol Med Microbiol. 2011;63:328–338. doi: 10.1111/j.1574-695X.2011.00862.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhuge X, Wang S, Fan H, Pan Z, Ren J, Yi L, Meng Q, Yang X, Lu C, Dai J, Agricultural N. Characterization and functional analysis of AatB, a novel autotransporter adhesin and virulence factor of avian pathogenic Escherichia coli. Infect Immun. 2013;81:2437–2447. doi: 10.1128/IAI.00102-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li MF, Hu YH, Zheng WJ, Sun BG, Wang CL, Sun L. Inv1: an Edwardsiella tarda invasin and a protective immunogen that is required for host infection. Fish Shellfish Immunol. 2012;32:586–592. doi: 10.1016/j.fsi.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Seo KS, Kim JW, Park JY, Viall AK, Minnich SSA, Rohde HN, Schnider DR, Lim SY, Hong JB, Hinnebusch BJ, O’Loughlin JL, Deobald CF, Bohach GA, Hovde CJ. Role of a new intimin/invasin-like protein in Yersinia pestis virulence. Infect Immun. 2012;80:3559–3569. doi: 10.1128/IAI.00294-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan Q, Zhou M, Liang H, Zhu X, Guo Z, Li Y, Hardwidge PR, Zhu G. Contribution of flagellin subunit FliC to piglet epithelial cells invasion by F18ab E. coli. Vet Microbiol. 2013;166:220–224. doi: 10.1016/j.vetmic.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 45.Smith EJ, Thompson AP, Clarke DJ: Pathogenesis of adherent–invasive. Future Microbiol. 2013;8:1289–1300 [DOI] [PubMed]
- 46.Wang S, Shi Z, Xia Y, Li H, Kou Y, Bao Y, Dai J, Lu C. IbeB is involved in the invasion and pathogenicity of avian pathogenic Escherichia coli. Vet Microbiol. 2012;159:411–419. doi: 10.1016/j.vetmic.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 47.Beloin C, Roux A, Ghigo J-M. Escherichia coli Biofilms. Curr Top Microbiol Immunol. 2008;322:249–289. doi: 10.1007/978-3-540-75418-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Branda SS, Vik S, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13:20–6. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 49.de Pace F, Boldrin de Paiva J, Nakazato G, Lancellotti M, Sircili MP, Guedes Stehling E, Dias da Silveira W, Sperandio V. Characterization of IcmF of the type VI secretion system in an avian pathogenic Escherichia coli (APEC) strain. Microbiology. 2011;157:2954–2962. doi: 10.1099/mic.0.050005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong X, Fan X, Wang B, Shi X, Zhang XH. Invasin of Edwardsiella tarda is essential for its haemolytic activity, biofilm formation and virulence towards fish. J Appl Microbiol. 2013;115:12–19. doi: 10.1111/jam.12198. [DOI] [PubMed] [Google Scholar]
- 51.Dozois CM, Daigle F, Curtiss R. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc Natl Acad Sci U S A. 2003;100:247–52. doi: 10.1073/pnas.232686799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parreira VR, Gyles CL. Shiga toxin genes in avian Escherichia coli. Vet Microbiol. 2002;87:341–52. doi: 10.1016/S0378-1135(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 53.Knöbl T, Gomes TAT, Vieira MAM, Bottino JA, Ferreira AJP. Occurrence of adhesin-encoding operons in Escherichia coli isolated from breeders with salpingits and chicks with omphalitis. Brazilian J Microbiol. 2006;37:140–143. [Google Scholar]
- 54.McPeake SJW, Smyth J, Ball HJ. Characterisation of avian pathogenic Escherichia coli (APEC) associated with colisepticaemia compared to fecal isolates from healthy birds. Vet Microbiol. 2005;110:245–53. doi: 10.1016/j.vetmic.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Kostakioti M, Stathopoulos C. Functional analysis of the tsh autotransporter from an avian pathogenic Escherichia coli Strain. Infect Immun. 2004;72:5548–5554. doi: 10.1128/IAI.72.10.5548-5554.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amabile de Campos T, Stehling EG, Ferreira A, Pestana de Castro AF, Brocchi M, Dias da Silveira W. Adhesion properties, fimbrial expression and PCR detection of adhesin-related genes of avian Escherichia coli strains. Vet Microbiol. 2005;106:275–85. doi: 10.1016/j.vetmic.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 57.Kariyawasam S, Johnson TJ, Nolan LK. The pap operon of avian pathogenic Escherichia coli strain O1:K1 is located on a novel pathogenicity island. Infect Immun. 2006;74:744–749. doi: 10.1128/IAI.74.1.744-749.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mellata M, Dho-moulin M, Dozois CM, Iii RC, Brown PK, Arné P, Brée A, Desautels C, Fairbrother JM, Arne P, Bre A. Role of virulence factors in resistance of avian pathogenic Escherichia coli to serum and in pathogenicity. Infect Immun. 2003;71:536–540. doi: 10.1128/IAI.71.1.536-540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janßen T, Schwarz C, Preikschat P, Voss M, Philipp H-C, Wieler LH: Virulence-associated isolated genes in avian pathogenic Escherichia coli (APEC) from internal organs of poultry having died from colibacillosis. Int J Med. Microbiol. 2001;378:371–378. [DOI] [PubMed]
- 60.World Organization for Animal Health . Terrestrial Animal Health Code. Paris: World Organization for Animal Health; 2011. [Google Scholar]
- 61.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walters M, Sircili MP, Sperandio V. AI-3 Synthesis Is Not Dependent on luxS in Escherichia coli. J Bacteriol. 2006;188:5668–5681. doi: 10.1128/JB.00648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abgottspon D, Rölli G, Hosch L, Steinhuber A, Jiang X, Schwardt O, Cutting B, Smiesko M, Jenal U, Ernst B, Trampuz A. Development of an aggregation assay to screen FimH antagonists. J Microbiol Methods. 2010;82:249–255. doi: 10.1016/j.mimet.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 65.Zaas DW, Duncan MJ, Li G, Wright JR, Abraham SN. Pseudomonas invasion of Type I pneumocytes is dependent on the expression and phosphorylation of caveolin-2. J Biol Chem. 2005;280:4864–4872. doi: 10.1074/jbc.M411702200. [DOI] [PubMed] [Google Scholar]
- 66.Kaaden OR, Lange S, Stiburek B. Establisment and characterization of chicken embryo fibroblast clone LSCC-H32. In Vitro. 1982;18:0827–0834. doi: 10.1007/BF02796323. [DOI] [PubMed] [Google Scholar]
- 67.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH. Adherence of coagulase-negative Staphylococci to plastic tissue culture plates: a quantitative model for the adherence of Staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Meth. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 70.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]


