Abstract
Introduction
The literature on the effect of head injuries on the risk of PD is inconclusive. Some researchers have hypothesized that studies that have seen an effect are simply capturing injury related to pre-clinical PD. However in animal models brain inflammation, which can be initiated by head trauma, has been shown to produce PD-like effects. Furthermore, animal studies have found that early life inflammation in particular is of relevance for PD pathology.
Methods
We conducted an unmatched case-control study of 379 neurologist confirmed PD patients and 230 controls from the greater Boston, Massachusetts area with questionnaire data on history of head injury and other covariates. We used multivariable logistic regression to estimate adjusted odds ratios (OR) and their corresponding 95% confidence intervals (CI) for PD.
Results
When we excluded injuries that occurred less than 10 years prior to the diagnosis of PD (in order to avoid reverse causation), we found an increased risk of PD associated with a head injury that resulted in a loss of consciousness, but it did not reach statistical significance (OR= 1.57; 95% CI= 0.89-2.80). We found a significant (p=0.04) effect of age at first head injury. For every 5 year earlier age at first head injury with loss of consciousness the OR for PD was 1.37 (95% CI: 1.01-1.86).
Conclusion
Our results suggest that head injury in early life increases the risk of PD.
Keywords: head injury, Parkinson's disease, traumatic brain injury
Introduction
Parkinson's disease [PD] is a progressive neurodegenerative disease with an insidious onset.[1] Up to 95% of cases are considered sporadic or without a genetic cause.[2] It has been suggested that brain inflammation is a risk factor for PD.[3-5] In animal experiments, head trauma resulted in chronic brain inflammation through disturbance of the blood brain barrier and evolving white matter damage.[4, 6, 7] These findings have spurred a great interest in the impact that head injuries may have on the risk of PD.
Several studies have demonstrated an association between head injury and PD.[8-13] However, there is concern that these findings could be attributed to reverse causation, that is instability caused by prodromal PD could result in a higher risk of head injuries rather than the head injury causing PD. The few studies that have evaluated the timing of head injury have found that having a head injury closer to PD diagnosis was strongly associated with PD. This association decreased as the time between the head injury and PD diagnosis increased.[11, 14, 15] These findings suggest that reverse causation is a concern and needs to be considered in any analysis.
Animal experiments have found that early life brain inflammation caused by environmental exposures can result in persistent changes in the nigrostriatal pathway, accumulation of proinflammatory factors in the brain, and increased neurologic susceptibility to other environmental exposures.[3, 4, 7, 16] Only one study has considered whether the age at which head injuries occur is related to PD risk.[17] Therefore, we examined the question of age at head injury and risk of PD in an unmatched case-control study based in Boston, Massachusetts.
Methods
Subject recruitment
Between 2003 and 2007, patients with PD were recruited from four movement disorder clinics in the Boston, Massachusetts area. Cases were evaluated twice by a neurologist with at least 6 months between each evaluation. Case status was confirmed using U.K. Brainbank criteria.[18] Individuals deemed eligible were enrolled and completed questionnaires that included questions about past head injuries. Controls were recruited from family, friends and in-laws of the cases, community targeted advertisements, and through recruitment of participants in the Harvard Cooperative Program on Aging study (HCPOA). HCPOA is a registry of elderly volunteers who have agreed to participate in studies. A total of 379 cases and 230 controls answered questions on head injury including the timing of those injuries.
This study was reviewed and approved by the Human Research Committees at the Harvard School of Public Health, the BWH, and the VA Boston Healthcare System. All participants gave written informed consent prior to participating in the study.
Exposure and Covariate Assessment
Head injury status was assessed via questionnaire. Our primary exposure was head injury with loss of consciousness. Both cases and controls were asked to respond to the following questions: “Have you ever lost consciousness as a result of a head injury?,” and “At what age was your first head injury that resulted in loss of consciousness.” Those reporting a head injury were also asked about the total number of head injuries. From these responses we created a dichotomous variable for head injury (ever/never). A question on head injuries without loss of consciousness was introduced after the start of recruitment and so was answered by only 241 cases and 219 controls. This variable was used only in sensitivity analyses either only among those participants with this variable or among all participants and including a missing indicator for those without it. Unless otherwise specified, we use the term head injury to refer to head injury with loss of consciousness. In our base analyses, to help avoid reverse causation, we lagged exposure by 10 years prior to PD diagnosis. That is, we excluded any head injuries that occurred less than 10 years prior to PD diagnosis for the cases and less than 19 years prior to the questionnaire date for the controls, which was on average the same overall time before the questionnaire among the cases (because the average time from diagnosis to the questionnaire among cases was 9 years). In sensitivity analyses we also conducted additional analyses after excluding head injuries in the 15 and 20 years prior to PD diagnosis (and equivalent extensions in the controls) to reduce the chance of reverse causation even further. Additional data included in these analyses and obtained via questionnaire, were age, gender, race, education, and smoking status.
Statistical Analysis
Odds ratios (OR) and their corresponding 95% confidence intervals (CI) were estimated using multivariable logistic regression. Total number of head injuries was analyzed both as a continuous variable and as a categorical variable (0, 1, >1). Age at first head injury was analyzed as a categorical variable (no head injury and tertiles of age at first head injury based on the distribution among the controls). The significance of the trend over age at first injury was assessed in models treating age at first injury as a continuous variable and including an indicator for ever having a head injury, with all those without one assigned a constant for age, in order to keep all subjects in the analysis. To test the potential non-linearity of associations with these continuous variables, we used natural splines and selected the best model fit using the Akaike information criterion (AIC). For both age at first head injury and the total number of head injuries, better fit was obtained with linear models in all cases. Thus, only the linear results are reported.
Models were adjusted for age at the date of visit (years), its square, sex, race (white, non-white), highest level of education (high school or less, attended college, graduated college, postgraduate school), and smoking status (ever, never). In sensitivity analyses we adjusted for ever having a head injury without loss of consciousness, so that the reference group was those who never had any head injury with or without loss of consciousness. In a sensitivity analysis for our model evaluating the effect of age at first head injury, we also controlled for number of head injuries. We used missing indicator variables when covariate data was missing. Analyses were conducted using SAS software (version 9.3; SAS Institute, Inc. Cary, NC)[19] and R statistical software (version 3.1.1; R Development Core Team; 2013)[20] for models that utilized splines.
Results
Patients with PD were more likely to be male, white, have a higher level of education, and less likely to have ever smoked compared with the controls (Table 1). On average the cases were diagnosed with Parkinson's disease 9.4 years (sd=6.8 years) prior to answering the questionnaire. The median age at first head injury was 15 (Interquartile range= 10-18) among the cases and 18.5 (Interquartile range= 12-44.5) among the controls (Table 2).
Table 1.
Characteristics of study population by case status.
| Characteristics | Controls (N=230) | Cases (N=379) | P-value* |
|---|---|---|---|
| Years since diagnosis (mean ± SD) | NA | 9.4±6.8 | |
| Age (years) at data of visit (mean± SD) | 68.6±10.7 | 66.9±9.5 | 0.048 |
| Gender | <0.001 | ||
| Male (%) | 79 (34.4) | 242 (63.9) | |
| Female (%) | 151 (65.6) | 137 (36.1) | |
| Race | <0.001 | ||
| White (%) | 188 (81.7) | 362 (95.5) | |
| Non-White (%) | 42 (18.3) | 15 (4.0) | |
| Missing | 0 (0) | 2 (0.5) | |
| Education | <0.134 | ||
| High School or Less (%) | 35 (15.2) | 56 (14.7) | |
| Attended College (%) | 53 (23.0) | 58 (15.3) | |
| Graduated College (%) | 67 (29.1) | 112 (29.6) | |
| Attended Grad School (%) | 73 (31.7) | 149 (39.3) | |
| Missing (%) | 2 (0.9) | 4 (1.1) | |
| Smoking Status | |||
| Never (%) | 102 (44.4) | 181 (47.8) | 0.413 |
| Ever (%) | 111 (48.3) | 157 (41.4) | |
| Missing (%) | 17 (7.4) | 41 (10.8) |
NA, not applicable.
Produced using T-tests for continuous variables and Chi-Squared Tests for categorical and binary variables
Table 2.
Characteristics of study population by head injury status
| Never (N=516) | Ever (N=93) | |||
|---|---|---|---|---|
| Characteristics | Cases (N=310) | Controls (N=206) | Cases (N=69) | Controls (N=24) |
| Age at first head injury (years) at date of visit (median) | NA | NA | 15 | 18.5 |
| Interquartile range of age (years) at first head injury | NA | NA | 10-18 | 12-44.5 |
| Number of head injuries (median) | NA | NA | 1 | 1 |
| Gender | ||||
| Male (%) | 191 (61.6) | 64 (31.1) | 51 (73.9) | 15 (62.5) |
| Female (%) | 120 (38.4) | 142 (68.9) | 18 (26.1) | 9 (37.5) |
| Race | ||||
| White (%) | 295 (95.2) | 166 (80.6) | 67 (97.1) | 22 (91.7) |
| Non-White (%) | 13 (4.2) | 40 (19.4) | 2 (2.9) | 2 (8.3) |
| Missing (%) | 2 (0.6) | 0 (0) | 0 (0) | 0(0) |
| Education | ||||
| High School or Less (%) | 50 (16.1) | 32 (15.5) | 6 (8.7) | 3 (12.5) |
| Attended College (%) | 44 (14.2) | 50 (24.3) | 14 (20.3) | 3 (12.5) |
| Graduated College (%) | 93 (30.0) | 58 (28.2) | 19 (27.5) | 9 (37.5) |
| Attended Grad School (%) | 119 (38.4) | 64 (31.1) | 30 (43.5) | 9 (37.5) |
| Missing (%) | 4 (1.3) | 2 (1.0) | 0 (0) | 0 (0) |
| Smoking Status | ||||
| Never (%) | 151 (48.7) | 93 (45.2) | 30 (43.5) | 9 (37.5) |
| Ever (%) | 126 (40.6) | 100 (48.5) | 31 (44.9) | 11 (45.8) |
| Missing (%) | 33 (10.7) | 13 (6.3) | 8 (11.6) | 4 (16.7) |
NA, not applicable
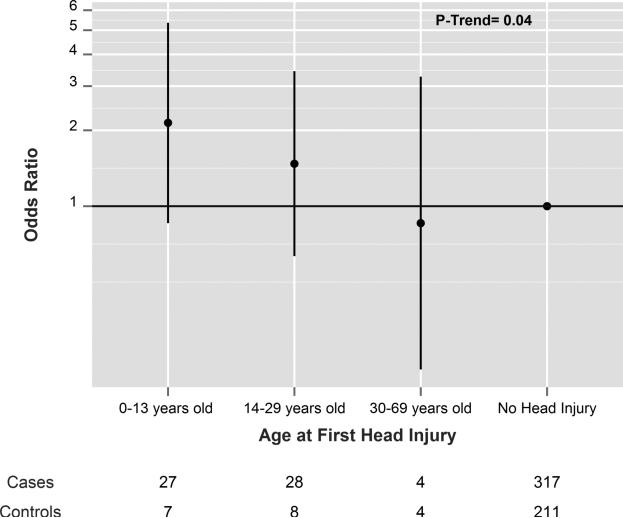
The odds ratio (OR) for PD among those with a head injury at any age was elevated, but did not reach statistical significance (Table 3). When head injuries within the 15 or 20 years prior to PD diagnosis (and equivalent periods among controls) were excluded to more stringently avoid reverse causation, the OR for PD were similar, although none of the OR quite reached statistical significance (Table 3). The OR for PD was 1.46 (95% CI= 0.75-2.86) for those with one head injury and 1.54 (95% CI= 0.53-4.52) for those with more than one. When treating head injuries as a continuous variable, the OR per injury was 0.99 (95% CI= 0.74-1.31). The OR for PD increased with earlier age of injury and this association was significant (p=0.04). The OR per 5 years younger age at first head injury was 1.40 (95% CI: 1.15-1.91). In the same model, when additionally controlling for total number of head injuries, results were similar (OR=1.37; 95% CI=1.01-1.86). The OR by categories of age at first head injury compared with those who never had a head injury from our base model are shown in figure 1.
Table 3.
Adjusted* OR for PD by history of head injury with loss of consciousness, excluding injuries in different time periods before the diagnosis date for cases and the equivalent time period for the controls.
| Duration of Lagged Exposure | Cases | Controls | OR (95% CI) | p-value |
|---|---|---|---|---|
| Exposure lagged 10 years | ||||
| Never having a head injury | 318 | 211 | 1.00 (Ref) | |
| Ever having a head injury with a loss of consciousness | 61 | 19 | 1.57 (0.89, 2.80) | 0.13 |
| Exposure lagged 15 years | ||||
| Never having a head injury | 320 | 213 | 1.00 (Ref) | |
| Ever having a head injury with a loss of consciousness | 59 | 17 | 1.68 (0.92, 3.07) | 0.09 |
| Exposure lagged 20 years | ||||
| Never having a head injury | 324 | 214 | 1.00 (Ref) | |
| Ever having a head injury with a loss of consciousness | 55 | 16 | 1.58(0.85, 2.95) | 0.14 |
Adjusted for ever having a head injury without loss of consciousness, gender, age, age squared, race, education, smoking status.
Figure 1.
Association between head injury and PD by the age at which the first head injury occurred when lagging injuries by 10 years. Odds ratios are indicated by black circles and the 95% confidence intervals by the vertical lines.
In sensitivity analyses that included additional adjustment for head injury without loss of consciousness, results were generally similar. For example, the OR for PD among those with a head injury with loss of consciousness in analyses excluding injuries in the ten years before PD diagnosis (and the equivalent period among controls) was 1.41 (95% CI: 0.78-2.53) when including all participants and using a missing indicator for those without data on head injury without loss of consciousness. The OR was 1.72 (95% CI: 0.88-3.35) when restricting to those participants with data on head injury without loss of consciousness and adjusting for it. Similarly, the trends for age at head injury with loss of consciousness were similar when adjusting for head injury without loss of consciousness either among everyone using a missing indicator (p=0.03) or just among those with data on head injury without loss of consciousness (p=0.02).
Discussion
Our study found an association between earlier age at first head injury and PD. The overall association between head injury and PD was elevated, but not significant. There was no association seen with increasing numbers of head injuries. The association with earlier age at head injury was not reduced when adjusted for total number of head injuries.
The prior literature on head injury and PD is mixed. Several studies have reported relationships between head injury and PD.[10, 11] More recent studies, however, have suggested that the association between head injuries and PD is driven by head injuries in the years just before diagnosis, likely the result of reverse causation[11, 14, 15]—that is that head injuries in a period prior to PD diagnosis may be the result of underlying prodromal PD rather than a cause of PD. However, only one other recent study evaluated age at first head injury and it came to similar conclusions as our current study.[17] These findings may help explain some of the discrepancy between studies of head injury that ignore age at injury. The large registry studies that demonstrated reverse causation had objectively identified occurrences of head injury, but they did not explore head injuries in early life—possibly because the data did not go back far enough. Elevated risk seen with self-report of head injury in other studies may have been found because this exposure assessment also captured earlier life head injuries.
There are several proposed mechanisms that have been hypothesized as to how head injury could contribute to PD risk. Evidence suggests that head injuries can result in long-term microglial activation.[7, 21] When these cells are over-activated or go unregulated there can be low level chronic neurodegeneration and it is this self-perpetuating cycle which can be initiated by head injuries that has been linked to PD.[4, 22] Brain inflammation as a result of head injury has been shown to produce PD-relevant pathology in animal experiments.[4, 6] Furthermore, these studies have indicated that early life is an important time window to consider when evaluating the neurotoxicity resulting from inflammation. Early life, even in utero, exposure to lipopolysaccharide and several pesticides in animal studies have all been associated with progressive degeneration of dopaminergic neurons,[23-25] with the suggestion that the effects are caused, at least in part, by the induced inflammation.
A limitation of our study was the collection of head injury data through self-report, which raises the possibility of recall bias. However, while preferential head injury reporting by cases is possible, it is difficult to theorize why participants would specifically over report head injuries in early life, and do so in a way that produces a significant linear dose-response with age at first injury. Furthermore, in at least one study,[9] perfect agreement was found between self-reported head injury and medical record verification in a validation subset. We also relied on the recruitment of many spouse, relative, and friend controls, which skewed our controls to be more often female since the cases tended to be male. However, adjustment for sex avoids possible bias from this. Spouse, relative, and friend controls are not always ideal because they tend to have characteristics more similar to the cases than the general population, but, as a result, potential bias from this is usually towards the null. Furthermore, similarity between cases and controls in covariates improves the control for confounding.
In conclusion, our findings suggest that the age at which a head injury occurs is relevant for subsequent risk of PD: specifically, the earlier the age at head injury, the greater the risk of PD. This new finding could explain some of the discrepancy between prior studies of head injury and risk of PD, and suggests that the younger brain may be particularly vulnerable to traumatic insult. These findings are of particular concern, given that on average over half a million children between the ages of 0-14 are hospitalized for traumatic brain injuries each year.[26]
Brain inflammation in animals in early life is linked with increased PD-like pathology
Head injury is a known cause of brain inflammation
The association between head injury and an increase in the risk of PD is debated
Robust methodology shows an increased risk of PD with head injuries in early childhood
This could explain inconsistencies in the association between head injury and PD
Acknowledgments
This work was supported by the National Institute of Health, National Institute of Environmental Health Sciences (R01-ES010798 and P01-ES000002 (M.W.)), National Institute of Health: IMSD Academic Training grant (R25 GM055353 (K.T.)), the National Institute for Occupational Safety and Health: ERC training grant (T42 OH008416 (K.T.)) and VA Research Career Scientist award (D.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Kathryn Taylor: Analysis and interpretation of data; Drafting the article; Final approval of the version to be published;
Marie-Helene Saint-Hilaire: Acquisition of the data; Revising and critically appraising manuscript for important intellectual content; Approving final published version;
Lewis Sudarsky: Acquisition of the data; Revising and critically appraising manuscript for important intellectual content; Approving final published version;
David K. Simon: Acquisition of the data Revising and critically appraising manuscript for important intellectual content; Approving final published version;
Bonnie Hersh: Acquisition of the data; Revising and critically appraising manuscript for important intellectual content; Approving final published version;
David Sparrow: Acquisition of the data; Revising and critically appraising manuscript for important intellectual content; Approving final published version;
Howard Hu: Substantial contributions to the conception or design of the work, acquisition of the data; Revising and critically appraising manuscript for important intellectual content; Approving final published version;
Marc G. Weisskopf: Substantial contributions to the conception or design of the work, acquisition of the data and interpretation of data; Drafting the work and Revising and critically appraising manuscript for important intellectual content;
Conflicts of Interests
None.
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–66. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Tanner CM. Is the cause of Parkinson's disease environmental or hereditary? Evidence from twin studies. Adv Neurol. 2003;91:133–42. [PubMed] [Google Scholar]
- 3.Cai Z, Fan LW, Kaizaki A, Tien LT, Ma T, Pang Y, et al. Neonatal systemic exposure to lipopolysaccharide enhances susceptibility of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Dev Neurosci. 2013;35:155–71. doi: 10.1159/000346156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu B, Gao HM, Hong JS. Parkinson's disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect. 2003;111:1065–73. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Glushakova OY, Johnson D, Hayes RL. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J Neurotrauma. 2014;31:1180–93. doi: 10.1089/neu.2013.3080. [DOI] [PubMed] [Google Scholar]
- 7.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–83. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 8.Goldman SM, Kamel F, Ross GW, Jewell SA, Bhudhikanok GS, Umbach D, et al. Head injury, alpha-synuclein Rep1, and Parkinson's disease. Ann Neurol. 2012;71:40–8. doi: 10.1002/ana.22499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson's disease risk in twins. Ann Neurol. 2006;60:65–72. doi: 10.1002/ana.20882. [DOI] [PubMed] [Google Scholar]
- 10.Lee PC, Bordelon Y, Bronstein J, Ritz B. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology. 2012;79:2061–6. doi: 10.1212/WNL.0b013e3182749f28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris MA, Shen H, Marion SA, Tsui JK, Teschke K. Head injuries and Parkinson's disease in a case-control study. Occup Environ Med. 2013;70:839–44. doi: 10.1136/oemed-2013-101444. [DOI] [PubMed] [Google Scholar]
- 12.Marras C, Hincapie CA, Kristman VL, Cancelliere C, Soklaridis S, Li A, et al. Systematic review of the risk of Parkinson's disease after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2014;95:S238–44. doi: 10.1016/j.apmr.2013.08.298. [DOI] [PubMed] [Google Scholar]
- 13.Jafari S, Etminan M, Aminzadeh F, Samii A. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord. 2013;28:1222–9. doi: 10.1002/mds.25458. [DOI] [PubMed] [Google Scholar]
- 14.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson's disease after hospital contact for head injury: population based case-control study. Bmj. 2008;337:a2494. doi: 10.1136/bmj.a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang F, Chen H, Feldman AL, Kamel F, Ye W, Wirdefeldt K. Head injury and Parkinson's disease: a population-based study. Mov Disord. 2012;27:1632–5. doi: 10.1002/mds.25143. [DOI] [PubMed] [Google Scholar]
- 16.De Lella Ezcurra AL, Chertoff M, Ferrari C, Graciarena M, Pitossi F. Chronic expression of low levels of tumor necrosis factor-alpha in the substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiol Dis. 2010;37:630–40. doi: 10.1016/j.nbd.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Gao J, Liu R, Zhao E, Huang X, Nalls MA, Singleton AB, et al. Head injury, potential interaction with genes, and risk for Parkinson's disease. Parkinsonism Relat Disord. 2015;21:292–6. doi: 10.1016/j.parkreldis.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inc. SI. SAS Online Doc. SAS Institute Inc; Cary, NC: 2012. [Google Scholar]
- 20.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Viena, Austria: 2013. [Google Scholar]
- 21.Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. Journal of neuropathology and experimental neurology. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, et al. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–24. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- 23.Ling Z, Zhu Y, Tong C, Snyder JA, Lipton JW, Carvey PM. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol. 2006;199:499–512. doi: 10.1016/j.expneurol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Cory-Slechta DA, Thiruchelvam M, Richfield EK, Barlow BK, Brooks AI. Developmental pesticide exposures and the Parkinson's disease phenotype. Birth Defects Res A Clin Mol Teratol. 2005;73:136–9. doi: 10.1002/bdra.20118. [DOI] [PubMed] [Google Scholar]
- 25.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 2002;81:1285–97. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 26.Faul M XL, Wald MM, Coronado VG. In: Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention NCfIPaC, editor. Atlanta, GA: 2010. [Google Scholar]