Abstract
Endoplasmic reticulum (ER) stress triggered under hyperglycemic, hypoxic, and oxidative conditions has been implicated in cellular dysfunction through activation of the unfolded protein response (UPR). Recent clinical studies have documented that the release of soluble cellular and host factors following HIV infection in the central nervous system (CNS) results in induction of the ER stress response. Herein, we demonstrate that exposure of human brain microvascular endothelial cells (HBMECs) to HIV transactivator protein Tat101 resulted in early induction of several major ER stress regulators including ER chaperones Bip/GRP78 and ER stress sensors ATF6, p-PERK, and downstream mediators p-eIF2α and ATF4. Upregulation of the ER stress mediators was accompanied by decreased cell viability and increased apoptosis as evidenced by MTT and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assays, respectively. Pretreatment of HBMECs with either ER inhibitor or knockdown of the effector C/EBP homologous protein (CHOP) resulted in increased cell viability and abrogation of apoptosis following Tat exposure. Notably, Tat-mediated activation of the UPR response involved reactive oxygen species. Furthermore, treatment of Tat also resulted in mitochondrial dysfunction, evidenced by decrease in Bcl2/Bax ratio, dysfunction of mitochondrial membrane potential, and release of cytochrome c, all of which could be partially reversed by the ER stress inhibitor. The current study demonstrates that exposure of HBMECs to Tat induces multiple stress responses, including ER stress and mitochondrial dysfunction which in turn lead to apoptosis.
Keywords: ER stress, HIV transactivator protein, Apoptosis, Endothelial cells
Introduction
The blood-brain barrier (BBB) tightly regulates the movement of molecules, ions, and cells between the blood and the neural tissue and plays an important role in maintaining the central nervous system (CNS) stability [1, 2]. Breakdown of the BBB plays a key role in the pathogenesis of various neurological diseases [3, 4]. Following the disruption of BBB, there is entry of various plasma components, immune molecules, and cells within the CNS, which eventually culminates into neuroinflammation, ultimately leading to neuronal degeneration [4, 5].
Human immunodeficiency virus (HIV)-associated neurological disorders (HAND) are a complication of acquired immune deficiency syndrome (AIDS) and continue to rise as individuals live longer following antiretroviral therapy. The underlying mechanisms of HAND are, in part, attributable to disruption of the BBB which results in increased influx of activated/infected monocytes from the periphery to the CNS leading to neuroinflammation [6]. Various mechanisms including both direct proapoptotic effects on the endothelial cells as well as indirect paracrine effects manifested by pro-inflammatory modulators such as chemokines and cytokines are thought to play key roles in regulating BBB permeability [7].
Endoplasmic reticulum (ER) is a principal site for protein synthesis, folding, and calcium storage. ER relies on numerous resident chaperone proteins, high levels of calcium, and an oxidative environment to carry out these functions efficiently [8, 9]. ER is sensitive to homeostatic alterations induced by various stimuli (oxidative stress, calcium homeostasis, chemical toxins, and accumulation of misfolded proteins), resulting in, what is classically referred to as “ER stress” [10]. ER stress triggers multiple signaling pathways including the unfolded protein response (UPR), the ER-overload response (EOR), and the ER-associated degradation (ERAD) as a means to combat the stressors [11]. UPR is characterized by the coordinated activation of three transmembrane ER proteins: inositol requiring ER-to-nucleus signal kinase (IRE) 1, activating transcription factor (ATF) 6, and double-stranded RNA-activated kinase (PKR)-like ER kinase (PERK). Following accumulation of unfolded proteins in the lumen of the ER, these proteins get activated which, in turn, leads to modulation of expression of key genes and proteins, such as the genes encoding ER chaperones and folding enzymes, to subsequently increase the folding capacity of the ER. Collectively, UPR is a coordinated pro-survival response that reduces the accumulation of unfolded proteins and restores normal ER functioning. If, however, the protein aggregation persists and the stress continues to mount, signaling switches from a pro-survival to a pro-apoptotic phenotype [12]. At this stage, the pro-apoptotic gene C/EBP homologous protein (CHOP)/GADD153 is upregulated triggering cell death. Intriguingly, endothelial apoptosis has been implicated as a characteristic feature of BBB breach in patients with HAND compared with non-demented infected individuals or HIV-negative controls [13].
Although antiretrovirals have resulted in successful suppression of viremia, the inability of these drugs to both impact expressions of early viral proteins as well as to penetrate the CNS effectively leads to the persistence of proteins including HIV-1 Tat, in tissues such as the CNS and lymph nodes. Toxicity of HIV Tat on various cells of the CNS has been well documented [14, 15], and it has also been shown that intraventricular injection of the viral protein in rodents results in inflammation, gliosis, apoptosis, and ventricular enlargement [16, 17], with induction of reactive oxygen species (ROS) as a possible mediator of cell damage. Recent reports have implicated induction of ER stress as a major response in various cell types including neurons, astrocytes, and macrophages/microglia in the brains of HIV-positive patients [17]. Extensive evidence implicates a close link of ER stress with generation of ROS [18]. ROS, in turn, can be either upstream or downstream of the UPR targets [19].
The aim of the present study was to examine the mechanism(s) by which ER stress, ROS, and mitochondrial dysfunction contribute to Tat-mediated apoptosis of human brain microvascular endothelial cells (HBMECs).
Materials and Methods
Cell Culture
Primary HBMECs were obtained from Dr. Monique Stins (The Johns Hopkins University, Baltimore, MD) and were cultured in RPMI 1640 containing 10 % heat-inactivated fetal bovine serum, 10 % Nu-Serum, 2 mM glutamine, 1 mM pyruvate, penicillin (100 U/mL), streptomycin (100 μg/mL), essential amino acids, and vitamins. All cell-culture dishes and flasks were coated with rat-tail collagen type I. For pharmacological inhibition studies, cells were pretreated for 1 h with ER stress inhibitor, salubrinal (SB, 10 μM, Santa Cruz) or sodium 4-phenylbutyrate acid (4PBA, 10 μM, Sigma), ROS inhibitor, N-tert-butyl-α-phenylnitrone (NBP, 100 μM, Sigma), or apocynin (200 μM, Sigma), prior to Tat treatment. All experiments were conducted under serum-free conditions.
MTT Assay
HBMECs were seeded at a density of 1×104 cells/well in 96-well plates, and the cell viability was determined by MTT assay, which is based on the conversion of MTT into purple formazan by mitochondrial dehydrogenases. After incubation, HBMECs were treated with 5 mg/mL MTT at 37 °C for 4 h. The medium was then removed, and dimethylsulfoxide was added to each well. Absorbance was determined at 570 nm on a microplate reader, and cell viabilities were calculated. Results were expressed as percentage of values, assuming that the cell viability of the control cells was 100 %. Each experiment was repeated at least three times with each treatment given in triplicate. Data were presented as an average of the results from individual experiments.
siRNA Transfection
Short interfering RNA (siRNA) targeted against CHOP messenger RNA (mRNA) was obtained from Thermo Scientific Dharmacon RNAi Technologies (ONTARGETplus SMARTpool). siRNA transfection protocols were followed according to the manufacturer’s instructions. Briefly, HBMECs were seeded at 50 % confluence into 24-well plates. Dharma-FECT 1 transfection reagent (Dharmacon) was combined with serum-free DMEM medium (Invitrogen Life Technologies) for 5 min at room temperature. CHOP or scrambled siRNA were then added into the respective mixtures (described above) and incubated for 20 min at room temperature, following which, the mixture was added to the cells to have the final concentration of 100 nM of the respective siRNAs. The cell culture plate was shaken gently for 5 s and incubated for 24 h at 37 °C. Knockdown efficiencies of the proteins were determined by Western blotting.
Detection of ROS
The Image-iT™ LIVE Green Reactive Oxygen Species (ROS) Detection Kit obtained from Invitrogen was used to estimate ROS in live HBMEC cells. Following treatment of cells according to the experimental conditions, cells were incubated with 15 mM DCF-DA for 45 min, briefly centrifuged to remove the dye and resuspended in HEPES buffer. The change in fluorescence was measured in a spectrofluorimeter set at 485-nm excitation and 530-nm emission. Change in fluorescence intensity was represented in arbitrary units.
TUNEL Assay
Apoptosis was determined by the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay. The procedure was carried out according to the protocol provided in the TUNEL kit (Roche, Germany). Briefly, after fixation, cells were incubated in the TUNEL reaction mixture containing deoxynucleotidyl transferase (TdT) buffer with TdT and biotinylated dUTP or FITC-dUTP in a humid atmosphere at 37 °C for 90 min and then washed with PBS. HBMECs were incubated at room temperature for 30 min with anti-HRP antibody and visualized with diaminobenzidine. Transfected HBMECs were observed by fluorescence microscopy. TUNEL-positive apoptotic cells were quantified by counting the positively stained cells.
Preparation of Cytosolic and Membrane Fractions
Treated cells were harvested and washed in ice-cold phosphate-buffered saline (PBS). Briefly, total cellular protein was extracted from the cells. The lysates were spun at 10,000g for 30 min to pellet the mitochondria. And, the mitochondrial and cytosolic proteins were isolated using a commercial Mitochondrial/Cytosol Fraction Kit according to the manufacturer’s instructions (BioVision Inc., Mountain View, CA).
Western Blot Analysis
Western blot analysis was performed using protein extracts of HBMECs. Proteins were separated by electrophoresis and transferred to PVDF membranes. The membranes were blocked with either 5 % nonfat dry milk or 5 % BSA in Tris-buffered saline with Tween-20 (TBST) at room temperature for 1 h to block nonspecific immunoreactivity. Subsequently, the membranes were incubated with the corresponding primary antibodies overnight at 4 °C. After four washes in TBST, the membranes were incubated with secondary antibodies for 1 h and detected by the enhanced chemiluminescence detection kit. In some cases, the blots were stripped and reprobed with antibodies specific for Bax, Bcl-2, cytochrome c, BiP/GRP78, p-PERK/PERK, p-IRE1/IRE1 (Santacruz Bio-technology, 1:500), p-eIF2α/eIF2α, caspases 12, and CHOP/AGDD153 (Cell Signaling, Danvers, MA). All of the westerns were repeated at least three times.
Immunocytochemical Staining
For immunocytochemistry, HBMECs were plated on cover slips for 24 h, treated with the respective agents for 12 h, and fixed with 4 % paraformaldehyde for 15 min at room temperature followed by permeabilization with 0.3 % Triton X-100 in PBS. Cells were then incubated in a blocking buffer for 1 h at room temperature followed by addition of anti-CHOP/AGDD153 (1:1000; Abcam) overnight at 4 °C. This was followed by addition of the secondary Alexflour 488 goat anti-mouse IgG for 2 h to detect expression of CHOP. Cells were mounted with prolong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA) onto slides. Slides were examined using fluorescence microscope (Carl Zeiss). To visualize the ER, cells were cultured with 250 nmol/L of ER Tracker Red CMXRos (Molecular Probes, Eugene, OR, USA) for 30 min at 37 °C prior to fixation. Primary anti-caspase 12 antibody diluted at 1:500 was added to cells for 3 h at room temperature and then incubated with fluorescence-conjugated secondary antibody for 30 min. After the slides were covered with glycerol buffer, the cells were observed under fluorescence microscopy and images were taken.
Analysis of Mitochondrial Membrane Depolarization
The change in mitochondrial membrane potential in the HBMECs was monitored using the mitochondrial membrane potential detection kit (Cayman Chemical Company) according to the manufacturer’s instructions. Briefly, HBMECs cultured in either 24-well plate (1×105 cells per well) or 96-well plate (3×104 cells per well) were treated with Tat followed by treatment with 1× JC-1 reagent diluted in serum-free culture medium for 20 min at 37 °C in 5 % CO2. Thereafter, cells were rinsed once in 1× rinsing buffer provided in the kit. Fluorescence was measured using the FL600 fluorescent plate reader (Bio-Tek Instruments, Winooski, VT) at the excitation wavelengths of 485 and 535 nm. All experiments were repeated at least three times.
Statistical Analysis
All the data are expressed as the mean±SEM. Statistical significance was evaluated with Student’s t test for between two groups or ANOVA followed by the Newman-Keuls’ test for multiple groups. P<0.05 was considered as statistically significant difference.
Results
HIV Tat Mediated Reduction of Cell Viability and Tat Induced Apoptosis of HBMECs
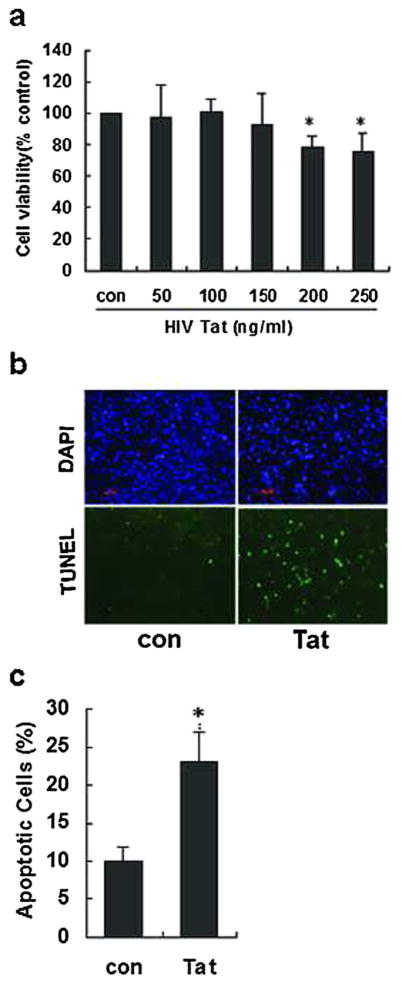
Herein, we sought to examine whether HIV Tat was toxic for brain endothelial cells. For this, HBMECs were exposed to HIV Tat (0–250 ng/mL) for 36 h and cell viability assessed using MTT assay. The rationale for choosing Tat concentration to perform dose response curve in the range of 50–250 ng/ mL is in keeping with the physiological levels of Tat found in the cerebrospinal fluid and in the serum of HIV-infected patients, as reported previously [20, 21]. As shown in Fig. 1a, while exposure of HBMECs to 150 ng/mL Tat did not induce a significant decrease in cell viability compared with the control cells, exposure of cells to 200 ng/mL Tat markedly reduced cell viability (80.9 %, P<0.05).
Fig 1.
Dose-dependent effect of HIV Tat on viability of HBMECs. a HBMECs exposed to varying concentrations of HIV Tat (0–250 ng/mL) were assessed for cell viability using the MTT assay. b A representative image demonstrating HIV Tat-mediated induction of apoptosis as shown by TUNEL-positive cells (green). Nucleus is stained with DAPI (blue). Scale bars=20 μm. c Apoptotic cells were counted by positive TUNEL staining, and apoptotic ratio was calculated by plotting TUNEL-positive cell number against total cell number. Data are presented as mean±SEM of four individual experiments. *P<0.05 compared with control
Further validation of these findings was done using TUNEL assay. Briefly, cells were exposed to HIV Tat (200 ng/mL) for 36 h and assessed for apoptosis using TUNEL staining. As shown in Fig. 1b, c, following Tat exposure, there was an increase in apoptotic TUNEL-positive (green) cells (23 %, P<0.05). For further studies, we chose Tat at a concentration of 200 ng/mL. This is also in agreement with our previous work [22].
Tat Induced ER Stress and Activated UPR in HBMECs
It is well known that prolonged ER stress leads to induction of apoptosis. To determine whether exposure of Tat resulted in induction of ER stress in HBMECs, we investigated expression of several critical ER stress markers such as Bip/GRP78, p-IRE1, p-PERK, and ATF6. Briefly, HBMECs were exposed to HIV Tat (200 ng/mL) for 0.5, 1, 3, 6, 12, and 24 h followed by assessment of cell lysates for expression of the ER stress proteins by Western blot analysis. As shown in Fig. 2, exposure of HBMECs to HIV Tat resulted in a significant and time-dependent increase in the expression of the ER stress sensor proteins p-IRE1, p-PERK, and ATF6. Both p-PERK and ATF6 were induced as early as 0.5 h with the upregulation persisting for 24 h. The total protein levels of PERK and IRE1 did not change throughout the exposure period. HIV Tat thus induced time-dependent expression of ER stress proteins in HBMECs.
Fig 2.
Time-dependent effects of Tat on activation of ER stress sensors and ER chaperones. a Western blot analysis of lysates of HBMECs treated with HIV Tat demonstrating time-dependent increase in expression of p-PERK, p-IRE1a, ATF6, and GRP78/Bip. Shown here are representative results from at least three independent experiments. b Densitometric analysis of Tat-mediated induction of ER stress markers in HBMECs. Data are presented as mean±SEM of four individual experiments. *P<0.05; **P<0.01 compared with control
HIV Tat Induced Generation of ROS That Was Involved in ER Stress
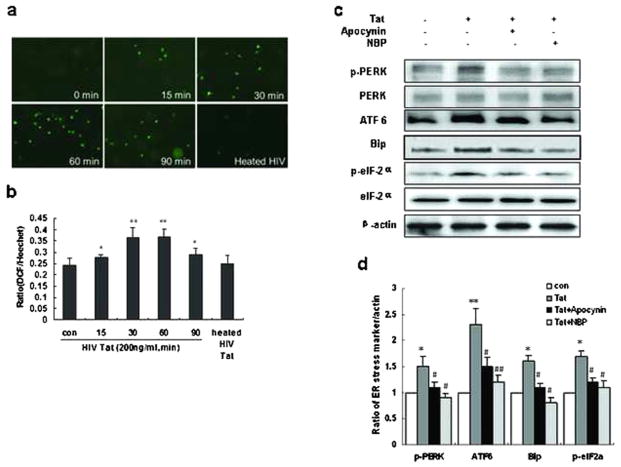
ROS generation is central to activation of the ER stress pathway [23]. We thus sought to examine whether exposure of HBMECs to Tat could also result in induction of ROS. Following treatment of HBMECs to Tat (200 ng/mL), levels of intracellular ROS were assessed using the DCF fluorescence assay. We found that the levels of DCF fluorescence were significantly and time-dependently increased following exposure of HBMECs to Tat (Fig. 3a, b). As expected, heated Tat failed to induce generation of ROS.
Fig 3.
Tat-mediated induction of ER stress involves ROS. a, b Tat-induced time-dependent generation of ROS in HBMECs. HBMECs were incubated with HIV Tat for indicated time periods (15–90 min) and assessed for production of ROS production using the DCF-DA assay. c Western blot analysis of the ER stress markers in the lysates of HBMECs exposed to HIV Tat in the presence or absence of ROS inhibitors apocynin or NBP. The results shown here are representative of three independent experiments. d Quantification analysis of HBMECs were monitored for ER stress markers expression using Western blot. Data are presented as mean±SEM of four individual experiments. *P<0.05; **P<0.01 compared with control; #P<0.05; ##P<0.01 compared with Tat-treated cells
To further investigate the relationship between ROS generation and ER stress response, HBMECs were pretreated with the ROS inhibitors apocynin (a NADPH oxidase inhibitor) or NBP (a ROS scavenger), followed by treatment with Tat. As shown in Fig. 3c, both the ROS inhibitors significantly ameliorated Tat-induced expression of ER stress markers, p-PERK, ATF6, Bip, and p-elF2α. These results thus underpin the role of upstream induction of ROS in Tat-mediated upregulation of ER stress in HBMECs.
HIV Tat Induced ER Stress Pro-Apoptotic Proteins—CHOP and Caspase 12
Having determined that ER stress pathway was upregulated following Tat exposure, the next step was to investigate whether the downstream pro-apoptotic pathways of the ER stress pathway were also activated following Tat exposure. It has been well documented that CHOP/GADD153 (growth arrest and DNA damage 153) belong to the family of bZip transcription factors and are induced via the ATF6 and PERK pathways [12]. Tat exposure resulted in a time-dependent expression of CHOP (Fig. 4a, d). Validation of Tat-mediated induction of CHOP was further demonstrated by pretreating HBMECs with two different ER stress inhibitors SB or 4PBA followed by exposure of cells to Tat. As shown in Fig. 4d, cells pretreated with the ER stress inhibitors failed to demonstrate Tat-mediated induction of CHOP, thus underpinning the role of CHOP as a downstream mediator of the ER stress pathway. Validation of these findings was also carried out by immunostaining HBMECs that were exposed to Tat in the presence or absence of ER stress inhibitors, for CHOP (Fig. 4b).
Fig 4.
Tat induced expression of the pro-apoptotic ER stress protein CHOP and caspase 12. a Representative Western blot demonstrating Tat-mediated time-dependent upregulation of both CHOP and cleaved caspase 12 in HBMECs. b, c HBMECs grown on cover slips were exposed to Tat (12 h) in the presence or absence of ER stress inhibitor and immunostained using the CHOP (b) or caspase 12 (c) antibody. As shown in the representative image, Tat treatment resulted in increased expression of CHOP and caspase 12 compared to untreated cells and exposure in the presence of the ER stress inhibitor and reduced the level of CHOP and caspase 12. DAPI (blue) was used to counterstain the nuclei. Shown here are representative results from at least three independent experiments and significant quantification. d Western blot analysis of CHOP and caspase 12 using lysates of HBMECs exposed to HIV Tat in the presence or absence of ER stress inhibitors SB or 4PBA. Shown here are representative results from at least three independent experiments and significant quantification. Scale bars=20 μm. e Quantification analysis of cells was monitored for CHOP and caspase 12 expression using Western blot. Data are presented as mean±SEM of four individual experiments. *P<0.05 compared with control; #P<0.05; ##P<0.01 compared with Tat-treated cells
Since caspases are important effector components of the apoptotic pathway and are activated via sequential processing of the caspase family members, we next sought to determine whether caspases were also critical for Tat-mediated induction of apoptosis. Caspase 12 is specifically localized on the cytoplasmic side of the ER and is thought to play a pivotal role in ER stress-mediated cell death. HBMECs were exposed to Tat for various times followed by assessment of caspase 12 expression by Western blot analysis. As shown in Fig. 4a, Tat induced activation of caspase 12 in HBMECs, and this effect was reversed in cells pretreated with the ER stress inhibitors. Furthermore, double labeling of cleaved caspase 12 with ER tracker also confirmed localization of caspase 12 to the ER in Tat-treated HBMECs (Fig. 4c).
ER Stress Is Involved in Tat-Induced Apoptosis
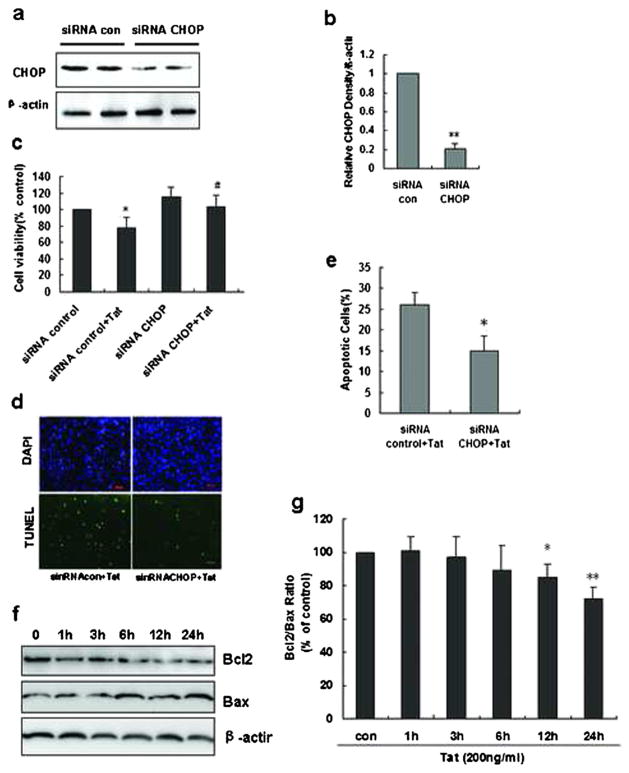
To further examine the role of ER stress in Tat-induced cellular apoptosis, HBMECs were transfected with siRNA specific for CHOP. Transfection efficiency was determined by Western blot analysis. Following transfection with 100 nM of CHOP siRNA, there was about 80 % decrease in expression of CHOP as determined by Western blot (Fig. 5a, b). Twenty-four hours following transfection with siRNA-CHOP, cells were treated with Tat for 36 h and assessed for cell viability using MTTassay. Consistent with the results obtained with the ER stress inhibitor, cells transfected with the siRNA-CHOP and exposed to Tat demonstrated increased viability compared with cells transfected with control siRNA, thereby suggesting that Tat-mediated induction of apoptosis of HBMECs was, at least, partially dependent on ER stress.
Fig 5.
Role of CHOP in mediating Tat-induced apoptosis of HBMECs. a HBMECs were transfected with either nonsense or CHOP siRNAs and examined for CHOP knockdown efficiency. b Quantification analysis of cells was monitored for CHOP expression using Western blot. **P<0.01 compared with siRNA control. c HBMECs transfected with the respective siRNAs were exposed to HIV Tat and assessed for cell viability 36 h later using the MTT assay. *P<0.05 compared with siRNA control; #p<0.05 compared with siRNA control + Tat. d Representative image of HBMECs transfected with the respective siRNAs was exposed to HIV Tat and assessed for cell viability 36 h later using TUNEL assay. e Quantification of percentage of apoptotic cells after exposure to Tat in the absence or presence of negative or CHOP siRNA. *P<0.05 compared with siRNA control + Tat. f Representative Western blots showing the protein levels of Bcl2 and Bax in HBMECs treated with Tat at indicated time points. g The fold changes calculated by normalization of band density with GAPDH from three independent experiments. *P<0.05; **P<0.01 compared with control
These findings were further confirmed by TUNEL staining, and as shown in Fig. 5d, knockdown of CHOP significantly protected the cells against toxicity mediated by Tat.
Tat Triggered Mitochondrial Dysfunction in HBMECs
Mitochondrial dysfunction plays a key role in various forms of apoptotic cell death pathways that are regulated by the Bcl-2 family of proteins. Increased expression of pro-apoptotic protein Bax and a concomitant decreased expression of the anti-apoptotic protein, mitochondrial permeabilization, loss of mitochondrial membrane potential (MMP), and the subsequent release of pro-apoptotic proteins such as cytochrome c are all hallmarks of apoptosis. To next investigate the involvement of mitochondrial dysfunction in Tat-induced apoptosis of HBMECs, we sought to determine the effects of HIV Tat on the expression of Bcl2/Bax proteins, MMP (Δψm), and cytochrome c release. As expected, the ratio of Bcl2/Bax was significantly decreased in a time-dependent manner in cells exposed to Tat (Fig. 6a, b).
Fig 6.
Tat-mediated mitochondrial dysfunction and apoptosis in HBMECs. a Tat-mediated time-dependent dysfunction of the mitochondria in HBMECs using the JC-1 fluorescence assay. Briefly, cells were exposed to HIV Tat for varying times (6–24 h) and assessed for mitochondrial dysfunction using the mitochondrial membrane potential detection kit. *P<0.05; **P<0.01 compared with control. b HBMECs transfected with either nonsense or CHOP siRNA were exposed to Tat and assessed for mitochondrial dysfunction using the JC-1 assay. Shown here are representative results from at least three independent experiments and significant quantification. *P<0.05 compared with siRNA control; #P<0.05 compared with siRNA control + Tat. c, d Western blot analysis of cytochrome c in isolated mitochondrial and cytosolic fractions of HBMECs exposed to HIV Tat for 6 and 24 h. Data are from three independent experiments. *P<0.05 compared with control. e, f HBMECs transfected with either nonsense or CHOP siRNA were exposed to Tat and assessed for cytochrome c in isolated fractions of mitochondria and cytoplasm. Data are presented as mean±SEM of four individual experiments. *P<0.05 compared with siRNA control; #P<0.05 compared with siRNA control + Tat
We next assessed mitochondrial membrane depolarization in HBMECs exposed to HIV Tat (12 h) using the fluorescent lipophilic cationic dye JC-1 that accumulates in the mitochondria in proportion to the Δψm and that exists across the inner mitochondria membrane. As shown in Fig. 6c, treatment of HBMECs with Tat caused a significant depolarization of MMP compared with the untreated controls, and this effect was reversed in cells knocked down for CHOP.
Impairment of MMP leads to release of cytochrome c from the mitochondria to the cytoplasm, with activation of the downstream apoptotic cascades. We thus next sought to assess the effect of Tat exposure on the release of cytochrome c. As shown in Fig. 6e, exposure of HBMECs to HIV Tat resulted in increased release of cytochrome c in the cytosolic fraction, with a concomitant decrease in the mitochondrial fraction. Interestingly, knockdown of CHOP in siRNA-transfected HBMECs failed to release cytochrome c in the cytoplasm following exposure to Tat, thereby implicating Tat-induced ER stress effector as an upstream mediator of mitochondrial dysfunction.
Discussion
HAND is a common complication of HIV infection. Among the factors involved in the pathogenesis of HAND, loss of integrity of the endothelial cell layer of the BBB is one of the key mechanisms by which there occurs an increased influx of immune cells, toxins, and pathogens into the neural tissue [24]. Our findings for the first time demonstrate that HIV Tat-mediated apoptosis of brain endothelial cells involves both ER stress and mitochondrial dysfunction. Activated ER stress, as indicated by an increase in the UPR, results in impairment of BBB by promoting vascular endothelial cell apoptosis. Treatment of cultured HBMECs with either the ER stress inhibitor or silencing of CHOP using specific siRNAs abrogated HIV Tat-induced apoptosis. Furthermore, our findings also implicated the role of mitochondrial dysfunction that was downstream of ER stress pathway, as a contributor of HIV Tat-induced brain endothelial cell apoptosis.
HIV-associated protein Tat has been well recognized to trigger both the intrinsic and extrinsic apoptotic pathways, in various kinds of cells thereby contributing to disease pathogenesis in the CNS. HIV Tat has been shown to exert toxicity to the dopaminargic [25] and hippocampal neurons [26] and is also cytotoxic to the intestinal epithelial [27] and retinal epithelial cells [28] likely triggering cellular damage and death via the apoptotic pathways. Consistent with these reports, we also demonstrate that HBMECs exposed to HIV Tat exhibited significant decrease in cell viability with increased apoptosis, compared to cells not treated with Tat.
In the HIV-infected brain, upregulation of ER stress sensor proteins has been documented in various cells of the CNS such as the neurons and astrocytes [29]. The present study was aimed at investigating whether the treatment of HBMECs with HIV Tat101 could induce ER stress in HBMECs, which in turn, could lead to Tat-mediated apoptosis. Immunoblotting was used to assess the expression of ER chaperon BiP/GRP78 and the ER stress sensor proteins: IRE1, PERK, and ATF6, which are the crucial ER stress signature markers. In the physiological state, when ER-resident protein folding and processing capacity balances the ER load of newly synthesized proteins, the ER chaperon GRP78 is bound to IRE1, PERK, and ATF6, thus blocking activation. Under conditions of ER stress, however, when unfolded proteins accumulate in the ER lumen, GRP78 dissociates from PERK, IRE1, and ATF6 and binds to the unfolded proteins to assist in refolding. PERK is an ER-associated transmembrane serine/threonine protein kinase. Following accumulation of unfolded or misfolded proteins in the ER lumen, PERK dimerizes and trans-autophosphorylates, leading in turn, to activation of eIF2a. Phosphorylation of eIF2a inhibits global translation initiation, while promoting activation of ATF4 [10]. Under physiological conditions, ATF6, another key ER stress sensor, remains localized at the ER membrane and bound to BiP. Following ER stress triggers, however, BiP dissociates from ATF6 leading to release of the latter into the Golgi complex, where ATF6 is subsequently cleaved by proteases. The processed form of ATF6 translocates to the nucleus and binds to its target genes [8]. The findings reported herein demonstrated that treatment of HBMECs with HIV Tat101 increased a number of signature ER stress markers GRP78/Bip, ATF6, p-PERK, and the downstream mediators, p-eIF2a and ATF4. The current study provides important evidence to support the involvement of ER stress in HIV Tat-mediated apoptosis of HBMECs. Another key ER stress sensor is IRE1. Activated IRE1 acts as an endonuclease that cleaves a sequence of 26 bases from the coding region of xbp1 mRNA. This in turn causes a frame-shift leading to the processing of the mRNA into a newly spliced protein that functions as a transcription factor for genes involved in the ER resident folding and processing reactions. Intriguingly, our findings demonstrate that HIV Tat101 also significantly induced expression of IRE1 as well as that of ATF6 and p-PERK proteins. Taken together, the findings suggest that HIV Tat101-mediated induction of UPR response involves the p-PERK, ATF6, and IRE1 signaling pathways.
It is well documented that if the UPR-mediated efforts to correct the protein-folding defect fail, apoptosis ensues [28, 30]. Several mechanisms have been proposed by which apoptotic signals are generated following ER stress [31, 32]. The most important ER stress-induced apoptotic pathway is known to be mediated through the CHOP/GADD153 signaling [33, 34]. CHOP/GADD153 is a bZiP transcription factor that is induced via the ATF6 and PERK/eIF2á UPR pathways. As expected, our findings demonstrated that HIV Tat mediated activation of PERK and the ATF6 pathways, leading subsequently to upregulation of the pro-apoptotic protein CHOP. Furthermore, we also demonstrated that blocking either the PERK pathway or CHOP expression abrogated HIV Tat101-induced apoptosis of HBMECs.
Since caspase 12, a member of the caspase family is well known to play a special role in ER-related cellular apoptosis, we next sought to examine whether it was also involved in HIV Tat-mediated apoptosis of HBMECs. Treatment of HBMECs with HIV Tat resulted in activation of caspase 12, and this effect could be reversed by the ER inhibitor, thereby lending further credence to the involvement of ER stress-related signaling pathways in HIV Tat-induced apoptosis of brain endothelial cells. These findings are in agreement with a previous study on embryonic fibroblasts isolated from caspase 12−/− mice that were reported to exhibit resistance against ER stress-inducing agents [34].
Furthermore, it was of interest that HIV Tat also stimulated generation of ROS in HBMECs. It has been reported that generation of ROS can lead to protein misfolding in the ER, with the accumulated misfolded proteins culminating ultimately into the ER stress response [35, 36]. Our data clearly demonstrated a link between ROS production and ER stress as evidenced by the fact that ROS inhibitor downregulated ER stress-associated protein expression. These findings are in agreement with the previous report indicating that ER is sensitive to oxidative stress [37]. It is worth mentioning that ROS can not only act as a trigger but is also generated as a result of ER stress [38]. Accumulating evidence suggests that protein folding and generation of ROS as a byproduct of protein oxidation in the ER are closely linked events. There may thus be a vicious cycle between ER stress and oxidative stress, leading to cellular apoptosis.
In addition to ER, mitochondria are also key cellular organelles involved in the initiation of cell death [39]. Mitochondrial apoptosis pathway is regulated by the integrity of the mitochondrial outer membrane, which in turn is tightly regulated by the ratio of the anti-versus the pro-apoptotic family of proteins. In particular, a decrease in the ratio of anti-apoptotic Bcl-2 to pro-apoptotic Bax results in the disruption of the mitochondrial membrane potential with the consequent release of cytochrome c from the mitochondria into the cytoplasm. This in turn triggers initiation and activation of the caspase cascade [40, 41]. Our findings demonstrated that exposure of HIV Tat to HBMECs induced upregulation of the pro-apoptotic protein Bax with a concomitant decrease in the expression of the anti-apoptotic protein Bcl-2, with a significant increase in Bax to Bcl-xl ratio. HIV Tat exposure of HBMECs also resulted in a loss of mitochondrial membrane potential and release of cytochrome c from the mitochondria. Notably, mitochondria and ER networks are fundamental for the maintenance of cellular homeostasis. Our findings clearly suggest the involvement of ER stress in mitochondrial dysfunction leading to HIV Tat-mediated apoptosis of HBMECs. These findings can have ramifications in HIV-infected individuals that are exposed to the viral protein Tat. Exposure of brain endothelial cells to the viral protein in the systemic compartment could lead to cellular apoptosis, thereby disrupting the barrier permeability and leading to increased inflammation within the CNS.
In summary, our findings demonstrate that exposure of HBMECs to HIV Tat induced ROS, ER stress, and mitochondrial dysfunction resulting in cell death. Moreover, our data also suggest that the use of ER stress inhibitor or antioxidants prevented cell death. Therapies aimed at abrogating ER stress markers could be considered as approaches to inhibit endothelial cell apoptosis and the ensuing neuroinflamamtion.
Acknowledgments
This work was supported by grants DA020392, DA023397, and DA024442 from the National Institutes of Health.
Footnotes
Conflict of Interest The authors declare no competing financial interests.
Contributor Information
Rong Ma, Department of Pharmacology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China.
Lu Yang, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE 68198, USA.
Fang Niu, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE 68198, USA.
Shilpa Buch, Email: sbuch@unmc.edu, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE 68198, USA, Department of Pharmacology and Experimental Neuroscience, 985880 Nebraska Medical Center (DRC 8011), University of Nebraska Medical Center, Omaha, NE 68198-5880, USA.
References
- 1.Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 2.Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K. Molecular and cellular permeability control at the blood-brain barrier. Brain Res. 2001;36:258–264. doi: 10.1016/s0165-0173(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 4.Kraft P, Schwarz T, Gob E, Heydenreich N, Brede M, Meuth SG, Kleinschnitz C. The phosphodiesterase-4 inhibitor rolipram protects from ischemic stroke in mice by reducing blood-brain-barrier damage, inflammation and thrombosis. Exp Neurol. 2013;247:80–90. doi: 10.1016/j.expneurol.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Palmer AM. The role of the blood brain barrier in neurodegenerative disorders and their treatment. J Alzheimers Dis. 2011;24:643–656. doi: 10.3233/JAD-2011-110368. [DOI] [PubMed] [Google Scholar]
- 6.Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev. 2009;19:169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- 7.Feng S, Cen J, Huang Y, Shen H, Yao L, Wang Y, Chen Z. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS One. 2011;6:e20599. doi: 10.1371/journal.pone.0020599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 9.Mori K, Sant A, Kohno K, Normington K, Gething MJ, Sambrook JF. A 22 bp cis-acting element is necessary and sufficient for the induction of the yeast KAR2 (BiP) gene by unfolded proteins. EMBO J. 1992;11:2583–2593. doi: 10.1002/j.1460-2075.1992.tb05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 11.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 12.Upton JP, Austgen K, Nishino M, Coakley KM, Hagen A, Han D, Papa FR, Oakes SA. Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol Cell Biol. 2008;28:3943–3951. doi: 10.1128/MCB.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Silva EF, Fonseca FA, França CN, Ferreira PR, Izar MC, Salomão R. Imbalance between endothelial progenitors cells and microparticles in HIV-infected patients naive for antiretroviral therapy. AIDS. 2011;13:1595–1601. doi: 10.1097/QAD.0b013e32834980f4. [DOI] [PubMed] [Google Scholar]
- 14.Bethel-Brown C, Yao H, Callen S, Lee YH, Dash PK, Kumar A, Buch S. HIV-1 Tat-mediated induction of platelet-derived growth factor in astrocytes: role of early growth response gene 1. J Immunol. 2011;186:4119–4129. doi: 10.4049/jimmunol.1002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toborek M, Lee YW, Pu H, Malecki A, Flora G, Garrido R, Hennig B, Bauer HC, Nath A. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J Neurochem. 2003;84:169–179. doi: 10.1046/j.1471-4159.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 16.Patel HH, Hamuro LL, Chun BJ, Kawaraguchi Y, Quick A, Rebolledo B, Pennypacker J, Thurston J, Rodriguez-Pinto N, Self C, Olson G, Insel PA, Giles WR, Taylor SS, Roth DM. Disruption of protein kinase A localization using a trans-activator of transcription (TAT)-conjugated A-kinase-anchoring peptide reduces cardiac function. J Biol Chem. 2010;285:27632–27640. doi: 10.1074/jbc.M110.146589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kline ER, Sutliff RL. The roles of HIV-1 proteins and antiretroviral drug therapy in HIV-1-associated endothelial dysfunction. J Investig Med. 2008;56:752–769. doi: 10.1097/JIM.0b013e3181788d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 20.Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toborek M, Lee YW, Flora G, Pu H, András IE, Wylegala E, Hennig B, Nath A. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol. 2005;25:181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng F, Yao H, Akturk HK, Buch S. Platelet-derived growth factor CC-mediated neuroprotection against HIV Tat involves TRPC-mediated inactivation of GSK 3beta. PLoS One. 2012;7:e47572. doi: 10.1371/journal.pone.0047572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maingat F, Halloran B, Acharjee S, Van MG, Church D, Gill MJ, Uwiera RR, Cohen EA, Meddings J, Madsen K, Power C. Inflammation and epithelial cell injury in AIDS enteropathy: involvement of endoplasmic reticulum stress. FASEB J. 2011;25:2211–2220. doi: 10.1096/fj.10-175992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahajan SD, Alinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Fernandez SF. Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J Clin Immunol. 2008;28:528–541. doi: 10.1007/s10875-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 25.Midde NM, Gomez AM, Zhu J. HIV-1 Tat protein decreases dopamine transporter cell surface expression and vesicular monoamine transporter-2 function in rat striatal synaptosomes. J Neuroimmune Pharmacol. 2012;7:629–639. doi: 10.1007/s11481-012-9369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- 27.Buccigrossi V, Laudiero G, Nicastro E, Miele E, Esposito F, Guarino A. The HIV-1 transactivator factor (Tat) induces enterocyte apoptosis through a redox-mediated mechanism. PLoS One. 2011;6:e29436. doi: 10.1371/journal.pone.0029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai L, Zhu X, Ma T, Wang J, Wang F, Zhang S. The p38 MAPK NF-kappaB pathway, not the ERK pathway, is involved in exogenous HIV-1 Tat-induced apoptotic cell death in retinal pigment epithelial cells. Int J Biochem Cell Biol. 2013;45:1794–1801. doi: 10.1016/j.biocel.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Lindl KA, Akay C, Wang Y, White MG, Jordan-Sciutto KL. Expression of the endoplasmic reticulum stress response marker, BiP, in the central nervous system of HIV-positive individuals. Neuropathol Appl Neurobiol. 2007;33:658–669. doi: 10.1111/j.1365-2990.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- 30.Moserova I, Kralova J. Role of ER stress response in photo-dynamic therapy: ROS generated in different subcellular compartments trigger diverse cell death pathways. PLoS One. 2012;7:e32972. doi: 10.1371/journal.pone.0032972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 32.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 33.Yaman I, Fernandez J, Liu H, Caprara M, Komar AA, Koromilas AE, Zhou L, Snider MD, Scheuner D, Kaufman RJ, Hatzoglou M. The zipper model of translational control: a small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell. 2003;113:519–531. doi: 10.1016/s0092-8674(03)00345-3. [DOI] [PubMed] [Google Scholar]
- 34.Kadowaki H, Nishitoh H, Ichijo H. Survival and apoptosis signals in ER stress: the role of protein kinases. J Chem Neuroanat. 2004;28:93–100. doi: 10.1016/j.jchemneu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Tsai CF, Yeh WL, Huang SM, Tan TW, Lu DY. Wogonin induces reactive oxygen species production and cell apoptosis in human glioma cancer cells. Int J Mol Sci. 2012;13:9877–9892. doi: 10.3390/ijms13089877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsukiyama-Kohara K. Role of oxidative stress in hepatocarcinogenesis induced by hepatitis C virus. Int J Mol Sci. 2012;13:15271–15278. doi: 10.3390/ijms131115271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 38.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Investig. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chami M, Oules B, Szabadkai G, Tacine R, Rizzuto R, Paterlini-Brechot P. Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol Cell. 2008;32:641–651. doi: 10.1016/j.molcel.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giorgi C, De SD, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]