Abstract
Allogeneic hematopoietic cell transplantation is often complicated by graft-versus-host disease (GVHD). We analyzed the incidence and risk factors for acute and chronic graft-versus-host disease (GVHD), and their impact on disease relapse and survival, among recipients of single umbilical cord blood (sUCB, n=295), double umbilical cord blood (dUCB, n=416), and matched sibling donor (MSD, n=469) allografts. The incidence of grade II-IV aGVHD and chronic GVHD among dUCB, sUCB, and MSD was 56%, 26% and 37% and 26%, 7%, and 40%, respectively. Development of acute GVHD had no effect on relapse, non-relapse mortality, or overall survival among UCB recipients, but was associated with worse non-relapse mortality and survival in MSD recipients. Development of cGVHD was only associated with lower relapse in dUCBT. In multivariate analysis of GVHD incidence, age > 18 years was associated with higher incidence of aGVHD and cGVHD across all cohorts, while worse HLA match and prior aGVHD were associated with higher risks of aGVHD in both UCB cohorts. Non-myeloablative conditioning limited the risk of aGVHD compared to myeloablative conditioning in dUCB recipients. Cyclosporine A and mycophenolate mofetil as GVHD prophylaxis lowered the risk of cGVHD compared to steroids with cyclosporine A among sUCB recipients. This large contemporary analysis suggests similarity of risks and consequences of GVHD for UCB and MSD recipients. Limiting the severity of aGVHD remains important in all groups. Increasing the UCB inventory or developing strategies that reduce the cell-dose threshold and thereby increase the chance of identifying an adequately dosed, better HLA-matched single UCB unit may further limit risks of acute GVHD after UCB transplantation.
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is a potentially curative treatment modality for a spectrum of hematologic malignancies, bone marrow failure syndromes, and inherited metabolic and immune disorders. For transplant candidates without a suitable matched sibling donor (MSD), umbilical cord blood (UCB) has emerged as an effective alternative donor source with its recent clinical outcomes approaching, and in certain circumstances surpassing, those of matched unrelated donor (MUD) allografting (1-5). Despite continued improvements in outcomes after allo-HCT (6), acute (aGVHD) and chronic GVHD (cGVHD) remain major determinants of post-transplant morbidity, non-relapse mortality (NRM), and health-related quality of life. Even among recipients of MSD allo-HCT, incidence rates of aGVHD and cGVHD reach 40-50% and 30-70%, respectively (7, 8). Prior studies have suggested important differences in risk factors for GVHD after UCB transplantation (UBCT) (9, 10). While the incidence of cGVHD after single or double unit UCBT was lower than in MUD allo-HCT, despite mismatch in up to two HLA loci across multiple studies (2-5, 11-15), the incidence of aGVHD after dUCBT appeared to be higher than with sUCBT (16-18). Only a few smaller previous studies combining sUCBT and dUCBT recipients evaluated prognostic factors of GVHD (19-22), and only one of those to our knowledge reported the impact of GVHD on relapse and survival (21). Existing data on factors that determine acute and chronic GVHD in dUCBT are incomplete, including the implications of conventional HLA disparity (i.e., antigen-level match at HLA–A, –B, and allele-level match at –DRB1). We therefore performed a comprehensive analysis of GVHD incidence and risks factors among sUCB, dUCB, and MSD allograft recipients with particular focus on HLA disparity and impact of GVHD on post-transplant relapse and survival.
METHODS
Study design
All consecutive patients undergoing their first MSD (n=469), sUCB (n=295), or dUCB (n=416) transplantation for a malignant or non-malignant condition between 2000 and 2012 were studied. By taking advantage of the homogeneity in GVHD grading criteria, treatment plans, and graft selection criteria for MSD and UCB at a single transplant center, we designed this study to evaluate the cohort-specific GVHD outcomes of UCBT in parallel to the current gold-standard outcomes of MSD allo-HCT. Patient demographic and clinical information was retrieved from the transplant database at the University of Minnesota. The primary endpoints were onset of aGVHD and cGVHD after allo-HCT and their associated risk factors within each individual cohort. Secondary study endpoints included non-relapse mortality (NRM), disease relapse, and overall survival, as influenced by GVHD. The diagnoses of acute and chronic GVHD were made according to standard clinical criteria (23-25). HLA disparity and gender mismatch within the dUCB cohort were established based on degree of HLA and gender matching between the predominant cord blood unit of donor and recipient. The cumulative incidences of aGVHD and cGVHD along with their risk factors were assessed within individual cohorts based on their distinct underlying patient populations. The analysis of NRM and relapse was restricted to patients with hematologic malignancies across all 3 cohorts (MSD, n=423; sUCB, n=161; sUCB, n=391). All patients signed informed consent prior to their transplantation, and this study was approved by the Institutional Review Board at the University of Minnesota.
Donor selection, conditioning regimens, GVHD prophylaxis, and supportive care
Our donor selection algorithm conformed to the general practice of using an HLA-identical sibling as the first-choice donor. In the absence of suitable MSD, a UCB donor graft was frequently used, particularly for patients with an urgent need for allografting. UCB donor selection was based on both cell dose and conventional HLA-matching including antigen-level matching at HLA-A and -B and allele-level matching at HLA-DRB1 as previously described (26). Non-myeloablative conditioning (NMA) regimen was defined by established criteria (27), and it was used on the basis of patient age (≥ 55 for MSD and ≥ 45 years for UCB), presence of comorbidities, and extent of prior therapy. The details of conditioning regimens for MSD and UCB allograft recipients were previously reported (21, 28, 29). In brief, most patients with hematologic malignancies who underwent myeloablative conditioning (MAC) MSD allo-HCT received cyclophosphamide 120 mg/kg and total body irradiation (TBI) 1320 cGy followed by cyclosporine A (CsA)/methotrexate or CsA/mycophenolate mofetil (MMF) for GVHD prophylaxis. Most MAC UCBT recipients received cyclophosphamide 120 mg/kg, fludarabine 75 mg/m2, and total body irradiation (TBI) 1320 cGy with CsA/MMF used for GVHD prophylaxis (26). Non-TBI-based conditioning regimens (17.5%) for MAC MSD allo-HCT included Bu/Cy (9%) ± antithymocyte globulin (ATG), Flu-based (3%) ± ATG, and other less frequent regimens (5.5%). The NMA regimen for MSD and UCB allo-HCT primarily consisted of cyclophosphamide (50 mg/kg Day −6), fludarabine (30-40 mg/m2 Day −6 through −2), and TBI (200 cGy Day −1) ± ATG for those at higher risk for graft failure due to limited recent chemotherapy (21). For GVHD prophylaxis, most dUCBT recipients were given CsA and MMF, and two-thirds of sUCBT recipients were given CsA/MMF and one-third CsA/methylprednisolone. From 2006 onward, UCBT protocols were modified to use a higher MMF dose (3 g/day vs. 2 g/day). Stem cell infusion procedure and post-transplant supportive care conformed to our institutional practice guidelines as described (21, 26). All patients received filgrastim after allo-HCT until absolute neutrophil count was ≥2.5 × 109/L for two consecutive days.
Statistical analysis
Demographic and clinical characteristics were compared across all three cohorts by continuous (general Wilcoxon test) and categorical (Chi-square test) data analyses. The major study outcomes were not directly compared across the cohorts because each individual cohort had distinct patient population-, disease-, and transplantation-related characteristics. Instead, cohort-specific analyses were conducted throughout the study. Cumulative incidence of aGVHD and cGVHD was estimated with non-GVHD deaths or relapse modeled as competing risks (30). Fine and Gray proportional hazards regression was used to assess the independent effect of an individual variable on development of GVHD (31). Proportional hazard assumptions were verified using Martingale residuals. Cox regression analysis was used in instances when GVHD was treated as a time-dependent covariate in regression models of secondary endpoints (32). Regression analyses were performed in a step-wise fashion declaring factors with p-values < 0.05 as statistically significant. There was no correction for multiple comparisons. Confounding factors or variables that showed significance across similar endpoints or subgroups may have also been included in the final models. Factors tested in regression models included age, disease risk, conditioning regimen (MAC vs. NMA), donor/recipient CMV serostatus, donor-recipient gender match (female-to-male vs. other), year of transplant (<2006 vs. ≥2006), GVHD prophylaxis, ATG use, and stem cell source (marrow vs. PBSC) for MSD. Disease risks at the time of allo-HCT for patients with hematologic malignancies were classified as standard (i.e., acute leukemia in first or second complete remission, CML in first chronic phase, Hodgkin or non-Hodgkin lymphoma in complete or partial chemotherapy sensitive remission, CLL in first remission, MDS or myeloproliferative neoplasm without excess blasts) or high (all other conditions) risks based on the ASBMT RFI 2006 risk-scoring schema (http://www.asbmt.org). All statistical analyses were performed with SAS 9.3 (SAS Institute, Cary, NC) and R 3.0.2.
RESULTS
Patient and clinical characteristics across all cohorts
Patient and transplant-specific characteristics in all three cohorts are detailed in Table 1. Although sUCBT recipients were younger, the median age of MSD and dUCB allograft recipients was comparable. While gender mismatched allografts were balanced among all cohorts, the recipients of sUCB, dUCB, and MSD differed from each other according to conditioning intensity (myeloablative in 82%, 42%, 55%, respectively), GVHD prophylaxis (CsA+MMF in 66%, 99%, 48%, respectively), use of ATG, underlying diagnosis, and disease risk. The dUCBT cohort was also enriched with recipients of 4/6 HLA-mismatched engrafted units compared to the sUCBT cohort. The median follow-up for survivors across all cohorts exceeded 5 years. In addition, we observed distinct patterns of organ-specific involvement by acute and chronic GVHD across all cohorts (Supplemental Figure 1).
Table 1.
Patient and clinical characteristics across all cohorts
|
| |||
| Variables* | MSD (N=469) |
sUCB (N=295) |
dUCB (N=416) |
|---|---|---|---|
|
| |||
| Age, years | |||
| Median (range) | 46.9 (0-74) | 8.1 (0-65) | 43.9 (1-72) |
| < 18 | 83 (17.7) | 232 (78.6) | 64 (15.4) |
| Gender | |||
| Male | 290 (61.8) | 159 (53.9) | 261 (62.7) |
| Female-to-male mismatch | 133 (28.4) | 74 (25.1) | 128 (30.8) |
| Year of allo-HCT | |||
| 2000-2005 | 242 (51.6) | 154 (52.2) | 156 (37.5) |
| 2006-2012 | 227 (48.4) | 141 (47.8) | 260 (62.5) |
| Diagnosis | |||
| Non-malignant | 46 (9.8) | 134 (45.4) | 25 (6) |
| Leukemia‡/MDS/MPN | 257 (54.8) | 133 (45.1) | 293 (70.4) |
| Lymphoma§/CLL/Other | 166 (35.4) | 28 (9.5) | 98 (23.6) |
| Disease risk¥ | |||
| Standard | 224 (47.8) | 113 (38.3) | 250 (60.1) |
| High-risk | 199 (42.4) | 48 (16.3) | 141 (33.9) |
| CMV seropositive recipient | 260 (55.4) | 133 (45.1) | 233 (56) |
| Conditioning regimen | |||
| MAC | 260 (55.4) | 242 (82) | 173 (41.6) |
| NMA | 209 (44.6) | 53 (18) | 243 (58.4) |
| ATG (with conditioning) | 77 (16.4) | 175 (59.3) | 95 (22.8) |
| GVHD prophylaxis | |||
| CsA+MMF | 224 (47.8) | 194 (65.8) | 411 (98.8) |
| CsA+Methotrexate | 245 (52.2) | 0 | 0 |
| CsA+Steroid ± ATG | 0 | 101 (34.2) | 5 (1.2) |
| HLA disparityǂ | |||
| 6/6 | 469 (100) | 66 (22.4) | 46 (11.1) |
| 5/6 | 0 | 147 (49.8) | 183 (44) |
| 4/6 | 0 | 82 (27.8) | 187 (45) |
| TNC (× 108/kg) | |||
| Median (range) | 7.7 (1-31) | 0.5 (0-5) | 0.4 (0-5) |
| Follow-up time | |||
| Median (range), months | 72.4 (0.4-171) | 63.7 (0.5-171) | 67.5 (0.2-149) |
|
| |||
Corresponding to median (range) as outlined or N (%)
Includes AML, ALL, CML
Includes Hodgkin and Non-Hodgkin lymphomas
According to ASBMT RFI 2006 definitions; remaining % corresponds to non-malignant disorders
Based on HLA matching between the predominant cord unit and recipient
Abbreviations: CsA, cyclosporine A; MMF, mycophenolate mofetil; ATG, anti-thymocyte globulin; CMV, cytomegalovirus; MDS, myelodysplastic syndromes; MPN, myeloproliferative neoplasms; CLL, Chronic lymphocytic leukemia; MAC, myeloablative conditioning; NMA, non-myeloablative conditioning; TNC, total nucleated cells
Incidence and risk factors of aGVHD
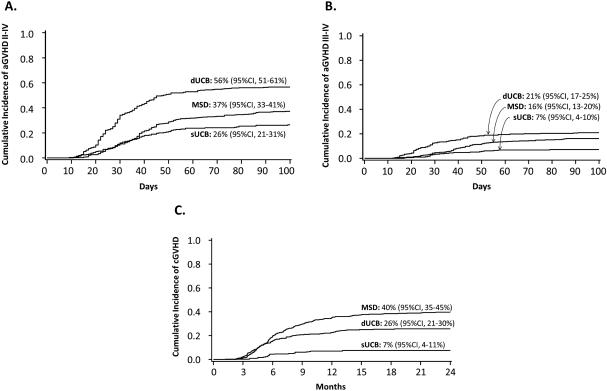
The cumulative incidence (100 days) of grade II-IV was 37% (MSD), 26% (sUCB), and 56% (dUCB), and the cumulative incidence (100 days) of grade III-IV aGVHD was 16% (MSD), 7% (sUCB), and 21% (dUCB) (Figure 1). Cohort-specific multivariate analyses of aGVHD risks are shown in Table 2.
Figure 1.
Cumulative incidence of GVHD by donor type
Table 2.
Cohort-specific risk factors of GVHD: Multivariate regression analysis
| Cohort/Risk factor | Relative risk (95% CI) | ||
|---|---|---|---|
| aGVHD II-IV | aGVHD III-IV | cGVHD | |
| MSD | |||
| Graft source/Age | |||
|
BM+PBSC/<18 BM/≥18 PBSC/≥18 |
1.0 2.0 (0.7-5.7) 3.7 (1.8-7.6) |
1.0 1.0 (0.1-7.9) 2.7 (0.8-8.9) |
1.0 4.8 (1.2-18.2) 10.0 (3.3-30.4) |
| Disease risk | |||
|
Standard High Non-malignant |
1.0 1.0 (0.7-1.4) 0.3 (0.1-1.1) |
1.0 1.1 (0.7-1.8) 0.3 (0.4-1.9) |
1.0 1.0 (0.8-1.4) 0.6 (0.2-2.2) |
| Conditioning | |||
|
MAC NMA (+ATG) NMA (-ATG) |
1.0 1.1 (0.7-1.8) 0.9 (0.7-1.3) |
1.0 2.9 (1.4-5.8) 2.1 (1.2-3.7) |
1.0 0.9 (0.5-1.4) 1.2 (0.9-1.6) |
| aGVHD II-IV‡ | |||
|
No Yes |
NA |
NA |
1.0 1.1 (0.8-1.4) |
| sUCB | |||
| Age | |||
|
<18 ≥18 |
1.0 1.9 (1.2-3.0) |
1.0 3.4 (1.6-7.2) |
1.0 5.7 (1.9-16.5) |
| HLA disparity | |||
|
6/6 5/6 4/6 |
1.0 1.9 (1.0-3.9) 1.9 (0.9-4.0) |
1.0 0.8 (0.2-2.4) 1.0 (0.3-3.2) |
1.0 0.6 (0.2-2.2) 1.1 (0.3-4.0) |
| GVHD prophylaxis | |||
|
CsA+Steroid CsA+MMF |
1.0 0.9 (0.5-1.4) |
1.0 0.4 (0.2-1.1) |
1.0 0.3 (0.1-0.9) |
| aGVHD II-IV‡ | |||
|
No Yes |
NA |
NA |
1.0 2.4 (1.0-6.0) |
| dUCB | |||
| Age | |||
|
<18 ≥18 |
1.0 1.6 (1.0-2.4) |
1.0 1.3 (0.7-2.5) |
1.0 5.4 (2.1-13.7) |
| HLA disparity | |||
|
6/6 5/6 4/6 |
1.0 1.1 (0.7-1.7) 1.1 (0.7-1.7) |
1.0 2.5 (0.9-6.9) 2.8 (1.0-7.7) |
1.0 0.9 (0.4-1.8) 1.3 (0.7-2.7) |
| Conditioning | |||
|
MAC NMA (+ATG) NMA (-ATG) |
1.0 0.5 (0.3-0.7) 0.8 (0.6-1.1) |
1.0 0.5 (0.3-1.0) 0.6 (0.4-1.0) |
1.0 0.7 (0.4-1.1) 0.7 (0.5-1.1) |
| Year of allo-HCT | |||
|
<2006 ≥2006 |
1.0 0.6 (0.5-0.8) |
1.0 0.8 (0.5-1.2) |
1.0 0.9 (0.6-1.3) |
| aGVHD II-IV‡ | |||
|
No Yes |
NA |
NA |
1.0 2.0 (1.3-3.2) |
Note: Bolded estimates denote differences with p≤0.05
Time-dependent covariate
Abbreviations: BM, bone marrow; PBSC, peripheral blood stem cell; CI, confidence interval
MSD cohort
Both older age and PBSC grafts were associated with a higher risk of aGVHD. Owing to the significant correlation between age and graft source, we report the data for graft source stratified by age. Older recipients of PBSC grafts had significantly higher incidence rates of grade II-IV (HR=3.7, 95% CI 1.8-7.6) aGVHD (Table 3) compared to younger patients. The use of NMA allo-HCT with (HR=2.9, 95% CI 1.4-5.8) or without ATG (HR=2.1, 95% CI 1.2-3.7) was also associated with increased risk of grade III-IV aGVHD as compared to MAC allo-HCT. We further explored the independent effect of conditioning intensity on aGVHD and found that NMA conditioning significantly increased aGVHD risk, even after restricting the analysis to PBSC recipients (HR=2.2, p<0.01 for NMA [n=190] vs. MAC [n=177]).
Table 3.
Time-dependent impact of GVHD on 2-year NRM, Relapse and OS
| Cohort/GVHD | NRM | Relapse | OS ‡ | |||
|---|---|---|---|---|---|---|
| 95% CI | p | 95% CI | p | 95% CI | p | |
| MSD | ||||||
| Grade II-IV aGVHD | 2.1 (1.3-3.2) | <0.01 | 1.3 (0.9-1.8) | 0.21 | 1.6 (1.2-2.2) | <0.01 |
| Grade III-IV aGVHD | 3.8 (2.4-6.0) | <0.01 | 1.0 (0.6-1.7) | 0.93 | 1.9 (1.3-2.7) | <0.01 |
| cGVHD | 1.8 (0.9-3.4) | 0.43 | 0.6 (0.4-1.2) | 0.14 | 0.8 (0.5-1.3) | 0.4 |
| sUCB | ||||||
| Grade II-IV aGVHD | 1.1 (0.5-2.2) | 0.78 | 1.0 (0.5-2.2) | 0.93 | 1.2 (0.8-1.8) | 0.51 |
| Grade III-IV aGVHD | 1.1 (0.4-3.3) | 0.85 | 0.8 (0.2-2.6) | 0.72 | 1.8 (1.0-3.3) | 0.06 |
| cGVHD | 3.5 (0.7-18.5) | 0.14 | 0.8 (0.2-3.5) | 0.75 | 1.4 (0.5-3.6) | 0.50 |
| dUCB | ||||||
| Grade II-IV aGVHD | 0.8 (0.5-1.2) | 0.31 | 0.8 (0.6-1.2) | 0.29 | 0.9 (0.7-1.2) | 0.49 |
| Grade III-IV aGVHD | 1.2 (0.7-1.9) | 0.55 | 0.8 (0.5-1.3) | 0.41 | 1.1 (0.8-1.6) | 0.49 |
| cGVHD | 1.3 (0.6-2.7) | 0.46 | 0.5 (0.3-0.9) | 0.03 | 0.9 (0.5-1.4) | 0.51 |
Note: All estimates were derived from the multivariate models adjusted for patient age, stem cell source, recipient CMV serostatus, HLA disparity, conditioning intensity, year of transplant, and disease risk, as necessary.
Estimates for OS correspond to relative risk of death from any cause
sUCB cohort
In the multivariate analysis, age ≥ 18 years was associated with higher incidence of grade II-IV aGVHD (HR=1.9, p=0.01) and grade III-IV aGVHD (HR=3.4, p<0.01). Worse HLA match influenced development of grade II-IV aGVHD with higher incidence among recipients of a 4-5/6-matched cord blood unit (HR=1.9 for 5/6; HR=1.9 for 4/6, all p≤0.05) as compared to those with 6/6 match. No association was found between HLA disparity and risk of grade III-IV aGVHD. No other factors influenced the incidence of aGVHD.
dUCB cohort
In the multivariate analysis, the use of ATG with NMA allo-HCT (HR=0.5, 95% CI 0.3-0.7) and transplants performed after 2006 (HR=0.6; 95% CI 0.5-0.8) were associated with lower risks of grade II-IV aGVHD, whereas age ≥ 18 years was associated with higher risks (HR=1.6; 95% CI 1.0-2.4). Greater HLA mismatch was associated with greater risks of grade III-IV aGVHD (HR=2.5, 95% CI 0.9-6.9 for 5/6 vs. HR= 2.8, 95% CI 1.0-7.7 for 4/6 vs. 6/6 match). NMA conditioning with ATG (HR=0.5; 95%CI 0.3-1.0) or without ATG (HR=0.6; 95% CI 0.4-1.0) led to lower risks of grade III-IV aGVHD as compared to MAC. There was a significant overlap in the use of ATG (total n=95) and NMA allo-HCT (n=82 with ATG). However, even among ATG-free dUCB NMA allografts, there was a trend towards less grade III-IV aGVHD and cGVHD.
Risk factors for cGVHD
The cumulative incidence of cGVHD (2 years) was 40% (MSD), 7% (sUCB), and 26% (dUCB) (Figure 1). Cohort-specific univariate and multivariate analyses of cGVHD risks are outlined in Table 2.
MSD cohort
In competing risk multivariate analysis, only age and graft source predicted onset of cGVHD, and older recipients of either marrow (HR=4.8; 95% CI 1.2-18.2) or PBSC (HR=10; 95% CI 3.3-30.4) grafts had a higher incidence of cGVHD. No other variables were associated with cGVHD.
sUCB cohort
After including grade II-IV aGVHD as a time-dependent covariate in the multivariate Cox regression, older age (HR=5.7; 95% CI, 1.9-16.5) was associated with higher incidence of cGVHD, whereas prophylaxis with CsA + MMF was associated with a lower incidence of cGVHD (HR=0.3; 95% CI, 0.1-0.9 vs. CsA + Steroid). Prior history of grade II-IV aGVHD also increased the risk of cGVHD (HR=2.4, 95% CI, 1.0-6.0), whereas HLA disparity did not influence risk of cGVHD.
dUCB cohort
In the multivariate analysis, prior grade II-IV aGVHD (HR=2.0; 95% CI, 1.3-3.2), as a time-dependent covariate, and older age (HR=5.4; 95% CI, 2.1-13.7) were associated with higher incidence of cGVHD (Table 2). HLA disparity had no appreciable influence on development of cGVHD.
Impact of GVHD on NRM and Relapse
MSD cohort
Two-year NRM for MSD allo-HCT was 22% (95% CI, 18-26%). In multivariate analysis, grade II-IV (HR=2.1; 95% CI, 1.3-3.2; p<0.01) and III-IV (HR=3.8; 95% CI, 2.4-6.0; p<0.01) aGVHD, but not cGVHD (HR=1.8; 95% CI, 0.9-3.4; p=0.43), were associated with higher 2-year NRM (Table 3). Probability of disease relapse within 2-years from allo-HCT was 27% (95% CI, 23-31%). There was no association between aGVHD (any grade) or cGVHD and relapse (all p>0.1).
sUCB cohort
Cumulative incidence of 2-year NRM for sUCB allograft recipients was 21% (95% CI, 15-27). Neither incidence of aGVHD nor cGVHD had a significant impact on NRM (all p>0.1). The probability of disease relapse within 2-years after allo-HCT was 29% (95% CI, 22-36%) and was not influenced by aGVHD or cGVHD (all p>0.7).
dUCB cohort
Cumulative incidence of 2-year NRM for dUCB allograft recipients was 23% (95% CI, 19-27). Neither grade II-IV nor III-IV aGVHD had any impact on NRM. Similarly, the development of cGVHD had no significant influence on NRM (HR=1.4; 95% CI, 0.7-2.7, p=0.4). Cumulative risk of relapse within 2 years after allo-HCT was 31% (95% CI, 27-35%). While relapse risk was not affected by grade II-IV or III-IV aGVHD, it was significantly reduced by development of cGVHD (HR=0.5; 95% CI, 0.3-0.9, p=0.03) in the multivariate analysis.
Impact of GVHD on OS
MSD Cohort
Two-year overall survival was 60% (95% CI, 55-64%) for the entire cohort. Grade II-IV (HR=1.6; 95% CI, 1.2-2.2, p<0.01) and III-IV (HR=1.9; 95% CI, 1.3-2.7, p<0.01) aGVHD, but not cGVHD (HR=0.8; 95% CI, 0.5-1.3, p=0.4), were associated with inferior OS in the multivariate analysis (Table 3).
sUCB Cohort
In the entire cohort, overall survival at 2-years from allo-HCT was 64% (95% CI, 58-69%). Onset of grade II-IV aGVHD or cGVHD had no impact on OS in the entire cohort. Grade III-IV aGVHD trended to an association with higher mortality (HR=1.8; 95% CI, 1.0-3.3, p=0.06) among all patients.
dUCB Cohort
Overall survival at 2-years from allo-HCT was 56% (95% CI, 51-60%). Neither aGVHD nor cGVHD was prognostic for OS, and no other significant predictors of OS were identified in multivariate analysis.
DISCUSSION
GVHD compromises the effectiveness of potentially curative HCT therapy for patients with life-threatening blood disorders. The preferred use of an HLA-identical sibling donor has emerged in part due to better donor-recipient histocompatibility and consequently less GVHD compared to the use of an unrelated donor. However, the necessity of stringent HLA matching has been successfully challenged by unrelated and usually partially HLA mismatched UCBT, with its major outcomes rivaling those after matched related and unrelated donor allo-HCT (1-5, 14, 29, 33-35). Our analysis has focused on establishing the incidence and prognostic determinants of acute and chronic GVHD in UCBT in parallel to benchmark MSD allo-HCT. Our observed lower incidence of cGVHD in both sUCBT and dUCBT and higher incidence of grade II-IV aGVHD in dUCBT is consistent with several prior studies (15, 19, 22, 36-38). Notably, despite a higher overall incidence of aGVHD in the dUCB cohort, aGVHD had no impact on 2-year NRM, relapse, or OS in the present study. This finding is in contrast to MSD where acute GVHD was associated with higher NRM and lower OS. This could be, in part, related to higher responsiveness among UCBT recipients with aGVHD to upfront therapy with systemic corticosteroids (39). The results of our analysis of risk factors for cGVHD are also in agreement with prior studies demonstrating older age (all three cohorts) and use of PBSC graft (MSD cohort) to be associated with higher risk of cGVHD (7, 16).
Historically, the lower incidence of cGVHD in UCBT has hampered investigation of prognostic determinants of cGVHD in multiple prior studies, most of which had fewer patients compared to ours. This study, due to its primary focus on GVHD incidence and risks, included broad populations of sibling donor and cord blood allograft recipients, including a large population of dUCBT recipients. By setting the MSD cohort as a reference, we provided comparative results of incidence and impact of acute and chronic GVHD on outcomes after UCBT.
Other noteworthy findings from our study pertain to risk factors of acute and chronic GVHD. Similar to our results, use of ATG as part of the conditioning or GVHD prophylaxis also has been shown to be associated with lower incidence of grade II-IV aGVHD in dUCBT (5, 21, 22). In our study, all patients in the dUCBT cohort receiving ATG underwent a NMA allo-HCT with a lower risk of aGVHD being possibly influenced by both factors. Surprisingly, however, in the MSD cohort we found a higher risk of grade II-IV aGVHD associated with NMA conditioning with or without ATG. This can be in part explained by the older age of the recipients and more frequent use of PBSC source with NMA allo-HCT in our center (n=192, 91%). HLA mismatch is a well-recognized risk factor associated with higher incidence of grade II-IV aGVHD following URD HCT as well as sUCBT, with the use of either high resolution(16) or conventional typing (16, 40, 41). Our study has now provided further confirmation of these results in the setting of dUCBT while utilizing conventional HLA-matching. While our observed association between donor-to-recipient HLA matching and grades II-IV aGVHD following sUCBT corroborate earlier studies (16, 40, 41), the impact of conventional HLA matching on onset of severe aGVHD after dUCBT is a unique finding. Moreover, it is consistent with another recent analysis, where better allele-level HLA match between engrafted cord blood unit and recipient predicted lower incidence of severe aGVHD (39). In concert with other reports (15, 19), we identified older age as an independent predictor of more frequent grade II-IV aGVHD across all three cohorts. Furthermore, UCB allograft recipients over 18 years old were at significantly higher risk for development of cGVHD in all adjusted models. Although a similar association between older age and higher risk of cGVHD was previously reported in the COBLT study of pediatric sUCB recipients (16), our study is the first to establish the significance of recipient age as a predictor of cGVHD in dUCBT. Finally, in our analysis, dUCBT procedures performed after 2005 were 40% less likely to associate with grade II-IV aGVHD. This observation can be most probably explained by the change in our clinical practice of using a higher MMF dose (3g after 2006 vs. 2g in the past) for GVHD prophylaxis. This resulted in a significant risk reduction of grade II-IV acute GVHD as demonstrated recently by our group (42).
The use of sUCB, dUCB, or MSD (bone marrow vs. peripheral blood) sources of hematopoietic stem cells often involves pre-specified package of distinct patient-, disease-, and allo-HCT-related factors that limit the ability of this study and others to delineate the true association between the donor source and GVHD outcomes. We attempted to eliminate this bias by focusing on cohort-specific associations and by foregoing direct comparison in GHVD incidence and risks between the cohorts.
In summary, this large analysis of contemporary cohorts establishes the incidence and prognostic determinants of GVHD for UCB in parallel to benchmark MSD allo-HCT. Our data argue that better HLA match may further mitigate the risks of grade III-IV aGVHD and thereby maximize the benefits of dUCBT. Increasing the UCB inventory or developing strategies that reduce the cell-dose threshold and thereby increase the chance of identifying an adequately dosed, better HLA-matched single UCB unit would further limit risks of acute GVHD after UCB transplantation.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported in part by the U54 Immune Mediated Disorders After Allogeneic HCT grant (AL) and by Biostatistics and Bioinformatics Shared Resource of the Masonic Cancer Center, University of Minnesota. The authors gratefully acknowledge Michael Franklin, MS, for assistance in editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP CONTRIBUTION
AL and MA conceived of the study. TED, AL, MA analyzed and interpreted study data. AL wrote and revised the manuscript. TED, CGB, SH, BRB, JW, DJW, and MA, edited the manuscript. All authors reviewed and approved the final manuscript.
DISCLOSURES OF CONFLICTS OF INTEREST
The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Weisdorf D, Eapen M, Ruggeri A, Zhang MJ, Zhong X, Brunstein C, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a center for international blood and marrow transplant research-eurocord analysis. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20(6):816–22. doi: 10.1016/j.bbmt.2014.02.020. doi: 10.1016/j.bbmt.2014.02.020. PubMed PMID: 24582782; PubMed Central PMCID: PMC4085692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. The New England journal of medicine. 2004;351(22):2265–75. doi: 10.1056/NEJMoa041276. doi: 10.1056/NEJMoa041276. PubMed PMID: 15564543. [DOI] [PubMed] [Google Scholar]
- 3.Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97(10):2957–61. doi: 10.1182/blood.v97.10.2957. PubMed PMID: 11342417. [DOI] [PubMed] [Google Scholar]
- 4.Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369(9577):1947–54. doi: 10.1016/S0140-6736(07)60915-5. doi: 10.1016/S0140-6736(07)60915-5. PubMed PMID: 17560447. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. The lancet oncology. 2010;11(7):653–60. doi: 10.1016/S1470-2045(10)70127-3. doi: 10.1016/S1470-2045(10)70127-3. PubMed PMID: 20558104; PubMed Central PMCID: PMC3163510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn T, McCarthy PL, Jr., Hassebroek A, Bredeson C, Gajewski JL, Hale GA, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(19):2437–49. doi: 10.1200/JCO.2012.46.6193. doi: 10.1200/JCO.2012.46.6193. PubMed PMID: 23715573; PubMed Central PMCID: PMC3691359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. doi: 10.1182/blood-2011-06-364265. doi: 10.1182/blood-2011-06-364265. PubMed PMID: 22010102; PubMed Central PMCID: PMC3251233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stem Cell Trialists' Collaborative G Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(22):5074–87. doi: 10.1200/JCO.2005.09.020. doi: 10.1200/JCO.2005.09.020. PubMed PMID: 16051954; PubMed Central PMCID: PMC1475795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsultan A, Giller RH, Gao D, Bathurst J, Hild E, Gore L, et al. GVHD after unrelated cord blood transplant in children: characteristics, severity, risk factors and influence on outcome. Bone marrow transplantation. 2011;46(5):668–75. doi: 10.1038/bmt.2010.174. doi: 10.1038/bmt.2010.174. PubMed PMID: 20676147. [DOI] [PubMed] [Google Scholar]
- 10.Newell LF, Flowers ME, Gooley TA, Milano F, Carpenter PA, Martin PJ, et al. Characteristics of chronic GVHD after cord blood transplantation. Bone marrow transplantation. 2013;48(10):1285–90. doi: 10.1038/bmt.2013.48. doi: 10.1038/bmt.2013.48. PubMed PMID: 23584444; PubMed Central PMCID: PMC3795867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocha V, Wagner JE, Jr., Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. The New England journal of medicine. 2000;342(25):1846–54. doi: 10.1056/NEJM200006223422501. doi: 10.1056/NEJM200006223422501. PubMed PMID: 10861319. [DOI] [PubMed] [Google Scholar]
- 12.Warlick ED, Peffault de Latour R, Shanley R, Robin M, Bejanyan N, Xhaard A, et al. Allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia: similar outcomes regardless of donor type. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(2):357–63. doi: 10.1016/j.bbmt.2014.10.030. doi: 10.1016/j.bbmt.2014.10.030. PubMed PMID: 25452032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora M, Nagaraj S, Wagner JE, Barker JN, Brunstein CG, Burns LJ, et al. Chronic graft-versus-host disease (cGVHD) following unrelated donor hematopoietic stem cell transplantation (HSCT): higher response rate in recipients of unrelated donor (URD) umbilical cord blood (UCB) Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(10):1145–52. doi: 10.1016/j.bbmt.2007.06.004. doi: 10.1016/j.bbmt.2007.06.004. PubMed PMID: 17889350. [DOI] [PubMed] [Google Scholar]
- 14.Ponce DM, Zheng J, Gonzales AM, Lubin M, Heller G, Castro-Malaspina H, et al. Reduced late mortality risk contributes to similar survival after double-unit cord blood transplantation compared with related and unrelated donor hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(9):1316–26. doi: 10.1016/j.bbmt.2011.01.006. doi: 10.1016/j.bbmt.2011.01.006. PubMed PMID: 21232625; PubMed Central PMCID: PMC3156939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scaradavou A, Brunstein CG, Eapen M, Le-Rademacher J, Barker JN, Chao N, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121(5):752–8. doi: 10.1182/blood-2012-08-449108. doi: 10.1182/blood-2012-08-449108. PubMed PMID: 23223509; PubMed Central PMCID: PMC3563363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtzberg J, Prasad VK, Carter SL, Wagner JE, Baxter-Lowe LA, Wall D, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112(10):4318–27. doi: 10.1182/blood-2007-06-098020. doi: 10.1182/blood-2007-06-098020. PubMed PMID: 18723429; PubMed Central PMCID: PMC2581998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115(9):1843–9. doi: 10.1182/blood-2009-07-231068. doi: 10.1182/blood-2009-07-231068. PubMed PMID: 20029048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caillat-Zucman S, Le Deist F, Haddad E, Gannage M, Dal Cortivo L, Jabado N, et al. Impact of HLA matching on outcome of hematopoietic stem cell transplantation in children with inherited diseases: a single-center comparative analysis of genoidentical, haploidentical or unrelated donors. Bone marrow transplantation. 2004;33(11):1089–95. doi: 10.1038/sj.bmt.1704510. doi: 10.1038/sj.bmt.1704510. PubMed PMID: 15077132. [DOI] [PubMed] [Google Scholar]
- 19.Ruggeri A, Sanz G, Bittencourt H, Sanz J, Rambaldi A, Volt F, et al. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2014;28(4):779–86. doi: 10.1038/leu.2013.259. doi: 10.1038/leu.2013.259. PubMed PMID: 24005245. [DOI] [PubMed] [Google Scholar]
- 20.Robin M, Sanz GF, Ionescu I, Rio B, Sirvent A, Renaud M, et al. Unrelated cord blood transplantation in adults with myelodysplasia or secondary acute myeloblastic leukemia: a survey on behalf of Eurocord and CLWP of EBMT. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011;25(1):75–81. doi: 10.1038/leu.2010.219. doi: 10.1038/leu.2010.219. PubMed PMID: 20882048. [DOI] [PubMed] [Google Scholar]
- 21.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–70. doi: 10.1182/blood-2007-04-067215. doi: 10.1182/blood-2007-04-067215. PubMed PMID: 17569820; PubMed Central PMCID: PMC2018678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–5. doi: 10.1182/blood-2008-07-163238. doi: 10.1182/blood-2008-07-163238. PubMed PMID: 18997171; PubMed Central PMCID: PMC2656268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15(6):825–8. PubMed PMID: 7581076. [PubMed] [Google Scholar]
- 24.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. The American journal of medicine. 1980;69(2):204–17. doi: 10.1016/0002-9343(80)90380-0. PubMed PMID: 6996481. [DOI] [PubMed] [Google Scholar]
- 25.Arora M, Burns LJ, Davies SM, Macmillan ML, Defor TE, Miller WJ, et al. Chronic graft-versus-host disease: a prospective cohort study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2003;9(1):38–45. doi: 10.1053/bbmt.2003.50003. doi: 10.1053/bbmt.2003.50003. PubMed PMID: 12533740. [DOI] [PubMed] [Google Scholar]
- 26.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–7. doi: 10.1182/blood-2004-07-2717. doi: 10.1182/blood-2004-07-2717. PubMed PMID: 15466923. [DOI] [PubMed] [Google Scholar]
- 27.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004. doi: 10.1016/j.bbmt.2009.07.004. PubMed PMID: 19896087; PubMed Central PMCID: PMC2861656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majhail NS, Brunstein CG, Shanley R, Sandhu K, McClune B, Oran B, et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone marrow transplantation. 2012;47(4):494–8. doi: 10.1038/bmt.2011.114. doi: 10.1038/bmt.2011.114. PubMed PMID: 21602900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–9. doi: 10.1182/blood-2010-05-285304. doi: 10.1182/blood-2010-05-285304. PubMed PMID: 20686119; PubMed Central PMCID: PMC2996124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Statistics in medicine. 1997;16(8):901–10. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. PubMed PMID: 9160487. [DOI] [PubMed] [Google Scholar]
- 31.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 32.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society. 1972:187–220. [Google Scholar]
- 33.Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20(10):1573–9. doi: 10.1016/j.bbmt.2014.05.029. doi: 10.1016/j.bbmt.2014.05.029. PubMed PMID: 24910379. [DOI] [PubMed] [Google Scholar]
- 34.Chen YB, Aldridge J, Kim HT, Ballen KK, Cutler C, Kao G, et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(5):805–12. doi: 10.1016/j.bbmt.2011.10.016. doi: 10.1016/j.bbmt.2011.10.016. PubMed PMID: 22015993. [DOI] [PubMed] [Google Scholar]
- 35.Atsuta Y, Morishima Y, Suzuki R, Nagamura-Inoue T, Taniguchi S, Takahashi S, et al. Comparison of unrelated cord blood transplantation and HLA-mismatched unrelated bone marrow transplantation for adults with leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(5):780–7. doi: 10.1016/j.bbmt.2011.10.008. doi: 10.1016/j.bbmt.2011.10.008. PubMed PMID: 22008851. [DOI] [PubMed] [Google Scholar]
- 36.Labopin M, Ruggeri A, Gorin NC, Gluckman E, Blaise D, Mannone L, et al. Cost-effectiveness and clinical outcomes of double versus single cord blood transplantation in adults with acute leukemia in France. Haematologica. 2014;99(3):535–40. doi: 10.3324/haematol.2013.092254. doi: 10.3324/haematol.2013.092254. PubMed PMID: 24143000; PubMed Central PMCID: PMC3943318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner JE, Jr., Eapen M, Carter S, Wang Y, Schultz KR, Wall DA, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. The New England journal of medicine. 2014;371(18):1685–94. doi: 10.1056/NEJMoa1405584. doi: 10.1056/NEJMoa1405584. PubMed PMID: 25354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114(19):4293–9. doi: 10.1182/blood-2009-05-220525. doi: 10.1182/blood-2009-05-220525. PubMed PMID: 19706886; PubMed Central PMCID: PMC2774557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ponce DM, Gonzales A, Lubin M, Castro-Malaspina H, Giralt S, Goldberg JD, et al. Graft-versus-host Disease After Double-Unit Cord Blood Transplantation Has Unique Features and an Association with Engrafting Unit-Recipient HLA-match. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013 doi: 10.1016/j.bbmt.2013.02.008. doi: 10.1016/j.bbmt.2013.02.008. PubMed PMID: 23416854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. The New England journal of medicine. 1997;337(6):373–81. doi: 10.1056/NEJM199708073370602. doi: 10.1056/NEJM199708073370602. PubMed PMID: 9241126. [DOI] [PubMed] [Google Scholar]
- 41.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. The New England journal of medicine. 1998;339(22):1565–77. doi: 10.1056/NEJM199811263392201. doi: 10.1056/NEJM199811263392201. PubMed PMID: 9828244. [DOI] [PubMed] [Google Scholar]
- 42.Bejanyan N, Rogosheske J, DeFor T, Lazaryan A, Esbaum K, Holtan S, et al. Higher Dose of Mycophenolate Mofetil Reduces Acute Graft-versus-Host Disease in Reduced-Intensity Conditioning Double Umbilical Cord Blood Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015 doi: 10.1016/j.bbmt.2015.01.023. doi: 10.1016/j.bbmt.2015.01.023. PubMed PMID: 25655791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.