Abstract
Several distinct graft-versus-host disease (GVHD)-related syndromes have been defined by the NIH Consensus Conference. We enrolled a prospective cohort of 911 hematopoietic cell transplantation (HCT) recipients at 13 centers between March 2011 and May 2014 to evaluate four GVHD syndromes: late acute GVHD, chronic GVHD, bronchiolitis obliterans syndrome, and cutaneous sclerosis. The median age at HCT was 53.7 years. Most patients received peripheral blood stem cell transplant (81%) using a non myeloablative or reduced intensity conditioning (55%). Pediatric age group and use of bone marrow and umbilical cord blood were underrepresented in our cohort (<=11%). The cumulative incidence of late acute GVHD (late onset and recurrent) was 10% at a median of 5.5 months, chronic GVHD was 47% at a median of 7.4 months, bronchiolitis obliterans was 3% at a median of 12.2 months, and cutaneous sclerosis was 8% at a median onset of 14.0 months after HCT. Late acute GVHD and bronchiolitis obliterans had particularly high non-relapse mortality of 23% and 32% by 2 years after diagnosis. The probability of late acute- and chronic-GVHD-free, relapse-free survival at one and two years after HCT was 38% and 26%. This multi-center, prospective study confirms the high rate of late acute and chronic GVHD syndromes and supports the need for continuous close monitoring and development of more effective GVHD treatment strategies to improve HCT success.
Keywords: Hematopoietic cell transplant, graft-versus-host disease syndromes, late acute GVHD, chronic graft-versus-host disease, bronchiolitis obliterans syndrome, cutaneous sclerosis
Introduction
Chronic graft-versus host disease (GVHD) is one of the leading causes of late mortality and morbidity after allogeneic hematopoietic cell transplant (HCT).(1, 2) The clinical presentation of late acute and chronic GVHD is heterogeneous with several distinct syndromes as defined by the NIH Consensus conference in 2005(3) and confirmed in the 2014 update.(4) The Chronic GVHD Consortium enrolled allogeneic HCT recipients in a prospective multi-center, longitudinal observational cohort study and followed them closely for development of four GVHD syndromes. These include late acute GVHD, bronchiolitis obliterans syndrome, cutaneous sclerosis and chronic GVHD. Although bronchiolitis obliterans and cutaneous sclerosis are considered part of the chronic GVHD syndrome, they are also reported separately since they are of particular interest because of their unique clinical manifestations and poor response to available therapies.
As one of the goals of this study was to develop a biorepository of biologic material for future studies, research samples were collected at enrollment and during the first year. Detailed clinical evaluations were performed and additional research samples were collected when GVHD syndromes developed. This report focuses on the incidence, clinical manifestations, and outcomes of the four syndromes observed in this cohort. This information is important for future biomarker studies and to understand the clinical features of these syndromes, especially since a growing number of novel therapeutic interventions are now available for testing as GVHD prophylaxis agents, and for the treatment of GVHD once the diagnosis is established.
Methods
Study design
The Chronic GVHD Consortium includes 13 centers that enrolled 911 allogeneic HCT recipients over a period of three years (March 2011 to May 2014), and followed them prospectively. Enrollment was allowed up to 4 months after HCT (121 days) as long as patients had not already developed late acute GVHD or chronic GVHD. 413 patients were enrolled before HCT and 498 patients were enrolled after HCT. The median time to enrollment was 1.9 months (interquartile range −0.6 to 2.8 months). All sites obtained IRB approval, and all participants provided written informed consent.
The late acute and chronic GVHD syndromes were defined according to the NIH Consensus Criteria.(3) In patients who did not develop a GVHD syndrome, research samples were collected at two time points (day 100 and either day 180 or day 365). If a late GVHD syndrome developed, samples were collected at onset and either 3 or 6 months later. Supplemental Table S1 provides details on the number of samples available for each late GVHD syndrome, and readers may request access to these samples by contacting the authors.
Statistical analysis
Cumulative incidence for each syndrome was calculated treating recurrent malignancy/progression or death as competing risks.(5) For analysis of late acute GVHD, onset of chronic GVHD was also considered a competing risk since by definition, late acute GVHD cannot be diagnosed once chronic GVHD is diagnosed. Overall survival was calculated using Kaplan-Meier estimates, and 95% confidence intervals (CIs) were calculated using the variance derived from the formula of Greenwood.(6)
Late acute and chronic GVHD-free, relapse-free survival was calculated since patients who did not develop any late acute or chronic GVHD-related syndrome and survived without recurrent malignancy or progression represent the most successful outcome of HCT. In this composite endpoint, death, recurrent or progressive underlying malignancy, and development of late acute GVHD or any chronic GVHD syndrome were considered events. A second analysis evaluated the same endpoint but also considered grade III–IV classic acute GVHD as an event.(7)
Multivariate analyses were performed using stepwise forward selection. P ≤ 0.05 was the criterion for inclusion in the final models.(8) The potential variables were: recipient age at transplant, sex, sex mismatch, race, diagnosis, disease status at transplant, center location, use of TBI in conditioning regimen, conditioning intensity, recipient/ donor CMV status, prior acute GVHD, donor type, graft type and Karnofsky performance status (KPS).
Results
This analysis included 911 allogeneic HCT recipients. Patient and transplant characteristics are described in Table 1. The median recipient age at transplant was 53.7 years (range: 19–77.9 years). Thirty three percent received HCT from an HLA-identical sibling donor, 44% from an HLA-matched unrelated donor (URD), and 20% from an HLA-mismatched URD that includes cord blood. Transplants from HLA-mismatched related (<1%) and haplo-identical donors (2%) were rare. Most patients received a peripheral blood stem cell (PBSC) graft (81%), while fewer received umbilical cord blood (UCB, 11%) or bone marrow grafts (8%). Slightly less than half (45%) received myeloablative conditioning. The majority (65%) of recipients were treated for acute leukemia or myelodysplastic syndrome (MDS), and 88% had early or intermediate disease status. In 21% of the cases, donors were females and recipients were males. The median follow up of survivors was 26.3 months (interquartile range 20–35.3 months).
Table 1.
Demographics (N=911)
| % | ||
|---|---|---|
| Recipient age at transplant, median (range) | 53.7 (19–77.9) | |
| 11–30 | 95 | 11 |
| 31–50 | 268 | 29 |
| > 50 | 548 | 60 |
| Donor type (n=898), n (%) | ||
| HLA identical sibling | 297 | 33 |
| HLA-matched other relative | 8 | 1 |
| HLA-matched unrelated donor | 393 | 44 |
| HLA-mismatched relative (single antigen or allele mismatched) | 3 | <1 |
| HLA-mismatched unrelated donor | 181 | 20 |
| Haplo-identical relative (2 or more antigen or allele mismatched) | 16 | 2 |
| Graft Type (n=910), n (%) | ||
| Bone Marrow | 77 | 8 |
| Cord Blood | 102 | 11 |
| Peripheral Blood | 731 | 81 |
| Conditioning Intensity (n=910) | ||
| Myeloablative | 405 | 45 |
| Not myeloablative | 505 | 55 |
| Total Body Irradiation (TBI) | ||
| Yes | 408 | 45 |
| No | 503 | 55 |
| Graft-vs.-host disease prophylaxis, n (%)* | ||
| Calcineurin inhibitor + methotrexate +/− other | 390 | 43 |
| Calcineurin inhibitor + mycophenolate mofetil | 225 | 25 |
| Calcineurin inhibitor + sirolimus | 97 | 11 |
| Calcineurin inhibitor + mycophenolate mofetil + sirolimus | 64 | 7 |
| Anti-thymoctye globulin/ T-cell depletion +/− other | 23 | 3 |
| Other | 112 | 12 |
| Center location, n (%) | ||
| Pacific/ Mountain | 279 | 31 |
| Central | 260 | 28 |
| Eastern | 372 | 41 |
| Donor-recipient sex match (n=896), n (%) | ||
| Female into male | 190 | 21 |
| Other | 708 | 79 |
| Time from transplant to study consent, median months (range) | 1.9 (−5.5 ~ 4.0) | |
| Median follow up of survivors since HCT, median months (range) | 26.3 (5.1–53.8) |
Total >100% due to rounding
Since enrollment was allowed until 4 months after transplant, we performed a sensitivity analysis limited to the 413 patients who were enrolled pre-HCT to evaluate cumulative incidence of each syndrome. The estimates were compared to the 498 who were enrolled after transplant. Since both estimates were similar (data not shown) suggesting that the population enrolled after HCT was not unduly biased by early deaths, relapses or development of late acute GVHD, we report estimates for the entire cohort in this analysis.
Late acute GVHD
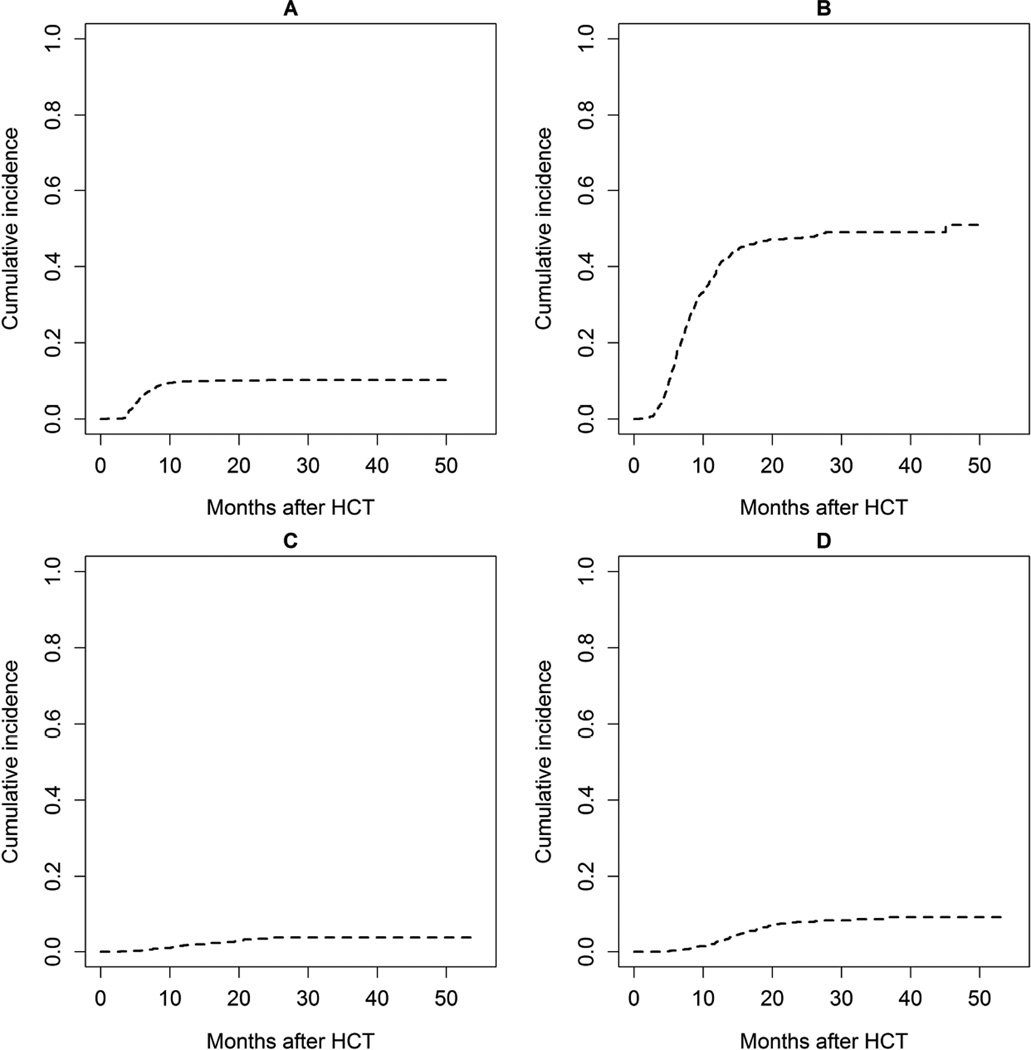
By 2 years post HCT, recurrent or late onset acute GVHD developed in 10% (95% CI: 8–12%) of HCT recipients (Table 2, Figure 1A). The median time to onset was 5.5 months (range: 0.9–24.0 months) and was similar between myeloablative and reduced intensity conditioning regimens. 58% had recurrent late acute GVHD (recurrent symptoms after day 100 after previously having resolved classic acute GVHD), and 42% de-novo late acute GVHD (acute GVHD developing after day 100). The cumulative incidence of NRM was 23% (95% CI: 15%–35%) by two years after onset of the syndrome (Table 2) and not different between recurrent and de-novo onset (p=0.72). The probability of overall survival was 70% (95% CI: 57%–79%) at two years after onset of late acute GVHD.
Table 2.
Cumulative Incidence and Outcomes in GVHD syndromes
| Syndrome | N | Cumulative incidence at 2 years (95% CI) |
Median time to onset, mos (range) |
Non-relapse mortality 2 years after onset (95% CI) |
Survival 2 years after onset (95% CI) |
|---|---|---|---|---|---|
| Late acute GVHD | 92 | 10% (8%–12%) | 5.5 (0.9–24.0) | 23% (15%–35%) | 70% (57%–79%) |
| Chronic GVHD* | 428 | 47% (44%–51%) | 7.4 (0.8–45.1) | 12% (9%–16%) | 81% (76%–85%) |
| Bronchiolitis obliterans | 30 | 3% (2%–5%) | 12.2 (2.8–24.3) | 32% (18%–57%) | 65% (43%–80%) |
| Cutaneous sclerosis | 68 | 8% (6%–10%) | 14.0 (4.0–36.9) | 20% (10%–39%) | 71% (53%–83%) |
Chronic GVHD includes bronchiolitis obliterans syndrome and cutaneous sclerosis
Figure 1.
Cumulative Incidence of late GVHD syndromes A: Late acute GVHD, B: Chronic GVHD, C: Bronchiolitis obliterans syndrome, D: Cutaneous sclerosis
In multivariate analysis of incidence of late acute GVHD, female donor/male recipient was associated with a 1.6-fold higher risk of developing late acute GVHD (HR: 1.64, 95% CI: 1.01–2.56, p = 0.04) (Table 3). Of note, recipient age at HCT, conditioning regimen, donor match, prior classic acute GVHD, and graft type were not predictive of late acute GVHD.
Table 3.
Multivariate Analysis of Incidence of Each Syndrome
| Variable | Hazard Ratio |
95% CI | P- value |
|---|---|---|---|
| Late acute GVHD | |||
| Sex combination | |||
| Others | Ref=1 | ||
| Female donor/male recipient | 1.64 | 1.01– 2.56 | 0.04 |
| Chronic GVHD | |||
| Donor type | Overall p<0.001 | ||
| Cord | Ref=1 | ||
| HLA-identical related | 2.03 | 1.37–3.14 | 0.001 |
| HLA-matched adult unrelated | 2.41 | 1.63–3.71 | <0.001 |
| HLA-mismatched unrelated | 2.09 | 1.30–3.43 | 0.003 |
| HLA-mismatched relative | 1.62 | 0.72–3.35 | 0.21 |
| Patient / Donor cytomegalovirus | |||
| Both negative | Ref=1 | ||
| Either positive | 0.80 | 0.65–0.98 | 0.03 |
| Disease diagnosis | Overall p = 0.004 | ||
| AA/ALL/AML | Ref=1 | ||
| CLL/CML | 1.53 | 1.10–2.09 | 0.009 |
| HD/NHL | 1.30 | 0.96–1.74 | 0.09 |
| MDS | 0.99 | 0.72–1.32 | 0.92 |
| Others | 1.57 | 1.18–2.06 | 0.002 |
| Race / Ethnicity | |||
| Others | Ref=1 | ||
| Non-Hispanic Caucasian | 0.74 | 0.57–0.96 | 0.03 |
| Bronchiolitis Obliterans Syndrome | |||
| Center location | Overall p=0.002 | ||
| Pacific/ Mountain | Ref=1 | ||
| Eastern | 0.67 | 0.20–2.10 | 0.49 |
| Central | 3.03 | 1.32–7.81 | 0.01 |
| Recipient sex | |||
| Male | Ref=1 | ||
| Female | 2.56 | 1.24–5.56 | 0.01 |
| Cutaneous sclerosis | |||
| Center location | Overall p = 0.008 | ||
| Pacific/ Mountain | Ref=1 | ||
| Eastern | 0.49 | 0.28–0.84 | 0.01 |
| Central | 0.44 | 0.22–0.83 | 0.01 |
| Graft type | Overall p = 0.05 | ||
| Cord | Ref=1 | ||
| Bone marrow | 3.36 | 0.72–23.6 | 0.15 |
| Peripheral blood | 4.13 | 1.27–25.3 | 0.05 |
| GVHD-free-relapse-free-survival | |||
| Conditioning regimen | |||
| Non-Myeloablative | Ref=1 | ||
| Myeloablative | 0.83 | 0.71–0.97 | 0.02 |
| Sex combination | |||
| Others | Ref=1 | ||
| Female donor/male recipient | 1.40 | 1.16–1.68 | <0.001 |
| Donor type | Overall p<0.001 | ||
| Cord | Ref=1 | ||
| HLA-identical related | 1.74 | 1.29–2.38 | <0.001 |
| HLA-matched adult unrelated | 2.08 | 1.56–2.82 | <0.001 |
| HLA-mismatched unrelated | 1.79 | 1.25–2.59 | 0.002 |
| HLA-mismatched relative | 1.42 | 0.74–2.52 | 0.26 |
| Prior classic acute GVHD | |||
| No | Ref=1 | ||
| Yes | 1.19 | 1.02–1.39 | 0.03 |
Abbreviations: GVHD: graft-versus-host disease
Chronic graft-versus-host disease
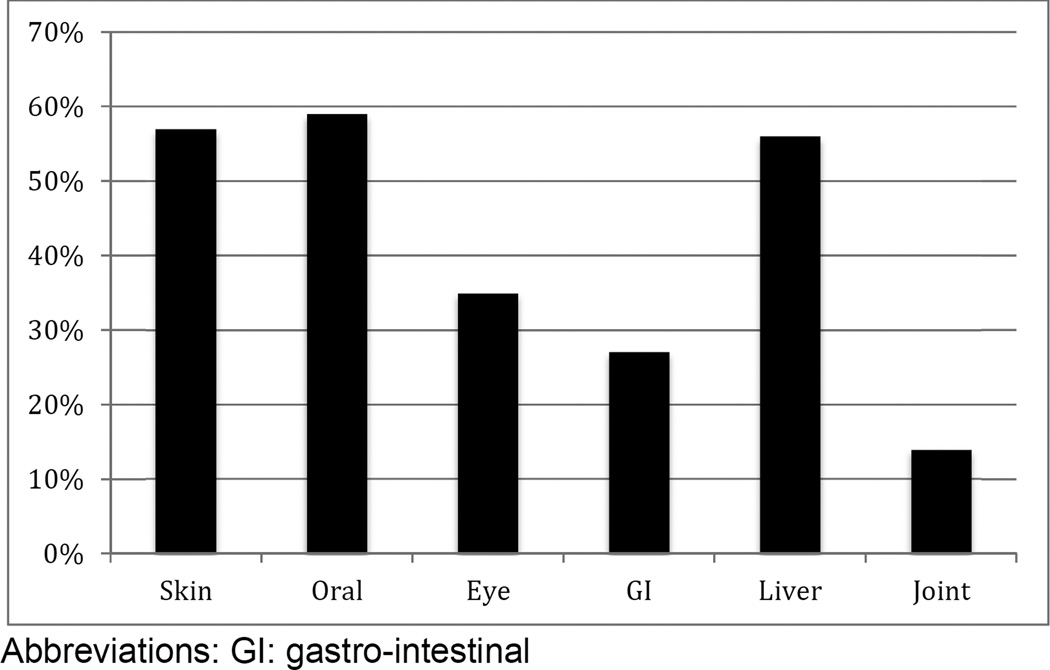
The cumulative incidence of chronic GVHD was 47% (95% CI: 44%–51%) by two years after HCT (Table 2, Figure 1B). The median time to onset of chronic GVHD was 7.4 months (range: 0.8–45.1 months). Amongst donor types, the lowest incidence was seen in UCB recipients (cumulative incidence 28% (95% CI: 20% to 38%), while the incidence was approximately 50% in recipients from HLA-identical sibling donors (cumulative incidence 47% (95% CI: 42% to 53%), HLA-matched URD (cumulative incidence 53% (95% CI: 48% to 58%) and HLA-mismatched adult URD (cumulative incidence 49% (95% CI: 39% to 61%). A lower incidence was seen in recipients of bone marrow grafts (cumulative incidence 43% (95% CI: 33% to 56%) as compared to PBSC grafts (cumulative incidence 51% (95% CI: 47% to 55%). Organ involvement amongst patients with chronic GVHD is shown in Figure 2. Mouth was the most common site involved (59%) followed by skin (57%) and liver (56%). At onset, the NIH global severity of the disease was mild in 19%, moderate in 53% and severe in 28%. Once chronic GVHD was diagnosed the proportion of mild-moderate GVHD did not differ among donor types and was 64% in cord blood recipients as compared to HLA-identical sibling donor recipients (70%), HLA-mismatched URD recipients (72%), and HLA-matched URD (72%).
Figure 2.
Organ involvement amongst patients with chronic GVHD
Among the 428 patients with chronic GVHD, NRM was 12% (95% CI: 9%–16%) and the probability of overall survival was 81% (95% CI: 76%–85%) at two years after the diagnosis of chronic GVHD. Based on initial presentation, the 2-year NRM was 11% (95% CI: 5%–24%) for mild, 8% (95% CI: 5%–13%) for moderate, and 18% (95% CI: 12%–28%) for severe chronic GVHD.
Amongst all patients with chronic GVHD, only 11% (95% CI: 8%–16%) were able to discontinue all immune suppression one year after chronic GVHD diagnosis. Patients with severe chronic GVHD were less likely (9%) to discontinue immune suppression as compared to moderate (12%) or mild chronic GVHD (18%). Amongst donor types, UCB recipients were more likely to discontinue immune suppression (30%) at one year after diagnosis as compared to HLA identical sibs (7%), HLA-matched URD (7%) or HLA mismatched URD (3%).
In multivariate analysis of incidence of chronic GVHD, recipients of UCB were less than 50% as likely to develop chronic GVHD as other donor types (HR: 0.46, p<0.001), whereas all other donor types had a similar risk (data not shown). Trends of association were also observed for patient/donor CMV (either positive versus both negative), HR: 0.80, 95% CI: 0.65–0.98, p = 0.03), disease diagnosis (overall p = 0.004) and race/ethnicity (p = 0.03) (Table 3). None of the other variables, including age, conditioning regimen, graft source, gender matching or prior classic acute GVHD were predictive of chronic GVHD.
Bronchiolitis obliterans syndrome
Bronchiolitis obliterans syndrome was seen in 3% (95% CI: 2%–5%) of HCT recipients by two years after HCT (Table 2, Figure 1C). The median time to onset of bronchiolitis obliterans was 12.2 months (range: 2.8–24.3 months). At onset, the median percent predicted FEV1 was 42.0% (range: 21.4%–70.8%), and the median FEV1/FVC was 0.44 (range: 0.35–0.75). Among these 30 patients, the NRM was high at 32% (95% CI: 18%–57%) by two years after diagnosis of bronchiolitis obliterans, and the probability of overall survival was 65% (95% CI: 43%–80%).
At the time of bronchiolitis obliterans diagnosis, many patients were already on steroids (n = 21, 81%), calcineurin inhibitors (n = 14, 54%) and sirolimus (n = 5, 19%). After diagnosis, 77% (95% CI: 56%–91%) patients with bronchiolitis obliterans were treated with FAM (fluticasone, azithromycin and montelukast). Amongst 23 patients alive at one year after diagnosis of bronchiolitis obliterans, 22 (96%) are still on immune suppression.
In multivariate analysis of incidence of bronchiolitis obliterans (Table 3), only female sex (HR: 2.56, 95% CI: 1.24–5.56, p = 0.01) and HCT site (central versus pacific / mountain, HR: 3.03, 95% CI: 1.32–7.81, p = 0.01, overall p = 0.002) were associated with higher risk of bronchiolitis obliterans. Prior reported risk factors such as use of PBSC, conditioning regimens or sex mismatch were not identified as predictors in our cohort.
Cutaneous sclerosis
The cumulative incidence of cutaneous sclerosis was 8% (95% CI: 6%–10%) by two years after HCT (Table 2, Figure 1D). The median time to onset of cutaneous sclerosis was 14.0 months (range 4.0–36.9 months). Amongst these 68 patients, the NRM was 20% (range 10–39%) by two years and the probability of overall survival was 71% (95% CI: 53%–83%) at two years after onset of cutaneous sclerosis.
At the time of diagnosis of cutaneous sclerosis, many patients were already on steroids (n = 20, 42%) and calcineurin inhibitors (n = 20, 42%). After diagnosis, patients with cutaneous sclerosis were treated with calcineurin inhibitors (60%), sirolimus (44%), B cell depleting antibodies (19%), tyrosine kinase inhibitors (19%), mycophenolate mofetil (17%), extracorporeal photopheresis (8%), and other agents (15%). One year after diagnosis of cutaneous sclerosis, 45 (94%) patients are alive and all of them are still on immune suppression.
In multivariate analysis of incidence of cutaneous sclerosis (Table 3), use of PBSC as a graft source was associated with a 4.1-fold higher risk of developing cutaneous sclerosis comparing to umbilical cord blood (HR: 4.13, 95% CI: 1.27–25.3, p = 0.05). HCT site was also associated with cutaneous sclerosis development (overall p = 0.008). Use of total body irradiation (TBI) and the amount of TBI exposure in conditioning were not identified as potential predictors in this analysis.
Patterns of involvement with multiple syndromes
Among 92 patients with late acute GVHD, the cumulative incidence of development of chronic GVHD was 30% at one year after diagnosis of late acute GVHD. Among these 29 patients, 7/29 (24%) also developed cutaneous sclerosis and 4/29 (14%) developed bronchiolitis obliterans at medians of 13.1 and 15.6 months after HCT, respectively. Among all 911 patients, concurrent cutaneous sclerosis and bronchiolitis obliterans were seen in only 3 patients. Prior classic acute skin GVHD was not associated with risk of later cutaneous sclerosis (HR=0.82, p = 0.46).
GVHD-free, relapse-free survival
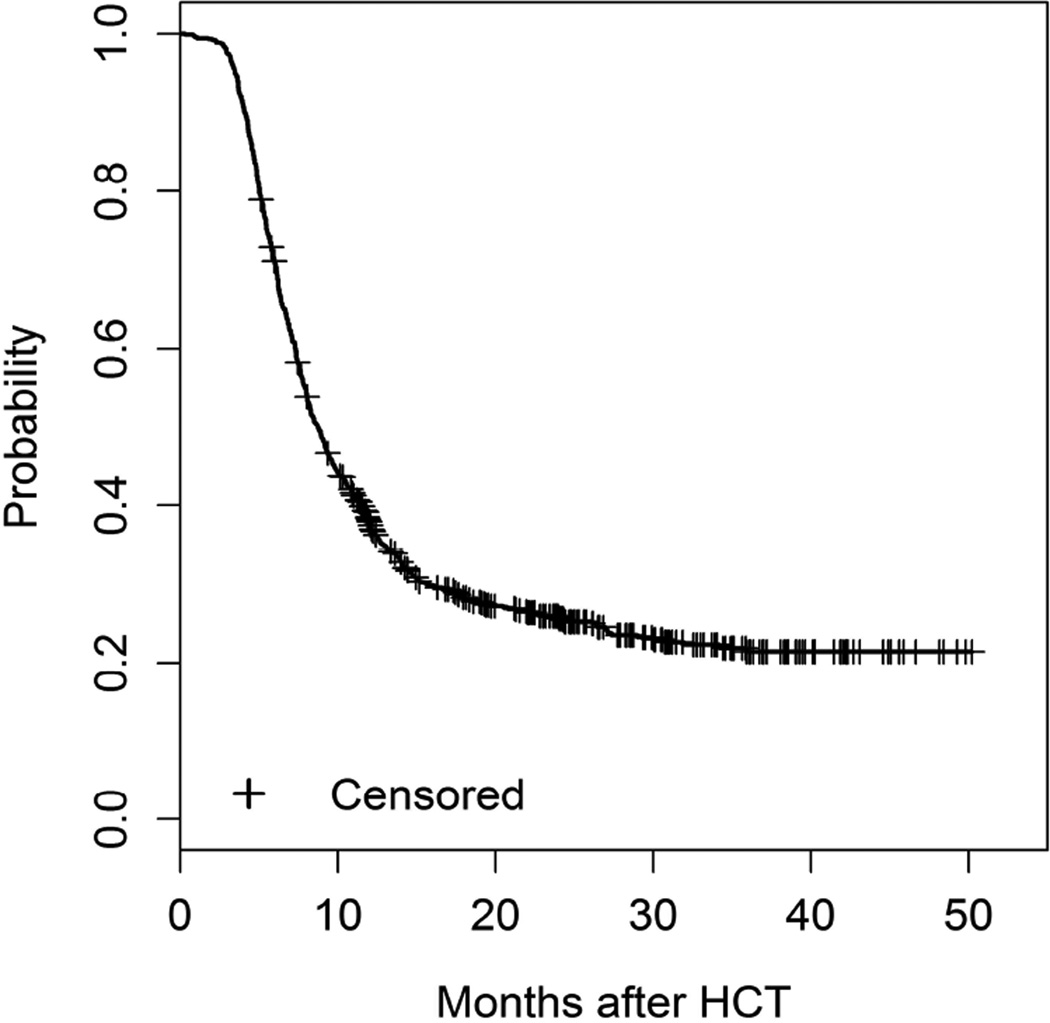
We also evaluated GVHD-free, relapse-free survival in all recipients. The probability of GVHD-free, relapse–free survival at 1 year was 38% (95% CI 35%–41%) and was 26% (95% CI: 23%–29%) at 2 years including only late acute GVHD and chronic GVHD) (Figure 3). Including grade III–IV classic acute GVHD as an event did not substantively change the estimates: 36% (95% CI 33%–39%) at one year, and 25% (95% CI: 22%–28%) at two years. Due to concern that enrollment after transplant could bias these estimates, we also performed a sensitivity analysis, where the endpoint was evaluated only in patients who were enrolled before HCT (n = 413). The probability of GVHD-free, relapse-free survival was 40% (95% CI: 35%–44%) and 28% (95% CI: 24%–33%) at 1 and 2 years in this subset, respectively. Since the results were similar to the overall cohort, subsequent multivariate analysis was performed in the entire cohort.
Figure 3.
GVHD-free-relapse-free-survival including only late acute and chronic GVHD syndromes
In multivariate analysis (including only late acute GVHD, chronic GVHD, recurrent malignancy and death as events) UCB transplant recipients were at significantly lower risk (HR: 0.53, overall p<0.001) when compared to other donor types. Recipients with a sex mismatched HCT (female donor to male recipient versus others) or prior classic acute GVHD were 1.40 (95% CI: 1.16–1.68, p<0.001) and 1.19 (95% CI: 1.02–1.39, p = 0.03)-fold more likely to experience late acute GVHD syndrome, chronic GVHD, recurrent malignancy or death, while recipients with myeloablative conditioning regimen were less likely to develop late acute GVHD syndrome, chronic GVHD, recurrent malignancy or death (HR: 0.83, 95% CI: 0.71–0.97, p = 0.02) (Table 3).
Discussion
Our data represent the first large prospective multicenter study analyzing the natural history, incidence, clinical presentation, outcomes and risk factors for late acute GVHD and chronic GVHD syndromes after HCT using the NIH Consensus Criteria definitions. Although the diagnostic criteria were updated in 2014, the diagnostic criteria for these GVHD syndromes were not changed so our results remain valid.(4)
Retrospective, single institution studies reclassifying chronic GVHD into late acute GVHD and chronic GVHD based on NIH consensus criteria have reported prevalences of late acute GVHD ranging from 15–45% and NRM rates ranging from 21%–72.5%.(9–13) In our prospective, multi-center cohort, the incidence of recurrent and late onset acute GVHD was slightly lower at 10%. This may be explained by our exclusion of persistent acute GVHD that may have been captured in other studies (as we only captured patients developing new late acute GVHD or with recurrent acute GVHD). Regardless, our analysis revealed a high NRM in this group, indicating a need for trials targeting newer treatment strategies for this subgroup. In multivariate analysis, we identified only a female donor for a male patient as predictive of late acute GVHD.
Our observed incidence and outcomes of chronic GVHD are similar to previously reported studies.(14, 15) Consistent with these studies, UCB was identified to be significantly associated with a lower risk of chronic GVHD.(15) However, PBSC and female donor into male recipient matching were not associated with chronic GVHD in our cohort. Since these associations have been observed in multiple prior studies, our inability to find an association may be due to our cohort size and heterogeneity and should not be taken as strong evidence against the importance of these predictive factors.
Bronchiolitis obliterans syndrome is a well-known complication of allogeneic HCT. The syndrome has been described in approximately 5.5% of all HCT recipients(16–18) and has been associated with a higher risk of mortality.(18) Consistent with prior studies, our estimated cumulative incidence rate was 3%, and the NRM was high at 32% at 2 years after diagnosis. Prior studies have identified several predictors of bronchiolitis obliterans including use of methotrexate in GVHD prophylaxis, busulfan-based conditioning regimen, respiratory viral infections during the first 100 days post HCT, or use of PBSC as a graft source.(16–18) Using NIH criteria for diagnosis of bronchiolitis obliterans, Au et al evaluated risk factors for bronchiolitis obliterans development and identified only the presence of chronic GVHD and lower immunoglobulin G levels as associated with a higher risk of this syndrome.(18) In our analysis, all patients diagnosed with bronchiolitis obliterans were also categorized as chronic GVHD, removing GVHD as a potential risk factor. We also found female sex and transplant center location (central versus Pacific/ Mountain) to be associated with higher risk of bronchiolitis obliterans. We did not confirm medication and graft source risk factors in our population. The underlying reasons behind these differences in risk factor identification are unclear. We did not have sufficient data on respiratory viral infections to evaluate this potential predictor. The geographical differences could reflect ascertainment bias, where patients may be seen more frequently at some sites or evaluated differently and hence have a higher frequency of diagnosis of subtle onset of bronchiolitis obliterans. Alternatively, our observations could reflect different patterns of viral infections or other biologic factors.
Our observed incidence of cutaneous sclerosis was 8%. Prior studies have reported cumulative incidence frequencies between 3–20%.(19–22) Previously observed risk factors include use of PBSC as a graft source, pre-HCT conditioning with TBI at a dose of > 450 cGy,(19), previous skin involvement, a higher CD3 cell dose, autoimmune markers, and eosinophilia.(21) Although we tested for some of these risk factors (TBI, and prior skin involvement with acute GVHD), none was significant in our analysis, only PBSC was confirmed as a risk factor. This discrepancy could be due to differences in the patient population, since our study had higher proportions of patients who received reduced intensity conditioning and UCB grafts. Also, some prior reports drew conclusions based on univariable regression analysis, perhaps due to small numbers.
We also evaluated the GVHD-free, relapse-free survival in this cohort, where development of late acute GVHD or any chronic GVHD syndrome (evaluated including or excluding classic acute grade III–IV GVHD), disease relapse or progression and death were considered events. This composite endpoint identifies the most favorable group post HCT. Similar to a previous center study(7), we noted a GVHD-free, relapse-free survival rate of 36% at one year post HCT. Multivariate analysis (without including grade III–IV classic acute GVHD) revealed that UCB HCT and myeloablative conditioning were associated with lower risk of the composite endpoint, and female donors for male recipients and previous classic acute GVHD were associated with a higher risk.
This study has a number of limitations. First, some patients were excluded from the study based on active malignancy at transplant, pre-existing autoimmune disorders or diagnosis of a GVHD syndrome of interest prior to enrollment. They were excluded if they were non-compliant with study procedures or did not plan to return to the transplant center for at least some of their follow-up after day 100. In addition, patients had to sign informed consent for study participation, and some declined citing participation in too many studies or lack of interest. These exclusions mean that our study is not truly population-based. There was also missing information in some baseline variables in < 5% of our study cohort. However, our study population is very large and exploited the clinical heterogeneity across multiple centers to enhance its applicability to the general population of HCT transplanted today. Sensitivity analyses did not detect any differences between those enrolled before transplantation versus afterwards. Although it would be ideal to have analyzed all transplanted patients rather than only those who consented, most sites including those in our Consortium do not capture robust data about these syndromes as defined by NIH criteria outside of a clinical study. Second, we did not capture persistent late acute GVHD, since we felt that this subgroup experiencing continuous acute GVHD past day 100 likely had the same pathophysiology as classic acute GVHD. In contrast, recurrent or late onset acute GVHD are of interest since these phenotypes were recognized only with the 2005 NIH criteria, and the epidemiology of this syndrome has not been described. If persistent late acute GVHD were also included, the incidence of any late acute GVHD would necessarily be greater. Finally, there were essentially no pediatric patients as well as an underrepresentation of UCB and bone marrow as graft source in this cohort and very few patients received a T-cell depleted graft, preventing our ability to comment on these populations.
In summary, the burden of late acute and chronic GVHD is high in allogeneic HCT recipients. As most patients survive the first 3–12 months after HCT, these GVHD syndromes assume greater importance in determining the long-term success of HCT in terms of whether or not health and quality of life is restored.
Supplementary Material
Highlights.
The cumulative incidence of CGVHD according to the NIH consensus criteria was 47%.
The probability of late GVHD-free-relapse-free survival was 38% at 1 year.
Late acute GVHD and bronchiolitis obliterans had high 2 year NRM of 23% and 32%.
Acknowledgments
The Chronic GVHD Consortium (U54 CA163438) is part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Disease Research (ORDR), NCATS, funded through a collaboration between NCATS and the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship contributions
Contribution: M.A and S.J.L. proposed the study concept, analysis plan, and wrote the manuscript; X.C. performed statistical analyses and contributed to writing the manuscript; C.S.C, M.H.J., J.P., P.J.M., M.E.D.F., Y.I., G.L.C., W.A.W., N.K., J.P., H.D., S.A., S.M. and I.P. contributed to data analysis and critical review of the manuscript.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Fraser CJ, Bhatia S, Ness K, Carter A, Francisco L, Arora M, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006 Oct 15;108(8):2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socie G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341(1):14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 3.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005 Dec;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014 Dec 18; doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooley TALW, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan ELMP. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 7.Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite endpoint of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015 Jan 15; doi: 10.1182/blood-2014-10-609032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D. C. Regression models and life tables. J R Stat Soc B. 34(2):187–220. 972. [Google Scholar]
- 9.Aisa Y, Mori T, Kato J, Yamane A, Kohashi S, Kikuchi T, et al. Validation of NIH consensus criteria for diagnosis and severity-grading of chronic graft-versus-host disease. International journal of hematology. 2013 Feb;97(2):263–271. doi: 10.1007/s12185-013-1268-1. [DOI] [PubMed] [Google Scholar]
- 10.Arora M, Nagaraj S, Witte J, DeFor TE, MacMillan M, Burns LJ, et al. New classification of chronic GVHD: added clarity from the consensus diagnoses. Bone marrow transplantation. 2009 Jan;43(2):149–153. doi: 10.1038/bmt.2008.305. [DOI] [PubMed] [Google Scholar]
- 11.Cho BS, Min CK, Eom KS, Kim YJ, Kim HJ, Lee S, et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia. 2009 Jan;23(1):78–84. doi: 10.1038/leu.2008.276. [DOI] [PubMed] [Google Scholar]
- 12.Jagasia M, Giglia J, Chinratanalab W, Dixon S, Chen H, Frangoul H, et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007 Oct;13(10):1207–1215. doi: 10.1016/j.bbmt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Vigorito AC, Campregher PV, Storer BE, Carpenter PA, Moravec CK, Kiem HP, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009 Jul 16;114(3):702–708. doi: 10.1182/blood-2009-03-208983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2003 Apr;9(4):215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 15.Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing Incidence of Chronic Graft-versus-Host Disease in Allogeneic Transplantation - A Report from CIBMTR. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014 Oct 30; doi: 10.1016/j.bbmt.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer J, Williams K, Inamoto Y, Chai X, Martin PJ, Tomas LS, et al. Pulmonary symptoms measured by the national institutes of health lung score predict overall survival, nonrelapse mortality, and patient-reported outcomes in chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014 Mar;20(3):337–344. doi: 10.1016/j.bbmt.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010 Jan;16(1 Suppl):S106–S114. doi: 10.1016/j.bbmt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011 Jul;17(7):1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inamoto Y, Storer BE, Petersdorf EW, Nelson JL, Lee SJ, Carpenter PA, et al. Incidence, risk factors, and outcomes of sclerosis in patients with chronic graft-versus-host disease. Blood. 2013 Jun 20;121(25):5098–5103. doi: 10.1182/blood-2012-10-464198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penas PF, Jones-Caballero M, Aragues M, Fernandez-Herrera J, Fraga J, Garcia-Diez A. Sclerodermatous graft-vs-host disease: clinical and pathological study of 17 patients. Archives of dermatology. 2002 Jul;138(7):924–934. doi: 10.1001/archderm.138.7.924. [DOI] [PubMed] [Google Scholar]
- 21.Skert C, Patriarca F, Sperotto A, Cerno M, Fili C, Zaja F, et al. Sclerodermatous chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: incidence, predictors and outcome. Haematologica. 2006 Feb;91(2):258–261. [PubMed] [Google Scholar]
- 22.Chosidow O, Bagot M, Vernant JP, Roujeau JC, Cordonnier C, Kuentz M, et al. Sclerodermatous chronic graft-versus-host disease. Analysis of seven cases. Journal of the American Academy of Dermatology. 1992 Jan;26(1):49–55. doi: 10.1016/0190-9622(92)70005-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.