Abstract
Methylphenidate (MPD) is a central nervous system (CNS) stimulant, which belongs to the phenethylamine group and is mainly used in the treatment of attention deficit hyperactive disorder (ADHD). However, a growing number of young individuals misuse or abuse MPD to sustain attention, enhance intellectual capacity and increase memory. Recently, the use of MPD as a cognitive enhancement substance has received much attention and raised concerns in the literature and academic circles worldwide. The prescribing frequency of the drug has increased sharply as consequence of the more accurate diagnosis of the ADHD and the popularity of the drug itself due to its beneficial short-term effect. However, careful monitoring is required, because of possible abuse.
In this review different aspects concerning the use of MPD have been approached. Data showing its abuse among college students are given, when the drug is prescribed short term beneficial effects and side effects are provided; moreover studies on animal-models suggesting long lasting negative effects on healthy brains are discussed. Finally, emphasis is given to the available formulations and pharmacology.
Keywords: Abuse, ADHD, cognitive enhancement, long-term effects, methylphenidate.
1. INTRODUCTION
Methylphenidate (MDP) is a central nervous system (CNS) stimulant, of the phenethylamine class, available on the market with the following trade names: Ritalin, Equasym XL, Concerta, Quillivant XR, Methylin, Metadate and Focalin. Its street names are the following: Bennies, Black Beauties, Cat, Coke, Crank, Crystal, Flake, Ice, Pellets, R-Ball, Skippy, Snow, Speed, Uppers, and Vitamin R. The drug was first synthesized in 1944 and launched as Ritalin by Ciba-Geigy Pharmaceutical Company in 1954. It was originally used, after licensed in 1955 by the U.S. Food and Drug Administration (FDA), for the treatment of lethargy, narcolepsy, chronic fatigue, disorders associated with depression and what was then known as hyperactivity [1]. However, its most impressive beneficial effect has been the decrease of the symptoms noticed in attention deficit hyperactivity disorder (ADHD), which is one of the most common behavioral disorders in childhood and may persist into adulthood [2, 3] found in approximately 3% to 5% of the general population of school-age children, occurring more frequently in boys [4-6]. Its prevalence may rise up to 17% when less strict criteria are used [7]. In 1990, when diagnosis of the ADHD became more broadly accepted the MDP became the drug of choice for the treatment of this disorder with its prescription exceedingly increasing [4, 8-11]. In general, MPD is safe when used as prescribed and produces limited side effects when used orally in therapeutic doses [12-15]. Although, MPD’s safety, efficacy and cost effectiveness have been documented in many studies [16, 17] there is still a gap of knowledge regarding its long-term effects on brain function and structure and its influence on brain development.
Historically, in 1937, Charles Bradley reported a positive effect of stimulant medication in children with various behavior disorders [18]. Many of the children previously mentioned would probably be diagnosed with ADHD today [19]. Bradley discovered the beneficial effects of stimulants on children behavior symptomatically, in his attempt to treat their headaches produced after pneumoencephalograms as result of a considerable loss of spinal fluid. Benzedrine, “the most potent stimulant available at the time” was used by Bradley to treat the headaches. Although benzedrine had only a tiddley effect on the headaches, it caused an astonishing improvement in behavior and school performance in some of the children. Subsequent trials of Bradley led to the same conclusion i.e. the use of benzedrine improved the school performance of approximately half the children and they “were more interested in their work and performed it more quickly and accurately” [19]. However, his revolutionary observations had no influence on practice at the time since the assumption that behavioral disorders require psychological interventions was predominant [20]. Posterior studies for example by Denhoff et al. [21] produced growing interest in stimulant treatment of hyperkinetic children. At present, stimulant medication is the most frequently used treatment for children with ADHD and MPD is the stimulant of first choice, [9, 22] while benzedrine, which was the first stimulant used for the same purpose, is no longer in use [8].
2. MATERIALS AND METHODS
Some databases, from 1957 to 2015, were searched: Medline, Cochrane Central, Scopus, Web of Science, ScienceDirect, EMBASE and Google Scholar, using the following keywords: Methylphenidate, cognitive enhancement, ADHD, abuse, long-term effects, Ritalin, Concerta, Metadate, Methylin and Focalin. The main key word “Methylphenidate” was individually searched in association to each of the others. Among the 7305 sources found after the initial screening in order to exclude duplicate sources, 134 references, taking into consideration the aims of the paper, were selected.
3. PHARMACOLOGY AND AVAILABLE FORMULA-TIONS
ADHD and other related disorders are considered to be associated with dopamine and norepinephrine sub-performance in the brain, particularly in the prefrontal cortex (PFC) [23]. As already mentioned, ADHD is a common developmental disorder that affects school–age children [24] and impairs the functioning of the frontal lobes, specifically the PFC, which is responsible for self-regulatory functions, including among others motivation, memory and inhibition. Executive function (EF) refers to the mental control procedures, encompassing cognitive, physical and emotional control, that are requested to maintain effective goal-directed behavior, which includes problem solving, planning and organizing skills [23]. Deficits in EFs have been suggested to lead to cognitive difficulties experienced by children with ADHD [25, 26]. Deficits in working memory (WM), which is a key EF, have been cited in individuals with ADHD [27, 28]. Moreover, a lot of studies have indicated that WM impairments are central to ADHD [29, 30].
Since MPD’s mechanism of action implicates the inhibition of catecholamine reuptake, principally as a dopamine reuptake inhibitor, the drug is considered efficient for the control of the symptomatology of ADHD, which is mostly consistent with the dysfunction of the PFC. MPD acts by blocking both dopamine and norepinephrine transporters, which leads to increased extracellular dopamine and norepinephrine concentrations in PFC [31] and dopamine in the striatum [32-36]. It has been shown that working memory performance is facilitated after a low dose of MPD infusion in PFC, whereas infusion of MPD into striatum has no reaction on this PFC-dependent cognition task [37], suggesting that PFC is a main site for MPD’s therapeutic action [38, 39]. In vivo, acute administration of MPD exerts excitatory actions on the PFC neurons by an indirect activation of alpha2-adrenoceptors and D1 receptors [38, 40-42], while in vitro, the drug could enhance excitability of pyramidal PFC neurons by activating alpha-2 receptors located in interneurons [43]. Zhang et al. found that MPD facilitates NMDA-receptor mediated excitatory synaptic transmission through σ1 receptors via PLC/PKC signaling [44]. Evidence also suggests that alpha2A adrenoceptor gene is involved with the MPD-induced improvement in ADHD [45].
MPD is highly effective in improving the core symptoms of ADHD [46]. Pietrzak et al. suggested that MPD improved response inhibition, attention control and sustained attention in approximately 70% of the studies examined [47]. Moreover, WM is improved by MPD through dopaminergic transmission facilitation [48]. Although, the most recent findings suggest that impairments in visuospatial WM (VSWM) is common to individuals with ADHD [29, 49], there is a limited number of studies investigating the effectiveness of MPD on VSWM in children with ADHD [50, 51].
MPD (structure is shown in Fig. 1) can be found on the market in various forms under numerous brand names and formulations including tablets, capsules, oral suspension (liquid syrup) and adhesive-based matrix transdermal system (patch). The drug is currently administered either as extended [52-54], instant-release or as an osmotically controlled-released formulation [55, 56].
Fig. (1).
Methylphenidate chemical structure.
Newer long-acting formulations include an immediate-release ingredient to ensure the instant onset of action and an extended-release ingredient, which continues acting throughout the day. Thus, a rapid onset of action can be achieved with one dose per day. Different technologies are used for the numerous MPD formulations aiming to control the symptoms for at least 8 hours and incorporate different proportions of instant and extended-release MPD. For instance, Focalin XR® and Ritalin LA® use Spheroidal Oral Drug Absorption System (SODAS®) technology to supply 50% of the MPD dose instantly and 50% as extended release [57]. dl-threo-Methylphenidate exists as two enantiomers, l-threo-methylphenidate (l-MPD) and d-threo-methylphenidate (d-MPD). d-MPD has been developed as a medicine to treat ADHD itself. dl-threo-Methylphenidate undergoes enantioselective metabolism in the liver, resulting in remarkable variations in the plasma concentrations of its isomers, depending on both the formulation administered and the route of administration. Plasma d-MPD concentrations are higher than those of l-MPD when dl-threo-methylphenidate is taken orally. Nevertheless, with the newly developed methylphenidate transdermal system (MTS), 'first-pass' metabolism is circumvented and consequently, plasma d-MPD concentrations are consistent with those reached after oral administration, but the relative l-MPD concentrations are much higher, i.e. 50-60% of those of d-MPD. However, even in this case, it is more possible that the contribution of l-MPD to both the adverse effects and effectiveness of the racemate is no higher than 5-10% of the total. Transdermal drug delivery is considered an effective and safe mean of administering MPD to individuals with ADHD [58].
According to Heal and Pierce [36] d-MPD and l-MPD have the same pharmacological profile as the parent racemate, i.e. acting as catecholamine-selective reuptake inhibitors. However, d-MPD is approximately 10 times more abundant than l-MPD in this regard. Ritalin LA® contains racemic MPD (ie. both d-MPH and l-MPH isomer), like most MPD formulation do. Taking into account that when the l-MPH is orally administered it is metabolized promptly via first pass through hepatic circulation, d-isomer is thought to be the main pharmacological contributor in the treatment of the disorder. Taking all this into consideration Focalin XR® developed a formulation containing only the d-MPH isomer.
The abilities of these drugs not only to improve the cognitive and behavioral impairment but also to modulate the common side effects is due to their capacity to potentiate noradrenergic and/or dopaminergic function in the central and peripheral nervous systems. The authors concluded that between the two isomers, d-MPD, which is more abundant and potent, contributes more to both the effectiveness and the adverse effects, irrespective of the route of administration or the formulation of the racemate [36].
MPD’s, formulated as hydrochloride salt, high solubility in the fluids of the gastrointestinal tract, leads to rapid and extensive absorption from the intestine to the colon [59, 60]. Thus, it is more likely that the gastric emptying time is the main factor in controlling MPD absorption after immediate-release intake, whereas for the various controlled-release formulations, programmed drug release and the dissolution pattern are considered to be the factors which control the drug’s absorption. Because of extensive first-pass metabolism followed by oral administration, the absolute bioavailability is low and variable [61].
Once MPD reaches the systemic circulation, it is promptly distributed to various tissues, with a steady-state volume of distribution of approximately 2 L/kg [62].
MPD is primarily metabolized to the inactive [63] metabolite ritalinic acid [59, 64, 65] by deesterification. This simple process leads to absolute bioavailability of 11-53% [66]. The circulating concentrations of the metabolite significantly exceed the concentrations of the parent drug [67-70]. Urinary elimination of ritalinic acid accounts for 60-80% of the dose [59, 64, 65].
Clearance of the drug is also speedy, with little or no accumulation of MPD from day to day, even with the controlled-release formulations [68]. Nonlinearity, possibly related to first-pass metabolism saturation may be noticed at higher oral doses [71]. Half-life has been reported to be 2-6 hours after immediate-release or intravenous dosing with most studies reporting an average of 2-3 hours. Longer half-life is reported for extended-release formulations, but this is most probably related to prolonged absorption [72].
4. ADVERSE SIDE EFFECTS
Although MPD is effective in the majority of children in the short term, there is considerable variation in individual response to treatment, with a minority not managing sufficient symptom control whereas others are unable to tolerate the adverse effects of the drug [73-75].
The adverse effects of MPD include among others: pupil dilation, [76] loss of hair, depression, anorexia, headaches, impairment of libido, insomnia, restlessness, anxiety and hypersensitivity [77-80]. Data obtained by Auger et al. [81], demonstrate that patients treated with high-dose stimulants, used in the treatment of excessive somnolence disorders, showed a significant increase of the occurrence of psychosis, psychiatric hospitalizations and substance misuse compared to the patients treated with standard doses of the same stimulants. In addition, anorexia and tachyarrhythmia were more common in the high-dose treated group compared to controls. Occasionally, stimulant psychosis can occur during long-term therapy with MPD. Thus, regular psychiatric monitoring of individuals treated with MPD has been recommended [82].
Recent large-scale studies by the US FDA suggest that, serious adverse cardiovascular events such as sudden death, myocardial infarction, and stroke are not associated with the medical use of MPD, amphetamine or other customarily prescribed ADHD stimulants in children, young adults, and adults [83, 84]. On the other hand, some studies suggest a small but significant impact of MPD on the cardiovascular system including increases in heart rate and blood pressure and sudden cardiac death [85, 86].
Aktepe et al. [87], investigated the side effects of MPD in ADHD children (n=126). In 55.5% of cases, MPD was used alone, whereas in 26.9% of cases, it was used in combination with other medications. Side effects were recorded in 51.6% of cases and no side effects in 48.4%. MPD treatment had to be ceased in only 5.5% of cases. The side effects resulting in the cessation of the treatment with MPD are as follows: weight loss, irritability, hyperactivity, exacerbation of tics and papular rash. The side effects reported are shown in Fig. 2. MPD was used at a dose of 10-20 mg/day in 89.6% of cases.
Fig. (2).
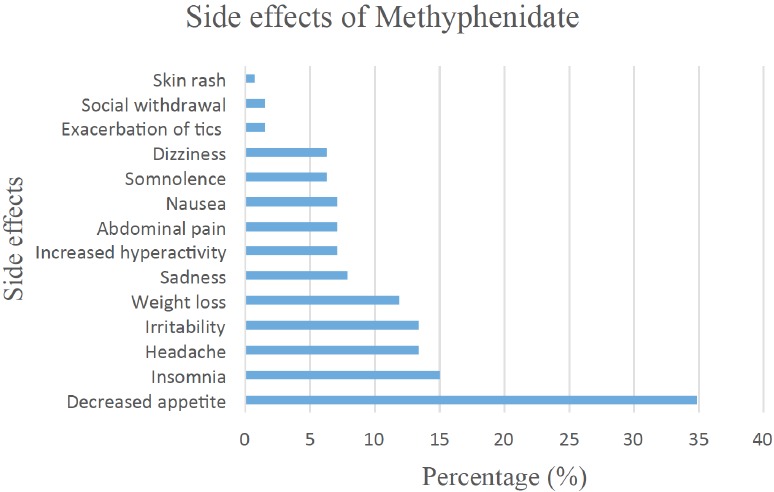
Adverse side effects of Methylphenidate reported by Aktepe et al. [87].
Moreover, MPD has been associated with the risk of long lasting and often painful erections, known as priapism. Thus, FDA updated patient medication guides and MPD labels to provide information about the rare but important risk of priapism. If the previously mentioned disorder is not treated immediately, it can result in permanent harm to the penis. Priapism is more likely to occur in male patients treated with atomoxetine than those treated with MPD. However, due to limitations in available information, it is not known how frequently priapism appears in patients treated with MPD. Cases of a prepubertal child and a 14-year-old male who developed priapism after therapy with MPD have been reported in literature [88, 89]. It is recommended that healthcare professionals should inform male patients about the symptoms and signs of priapism and emphasize the necessity for immediate treatment, since younger males may not recognize the problem or be too embarrassed to tell anyone if it happens [90].
Hepatotoxicity is a rare adverse reaction to MPD. The literature review indicates some cases of liver failure attributed to MPD, that recovered after the treatment cessation. However, a recent study reported a liver failure case attributed to MPD, where liver transplantation was required. The probable mechanism of liver injury was MPD direct toxicity to hepatocytes. Thus, the monitoring of liver function is highly recommended in these cases [91].
5. ABUSE/MISUSE
MPD has been broadly used as drug of abuse because of its psychostimulant properties. Moreover, MPD has been used by college students in order to achieve the so called cognitive enhancement (CE) which is defined as the usage (by healthy individuals) of psychoactive drugs aiming at enhancing cognition which includes among others concentration and memory. CE substances commonly used can be divided into three groups: 1) over-the-counter, such as caffeine tablets, energy drinks etc.; 2) drugs approved for the treatment of certain disorders, such as amphetamines and MPD and 3) illicit drugs, such as ecstasy and crystal meth [92]. According to users’ reports on ‘self-experiments’ Internet forums, abusers either grind the commercially available tablets into powder and snort it or they convert it into liquid form, since the drug is water soluble, making it injectable (intravenous administration). Smoking and oral administration have also been reported. Moreover as referred in the same forum MPD can be abused in combination with other drugs, such as cannabis, oxycodone, amphetamines, alprazolam, alcohol and others [93].
Unlike other potent stimulants, there is no clandestine production of MPD and diverted pharmaceutical products are the only source for abuse purposes. MPD is obtained from fraudulent prescriptions, pharmacy theft, doctor shopping, and from friends or associates who have obtained the drug through a prescription [94].
The potentiality of abuse of MPD was considered in the early 1960s in a case report of a patient who was taking 125 tablets of MPD per day [95]. Reports of oral MPD abuse including reports of MPD paranoia [96], hallucinations [96, 97], delusional disorder [98] and euphoria [99], appeared later in the literature. Intravenous abuse of MPD related to psychosis was indicated in 1963 and subsequently intravenous abuse was reported in several studies in the early 1970s [96, 97, 100]. Afterward, a study depicted the intravenous abuse patterns, mortality, and morbidity related to MPD [101].
Studies on animals for evaluation of the long-term effects of abuse resulted in ambiguous outcomes; negative effects like the ones of methamphetamine in low doses and no effect in high doses. Additional studies to evaluate potential neurotoxicity under such conditions is necessary. It has been proved that, the abuse of MPD, when in rare cases intravenously administered can even produce dopaminergic fibers maturation impairment in subcortical brain areas [102].
Many surveys have been conducted suggesting wide abuse of the drug as a cognitive enhancer among healthy college students. However, it is hard to define the actual trend of abuse [103]. According to Teter et al., who conducted a research to investigate the motives, prevalence and routes of administration of illicitly used prescription stimulants among college students, 24.5% of the 269 students who declared past-year illicit use of prescription stimulants, reported MPD use. In the same survey, it was found that the motives for illicit use of prescription stimulants are the following: to assist with concentration, help study, heighten alertness, get high and experimentation. Although most users reported oral administration, a significant percentage (38%) of users reported intranasal administration [104].
Another study, reported that 2.3% of high school seniors declared past-year use of Ritalin, while 1.9% used methamphetamine [105]. A 2000 study, conducted within a liberal art college stated that more than 16% of the students who participated had tried MPD recreationally and approximately 13% had administrated the substance intranasally [106].
As reported by the U.S. Department of Health and Human Services, in Monitoring the Future National Survey Results on Drug Use, 1975-2006 [107], the use of MPD among young adults and college students in 2006 was 2.6% and 3.9%, respectively. A slight decrease in the use of MPD in both groups from 2002 to 2006 was also noted. However, compared to the trend of use of other phenethylamines commonly abused, such as amphetamines, MPD is less commonly abused by both categories.
A large study carried out at the University of Michigan; found that approximately 3% of the students (out of 2250 students who completed the survey) had declared past year illicit use of the drug. No significant differences between males and females percentages of misuse/abuse were found. In addition, an association between MPD misuse and use of alcohol and drugs was found. In particular, MPD misusers were considerably more likely to use drugs and alcohol [108].
According to White et al. [109] 16% of students of a northeastern US university have misused or abused stimulant medications. Of this category, 96% of those specified a medication, reported Ritalin as their stimulant of choice. More than 50% of the students misusing the drug, reported administration of the drug 2-3 times per year, 34% 1-2 times per month, while 15.5% of the misusers take the drug 2-3 times per week. Similar use patterns were reported by the two sexes. Reducing hyperactivity, improving attention and improving grades were the reasons which lead to misuse or abuse of the drug according to the participants.
When MPD is intranasally abused similar effects to intranasal use of crack cocaine and amphetamines are produced [110, 111]. Doses as high as 200mg have been reported for intranasal MPD abuse and 40mg-1000mg for intravenous abuse [4, 111, 112].
To sum up, comparative analysis is not recommended since the methodology used differs from study to study. However, data obtained suggest broad abuse/misuse of MPD as cognitive enhancer among college students, teenagers and young adults [113-115]. Table 1 summarizes the outcomes of studies conducted to determine the prevalence of MPD’s abuse.
Table 1.
Prevalence of MPD’s abuse and common routes of administration when abused.
| Author | Type of Survey | Percentage of Individuals Used MPD | Routes of Administration |
|---|---|---|---|
| Teter et al. [104] | Web-based | 24,5% out of 269 past-year illicit users of prescription stimulants | Oral, intranasal |
| Babcock and Byrne [106] | Self -reported survey (10 yes-and-no questions) | 16.6% out of 283 students | 12.7% intranasal |
| Teter et al. [108] | Internet survey | 3% out of 2250 students reported past-year illicit use | |
| White et al. [109] | Internet survey | 16% reported abuse/misuse of stimulant medication. 96% of those reported Ritalin as the stimulant of choice | Orally, 40% intranasal |
6. LONG-TERM EFFECTS OF MPD ON THE BRAIN
Several studies, involving both animals, mostly rats, and humans have been conducted in order to assess the possible enhanced cognitive effects of MPD on the normal brain [116-118]. It has been reported that high doses (5–10 mg/kg) when intraperitoneally administered in rats increase the locomotor activity and impair both performance and attention. On the other hand, improvement of the cognitive performance and reduced motor activity was noted after rats were intraperitoneally administered a low dose (0.5–2 mg/kg) of the drug. Moreover, the administration of even lower doses (0.25–1 mg/kg) of MPD in healthy rats heightens the attention skills without influencing motor activity [119].
MPD improves the performance of prefrontal cortex tasks in both “normal” college students [120] and in patients diagnosed with ADHD [121].
Since MPD is most commonly prescribed to children and adolescents with ADHD at a moment of crucial importance for the adolescent because of the development and the maturation of the brain, it is thought that drug exposure at this period of life could result in lasting alterations that will persist into the adulthood. Lee et al. investigated the effects of the repeated drug administration on the locomotor diurnal rhythm activity patterns of female Sprague-Dawley (SD) rats during adolescence. The experiment involved 31 rats divided into the following groups: control, 0.6 mg/kg, 2.5 mg/kg, and 10 mg/kg MPD group. Saline was injected to all groups on the first day of experiment, whereas on days 2-7 rats were administered with either saline, 0.6 mg/kg, 2.5 mg/kg, or 10 mg/kg of MPD. A washout period followed (i.e. Days 8-10). The same dose used on days 2-7 was injected again on day 11 to the four groups, respectively. The obtained data illustrated that repeated administrations of both 2.5 mg/kg and 10 mg/kg of the drug could alter the locomotor diurnal rhythm patterns, suggesting that the previously mentioned doses exert long-term effects [122].
The cellular mechanisms of action of MPD and its eventual effect on prefrontal cortical circuitry are not fully established, in particular within the developing brain system. Urban et al. [123], involve in their experiments both adult SD rats and Juvenile (postnatal day [PD] 15), that were administered with either saline or MPD. Both neuronal excitability and synaptic transmission in pyramidal neurons of prefrontal cortex were examined. Moreover, recovery from the drug treatment was tested 1, 5 and 10 weeks after the last administration of the drug.
It was concluded, that either chronic treatment or a single dose of 1 mg/kg intraperitoneal MPD, could generate considerable depressive effects on pyramidal neurons in juvenile rat prefrontal cortex. Doses of 0.03 to 0.3 mg/kg also generated depressive effects in juvenile rats, in a linear dose-dependent way. Function recovery achieved within 1 week from chronic 1 mg/kg treatment, while depression of prefrontal neurons noticed on rats chronically treated with 3 and 9 mg/kg lasted 10 weeks and beyond. The obtained data suggest that the prefrontal cortex of the juvenile is supersensitive to the drug and the acceptable therapeutic range for adults is overestimated, since the chronic treatment with 1 mg/kg MPD is well correlated with the acceptable therapeutic range for adults. In general, juvenile treatment with MPD can produce long-lasting and potentially permanent changes to excitatory neuron function in the prefrontal cortex of juvenile rats [123].
Both meta-analyses and systematic reviews of magnetic resonance imaging studies suggest that long-term treatment with ADHD stimulants (particularly, MPD and amphetamine) reduces abnormalities in brain function and structure found in individuals with ADHD [124-126]. Furthermore, both the efficacy and the safety of long-term use of ADHD stimulants for subjects with ADHD have been established [127]. Specifically, the continual treatment efficacy and safety of both MPD and amphetamine have been evidenced in controlled drug trials with duration of several years [127].
Rats with ADHD-like behavior were employed in animal studies aiming to evaluate the safety of MPD on the developing brain. It was noted that both psychomotor impairments and liturgical and structural parameters of dopaminergic system were improved with treatment. Nevertheless, MPD produced long lasting alterations to the dopaminergic system of healthy control animals. Moreover, it was shown that rats treated with MPD grew up to be more emotional and stressed [102].
Bolaños et al. [128] observed that animals treated with MPD showed increased anxiety-like behavior, were considerably more sensitive to stressful situations, and had enhanced plasma levels of corticosterone, compared to controls. However, for the latest study, healthy rats were used thus remaining unclear whether MPD could cause adverse emotional effects on ADHD animal models [128].
7. CONCLUSIONS
Data suggest that MPD is a drug of great value that nicely fits the pharmacological demands to control dopamine dysfunctions in ADHD. However, long lasting alterations to the dopaminergic system in normal control animals suggest that if a child is misdiagnosed with ADHD he or she may be at risk of long lasting unfavorable effects in brain development. Thus, careful clinical diagnosis and assessment of ADHD symptomatology is required to assure that the drug is only prescribed to children with comprehensible ADHD symptomatology. Data obtained from animal studies illustrate that under the latest conditions MPD is supportive for the related behavior and brain development in children with ADHD. It is worth pointing out that medical malpractice suits, charging physicians with negligent misdiagnosis of ADHD and failure to obtain sufficient informed consent for the use of stimulants and inadequate information on side effects have been raised [129].
In addition, clinicians should be very attentive concerning the prescribing of dosages higher than the maximum guidelines since the latter could result in undesired effects. Moreover, due to the numerous formulations commercially available, clinicians should be very cautious in choosing the most suitable one, taking into account the patients’ response to the drug and the different treatment needs of each individual. In general, a tailored approach is required in order to optimize the treatment of ADHD symptoms.
Summing up, the wealth of both human and animal data of MPD indicates the great significance of the drug, which has to be carefully handled in the right way. However, it is extremely difficult to compare the studies investigating long term effects because of the heterogeneity in data and duration which suggests the necessity of systematic monitoring of long-term safety of the drug [130].
Nevertheless, the use of MPD and other stimulants for the treatment of ADHD symptoms has been controversial [131, 132]. One such criticism is the prescription of psychostimulants medication to children in order to reduce ADHD symptoms [133]. The claim that MPD acts as a gateway drug has been discredited by multiple sources, according to which MPD rarely leads to addiction or abuse when taken appropriately as treatment for ADHD.
Data obtained from numerous studies (comparative analysis is not recommended, though) suggest broad abuse/misuse of MPD as cognitive enhancer among college students, teenagers and young adults [134]. Results obtained from studies concerning the effects of the stimulant on healthy individuals suggest its abuse is a concern on college campuses.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Challman T.D., Lipsky J.J. Methylphenidate: its pharmacology and uses. Mayo Clin. Proc. 2000;75(7):711–721. doi: 10.1016/S0025-6196(11)64618-1. [DOI] [PubMed] [Google Scholar]
- 2.Hamedi M., Mohammdi M., Ghaleiha A., Keshavarzi Z., Jafarnia M., Keramatfar R., Alikhani R., Ehyaii A., Akhondzadeh S. Bupropion in adults with Attention-Deficit/Hyperactivity Disorder: a randomized, double-blind study. Acta Med. Iran. 2014;52(9):675–680. [PubMed] [Google Scholar]
- 3.Mannuzza S., Klein R.G., Bessler A., Malloy P., LaPadula M. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch. Gen. Psychiatry. 1993;50(7):76–565. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- 4.Morton W.A., Stockton G.G. Methylphenidate abuse and psychiatric side effects. Prim. Care Companion J. Clin. Psychiatry. 2000;2(5):159–164. doi: 10.4088/PCC.v02n0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polanczyk G., de Lima M.S., Horta B.L., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am. J. Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 6.Kooij S.J., Bejerot S., Blackwell A., Caci H., Casas-Brugué M., Carpentier P.J., Edvinsson D., Fayyad J., Foeken K., Fitzgerald M., Gaillac V., Ginsberg Y., Henry C., Krause J., Lensing M.B., Manor I., Niederhofer H., Nunes-Filipe C., Ohlmeier M.D., Oswald P., Pallanti S., Pehlivanidis A., Ramos-Quiroga J.A., Rastam M., Ryffel-Rawak D., Stes S., Asherson P. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry. 2010;10:67. doi: 10.1186/1471-244X-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbaresi W.J., Katusic S.K., Colligan R.C., Pankratz V.S., Weaver A.L., Weber K.J., Mrazek D.A., Jacobsen S.J. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch. Pediatr. Adolesc. Med. 2002;156(3):217–224. doi: 10.1001/archpedi.156.3.217. [DOI] [PubMed] [Google Scholar]
- 8.Lange K.W., Reichl S., Lange K.M., Tucha L., Tucha O. The history of attention deficit hyperactivity disorder. Atten. Defic. Hyperact. Disord. 2010;2(4):241–255. doi: 10.1007/s12402-010-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leonard B.E., McCartan D., White J., King D.J. Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse clinical effects. Hum. Psychopharmacol. 2004;19(3):151–180. doi: 10.1002/hup.579. [DOI] [PubMed] [Google Scholar]
- 10.Zito J.M., Safer D.J., dosReis S., Gardner J.F., Boles M., Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. 2000. [DOI] [PubMed]
- 11.Safer D.J., Zito J.M., Fine E.M. Increased methylphenidate usage for attention deficit disorder in the 1990s. Pediatrics. 1996;98(6 Pt 1):1084–1088. [PubMed] [Google Scholar]
- 12.Diagnosis and treatment of attention deficit hyperactivity disorder (ADHD). NIH Consens. Statement. 1998;16(2):1–37. [PubMed] [Google Scholar]
- 13.Findling R.L., Dogin J.W. Psychopharmacology of ADHD: children and adolescents. J. Clin. Psychiatry. 1998;59(Suppl. 7):42–49. [PubMed] [Google Scholar]
- 14.Wender P.H. Pharmacotherapy of attention-deficit/hyperactivity disorder in adults. J. Clin. Psychiatry. 1998;59(Suppl. 7):76–79. [PubMed] [Google Scholar]
- 15.Biederman J. Attention-deficit/hyperactivity disorder: a life-span perspective. J. Clin. Psychiatry. 1998;59(Suppl. 7):4–16. [PubMed] [Google Scholar]
- 16.Gilmore A., Milne R. Methylphenidate in children with hyperactivity: review and cost-utility analysis. Pharmacoepidemiol. Drug Saf. 2001;10(2):85–94. doi: 10.1002/pds.564. [DOI] [PubMed] [Google Scholar]
- 17.Mott T.F., Leach L., Johnson L. Clinical inquiries. Is methylphenidate useful for treating adolescents with ADHD? J. Fam. Pract. 2004;53(8):659–661. [PubMed] [Google Scholar]
- 18.Bradley C. The behavior of children receiving benzedrine. Am. J. Psychiatry. 1937;94(3):577–585. doi: 10.1176/ajp.94.3.577. [DOI] [Google Scholar]
- 19.Gross M.D. Origin of stimulant use for treatment of attention deficit disorder. Am. J. Psychiatry. 1995;152(2):298–299. doi: 10.1176/ajp.152.2.298b. [DOI] [PubMed] [Google Scholar]
- 20.Conners C.K. Attention-deficit/hyperactivity disorder—historical development and overview. J. Atten. Disord. 2000;3(4):173–191. [Google Scholar]
- 21.Denhoff E., Laufer M.W., Solomons G. Hyperkinetic impulse disorder in children’s behavior problems. Psychosom. Med. 1957;19(1):38–49. doi: 10.1097/00006842-195701000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Giacobini M., Medin E., Ahnemark E., Russo L.J., Carlqvist P. Prevalence, patient characteristics, and pharmacological treatment of children, adolescents, and adults diagnosed with ADHD in Sweden. J. Atten. Disord. 2014:1087054714554617. doi: 10.1177/1087054714554617. [DOI] [PubMed] [Google Scholar]
- 23.Arnsten A.F., Li B.M. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol. Psychiatry. 2005;57(11):84–1377. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and statistical manual of mental disorders - DSM-IV-TR. 4th ed., text rev. Washington, DC: APA; 2000. [Google Scholar]
- 25.Barkley R.A. Attention-deficit/hyperactivity disorder, self-regulation, and time: toward a more comprehensive theory. J. Dev. Behav. Pediatr. 1997;18(4):271–279. doi: 10.1097/00004703-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Corbett B.A., Constantine L.J., Hendren R., Rocke D., Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 2009;166(2-3):210–222. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alloway T.P., Gathercole S.E., Holmes J., Place M., Elliott J.G., Hilton K. The diagnostic utility of behavioral checklists in identifying children with ADHD and children with working memory deficits. Child Psychiatry Hum. Dev. 2009;40(3):353–366. doi: 10.1007/s10578-009-0131-3. [DOI] [PubMed] [Google Scholar]
- 28.Rapport M.D., Alderson R.M., Kofler M.J., Sarver D.E., Bolden J., Sims V. Working memory deficits in boys with attention-deficit/hyperactivity disorder (ADHD): the contribution of central executive and subsystem processes. J. Abnorm. Child Psychol. 2008;36(6):825–837. doi: 10.1007/s10802-008-9215-y. [DOI] [PubMed] [Google Scholar]
- 29.Dovis S., Van der Oord S., Wiers R.W., Prins P.J. Can motivation normalize working memory and task persistence in children with attention-deficit/hyperactivity disorder? The effects of money and computer-gaming. J. Abnorm. Child Psychol. 2012;40(5):669–681. doi: 10.1007/s10802-011-9601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barkley R.A. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol. Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 31.Berridge C.W., Devilbiss D.M., Andrzejewski M.E., Arnsten A.F., Kelley A.E., Schmeichel B., Hamilton C., Spencer R.C. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry. 2006;60(10):1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 32.Bymaster F.P., Katner J.S., Nelson D.L., Hemrick-Luecke S.K., Threlkeld P.G., Heiligenstein J.H., Morin S.M., Gehlert D.R., Perry K.W. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 33.Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br. J. Pharmacol. 2006;147(Suppl. 1):S82–S88. doi: 10.1038/sj.bjp.0706428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatley S.J., Volkow N.D., Gifford A.N., Fowler J.S., Dewey S.L., Ding Y.S., Logan J. Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology (Berl.) 1999;146(1):93–100. doi: 10.1007/s002130051093. [DOI] [PubMed] [Google Scholar]
- 35.Volkow N.D., Wang G.J., Fowler J.S., Logan J., Gatley S.J., Wong C., Hitzemann R., Pappas N.R. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J. Pharmacol. Exp. Ther. 1999;291(1):409–415. [PubMed] [Google Scholar]
- 36.Heal D.J., Pierce D.M. Methylphenidate and its isomers: their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system. CNS Drugs. 2006;20(9):713–738. doi: 10.2165/00023210-200620090-00002. [DOI] [PubMed] [Google Scholar]
- 37.Spencer R.C., Klein R.M., Berridge C.W. Psychostimulants Act Within the Prefrontal Cortex to Improve Cognitive Function. 2012. [DOI] [PMC free article] [PubMed]
- 38.Arnsten A.F. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- 39.Wilens T.E. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 2008;28(3) 2:S46–53. doi: 10.1097/JCP.0b013e318173312f. [DOI] [PubMed] [Google Scholar]
- 40.Gamo N.J., Wang M., Arnsten A.F. Methylphenidate and atomoxetine enhance prefrontal function through α2-adrenergic and dopamine D1 receptors. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(10):1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnsten A.F., Dudley A.G. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav. Brain Funct. 2005;1(1):2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gronier B. In vivo electrophysiological effects of methylphenidate in the prefrontal cortex: involvement of dopamine D1 and alpha 2 adrenergic receptors. Eur. Neuropsychopharmacol. 2011;21(2):192–204. doi: 10.1016/j.euroneuro.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Andrews G.D., Lavin A. Methylphenidate increases cortical excitability via activation of alpha-2 noradrenergic receptors. Neuropsychopharmacology. 2006;31(3):594–601. doi: 10.1038/sj.npp.1300818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C.L., Feng Z.J., Liu Y., Ji X.H., Peng J.Y., Zhang X.H., Zhen X.C., Li B.M. Methylphenidate enhances NMDA-receptor response in medial prefrontal cortex via sigma-1 receptor: a novel mechanism for methylphenidate action. PLoS One. 2012;7(12):e51910. doi: 10.1371/journal.pone.0051910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polanczyk G., Zeni C., Genro J.P., Guimarães A.P., Roman T., Hutz M.H., Rohde L.A. Association of the adrenergic alpha2A receptor gene with methylphenidate improvement of inattentive symptoms in children and adolescents with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2007;64(2):218–224. doi: 10.1001/archpsyc.64.2.218. [DOI] [PubMed] [Google Scholar]
- 46.Solanto M., Newcorn J., Vail L., Gilbert S., Ivanov I., Lara R. Stimulant drug response in the predominantly inattentive and combined subtypes of attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2009;19(6):663–671. doi: 10.1089/cap.2009.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pietrzak R.H., Goldstein R.B., Southwick S.M., Grant B.F. Physical health conditions associated with posttraumatic stress disorder in U.S. older adults: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J. Am. Geriatr. Soc. 2012;60(2):296–303. doi: 10.1111/j.1532-5415.2011.03788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams G.V., Goldman-Rakic P.S. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376(6541):572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 49.Bedard A.C., Martinussen R., Ickowicz A., Tannock R. Methylphenidate improbe visual-spatial memory in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(3):260–268. doi: 10.1097/00004583-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Westerberg H., Hirvikoski T., Forssberg H., Klingberg T. Visuo-spatial working memory span: a sensitive measure of cognitive deficits in children with ADHD. Child Neuropsychol. 2004;10(3):155–161. doi: 10.1080/09297040409609806. [DOI] [PubMed] [Google Scholar]
- 51.Matsuura N., Ishitobi M., Arai S., Kawamura K., Asano M., Inohara K., Fujioka T., Narimoto T., Wada Y., Hiratani M., Kosaka H. Effects of methylphenidate in children with attention deficit hyperactivity disorder: a near-infrared spectroscopy study with CANTAB®. Child Adolesc. Psychiatry Ment. Health. 2014;8(1):273. doi: 10.1186/s13034-014-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Childress A., Sallee F.R. The use of methylphenidate hydrochloride extended-release oral suspension for the treatment of ADHD. Expert Rev. Neurother. 2013;13(9):979–988. doi: 10.1586/14737175.2013.833002. [DOI] [PubMed] [Google Scholar]
- 53.Maldonado R. Comparison of the pharmacokinetics and clinical efficacy of new extended-release formulations of methylphenidate. Expert Opin. Drug Metab. Toxicol. 2013;9(8):1001–1014. doi: 10.1517/17425255.2013.786041. [DOI] [PubMed] [Google Scholar]
- 54.Mardomingo-Sanz M.J. [Clinical use of 30:70 controlled-release methylphenidate in the treatment of attention deficit hyperactivity disorder]. Rev. Neurol. 2012;55(6):359–369. [PubMed] [Google Scholar]
- 55.Katzman M.A., Sternat T. A review of OROS methylphenidate (Concerta(®)) in the treatment of attention-deficit/hyperactivity disorder. CNS Drugs. 2014;28(11):1005–1033. doi: 10.1007/s40263-014-0175-1. [DOI] [PubMed] [Google Scholar]
- 56.Sugrue D., Bogner R., Ehret M.J. Methylphenidate and dexmethylphenidate formulations for children with attention-deficit/hyperactivity disorder. Am. J. Health Syst. Pharm. 2014;71(14):1163–1170. doi: 10.2146/ajhp130638. [DOI] [PubMed] [Google Scholar]
- 57.Coghill D., Banaschewski T., Zuddas A., Pelaz A., Gagliano A., Doepfner M. Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies. BMC Psychiatry. 2013;13:237. doi: 10.1186/1471-244X-13-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Findling R.L., Dinh S. Transdermal therapy for attention-deficit hyperactivity disorder with the methylphenidate patch (MTS). CNS Drugs. 2014;28(3):217–228. doi: 10.1007/s40263-014-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faraj B.A., Israili Z.H., Perel J.M., Jenkins M.L., Holtzman S.G., Cucinell S.A., Dayton P.G. Metabolism and disposition of methylphenidate-14C: studies in man and animals. J. Pharmacol. Exp. Ther. 1974;191(3):535–547. [PubMed] [Google Scholar]
- 60.Food and Drug Administration. Concerta (methylphenidate HCI) extended-release tablets. Company: Alza Corporation. Application N. 21-121. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21-121_Concerta.cfm . [Accessed Mar 24, 2015].
- 61.Chan Y.M., Soldin S.J., Swanson J.M., Deber C.M., Thiessen J.J., Macleod S. Gas chromatographic/mass spectrometric analysis of methylphenidate (ritalin) in serum. Clin. Biochem. 1980;13(6):266–272. doi: 10.1016/S0009-9120(80)80007-5. [DOI] [PubMed] [Google Scholar]
- 62.Ding Y.S., Fowler J.S., Volkow N.D. Is the l-threo enantiomer of methylphenidate (Ritalin) inactive in the brain when given orally? Am. Col. Neuropsychopharmacol. 2002;12:255. [Google Scholar]
- 63.Patrick K.S., Kilts C.D., Breese G.R. Synthesis and pharmacology of hydroxylated metabolites of methylphenidate. J. Med. Chem. 1981;24(10):1237–1240. doi: 10.1021/jm00142a021. [DOI] [PubMed] [Google Scholar]
- 64.Bartlett M.F., Egger H.P. Disposition and metabolism of methylphenidate in dog and man. Fed. Proc. 1972;31:537. [PubMed] [Google Scholar]
- 65.Redalieu E., Bartlett M.F., Waldes L.M., Darrow W.R., Egger H., Wagner W.E. A study of methylphenidate in man with respect to its major metabolite. Drug Metab. Dispos. 1982;10(6):708–709. [PubMed] [Google Scholar]
- 66.Chan Y.P., Swanson J.M., Soldin S.S., Thiessen J.J., Macleod S.M., Logan W. Methylphenidate hydrochloride given with or before breakfast: II. Effects on plasma concentration of methylphenidate and ritalinic acid. Pediatrics. 1983;72(1):56–59. [PubMed] [Google Scholar]
- 67.Wargin W., Patrick K., Kilts C., Gualtieri C.T., Ellington K., Mueller R.A., Kraemer G., Breese G.R. Pharmacokinetics of methylphenidate in man, rat and monkey. J. Pharmacol. Exp. Ther. 1983;226(2):382–386. [PubMed] [Google Scholar]
- 68.Modi N.B., Lindemulder B., Gupta S.K. Single- and multiple-dose pharmacokinetics of an oral once-a-day osmotic controlled-release OROS (methylphenidate HCl) formulation. J. Clin. Pharmacol. 2000;40(4):379–388. doi: 10.1177/00912700022009080. [DOI] [PubMed] [Google Scholar]
- 69.Modi N.B., Wang B., Hu W.T., Gupta S.K. Effect of food on the pharmacokinetics of osmotic controlled-release methylphenidate HCl in healthy subjects. Biopharm. Drug Dispos. 2000;21(1):23–31. doi: 10.1002/1099-081X(200001)21:1<23::AID-BDD212>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 70.Modi N.B., Wang B., Noveck R.J., Gupta S.K. Dose-proportional and stereospecific pharmacokinetics of methylphenidate delivered using an osmotic, controlled-release oral delivery system. J. Clin. Pharmacol. 2000;40(10):1141–1149. [PubMed] [Google Scholar]
- 71.Wong Y.N., King S.P., Laughton W.B., McCormick G.C., Grebow P.E. Single-dose pharmacokinetics of modafinil and methylphenidate given alone or in combination in healthy male volunteers. J. Clin. Pharmacol. 1998;38(3):276–282. doi: 10.1002/j.1552-4604.1998.tb04425.x. [DOI] [PubMed] [Google Scholar]
- 72.Markowitz J.S., Straughn A.B., Patrick K.S. Advances in the pharmacotherapy of attention-deficit-hyperactivity disorder: focus on methylphenidate formulations. Pharmacotherapy. 2003;23(10):1281–1299. doi: 10.1592/phco.23.12.1281.32697. [DOI] [PubMed] [Google Scholar]
- 73.Froehlich T.E., Epstein J.N., Nick T.G., Melguizo Castro M.S., Stein M.A., Brinkman W.B., Graham A.J., Langberg J.M., Kahn R.S. Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50(11):1129–1139.e2. doi: 10.1016/j.jaac.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greenhill L.L., Abikoff H.B., Arnold L.E., Cantwell D.P., Conners C.K., Elliott G., Hechtman L., Hinshaw S.P., Hoza B., Jensen P.S., March J.S., Newcorn J., Pelham W.E., Severe J.B., Swanson J.M., Vitiello B., Wells K. Medication treatment strategies in the MTA Study: relevance to clinicians and researchers. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35(10):1304–1313. doi: 10.1097/00004583-199610000-00017. [DOI] [PubMed] [Google Scholar]
- 75.Vitiello B., Severe J.B., Greenhill L.L., Arnold L.E., Abikoff H.B., Bukstein O.G., Elliott G.R., Hechtman L., Jensen P.S., Hinshaw S.P., March J.S., Newcorn J.H., Swanson J.M., Cantwell D.P. Methylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTA. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(2):188–196. doi: 10.1097/00004583-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 76.Jaanus S.D. Ocular side effects of selected systemic drugs. Optom. Clin. 1992;2(4):73–96. [PubMed] [Google Scholar]
- 77.Cascade E., Kalali A.H., Wigal S.B. Real-world data on: Attention Deficit Hyperactivity Disorder medication side effects. Psychiatry (Edgmont) 2010;7(4):13–15. [Edgmont]. [PMC free article] [PubMed] [Google Scholar]
- 78.Ghuman J.K., Aman M.G., Lecavalier L., Riddle M.A., Gelenberg A., Wright R., Rice S., Ghuman H.S., Fort C. Randomized, placebo-controlled, crossover study of methylphenidate for attention-deficit/hyperactivity disorder symptoms in preschoolers with developmental disorders. J. Child Adolesc. Psychopharmacol. 2009;19(4):329–339. doi: 10.1089/cap.2008.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rappley M.D. Safety issues in the use of methylphenidate. An American perspective. Drug Saf. 1997;17(3):143–148. doi: 10.2165/00002018-199717030-00001. [DOI] [PubMed] [Google Scholar]
- 80.Firestone P., Musten L.M., Pisterman S., Mercer J., Bennett S. Short-term side effects of stimulant medication are increased in preschool children with attention-deficit/hyperactivity disorder: a double-blind placebo-controlled study. J. Child Adolesc. Psychopharmacol. 1998;8(1):13–25. doi: 10.1089/cap.1998.8.13. [DOI] [PubMed] [Google Scholar]
- 81.Auger R.R., Goodman S.H., Silber M.H., Krahn L.E., Pankratz V.S., Slocumb N.L. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep. 2005;28(6):667–672. doi: 10.1093/sleep/28.6.667. [DOI] [PubMed] [Google Scholar]
- 82.Kraemer M., Uekermann J., Wiltfang J., Kis B. Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature. Clin. Neuropharmacol. 2010;33(4):204–206. doi: 10.1097/WNF.0b013e3181e29174. [DOI] [PubMed] [Google Scholar]
- 83.Cooper W.O., Habel L.A., Sox C.M., Chan K.A., Arbogast P.G., Cheetham T.C., Murray K.T., Quinn V.P., Stein C.M., Callahan S.T., Fireman B.H., Fish F.A., Kirshner H.S., O’Duffy A., Connell F.A., Ray W.A. ADHD drugs and serious cardiovascular events in children and young adults. N. Engl. J. Med. 2011;365(20):1896–1904. doi: 10.1056/NEJMoa1110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Habel L.A., Cooper W.O., Sox C.M., Chan K.A., Fireman B.H., Arbogast P.G., Cheetham T.C., Quinn V.P., Dublin S., Boudreau D.M., Andrade S.E., Pawloski P.A., Raebel M.A., Smith D.H., Achacoso N., Uratsu C., Go A.S., Sidney S., Nguyen-Huynh M.N., Ray W.A., Selby J.V. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673–2683. doi: 10.1001/jama.2011.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arcieri R., Germinario E.A., Bonati M., Masi G., Zuddas A., Vella S., Chiarotti F., Panei P. Italian Attention-Deficit/Hyperactivity Disorder Regional Reference Centers. Cardiovascular measures in children and adolescents with attention-deficit/hyperactivity disorder who are new users of methylphenidate and atomoxetine. J. Child Adolesc. Psychopharmacol. 2012;22(6):423–431. doi: 10.1089/cap.2012.0014. [DOI] [PubMed] [Google Scholar]
- 86.Martinez-Raga J., Knecht C., Szerman N., Martinez M.I. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15–30. doi: 10.1007/s40263-012-0019-9. [DOI] [PubMed] [Google Scholar]
- 87.Aktepe E., Ozkorumak E., Tanriöver-Kandil S. Pregnancy and delivery complications and treatment approach in attention deficit hyperactivity disorder. Turk. J. Pediatr. 2009;51(5):478–484. [PubMed] [Google Scholar]
- 88.Kelly B.D., Lundon D.J., McGuinness D., Brady C.M. Methylphenidate-induced erections in a prepubertal child. J. Pediatr. Urol. 2013;9(1):e1–2. doi: 10.1016/j.jpurol.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 89.Cakin-Memik N., Yildiz O., Sişmanlar S.G., Karakaya I., Ağaoğlu B. Priapism associated with methylphenidate: a case report. Turk. J. Pediatr. 2010;52(4):430–434. [PubMed] [Google Scholar]
- 90.Eiland L.S., Bell E.A., Erramouspe J. Priapism associated with the use of stimulant medications and atomoxetine for attention-deficit/hyperactivity disorder in children. Ann. Pharmacother. 2014;48(10):1350–1355. doi: 10.1177/1060028014541791. [DOI] [PubMed] [Google Scholar]
- 91.Tong H.Y., Díaz C., Collantes E., Medrano N., Borobia A.M., Jara P., Ramírez E. Liver transplant in a patient under methylphenidate therapy: a case report and review of the literature. Case Rep. Pediatr. 2015;2015:437298. doi: 10.1155/2015/437298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Franke A.G., Bagusat C., Rust S., Engel A., Lieb K. Substances used and prevalence rates of pharmacological cognitive enhancement among healthy subjects. Eur. Arch. Psychiatry Clin. Neurosci. 2014;264(Suppl. 1):S83–S90. doi: 10.1007/s00406-014-0537-1. [DOI] [PubMed] [Google Scholar]
- 93.Erowid Experience Vaults. Pharms - Methylphenidate Reports. Available from: https://www.erowid.org/experiences/subs/exp_Pharms_Methylphenidate.shtml . [Accessed Mar 24, 2015].
- 94.U.S. Department of Justice. METHYLPHENIDATE (Trade Names: Ritalin- (IR, LA, and SR), Concerta, Metadate- (CD and ER), Methylin- (IR and ER) and Focalin- (IR and ER)). Drug Enforcement Administration - Office of Diversion Control - Drug & Chemical Evaluation Section. Available from: http://www.deadiversion.usdoj.gov/drug_chem_info/methylphenidate.pdf . 2013. [Accessed Mar 24, 2015].
- 95.Rioux B. Is ritalin an addiction-producing drug? Dis. Nerv. Syst. 1960;21:346–349. [PubMed] [Google Scholar]
- 96.Spensley J., Rockwell D.A. Psychosis during methylphenidate abuse. N. Engl. J. Med. 1972;286(16):880–881. doi: 10.1056/NEJM197204202861607. [DOI] [PubMed] [Google Scholar]
- 97.Lucas A.R., Weiss M. Methylphenidate hallucinosis. Jama. 1971;217(8):81–1079. [PubMed] [Google Scholar]
- 98.Bloom A.S., Russell L.J., Weisskopf B., Blackerby J.L. Methylphenidate-induced delusional disorder in a child with attention deficit disorder with hyperactivity. J. Am. Acad. Child Adolesc. Psychiatry. 1988;27(1):88–89. doi: 10.1097/00004583-198801000-00013. [DOI] [PubMed] [Google Scholar]
- 99.Corrigall R., Ford T. Methylphenidate euphoria. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35(11):1421. doi: 10.1097/00004583-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 100.Willey R.F. Abuse of methylphenidate (Ritalin). N. Engl. J. Med. 1971;285(8):464. doi: 10.1056/NEJM197108192850819. [DOI] [PubMed] [Google Scholar]
- 101.Parran T.V., Jr, Jasinski D.R. Intravenous methylphenidate abuse. Prototype for prescription drug abuse. Arch. Intern. Med. 1991;151(4):781–783. doi: 10.1001/archinte.1991.00400040119027. [DOI] [PubMed] [Google Scholar]
- 102.Grund T., Lehmann K., Bock N., Rothenberger A., Teuchert-Noodt G. Influence of methylphenidate on brain development--an update of recent animal experiments. Behav. Brain Funct. 2006;2:2. doi: 10.1186/1744-9081-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Outram S.M. The use of methylphenidate among students: the future of enhancement? J. Med. Ethics. 2010;36(4):198–202. doi: 10.1136/jme.2009.034421. [DOI] [PubMed] [Google Scholar]
- 104.Teter C.J., McCabe S.E., LaGrange K., Cranford J.A., Boyd C.J. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26(10):10–1501. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnston L.D., O'Malley P.M., Bachman J.G., Schulenberg J.E. Monitoring the future: national survey results on drug use, 1975- 2003. Volume II. College students and adults ages. 2003:19–45. doi: 10.1080/07448480009596296. US Department of Health and Human Services. [DOI] [Google Scholar]
- 106.Babcock Q., Byrne T. Student perceptions of methylphenidate abuse at a public liberal arts college. J. Am. Coll. Health. 2000;49(3):143–145. doi: 10.1080/07448480009596296. [DOI] [PubMed] [Google Scholar]
- 107.Johnston L.D., O’Malley P.M., Bachman J.G., Schulenberg J.E. Monitoring the future: national survey results on drug use, 1975 - 2006. Vol. II. College students and adults ages. 2007:19–45. National Institutes of Health. U.S. Department of Health and Human Services. [Google Scholar]
- 108.Teter C.J., McCabe S.E., Boyd C.J., Guthrie S.K. Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy. 2003;23(5):609–617. doi: 10.1592/phco.23.5.609.34187. [DOI] [PubMed] [Google Scholar]
- 109.White B.P., Becker-Blease K.A., Grace-Bishop K. Stimulant medication use, misuse, and abuse in an undergraduate and graduate student sample. J. Am. Coll. Health. 2006;54(5):261–268. doi: 10.3200/JACH.54.5.261-268. [DOI] [PubMed] [Google Scholar]
- 110.Volkow N.D., Ding Y.S., Fowler J.S., Wang G.J., Logan J., Gatley J.S., Dewey S., Ashby C., Liebermann J., Hitzemann R., Wolf A.P. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch. Gen. Psychiatry. 1995;52(6):456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- 111.Jaffe S.L. Intranasal abuse of prescribed methylphenidate by an alcohol and drug abusing adolescent with ADHD. J. Am. Acad. Child Adolesc. Psychiatry. 1991;30(5):773–775. doi: 10.1097/00004583-199109000-00012. [DOI] [PubMed] [Google Scholar]
- 112.Levine B., Caplan Y.H., Kauffman G. Fatality resulting from methylphenidate overdose. J. Anal. Toxicol. 1986;10(5):209–210. doi: 10.1093/jat/10.5.209. [DOI] [PubMed] [Google Scholar]
- 113.Clemow D.B., Walker D.J. The potential for misuse and abuse of medications in ADHD: a review. Postgrad. Med. 2014;126(5):64–81. doi: 10.3810/pgm.2014.09.2801. [DOI] [PubMed] [Google Scholar]
- 114.Shanks R.A., Ross J.M., Doyle H.H., Helton A.K., Picou B.N., Schulz J., Tavares C., Bryant S., Dawson B.L., Lloyd S.A. Adolescent exposure to cocaine, amphetamine, and methylphenidate cross-sensitizes adults to methamphetamine with drug- and sex-specific effects. Behav. Brain Res. 2015;281:116–124. doi: 10.1016/j.bbr.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 115.Pauly V., Lapeyre-Mestre M., Braunstein D., Rueter M., Thirion X., Jouanjus E., Micallef J. Detection of signals of abuse and dependence applying disproportionality analysis. Eur. J. Clin. Pharmacol. 2015;71(2):229–236. doi: 10.1007/s00228-014-1783-x. [DOI] [PubMed] [Google Scholar]
- 116.Viggiano D., Vallone D., Sadile A. Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling. Neural. Plast. 2004;11(1-2):97–114. doi: 10.1155/NP.2004.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller G.M. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. 2011;116(2):164–176. doi: 10.1111/j.1471-4159.2010.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Markowitz J.S., DeVane C.L., Pestreich L.K., Patrick K.S., Muniz R. A comprehensive in vitro screening of d-, l-, and dl-threo-methylphenidate: an exploratory study. J. Child Adolesc. Psychopharmacol. 2006;16(6):687–698. doi: 10.1089/cap.2006.16.687. [DOI] [PubMed] [Google Scholar]
- 119.Mehta M.A., Sahakian B.J., Mavaddat N., Pickard J.D., Robbins T.W., Solanto M.V., Arnstenand A.F., Castellanos F.X. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience, Comparative psychopharmacology of methylphenidate and related drugs in human volunteers, patients with ADHD and experimental animals. New York: Oxford University Press; 2001. pp. 303–331. [Google Scholar]
- 120.Mehta M.A., Owen A.M., Sahakian B.J., Mavaddat N., Pickard J.D., Robbins T.W. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J. Neurosci. 2000;20(6):RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aron A.R., Dowson J.H., Sahakian B.J., Robbins T.W. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2003;54(12):1465–1468. doi: 10.1016/S0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 122.Lee M.J., Yang P.B., Wilcox V.T., Burau K.D., Swann A.C., Dafny N. Does repetitive Ritalin injection produce long-term effects on SD female adolescent rats? Neuropharmacology. 2009;57(3):201–207. doi: 10.1016/j.neuropharm.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 123.Urban K.R., Waterhouse B.D., Gao W.J. Distinct age-dependent effects of methylphenidate on developing and adult prefrontal neurons. Biol. Psychiatry. 2012;72(10):880–888. doi: 10.1016/j.biopsych.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hart H., Radua J., Nakao T., Mataix-Cols D., Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 125.Spencer T.J., Brown A., Seidman L.J., Valera E.M., Makris N., Lomedico A., Faraone S.V., Biederman J. Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of MRI-based neuroimaging studies. J. Clin. Psychiatry. 2013;74(9):902–917. doi: 10.4088/JCP.12r08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Frodl T., Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr. Scand. 2012;125(2):114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- 127.Huang Y.S., Tsai M.H. Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge. CNS Drugs. 2011;25(7):54–539. doi: 10.2165/11589380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 128.Bolaños C.A., Barrot M., Berton O., Wallace-Black D., Nestler E.J. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol. Psychiatry. 2003;54(12):1317–1329. doi: 10.1016/S0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- 129.Ouellette E.M. Legal issues in the treatment of children with attention deficit hyperactivity disorder. J. Child Neurol. 1991;6(Suppl.):S68–S75. doi: 10.1177/0883073891006001s08. [DOI] [PubMed] [Google Scholar]
- 130.Clavenna A., Bonati M. Safety of medicines used for ADHD in children: a review of published prospective clinical trials. Arch. Dis. Child. 2014;99(9):866–872. doi: 10.1136/archdischild-2013-304170. [DOI] [PubMed] [Google Scholar]
- 131.Bihlar M.B., Jokinen J., Bolte S., Hirvikoski T. Long-term outcomes of pharmacologically treated versus non-treated adults with ADHD and substance use disorder: a naturalistic study. J. Subst. Abuse Treat. 2014;51:82–90. doi: 10.1016/j.jsat.2014.11.005. pii: S0740-5472(14)00230-X. [DOI] [PubMed] [Google Scholar]
- 132.Abel K.F., Bramness J.G., Martinsen E.W. Stimulant medication for ADHD in opioid maintenance treatment. J. Dual Diagn. 2014;10(1):32–38. doi: 10.1080/15504263.2013.867657. [DOI] [PubMed] [Google Scholar]
- 133.Lakhan S.E., Hagger-Johnson G.E. The impact of prescribed psychotropics on youth. Clin. Pract. Epidemol Ment. Health. 2007;3:21. doi: 10.1186/1745-0179-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Frati P., Kyriakou C., Del Rio A., Marinelli E., Vergallo G.M., Zaami S., Busardò F.P. Smart drugs and synthetic androgens for cognitive and physical enhancement: revolving doors of cosmetic neurology. Curr. Neuropharmacol. 2015;13(1):5–11. doi: 10.2174/1570159X13666141210221750. [DOI] [PMC free article] [PubMed] [Google Scholar]


