Tregs generated in BCG-vaccinated mice focus on dampening immunity rather than controlling immunopathology.
Keywords: Tregs, BCG vaccination, inflammation
Abstract
Increasing information has shown that many newly emerging strains of Mycobacterium tuberculosis, including the highly prevalent and troublesome Beijing family of strains, can potently induce the emergence of Foxp3+ CD4 Tregs. Although the significance of this is still not fully understood, we have previously provided evidence that the emergence of this population can significantly ablate the protective effect of BCG vaccination, causing progressive fatal disease in the mouse model. However, whether the purpose of this response is to control inflammation or to directly dampen the acquired immune response is still unclear. In the present study, we have shown, using both cell depletion and adoptive transfer strategies, that Tregs can have either properties. Cell depletion resulted in a rapid, but transient, decrease in the lung bacterial load, suggesting release or temporary re-expansion of effector immunity. Transfer of Tregs into Rag2−/− or marked congenic mice worsened the disease course and depressed cellular influx of effector T cells into the lungs. Tregs from infected donors seemed to preferentially depress the inflammatory response and granulocytic influx. In contrast, those from BCG-vaccinated and then challenged donors seemed more focused on depression of acquired immunity. These qualitative differences might be related to increasing knowledge reflecting the plasticity of the Treg response.
Introduction
More than 2 million new cases of disease caused by Mycobacterium tuberculosis are diagnosed annually, with >8 million deaths [1, 2]. The incidence of multidrug resistant strains is clearly increasing and has been estimated to now be in excess of 650,000 cases annually [3–5]. Tuberculosis has now supplanted other infections and is the predominant opportunistic infection in people who are HIV-positive. Thus, during the past 2 decades, a substantial effort has been made to develop more effective vaccines against tuberculosis, with several promising candidates forming a pipeline [6, 7] and 1 candidate reaching a phase IIb efficacy trial [8]. Although not successful, this was still a major achievement.
In terms of vaccine screening, however, concern exists that most candidates will be tested using short-term assays in which they are challenged using laboratory-adapted strains of M. tuberculosis (H37Rv or Erdman). Laboratory-adapted strains were isolated >60 years ago and have been passaged many times since. Thus, they do not seem to possess the virulence of the newly emerging clinical strains, such as the highly prevalent Beijing strains [9–11]. As we have previously shown [12, 13], some of these latter strains are potent inducers of CD4+Foxp3+ Tregs, the numbers of which increase in the lungs of infected mice over time. If the mouse is first vaccinated with BCG, accelerated acquired immunity is generated; however, this merely delays the emergence of the Treg response, followed by a loss of protection and progressive pulmonary bacterial growth. Thus, the capacity of a given isolate to induce this response could be a serious impediment to vaccine efficacy.
Our earlier studies of this phenomenon demonstrated the kinetics of the Treg response and showed that it was a potent event in both mice [13] and guinea pigs [14, 15] infected with several emerging clinical strains. It was not clear, however, whether the Treg response was directed at the effector T cell response or simply induced with the purpose of dampening the damaging inflammatory response in the lungs of mice infected with these virulent strains of M. tuberculosis. Treg infiltration in pulmonary granulomas has been reported, although their role is still not completely understood [16, 17]. Moreover, depletion of Tregs during early infection, as well as before and after BCG vaccination, reduces the lung bacterial burden; however, Tregs are also required to control inflammatory immunopathology during chronic infection [18–20]. These results suggest that Tregs have a dual role in the immune response to tuberculosis. That is, they can restrict the protective T cell response and also can act as regulators of the inflammatory response to abate tissue damage to the host. Therefore, the purpose of the present study was to evaluate the role of Foxp3+ T cells generated after M. tuberculosis infection on previously BCG immunized or nonimmunized mice.
To address this, in a holistic way, we decided to perform both depletion and adoptive transfer of Foxp3 T cells generated under immunized and nonimmunized infected mice. For the depletion studies, we used 2 strategies: the first, depletion of CD25hi cells by infusion of monoclonal antibody; and the second, by selectively depleting Foxp3+ cells using mice in which Foxp3 expression is linked to expression of the DTR. In both cases, cell depletion reduced the bacterial load in the lungs, suggesting release or re-expansion of acquired immunity. However, this effect was transient and resulted in mortality. In further studies, we used high-speed cell sorting to purify CD25hiFoxp3+ CD4 T cells from mice during the peak of acquired immunity, in which Tregs are the predominant population, and used these for adoptive transfer studies. When intravenously transferred into Rag2−/− mice, a significant number of these cells returned to the lungs and a major population expressed IL-10. The group of mice showing worsening bacterial burden, pulmonary pathology, and effector immunity were the recipients of CD25hiFoxp3+ cells from BCG-vaccinated M. tuberculosis-exposed mice.
Having shown that such cells could return to the lungs, we then found similar results when CD25hiFoxp3+ cells were transferred into marked congenic mice. Again, the recipients of the CD25hiFoxp3+ cells from BCG-vaccinated M. tuberculosis-exposed mice demonstrated an increased bacterial burden, and the influx of IFN-γ+ effector cells was diminished. Finally, because most lesions in these mice, who had received cells from BCG-vaccinated and M. tuberculosis-infected donors, became necrotic, we also measured the influx of Gr-1+ cells. Interestingly, it appeared that Tregs harvested from M. tuberculosis-infected donors significantly reduced the influx of both Gr-1hi and Gr-1int cells during chronic infection. However, the recipient mice of the CD25hiFoxp3+ cells harvested from BCG-vaccinated and M. tuberculosis-infected donors resulted in increased levels of both Gr-1hi and Gr-1int cells during chronic disease. Collectively, our observations raise the intriguing possibility that Tregs from the 2 sources might have qualitative differences. Those from the BCG-vaccinated and M. tuberculosis-infected donors were more directed toward dampening the effector T cell response, given that they become induced in mice with a substantial protective effector T cell response engendered by the BCG vaccine. In contrast, those from the nonvaccinated group were directed more toward suppression of the granulocytic response.
MATERIALS AND METHODS
Animals
The C57BL/6, B6.PL-Thy1a/CyJ and Rag2−/− mice, aged 6–8 wk, were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Foxp3gfp/DTR transgenic mice were a gift from Dr. A. Rudensky (Howard Hughes Medical Institute and Immunology Program, Memorial Sloan-Kettering Cancer Center, New York, NY, USA). The mice were maintained in a BSL-3 facility at Colorado State University and had ad libitum access to water and chow. They were kept under barrier conditions in an ABL-III laboratory and fed sterile water and chow. The animal care and usage committee of Colorado State University approved all experimental protocols.
Experimental infections
W-Beijing M. tuberculosis strains HN878 or SA161 were grown in Proskauer-Beck liquid medium containing 0.05% Tween 80 to midlog phase and then frozen in aliquots at −70°C until needed. For low-dose aerosol infections, bacterial stocks were diluted in 5 ml of sterile distilled water to 2 × 106 CFU/ml and placed in a nebulizer attached to an airborne infection system (Glass-Col, Terre Haute, IN, USA) [21, 22]. The mice were exposed to an aerosol infection in which approximately 100 bacteria were deposited in the lungs of each mouse. In our experiments, we used HN878 or SA161, because they both induced similar results in our mode [13]. This established a chronic disease in the range of 5.0–5.6 log10 bacilli in the lungs during the course of the study. In the vaccination studies, the mice were immunized with 106 CFU BCG Pasteur subcutaneously.
Preparation of cells
The mice were euthanized by CO2 asphyxiation, and the thoracic cavity was opened. The lung was cleared of blood by perfusion through the pulmonary artery with 10 ml of ice-cold PBS containing 50 U/ml heparin (Sigma-Aldrich, St. Louis, MO, USA). The lungs were aseptically removed, teased apart, and treated with a solution of DNase IV (30 μg/ml; Sigma-Aldrich) and collagenase XI (0.7 mg/ml; Sigma-Aldrich) for 30 min at 37°C. Erythrocytes were lysed with Gey's solution (0.15 M NH4Cl, 10 mM HCO3), and the cells were washed with Dulbecco's modified Eagle's minimal essential medium. The total cell numbers were determined by flow cytometry using liquid counting beads (BD Pharmingen, San Jose, CA, USA), as described by the manufacturer.
Flow cytometry for surface markers and intracellular cytokines
For flow cytometry analysis, single-cell suspensions of lung from each mouse were resuspended in PBS (Sigma-Aldrich) containing 0.1% sodium azide. Fc receptors were blocked with purified anti-mouse CD16/32. The cells were incubated in the dark for 25 min at 4°C with predetermined optimal titrations of specific antibodies. Cell surface expression was analyzed for CD44, Thy1.1, CD4, CD62L, CD127, CD11b, CD11c, and Gr-1. All antibodies and reagents were purchased from BD Pharmingen or eBioscience (San Diego, CA, USA). The samples were analyzed using a Becton Dickinson LSR-II instrument (Franklin Lakes, NJ, USA), and data were analyzed using FACSDiva, version 7.0, software. Individual cell populations were identified according to the presence of specific fluorescence-labeled antibodies. All the analyses were performed with acquisition of a minimum of 300,000 events [23].
Intracellular cytokine staining
The cells were initially stimulated for 4 h at 37°C with a 1× cell stimulation cocktail (eBioscience) diluted in complete DMEM. Thereafter, the cells were stained for cell surface markers, as indicated, fixed, and permeabilized using the instructions provided by the manufacturer of the Fix/Perm and Perm wash kit (eBioscience). Thereafter, the cells were incubated for 30 min at 4°C with FcBlock plus anti–IL-10 (clone JES5-16E3; eBioscience), anti–IFN-γ (clone XMG1.2; eBioscience), and anti-Foxp3 (clones FJK-16s and NRRF-30; eBioscience) or with the respective isotype control. Data acquisition and analysis was performed as described.
Cell sorting
To sort CD4+CD25hiCD127− and CD4+CD25− cells, single cell suspensions were obtained from the lungs of the infected mice as described. An insufficient number of cells were sorted from naïve mice; thus, it was not possible to use this control. After incubating with 5 μg/ml Fc block (eBioscience) for 20 min at 4°C, the cells were stained with APC-labeled anti-CD4 (clone GK1.5; eBioscience), PeCy7-labeled anti-CD25 (clone PC61.5; eBioscience), and Alexa 700-labeled CD127 (clone A7R43; eBioscience), as described. Thereafter, the cells were stained with 0.5 μg/ml 7-AAD (eBioscience) for 5 min and sorted on a FACS Aria III (BD Biosciences) with a 70-μm nozzle, using the following strategy. Doublets were gated out by FSC-A vs. FSC-H and 7-AAD+ dead cells were excluded from the singlet population using FL3. Thereafter, live CD4+CD25hiCD127− or CD4+CD25− were individually sorted.
Anti-CD25 and diphtheria toxin treatment
CD25+ cells were depleted by intraperitoneal injection of 100 μg of anti-CD25 on d 33, 43, and 53 after infection. Depletion of Foxp3+ cells in Foxp3gfp/DTR mice was performed by intraperitoneal injection of diphtheria toxin (Merck, Readington Township, NJ, USA) at 50 mg/kg on d 28 and 30 after infection, followed by 10 mg/kg at d 32 and 36 after infection.
Histopathologic evaluation and lesion burden determination
Lung lobes were fixed in 4% buffered paraformaldehyde. The tissues were embedded in paraffin, and 5-μm sections were stained with H&E for histopathologic evaluation using routine methods. Standardized sampling of the lung was performed by midsagittal sectioning of the right caudal lung lobe from each mouse at a predetermined anatomic location, irrespective of the presence of visible lung lesions. Morphometric analysis was performed using an Eclipse 80i microscope (Nikon Instruments, Melville, NY, USA) and Stereo Investigator software, version 10.02 (MBF Bioscience, Williston, VT, USA), with the tissue area and lesion area estimated using the area fraction fractionator method and expressed as a percentage ratio of the lesion to total tissue area, as previously described [24].
Immunohistochemistry and image analysis
Staining was performed using the Leica Bond III fully automated immunohistochemical stainer with Gr-1 at 1:50 dilution, AP-conjugated anti-rat at 1:50 dilution, and Fast Red chromogen detection reagent. The slides were counterstained with hematoxylin. For image analysis, images were acquired at 400× magnification using an Olympus BX50 microscope equipped with an Olympus DP70 camera (Olympus, Center Valley, PA, USA) and then calibrated for size according to the number of pixels per nm2. Image analysis was performed using Nikon NIS Elements software (Nikon Instruments). Images from each of 4 groups and an isotype control from group 3 were subjected to white subtraction, and then thresholds were set for detection of Gr-1 immunoreactivity based on Fast Red chromogen staining. The thresholds for red, green, and blue intensity were set at 14–107, 0, and 7–72, respectively. The area of tissue with detected Fast Red reactivity was quantified and is expressed as a ratio of the total area captured in each image. The areas quantified were compared with background threshold detection according to the isotype control.
Statistical analysis
Statistical significance was determined using 2-way ANOVA with Bonferroni post tests using GraphPad Prism, version 4.00, for Windows (GraphPad Software, San Diego, CA, USA).
RESULTS
Effect of depletion of CD25hi T cells by monoclonal antibody on disease course
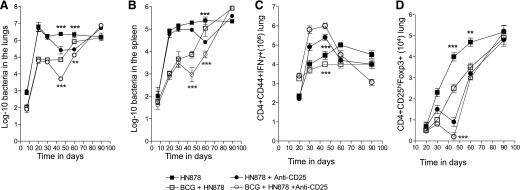
Previous studies by our group [12, 13] demonstrated that after W-Beijing M. tuberculosis infection, CD4+ Foxp3+ Tregs increase in the lungs of infected mice over time. If the mouse has been vaccinated with BCG and exposed to a W-Beijing M. tuberculosis strain, the BCG-induced efficacy wanes during chronic infection. However, BCG vaccination and exposure with a laboratory adoptive strain (H37RV) results in BCG vaccine-derived efficacy. Thus, experiments to ablate the Treg response were conducted to further understand the role of Tregs in BCG-induced immunity during infection. As shown in Fig. 1A and B, mice infected by low-dose aerosol exposure with the highly virulent strain HN878 were able to contain the infection after 30 d of the infection. In contrast, mice previously vaccinated with BCG were initially substantially protected at d 30; however, by d 60, this protection was lost in the lungs and spleen. This is consistent with earlier results from our laboratory [13]. After 33 d of infection, the groups of mice were infused via the intraperitoneal route with 100 μg of anti-CD25 mAb, which was given every 10 d for 30 d. Initially, this treatment resulted in a ≥10-fold reduction in the bacterial load (P = 0.006) for both vaccinated and nonvaccinated groups, indicating it had increased or promoted acquired immunity. However, this effect was quickly lost—18 d after anti-CD25 treatment was stopped. By d 70 of the infection, no differences could be seen between the groups (Fig. 1A). The beneficial effects of BCG vaccination were also lost in the spleen (Fig. 1B). It was also noted that the course of the infection in the infected mice retained a chronic appearance in terms of CFU levels; however, depletion in the BCG-vaccinated mice caused a progressive increase in CFU of approximately 3.5-log from d 40 to 90.
Figure 1. Effects of CD25 depletion on the course of infections in C57BL/6 mice.
Course of infection in the lungs (A) and spleens (B) of mice infected by low-dose aerosol with the Beijing strain HN878. Bacterial numbers are shown in nonvaccinated (solid symbols)] or BCG-vaccinated mice (open symbols). The effects of administration of monoclonal anti-CD25 antibody is shown (circles) compared with saline controls (squares). Data shown as log10 mean values ± sem (n = 5). (C) Treatment with monoclonal anti-CD25 temporarily increased total CD4+CD44+IFN-γ+ cell numbers in the lungs, parallel with the reduction in total CD4+CD25hiFoxp3+ cells (D). Statistical analysis performed for each group, nonvaccinated treated compared with nonvaccinated, nontreated (solid symbols). BCG-vaccinated groups were analyzed separately (open symbols). The differences in bacterial counts and cell numbers were analyzed at each specified time point. Only statistically significant results are shown. Statistical analysis using 2-way ANOVA (GraphPad Prism). **P < 0.010, ***P < 0.001.
To investigate the probable cause of such changes, we analyzed the temporal changes in IFN-γ+-producing cells and in Foxp3+ cells in the lungs. Decreased bacterial loads coincided with a steep increase in CD4+CD44+IFN-γ+ total cells and a temporary decrease in CD4+CD25hiFoxp3+ cells in the lungs of the treated mice (Fig. 1C and D).
Targeted depletion of Foxp3+ Tregs during the disease course
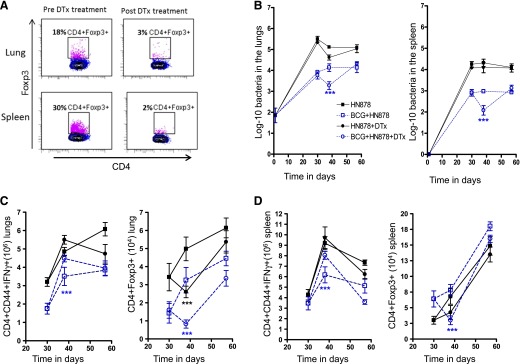
Because treatment with anti-CD25 antibodies can also target early activated T and B cells, in our next study, we used a more specific approach to test the hypothesis that removal of Foxp3+ Tregs would increase acquired immunity and promote antimicrobial immunity. To test this hypothesis, we repeated our earlier studies using C57BL/6 mice in which expression of the Foxp3 gene is directly linked to the DTR, as described previously [18]. Because Foxp3+ cells in these mice constitutively express this receptor, they can be specifically eliminated by intraperitoneal injection of the toxin. In the studies shown in Fig. 2A, depletion of Foxp3+ cells in Foxp3gfp/DTR mice was performed by intraperitoneal injection of diphtheria toxin at 50 mg/kg on d 28 and 30 after infection, followed by 10 mg/kg at d 32 and 36 after infection. As a result of this treatment, the numbers of Foxp3+ cells in the lungs and spleen on d 28 were reduced from 18% and 30% to 3% and 2% on d 40, respectively. The outcome of this treatment in relation to bacterial burden is shown in Fig. 2B. Within 7–10 d, in the mice that had been vaccinated first with BCG, the bacterial load in the toxin-depleted mice had reduced by 10-fold; however, similar to the results of CD25-targeted depletion, this protective effect was transient, and this benefit was completely lost by d 55. In contrast, in mice that were not BCG vaccinated, the effect of Foxp3+ cell depletion on CFU levels was minor and not significant. However, the prohibitive cost of this model abrogated the evaluation of later time points.
Figure 2. Effects of targeted depletion of Foxp3+ cells.
Foxp3+ T cells expressing the “knocked-in” DTR (Foxp3gfp/DTR) were specifically targeted by intraperitoneal injection of diphtheria toxin at 50 mg/kg on d 28 and 30 after infection, followed by 10 mg/kg at d 32 and 36 after infection. (A) Flow cytometric dot plots demonstrate that this reduced the numbers of Foxp3+ cells on d 40 down to significantly lower levels (P = 0.0091) in M. tuberculosis-infected animals. (B) The effects of this depletion on the course of low-dose aerosol with the Beijing strain HN878. Bacterial numbers are shown in M. tuberculosis-infected mice (solid symbols, black) and BCG-vaccinated mice (open symbols, blue). The effects of administration of diphtheria toxin are shown (circles) compared with saline controls (squares). Data are shown as log10 mean values ± sem (n = 5). Depletion of Foxp3+ cells by administration of diphtheria toxin significantly increased the numbers of CD4+CD44+IFN-γ+ cells in lungs (C, left) and spleens (D, left) in BCG-vaccinated mice. CD4+Foxp3+ cell numbers significantly decreased in the lungs of both groups injected with diphtheria toxin (C, right), although in the spleens, only the BCG-vaccinated group showed a significant reduction (D, right). Statistical analysis was performed for each group, nonvaccinated treated compared with nonvaccinated, nontreated (black). BCG-vaccinated groups were analyzed separately (blue). The differences in bacterial counts and cell numbers were analyzed at each specified time point; only statistically significant results are shown. Statistical analysis using 2-way ANOVA (GraphPad Prism), ***P < 0.001, or Mann-Whitney U test, **P < 0.01.
For this experiment, we evaluated the total number of Foxp3+ and the IFN-γ producing cells in the lungs and spleens of mice treated with diphtheria toxin compared with controls (Fig. 2C and D). A temporary increase in CD4+CD44+-IFN-γ+ cells was only observed in the diphtheria toxin-treated mice previously vaccinated with BCG (Fig. 2C, left, and 2D, left). However, treatment with diphtheria toxin induced a significant reduction in CD4+Foxp3+ cell numbers in both groups, BCG-vaccinated and BCG-unvaccinated mice (Fig. 2C, right).
Adoptive transfer studies using CD25hiFoxp3+ purified CD4 T cells
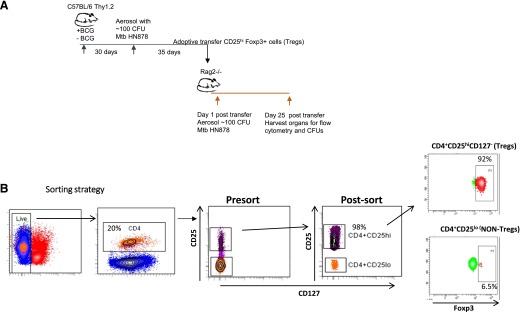
A second approach that we used to investigate the effect of BCG vaccination on Tregs was adoptive cell transfer. In a first series of studies, we addressed the issue of whether CD25hiFoxp3+ cells harvested from mice at a time at which Tregs predominated could migrate back to the lungs after intravenous transfer. As shown in Fig. 3A, we obtained CD25hiFoxp3+ cells from both M. tuberculosis-infected and BCG-vaccinated and then infected donors. In each group, we obtained 98% purity after cell sorting (Fig. 3B). We then infused 1 × 106 of these cells into each Rag2−/− mouse that were then infected with the virulent Beijing strain SA161. For comparison, we transferred CD25loFoxp3− cells from the same 2 groups of mice.
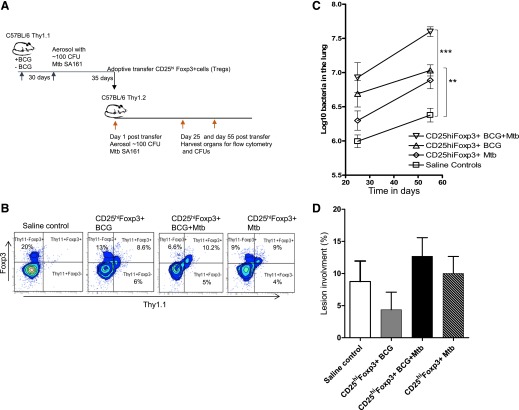
Figure 3. Adoptive transfer of sorted cells into Rag2−/− mice.
(A) The experimental design is shown using C57BL/6 mice as the source of donor cells. Cells were sorted on d 35 of the infection in the C57BL/6 donors, then 1 × 106 cells were transferred into Rag2−/− recipients that were then infected with SA161. (B) The gating strategy for cell sorting was as follows: dead cells were gated out after staining with 7AAD, and then CD4+ cells stained with anti-CD25 and anti-CD127. After high-speed sorting, highly purified CD25hiCD127− cells were obtained (98%), with 92% of them expressing Foxp3+. Cell populations were harvested from infected donors and donors that had been BCG vaccinated and then M. tuberculosis infected; their Foxp3 percentage was 92%.
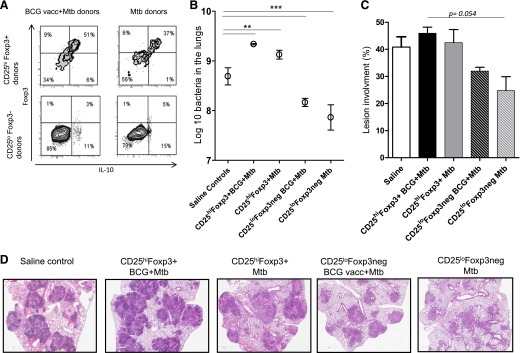
The results of the analysis of the cells in the lungs on d 25 of the infection in the Rag2−/− recipients is shown in Fig. 4A. Large numbers of Foxp3+ cells could be found in the lungs at this time, some of which were also positive for IL-10. The mice transferred with CD25hiFoxp3+ cells from BCG-vaccinated M. tuberculosis-infected donors had significantly higher percentages of these cells 25 d after adoptive transfer (P = 0.0079) compared with recipients of CD25hiFoxp3+ cells from M. tuberculosis-infected donors. Importantly, the high susceptibility of immunocompromised Rag2−/− mice was further aggravated in recipients of CD25hiFoxp3+ cells, as evidenced by a higher bacterial burden than in saline controls (Fig. 4B). In contrast, mice infused with CD25loFoxp3− cells had significant reductions in the bacterial load (Fig. 4B), indicating that some degree of protective T cell activity remained in the CD25loFoxp3− purified subset. At the histologic level, the lungs of the saline controls and recipients infused with CD25hiFoxp3+ cells from BCG-vaccinated M. tuberculosis-infected mice were dominated by very large necrotic lesions full of very dark blue staining granulocytes forming circular abscess-like structures (Fig. 4D), less evident in recipients of CD25hiFoxp3+ cells form M. tuberculosis-exposed animals. Smaller lesions and less necrosis was seen in mice receiving CD25loFoxp3− cells, consistent with the reduction in CFU levels seen above; however, the percentage of lesion involvement was not statistically significant (P = 0.054; Fig. 4C). Because Rag2−/− mice are highly susceptible to M. tuberculosis infection and develop severe necrotic lesions after M. tuberculosis infection [25–27], we decided to further evaluate the effects of the CD25hiFoxp3+ cells originated under different conditions using immunocompetent mice as described in the subsequent sections.
Figure 4. Bacterial load and lung pathology of Rag2−/− recipients.
(A) Flow cytometry contour dot plots showing the dual expression of CD25hiFoxp3+ and Foxp3− populations sorted. Mice transferred with CD25hiFoxp3+ cells from BCG-vaccinated M. tuberculosis-infected (BCG vacc+Mtb) donors had significantly higher percentages of these phenotype of cells 25 d after adoptive transfer and aerosol infection (0.0079). (B) Bacterial loads in the lungs of recipient mice (log10 mean values ± sem (n = 5). Recipient mice of CD25hiFoxp3+CD4+ cells from BCG-vaccinated and M. tuberculosis-exposed mice had significant increases in bacterial burden, followed by the recipients of CD25hiFoxp3+CD4+ cells from M. tuberculosis-exposed mice compared with saline controls. In contrast, the mice receiving the CD25loFoxp3− cells from both BCG-vaccinated and M. tuberculosis-infected and M. tuberculosis-exposed mice still retained some protective activity, denoted by reduced bacterial burdens. (C) Lung lesion burden was increased in recipient mice of CD25hiFoxp3+CD4+ cells from any donor compared with mice receiving CD25loFoxp3− cells. (D) H&E staining of harvested lungs (10× magnification). Saline controls and recipients of CD25hiFoxp3+ cells from BCG-vaccinated then M. tuberculosis-infected donors had increased granuloma size, indicative of worsening clinical outcomes, and severe abscess-like formation containing granulocytes and severe necrosis. This was less severe in mice given CD25hiFoxp3+ cells from M. tuberculosis-infected donors and in mice given CD25loFoxp3− cells from the BCG-vaccinated and M. tuberculosis-infected and M. tuberculosis-exposed mice. Statistical analysis using 2-way ANOVA (GraphPad Prism), **P < 0.010, ***P < 0.001, or Mann-Whitney U test, **P < 0.010.
Adoptive transfer of Tregs into congenic recipient
Having established that Tregs acquired in response to M. tuberculosis SA161 infection could return to the lungs after cell transfer, we repeated these studies in immunocompetent mice, using Thy-1.1 congenic mice as the source of the donor cells. These were sorted as above on d 35 of the infection in the donors. Next, 1 × 106 cells were transferred into recipients that were then infected with M. tuberculosis SA161 (Fig. 5A). Importantly, transferred CD25hiFoxp3+ T cells were present in comparable percentages in the Thy-1.2 recipient mice 55 d after adoptive transfer (Fig. 5B).
Figure 5. Cell transfer studies using congenic recipients.
(A) Thy-1.1 congenic mice were the source of donor cells. These were sorted as above on d 35 of the infection in the donors, then 1 × 106 cells were transferred into recipients that were then infected with SA161. (B) Percentages of transferred Thy1.1+CD25hiFoxp3+ T cells present in the lungs of Thy-1.2 recipient mice 55 d after adoptive transfer from different sources. (C) Bacterial loads in the lungs of recipient Thy-1.2 mice on d 25 and 55 of infection (log10 mean values ± sem; n = 5). Cells from M. tuberculosis-infected mice resulted in higher CFU levels than in control recipients at d 55 after transfer, but CD25hiFoxp3+ cells from BCG-vaccinated and then infected donors were even higher. (D) Lung lesion burden in recipient mice of CD25hiFoxp3+CD4+ cells from 3 different sources compared with saline controls on d 55 after infection. Statistical analysis using 2-way ANOVA (GraphPad Prism), **P < 0.010, ***P < 0.001.
In terms of the effects on the bacterial load (Fig. 5C), similar results to the ones obtained using Rag2−/− mice were present when using the congenic recipient mice. The congenic mice receiving CD25hiFoxp3+ cells from M. tuberculosis-infected donors showed an increase in the bacterial load from d 25 to 55 compared with the saline controls, which was presumably an additive effect on the Tregs being generated by the recipients. In the mice infused with CD25hiFoxp3+ cells from BCG-vaccinated and then M. tuberculosis-infected donors, an even higher bacterial load was attained, which was significantly higher than that in the infected donors.
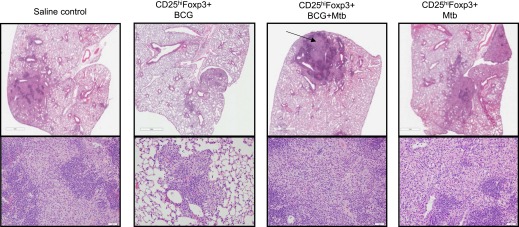
Similar to the results in the Rag2−/− mice, the histologic differences between mice infused with CD25hiFoxp3+ cells were more qualitative than quantitative. Again, when lesions were scored, the recipients of CD25hiFoxp3+ cells from BCG-vaccinated and then infected mice showed higher percentages of lung involvement, although the differences were not statistically significant (Fig. 5D). The lung lesions in the C57BL/6 mice receiving CD25hiFoxp3+ cells from congenic BCG-vaccinated M. tuberculosis-infected donors consisted of consolidating necrotic lesions, with an abnormally increased accumulation of granulocytes, a highly unusual finding in immunocompetent C57BL/6 mice, which do not develop necrotic lesions after M. tuberculosis infection (Fig. 6). In contrast, mice receiving CD25hiFoxp3+ cells from BCG-vaccinated only or M. tuberculosis-infected donors did not develop necrosis, and their lesions were predominantly composed of lymphocytes. In contrast, saline controls developed lesions with lymphocytic infiltrates with a normal accumulation of granulocytes (Fig. 6).
Figure 6. Lung histopathology in immunocompetent congenic recipients.
Lung immunopathology 55 d after adoptive transfer and M. tuberculosis infection in C57BL/6 congenic recipient. Lung lesions in mice infused with CD25hiFoxp3+ cells from BCG-vaccinated then infected donors were characterized by large fields of inflammation and necrosis (arrow) with much higher granulocytic infiltrates. Saline controls and recipients of CD25hiFoxp3+ cells from BCG-vaccinated donors or M. tuberculosis-infected donors developed lymphocytic predominant lesions. H&E staining. Top row, 10× magnification; bottom row, 20× magnification.
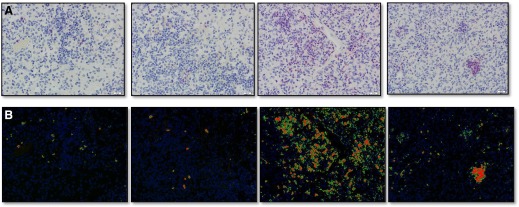
In agreement with the observations present in Rag2−/− mice, the lung lesions in mice receiving CD25hiFoxp3+ cells from BCG-vaccinated M. tuberculosis-infected donors consisted of large consolidating necrotic lesions, with an abnormally increased accumulation of granulocytes, a highly unusual finding in immunocompetent C57BL/6 mice, which do not develop necrotic lesions after M. tuberculosis infection (Fig. 6). Mice receiving CD25hiFoxp3+ cells from BCG-vaccinated only or M. tuberculosis-infected donors did not develop necrosis, and their lesions were predominantly composed of lymphocytes. In contrast, the saline controls developed lesions with lymphocytic infiltrates with a normal accumulation of granulocytes (Fig. 6). To perform a quantitative analysis of granulocytic infiltration in mice receiving CD25hiFoxp3+ cells and controls, we performed anti–Gr-1 immunohistochemistry (Fig. 7A) and image analysis using Nikon NIS Elements software (Fig. 7B). The results confirmed that mice receiving CD25hiFoxp3+ cells from BCG-vaccinated M. tuberculosis-infected donors had the highest percentage of area stained for Gr-1+ cells (Table 1).
Figure 7. Anti–Gr-1 immunohistochemistry and image analysis.
Anti–Gr-1 immunohistochemistry was performed to determine the location of Gr-1+ cells. (A) Magnified images (400×) were acquired using Olympus Bx50 microscope with an Olympus DP70 camera. (B) Image analysis was performed using Nikon NIS Elements software. Images from each of 4 groups and an isotype control from group 3 were subjected to white subtraction. Then, thresholds for detection of Gr-1 immunoreactivity were set. The area of tissue with detected Fast Red reactivity was quantified and expressed as a ratio of the total area captured in each image. See Table 1 for numerical data.
TABLE 1.
Areas quantified compared with background threshold detection based on the isotype control
| Group | Total Area (nm2) | Labeled Area (nm2) | Percent Area |
|---|---|---|---|
| Saline Control | 95010 | 364.16 | 0.38 |
| CD25hiFoxp3+BCG | 95010 | 395.20 | 0.42 |
| CD25hiFoxp3+BCG+Mtb | 95010 | 10071.55 | 10.60 |
| CD25hiFoxp3+Mtb | 95010 | 1965.08 | 2.07 |
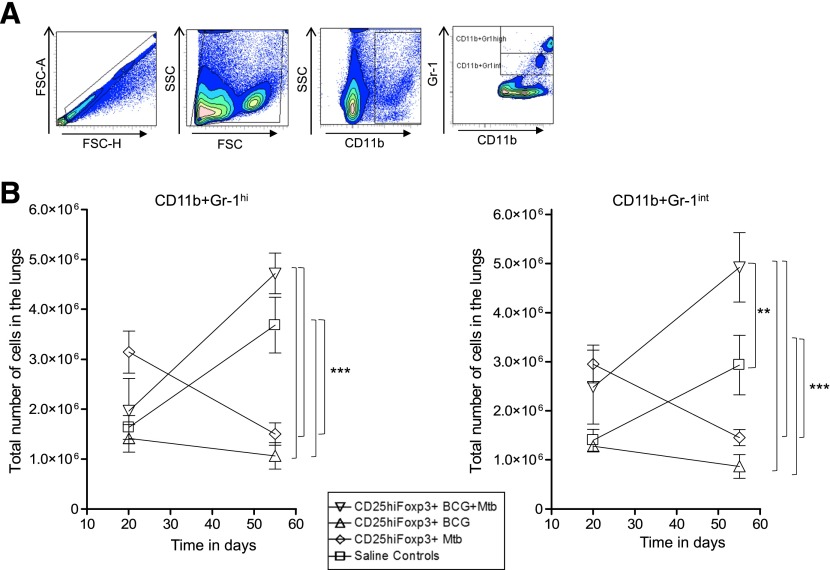
Our group, and others, has previously reported the influx of Gr-1hi and Gr-1int populations in mice that develop lung necrosis owing to M. tuberculosis infection [28, 29]. Figure 8A shows the gating strategy for tracking the effect of the various Gr-1+ populations from the different groups of mice. In the saline control recipient mice, we observed an increase in Gr-1hi cells during the course of the infection. In contrast, in mice infused with CD25hiFoxp3+ cells from M. tuberculosis-infected mice, the numbers of these cells was reduced by a factor of 5-fold by d 55 (Fig. 8B). In contrast, in mice receiving cells from BCG-vaccinated and then M. tuberculosis-infected donors, no such reduction in Gr-1hi cells was seen; in fact, higher numbers were recorded (P = 0.009). A similar effect was seen in the context of Gr-1int cells, in which the numbers were reduced 4-fold in mice receiving cells from infected donors. In contrast, the numbers of these cells doubled compared with the number in the saline controls in mice receiving cells from BCG-vaccinated and then infected donors (P = 0.0007). Our flow cytometry corroborated our histopathologic findings (Fig. 8). This seems to indicate a qualitative difference in Tregs from the 2 donor sources in terms of their ability to alter or respond to Gr-1+ cell influx into the lungs.
Figure 8. Influence of cell transfer on lung cell dynamics in congenic recipients.
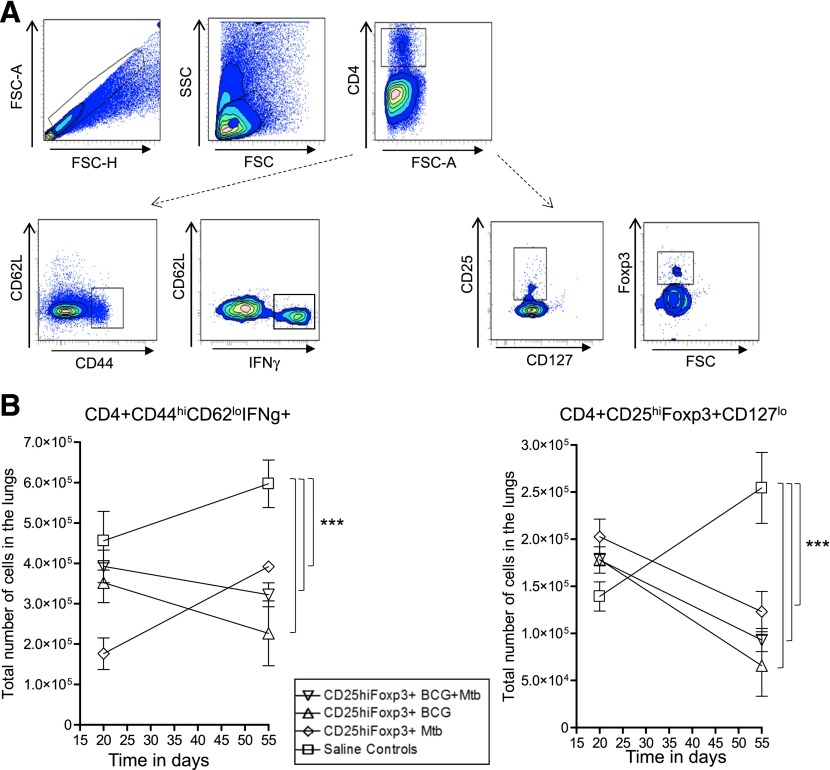
(A) Gating strategy used to analyze CD4+CD44hiCD62LhiIFN-γ+ cells (A, left) and CD4+CD25hiFoxp3+CD127lo (A, right) in the lungs of recipient mice. (B, left) CD25hiFoxp3+ cells from BCG-vaccinated and BCG-vaccinated M. tuberculosis-infected donors reduced the number of CD4+CD44hiCD62LhiIFN-γ+ effector T cells as the infection progressed. In contrast, cells from M. tuberculosis (Mtb)-infected donors did not have this capability. (B, right) Recipients of CD25hiFoxp3+ cells from all groups showed evidence of a small contraction or loss of the CD4+CD25hiFoxp3+CD127lo population in the lungs over time. Saline controls (squares), cells from Mtb-infected donors (diamonds), cells from BCG-vaccinated then infected donors (downward triangles), cells from BCG-vaccinated donors (triangles). Data points shown as mean numbers ± sem (n = 5). Statistical analysis was performed comparing all groups against each other at both time points evaluated; only significant differences are presented. Statistical analysis using 2-way ANOVA (GraphPad Prism, version 11), **P < 0.010, ***P < 0.001.
Finally, we evaluated the effect of the adoptive transfer of CD25hiFoxp3+ cells on the number of CD4 effector T cells and in the overall (both Thy1.1 and Thy1.2) Treg numbers. Figure 9A, left, shows the gating strategy for the protective immunity of CD4+CD44hiCD62loIFN-γ+ effector T cells. Figure 9A, right, shows the gating strategy for CD4+CD25hiFoxp3+CD127− Tregs. Although the cells transferred from BCG-vaccinated donors and BCG-vaccinated and then M. tuberculosis-infected donors depressed the influx of CD4+CD44hiCD62loIFN-γ+ effector cells into the lungs, the cells from infected donors had an opposite effect (Fig. 9B, left). Further analysis then showed evidence of a small decrease in the Tregs themselves in these mice, with a 50% reduction seen in the mice given CD25hiFoxp3+ from either donor source (Fig. 9B, right).
Figure 9. Influence of cell transfer on the influx of Gr-1+ cells in congenic recipients.
(A) The gating strategy for tracking the impact of the various Gr-1+ populations from the different groups of mice. (B) In mice receiving CD25hiFoxp3+ cells from BCG-vaccinated then M. tuberculosis (Mtb)-infected donors, the influx of both Gr-1hi and Gr-1int cells was enhanced over saline controls. In contrast, the levels of both of these cells were diminished by the transfer of CD25hiFoxp3+ cells from infected donors. Saline controls (squares), cells from Mtb infected donors (diamonds), cells from BCG-vaccinated then infected donors (downward triangles), cells from BCG-vaccinated donors (triangles). Data points shown as mean numbers ± sem (n = 5). Statistical analysis was performed comparing all groups against each other at both time points evaluated; only significant differences are presented. Statistical analysis using 2-way ANOVA (GraphPad Prism), **P < 0.010, ***P < 0.001.
DISCUSSION
Recent studies in our laboratory, and in others, using small animal models have shown that infection with M. tuberculosis can induce the generation of Foxp3+ Tregs [12, 13, 15, 17, 20, 30, 31]. Some strains, in particular, newly emerging clinical isolates from within the W-Beijing family, are very potent inducers of Foxp3+ cells, but whether this is a general phenomenon in the context of these new strains remains unknown. In our studies, BCG delayed the emergence of Tregs in mouse models; however, this effect was transient, and any benefits of the vaccination were then lost during chronic disease [13]. Moreover, it is not known whether these Tregs can influence different categories of vaccines or to what degree; however, considering that new BCG vaccines represent a major component of the current tuberculosis vaccine pipeline [6], the potential interference with vaccine-induced immunity by these cells should be considered during vaccine development.
In mice infected with the US-outbreak Beijing strain HN878, an initially potent CD4+IFN-γ+ effector T cell response gradually contracted at a time associated with an influx into the lungs of CD25hiFoxp3+ CD4 Tregs [12]. In subsequent studies [13], we then demonstrated that previous BCG vaccination resulted in an accelerated protective response in the lungs to the Beijing strains HN878 and SA161. However, this merely delayed the emergence of the Treg response. The latter expanded from d 30 to 60 and was associated with an increase in the bacterial load to a level indistinguishable from nonvaccinated controls. The primary importance of these studies was that at the d-30 time point, which is routinely used in vaccine screening studies [7, 32], BCG appeared highly protective. However, this benefit was then lost thereafter during chronic infection (a period not routinely evaluated).
Tregs were identified by their cell expression and suppressive properties [33, 34]. In the context of tuberculosis infection, given the highly inflammatory nature of the clinical strains we have tested to date, it was reasonable to hypothesize that one role of the Treg response was to dampen the inflammation and damage to the infected lung tissue. However, whether these cells also directly contributed to the contraction of the effector T cell response occurring at that time remains far from clear.
To address this issue, we used 2 approaches: cell depletion and cell transfer studies. In a first set of studies, we used the conventional approach [19, 20, 30] of depleting Tregs by administering monoclonal antibody to CD25, given that most Foxp3+ cells during the chronic phase of infection with high virulence W-Beijing strains we have tested to date are CD25hi [12]. This was applied from d 33 to 53 of an HN878 infection, when Foxp3+ cells are steadily becoming the predominant CD4 population in the lungs. We reasoned that if Foxp3+ cells were actively interfering with the protective capacity of effector T cell populations at that time, their depletion should have an effect on the bacterial load in the lungs. This was indeed the case, with a rapid 10-fold decrease in the lung CFUs soon after CD25 depletion was started. It is reasonable to hypothesize that this indicates that the remaining effector immunity in these mice was released or allowed to re-expand after removal of the Tregs.
Not long afterward, however, this beneficial effect was lost. One possibility is that the anti-CD25 antibody in the system also destroyed these effector cells or that they were driven into exhaustion by the sudden surge in available bacterial antigens. That the bacterial load did not resume as a chronic infection but seemed to be growing progressively tends to support this. Moreover, at least some of the cells in the lungs by this time showed the characteristics of effector memory cells [23], which are very susceptible to exhaustion [35, 36]. Additionally, the rapid rebound of Tregs after anti-CD25 treatment stopped, suggesting a strong Treg suppression of protective immunity, leading to an increased bacterial burden. Regardless, the practical issue of whether Treg activity could be removed immunotherapeutically and the infection then gradually cleared seems unlikely based on these results. This finding emphasizes the point that effector immunity and regulatory immunity is not “good vs. bad” but a physiologic homeostatic system [33, 37] designed to balance inflammatory and protective events in the lungs.
Depletion studies using a more direct tactic of lysing Foxp3+ cells in vivo using diphtheria toxin gave similar results, with an initial sharp decrease in the bacteria load after toxin administration. Because the toxin would have no action on effector T cells present at the time, these data further support the idea that effector immunity is released to drive antimicrobial immunity. However, this immunity is not retained over time. According to our results, the transient reduction in bacterial counts can be attributed to an increase in IFN-γ–producing CD4 T cells associated with a temporary reduction in CD4+Foxp3+ cell numbers residing in the lung and spleen. Despite observing a reduction in Foxp3+ cells in both groups treated with diphtheria toxin (BCG-vaccinated and nonvaccinated), the total numbers of IFN-γ–producing T cells were significantly increased only in the diphtheria toxin-treated BCG group. One possible explanation for this might be the suppressive capabilities of Tregs, which depend on their nature and origin and, significantly, on the inflammatory microenvironment [37, 38]. This microenvironment obviously differs between BCG-vaccinated mice exposed to M. tuberculosis and mice only exposed to M. tuberculosis. Alternatively, the depletion using diphtheria toxin targeted a specific “subtype” of Tregs that suppress CD4 T cell responses in an injury-specific manner [39]; or simply, the diphtheria toxin-induced Treg depletion was insufficient to recuperate a significant number of IFN-γ–producing cells in nonvaccinated mice infected with M. tuberculosis. Further experiments are needed to determine whether it is possible to keep the effector response sustained and stable and to elucidate the factors that differentiate the outcome of Treg depletion in vaccinated and nonvaccinated, M. tuberculosis-infected mice.
To directly investigate the role of Tregs generated in BCG-vaccinated M. tuberculosis-infected mice and infected-only mice, we transferred lymphocytes enriched for Tregs into recipients infected with virulent Beijing strains, either HN878 or SA161, because they induced similar results in our model [13]. We first needed to demonstrate that these cells could migrate to the lung in Rag2−/− mice after intravenous transfer. This was the case, and transfer of CD25hi Foxp3+CD4+ cells established a sizeable population of Foxp3+ cells, most of which were also positive for the cytokine IL-10, as we have previously observed [12]. As expected, transfer of these cells further increased the bacterial load in the lungs. In contrast, in the control animals infused with CD25lo Foxp3− cells, a significant reduction in CFUs was observed, indicating that this sorted subpopulation still retained some CD4 cells capable of protection.
As previously described by others [26, 27], and reported in the present study (Fig. 4D), infection in the lungs of Rag2−/− mice generates severe granulocytic abscess-like lesions. Adoptive transfer of CD25hi Foxp3+CD4+ cells harvested from donors that were vaccinated with BCG and then infected had little effect on the appearance of these lesions. However, if the transferred cells were harvested from nonvaccinated mice, a beneficial effect, with less granulocytic infiltration ensued. This observation seemed to provide the first hint that Tregs from the 2 sources had some qualitative differences, with cells harvested from infected donors appearing to influence inflammation and necrosis development and the same cells from vaccinated mice not appearing to have this capacity and, instead, having some influence on the expression of acquired T cell immunity.
We then addressed what would happen if CD25hiFoxp3+ cells were infused into immunocompetent mice capable of making their own response to M. tuberculosis infection. We found that the infusion of cells from infected donors significantly increased the bacterial load in the lungs of the recipients compared with that of the controls. When cells from BCG-vaccinated and then infected donors were infused, the bacterial load increased even further, a 10-fold increase compared with the controls, but also significantly higher than in the other recipient groups. Although the mice infused with CD25hiFoxp3+ cells from BCG-vaccinated only or M. tuberculosis-infected donors developed lymphocytic predominant lesions, granulocytes predominated in the mice infused with cells from vaccinated and then infected donors. However, the most remarkable observation was that this group developed necrotic granulomas 55 d after infection.
Flow cytometric analysis showed that the transfers established Thy-1.1+Foxp3+ cells in the lungs of the recipients in reasonable numbers. When the total cell numbers of the Foxp3+ cells in the lungs were analyzed, these increased in the saline-infused recipient controls but decreased in the mice receiving CD25hiFoxp3+ donor cells from either source, perhaps reflecting a reduction in cell influx owing to their secretion of IL-10 [40–42]. A similar depression was observed in the numbers of CD4+CD44hiCD62LloIFN-γ+ cells in mice given cells from BCG-vaccinated and then infected donors. However, this was not observed in the mice infused with cells from infected donors, again consistent with the idea that this source of Tregs is less focused on influencing the effector T cell response.
Because inflammation is a major driver of Gr-1+ cell influx into the lungs [28, 29], we measured the influx in the recipients of CD11b+Gr-1hi and CD11b+Gr-1int cells. In both cases, the numbers of these cells was significantly depressed in the mice receiving cells from infected donors. However, the reverse occurred in the mice receiving cells from BCG-vaccinated and then infected donors. Our group, and others, have reported that cells expressing intermediate levels of Gr-1 are a predominant subset of cells accumulating in the lungs of mice developing lung necrosis [28, 29, 43]. These results suggest that different subsets of Tregs might expand, depending on the cellular composition of the microenvironment in which they reside, and their role most likely varies according to the presence of these cellular cues [37, 44].
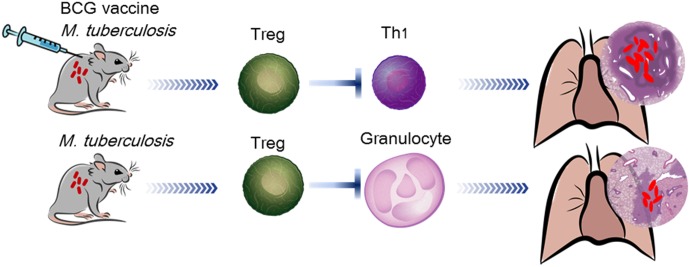
These data collectively allow the speculation that the Tregs induced in mice expressing strong effector immunity by vaccination are more focused on directly dampening this immunity and that Tregs from mice in which effector immunity develops more slowly and pathologic damage to the lungs is more serious seems to be more focused on dampening the inflammatory response, including the influx of granulocytes (Fig. 10). This divergence in regulatory control is hardly surprising, given the increasing knowledge of the plasticity of the Foxp3 (and TH17) response [45–47]. To establish qualitative differences between Tregs generated under different conditions, we have proposed studies using global gene profiling, which will provide valuable information in this regard [48, 49].
Figure 10. Differential induction of Tregs.
Tregs induced in mice vaccinated and subsequently infected were more focused on directly dampening the Th1 immune response. In contrast, Tregs from nonvaccinated mice infected with M. tuberculosis were more focused on dampening the inflammatory response, including the influx of granulocytes.
Although the present study focused on Treg activity and accumulation during the chronic and, eventually, fatal course of the disease, strong evidence also exists that this cell population can start to be generated very early in M. tuberculosis infection [31], soon after bacilli are carried to regional lymph nodes, which is a very early and key event in the disease process [50, 51]. Furthermore, to compound the matter, we have shown that certain W-Beijing strains are very potent Treg inducers [14] and that other clinical strains from South Africa, recently tested, are not [52].
Finally, from a practical viewpoint, the consequences of our collective studies, as well as others, on the pressing matter of developing new vaccines are still unclear. Certainly, our results highlight the necessity of including multiple variables (BCG vaccination, environmental mycobacteria exposure, and different M. tuberculosis strains) when screening for new vaccines, because the successful advance to clinical trials depends on it.
AUTHORSHIP
M.I.H.-T. designed and performed the experiments, analyzed the data, and prepared the manuscript. A.O.-H. designed and performed the experiments and analyzed the data and helped correct the manuscript. K.A. and C.A.S. helped perform the experiments. B.P. evaluated histopathology, performed morphometric analysis and anti–Gr-1 immunohistochemistry with image analysis. I.M.O. collaborated and helped correct manuscript. D.J.O. was the primary investigator and helped to design the experiments and correct the manuscript.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health innovator award (Grants 1DP2OD006450–01 and AI081959). This study was also funded under the American Recovery and Reinvestment Act of 2009. We are very grateful to Dr. A. Rudensky for his provision of the gene knock-in mice used in these studies.
Glossary
- BCG
bacillus Calmette-Guérin
- DTR
diphtheria toxin receptor
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Dye C., Glaziou P., Floyd K., Raviglione M. (2013) Prospects for tuberculosis elimination. Annu. Rev. Public Health 34, 271–286. [DOI] [PubMed] [Google Scholar]
- 2.Glaziou P., Falzon D., Floyd K., Raviglione M. (2013) Global epidemiology of tuberculosis. Semin. Respir. Crit. Care Med. 34, 3–16. [DOI] [PubMed] [Google Scholar]
- 3.Falzon D., Jaramillo E., Wares F., Zignol M., Floyd K., Raviglione M. C. (2013) Universal access to care for multidrug-resistant tuberculosis: an analysis of surveillance data. Lancet Infect. Dis. 13, 690–697. [DOI] [PubMed] [Google Scholar]
- 4.Falzon D., Weyer K., Raviglione M. C. (2013) Drug-resistant tuberculosis: latest advances. Lancet Respir. Med. 1, e9–e10. [DOI] [PubMed] [Google Scholar]
- 5.Ford C. B., Shah R. R., Maeda M. K., Gagneux S., Murray M. B., Cohen T., Johnston J. C., Gardy J., Lipsitch M., Fortune S. M. (2013) Mycobacterium tuberculosis mutation rate estimates from different lineages predict substantial differences in the emergence of drug-resistant tuberculosis. Nat. Genet. 45, 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans T. G., Brennan M. J., Barker L., Thole J. (2013) Preventive vaccines for tuberculosis. Vaccine 31(Suppl 2), B223–B226. [DOI] [PubMed] [Google Scholar]
- 7.Orme I. M. (2013) Vaccine development for tuberculosis: current progress. Drugs 73, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tameris M. D., Hatherill M., Landry B. S., Scriba T. J., Snowden M. A., Lockhart S., Shea J. E., McClain J. B., Hussey G. D., Hanekom W. A., Mahomed H., McShane H.; MVA85A 020 Trial Study Team (2013) Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381, 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ordway D. J., Sonnenberg M. G., Donahue S. A., Belisle J. T., Orme I. M. (1995) Drug-resistant strains of Mycobacterium tuberculosis exhibit a range of virulence for mice. Infect. Immun. 63, 741–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palanisamy G. S., DuTeau N., Eisenach K. D., Cave D. M., Theus S. A., Kreiswirth B. N., Basaraba R. J., Orme I. M. (2009) Clinical strains of Mycobacterium tuberculosis display a wide range of virulence in guinea pigs. Tuberculosis (Edinb.) 89, 203–209. [DOI] [PubMed] [Google Scholar]
- 11.Palanisamy G. S., Smith E. E., Shanley C. A., Ordway D. J., Orme I. M., Basaraba R. J. (2008) Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis (Edinb.) 88, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ordway D., Henao-Tamayo M., Harton M., Palanisamy G., Troudt J., Shanley C., Basaraba R. J., Orme I. M. (2007) The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 179, 522–531. [DOI] [PubMed] [Google Scholar]
- 13.Ordway D. J., Shang S., Henao-Tamayo M., Obregon-Henao A., Nold L., Caraway M., Shanley C. A., Basaraba R. J., Duncan C. G., Orme I. M. (2011) Mycobacterium bovis BCG-mediated protection against W-Beijing strains of Mycobacterium tuberculosis is diminished concomitant with the emergence of regulatory T cells. Clin. Vaccine Immunol. 18, 1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato-Maeda M., Shanley C. A., Ackart D., Jarlsberg L. G., Shang S., Obregon-Henao A., Harton M., Basaraba R. J., Henao-Tamayo M., Barrozo J. C., Rose J., Kawamura L. M., Coscolla M., Fofanov V. Y., Koshinsky H., Gagneux S., Hopewell P. C., Ordway D. J., Orme I. M. (2012) Beijing sublineages of Mycobacterium tuberculosis differ in pathogenicity in the guinea pig. Clin. Vaccine Immunol. 19, 1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang S., Harton M., Tamayo M. H., Shanley C., Palanisamy G. S., Caraway M., Chan E. D., Basaraba R. J., Orme I. M., Ordway D. J. (2011) Increased Foxp3 expression in guinea pigs infected with W-Beijing strains of M. tuberculosis. Tuberculosis (Edinb.) 91, 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kursar M., Koch M., Mittrücker H.-W., Nouailles G., Bonhagen K., Kamradt T., Kaufmann S. H. E. (2007) Cutting edge: regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J. Immunol. 178, 2661–2665. [DOI] [PubMed] [Google Scholar]
- 17.Scott-Browne J. P., Shafiani S., Tucker-Heard G., Ishida-Tsubota K., Fontenot J. D., Rudensky A. Y., Bevan M. J., Urdahl K. B. (2007) Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 204, 2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBride A., Konowich J., Salgame P. (2013) Host defense and recruitment of Foxp3⁺ T regulatory cells to the lungs in chronic Mycobacterium tuberculosis infection requires toll-like receptor 2. PLoS Pathog. 9, e1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaron B., Maranghi E., Leclerc C., Majlessi L. (2008) Effect of attenuation of Treg during BCG immunization on anti-mycobacterial Th1 responses and protection against Mycobacterium tuberculosis. PLoS One 3, e2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn K. M., McHugh R. S., Rich F. J., Goldsack L. M., de Lisle G. W., Buddle B. M., Delahunt B., Kirman J. R. (2006) Inactivation of CD4+ CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunol. Cell Biol. 84, 467–474. [DOI] [PubMed] [Google Scholar]
- 21.Kelly B. P., Furney S. K., Jessen M. T., Orme I. M. (1996) Low-dose aerosol infection model for testing drugs for efficacy against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 40, 2809–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ordway D. J., Orme I. M. (2011) Animal models of mycobacteria infection. In Current Protocols in Immunology (J. E. Coligan, B. E. Bierer, D. H. Margulies, E. M. Shevach, W. Strober, eds.), Chapter 19, Unit 19.5, Wiley, Hoboken, NJ. doi:10.1002/0471142735.im1905s94. [DOI] [PubMed] [Google Scholar]
- 23.Henao-Tamayo M. I., Ordway D. J., Irwin S. M., Shang S., Shanley C., Orme I. M. (2010) Phenotypic definition of effector and memory T-lymphocyte subsets in mice chronically infected with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 17, 618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ordway D. J., Shanley C. A., Caraway M. L., Orme E. A., Bucy D. S., Hascall-Dove L., Henao-Tamayo M., Harton M. R., Shang S., Ackart D., Kraft S. L., Lenaerts A. J., Basaraba R. J., Orme I. M. (2010) Evaluation of standard chemotherapy in the guinea pig model of tuberculosis. Antimicrob. Agents Chemother. 54, 1820–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng C. G., Kaviratne M., Rothfuchs A. G., Cheever A., Hieny S., Young H. A., Wynn T. A., Sher A. (2006) NK cell-derived IFN-γ differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J. Immunol. 177, 7086–7093. [DOI] [PubMed] [Google Scholar]
- 26.Kipnis A., Irwin S., Izzo A. A., Basaraba R. J., Orme I. M. (2005) Memory T lymphocytes generated by Mycobacterium bovis BCG vaccination reside within a CD4 CD44lo CD62 ligand(hi) population. Infect. Immun. 73, 7759–7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nandi B., Behar S. M. (2011) Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J. Exp. Med. 208, 2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyadova I. V., Tsiganov E. N., Kapina M. A., Shepelkova G. S., Sosunov V. V., Radaeva T. V., Majorov K. B., Shmitova N. S., van den Ham H. J., Ganusov V. V., De Boer R. J., Racine R., Winslow G. M. (2010) In mice, tuberculosis progression is associated with intensive inflammatory response and the accumulation of Gr-1 cells in the lungs. PLoS One 5, e10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obregón-Henao A., Henao-Tamayo M., Orme I. M., Ordway D. J. (2013) Gr1(int)CD11b+ myeloid-derived suppressor cells in Mycobacterium tuberculosis infection. PLoS One 8, e80669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn K. M., Rich F. J., Goldsack L. M., de Lisle G. W., Buddle B. M., Delahunt B., Kirman J. R. (2008) Accelerating the secondary immune response by inactivating CD4(+)CD25(+) T regulatory cells prior to BCG vaccination does not enhance protection against tuberculosis. Eur. J. Immunol. 38, 695–705. [DOI] [PubMed] [Google Scholar]
- 31.Shafiani S., Tucker-Heard G., Kariyone A., Takatsu K., Urdahl K. B. (2010) Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J. Exp. Med. 207, 1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McShane H., Williams A. (2014) A review of preclinical animal models utilised for TB vaccine evaluation in the context of recent human efficacy data. Tuberculosis (Edinb.) 94, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaguchi S., Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T. (2009) Regulatory T cells: how do they suppress immune responses? Int. Immunol. 21, 1105–1111. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi T., Kuniyasu Y., Toda M., Sakaguchi N., Itoh M., Iwata M., Shimizu J., Sakaguchi S. (1998) Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10, 1969–1980. [DOI] [PubMed] [Google Scholar]
- 35.Henao-Tamayo M., Obregón-Henao A., Ordway D. J., Shang S., Duncan C. G., Orme I. M. (2012) A mouse model of tuberculosis reinfection. Tuberculosis (Edinb.) 92, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behar S. M., Carpenter S. M., Booty M. G., Barber D. L., Jayaraman P. (2014) Orchestration of pulmonary T cell immunity during Mycobacterium tuberculosis infection: immunity interruptus. Semin. Immunol. 26, 559–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shevach E. M. (2011) Biological functions of regulatory T cells. Adv. Immunol. 112, 137–176. [DOI] [PubMed] [Google Scholar]
- 38.Chow Z., Banerjee A., Hickey M. J. (2015) Controlling the fire--tissue-specific mechanisms of effector regulatory T-cell homing. Immunol. Cell Biol. 93, 355–363. [DOI] [PubMed] [Google Scholar]
- 39.MacConmara M. P., Tajima G., O’Leary F., Delisle A. J., McKenna A. M., Stallwood C. G., Mannick J. A., Lederer J. A. (2011) Regulatory T cells suppress antigen-driven CD4 T cell reactivity following injury. J. Leukoc. Biol. 89, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins D. M., Sanchez-Campillo J., Rosas-Taraco A. G., Lee E. J., Orme I. M., Gonzalez-Juarrero M. (2009) Lack of IL-10 alters inflammatory and immune responses during pulmonary Mycobacterium tuberculosis infection. Tuberculosis (Edinb.) 89, 149–157. [DOI] [PubMed] [Google Scholar]
- 41.Pitt J. M., Stavropoulos E., Redford P. S., Beebe A. M., Bancroft G. J., Young D. B., O'Garra A. (2012) Blockade of IL-10 signaling during bacillus Calmette-Guérin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J. Immunol. 189, 4079–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner J., Gonzalez-Juarrero M., Ellis D. L., Basaraba R. J., Kipnis A., Orme I. M., Cooper A. M. (2002) In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J. Immunol. 169, 6343–6351. [DOI] [PubMed] [Google Scholar]
- 43.Knaul J. K., Jörg S., Oberbeck-Mueller D., Heinemann E., Scheuermann L., Brinkmann V., Mollenkopf H. J., Yeremeev V., Kaufmann S. H., Dorhoi A. (2014) Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 190, 1053–1066. [DOI] [PubMed] [Google Scholar]
- 44.Cooper A. M. (2009) Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27, 393–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hori S. (2010) Developmental plasticity of Foxp3+ regulatory T cells. Curr. Opin. Immunol. 22, 575–582. [DOI] [PubMed] [Google Scholar]
- 46.Kleinewietfeld M., Hafler D. A. (2013) The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 25, 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geiger T. L., Tauro S. (2012) Nature and nurture in Foxp3(+) regulatory T cell development, stability, and function. Hum. Immunol. 73, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curotto de Lafaille M. A., Lafaille J. J. (2002) CD4(+) regulatory T cells in autoimmunity and allergy. Curr. Opin. Immunol. 14, 771–778. [DOI] [PubMed] [Google Scholar]
- 49.Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., Mesirov J. P. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chackerian A. A., Alt J. M., Perera T. V., Dascher C. C., Behar S. M. (2002) Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 70, 4501–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urdahl K. B., Shafiani S., Ernst J. D. (2011) Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 4, 288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shanley C. A., Streicher E. M., Warren R. M., Victor T. C., Orme I. M. (2013) Characterization of W-Beijing isolates of Mycobacterium tuberculosis from the Western Cape. Vaccine 31, 5934–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]