Summary
Point-of-care technologies (POC or POCT) are enabling innovative cardiovascular diagnostics that promise to improve patient care across diverse clinical settings. The National Heart, Lung, and Blood Institute convened a working group to discuss POCT in cardiovascular medicine. The multidisciplinary working group, which included clinicians, scientists, engineers, device manufacturers, regulatory officials, and program staff, reviewed the state of the POCT field; discussed opportunities for POCT to improve cardiovascular care, realize the promise of precision medicine, and advance the clinical research enterprise; and identified barriers facing translation and integration of POCT with existing clinical systems. A POCT development roadmap emerged to guide multidisciplinary teams of biomarker scientists, technologists, health care providers, and clinical trialists as they: 1) formulate needs assessments; 2) define device design specifications; 3) develop component technologies and integrated systems; 4) perform iterative pilot testing; and 5) conduct rigorous prospective clinical testing to ensure that POCT solutions have substantial effects on cardiovascular care.
Key Words: biomarkers, clinical trials, precision medicine
Abbreviations and Acronyms: cTn, cardiac troponin; FDA, Food and Drug Administration; NHLBI, National Heart, Lung, and Blood Institute; POC, point-of-care; POCT, point-of-care technology; WG, working group
The prevention and management of cardiovascular (CV) disease increasingly demands effective diagnostic testing. Consensus defines a diagnostic as a method and an associated device that performs a physical measurement from a patient or associated biological sample and produces a quantitative or descriptive output, known as a biomarker. The definition of a biomarker, in turn, encompasses “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (1). Diagnostics, because of their strategic position at the intersection between patients and their clinically actionable data, directly affect the patient experience and the quality of care that individuals receive (Figure 1). They also furnish valuable tools for clinical investigation. Diagnostics enable providers to improve upon “one-size-fits-all” treatment strategies and instead provide personalized care on the basis of factors such as genetic makeup, comorbidities, real-time serologic assessments, and responses to therapy.
Figure 1.

Diagnostics
Diagnostics obtain measurements from a patient or associated biological sample and produce quantitative or descriptive outputs known as biomarkers, which enable clinical care, patient communication, and clinical research.
Historically, during the days of “house calls,” diagnostic testing relied primarily on physical examination and bedside analysis of urine (2). As methods for biochemical and cellular biofluid analysis advanced, the portfolio of available tests expanded and central laboratories emerged to standardize sample acquisition and measurement quality while offering economies of scale (3). Today, technology is expanding the number of diagnostic tests that can reach beyond the walls of centralized laboratories and back to the point of care (POC) for use across a broad range of clinical settings. Yet, despite the intuitive appeal of miniaturization and immediate test resulting, point-of-care technologies (POCTs) face important practical questions about their integration into clinical workflows, objective measurement of clinical benefit, standards necessary to ensure quality despite decentralization, and what reimbursement models will engender mutual enthusiasm by payers and providers.
POCTs promise to provide high-quality biomarker measurements optimized for the special constraints of diverse clinical settings including acute care, outpatient clinics, clinical research centers, homes, rural areas, and the developing world (Figure 2). In acute care settings such as the operating room (OR), cardiac catheterization suite, intensive care unit (ICU), or emergency room (ER), physicians seek real-time feedback to optimize care and tailor therapies to the dynamic circumstances they confront. In outpatient clinics, providers look for opportunities to replace reactive medicine with prevention, and to implement “precision medicine,” a national initiative that includes mobile and personal technologies as key components (4). In the home, care teams seek minimally invasive devices that seamlessly integrate health monitoring into daily living. The hope is that longitudinal measurements of home health will supplement episodic clinic visits and transform outpatient care into a data-driven practice. Independent of their health care providers, the public is adopting diverse POC-like self-tracking devices such as sleep monitors, Wi-Fi–connected scales, blood pressure cuffs, finger-stick blood tests, and wearable wristbands and watches linked to cloud storage, analytics, and opportunities for sharing. The degree to which such technologies will improve health care delivery and clinical outcomes remains hotly debated. Ultimately, only rigorous testing will determine their actual clinical utility.
Figure 2.

Point-of-Care Technologies
Point-of-care technologies (POCTs) are positioned to address the special constraints of diverse clinical settings including acute care, outpatient clinics, clinical research centers, homes, rural areas, and the developing world.
In clinical research, POCTs can expand quantitative data collection to broader populations. By fostering inclusion of under-represented groups in rural areas and the developing world often beyond the reach of traditional clinical trials, POCTs promise to improve the generalizability of study results 5, 6, 7.
The National Heart, Lung, and Blood Institute (NHLBI) convened a working group (WG) to examine the translation of CV POCT to precision medicine and clinical research (8). The meeting aimed to provide guidance to the NHLBI regarding the development, evaluation, and dissemination of high-impact POCT in research and treatment. This report summarizes and expands upon the WG discussions by: 1) describing examples of how POCT can address some of the most commonly faced problems in CV disease management; 2) identifying barriers and challenges to clinical translation; 3) calling for rigorous clinical testing and validation before integrating new POCTs into routine clinical care; and 4) outlining a POCT development roadmap that articulates specific recommendations to guide NHLBI research priorities.
IPOC Examples, Challenges, and Opportunities
POCT in acute care settings
Practitioners in acute care settings such as the ER, OR, ICU, hemodialysis unit, or cardiac catheterization suite face highly dynamic situations. Real-time POCT promise to improve patient care in these environments by supplying data rapidly to support decision-making, as illustrated in the following examples.
Example 1: rapid evaluation of ER patients with chest pain—“rule out myocardial infarction”
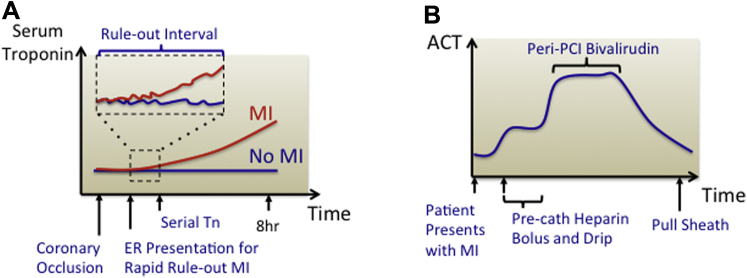
In ambulances and ERs, POCT can improve the efficiency of care by enabling rapid assessment and triage of patients with chest discomfort. Cardiac troponin (cTn), a highly sensitive and specific biomarker of myocardial injury, guides triage and management of patients presenting with symptoms suggestive of acute coronary syndrome (9). ERs already use commercial POC cTn assays, but parallel efforts are exploring whether central laboratory cTn assays can perform serial measurements at progressively shorter intervals to discriminate cardiac from noncardiac causes of chest discomfort and enable rapid patient triage. Historically, stable serial measurements of cTn taken at 6- to 12-h intervals served to “rule out” cardiac injury 10, 11. More recently, high-sensitivity cTn assays, available only in the central laboratory, permit exclusion of clinically important myocardial injury with high confidence at initial sampling as well as after only 2 serial measurements performed at 1- to 2-h intervals 12, 13, 14, 15. POC devices that can match this performance without sending samples to a central laboratory may become mainstream frontline CV diagnostics (Figure 3A).
Figure 3.
Dynamic Biomarkers for POCT Measurement
(A) In the emergency room, high-sensitivity troponin assays resolve small differences in troponin levels to rule out clinically important myocardial injury after serial measurements performed at increasingly short time intervals. (B) In the cardiac catheterization laboratory and the cardiac surgery operative room, activated clotting time (ACT) measurements are used to follow the dynamics of patient coagulation.
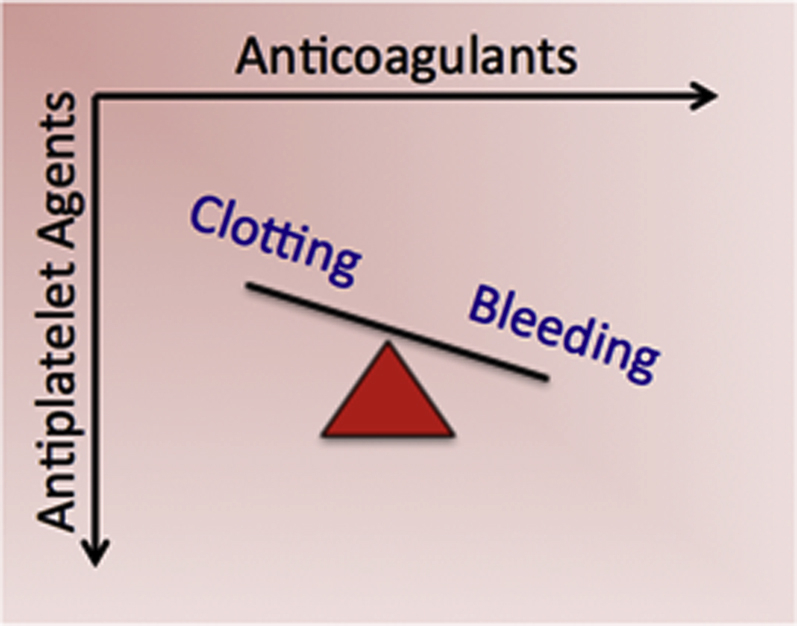
Example 2: management of bleeding and clotting risks
The quandary of balancing the risks of bleeding and clotting concerns practitioners of many specialties. Clot formation involves complex interactions among coagulation factors, platelets, and tissues (16). Surprisingly, a limited number of coagulation diagnostics guide routine outpatient and inpatient management 17, 18. Central laboratories typically measure 2 key coagulation parameters: prothrombin time and activated prothromboplastin time. Yet, delays of ∼1 h limit the utility of central laboratory measurements for acute care settings such as the ICU or OR, where thrombotic risk can vary moment to moment due to administration of anticoagulant boluses and pharmacological reversal agents 19, 20, 21. In these settings, activated clotting time (ACT), a whole blood measurement that integrates intrinsic and extrinsic coagulation with platelet function, commonly serves to quantify thrombotic potential (21). In the case of ACT measurements, procedural technicians, within steps of patient and proceduralist, perform POC testing independent of the central laboratory. This example illustrates the feasibility of integrating real-time POC diagnostics into acute clinical workflow (Figure 3B).
Platelet function complements coagulation in regulating thrombotic risk. Yet, despite extensive studies of platelet function assays in both central laboratory and POC formats, questions remain regarding their incremental benefits. Measures of platelet function do identify populations at higher risk of thrombotic events, but the demonstration that therapy guided by such assays improves outcomes has proven elusive 22, 23, 24. This apparent paradox underscores the need to subject any POC diagnostic, no matter how plausible, to rigorous research to evaluate its efficacy and added value. Coagulation and platelet function biomarkers exhibit variability and context-dependence, adding complexity to their clinical use 25, 26. POC diagnostics offer the potential to capture these variations through more frequent measurement, but whether doing so substantially and cost-effectively improves outcomes will require additional research (Figure 4).
Figure 4.
POCT for Antithrombotic Therapy
Many patients have indications for both anticoagulation (e.g., atrial fibrillation, venous thromboembolism, mechanical heart valve) and antiplatelet therapy (e.g., myocardial infarction or percutaneous coronary intervention). There is limited clinical trial data to guide the use of these agents in combination therapy. New diagnostics could aid selection and titration of combination regimens to personalize care by optimizing the risks of bleeding and clotting.
Exploratory thrombosis assays aim to complement existing assays of thrombotic risk. Examples include clot relaxation (27) or thromboelastography (16), which evaluate viscoelastic properties of clot formation. Although such assays were initially considered to be too complex for routine clinical use, recent POC adaptations of these measurements aim to improve usability 18, 28.
Example 3: future acute care POC assays
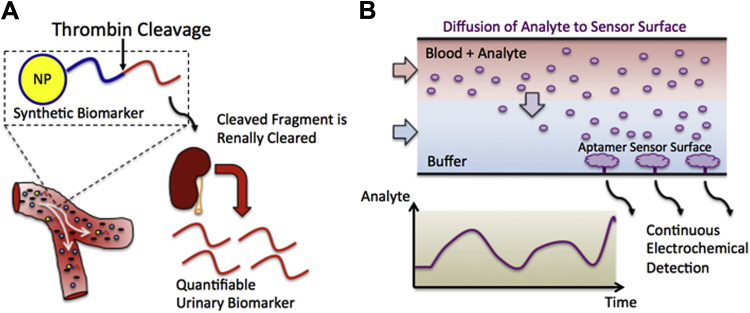
Reliable detection of thrombosis presents a diagnostic challenge. Available POC devices can measure thrombosis serum biomarkers such as D-dimer, which notoriously lacks specificity. Often, acute D-dimer elevation due to thrombosis cannot be distinguished from chronic elevation related to comorbid conditions. Instead, modern diagnosis of deep vein thromboses and pulmonary emboli relies primarily on imaging modalities such as ultrasound or contrast chest computed tomography, respectively. Recently, exogenous “synthetic biomarkers” were engineered to supplement endogenous biomarkers and enable more flexible remote monitoring of thrombosis. In concept, an intravascular nanoparticle-conjugated peptide, when cleaved by activated thrombin, liberates a peptide fragment that undergoes renal clearance detectable in the urine centrally or by POC platforms, such as novel paper-based microfluidic assays 29, 30, 31 (Figure 5A). Similar synthetic biomarker strategies are under development for a broad range of analytes.
Figure 5.
Next-Generation POCT Sensing Strategies
(A) Synthetic urinary biomarkers are being developed to augment endogenous biomarkers and enable noninvasive monitoring of intravascular thrombosis. A peptide conjugated to a nanoparticle is cleaved in the presence of thrombin and liberates a peptide fragment that is cleared by the kidney and can be detected in the urine. (B) Microfluidic continuous biomarker monitoring devices allow analytes to diffuse from blood to an electrochemical sensing surface modified with aptamers that reversibly bind them enabling continuous monitoring.
In principle, continuous biomarker monitoring would provide the most complete picture of an individual’s physiological state. Historically, the ability to measure continuously physicochemical biomarkers such as blood pressure, pulse, electrocardiogram, respiration rate, and oxygen saturation revolutionized critical care and substantially improved the safety of general anesthesia. Continuous vital sign measurements have become the standard of care for periprocedural CV monitoring; however, efforts to engineer continuous blood biomarker measurement platforms that monitor “biomolecular vital signs” presents more challenging problems, whose solutions are only nascent (32). The most notable clinically relevant analyte adapted for continuous measurement is glucose. Glucose is readily detected by diverse electrochemical-sensing platforms coupled to an immobilized enzyme (glucose oxidase); yet, the lack of broadly available analyte-enzyme pairs limits the generalizability of this approach. More recently, reversible affinity sensors have promised to expand the portfolio of analytes subject to continuous monitoring. For example, a microfluidic device containing a sensing surface functionalized with nucleic acid-based aptamers can reversibly bind corresponding analytes. The fluidic device directs blood across the planar sensing surface separated by a layer of buffer solution (Figure 5B) such that only biomolecules below a critical molecular weight can diffuse across the buffer layer and encounter the sensing surface. The reversible binding of analytes to their corresponding immobilized aptamer then generates an electrochemical signal proportional to blood analyte concentration (33). The flexibility of such platforms is expanding as the number of available analyte-aptamer pairs grows. This example also illustrates the exciting potential enabled by integrated microfluidic platforms, which operate at flow rates less than microliters per minute, and can in theory enable continuous blood draws over hours and days while keeping total blood volumes below that of a single conventional blood draw.
POCT in ambulatory settings
In outpatient clinics, the brevity of the patient visit rather than the dynamics of the physiological state provide the motivation for POC diagnostic testing. Patients frequently have blood drawn for diagnostic testing after a clinic visit. Unfortunately, the ad-hoc follow-up discussions of testing results can lead to undesirable breaks in patient-provider communication. A current movement calling for more diagnostic testing in the clinic aims to resolve such inefficiencies. POC diagnostic platforms in development aim to answer these challenges by enabling measurement of existing biomarkers as well as fundamentally new biomarkers that can enhance the outpatient practitioner’s diagnostic toolkit.
Example 1: miniaturization and mobilization of existing laboratory diagnostics
The earliest examples of POC diagnostics aimed to miniaturize and make portable the measurement of established biomarkers such as the complete blood count or basic metabolic panel. Yet, clinical use of such POC diagnostics has not kept pace with the number of commercially available testing platforms. Instead, there is continued reliance on central laboratories, which likely points to challenges presented by new POCT, including cost, uncertain reimbursement, requirements for calibration with legacy central laboratories, standardization of testing procedures, verification of testing expertise, maintenance of the decentralized testing equipment and procurement of disposables, and establishment of good data management practices including security and privacy. Despite these challenges, miniaturized and mobilized versions of existing diagnostic tests, being the first POCT diagnostics to enter clinical CV care and research, will likely serve as vehicles for addressing these challenges.
Example 2: personalized CV care using nucleic acid assays
Whether nucleic acid-based assays should be adapted to POC formats remains an area of ongoing investigation. Deoxyribonucleic acid (DNA)–based diagnostics have enjoyed success in oncology because particular DNA mutations inform therapy efficacy. Similarly, in infectious disease, detection of the DNA from an invading pathogen carries clear diagnostic information. Cardiologists, however, have used DNA diagnostics primarily for monogenic conditions. One exception is the assessment of rejection of transplanted hearts, where cell-free (cf) DNA sequences can selectively detect donor heart damage. Indeed, circulating donor cfDNA levels correlate with episodes of acute rejection as determined by invasive endomyocardial biopsy 34, 35. Detection of donor cfDNA enabled prospective noninvasive diagnosis of acute rejection with sensitivity and specificity comparable to the biopsy alone (35). A similar cfDNA sequencing approach examined the evolving pathogen landscape in heart transplant recipients in response to changes in their immunosuppressant and antiviral regimens (36). Determining the utility of POC genomic assays for CV transplant rejection or infection assessment will require additional research.
RNA, in contrast to DNA, can change dynamically during disease, making it an attractive biomarker for next-generation CV diagnostics. The complex and multifactorial nature of CV diseases has motivated exploration of transcriptional profiling. One approach uses a 23-gene expression assay platform on the basis of microfluidics and dehydrated primers, which measures gene expression fingerprints from circulating cells 37, 38 to provide negative predictive power to limit the need for more elaborate CV testing. The assay, which in its current instantiation is still far from POC, requires shipment of samples to a central facility. Nonetheless, this early example demonstrates the feasibility of using gene expression fingerprinting as a discriminatory CV biomarker.
Example 3: next-generation integrated diagnostics platforms
In addition to soluble proteins and cell-based or cell-free nucleic acids, emerging biomarkers such as rare circulating cells, mRNA, and exosomes, and their contents hold diagnostic promise 39, 40, 41, 42. Platforms based on microtechnology and nanotechnology can capture rare analytes from crude patient samples, fractionate specimens, and quantify biomarkers using signal amplification and integrated detection schemes.
Microtechnology uses devices with dimensions on the order of the thickness of a human hair. Built using fabrication techniques originally developed for the microelectronics industry and extended to microelectromechanical systems, these devices are ideally suited for POC handling and analysis of small volumes of complex biological fluid specimens such as blood or urine 43, 44. Fabrication in transparent biocompatible polymers renders these devices compatible with conventional optical detectors. Engineers have developed increasingly powerful integrated fluid handling components that now enable dense arrays of highly efficient pumps and valves to precisely control movement of fluids, solutes, and cells 45, 46. This powerful toolkit has enabled the design of diagnostic devices that perform a variety of functions, including particle and cell sorting, rare cell capture, and massively parallel and sequential biochemical reactions (41). The design flexibility enabled by microtechnologies offers broad utility for the development of POCT devices. Defining clear diagnostic problems in CV medicine that can harness the creativity of this community holds great potential.
Nanoscale devices have a length scale 3 orders of magnitude smaller than that of microfabricated devices. Nanoparticles are key components of these technologies. Among the many ways to detect nanoparticles, strategies on the basis of magnetic properties of the particles or surface plasmon resonance represent particularly elegant examples 47, 48, 49. Extension of the size scale of particles can allow sensing by commercially available detectors such as smartphones. For example, in an application requiring counting of cell subsets, investigators bathed biological samples in microbeads conjugated to cell-specific antibodies and used the diffraction pattern of cells decorated with antibody-conjugated beads to identify and count the cell population of interest using a custom dongle attached to a commercially available smartphone (50). Several excellent reviews describe these technologies in further detail and describe examples of additional POC applications (5).
POCT in the home
Patients spend <1% of their lives interacting with the health care system in the traditional sense. They spend the remaining >99% in the outside world, “at home.” Innovative technologies such as the Internet of Things, wearable devices, mobile communication devices, and social networks promise to improve fundamentally our understanding of human health and transform the home into the next frontier of outpatient medicine.
Outpatient clinical visits frequently begin with open-ended questions such as “have you been taking care of yourself at home?” This question calls on patients to summarize months of post-prandial glucose levels and blood pressure measurements, as well as adherence to recommended exercise, diet, or prescription medications. The availability of more objective and quantitative data can paint a dynamic and unbiased picture of home health across time. Balancing the desire to inform but not overburden providers with extraneous or unactionable information will require thoughtful data synthesis and analytics to traverse efficiently the voluminous data. Third-party disease management businesses may serve as intermediates. Nevertheless, successful navigation of the “big data” problem will transform the home into an informative lens through which one can observe patient health.
The following examples demonstrate the breadth of home health monitoring devices currently available or in development. Together, these technologies promise to create a more comprehensive picture of patient health and behavior that can complement patient self-reporting during in-person health care visits.
Example 1: self-testing POC diagnostics
Blood glucose testing remains one of the oldest and most widely accepted POC applications. Yet, despite substantial investment and widespread use, few sufficiently powered studies have examined the clinical utility and cost-effectiveness of glucose self-monitoring 51, 52. Meta-analyses estimate glycated hemoglobin declines of 0.22% to 0.40% in patients using blood glucose self-monitoring compared with control subjects 52, 53. Although these studies did not directly evaluate the effect on clinical outcome, this magnitude of reduction associates with a significantly reduced risk of microvascular complications in other clinical trials (54). Studies that involved the inclusion of glucose self-monitoring as a component of a structured therapeutic management program, including education and follow-up, yielded the greatest improvements in outcomes (55).
Monitoring of anticoagulation in warfarin-treated individuals with POC international normalized ratio measurements furnishes another example of self-testing. A meta-analysis of 11 randomized controlled trials showed significant reductions in the risk for thromboembolic events (hazard ratio: 0.51; 95% confidence interval [CI]: 0.31 to 0.85), with no increase in major hemorrhagic events or death (hazard ratios: 0.88; 95% CI: 0.74 to 1.06; and 0.82; 95% CI: 0.62 to 1.09, respectively) in patients who self-monitored compared with patients who did not (56). In addition, the coupling of self-monitoring with self-management and dosing was associated with greater risk reductions. Thus, although self-monitoring of the international normalized ratio may not benefit all patients, wider access and availability of testing in the home can strengthen the effectiveness of care.
Example 2: connected diagnostics—wearables, smart phones, and the “Internet of Things.”
Wearables
The ability to position sensors onto a patient and into the clothing they wear has powerful potential to transform our understanding of CV health and disease. These categories of POC devices, sometimes termed “wearables,” a subset of the “internet of things,” the “Internet of Things,” have captured the imagination of physicians and patients alike. The earliest versions of internet-connected home health devices simply adapted conventional diagnostics previously used in the clinic or hospital, such as blood pressure cuffs, heart rate monitors, scales, pedometers, oximeters, and positive pressure ventilation controllers. Telehealth programs and chronic disease management practices developed systems to monitor data from these sensors, provide feedback regarding results to providers and/or patients, and encourage compliance using reminders. More recently, wearable technologies such as wristbands and watches equipped with integrated microscale accelerometers have enabled activity monitoring, performance feedback, and the ability to annotate data with subjective measures of health for sharing with friends, family, health care providers, or the world.
High-performance electronic circuits play an essential role in home health devices, but rigid circuit boards and wires present a barrier to miniaturization and integration into ambulatory-sensing solutions. The recent development of a flexible electronics platform permits the fabrication of high-functioning electronic devices into thin flexible tattoo-like transparent films that adhere to the skin 57, 58, 63, 64. The durability of initial prototypes underwent testing in challenging locations such as the elbow without signal degradation over days despite repeated extension and flexion. Transmission of the electrocardiogram and electroencephalogram confirmed the functional utility of the device. Optimization of such technologies should permit continuous remote measurements in outpatients. Monitoring of atrial fibrillation could benefit from such advances. The burden of atrial fibrillation and the frequency of atrial fibrillation paroxysms likely correlate with stroke risk 59, 60. The emergence of minimally invasive heart rate and rhythm-monitoring devices on the basis of flexible electronics and other technologies offers a unique opportunity to document longitudinally patient rhythms in relation to other life events (61). Such information would enhance our understanding of triggers associated with arrhythmia onset and termination and aid patient management.
Internet of Things
A movement termed the “Internet of Things” aims to convert the home into a densely interconnected environment with embedded sensors in everyday objects that can monitor, communicate, and connect the environments in which we live. As an example of this technology, bedroom-embedded sleep monitors aim to optimize rest and detect sleep-disturbed breathing. The embedded sensing concept, although still in its earliest stages, has excited CV care providers with the possibility of devices that will monitor high-risk patients, identify early warning signs of decline, and prompt early intervention that may avoid more severe decompensation.
Management of heart failure is particularly poised to benefit from emerging home health technologies (62). Heart failure affects more than 5 million U.S. patients, triggers >1 million hospitalizations annually, and associates with remarkably high rehospitalization rates (∼25% at 30 days and ∼50% by 6 months) (63). Because weight gain often precedes hospitalization by days to weeks, some guidelines recommend that patients weigh themselves daily at home. Unfortunately, adherence to this recommendation remains poor; in a recent large-scale clinical trial, compliance with telemonitoring fell from 90% to 55% by 6 months despite implementation of an aggressive reminder system (64). This deficiency challenges disease management teams and practitioners caring for patients at home. Hence, a need exists to develop devices that monitor activity and/or weight without proactive patient participation. Doing so should improve the regularity of home testing and may avert unnecessary hospitalizations through early detection of volume overload (Figure 6).
Figure 6.
Management of Patients With Heart Failure Will Benefit Tremendously From POCT in the Home
This patient population is benefiting from new point-of-care technologies (POCTs) that longitudinally monitor biomarkers of heart failure decompensation (e.g., symptoms, weight, and ventricular filling pressures) to guide adjustments in diuretic dosing and avoid unnecessary hospitalizations. BBQ = barbecue, a high-salt meal.
Medication compliance also received early attention. Adherence to recommended pharmacological therapy remains an important but often unappreciated challenge of outpatient CV care. This challenge spurred the development of Wi-Fi–connected pill bottle caps and internet-connected sealable blister packs, inhalers, or injectables to provide new windows into patient medication compliance. This capability enables study of whether incentivization strategies and gamification can improve adherence to daily medications such as statins. Although recording patient medication access times does not directly reveal ingestion, these data nevertheless provide previously unobtainable information about patient medication habits at home. To measure more directly medication adherence, developers have created a microchip sensor-enabled pill with Food and Drug Administration (FDA) clearance that communicates with an adhesive patch worn on the torso that records when the pill is ingested (65). Early studies using this technology reportedly suggest that patients with greater irregularity in the timing of their morning medication were more likely to miss doses altogether and had lower medication adherence rates across time. This result suggested that interventions, such as incorporating therapy into a different facet of a daily routine, might improve compliance. Combining integrated sensing technologies such as these with behavioral studies is a fertile area for future research.
Smartphones
Smartphones serve as powerful platforms for software and hardware developers to collect, store, manage, and communicate health sensor data. In the CV space, a smartphone case with integrated contact electrodes allows a user to measure continuously an electrocardiographic rhythm strip simply by holding the case with 2 hands. Combined with rhythm detection software and the ability to save and share tracings, this technology has the potential to expand greatly patient self-recording of single-lead heart rhythms. For diabetic individuals, an electrochemical blood glucose meter that attaches to commercial smartphones as a dongle can measure, store, and analyze glycemic control. Similarly, full laboratory-quality immunoassays have also been miniaturized and adapted to a custom dongle attached to a commercial smartphone (66).
Smartphones have generated tremendous excitement for use in clinical research. Apple’s ResearchKit (Apple, Cupertino, California), which was downloaded with great enthusiasm upon its initial release, allows users to participate in clinical research via their smartphones and iPads. Applications focusing on CV disease (MyHeart Counts) and diabetes management (GlucoSuccess) were among the initial offerings and feature the ability to monitor activity, self-record a 6-min walk test, and record dietary habits and medication adherence. Google X, Duke, and Stanford also recently announced an ambitious project, the Baseline Study, which aims to understand what keeps people healthy and what determines disease trajectories. As the pilot phase of the study gives way to larger cohorts, reports suggest that the study will collect genomic information and employ more complex human phenotyping from the “Study Kit” application, associated devices, and even wearables such as the much anticipated “smart” contact lenses. These innovations extend the POC concept to everyday life and provide enormous potential for mining “big data” for health purposes at a population level, but also enabling precision medicine or personalized management for the individual.
Example 3: invasive outpatient health monitors
Implantable monitors are inherently invasive and, therefore, require careful consideration of safety before use. Once placed, however, this class of monitoring devices makes several potentially powerful biomarkers available to providers longitudinally across time. For example, implantable rhythm recorders as well as conventional pacemakers and implantable cardiac-defibrillators can detect rare but concerning paroxysmal ventricular arrhythmias as well as exhaustively profile the timing and burden of chronic arrhythmias such as atrial fibrillation. An ambulatory intrathoracic impedance monitor and associated algorithm attempt to identify thresholds and temporal signatures of impedance changes that predict worsening heart failure 67, 68, 69. Another device still in development directly measures left ventricle filling pressures in the left atrial appendage, but requires transseptal puncture for device placement. The data, which are transmitted to a hand-held patient advisory tool, can then guide medication dosing changes according to an algorithm. Taking a different approach, a new FDA-approved implantable pulmonary artery pressure monitor can be placed during a right heart catheterization and does not require a transseptal puncture. In the COMPASS-HF (Chronicle Offers Management to Patients With Advanced Signs and Symptoms of Heart Failure) trial that evaluated this technology, increases in PA pressure were reportedly more sensitive and specific, and they anticipated weight increase associated with decompensation 70, 71. Support for device approval largely stems from the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) trial, which demonstrated that in New York Heart Association functional class III heart failure patients hospitalized in the past 12 months, management guided by the pulmonary artery pressure monitor significantly reduced heart failure admissions (72).
Practical Aspects of POCT Implementation
Clinical testing and validation of POCT
POCT development must balance the enthusiasm for promising new diagnostic platforms with the need for rigorous validation studies. Adoption of a new device should depend on demonstrated performance compared with reference standards of care to ensure that it provides similar or improved clinical utility. Even if POC diagnostics cannot demonstrably alter hard outcomes such as survival, they may provide added value by limiting lengths of stay, reducing readmissions, avoiding unnecessary invasive tests, boosting physician and/or patient satisfaction, improving quality of life, or benefiting other aspects of health care delivery, cost, or comparable metrics. The success or failure of a POC diagnostic depends critically on establishing clearly stated goals and conducting rigorous research to evaluate its ability to meet pre-specified objectives. Anticipating potential risks of POCT has equal importance. For example, more diagnostic availability could conceivably increase testing volume and cost. More testing may also trigger more false positive results and lead to more invasive downstream testing, which would needlessly alarm patients and practitioners alike. Research that addresses such health systems and patient-provider communication issues associated with POCT could vitally and meaningfully affect patient care.
Ethical and regulatory considerations
Although POC testing offers many advantages as a clinical tool, decentralization of diagnostic testing may require new regulations to maintain procedural standardization, adherence to calibration standards, and maintenance of patient privacy. The FDA, in its oversight role, has provided related guidance such as the “regulatory oversight framework for laboratory developed tests” and the “mobile medical application policy” 73, 74. Practitioners should also be aware that under current regulatory requirements, POC devices are not necessarily waived under CLIA (Clinical Laboratory Improvement Amendments) simply because of use at the point of care. The use of POC devices for clinical research requires development of a policy regarding sharing of results with participants.
Protecting the health information of patients remains fundamental to clinical care by ethics and statute. The design, ownership, and operation of new POC devices requires cooperation by multiple partners to capitalize on the data collected without compromising patient privacy. These concerns mirror those currently encountered with widespread adoption of electronic health records. Yet, the mobile nature of POC devices decentralizes privacy preservation and entrusts sensitive patient data to a broad range of individuals spanning health care specialists to POC data management service providers. The active research of the HHS suggests the need to update provisions of the Health Insurance Portability and Accountability Act of 1996, now influenced by challenges related to the rapid developments of health information technology, including implementation of electronic medical records and mobile technologies for health care (75).
POCT as a Clinical Research Tool
POCT have considerable potential to enhance clinical research. Biomarkers can contribute to clinical trials by complementing clinical endpoints with interim measurements that can deepen understanding of interventions. In this context, POC diagnostics offer powerful opportunities to enrich biomarker collection during the conduct of well-controlled clinical trials with carefully adjudicated endpoints. Ambulatory monitoring devices such as mobile device applications, wearable monitors, and home-based sensors remain some of the most attractive POCT categories as aids to clinical investigation. Unlocking the vast assortment of uncaptured ambulatory data presents an opportunity for POC devices to enrich substantially clinical trial data collection. Similarly, biofluid sampling during clinical trials remains a valuable resource when paired with carefully curated patient populations meeting well-defined entry criteria that can correlate with carefully adjudicated endpoints. Yet, limits pertain to the number of biofluid biomarkers that conventional assays can measure. POC technologies that seek to multiplex biomarker measurements have a tremendous potential to maximize the information yield from these scarce samples and facilitate biomarker discovery, biomarker validation, and mechanistic insight.
Additional benefits of POCT in clinical research include enabling novel patient recruitment pathways by facilitating screening for eligibility criteria without requiring centralized testing or return visits. POCTs also offer flexible pathways for baseline and follow-up data collection, providing opportunities to improve clinical trial quality control through longitudinal and site-specific monitoring of study protocol compliance. POC diagnostics could expand opportunities for clinical trial participation beyond heavily populated urban areas, where trial coordination is typically centered. Doing so will reduce the inherent selection bias associated with existing geographic constraints and make trial results more relevant to a broader population. The National Institutes of Health has initiated funding for several clinical trials to test such technologies; continued development of this approach will represent an important advance in CV clinical research (see Table 1 for examples).
Clinical research in the developing world often focuses on communicable diseases; however, CV disease remains the leading cause of death worldwide and does not spare low- and middle-income countries (6). In resource-limited environments where geographic and financial constraints limit the availability of centralized laboratories, catheterization facilities, and specialty CV care, the relationship between diagnostic testing and CV disease management differs dramatically from heavily populated urban centers. Thus, these settings will require dedicated clinical research to understand how POCT can answer the special needs of these environments. In principle, POCTs that minimize instrumentation and infrastructure demands offer particular promise in these settings. Several platforms in development that use “lab-on-a-chip” technologies featuring integrated sample acquisition, processing, and measurement may address these needs. Those that interface with smart phone technologies are particularly attractive given the broad availability of cell phones in rural areas and the developing world (50).
Roadmap for CV Clinical Research POCT Device Development
POCTs have tremendous potential to advance CV care. Realizing this potential will require a funding environment that incentivizes engineering solutions beyond mere proof of concept demonstrations and cultivates them throughout the full technology development life cycle. Through close collaboration, engineers and health care providers must work together to ensure that innovative POC devices have a path for clinical testing, regularly undergo quantitative comparison to modern standards of care, and require careful monitoring to achieve POC goals without making excessive performance compromises. The NHLBI occupies an ideal position to incentivize formation of multidisciplinary teams comprised of health care providers, biomarker scientists, technologists, and clinical trialists to collaborate longitudinally throughout the development process. To guide the activities of these CV POCT teams, the NHLBI WG proposes the following 5-stage CV POCT Development Roadmap. This approach aims to capitalize on advances in biomarker science and emerging sensor technologies to create clinically relevant POC devices with a defined path for rigorous clinical testing and validation.
-
1.
Needs identification. Stage 1 goals include the identification of specific aspects of clinical care that have potential for substantial improvement by POCT “clinical needs” and the articulation of (well-posed) clinical problems that carefully describe the process of testing a specific POCT solution (which patient population, what clinical setting, the methods behind the quantitative measurement of benefits and risks, and the standard[s] of care that will serve as comparison).
-
2.
Biomarker selection and device design specification. Stage 2 will bring biomarker scientists and technologists together to take the “well-posed clinical problem” from stage 1 and use it to both select a relevant set of new and/or old biomarker(s) and define design criteria for a corresponding POC measurement device. Defining the clinical objectives early increases the likelihood of success once the technology exists.
-
3.
Device development. Stage 3 will focus on development of component technologies and system integration, leading to a functional POC measurement device. Guided by the specifications defined in stage 2, an iterative process of design, fabrication, and testing of key technologies and individual components will be undertaken. This will culminate with a system integration process that will require additional engineering and performance characterization using simplified models of the relevant human biomarkers.
-
4.
Pilot testing. Stage 4 will use the POC solution developed in stage 3 to perform pilot testing on clinical samples or small-scale patient populations. This effort will help identify obstacles associated with real-world biosamples, identify normal ranges and intervention thresholds, highlight data management issues, reveal unanticipated human factors, and provide initial data on device usability in a real-world clinical setting. An iterative process of device refinement and repeat pilot testing will prepare the technology for rigorous clinical testing in stage 5.
-
5.
Prospective clinical testing. Stage 5 will test and validate the technology in “real-world” health care settings. Assessment of device efficacy as well as liabilities and risks remains the overall goal. Because design tradeoffs are device- and application-specific, the criteria for success will need to be defined on a case-by-case basis (defined during stages 1 and 2). Acceptance of methodologies and validation studies for all new diagnostics including POC should require transparency and peer-reviewed publication.
Recommendations for NHLBI CV POCT Priorities
-
•
Support Stage 1 activities with the engagement of the broad CV and bioengineering communities in pre-competitive CV POCT needs-assessment activities. This action would ideally extend beyond a single meeting to include the creation of a forum for an ongoing electronic and living discussion that encourages broad participation from diverse backgrounds and spans multiple training levels. A multidisciplinary team of experts can consolidate, curate, and refine contributions to create a focused list of Cardiovascular POCT Grand Challenges that correspond to the most promising “well-posed clinical problems.” The most attractive Challenges will ideally extend beyond modest reductions in assay sample volume or increased portability of existing biomarker measurements, and instead pursue ambitious goals that ensure that novel CV POCT can improve dramatically CV care. Challenges that offer an opportunity for the CV POCT community to coalesce around a small number of high-impact problems are most likely to inspire development of high impact technologies.
-
•
Support stage 2 activities with the funding of biomarker science (basic discovery and validation of novel CV biomarkers) and novel sensing technologies (methods for detecting and quantifying a variety of biomarkers from diverse patient-derived samples). This fundamental work will cut across individual clinical POC devices and provide a scientific and technological basis for integrated POC solutions.
-
•
Support stage 3 activities with the funding of specific implementations of biomarker-sensing solutions that range from individual sensing components to complex, fully integrated POC sensing platforms that perform “sample-to-answer” measurements from clinically relevant specimens.
-
•
Support stage 4 activities by enabling pilot testing of integrated “sample-to-result” technologies developed in stage 3 via small-scale, proof-of-concept studies in preparation for larger-scale prospective POC testing in stage 5. Support could include facilitating access to annotated clinical specimens. Such pilot testing can also help determine reference values for POC tests in relevant populations, encourage harmonized data standards and reporting of POC tests, and determine the data and technical integration needs at different levels (e.g., technical, systems, patient data, and population).
-
•
Support stages 4 and 5 with core data storage, data processing, privacy, and analytics that will permit the transmittance of POCT results to and from patients and providers to realize their power without compromising patient privacy.
-
•
Integrate stage 4 and 5 POCT activities with ongoing or planned clinical studies funded by the NHLBI using funding mechanisms such as ancillary studies or Small Business Innovation Research/Small Business Technology Transfer grants, and make existing clinical data and relevant biobanks accessible to aid validation of POC technologies against established centralized laboratory measurements.
-
•
Assess standards for evaluation and clinical use of POC tests through the establishment of application-specific standards of quality, efficiency, affordability, accessibility, and safety of devices and data (in coordination with other appropriate agencies.)
-
•
Fund career development activities, program formation, and industry surrounding novel biomarker science and POC technology development that provides the foundation for new POCT opportunities on the basis of previously unrecognized or unmeasurable biomarkers.
POCT possess the potential to transform medical research and patient care. Implementation of a research and development program as proposed in the roadmap and recommendations that emerged from this WG can accelerate the realization of this promise.
Acknowledgments
The authors thank the following additional WG participants for their time and expertise: Robert Califf, MD, Food and Drug Administration; Paula Caposino, PhD, Food and Drug Administration; Jonathan Lindner, MD, Oregon Health and Science University; and Shuqui Chen, PhD, IQuum, Inc. National Institutes of Health staff participants included: Narasimhan Danthi, PhD, National Heart, Lung, and Blood Institute; William Riley, PhD, Office of Behavioral and Social Sciences Research; and Pothur Srinivas, PhD, MPH, National Heart, Lung, and Blood Institute.
Footnotes
Support for the Working Group was provided by the Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, National Institutes of Health (NIH). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. Dr. King has received the following grants: AHA 15MCPRP25690031, NIH-NHLBI 1K99HL129168, and Harvard Medical School LaDue Memorial Fellowship. Ms. Leeds, Ms. Iturriaga, and Drs. Paltoo, Rao, Adhikari, Desvigne-Nickens, and Galis are employees of the NIH. Dr. Grazette is a consultant for St. Jude, Amgen, and Relypsa; is an investigator for Novartis; and is a contractor for St. Jude. Dr. McDevitt serves as the scientific founder and has financial interests in LabNow and Force Diagnostics; and owns stock in SensoDx. Dr. Sia is a cofounder of Claros Diagnostics, acquired by OPKO Health. Dr. Barrett has received grant support from the U.S. NIH National Center for Advancing Translational Sciences (NCATS) grant (KL2TR000112). Dr. Apple has received grant support (nonsalaried) through the Minneapolis Medical Research Foundation, Abbott Diagnostics, Siemens, Ortho-Clinical Diagnostics, Roche Diagnostics, Radiometer, BRAHMS, Genentech, Arkray, BioMerieux, Alere, and Beckman Coulter; has been a paid consultant (<$10,000) for Instrumentation Laboratory T2 Biosystems and Philips Healthcare Incubator; and has served on the scientific advisory boards of Alere, Beckman Coulter, Instrumentation Laboratory, and Abbott Diagnostics. Dr. Gurbel has been a consultant for Daiichi Sankyo, Bayer, AstraZeneca, Boehringer Ingelheim, Merck, Medtronic, CSL, and Haemonetics; has received grant support from the NIH, Daiichi Sankyo, CSL, AstraZeneca, Coramed, Haemonetics, Medtronic, Harvard Clinical Research Institute, and Duke Clinical Research Institute; has been a speaker for Daiichi Sankyo and Merck; and holds a patent in personalized antiplatelet therapy in interventional cardiology. Dr. Weissleder is a founder of and consultant to T2Biosystems. Dr. Libby has received research support from the National Heart, Lung, and Blood Institute (R01 HL080472); has served as the Chair of this Working Group; is an unpaid consultant or involved in clinical trials for Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, DalCor, Genzyme, GlaxoSmithKline, Kowa, Merck, Novartis, Pfizer, Regeneron-Sanofi, and Takeda; and is a member of the scientific advisory boards for Athera Biotechnologies and Interleukin Genetics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. King and Grazette contributed equally to this work.
References
- 1.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong J.A. Urinalysis in Western culture: a brief history. Kidney Int. 2007;71:384–387. doi: 10.1038/sj.ki.5002057. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld L. Clinical chemistry since 1800: growth and development. Clin Chem. 2002;48:186–197. [PubMed] [Google Scholar]
- 4.National Institutes of Health. ACD Precision Medicine Initiative Working Group Public Workshop. Mobile and Personal Technologies in Precision Medicine Workshop. Available at: http://www.nih.gov/precisionmedicine/workshop-20150727.htm. Accessed February 12, 2016.
- 5.Chin C.D., Linder V., Sia S.K. Lab-on-a-chip devices for global health: past studies and future opportunities. Lab Chip. 2007;7:41–57. doi: 10.1039/b611455e. [DOI] [PubMed] [Google Scholar]
- 6.Reddy K.S. Cardiovascular disease in non-Western countries. N Engl J Med. 2004;350:2438–2440. doi: 10.1056/NEJMp048024. [DOI] [PubMed] [Google Scholar]
- 7.Chin C.D., Laksanasopin T., Cheung Y.K. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat Med. 2011;17:1015–1019. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 8.National Heart, Lung, and Blood Institute. Point-of-Care Technology for Cardiovascular Care. Available at: http://www.nhlbi.nih.gov/research/reports/2013-onsite-tools. Accessed February 12, 2016.
- 9.Amundson B.E., Apple F.S. Cardiac troponin assays: a review of quantitative point-of-care devices and their efficacy in the diagnosis of myocardial infarction. Clin Chem Lab Med. 2015;53:665–676. doi: 10.1515/cclm-2014-0837. [DOI] [PubMed] [Google Scholar]
- 10.Body R., Burrows G., Carley S. High-sensitivity cardiac troponin t concentrations below the limit of detection to exclude acute myocardial infarction: a prospective evaluation. Clin Chem. 2015;61:983–989. doi: 10.1373/clinchem.2014.231530. [DOI] [PubMed] [Google Scholar]
- 11.Carlton E.W., Cullen L., Than M., Gamble J., Khattab A., Greaves K. A novel diagnostic protocol to identify patients suitable for discharge after a single high-sensitivity troponin. Heart. 2015;101:1041–1046. doi: 10.1136/heartjnl-2014-307288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubini Gimenez M., Twerenbold R., Jaeger C. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am J Med. 2015;128:861–870.e4. doi: 10.1016/j.amjmed.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 13.Keller T., Zeller T., Ojeda F. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–2693. doi: 10.1001/jama.2011.1896. [DOI] [PubMed] [Google Scholar]
- 14.Shah A.S.V., Anand A., Sandoval Y. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481–2488. doi: 10.1016/S0140-6736(15)00391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palamalai V., Murakami M.M., Apple F.S. Diagnostic performance of four point of care cardiac troponin I assays to rule in and rule out acute myocardial infarction. Clin Biochem. 2013;46:1631–1635. doi: 10.1016/j.clinbiochem.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Furie B., Furie B.C. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 17.Dahlen J.R., Price M.J., Parise H., Gurbel P.A. Evaluating the clinical usefulness of platelet function testing: considerations for the proper application and interpretation of performance measures. Thromb Haemost. 2013;109:808–816. doi: 10.1160/TH12-08-0608. [DOI] [PubMed] [Google Scholar]
- 18.Lipets E.N., Ataullakhanov F.I. Global assays of hemostasis in the diagnostics of hypercoagulation and evaluation of thrombosis risk. Thromb J. 2015;13:4. doi: 10.1186/s12959-015-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsh K.J., Nedelcu E., Bai Y. How do we manage cardiopulmonary bypass coagulopathy? Transfusion. 2014;54:2158–2166. doi: 10.1111/trf.12751. [DOI] [PubMed] [Google Scholar]
- 20.Besser M.W., Klein A.A. The coagulopathy of cardiopulmonary bypass. Crit Rev Clin Lab Sci. 2010;47:197–212. doi: 10.3109/10408363.2010.549291. [DOI] [PubMed] [Google Scholar]
- 21.Chew D.P., Bhatt D.L., Lincoff A.M. Defining the optimal activated clotting time during percutaneous coronary intervention: aggregate results from 6 randomized, controlled trials. Circulation. 2001;103:961–966. doi: 10.1161/01.cir.103.7.961. [DOI] [PubMed] [Google Scholar]
- 22.Price M.J., Berger P.B., Teirstein P.S. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 23.Price M.J., Angiolillo D.J., Teirstein P.S. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation. 2011;124:1132–1137. doi: 10.1161/CIRCULATIONAHA.111.029165. [DOI] [PubMed] [Google Scholar]
- 24.Cohoon K.P., Heit J.A. Should platelet function testing guide antiplatelet therapy for patients with coronary artery stenting or acute coronary syndromes? Clin Chem. 2013;59:1299–1300. doi: 10.1373/clinchem.2012.200881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridker P.M., Manson J.E., Buring J.E., Muller J.E., Hennekens C.H. Circadian variation of acute myocardial infarction and the effect of low-dose aspirin in a randomized trial of physicians. Circulation. 1990;82:897–902. doi: 10.1161/01.cir.82.3.897. [DOI] [PubMed] [Google Scholar]
- 26.Muller J.E., Stone P.H., Turi Z.G. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 27.Skewis L.R., Lebedeva T., Papkov V. T2 magnetic resonance: a diagnostic platform for studying integrated hemostasis in whole blood—proof of concept. Clin Chem. 2014;60:1174–1182. doi: 10.1373/clinchem.2014.223735. [DOI] [PubMed] [Google Scholar]
- 28.Jeong Y.H., Bliden K.P., Shuldiner A.R., Tantry U.S., Gurbel P.A. Thrombin-induced platelet-fibrin clot strength: relation to high on-clopidogrel platelet reactivity, genotype, and post-percutaneous coronary intervention outcomes. Thromb Haemost. 2014;111:713–724. doi: 10.1160/TH13-08-0643. [DOI] [PubMed] [Google Scholar]
- 29.Lin K.Y., Kwong G.A., Warren A.D., Wood D.K., Bhatia S.N. Nanoparticles that sense thrombin activity as synthetic urinary biomarkers of thrombosis. ACS Nano. 2013;7:9001–9009. doi: 10.1021/nn403550c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warren A.D., Kwong G.A., Wood D.K., Lin K.Y., Bhatia S.N. Point-of-care diagnostics for noncommunicable diseases using synthetic urinary biomarkers and paper microfluidics. Proc Natl Acad Sci U S A. 2014;111:3671–3676. doi: 10.1073/pnas.1314651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren A.D., Gaylord S.T., Ngan K.C. Disease detection by ultrasensitive quantification of microdosed synthetic urinary biomarkers. J Am Chem Soc. 2014;136:13709–13714. doi: 10.1021/ja505676h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers M.L., Boutelle M.G. Real-time clinical monitoring of biomolecules. Annu Rev Anal Chem (Palo Alto Calif) 2013;6:427–453. doi: 10.1146/annurev.anchem.111808.073648. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson B.S., Hoggarth D.A., Maliniak D. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals. Sci Transl Med. 2013;5:213ra165. doi: 10.1126/scitranslmed.3007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder T.M., Khush K.K., Valantine H.A., Quake S.R. Universal noninvasive detection of solid organ transplant rejection. Proc Natl Acad Sci U S A. 2011;108:6229–6234. doi: 10.1073/pnas.1013924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Vlaminck I., Valantine H.A., Snyder T.M. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6:241ra77. doi: 10.1126/scitranslmed.3007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Vlaminck I., Khush K.K., Strehl C. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155:1178–1187. doi: 10.1016/j.cell.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elashoff M.R., Wingrove J.A., Beineke P. Development of a blood-based gene expression algorithm for assessment of obstructive coronary artery disease in non-diabetic patients. BMC Med Genomics. 2011;4:26. doi: 10.1186/1755-8794-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg S., Elashoff M.R., Beineke P. Multicenter validation of the diagnostic accuracy of a blood-based gene expression test for assessing obstructive coronary artery disease in nondiabetic patients. Ann Intern Med. 2010;153:425–434. doi: 10.7326/0003-4819-153-7-201010050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahoo S., Losordo D.W. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114:333–344. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 40.Rayner K., Dimmeler S., Calin G.A., Thum T., Raizman J.E., Diamandis E.P. Novel biomarkers for acute myocardial infarction: is microRNA the new kid on the block? Clin Chem. 2014;60:812–817. doi: 10.1373/clinchem.2013.215491. [DOI] [PubMed] [Google Scholar]
- 41.Nagrath S., Sequist L.V., Maheswaran S. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nahrendorf M., Swirski F.K., Aikawa E. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitesides G.M. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 44.Kumar A.A., Hennek J.W., Smith B.S. From the bench to the field in low-cost diagnostics: two case studies. Angew Chem Int Ed Engl. 2015;54:5836–5853. doi: 10.1002/anie.201411741. [DOI] [PubMed] [Google Scholar]
- 45.Thorsen T., Maerkl S.J., Quake S.R. Microfluidic large-scale integration. Science. 2002;298:580–584. doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- 46.Unger M.A., Chou H.P., Thorsen T., Scherer A., Quake S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 2000;288:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- 47.Im H., Shao H., Park Y.I. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol. 2014;32:490–495. doi: 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haun J.B., Castro C.M., Wang R. Micro-NMR for rapid molecular analysis of human tumor samples. Sci Transl Med. 2011;3:71ra16. doi: 10.1126/scitranslmed.3002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee H., Sun E., Ham D., Weissleder R. Chip-NMR biosensor for detection and molecular analysis of cells. Nat Med. 2008;14:869–874. doi: 10.1038/nm.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Im H., Castro C.M., Shao H. Digital diffraction analysis enables low-cost molecular diagnostics on a smartphone. Proc Natl Acad Sci U S A. 2015;112:5613–5618. doi: 10.1073/pnas.1501815112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans J.M.M., Mackison D., Swanson V., Donnan P.T., Emslie-Smith A., Lawton J. Self-monitoring among non-insulin treated patients with type 2 diabetes mellitus: patients’ behavioural responses to readings and associations with glycaemic control. Diabetes Res Clin Pract. 2013;100:235–242. doi: 10.1016/j.diabres.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Parkin C.G., Davidson J.A. Value of self-monitoring blood glucose pattern analysis in improving diabetes outcomes. J Diabetes Sci Technol. 2009;3:500–508. doi: 10.1177/193229680900300314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.St John A., Davis W.A., Price C.P., Davis T.M.E. The value of self-monitoring of blood glucose: a review of recent evidence. J Diabetes Complications. 2010;24:129–141. doi: 10.1016/j.jdiacomp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Stratton I.M., Adler A.I., Neil H.A. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St John A. Evidence to support point-of care-testing. Clin Biochem Rev. 2010;31:111–119. [PMC free article] [PubMed] [Google Scholar]
- 56.Heneghan C., Ward A., Perera R. Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet. 2012;379:322–334. doi: 10.1016/S0140-6736(11)61294-4. [DOI] [PubMed] [Google Scholar]
- 57.Kim D.H., Lu N., Ma R. Epidermal electronics. Science. 2011;333:838–843. doi: 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]
- 58.Xu S., Zhang Y., Jia L. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science. 2014;344:70–74. doi: 10.1126/science.1250169. [DOI] [PubMed] [Google Scholar]
- 59.Zimetbaum P., Waks J.W., Ellis E.R., Glotzer T.V., Passman R.S. Role of atrial fibrillation burden in assessing thromboembolic risk. Circ Arrhythm Electrophysiol. 2014;7:1223–1229. doi: 10.1161/CIRCEP.114.001356. [DOI] [PubMed] [Google Scholar]
- 60.Yiin G.S., Howard D.P., Paul N.L. Age-specific incidence, outcome, cost, and projected future burden of atrial fibrillation-related embolic vascular events: a population-based study. Circulation. 2014;130:1236–1244. doi: 10.1161/CIRCULATIONAHA.114.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimetbaum P., Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation. 2010;122:1629–1636. doi: 10.1161/CIRCULATIONAHA.109.925610. [DOI] [PubMed] [Google Scholar]
- 62.Chaudhry S.I., Phillips C.O., Stewart S.S. Telemonitoring for patients with chronic heart failure: a systematic review. J Card Fail. 2007;13:56–62. doi: 10.1016/j.cardfail.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Desai A.S., Stevenson L.W. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126:501–506. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 64.Chaudhry S.I., Mattera J.A., Curtis J.P. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hafezi H., Robertson T.L., Moon G.D., Au-Yeung K.Y., Zdeblick M.J., Savage G.M. An ingestible sensor for measuring medication adherence. IEEE Trans Biomed Eng. 2015;62:99–109. doi: 10.1109/TBME.2014.2341272. [DOI] [PubMed] [Google Scholar]
- 66.Laksanasopin T., Guo T.W., Nayak S. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci Transl Med. 2015;7:273re1. doi: 10.1126/scitranslmed.aaa0056. [DOI] [PubMed] [Google Scholar]
- 67.Yamokoski L.M., Haas G.J., Gans B., Abraham W.T. OptiVol fluid status monitoring with an implantable cardiac device: a heart failure management system. Expert Rev Med Devices. 2007;4:775–780. doi: 10.1586/17434440.4.6.775. [DOI] [PubMed] [Google Scholar]
- 68.Brachmann J., Bohm M., Rybak K. Fluid status monitoring with a wireless network to reduce cardiovascular-related hospitalizations and mortality in heart failure: rationale and design of the OptiLink HF Study (Optimization of Heart Failure Management using OptiVol Fluid Status Monitoring and CareLink) Eur J Heart Fail. 2011;13:796–804. doi: 10.1093/eurjhf/hfr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crozier I., Smith W. Modern device technologies. Heart Lung Circ. 2012;21:3206–3207. doi: 10.1016/j.hlc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Teerlink J.R. Learning the points of COMPASS-HF: assessing implantable hemodynamic monitoring in heart failure patients. J Am Coll Cardiol. 2008;51:1080–1082. doi: 10.1016/j.jacc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Bourge R.C., Abraham W.T., Adamson P.B. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073–1079. doi: 10.1016/j.jacc.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 72.Abraham W.T., Adamson P.B., Bourge R.C. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 73.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. Draft guidance for industry, Food and Drug Administration Staff, and Clinical Laboratories: framework for regulatory oversight of laboratory developed tests (LDTs). 2014. Available at: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm416685.pdf. Accessed February 5, 2016.
- 74.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, and Center for Biologics Evaluation and Research. Mobile medical applications: guidance for industry and Food and Drug Administration Staff. 2015. Available at: http://www.fda.gov/downloads/MedicalDevices/…/UCM263366.pdf. Accessed February 5, 2016.
- 75.HealthIT.gov. HIPAA and Health IT. Available at: http://www.healthit.gov/policy-researchers-implementers/hipaa-and-health-it. Accessed February 12, 2016.