Abstract
Importance
More than 80% of patients with heart failure with preserved ejection fraction (HFPEF), the most common form of HF among older persons, are overweight/obese. Exercise intolerance is the primary symptom of chronic HFPEF and a major determinant of reduced quality-of-life (QOL).
Objective
To determine whether caloric restriction (Diet), or aerobic exercise training (Exercise), improves exercise capacity and QOL in obese older HFPEF patients.
Design
Randomized, attention-controlled, 2x2 factorial trial conducted from February 2009 November 2014.
Setting
Urban academic medical center.
Participants
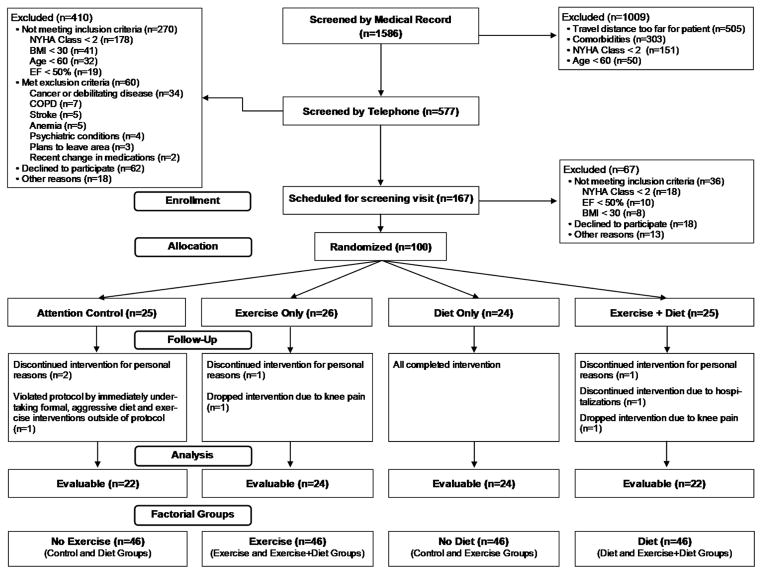
100 older (67±5 years) obese (BMI=39.3±5.6kg/m2) women (n=81) and men (n=19) with chronic, stable HFPEF enrolled from 577 patients initially screened (366 excluded by inclusion / exclusion criteria, 31 for other reasons, 80 declined participation). Twenty-six participants were randomized to Exercise alone, 24 to Diet alone, 25 to Diet+Exercise, and 25 to Control; 92 completed the trial.
Interventions
20 weeks of Diet and/or Exercise; Attention Control consisted of telephone calls every 2 weeks.
Main Outcomes and Measures
Exercise capacity measured as peak oxygen consumption (VO2, ml/kg/min; primary outcome) and QOL measured by the Minnesota Living with HF Questionnaire (MLHF) total score (co-primary outcome; score range: 0–105, higher scores indicate worse HF-related QOL).
Results
By main effects analysis, peak VO2 was increased significantly by both interventions: Exercise main effect 1.2 ml/kg/min (95%CI: 0.7,1.7; p<0.001); Diet main effect 1.3 ml/kg/min (95%CI: 0.8,1.8; p<0.001). The combination of Exercise+Diet was additive (complementary) for peak VO2 (joint effect 2.5 ml/kg/min). The change in MLHF total score was non-significant with Exercise (main effect −1 unit; 95%CI: −8,5; p=0.70) and with Diet (main effect −6 units; 95%CI: −12,1; p=0.078). The change in peak VO2 was positively correlated with the change in percent lean body mass (r=0.32; p=0.003) and the change in thigh muscle/intermuscular fat ratio (r=0.27; p=0.02). There were no study-related serious adverse events. Exercise attendance was 84±14%; Diet compliance was 99±1%. Body weight decreased by 7±1 kg (7%) in Diet, 4±1 kg (3%) in Exercise, 11±1 kg (10%) in Exercise+Diet, and 1±1 kg (1%) in Control.
Conclusion and Relevance
Among obese older patients with clinically stable heart failure and preserved ejection fraction, caloric restriction diet or aerobic exercise training increased peak oxygen consumption, and the effects may be additive. Neither intervention had a significant effect on quality of life as measured by the Minnesota Living with Heart Failure Questionnaire,
Clinical Trial Registration
Clinicaltrials.gov, NCT00959660; https://clinicaltrials.gov/ct2/show/NCT00959660
Keywords: Heart failure, Heart Failure with Preserved Ejection Fraction, Caloric restriction, Obesity, Exercise, Diet, Body Composition, Skeletal Muscle, Diastolic Function
INTRODUCTION
Heart failure with preserved ejection fraction (HFPEF) is the fastest growing form of HF, occurs primarily in older women, and is associated with high rates of morbidity, mortality, and health care expenditures.1 However, its pathophysiology is poorly understood, and medication trials to date have been neutral.
Most previous HFPEF trials focused on mediating the long-term consequences of hypertension. However, obesity is also an independent risk factor for development of HF2, 3 and >80% of HFPEF patients are overweight or obese.4, 5 Increased adiposity promotes inflammation, hypertension, insulin resistance, and dyslipidemia and impairs cardiac, arterial, skeletal muscle, and physical function,6–8 all of which are common in HFPEF and contribute to its pathophysiology.9 It was recently shown that the severity of exercise intolerance, the primary symptom and major contributor to reduced quality of life (QOL) in patients with chronic HFPEF, is significantly correlated with increased body adiposity and skeletal muscle adipose infiltration.6, 10
In obese older individuals without HF, weight loss via dietary caloric restriction (Diet) improves left ventricular (LV) hypertrophy and diastolic function, exercise capacity, glucose, lipid, and blood pressure control, inflammation markers, body composition, and skeletal muscle function.8, 11–13 However, Diet is controversial in HF patients; observational studies suggest overweight or mildly-to-moderately obese HF patients (including HFPEF specifically) survive longer than those who are normal- or under-weight.5 There have been no studies of Diet in any type of HF and current HFPEF management guidelines do not include Diet.14
The objective of this study was to conduct a randomized, single-blind, attention controlled trial to examine the effects of Diet, alone and combined with aerobic exercise training (Exercise), on exercise capacity measured as peak exercise oxygen consumption (VO2, primary outcome) and QOL (co-primary outcome), and exploratory outcomes of body composition, leg muscle function, cardiac function, and inflammation in obese older HFPEF patients.
METHODS
Study Overview
The trial was conducted at Wake Forest School of Medicine from February 2009 through November 2014, approved by the Institutional Review Board, and registered (NCT00959660). Participants provided written informed consent.
Participants
Participants were identified from search lists of medical records.15, 16 Inclusion criteria were: age ≥ 60 years; body mass index (BMI) ≥30kg/m2; symptoms and signs of HF defined by NHANES HF score ≥317 and/or the criteria of Rich et al.;18 LV ejection fraction ≥50%. Major exclusion criteria were: LV segmental wall motion abnormalities; significant ischemic or valvular heart disease, pulmonary disease, anemia, or other disorder that could explain the patients’ HF symptoms. Participants were clinically stable, had no significant change in cardiac medications for 4 weeks, and were not undergoing regular Exercise or Diet.
Outcomes
Primary Outcomes
Cardiopulmonary exercise testing was performed on a motorized treadmill using the modified Naughton protocol to the endpoint of exhaustion.19 Gas exchange was measured continuously during exercise (Medgraphics Ultima, St. Paul, Minnesota). Peak VO2 (ml/kg body mass/min), the co-primary outcome, was the average of measures from the last 30 seconds during peak exercise.19
The other co-primary outcome was disease-specific QOL assessed as the total score from the Minnesota Living with Heart Failure (MLHF) questionnaire.16,17,20 The MLHF score range is 0–105, higher scores indicate worse HF-related QOL.
Exploratory Outcomes
Exercise time, 6-minute walk distance (6MWD), ventilatory anaerobic threshold (VAT), and ventilation/carbon dioxide (Ve/VCO2) slope were assessed as previously described.15, 19
Total body lean mass and fat mass were measured by dual energy X-ray absorptiometry (DXA, Hologic Delphi QDR, Bedford MA) according to standardized protocols.10 Thigh muscle and fat areas and abdominal, epicardial and pericardial fat areas were measured using magnetic resonance imaging (MRI, General Electric Medical Systems, Milwaukee, Wisconsin), and an image analysis workstation (Tomovision, Montreal, Quebec).6
Leg press power (Watts) was assessed using the Nottingham power rig. Muscle quality was calculated as leg power/thigh muscle area (Watts/cm2) from MRI.
HF disease-specific QOL was assessed with the Kansas City Cardiomyopathy Questionnaire (KCCQ; range 0–100; higher scores indicate better QOL) and general QOL was assessed with the Medical Outcomes 36-item Short-Form Health Survey (SF-36; range 0–100, average is 50; higher scores indicate better QOL).15, 16, 21, 22
Doppler-echocardiograms were performed and analyzed per American Society of Echocardiography recommendations.16 Doppler LV filling patterns and pulse-wave velocity were assessed as described.16
LV mass and volumes were assessed by cardiac MRI from multi-slice, multi-phase gradient-echo sequences, traced manually, and calculated by summation.
Blood was collected after overnight fasting and stored at 80°C. B-type natriuretic peptide (BNP) was measured by radioimmunoassay (Phoenix Pharmaceuticals; Mountain View, Calif).15, 23 High-sensitivity C-reactive protein (hsCRP) and plasma interleukin-6 (IL-6) were measured by enzyme-linked immunosorbent assays; please see online supplement for details.
Blinding of Outcomes Assessments
The PI and all study investigators, except the biostatistician investigator were blinded to all study outcomes. Personnel performing the outcome measures were blinded to participant group. For practical considerations, an exception was for cardiopulmonary exercise testing where the supervising physician and staff were blinded to the baseline (pre-randomization) results. To minimize bias, standardized procedures known to elicit maximal exercise performance were used, including a standardized protocol, guidance by the respiratory exchange ratio (RER, an objective indicator of effort) and Borg scale, and reading of a standardized participant instruction script prior to each exercise test.
Randomization
After baseline assessments were completed, participants were randomized using a computer-generated list (SAS) maintained by the study statistician and stratified by beta-blocker medication and gender to one of four groups consisting of Exercise only, Diet only, combined Exercise and Diet (Exercise+Diet), or attention control (Control). No blocking across time was used.
Interventions
Participants randomized to either group receiving Exercise completed 1-hour supervised exercise sessions 3 times per week for 20-weeks consisting primarily of walking exercise using an individualized exercise prescription based on the exercise test results, and intensity level was progressed as tolerated and based primarily on heart rate reserve.15, 16
Participants randomized to either group receiving Diet were prescribed a hypocaloric diet using meals (lunch, dinner, snacks) prepared by the Wake Forest Clinical Research Metabolic Kitchen under direction of a registered dietician (RD). Participants prepared their own breakfast from a menu. Individual energy needs were calculated from resting metabolic rate (MedGraphics CCM) following an overnight fast and an activity factor based on self-reported daily activity. Prescribed calorie intake deficits were ~400 kcal/day for the Diet only group and ~350 kcal/day for the Exercise+Diet group (the difference between the groups allowed for the energy expenditure of the Exercise intervention), but not <1000 kcal/day. The diet provided ~1.2g protein/kg ideal body weight, 25–30% fat calories and the remainder as carbohydrate. Participants were provided daily calcium supplements (600 mg) and kept records of all food consumed which was monitored weekly.
Participants randomized to attention control (Control) received neither diet or exercise interventions and were requested and voluntarily agreed to not make diet or exercise changes during the 20-week study. They received telephone calls every 2 weeks from staff in an attempt to match that received by participants in the Diet and Exercise groups.
Statistical Analysis
All analyses were performed with SAS version 9.0. The study was a 2x2 factorial design to estimate the main effect of the two interventions, Exercise and Diet, and the primary and exploratory outcomes were tested at the 5% two-sided level of significance. The trial was designed to have two co-primary outcomes, the performance measure peak VO2 (ml/kg/min) and the MLHF questionnaire total score. All available outcome data were analyzed in an intention to treat analysis. The analysis testing the main effects of Diet and Exercise and their interaction was performed using analysis of covariance with the baseline measure of the outcome measure, gender, and beta blocker usage as covariates. This method adjusts for differences in the means of the baseline measure of the outcome and other predictor covariates to estimate what the mean in each level of the factor would be had both groups had the same overall mean of the covariates in the model. This method is equivalent to multiple imputation of missing data with the covariates as predictors and infinite iterations. We also performed sensitivity analyses to assess the impact of missing data. The least square means (LSMEAN) is presented along with either its standard error (SE) or 95% confidence intervals (CI). The main effect of each intervention, which is the difference in the LSMEANS between the two-levels of each of the factors (Exercise, Diet) is presented along with its 95% confidence interval and a p-value. Based on a previous study of HF patients, sample size calculations indicated that 80 evaluable participants would provide ≥80% power to detect a main effect of 6% in peak VO2 and effect size of 20% on MLHF total score. Allowing for up to 20% loss to follow-up, 100 participants were randomized to the four groups. Because the test for interaction between the two factors, which is a linear contrast between the four individual group means, has low power, the two interventions were only considered additive (complementary) if the p-value for intervention was ≥0.10.
Baseline participant characteristics are presented as mean and standard deviation or frequency and percent. Associations between changes in exercise capacity and other variables were made by Pearson correlations.
See the online supplemental text for additional details on sample size, effect size, testing for interaction, multiple comparisons, multiple stepwise regression, and missing data.
RESULTS
Participants
From 1,586 records reviewed, 577 patients were further screened by telephone; 167 were scheduled for a screening visit. Ultimately, 100 HFPEF patients (age 67±5 years) were enrolled and randomized: 26 Exercise, 24 Diet, 25 Exercise+Diet, 25 Control (Figure 1). Ninety-two participants (24 Exercise, 24 Diet, 22 Exercise+Diet, 22 Control) completed the intervention and follow-up testing (Figure 1). Participant characteristics were generally in accord with those observed in population studies, with predominantly women and high rates of hypertension, diabetes, LV hypertrophy and diastolic dysfunction (Table 1).
Figure 1.
CONSORT flow diagram.
Table 1.
Baseline Characteristics of Factorial Groups at Randomization
| Characteristic | Exercise (n=51) | No Exercise (n=49) | Diet (n=49) | No Diet (n=51) |
|---|---|---|---|---|
| Age (years) | 66.9 ± 5.5 | 66.0 ± 4.8 | 66.4 ± 5.0 | 66.6 ± 5.4 |
| Women | 41 (80%) | 40 (82%) | 40 (82%) | 41 (80%) |
| White | 28 (55%) | 27 (55%) | 24 (49%) | 31 (61%) |
| Body Weight (kg) | 109 ± 21 | 102 ± 13 | 105 ± 17 | 106 ± 19 |
| BSA (m2) | 2.12 ± 0.22 | 2.06 ± 0.15 | 2.09 ± 0.19 | 2.09 ± 0.19 |
| BMI (kg/m2) | 40.3 ± 7.1 | 38.4 ± 4.8 | 39.0 ± 5.0 | 39.7 ± 7.1 |
| Body fat (%) | 45 ± 6 | 46 ± 7 | 45 ± 6 | 45 ± 7 |
| NYHA class | ||||
| II | 27 (53%) | 33 (67%) | 31 (63%) | 29 (57%) |
| III | 24 (47%) | 16 (33%) | 18 (37%) | 22 (43%) |
| Ejection fraction (%) | 60 ± 6 | 62 ± 6 | 61 ± 6 | 62 ± 6 |
| LV Mass (g) | 213 ± 63 | 216 ± 57 | 218 ± 62 | 210 ± 58 |
| Relative wall thickness | 0.57 ± 0.12 | 0.57 ± 0.12 | 0.56 ± 0.13 | 0.58 ± 0.11 |
| Diastolic filling pattern | ||||
| Normal | 1 (2%) | 1 (2%) | 1 (2%) | 1 (2%) |
| Impaired Relaxation | 45 (88%) | 42 (88%) | 42 (88%) | 45 (88%) |
| Pseudonormal | 5 (10%) | 4 (8%) | 5 (10%) | 4 (8%) |
| Restrictive | 0 (0%) | 1 (2%) | 0 (0%) | 1 (2%) |
| e’ (cm/s) | 6.3 ± 1.4 | 6.1 ± 1.6 | 6.2 ± 1.7 | 6.2 ± 1.3 |
| E/ e’ ratio | 12.9 ± 3.4 | 13.4 ± 4.0 | 13.0 ± 3.9 | 13.2 ± 3.5 |
| B-type natriuretic peptide (pg/ml) | 24.9 (19.2, 39.4) | 21.6 (18.2, 26.5) | 22.0 (19.1, 33.0) | 22.2 (18.7, 33.6) |
| Current atrial fibrillation | 1 (2%) | 1 (2%) | 1 (2%) | 1 (2%) |
| History of diabetes mellitus | 21 (41%) | 14 (29%) | 16 (33%) | 19 (37%) |
| History of hypertension | 48 (94%) | 47 (96%) | 46 (94%) | 49 (96%) |
| Systolic BP (mmHg) | 137 ± 16 | 135 ± 16 | 136 ± 15 | 136 ± 16 |
| Diastolic BP (mmHg) | 78 ± 9 | 77 ± 7 | 78 ± 9 | 78 ± 7 |
| Current medications | ||||
| ACE-inhibitors | 20 (39%) | 17 (35%) | 18 (37%) | 19 (37%) |
| Diuretics | 38 (75%) | 38 (78%) | 35 (71%) | 41 (80%) |
| Beta-blockers | 20 (39%) | 20 (41%) | 19 (39%) | 21 (41%) |
| Calcium Antagonists | 18 (35%) | 17 (35%) | 18 (37%) | 17 (33%) |
| Nitrates | 3 (6%) | 6 (12%) | 4 (8%) | 5 (10%) |
| ARB’s | 19 (37%) | 16 (33%) | 15 (31%) | 20 (39%) |
| Peak VO2 (ml/kg/min) | 14.5 ± 2.9 | 14.5 ± 2.3 | 14.7 ± 2.9 | 14.3 ± 2.3 |
| Peak VO2 % of predicted | 58.1 ± 11.5 | 57.9 ± 9.2 | 58.9 ± 11.5 | 57.1 ± 9.2 |
| Peak VO2 (ml/min) | 1556 ± 347 | 1465 ± 268 | 1533 ± 346 | 1491 ± 279 |
| Peak RER | 1.12 ± 0.08 | 1.12 ± 0.10 | 1.13 ± 0.09 | 1.11 ± 0.08 |
| Exercise time (min) | 10.0 ± 2.6 | 10.3 ± 2.1 | 10.3 ± 2.4 | 9.9 ± 2.3 |
| 6 minute walk (feet) | 1337 ± 270 | 1368 ± 201 | 1359 ± 234 | 1346 ± 245 |
| 6 minute walk % of predicted | 72.4 ± 14.6 | 74.1 ± 10.9 | 73.6 ± 12.7 | 72.9 ± 13.2 |
Data are presented as mean ± SD or count (%), except for B-type natriuretic peptide which is presented as median (25th,75th percentile). Abbreviations: BSA, body surface area; BMI, body mass index; NYHA, New York Heart Association HF class; LV, left ventricular; EDV, end diastolic volume; e’, early mitral annulus velocity (septal); E, E-wave velocity; BP, blood pressure; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; RER, respiratory exchange ratio. Diastolic filling pattern determined according to ASE (American Society of Echocardiography) criteria. Peak VO2 and 6 minute walk % of predicted as compared to 60 healthy age and gender-matched sedentary controls (Stehle et al, J Gerontol Med Sci 2012; 11: 1212–1218).
Primary Outcomes
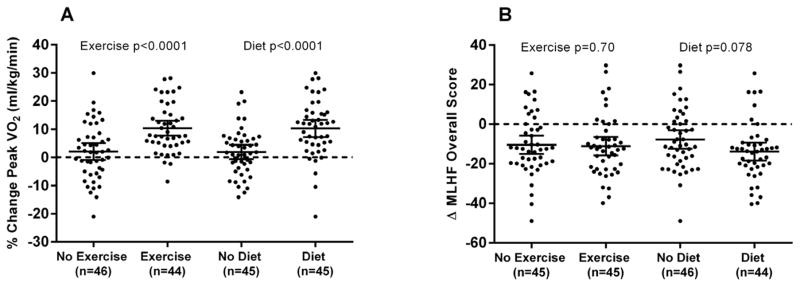
Both Diet and Exercise significantly increased exercise capacity as determined by the co-primary outcome, peak VO2 (Diet main effect 1.3 (0.8,1.8) ml/kg body mass/min, p<0.0001; Exercise main effect 1.2 (0.7,1.7) ml/kg body mass/min, p<0.0001). The change in the co-primary measure of QOL as measured by the MLHF total score was non-significant with Exercise (main effect −1 (−8,5) units, p=0.70) and with Diet (main effect −0.6 (−12,1) units, p=0.078) (Table 2).
Table 2.
Exercise Performance and Quality of Life by Factorial Group
| Variable | Exercise Factorial Groups | Diet Factorial Groups | Interaction between Exercise and Diet |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall Baseline |
Exercise (n=46) |
No Exercise (n=46) |
Exercise Main Effect |
P- Value |
Diet (n=46) |
No Diet (n=46) |
Diet Main Effect |
P- Value |
||
| Primary Outcomes | Mean ± SD | FU LSMean (95% CI) |
FU LSMean (95% CI) |
Difference (95% CI) |
FU LSMean (95% CI) |
FU LSMean (95% CI) |
Difference (95% CI) |
P-Value | ||
| Peak VO2 (ml/kg/min) | 14.5 ± 2.6 | 16.0 (15.6, 16.4) | 14.8 (14.4, 15.2) | 1.2 (0.7, 1.7) | <0.0001 | 16.1 (15.7, 16.5) | 14.8 (14.4, 15.2) | 1.3 (0.8, 1.8) | <0.0001 | 0.67 |
| MLHF Total Score | 29 ± 20 | 18 (14, 22) | 19 (15, 23) | −1 (−8, 5) | 0.70 | 16 (12, 20) | 21 (17, 25) | −6 (−12, 1) | 0.078 | 0.54 |
| Secondary/Exploratory Outcomes | ||||||||||
| Exercise Performance | ||||||||||
| Peak VO2 (ml/kglean/min) | 28.0 ± 4.3 | 30.3 (29.5, 31.1) | 28.2 (27.4, 29.0) | 2.1 (1.0, 3.1) | 0.0002 | 29.7 (28.9, 30.5) | 28.5 (27.7, 29.3) | 1.3 (0.2, 2.3) | 0.026 | 0.25 |
| Peak VO2 (ml/kgleglean/min) | 88.5 ± 15.4 | 95.3 (92.8, 97.8) | 89.1 (86.7, 91.5) | 6.2 (2.7, 9.7) | 0.0008 | 94.0 (91.6, 96.4) | 89.5 (87.0, 92.0) | 4.5 (0.9, 8.0) | 0.014 | 0.75 |
| Peak VO2 (ml/cm2muscle/min) | 12.7 ± 2.2 | 13.8 (13.6, 14.0) | 12.7 (12.5, 12.9) | 1.1 (0.7, 1.5) | <0.0001 | 13.6 (13.4, 13.8) | 12.9 (12.5, 13.3) | 0.6 (0.2, 1.1) | 0.002 | 0.15 |
| Peak VO2 (ml/min) | 1515 ± 321 | 1575 (1544, 1606) | 1483 (1452, 1514) | 91 (46, 137) | 0.0002 | 1537 (1506, 1568) | 1519 (1488, 1550) | 18 (−27, 64) | 0.44 | 0.42 |
| VO2 reserve (ml/min) | 1164 ± 289 | 1257 (1220, 1294) | 1160 (1123, 1197) | 97 (44, 151) | 0.0005 | 1238 (1201, 1275) | 1178 (1141, 1215) | 59 (6, 113) | 0.030 | 0.48 |
| Exercise Time (min) | 10.2 ± 2.4 | 12.9 (12.5, 13.3) | 10.9 (10.5, 11.3) | 2.0 (1.4, 2.6) | <0.0001 | 12.7 (12.3, 13.1) | 11.1 (10.7, 11.5) | 1.6 (1.0, 2.2) | <0.0001 | 0.53 |
| Workload (METS) | 5.8 ± 1.2 | 7.1 (6.9, 7.3) | 6.3 (6.1, 6.5) | 0.8 (0.4, 1.1) | <0.0001 | 7.1 (6.9, 7.3) | 6.3 (6.1, 6.5) | 0.7 (0.4, 1.1) | <0.0001 | 0.58 |
| Peak HR (bpm) | 139 ± 18 | 136 (134, 138) | 136 (134, 138) | 0 (−4, 4) | 0.90 | 136 (134, 138) | 136 (134, 138) | 0 (−4, 4) | 0.96 | 0.49 |
| Peak SBP (mmHg) | 178 ± 19 | 171 (167, 175) | 171 (167, 175) | 0 (−6, 5) | 0.92 | 171 (167, 175) | 172 (168, 176) | −1 (−7, 5) | 0.69 | 0.40 |
| Peak DBP (mmHg) | 78 ± 9 | 73 (71, 75) | 77 (75, 79) | −4 (−7, −1) | 0.005 | 73 (71, 75) | 78 (76, 80) | −5 (−8, −2) | 0.0002 | 0.54 |
| Peak RER | 1.12 ± 0.08 | 1.15 (1.13, 1.17) | 1.12 (1.10, 1.14) | 0.02 (0, 0.05) | 0.061 | 1.15 (1.13, 1.17) | 1.12 (1.10, 1.14) | 0.03 (0, 0.06) | 0.048 | 0.52 |
| VAT (ml/kg/min) | 9.7 ± 1.9 | 10.3 (9.9, 10.7) | 9.8 (9.4, 10.2) | 0.5 (−0.1, 1.0) | 0.097 | 10.2 (9.8, 10.6) | 9.9 (9.5, 10.3) | 0.3 (−0.3, 0.8) | 0.34 | 0.88 |
| VE/VCO2 Slope | 29.6 ± 3.9 | 29.2 (28.4, 30.0) | 29.6 (28.8, 30.4) | −0.4 (−1.6, 0.7) | 0.43 | 29.4 (28.6, 30.2) | 29.5 (28.7, 30.3) | −0.1 (−1.3, 1.0) | 0.82 | 0.44 |
| Variable | Exercise Factorial Groups | Diet Factorial Groups | Interaction between Exercise and Diet | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall Baseline | Exercise(n=46) | No Exercise (n=46) | Exercise Main Effect | P-Value | Diet (n=46) | No Diet (n=46) | Diet Main Effect | P-Value | ||
| Exploratory Outcomes | Mean ± SD | FU LSMean (95% CI) | FU LSMean (95% CI) | Difference (95% CI) | FU LSMean (95% CI) | FU LSMean (95% CI) | Difference (95% CI) | P-Value | ||
| 6 Minute walk (feet) | 1351 ± 226 | 1503 (1470, 1536) | 1397 (1366, 1428) | 106 (60, 152) | <0.0001 | 1488 (1457, 1519) | 1403 (1370, 1436) | 85 (39, 132) | 0.0005 | 0.09 |
| Leg Power (watts) | 111 ± 51 | 116 (108, 124) | 118 (110, 126) | −2 (−14, 10) | 0.76 | 122 (114, 130) | 112 (104, 120) | 11 (−2, 23) | 0.089 | 0.71 |
| Leg muscle quality(w/cm2) | 0.90 ± 0.32 | 0.97 (0.89, 1.05) | 1.00 (0.92, 1.08) | −0.03 (−0.15, 0.09) | 0.64 | 1.06 (0.98, 1.14) | 0.91 (0.83, 0.99) | 0.15 (0.03, 0.27) | 0.016 | 0.57 |
| Quality of Life | ||||||||||
| KCCQ Total Score | 62 ± 16 | 75 (71, 79) | 73 (69, 77) | 2 (−3, 7) | 0.43 | 78 (74, 82) | 70 (66, 74) | 7 (3, 12) | 0.004 | 0.96 |
| SF-36 PCS | 37 ± 9 | 42 (40, 44) | 42 (40, 44) | 0 (−3, 3) | 0.85 | 44 (42, 46) | 40 (38, 42) | 4 (1, 7) | 0.015 | 0.53 |
| NYHA Class | 2.4 ± 0.5 | 1.7 (1.5, 1.9) | 2.1 (1.9, 2.3) | −0.4 (−0.6, −0.2) | <0.0001 | 1.8 (1.6, 2.0) | 2.1 (1.9, 2.3) | −0.4 (−0.5, −0.2) | 0.0001 | 0.009 |
Data are presented as overall baseline mean ± SD and LSMean (95% CI) at follow-up visit for each of the factorial groups with main effect and 95% CI. P-value represents comparison of least square means at final visit following adjustment for overall baseline values, gender, and beta-blocker use.
Abbreviations: FU, follow-up;; VO2, oxygen consumption; SBP, systolic blood pressure; DBP, diastolic BP; RER, respiratory exchange ratio; VAT, ventilatory anaerobic threshold; VE, ventilatory equivalents; VCO2, carbon dioxide production; MLHF, Minnesota Living With Heart Failure Questionnaire; KCCQ, Kansas City Cardiomyopathy Questionnaire; SF-36PCS, Medical Outcomes Short Form 36 Health Survey Physical Component Score; NYHA, New York Heart Association HF Class. MLHF, score range is 0–105, higher scores indicate worse HF-related QOL. KCCQ; range 0–100; higher scores indicate better QOL. SF-36; range 0–100, average is 50; higher scores indicate better QOL. VO2 per kg of lean and per kg of leglean measured by DXA; cm2 muscle is area of thigh muscle measured by MRI. Leg muscle quality = leg power / thigh muscle area.
Exploratory Measures
Exercise Performance
Both Diet and Exercise significantly increased multiple other measures of exercise capacity as determined by peak VO2 expressed in ml/kg lean body mass/min (Exercise main effect: 2.1 (1.0,3.1) ml/kglean/min, p= 0.0002; Diet main effect: 1.36 (0.2,2.3), p=0.026), and ml/kg lean leg mass/min by DXA (Exercise main effect: 6.2 (2.7,9.7) ml/kgleglean/min, p= 0.0008; Diet main effect: 4.5 (0.9,8.0), p=0.014), and ml/cm2 thigh muscle area/min by MRI (Exercise main effect: 1.1 (0.7,1.5) ml/cm2muscle/min, p<0.0001; Diet main effect: 0.6 (0.2,1.1), p=0.002) as well as VO2 reserve (peak minus rest, ml/min (Exercise main effect: 97 (44,141) ml/min, p= 0.0005; Diet main effect: 59 (6,113), p=0.30), exercise time to exhaustion (Exercise main effect: 2.0 (1.4,2.6) min, p<0.0001; Diet main effect: 1.6 (1.0,2.2), p<0.0001), peak workload (METS; Exercise main effect: 0.8 (0.4,1.1) METS, p<0.0001; Diet main effect: 0.7 (0.4,1.1) p<0.0001), and 6MWD (Exercise main effect:106 (60,152) feet, p<0.0001; Diet main effect: 85 (39,132), p=0.0005) (Table 2). Mean peak RER values were >1.10 for all groups at baseline and follow-up, suggesting exhaustive effort. There was an Exercise by Diet super-additive (synergistic) interaction for 6MWD (p=0.09). There were no other significant Exercise by Diet interactions (Table 2), suggesting the interventions were additive (complementary) for other variables. With Diet, muscle quality significantly improved (main effect 0.15 (0.03,0.27) w/cm2) and leg press power showed non-significant change (main effect 11 (−2,23) watts, p=0.089) (Table 2).
Measures of QOL
Diet but not Exercise significantly improved the KCCQ score, a HF-specific QOL measure, by 7 (2.6,12.3) units (p=0.004), substantially greater than the accepted threshold (5 units) for clinical relevance.21 Diet also significantly improved the general QOL SF-36 physical component score (Diet main effect 4 (1,7) units, p=0.015) (Table 2). There were no significant Exercise by Diet interactions.
Weight and Body Composition
Body weight was significantly decreased by both Diet and Exercise (Table 3; Supplementary eFigure 2) (Exercise main effect: −3 (−5, −1) kg, p<0.0001; Diet main effect: −7 (−9, −5), p<0.0001). With Diet the DXA measures of lean body mass (main effect: −2 (−3, −1) kg, p<0.0001), fat mass (main effect: −5 (−6, −4) kg, p<0.0001), and percent fat mass (main effect: −2 (−3, −1) percent, p<0.0001) were significantly decreased while percent lean body mass was significantly increased (main effect 2 (1,3) percent, p<0.0001); in contrast with Exercise only fat mass was decreased (main effect: −2 (−3, −1) kg, p=0.001) (Table 3). With Diet, MRI measures of thigh subcutaneous fat (main effect: −16 (−22, −10) cm2, p<0.0001), thigh muscle (main effect: −6 (−9, −3) cm2, p<0.0001), abdominal subcutaneous fat (main effect: −5 (−20,11) cm2, p<0.0001), and visceral fat were significantly decreased (main effect: −31 (−43, −19) cm2, p<0.0001) (Table 3); there were no significant changes with Exercise. There was no change in pericardial or epicardial fat. There were no significant Exercise by Diet interactions. (Table 3).
Table 3.
Body Composition, Cardiac Function and Vascular Function by Factorial Group
| Variable | Exercise Factorial Groups | Diet Factorial Groups | Interaction between Exercise and Diet |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall Baseline |
Exercise (n=46) |
No Exercise (n=46) |
Exercise Main Effect |
P- Value |
Diet (n=46) |
No Diet (n=46) |
Diet Main Effect |
P- Value |
||
| Body composition | Mean ± SD | FU LSMean (95% CI) |
FU LSMean (95% CI) |
Difference (95% CI) |
FU LSMean (95% CI) |
FU LSMean (95% CI) |
Difference (95% CI) |
P-Value | ||
| Weight (kg) | 106 ± 18 | 99 (97, 101) | 102 (100, 104) | −3 (−5, −1) | <0.0001 | 97 (95, 99) | 104 (102, 106) | −7 (−9, −5) | <0.0001 | 0.82 |
| DXA measurements | ||||||||||
| Total non-bone lean (kg) | 53 ± 9 | 51 (50, 52) | 52 (51, 53) | −1 (−1, 0) | 0.25 | 50 (49, 51) | 52 (51, 53) | −2 (−3, −1) | <0.0001 | 0.24 |
| Total fat (kg) | 47 ± 10 | 42 (41, 43) | 44 (43, 45) | −2 (−3, −1) | 0.001 | 41 (40, 42) | 46 (45, 47) | −5 (−6, −4) | <0.0001 | 0.67 |
| Total non-bone lean (%) | 52 ± 6 | 54 (53, 55) | 53 (53, 53) | 1 (0, 1) | 0.066 | 54 (53, 55) | 52 (51, 53) | 2 (1, 3) | <0.0001 | 0.23 |
| Total fat (%) | 45 ± 6 | 44 (43, 45) | 45 (44, 46) | −1 (−1, 0) | 0.057 | 43 (42, 44) | 46 (45, 47) | −2 (−3, −1) | <0.0001 | 0.24 |
| MRI measurements | ||||||||||
| Thigh subcut fat (cm2) | 165 ± 78 | 149 (145, 153) | 152 (148, 156) | −3 (−9, 3) | 0.26 | 143 (139, 147) | 159 (155, 163) | −16 (−22, −10) | <0.0001 | 0.13 |
| Thigh skeletal muscle (cm2) | 122 ± 26 | 117 (115, 119) | 118 (116, 120) | −2 (−4, 1) | 0.26 | 115 (113, 117) | 120 (118, 122) | −6 (−9, −3) | <0.0001 | 0.91 |
| Thigh IM fat (cm2) | 25 ± 9 | 24 (22, 26) | 25 (23, 27) | −1 (−2, 0) | 0.19 | 24 (22, 26) | 25 (23, 27) | −1 (−2, 1) | 0.31 | 0.40 |
| Thigh SM/IM fat ratio | 5.4 ± 2.4 | 5.6 (5.4, 5.8) | 5.5 (5.3, 5.7) | 0.1 (−0.3, 0.5) | 0.55 | 5.6 (5.4, 5.8) | 5.5 (5.3, 5.7) | 0.1 (−0.3, 0.5) | 0.53 | 0.25 |
| Abd subcut fat (cm2) | 378 ± 152 | 341 (329, 353) | 346 (336, 356) | −5 (−20, 11) | 0.55 | 321 (311, 331) | 372 (360, 384) | −51 (−66, −35) | <0.0001 | 0.86 |
| Abd visceral fat (cm2) | 213 ± 108 | 188 (180, 196) | 198 (190, 206) | −10 (−22, 2) | 0.11 | 180 (172, 188) | 211 (203, 219) | −31 (−43, −19) | <0.0001 | 0.88 |
| Epicardial fat (cm3) | 36 ± 17 | 38 (36, 40) | 36 (34, 38) | 2 (−2, 6) | 0.33 | 37 (35, 39) | 36 (32, 40) | 1 (−3, 5) | 0.66 | 0.54 |
| Pericardial fat (cm3) | 64 ± 41 | 55 (51, 59) | 58 (54, 62) | −3 (−9, 2) | 0.24 | 55 (51, 59) | 58 (54, 62) | −3 (−9, 3) | 0.31 | 0.23 |
| Cardiac function | ||||||||||
| MRI measurements | ||||||||||
| Mass (g) | 95 ± 19 | 94 (92, 96) | 92 (90, 94) | 3 (−1, 6) | 0.10 | 91 (89, 93) | 95 (93, 97) | −4 (−7, 0) | 0.034 | 0.10 |
| End diastolic volume (ml) | 122 ± 25 | 122 (118, 126) | 124 (120, 128) | −1 (−8, 6) | 0.84 | 124 (120, 128) | 122 (116, 128) | 2 (−5, 9) | 0.64 | 0.051 |
| Ejection fraction (%) | 61 ± 6 | 61 (59, 63) | 61 (59, 63) | 0 (−2, 2) | 0.89 | 61 (59, 63) | 62 (60, 64) | −1 (−3, 1) | 0.53 | 0.04 |
| Variable | Exercise Factorial Groups | Diet Factorial Groups | Interaction between Exercise and Diet | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall Baseline | Exercise (n=46) | No Exercise (n=46) | Exercise Main Effect | P-Value | Diet (n=46) | No Diet (n=46) | Diet Main Effect | P-Value | ||
| Cardiac function | Mean ± SD | FU LSMean (95% CI) | FU LSMean (95% CI) | Difference (95% CI) | FU LSMean (95% CI) | FU LSMean (95% CI) | Difference (95% CI) | P-Value | ||
| Echo-Doppler measurements | ||||||||||
| LV Mass (g) | 212 ± 59 | 213 (205, 221) | 208 (200, 216) | 5 (−6, 16) | 0.36 | 211 (203, 219) | 210 (202, 218) | 0 (−10, 11) | 0.93 | 0.71 |
| Relative wall thickness | 0.57 ± 0.11 | 0.55 (0.53, 0.57) | 0.56 (0.54, 0.58) | −0.01 (−0.03, 0.01) | 0.25 | 0.54 (0.52, 0.56) | 0.57 (0.55, 0.59) | −0.03 (−0.05, −0.01) | 0.005 | 0.75 |
| LA Diameter (cm) | 4.0 ± 0.5 | 4.0 (4.0, 4.0) | 4.0 (4.0, 4.0) | 0 (−0.1, 0.1) | 0.75 | 4.0 (4.0, 4.0) | 4.0 (4.0, 4.0) | 0 (0, 0.1) | 0.42 | 0.84 |
| Cardiac index (L/min/m2) | 4.6 ± 1.1 | 4.7 (4.5–4.9) | 4.8 (4.6, 5.0) | −0.02 (−0.41, 0.36) | 0.90 | 4.8 (4.6, 5.0) | 4.7 (4.5, 4.9) | 0.05 (−0.33, 0.43) | 0.78 | 0.42 |
| E/A ratio | 0.87 ± 0.20 | 0.88 (0.82, 0.94) | 0.89 (0.83, 0.95) | −0.02 (−0.09, 0.06) | 0.73 | 0.93 (0.87, 0.99) | 0.83 (0.77, 0.89) | 0.10 (0.02, 0.17) | 0.014 | 0.13 |
| E’ (cm/s) | 6.2 ± 1.5 | 6.1 (5.7, 6.5) | 6.3 (5.9, 6.7) | −0.2 (−0.7, 0.4) | 0.56 | 6.2 (5.8, 6.6) | 6.2 (5.8, 6.6) | 0.1 (−0.5, 0.6) | 0.82 | 0.76 |
| E/ e’ ratio | 13.0 ± 3.6 | 13.1 (12.1, 14.1) | 12.9 (11.9, 13.9) | 0.1 (−1.3, 1.6) | 0.80 | 13.3 (12.3, 14.3) | 12.7 (11.7, 13.7) | 0.6 (−0.8, 2.0) | 0.39 | 0.28 |
| Vascular function | ||||||||||
| Arterial stiffness (cm/s) | 1047 ± 291 | 994 (925, 1063) | 1009 (938, 1080) | −15 (−115, 85) | 0.77 | 977 (906, 1048) | 1026 (957, 1095) | −50 (−150, 51) | 0.34 | 0.77 |
Data are presented as overall baseline mean ± SD and LSMean (95% CI) at follow-up visit for each of the factorial groups with main effect and 95% CI. P-value represents comparison of least square means at final visit following adjustment for overall baseline values, gender, and beta-blocker use. Abbreviations: FU, follow-up; DXA, dual x-ray absorptiometry; MRI, magnetic resonance imaging; subcut, subcutaneous; IM, intermuscular; SM, skeletal muscle; Abd, abdominal; LV, left ventricle; LA, left atrium; E/A, early to atrial filling velocity; e’, early mitral annulus velocity (septal). Relative wall thickness is the ratio of wall thickness divided by chamber size. Cardiac output measured by echo-Doppler LV outflow tract technique. Arterial stiffness determined using pulse wave velocity from carotid to femoral artery by Doppler.
Cardiovascular Function
With Diet, LV mass by MRI (main effect: −4 (−7,0) g, p=0.034) and LV relative wall thickness by echocardiography (main effect: −0.03 (−0.05, −0.01), p=0.005) were significantly decreased and mitral E/A velocity ratio (main effect: 0.10 (0.02,0.17), p=0.014) was significantly increased (Table 3). No other cardiac MRI or echo-Doppler measure was significantly changed (Table 3; Supplemental eTable 3). Arterial pulse-wave velocity was unchanged by either Diet or Exercise (Table 3).
Symptoms
With both Diet and Exercise, NYHA symptom class significantly improved (Exercise main effect: −0.4 (−0.6, −0.2) class, p<0.0001; Diet main effect: −0.4 (−0.5, −0.2) class, p=0.0001) (Table 2).
Inflammation and Lipids
With Diet but not Exercise, CRP was significantly reduced (Diet main effect: −2.8 (−4.9, −0.7) μg/L, p=0.023; Exercise main effect: −0.4 (−2.5,1.6), p=0.44); changes in IL-6 were non-significant (Diet main effect: −0.8 pg/ml (−1.5, −0.1), p=0.086; Exercise main effect: 0.4 (−0.3,1.1), p=0.51); there was no interaction (see also Supplementary eTable 4). The reduction in CRP correlated with the reduction in weight (r=0.29; p=0.005). With Diet but not Exercise, there were significant reductions in total cholesterol (Diet main effect: −14 (−24, −14) mg/dl, p=0.008; Exercise main effect: −4 (−14,6), p=0.40) and LDL cholesterol (Diet main effect: −13 (−21, −4) mg/dL, p=0.008; Exercise main effect: −4 (−12,4), p=0.35) (Supplementary eTable 4); these changes persisted after adjustment for lipid lowering medications.
Associations with Change in Exercise Capacity
In the overall groups combined, change in peak VO2 was inversely related to change in total mass and fat mass, and was positively related to change in percent lean body mass and thigh skeletal muscle/intermuscular fat ratio (SM/IMF) (Supplementary eFigure 3); there were also correlations with change in LV mass (r=−0.27, p=0.02) and CRP (r=−0.21; p=0.047). Similar results were observed with exercise time as the exercise capacity variable. Multiple stepwise regression showed that gender and change in total mass were the only independent predictors of peak VO2 (see online Supplemental Statistical Analysis section).
Intervention Fidelity
Participants completing the Exercise interventions attended a median of 84±14% of the exercise sessions, and together progressed from an average 19±6 minutes at a 2.8±0.4 metabolic equivalent (MET) level at week 1 to an average 49±10 minutes at a 3.8±1.2 MET level at week 20 Further details regarding attendance and progression are in the online supplement.
The average actual caloric intake deficit was 388±55 kcal/d for Diet-only participants and 355±23 kcal/d for Exercise+Diet participants. Dietary compliance (actual vs. prescribed calorie level) from recorded food logs was 99±1% for both Diet groups.
Adverse Events
Five adverse events judged as possibly related to the intervention occurred among 5 participants: hypoglycemia between meals in 2 participants (Diet, and Exercise+Diet groups), ankle pain and swelling later diagnosed as partial tendon tear (Exercise+Diet), stress foot fracture (Exercise), and an episode of unusual shortness of breath during exercise (Exercise). Three participants had a total of 6 hospitalizations, all judged unrelated to study participation: 1 participant was hospitalized for pancreatitis (Exercise), 1 participant had 3 hospitalizations for HF exacerbation/dyspnea (Exercise+Diet), and 1 participant had 2 hospitalizations for leg edema, pain, and erythema (Control). There were no deaths.
DISCUSSION
The major novel findings of this randomized controlled trial are that in older, obese patients with chronic, stable HFPEF intentional weight loss via caloric restriction Diet was feasible, appeared safe, and significantly improved the co-primary outcome of exercise capacity. The combination of Diet with Exercise, the only intervention previously shown to improve exercise capacity in HFPEF,15, 16, 24 produced a robust increase in exercise capacity. The co-primary outcome of QOL, as measured by the MLHF total score, did not show a significant change with either Exercise or Diet.
Diet significantly improved two other widely accepted, standardized measures of QOL, the KCCQ score (a HF-specific QOL instrument) and the SF-36 physical score (a general QOL instrument). The improvement in KCCQ score was greater than the accepted threshold for clinical relevance.21 The statistically significant, clinically meaningful improvement in KCCQ score with Diet occurred even though the change in MLHF total score did not achieve statistical significance. This suggests that the study may have been underpowered for the MLHF instrument and that the KCCQ instrument may be more sensitive to change in QOL in older HFPEF patients.
Diet also significantly improved body composition, leg muscle quality, lipid profile, and inflammation biomarkers. The improvement in exercise capacity was associated with improved body composition, particularly reduced total fat mass and thigh muscle/intermuscular fat ratio, and with reduced inflammation and LV mass.
These results are credible since studies of Diet alone or in combination with Exercise in non-HF clinical populations have shown similar overall findings.11, 12 In a randomized clinical trial with similar design and sample size of obese frail older adults, Diet, Exercise, and their combination significantly improved peak VO2 and other measures of physical function and the effects of Diet and Exercise were additive (complementary).11 Other studies also have shown significantly greater improvements in body composition with Diet than Exercise.11, 25 Our finding of more improvement in QOL measures with Diet than with Exercise is also credible based on prior Exercise-Diet studies in obese older persons11 and since Exercise has not consistently improved QOL in HFPEF.15, 16, 24 Further, preliminary reports have indicated that weight reduction via bariatric surgery can prevent the onset of HF, and can improve exercise capacity in patients with HF and reduced EF (HFREF).26, 27
As others have indicated, peak VO2 relative to body weight (ml/kg/min), the pre-planned co-primary outcome, is the most relevant measure of exercise capacity during weight-bearing (treadmill) exercise.11,28 A true increase in exercise tolerance with Diet is further supported by: a) significant increases in 4 other measures that are relatively independent of body mass (VO2 reserve, exercise time to exhaustion, workload, 6MWD); b) preservation of absolute peak VO2 (ml/min); c) improvement in leg power that occurred despite significant loss of muscle mass. The largest increase in exercise capacity was with Exercise+Diet combined.
What are the potential mechanisms underlying improved exercise capacity? Increased adipose tissue mass promotes inflammation, hypertension, insulin resistance, and dyslipidemia resulting in impaired cardiac, arterial, and skeletal muscle function, all of which contribute to reduced exercise capacity in HFPEF patients9, 29–33 and can be reversed with Diet.6, 8, 9, 13, 34 Using DXA, we recently reported that percent body fat and percent leg fat were significantly increased while percent body lean and leg lean mass were reduced in older HFPEF patients versus controls and were related to reduced exercise capacity.10 Using MRI, we found that older HFPEF patients have increased thigh IMF, despite a normal amount of subcutaneous fat.6 Furthermore, the SM/IMF ratio was increased and both IMF area and SM/IMF ratio were independent predictors of peak VO2.6 IMF may compete with muscle tissue for critical blood flow during exercise reducing perfusive O2.35 IMF may also reduce diffusive O2 transport by increasing the distance O2 must traverse from the capillary to the muscle mitochondria. Furthermore, increased IMF may reduce skeletal muscle capillary density and mitochondrial biogenesis and oxidative metabolism, all of which are abnormal in HFPEF.31, 33, 36 In our study, increased peak VO2 was associated with reduced fat mass, increased percent lean mass and thigh SM/IMF ratio, and reduced inflammation biomarkers. Thus, improvement in peak VO2 from Diet and Exercise may be due to reduced inflammation and enhanced mitochondrial function, attenuated reactive oxygen species generation, increased vascular oxidative stress resistance, increased nitric oxide bioavailability and improved microvascular function. Together, these may increase diffusive O2 transport and/or O2 utilization by the active muscles.37
With Diet, LV mass and relative wall thickness decreased and LV E/A ratio increased, but we observed no other improvements in resting cardiac function. We also observed no significant changes in epicardial or pericardial fat, in contrast to reduced adipose tissue elsewhere. Although we did not measure cardiac function during exercise, these data suggest that the improvements we observed with Diet and its combination with Exercise may be due primarily to favorable ‘non-cardiac’ peripheral adaptations, in accord with reports of Exercise in HFPEF.16, 32
Because of the reported ‘HF obesity paradox’ (lower mortality observed in overweight/obese),5 before Diet can be recommended for obese HFPEF patients, further studies likely are needed to determine whether these favorable changes are associated with reduced clinical events. However, a recent meta-analysis of randomized trials in older patients without HF indicates that intentional weight loss from Diet is associated with a 15% reduction in total mortality.38
As observed in other Diet studies and despite adequate protein intake, there was a significant decrease in muscle mass with Diet that was not prevented by Exercise. Although the long-term consequences of this are unclear, the muscle loss did not prevent increases in exercise capacity or leg power. Inclusion of strength training may have reduced loss of muscle mass during Diet.
Limitations
This was a randomized, clinical trial with frequent monitoring, professionally administered Diet, and medically supervised Exercise; safety and efficacy could differ under other conditions. The minimum BMI was 30 kg/m2 which includes most HFPEF patients.4, 5 However, our data do not address safety and efficacy of Diet in patients with lower BMI.
Our patients had typical clinical features of HFPEF (including severe exercise intolerance, LV hypertrophy and diastolic dysfunction, 76% on maintenance diuretics) and met pre-determined criteria for HFPEF utilized in prior publications and recommended by the AHA/ACC14 and ESC. The relatively modest BNP levels likely result from: 1) non-hospitalized, stable patients who were clinically well-compensated as required for exhaustive exercise testing and the 20-week intervention; 2) a strong, inverse relation that exists between BNP and BMI,39 such that when matched for other disease variables, BNP is much lower in obese than non-obese HFPEF patients, frequently <100pg/ml;39, 40 3) BNP levels are significantly lower in HFPEF than HFREF due to lower LV wall stress.23
Conclusions
Among obese older patients with clinically stable heart failure and preserved ejection fraction, caloric restriction diet or aerobic exercise training increased peak oxygen consumption, and the effects may be additive. Neither intervention had a significant effect on quality of life as measured by the Minnesota Living with Heart Failure Questionnaire.
Supplementary Material
Figure 2.
Adjusted individual changes and means with 95% CIs at the 20-week follow-up visit relative to baseline by factorial group of the primary study outcomes: peak VO2 (ml/kg/min, panel A) and Minnesota Living with Heart Failure Questionnaire (MLHF Overall Score, range 0–105, higher score indicates worse HF-related QOL, panel B). The p-values represent comparison of least square means of the outcome measure following adjustment for baseline values, gender, and beta-blocker use. By factorial group, peak VO2 data are missing in 4 cases: 2 in the Exercise group (due to gas leak and injury), 1 in the Diet group (due to injury), and 1 in the No Diet group (due to gas leak). By factorial group, MLHF data are missing in 4 cases: 2 in the Diet group, 1 in the Exercise group, and 1 in the No Exercise group all due to patient errors.
Acknowledgments
Funding: This study was supported by the following National Institutes of Health research grants: R01AG18915, P30AG021332, R01HL093713, and R01AG020583. Also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine at Wake Forest School of Medicine (Dr. Kitzman) and the Mortiz Chair in Geriatric Nursing Research in the College of Nursing and Health Innovation at The University of Texas at Arlington (Dr. Haykowsky).
Footnotes
Responsibility for data and analyses: Dalane W. Kitzman, MD, and Timothy Morgan, PhD had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Roles of the authors: Dalane W. Kitzman, MD: (1) substantial contributions to conception, design, acquisition, analysis, and interpretation of data; (2) drafting of the work and revising it critically for important intellectual content; (3) final approval of the version to be published; (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Peter Brubaker, PhD: 1) substantial contributions to conception, design, acquisition, analysis, and interpretation of data; (2) drafting of the work and revising it critically for important intellectual content; (3) final approval of the version to be published; (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Timothy Morgan, PhD: 1) substantial contributions to conception, design, acquisition, analysis, and interpretation of data; (2) drafting of the work and revising it critically for important intellectual content; (3) final approval of the version to be published; (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mark Haykowsky, PhD: 1) substantial contributions to conception, design, acquisition, analysis, and interpretation of data; (2) drafting of the work and revising it critically for important intellectual content; (3) final approval of the version to be published; (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Gregory Hundley, MD: 1) substantial contributions to conception, design, acquisition, analysis, and interpretation of data; (2) drafting of the work and revising it critically for important intellectual content; (3) final approval of the version to be published; (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
William E. Kraus, MD: 1) substantial contributions to conception, design, acquisition, analysis, and interpretation of data; (2) drafting of the work and revising it critically for important intellectual content; (3) final approval of the version to be published; (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Joel Eggebeen, MS: 1) substantial contributions to conception, design, acquisition, analysis, and interpretation of data; (2) drafting of the work and revising it critically for important intellectual content; (3) final approval of the version to be published; (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Barbara J. Nicklas, PhD: 1) substantial contributions to conception, design, acquisition, analysis, and interpretation of data; (2) drafting of the work and revising it critically for important intellectual content; (3) final approval of the version to be published; (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Role of sponsors: Sponsors and funding agencies had no role in conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Potential conflicts of Interest
Dr. Kitzman is a consultant for Relypsa Inc., Abbvie, Regeneron, GlaxoSmithKline, Merck, and Corvia Medical, receives grant support from Novartis, and owns stock in Gilead Sciences and stock options in Relypsa. No other members of the writing group have conflicts of interest to declare.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006 Jul 20;355(3):251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Morkedal B, Vatten L, Romundstad P, Laugsand L, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals. The HUNT Study, Norway. J Am Coll Cardiol. 2014 Jan 8; doi: 10.1016/j.jacc.2013.11.035. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002 Aug 1;347(5):305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 4.Redfield M, Chen H, Borlaug B, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013 Mar 11;309(12):1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haass M, Kitzman DW, Anand IS, et al. Body Mass Index and Adverse Cardiovascular Outcomes in Heart Failure Patients With Preserved Ejection Fraction / Clinical Perspective. Circ Heart Fail. 2011 May 1;4(3):324–31. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haykowsky M, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113(7):1211–6. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beavers K, Beavers D, Houston D, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013 Mar 1;97(3):552–60. doi: 10.3945/ajcn.112.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Normandin E, Houston DK, Nicklas BJ. Caloric restriction for treatment of geriatric obesity: Do the benefits outweigh the risks? Curr Opin Cardiol. 2015 Jun;4(2):143–55. doi: 10.1007/s13668-015-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma K, Kass D. Heart Failure With Preserved Ejection Fraction: Mechanisms, Clinical Features, and Therapies. Circ Res. 2014 Jun 20;115(1):79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haykowsky M, Brubaker P, Morgan T, Kritchevsky S, Eggebeen J, Kitzman D. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68(8):968–75. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villareal D, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011 Mar 30;364(13):1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beavers KM, Miller ME, Rejeski WJ, Nicklas BJ, Krichevsky SB. Fat mass loss predicts gain in physical function with intentional weight loss in older adults. J Gerontol A Biol Sci Med Sci. 2013 Jan 1;68(1):80–6. doi: 10.1093/gerona/gls092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prior S, Blumenthal J, Katzel L, Goldberg A, Ryan A. Increased Skeletal Muscle Capillarization After Aerobic Exercise Training and Weight Loss Improves Insulin Sensitivity in Adults With IGT. Diabetes Care. 2014 Mar 4;37(5):1469–75. doi: 10.2337/dc13-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the management of heart-failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Oct 15;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Kitzman D, Brubaker P, Morgan T, Stewart K, Little W. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010 Nov 1;3(6):659–67. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62(7):584–92. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–6. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 18.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney R. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–5. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 19.Scott JM, Haykowsky MJ, Eggebeen J, Morgan TM, Brubaker PH, Kitzman DW. Reliability of peak exercise testing in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2012 Dec 15;110(12):1809–13. doi: 10.1016/j.amjcard.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota living with heart failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Am Heart J. 1992;124:1017–25. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 21.Flynn K, Pina LI, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: Findings from the HF-ACTION Randomized controlled Study. JAMA. 2009;301(14):1451–9. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JEJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 23.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002 Nov 6;288(17):2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 24.Edelmann F, Gelbrich G, Dungen H, et al. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011 Oct 18;58(17):1780–91. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 25.Nicklas B, Wang X, You T, et al. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009 Apr 1;89(4):1043–52. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miranda W, Batsis J, Sarr M, et al. Impact of Bariatric Surgery on Quality of Life, Functional Capacity, and Symptoms in Patients with Heart Failure. OBES SURG. 2013;23(7):1011–5. doi: 10.1007/s11695-013-0953-8. [DOI] [PubMed] [Google Scholar]
- 27.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric Surgery and Long-term Cardiovascular Events. JAMA. 2012 Jan 4;307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 28.Wilms B, Ernst B, Thurnheer M, Weisser B, Schultes B. Differential changes in exercise performance after massive weight loss induced by bariatric surgery. OBES SURG. 2013 Mar;23(3):365–71. doi: 10.1007/s11695-012-0795-9. [DOI] [PubMed] [Google Scholar]
- 29.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of Exercise Intolerance in Elderly Heart Failure Patients With Preserved Ejection Fraction. J Am Coll Cardiol. 2011 Jul 12;58(3):265–74. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015 Mar;8(2):286–94. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306(9):H1364–70. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012 Jul 10;60(2):120–8. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott Bowen T, Rolim NP, Fischer T, et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail. 2015 Feb 1; doi: 10.1002/ejhf.239. [Epub ahead of print] http://dx.doi.org/10.1002/ejhf.239. [DOI] [PubMed]
- 34.Jensen M, Ryan D, Donato K, et al. Guidelines (2013) for managing overweight and obesity in adults. Obesity Res. 2014 Jul 1;22(S2):S1–S410. doi: 10.1002/oby.20819. [DOI] [PubMed] [Google Scholar]
- 35.Heinonen I, Bucci M, Kemppainen J, et al. Regulation of subcutaneous adipose tissue blood flow during exercise in humans. Jvf Applied Physiology. 2012 Mar 15;112(6):1059–63. doi: 10.1152/japplphysiol.00732.2011. [DOI] [PubMed] [Google Scholar]
- 36.Bharadwaj M, Nicklas B, Molina AJ. Obesity is accompanied by alterations in mitochondrial respiration in older adults. Gerontologist. 2013;53(S1):945–59. [Google Scholar]
- 37.Civitarese AE, Carling S, Heilbronn LK, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4(3):e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS ONE. 2015;10:e0121993. doi: 10.1371/journal.pone.0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stavrakis S, Pakala A, Thomas J, Chaudhry MA, Thadani U. Obesity, Brain Natriuretic Peptide Levels and Mortality in Patients Hospitalized With Heart Failure and Preserved Left Ventricular Systolic Function. Am J Med Sci. 2013;345(3):211–7. doi: 10.1097/MAJ.0b013e318271c012. [DOI] [PubMed] [Google Scholar]
- 40.Anjan VY, Loftus TM, Burke MA, et al. Prevalence, Clinical Phenotype, and Outcomes Associated With Normal B-Type Natriuretic Peptide Levels in Heart Failure With Preserved Ejection Fraction. Am J Cardiol. 2012 Sep 15;110(6):870–6. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.