Abstract
Primary Objective
To assess conversational synchrony in moderate to severe traumatic brain injury (TBI). Conversational synchrony, assessed by the similarity and coordination of words and words per turn, allows for effective and efficient communication and enhances the development of rapport.
Research Design
Eighteen participants with TBI (7 females) and nineteen healthy comparison participants (CP; 8 females) engaged in a 10-minute conversation with an unfamiliar partner.
Methods and Procedures
Conversational synchrony was assessed in these conversations by measuring the degree to which the participants’ productions of words and words per turn became more similar to one another over the course of the session
Main Outcomes and Results
Significantly more sessions with participants with TBI (11/18 for words, 9/18 for words per turn) compared to CP sessions (5/19 for words, 4/19 for words per turns) did not display conversational synchrony. Likewise, synchrony was significantly correlated with subjective ratings of the interaction from raters who were blind to participant status and the study hypotheses.
Conclusions
These results suggest that TBI can disrupt conversational synchrony and can, in turn, negatively impact social perceptions. The relationship between impaired conversational synchrony and other social communicative deficits in TBI warrants further study.
Keywords: interpersonal coordination, speech convergence, communication accommodation, discourse, traumatic brain injury
1. Introduction
Moderate to severe traumatic brain injury (TBI) frequently results in chronic and debilitating impairments in cognition, behaviour, and emotion. Of particular interest are the changes that occur in social behaviour and communication, as deficits in these areas are often the most common complaints from patients and family members and are major predictors of failure to reengage into society {1, 2, 3, 4]. Indeed, impairments in social communication are considered a hallmark of TBI although the majority of participants with TBI (an estimated 70%) do not have primary linguistic deficits, such as aphasia [5]. The reported impairments tend to be in higher level aspects of social interaction and conversation, including difficulty with maintaining topic, organizing discourse, reinforcing the conversational partner, regulating the timing of turn taking, and timing eye gaze [6, 7, 8]. Overall, conversations including an individual with traumatic brain injury are rated as less interesting, less rewarding, less appropriate, and more effortful compared to non-brain injured participants [9].
Despite recognition of the social and communicative nature of the deficits in TBI, the majority of research protocols examining communication impairments following TBI have focused on the productions of individuals with TBI in isolation [e.g., 10; although see 11]. Yet, communication is not accomplished by the acts of a single person, rather two or more participants work together to shape the nature of the interaction, even if one participant is simply listening [12]. We have argued that to understand the complex communication deficits that occur in real world social situations, clinical and research protocols should move away from examining the isolated productions of individuals with TBI and move towards understanding the ways in which partners jointly collaborate and coordinate together, as communication is shared and co-constructed between partners [13]. Such an approach offers greater ecological validity and increases the likelihood of capturing the full range of (the sometimes subtle yet disruptive) social communicative deficits in TBI. Furthermore, from the perspective that communication is a socially distributed cognitive activity [14, 15], studying the conversational interactions of individuals with TBI and communication partners provides an opportunity to understand how consequences of social communication deficits affect the communicative practices of both partners and the interaction as a whole.
Conversation is the cornerstone of social communication. Conversation requires significant coordination and synchrony of multiple aspects of behaviour. Not only do participants coordinate the content of their speech, they also tend to coordinate non-content aspects of speech and nonverbal behaviours. Participants tend to synchronize (i.e., become more similar over time) multiple behaviours across many aspects of conversation, such as length of speaking turn, total speaking time, accents, postures, gestures, emotional expressions, and actions with each other [16, 17, 18, 19]. These forms of conversational synchrony serve numerous purposes, including signaling active interest mutual understanding, and enhancing rapport and social bonds [16, 18]. This synchrony can be seen both when studying individual behaviours or the overall interaction, as blind raters can reliably distinguish between interactions of high and low synchrony by watching video recordings of the conversations, and these ratings correlate with the participant’s ratings of conversation satisfaction [20, 21].
Considering the importance of conversational synchrony for effective communication and the development of rapport, we suspect that many of the known deficits in social communication (e.g., regulating the timing of social behaviour, disorganized discourse, difficulties reinforcing conversational partners) in TBI may be related to disruptions in conversational synchrony. This hypothesized link between conversational synchrony and TBI is supported by our previous work examining neural structures involved in conversational synchrony. Although widespread neural dysfunction is common in TBI due to shearing of axonal connections, one of the most vulnerable brain regions in TBI is the ventromedial prefrontal cortex (vmPFC). The vmPFC is dorsal to the bony protuberances on the base of the skull, which can result in significant damage and tearing as the brain shifts during impact in TBI [22]. In previous work [23], we found that the vmPFC is a critical brain region for conversational synchrony. Non-content conversational synchrony was assessed by measuring the degree to which the participants’ productions of words and words per turn became more similar to one another across the course of the session. In healthy participant pairs, the target participants and their partners became more similar to each other in terms of their productions of both words and words per turn over the course of the interaction. In fact, by the end of the session, on average, both participants were contributing equally (each producing approximately 50% of the words). In striking contrast, vmPFC pairs did not become more similar to each other in terms of words or words per turn. Rather, the vmPFC participants tended to dominate the sessions and produced more words and words per turn than their partner throughout (on average, producing 69% more words). This lack of synchrony was replicated in two follow-up sessions where the vmPFC participants conversed with two new partners. These results indicate that dysfunction of the vmPFC can negatively impact conversational synchrony. Likewise, Body [24] argues that because conversation is largely unpredictable and requires rapidly processing information, dysfunction of the vmPFC in TBI may disrupt the selection of social appropriate outcomes and behaviours during conversation. This further supports our prediction that traumatic brain injury will disrupt conversational synchrony.
The goal of this study is to characterize the effect of TBI on conversational synchrony. In order to quantify conversational synchrony, we analyzed convergence of noncontent variables, specifically words and words per turn [19, 23]. Unlike traditional linguistic approaches that have focused on the content of the productions of the participant with brain injury alone, here we use a distributed cognition approach to examine the influence of both partners on the nature of dialogue [13]. Careful characterization of the types of social communication problems that can result from traumatic brain injury is particularly important as they are often subtle and may not be readily attributed to their brain damage [25].
2. Methods
2.1. Participants
Individuals with TBI were recruited through the Iowa Traumatic Brain Injury Registry at the University of Iowa. Participants included eighteen individuals (7 females) with traumatic brain injury. Etiological and neuropsychological data are presented in Tables 1 and 2. Fitting with the heterogeneity of the disorder, there is considerable variability in the neuropsychological profiles of the participants; many perform within normal limits across domains, in other cases there are notable deficits in general intellect, memory, and executive function across participants, shown in bold in Table 2. In order to assess the severity of the injury, we used a combination of the Glasgow Come Scale (GCS) [26] and available information on loss of consciousness (LOC), post-traumatic anterograde amnesia (PTA), and acute CT findings. In correspondence with the criteria described in the Mayo Classification System [27], participants were classified as mild when GCS was 13–15, positive acute CT findings were unremarkable, no focal lesions were visible on a chronic MRI, LOC was 30 minutes or less and PTA was shorter that 24 hours. Participants were classified as moderate-severe when GCS was less than 12, positive acute CT findings or chronic intracranial abnormality defined as focal lesions visible on MRI were present, LOC was longer than 30 minutes and PTA longer than 24 hours; if only one of these criteria was met, it was sufficient to classify the participant as moderate-severe (Table 1). Of the 18 participants with TBI in the current study, 2 were classified as mild, all others were classified as moderate-severe. All participants were free of dysarthria and aphasia (determined by a certified speech-language pathologist and/or the Western Aphasia Battery [28]). Nineteen healthy comparison participants (CP) (8 females), recruited from the Iowa City community, free of neurological and psychiatric conditions, were matched to the participants with TBI on sex, age (TBI, M=49.67; CP, M=49.74, t(35)=0.13, p=0.98), and education (TBI, M=14.22; CP, M=150, t(35)=1.12, p=0.27). All participants gave informed written consent approved by the Institutional Review Board of the University of Iowa.
Table 1.
Demographic and clinical information for participants with TBI
| Participant | Sex | Edu (yrs) |
Age at Testing (yrs) |
Chronicity (yrs) |
Etiology | GCS | RA* | PTA* | LOC | Acute CT findings | Focal Lesions (Chronic MRI) |
Severity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 13 | 76 | 27 | Baseball accident | n/a | None | 2 weeks | n/a | n/a | Bilateral frontal lobe | Moderate-severe |
| 2 | F | 12 | 66 | 29 | Unhelmeted bicycle accident | n/a | None | 3 days | n/a | n/a | Left temporal | Moderate-severe |
| 3 | F | 12 | 69 | 1 | Fall | 12 | 30 minutes | 22 days | 2 weeks | SAH (required hemicraniotomy) | Left temporal lobe, Right Frontal lobe | Moderate-severe |
| 4 | M | 13 | 48 | 6 | Unhelmeted MVA | n/a | n/a | No memory of accident | n/a | Right frontal EDH | n/a | Moderate-severe |
| 5 | M | 18 | 27 | 1 | Unhelmeted MVA | 13 | None | 3 days | None | n/a | n/a | Moderate-severe |
| 6 | M | 15 | 24 | 3 | Fall | 10 | None | 2 days | n/a | Bifrontal contusions (required craniotomy) | Bilateral frontal lobe, right temporal pole, left cerebellum | Moderate-severe |
| 7 | M | 11 | 38 | 3 | Unhelmeted MVA | 13 | None | 7 weeks | 3–4 months | SAH, hyperdensity in right temporal lobe | n/a | Moderate-severe |
| 8 | M | 16 | 25 | 6 | Fall | n/a | n/a | 1 day | ~2 days | Basilar Skull Fracture | Bilateral frontal pole and orbital regions | Moderate-severe |
| 9 | M | 20 | 70 | 14 | Fall | n/a | n/a | 1 day | 1–2 weeks | Small bleed | n/a | Moderate-severe |
| 10** | M | 12 | 51 | 25/5 | Fall/MVA | 15 | n/a/ n/a | 1 day/No | n/a / n/a | No | n/a / n/a | Moderate-severe |
| 11 | F | 18 | 48 | 2 | Unhelmeted MVA | 6 | n/a | Duration Unclear | Duration unclear | SAH | Left superior frontal gyrus | Moderate-severe |
| 12 | M | 12 | 59 | 5 | Fall | n/a | n/a | 1–2 months | A few seconds | Negative | No | Mild |
| 13 | F | 14 | 53 | 6 | Fall | 15 | A few minutes | A few minutes | A few minutes | Bifrontal hemorrhagic contusions | Bilateral frontal pole | Moderate-severe |
| 14 | F | 14 | 60 | 2 | Fall | n/a | n/a | 4–5 hours | n/a | SAH | No | Moderate-severe |
| 15 | M | 13 | 20 | 2 | Fall | 15 | n/a | n/a | Duration unclear? | EDH, right temporal bone fracture (required craniotomy) | No | Moderate-severe |
| 16 | M | 15 | 63 | 3 | MVA | n/a | 1 month | 22 months | No | n/a | No | Mild |
| 17 | F | 16 | 43 | 2 | Assault | n/a | 15 minutes | 1 day | 5 minutes | SAH, occipital skull fracture | Right frontal lobe | Moderate-severe |
| 18 | F | 12 | 54 | 2 | Bicycle accident, unhelmeted | n/a | None | A couple of weeks | 20 minutes | Negative | No | Moderate-severe |
Note:
via patient/collateral report;
sustained two TBIs, data is reported for first/second where applicable,
GCS=Glasgow Coma Scale; RA=duration of retrograde amnesia; PTA=duration of post-traumatic amnesia; LOC= Loss of Consciousness; SAH= Subarachnoid Hemorrhage; EDH= Epidural Hemorrhage; SDH= Subdural Hemorrhage; M=male; F=female; R=right; Edu=education; MVA=motor vehicle accident; n/a= not available
Table 2.
TBI participant performance on neuropsychological measures
| Participant | WAIS-IV FSIQ | WMS-III GMI | AVLT (TR5/DR) | CFT (DR) | BNT | TT | WAB | WCST (cat/PE) | BDI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 111* | 118 | 14/13 | 25 | 59 | 44 | 99 | 1/43 | 10 |
| 2 | 115* | 134 | 14/14 | 15 | 56 | 44 | 100 | 6/12 | 0 |
| 3 | 87 | 107 | 5/8 | 6 | 56 | 44 | 100 | 0/94 | 19 |
| 4 | 65 | n/a | 5/0 | 9 | 53 | 28 | 94.4 | n/a | 10 |
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | 99 | n/a | n/a |
| 6 | 117 | 132 | 15/12 | 27 | 55 | 44 | 100 | 6/5 | 14 |
| 7 | 89 | n/a | 14/11 | n/a | 53 | 43 | 99.4 | n/a | 15 |
| 8 | 120 | 111 | 15/14 | 22 | 58 | n/a | 100 | 3/20 | 0 |
| 9 | 113 | 115 | 14/11 | 18 | 60 | 44 | 100 | 6/14 | 10 |
| 10 | 61 | n/a | 4/5 | 5.5 | 55 | 29 | 92.5 | n/a | 22 |
| 11 | 85 | 75 | 3/4 | 10 | 58 | 40 | 97.6 | n/a | 16 |
| 12 | 80 | 103 | 12/11 | 14 | 58 | 44 | 97.4 | 1/23 | 18 |
| 13 | 112 | 111 | 13/12 | 23 | 60 | 44 | 99.6 | 6/11 | 0 |
| 14 | 112 | 135 | 14/14 | 15 | 59 | n/a | 100 | n/a | 1 |
| 15 | 104 | n/a | n/a | 27 | n/a | n/a | 100 | n/a | n/a |
| 16 | 107 | 114 | 8/3 | 14 | 57 | 44 | 95.8 | 6/9 | 14 |
| 17 | 113 | n/a | 15/15 | 20 | 56 | 44 | 100 | 6/4 | 26 |
| 18 | 82 | 83 | 11/6 | 10 | 50 | n/a | 95 | n/a | 10 |
Note: WAIS = Wechsler Adult Intelligence Scale; FIQ=Full Scale Intelligence Quotient; WMS = Wechsler Memory Scale-III; GMI = General Memory Index; AVLT = Auditory Verbal Learning Test; TR5/DR= Trial 5/Delayed Response; CFT (DR) = Complex Figure Test, Delayed Response; BNT = Boston Naming Test; TT = Token Test; WAB = Western Aphasia Battery; = WCST (cat/PE) = Wisconsin Card Sorting Test, Number of Categories successfully achieved/Number of Perservative Errors; BDI = Beck Depression Inventory;
denotes WAIS-III score. Bold scores indicate a deficit.
2.2. Conversational sample
Conversational samples were collected from each participant with TBI and each healthy comparison participant as they conversed with an unfamiliar partner. These samples were collected as part of the Mediated Discourse Elicitation Protocol (MDEP), a protocol developed to collect ecologically valid discourse samples in a clinical or laboratory environment [29]. The MDEP involves the collection of multiple types of discourse (e.g., conversation, storytelling, procedural discourse), however the analyses in the current study were restricted to the conversational sample. Conversational partners were a licensed speech-language pathologist, four graduate speech-language pathology students and one undergraduate speech-language pathology student (all female). The same set of conversational partners were involved in data collection for both the TBI and CP sessions. The conversational partners were blind to the hypotheses of the study and were not given any instruction or formal training for the conversation sample (e.g., they were not told what to talk about, or how much to talk). Participants were told they were going to have a conversation, just as they might with anyone in everyday life.
2.3. Coding interactional turns and words
All sessions were videotaped and transcribed, and coded for interactional turns and words following previous procedures [23, 30]. Briefly, interactional turns were defined as utterances produced by an individual and could include both verbal and nonverbal productions (e.g., head nod) alone. Turn boundaries were denoted by a change in speaker. Across all interactions, 7,034 total interactional turns were coded with no significant group differences (TBI, M=204.0 SD 101.2 turns; CP, M=166.2 SD 82.2 turns; t(35)=1.25, p=.22).
Words were broadly defined and included false starts (e.g., May- Maybe), fillers (i.e., uh) and backchannel responses (i.e., okay). Across all interactions, 78,192 total words were coded with no significant group differences (TBI interactions, M=2263.0 SD 1273.6 words; CP, M=1971.5 SD 895.0 words; t(35)=.81, p=0.42). The TBI and CP sessions were also similar in mean session length (12:28 SD 6:31, and 10:11 SD 5:09 (min:sec) respectively; t(35)=1.19, p=0.24).
2.4. Noncontent speech convergence analysis
In order to quantify conversational synchrony, noncontent speech convergence of words and words per turn was selected as it has been shown to be altered in participants with vmPFC damage, is independent of topic or content of the conversation, and characterizes the coordination of dialogue at a basic level. Following previous work [23], noncontent speech convergence coding was done by comparing the frequency of words and words per turn produced in the beginning compared to the end of the conversation. First, conversations were divided into segments of approximately 60 seconds, while respecting turn boundaries. Then, the percent difference between the participant and partners’ productions of words and words per turn was calculated for each segment, corrected for the length of the segment, and averaged over the first quarter (typically 2–3 minutes), and last quarter of the interaction. Finally, a convergence score was calculated in order to assess each dyad’s change over the course of the interaction, reflecting the relative change in their productions across the first quarter and last quarter of the interaction. For example, if Participant A spoke 36% more words than the partner in the first quarter of the interaction, and 5% more words than the partner in the last quarter, the convergence score is (5%–36%)/36%=−0.8. This score was then used to categorically classify dyads as those that exhibited convergence and those that did not. Convergence is displayed if the dyads reduced the differences in their productions across the interaction relative to the beginning of the interaction, reflected by convergence scores less than 0 but greater than or equal to −1. Convergence is not displayed if their productions do not become more similar (i.e., remain the same or become more different) by the end of the conversation, which is reflected by convergence scores of 0 or greater. Due to the heterogeneity of the TBI group (e.g., type of injury, neuropsychological profile, see Tables 1–2), we assessed the ability of each individual dyad to converge categorically, rather than using group level analyses of the average convergence scores, which obscured the variability of the dyads.
2.5. Ratings of conversations by blind raters
To see how well the non-content convergence score described above related to subjective impressions of gestalt synchrony in the conversation, three raters who were blind to participant status and the hypotheses of the study watched video recordings of 12 of the TBI interactions and 12 of the healthy comparison interactions (in randomized order) and rated each session on a variety of questions. The entire dataset of videos (18 TBI/19 CP) was not available at the time of the ratings. Of the 12 TBI interactions included in this portion of the study, 5 displayed convergence for words, 6 displayed convergence for words per turn. Of the 12 CP interactions, 8 displayed convergence for words, 7 displayed convergence for words per turn. Raters used a seven-point Likert scale to answer questions such as: Did the participants become more similar to each other over the course of the interaction in terms of the amount each was contributing to the conversation? If so, how much more similar? How natural did the conversation seem? Overall, how would you rate the interaction? The ratings for each conversation were averaged across the three raters and correlated with the convergence score for the dyad.
3. Results
3.1. Words
In the healthy comparison participant (CPs) interactions, 14 of 19 dyads, or 73.7%, displayed convergence for the number of words produced across the interaction. Thus, the majority of dyads became more similar to one another in terms of the number of words that each participant was producing over the course of the interaction. In contrast, in the TBI interactions, only 7 of 18 dyads, or 38.9%, displayed convergence, indicating the majority of dyads (11 dyads) did not become more similar to one another in terms of the number of words produced over the course of the interaction. A chi-squared test revealed significant group differences, χ²(2, n = 37) = 4.6, p = 0.03. An example of an individual CP and TBI participant’s data is shown in Table 3.
Table 3.
Examples of individual data for a participant with TBI and matched comparison participant showing the average number of words produced during the first quarter and the last quarter of the interaction.
| First quarter (average words) | Last quarter (average words) | |
|---|---|---|
| CP participant | 152.3 | 123.0 |
| Partner | 76.6 | 101.3 |
|
| ||
| TBI participant | 180.0 | 305.0 |
| Partner | 78.0 | 50.0 |
Note: CP=comparison participant; TBI= participant with traumatic brain injury
3.2. Words per turn
In the CP interactions, 15 of 19 dyads, or 78.9%, displayed convergence for the number of words per turn produced. Over the course of the interaction, the number of words in each turn that the participants produced became more similar, suggesting that they were having a back and forth mutually inclusive interaction. In interactions including participants with TBI, only 9 of 18 dyads, or 50%, displayed convergence, as half of the dyads (9 dyads) did not become more similar to one another. A chi-squared test revealed a group difference that approached significance, χ²(2, n = 37) = 3.4, p = 0.06. An example of an individual CP and TBI participant’s data is shown in Table 4.
Table 4.
Examples of individual data for a participant with TBI and matched comparison participant showing the average number of words per turn produced during the first quarter and the last quarter of the interaction.
| First quarter (average words/turn) | Last quarter (average words/turn) | |
|---|---|---|
| CP participant | 28.6 | 7.4 |
| Partner | 14.6 | 10.7 |
|
| ||
| TBI participant | 23.1 | 192.5 |
| Partner | 7.1 | 1.7 |
Note: CP=comparison participant; TBI= participant with traumatic brain injury.
3.3. Dynamics of the sessions
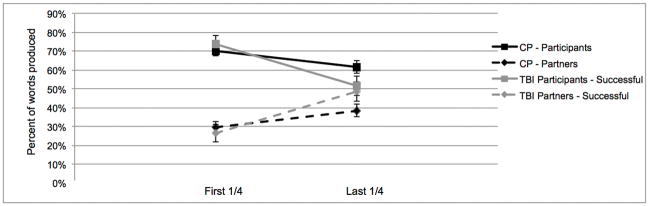
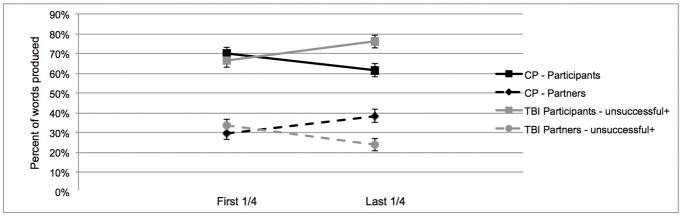
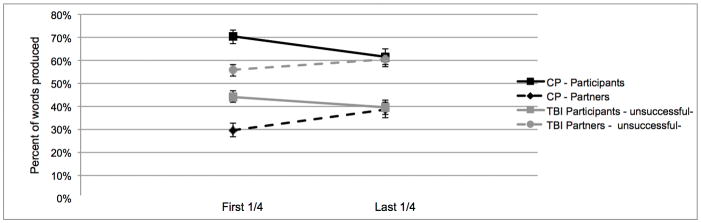
We explored the nature and dynamics of the sessions to better understand the contributions of the target participants (TBI or healthy comparison participants). Considering the heterogeneity of traumatic brain injury, discourse level impairments may manifest in different ways in individuals [7]. We looked at the percentage of words produced by the participant versus the partner in the first quarter and last quarter of the sessions. Recall that there were 11 pairs involving an individual with TBI who did not converge on the measure of words. In 6 of those pairs the participant with TBI was producing more words than the partner at the end of the interaction (on average 26.1% more words; Figure 1b), in the other 5 cases the participant with TBI was producing less words than the partner (on average 10.4% less words; Figure 1c). Average group data for the three subgroups of participants with TBI (successful, producing more words, producing less words) is displayed in Figures 1a, b, and c respectively. In the CP sessions, 5 participants did not display convergence for words, in all cases the CP participant was producing more words than the partner at the end of the interaction (on average 24.0% more words).
Figure 1.
The average percentage of words produced by both the participant and partner in the comparison (CP) and the traumatic brain injury (TBI) sessions. All figures below are in reference to the entire group of 19 CP participants. Figure 1a) shows the TBI sessions which successfully displayed convergence (n=7). There were two subgroups of TBI session that did not display convergence. Figure 1b) shows the sessions where the participant with TBI was producing more words than the partner (unsuccessful+) at the end of the interaction (n=6) and Figure 1c) shows the sessions where the participant with TBI was producing less words than the partner (unsuccessful−) at the end of the interaction (n=5).
3.4. Relationship between convergence score and blind ratings
Overall, raters judged the interactions involving participants with TBI to be significantly less natural (t(11)=3.02, p=0.01) and were less likely to want to converse with the participants in the future (t(11)=2.55, p=0.02) compared to the interactions of healthy participants. Figure 2 shows the significant positive correlation between the convergence score for words per turn for all participants included in the ratings (TBI and healthy comparison) and the raters’ judgment of how similar the participants became in terms of their contributions over the course of the interaction (r(22)=0.48, p=0.01) Note that lower scores on both measures represent more similarity across the interaction. Interestingly, this correlation was only significant for convergence score for words per turn; there was no correlation between the convergence score for words and the raters’ judgment of similarity (r(22)=0.23, p=0.27).
Figure 2.
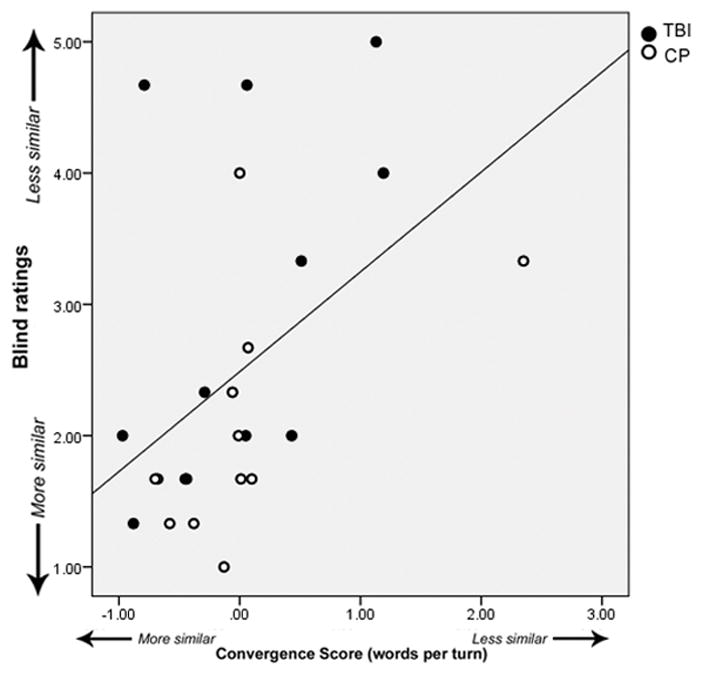
Correlation between convergence scores for TBI (filled circles) and comparison participants (open circles) and the ratings from blind judges regarding the similarity of the partners.
Furthermore, the raters’ answers to questions concerning similar concepts were highly correlated. For example, the higher the overall rating of the conversation, the more natural they perceived it (r(22)=0.91, p<0.01), and the more they saw the participants become more similar in their contributions (r(22)=0.615, p<0.01). Also, the more natural the conversation felt the more likely the raters were to say that they would like have a conversation with the participant in the future (r(22)=0.77, p<0.01).
4. Discussion
Conversational synchrony is ubiquitous across multiple behaviours in conversation and serves numerous purposes including signaling active interest in the conversational partner, and promoting the development of rapport [19, 20]. Here, we examined the effects of traumatic brain injury on conversational synchrony, as measured by convergence of words and words per turn. The primary finding was that the majority of interactions between participants with TBI and an unfamiliar partner did not become more similar to one another in terms of their productions of words or words per turn. By contrast, the majority of interactions of healthy comparison participants did display conversational synchrony as their productions of words or words per turn became more similar over the course of the interaction.
Further support for the disruption in conversational synchrony comes from ratings of the interactions by blind raters, who judged the interactions involving an individual with TBI as less natural than the CP interactions. Despite being blind to participant status, raters recognized that something was “off” in the conversations and reported being less likely to want to converse with the participants with TBI in the future if given the opportunity. Furthermore, regardless of group, convergence of words per turn was significantly positively correlated with subjective ratings. This provides further support that conversational synchrony, specifically the mutual back and forth of a conversation with equal words in each turn, is important to social perceptions. This may provide insight as to how deficits in conversational synchrony may contribute to the challenges that many participants with TBI face in creating and maintaining social relationships and reintegrating into society.
Deficits in a variety of aspects of conversational behaviour, including the timing and quality of gaze, turns, and talk time have been shown to be disrupted in the conversations of participants with TBI [8]. The findings of the current study support the idea that there are clear alterations in how participants with TBI interact in conversation. Conversations involve mutual dialogue, and by either dominating or not contributing sufficiently to the interaction, this can have significant deleterious effects on the development of rapport and on other people’s perceptions of their empathy and concern for others. As a result, conversational partners may not feel “in tune” with the participant with TBI. Our finding that many participants with TBI tend to produce fewer words than the partner over the course of the interaction are in line with previous work showing that participants with TBI have difficulty initiating and sustaining conversation and often rely on the conversational partner to compensate and carry the conversational burden (Figure 1c) [9, 31]. Importantly, this pattern of behaviour (fewer words at the end of the session) is only seen in the sessions with participants with TBI and is not seen in the CP sessions that do not display convergence. However, in our study we show that this is not the case for all participants with TBI, in fact, some perform in the opposite manner, in that they tend to dominate the interactions (Figure 1b) [8]. By studying individual dyads, we are able to carefully characterize the abilities of each dyad, and better understand how the heterogeneity of the disorder can affect social communication.
In contrast to many previous studies, this study took into account the behaviour of both participants in the interaction (see Figures 1a, b, c). The observed disruptions in conversational synchrony are likely due to the failure of participants with TBI to alter their behaviour, but also are likely combined with the negative influence these behaviours can have on the partner, such that they feel the need to compensate or withdraw from the conversation. If communication is distributed across partners, then disruptions should affect the behaviour of both partners [e.g., 32]. Indeed, previous research with patients with hippocampal damage has shown that the memory impairment of the patient with hippocampal damage can impact the language productions of their healthy partner [33]. In the current study, we speculate that the disruption in the partners’ behaviour occurs because of the cooperative, reciprocal nature of conversation: if one person is dominating the floor, it is more difficult for the partner to increase their talk time. Furthermore, we suspect that these disruptions are not limited to the nature of these particular interactions, or the lack of familiarity between partners; in previous research, we found that disruptions in conversational synchrony in ventromedial prefrontal cortex patients occurred across multiple partners and familiarity levels. Finally, as these same partners were involved in data collection for both the TBI and CP sessions, we do not believe these disruptions are primarily due to the behaviour of the partner, as they were successfully able to display synchrony in the majority of CP sessions.
Importantly, there were some TBI pairs in our dataset that did successfully display conversational synchrony on both words (7/18 pairs) and words per turn (9/18 pairs). One factor known to impact long-term social and behavioural outcomes in TBI is the severity of the injury. However, one of the two participants in our dataset with mild injuries did not display convergence, providing preliminary evidence that severity is not a critical predictor of success. All other participants with TBI were categorized as moderate-severe injuries, and there is not a consistent pattern from the demographic or neuropsychological profiles that would predict or support a particular explanation for why some were successful and others were not. For example, those with high IQs were not resistant to disruptions in convergence; likewise, some participants with memory impairments on the AVLT or perseverative errors on the WCST were able to successfully display convergence. Age and sex do not seem to be clear explanatory variables: convergence was disrupted in both the oldest and youngest participants and in the majority of both male and female participants. Importantly, our group is not large enough to formally determine predictive variables, and future research should focus on characterizing an interaction of multiple variables that lead to the most successful outcomes in TBI.
One potential difference that may elucidate why some participants with TBI were successful is the extent and location of brain damage. As full analysis of chronic neuroimaging scans is not available for all participants (see Table 1 for preliminary data), we are unable to attribute behavioral disruptions to particular brain regions. Furthermore, given the complexity of the phenomenon, it is most likely the case that conversational synchrony is not supported exclusively by a single brain region or even by a single network, but by the interplay between several large-scale brain networks [34, 35]. Our previous work has supported the idea that the ventromedial prefrontal cortex is one critical component of a neural network involved in conversational synchrony, such that participants with damage to the vmPFC consistently tend to dominate interactions and produce more words and words per turn throughout the conversation [23]. In the current study, participants with TBI were not as consistent as to the direction of the disruption as participants with damage to the vmPFC: instead of dominating the conversations, roughly half tended to produce less words and words per turn than the partner. However, the similarity of the disruption in synchrony that we see in some of the participants with TBI in the current study leads us to the preliminary speculation that the vmPFC, either through focal damage or diffuse white matter damage, may be disrupted in these participants with TBI. We are currently exploring the neuroimaging scans of these participants to see if there is focal damage to the vmPFC or other related areas in the action-observation network that are associated with behavioural deficits in social communication. Likewise, future research should focus on functional connectivity within brain networks as well as on those areas involved in mediating interaction between brain systems (e.g., anterior insula and anterior cingulate cortex).
Finally, it is important to consider the variability of this phenomenon in the healthy population. Not all of the healthy participant pairs successfully displayed conversational synchrony (5 for words (26%) and 4 for words per turn (21%)). Across the literature, it is common to find variability in conversational synchrony in healthy populations, but it is unclear what causes this variability (e.g., Cappella & Planalp [16] reported approximately 25% of healthy dyads in their study failed to converge). Despite the variability found in the healthy population and our small sample size, our results show that disruptions in conversational synchrony are significantly more likely to occur in interactions involving participants with TBI, particularly for convergence of words. Furthermore, unlike the sessions with a participant with TBI, the CP sessions in our sample that failed to display synchrony were highly consistent in the direction of their outcomes: in all cases the CP participant produced more words than the partner at the end of the session. On the other hand, sessions including a participant with TBI were much more variable as to which partner was producing more words at the end of the session. We believe this further supports the idea that the heterogeneity of communicative outcomes following TBI is reflected, in part, in the disruption in conversational synchrony [8, 9, 31]. We are also currently exploring how differences in conversational synchrony in healthy participants may be related to functional connectivity and the interaction of certain brain regions and networks. Such data may be informative in determining the difference, or similarity, between healthy individuals who fail to show the convergence effect and those individuals with TBI who demonstrate a lack of conversational synchrony as part of a larger set of cognitive-communication impairments.
In sum, the current study shows that traumatic brain injury can disrupt conversational synchrony, however, there is heterogeneity in how these disruptions manifest across individual participants. Furthermore, these findings support the notion that the unit of analysis for understanding complex communication deficits should include both communication partners in order to study the ways in which partners jointly collaborate and coordinate together. Likewise, better understanding of the neural basis of conversational synchrony, in both healthy participants and participants with brain injury, will better inform our understanding of social communication deficits and their consequences in traumatic brain injury and other disorders.
Acknowledgments
We thank the Duff Communication and Memory Laboratory for assistance with transcribing and coding the sessions. This study was supported by NIDCD RO1 DC011755 to MCD.
Footnotes
Declaration of Interests: The authors report no declarations of interest.
References
- 1.Brooks N, Campsie L, Symington C, Beattie A, McKinlay W. The five year outcome of severe blunt head injury: a relative's view. Journal of Neurology, Neurosurgery & Psychiatry. 1986;49(7):764–770. doi: 10.1136/jnnp.49.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks N, McKinlay W, Symington C, Beattie A, Campsie L. Return to work within the first seven years of severe head injury. Brain Injury. 1987;1(1):5–19. doi: 10.3109/02699058709034439. [DOI] [PubMed] [Google Scholar]
- 3.Finset A, Dyrnes S, Krogstad JM, Berstad J. Self-reported social networks and interpersonal support 2 years after severe traumatic brain injury. Brain Injury. 1995;9(2):141–150. doi: 10.3109/02699059509008187. [DOI] [PubMed] [Google Scholar]
- 4.Morton MV, Wehman P. Psychosocial and emotional sequelae of individuals with traumatic brain injury: a literature review and recommendations. Brain Injury. 1995;9(1):81–92. doi: 10.3109/02699059509004574. [DOI] [PubMed] [Google Scholar]
- 5.Sarno MT, Buonaguro A, Levita E. Characteristics of verbal impairment in closed head injured patients. Archives of Physical Medicine and Rehabilitation. 1986;67(6):400–405. [PubMed] [Google Scholar]
- 6.Hartley L. A Functional Approach. San Diego, CA: Singular Publishing; 1995. Cognitive-Communicative Abilities Following Brain Injury. [Google Scholar]
- 7.Snow P, Douglas J, Ponsford J. Conversational assessment following traumatic brain injury: a comparison across two control groups. Brain Injury. 1997;11(6):409–429. doi: 10.1080/026990597123403. [DOI] [PubMed] [Google Scholar]
- 8.Turkstra L, Brehm S, Montgomery E., Jr Analysing conversational discourse after traumatic brain injury: Isn't it about time? Brain Impairment. 2006;7(3):234–245. [Google Scholar]
- 9.Bond F, Godfrey HP. Conversation with traumatically brain-injured individuals: a controlled study of behavioural changes and their impact. Brain Injury. 1997;11(5):319–329. doi: 10.1080/026990597123476. [DOI] [PubMed] [Google Scholar]
- 10.Cherney LR, Coelho CA, Shadden BB. Analyzing discourse in communicatively impaired adults. Gaithersburg, MD: Aspen Publishers; 1998. [Google Scholar]
- 11.Jorgensen M, Togher L. Narrative after traumatic brain injury: a comparison of monologic and jointly-produced discourse. Brain Injury. 2009;23(9):727–740. doi: 10.1080/02699050903133954. [DOI] [PubMed] [Google Scholar]
- 12.Bavelas JB, Coates L, Johnson T. Listeners as co-narrators. Journal of Personality and Social Psychology. 2000;79(6):941. doi: 10.1037//0022-3514.79.6.941. [DOI] [PubMed] [Google Scholar]
- 13.Duff MC, Mutlu B, Byom L, Turkstra LS. Beyond utterances: Distributed cognition as a framework for studying discourse in adults with acquired brain injury. In Seminars in speech and language. 2012;33(1):44–54. doi: 10.1055/s-0031-1301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollan J, Hutchins E, Kirsh D. Distributed cognition: toward a new foundation for human-computer interaction research. ACM Transactions on Computer-Human Interaction (TOCHI) 2000;7(2):174–196. [Google Scholar]
- 15.Hutchins E. Cognition in the Wild. Cambridge, MA: MIT press; 1995. [Google Scholar]
- 16.Cappella JN, Planalp S. Talk and silence sequences in informal conversations: III Interspeaker influence. Human Communication Research. 1981;7:117–132. [Google Scholar]
- 17.Chartrand TL, Bargh JA. The chameleon effect: the perception-behaviour link and social interaction. Journal of Personality and Social Psychology. 1999;76(6):893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- 18.Giles H, Coupland J, Coupland N. Contexts of Accomodation. New York: Cambridge University Press; 1991. [Google Scholar]
- 19.Street RL., Jr Speech convergence and speech evaluation in fact-finding interviews. Human Communication Research. 1984;11(5):139–169. [Google Scholar]
- 20.Bernieri FJ, Rosenthal R. Interpersonal coordination: Behaviour matching and interactional synchrony. In: Feldman RS, Rime B, editors. Fundamentals of nonverbal behaviour. Cambridge: Cambridge University Press; 1991. pp. 401–432. [Google Scholar]
- 21.Cappella JN. Behavioural and judged coordination in adult informal social interactions: Vocal and kinesic indicators. Journal of Personality and Social Psychology. 1997;72:119–131. [Google Scholar]
- 22.Adams JH, Doyle D, Graham DI, Lawrence AE, McLellan DR, Gennarelli TA, et al. The contusion index: a reappraisal in human and experimental non-missile head injury. Neuropathol Appl Neurobiol. 1985;11(4):299–308. doi: 10.1111/j.1365-2990.1985.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 23.Gordon RG, Tranel D, Duff MC. The physiological basis of synchronizing conversational rhythms: The role of the ventromedial prefrontal cortex. Neuropsychology. 2014;8(4):624–630. doi: 10.1037/neu0000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Body R. Decision making and somatic markers in conversation after traumatic brain injury. Aphasiology. 2007;21(3–4):394–408. [Google Scholar]
- 25.Stone VE, Hynes C. Real-world consequences of social deficits: Executive functions, social competencies, and theory of mind in patients with ventral frontal damage and traumatic brain injury. In: Decety J, Cacioppo JT, editors. The Oxford Handbook of Social Neuroscience. New York: Oxford University Press; 2011. pp. 455–476. [Google Scholar]
- 26.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. The Lancet. 1974;304(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 27.Malec JF, Brown AW, Leibson CL, Flaada JT, Mandrekar JN, Diehl NN, Perkins PK. The Mayo classification system for traumatic brain injury severity. Journal of Neurotrauma. 2007;24(9):1417–1424. doi: 10.1089/neu.2006.0245. [DOI] [PubMed] [Google Scholar]
- 28.Kertesz A. Western Aphasia Battery. Orlando, FL: Grune & Stratton, Inc; 1982. [Google Scholar]
- 29.Hengst J, Duff MC. Clinicians as Communication Partners: Developing a Mediated Discourse Elicitation Protocol. Top Lang Disorders. 2007;27(1):37–49. [Google Scholar]
- 30.Duff MC, Hengst JA, Tranel D, Cohen NJ. Collaborative discourse facilitates efficient communication and new learning in amnesia. Brain and Language. 2008;106(1):41–54. doi: 10.1016/j.bandl.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coelho CA, Youse KM, Le KN. Conversational discourse in closed-head-injured and non-brain-injured adults. Aphasiology. 2002;16(4–6):659–672. [Google Scholar]
- 32.Togher L, Hand L, Code C. Analysing discourse in the traumatic brain injury population: Telephone interactions with different communication partners. Brain Injury. 1997;11(3):169–190. doi: 10.1080/026990597123629. [DOI] [PubMed] [Google Scholar]
- 33.Duff MC, Hengst JA, Gupta R, Tranel D, Cohen NJ. Distributed impact of cognitive-communication impairment: Disruptions in the use of definite references when speaking to individuals with amnesia. Aphasiology. 2011;25(6–7):675–687. doi: 10.1080/02687038.2010.536841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain injury. Nature Reviews Neurology. 2014;10:156–166. doi: 10.1038/nrneurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 35.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]