Abstract
Recent research in brain-machine interfaces and devices to treat neurological disease indicate that important network activity exists at temporal and spatial scales beyond the resolution of existing implantable devices. High density, active electrode arrays hold great promise in enabling high-resolution interface with the brain to access and influence this network activity. Integrating flexible electronic devices directly at the neural interface can enable thousands of multiplexed electrodes to be connected using many fewer wires. Active electrode arrays have been demonstrated using flexible, inorganic silicon transistors. However, these approaches may be limited in their ability to be cost-effectively scaled to large array sizes (8×8 cm). Here we show amplifiers built using flexible organic transistors with sufficient performance for neural signal recording. We also demonstrate a pathway for a fully integrated, amplified and multiplexed electrode array built from these devices.
I. Introduction
Electrocorticography (ECoG), the process of recording brain activity through electrodes placed directly on the cortical surface, is a common technique in the assessment and treatment of neurological disorders, such as epilepsy. ECoG offers a higher spatial and temporal resolution interface with the brain than extracranial EEG, since the electrodes are smaller and located at a much closer distance to the brain. Penetrating microelectrodes, such as the Utah array [1], can measure neural activity at a higher spatial resolution, including from single neurons, but are constrained to interface with small regions of the cortex. Further, the long-term feasibility of this interface is often limited to only 6–12 months [2], either due tissue damage caused by the inability of the rigid penetrating electrodes to flex and move as the brain swells and contracts [3] or by damage caused from hemorrhage and inflammation from the initial insertion [4].
ECoG does not appear to suffer from this stability issue and has demonstrated consistent signal quality over extended periods of time with minimized irritation and injury to brain tissue [5], [6]. Further, ECoG and higher resolution micro-ECoG (μECoG) recordings from flexible arrays of non-penetrating electrodes may offer comparable information content to the neural signals recorded from penetrating electrodes in some applications, such as BMI [7]–[9], decoding motor control signals [10] and decoding spoken words [11]. High resolution neural interface is also important to understanding pathologic brain signals [12].
In addition to high spatial resolution, developing electrode arrays with a high degree of conformality is also important. Highly flexible arrays of electrodes have the ability to conform to the uneven patterns of sulci and gyri on the surface of the brain, resulting in higher signal to noise ratios in recordings and more electrodes in contact with the brain [13].
Current clinical ECoG arrays use metal electrodes that typically have a diameter of 3mm on a grid with 1cm center-to-center spacing [14]. Because of these dimensions, the arrays spatially undersample the electrical signals of the brain. μECoG electrode arrays have been developed that utilize flexible silicon electronics to create a conformal, dense (800μm spacing) electrode array capable of covering large areas (14.4mm × 12.8mm) of the brain [15]. These devices offer recordings with high spatial and temporal resolution and utilize on-chip multiplexing to reduce the number of wires coming off the grid. Constructing these silicon devices can be a difficult and expensive process. Alternatively, organic electronics may be able to be fabricated at lower cost than flexible silicon electronics on the same type of plastic substrates.
Organic materials are considered a promising candidate for flexible electronics due to the low temperatures required for fabrication. Through intensive research in the past decades, organic materials have been shown to exhibit carrier mobility comparable to or higher than amorphous silicon used in modern active-matrix liquid-crystal displays [16]. In addition, synthetic chemistry is able to tailor organic materials in a way that cannot be done with inorganics. The increased functionality of organic transistors can enable a broad array of biological signal monitoring applications.
Pentacene, among the highest performance organic thin film semiconductor materials available, is insoluble in most organic solvents. A soluble pentacene precursor, developed by A. Afzali et al. [17], can be dissolved in a chlorinated solvent, such as chloroform, and subsequently thermally converted into pentacene. This production technique opens the door to large area fabrication of organic transistor circuits at low cost.
In this paper, we demonstrate solution processable organic thin film transistors that can be integrated directly into neural electrode arrays. We developed photolithographic methods to fabricate flexible organic thin film transistors. Combining these devices with custom circuits, we demonstrate common source and common drain amplifier topologies with performance sufficient for recording neural signals.
II. Materials and Methods
Pentacene thin film transistors (TFTs) were fabricated in bottom-gate, bottom-contact configuration on a Kapton® substrate according to the method described in [18] and [19]. A schematic of the device is illustrated in Fig. 1(a). Gold gate and source/drain contacts were patterned by photolithography with a thickness of 20nm. A 500nm parylene-C dielectric layer was deposited through physical vapor deposition. The pentacene precursor was applied by spin-coating and then thermally converted to pentacene to form the bottom-gate, bottom-contact configuration TFT. Fig. 1(b) shows photograph of the device electrodes before spin-coating the organic semiconductor.
Fig. 1.
(a) Schematic of the organic thin film transistor, a photographic example of which is seen in (b). (c) and (d) are the representative ID-VDS and ID-VGS properties of the devices, respectively. The channel width is 1500μm and length is 100μm. These properties were measured in an inert nitrogen environment.
Representative device characteristics measured under inert nitrogen environment are shown in Fig. 1(c) and Fig. 1(d). Hole accumulation can be seen in the ID-VDS curve, characterized by linearity at low absolute drain-source biases and saturation at large negative biases. The saturation mobility is 0.123 ± 0.038 cm2/(V·s), extracted from more than 50 transistors fabricated on different samples. The on/off current ratio is about 105, as shown in ID-VGS curve (Fig. 1d).
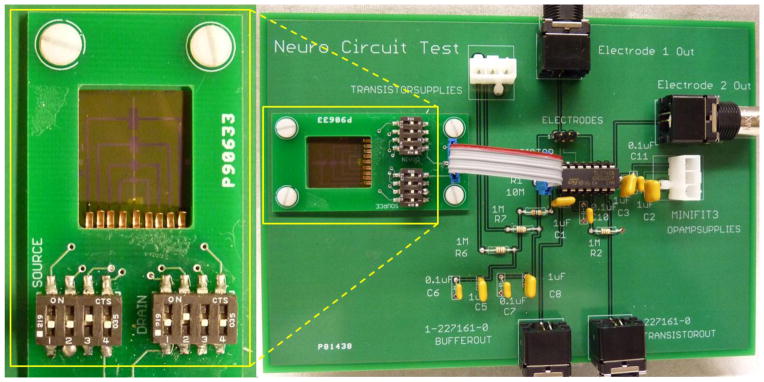
The pentacene TFTs were tested in both common source and common drain amplifier configurations. A circuit consisting of all elements of the amplifying configurations except the transistor was created using a custom printed circuit board (PCB), which is shown in Fig. 2. In order to make the electrical connection between the pentacene TFTs on a flexible substrate and the PCB, the buried bottom gate had to be revealed. This was done by removing the pentacene over the electrode pads with chloroform. The parylene dielectric was then selectively etched with oxygen plasma, while keeping the active area protected. An additional small PCB with switches and finger-shaped beryllium copper contacts on the underside was designed to make mechanical contact with the flexible substrate of the transistor array. By clamping the small PCB onto the larger PCB with the flexible transistor array in between, the beryllium copper fingers are able to make solid mechanical contact with the electrodes of the transistor array, forming the electrical connection to the PCB. The pressure contact PCB is highlighted in yellow and enlarged in Fig 2. A short ribbon cable was used to connect the gate, source and drain of the organic transistor to their respective locations on the main PCB.
Fig. 2.
The dual PCB board design. The large board contains all elements of the circuit excluding the transistor: the amplifying resistor, lowpass power supply filters, output buffer and highpass filter, and input/output components. The ribbon cable attaches the transistor to the rest of the circuit. The enlarged image on the left clearly shows the copper fingers used to make pressure contact with the organic TFT underneath.
The circuit on the main PCB was designed to be used for either common source or common drain amplifying circuits. Since the source and drain of the organic transistors are interchangeable, switching between the two configurations was possible by changing power supply polarity. A 10MΩ resistor was connected to the drain or source of the organic transistor depending on the desired circuit topology. This large resistance was used in order to yield higher gain. Power was supplied the organic transistor amplifier through 0.14 Hz low-pass filters in order to reduce noise coming from voltage supply electronics. The amplifier output was directly connected to a unity-gain buffer in order to reduce loading by subsequent measurement equipment.
III. Results
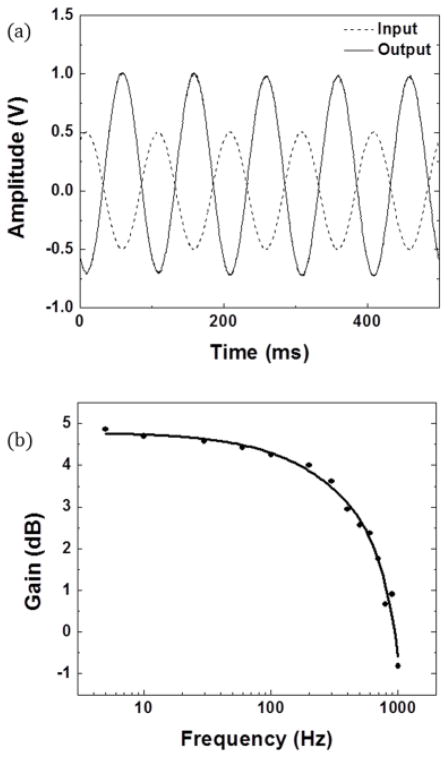
The common source amplifier configuration was tested with multiple pentacene TFTs on the dual PCB setup. The source/drain transistor power supplies were kept at a difference of 50V. The maximum gain was 5.0dB for the highest performance device, while the average gain was 2.3dB. Fig. 3(a) shows a characteristic input/output voltage response for the best transistor with a 100μm channel. The source supply was +28V and the drain supply was −22V. The input was a 1Vpp 10Hz sine wave. The output is inverted due to the common source topology.
Fig. 3.
Results from common source amplifier using pentacene TFT showing the (a) representative output versus input and (b) bode plot. Both results shown were taken from the same 100μm channel length device.
The frequency response for the same organic transistor amplifier in the common source configuration is shown in Fig. 3(b). The −3dB cutoff was observed between 700 and 850Hz, depending on the transistor used. The square wave response rise and fall time of the amplifier was found to be 0.5ms each.
The common drain topology was tested by reversing the polarity of the TFT power supplies, effectively exchanging the source and drain. The maximum output of the device with a 1Vpp input was 0.65Vpp, or an attenuation of 3.7dB. A frequency sweep revealed a −3dB bandwidth of 2.8kHz, much higher than the common source circuit, as expected.
IV. Discussion and Conclusions
The gain of the common source amplifier using pentacene TFTs (between 2.3 and 5dB) is promising for use in neural amplifiers. Current ECoG arrays use either passive electrodes or common drain amplifiers with unity gain. Integrating any level of gain directly at the electrode should improve the overall system performance. The attenuation seen in the organic transistor in common drain configuration, 3.7dB, would decrease the overall system performance, but would still be acceptable for many neural signal acquisition tasks.
The tradeoff for increased gain is decreased bandwidth: ~800Hz for the common source configuration versus ~2.8kHz for the common drain configuration. Given that the majority of clinically relevant, cortical surface brain activity occurs below 500Hz [20], a bandwidth of 800Hz would be sufficient for most applications. However, developing multiplexed electrode arrays may be difficult, given the low frequency response of the transistors.
One of the challenges faced while testing the amplifiers was the performance reduction of the pentacene TFTs in ambient atmosphere. Devices were initially characterized directly after fabrication, while still immersed in a pure nitrogen environment. The devices were subsequently retested after being exposed to open air. Carrier mobility, and likewise drain current, both continuously decreased as a result of exposure to moisture and oxygen. Several different encapsulating materials were applied to the TFTs in preliminary attempts to prevent this degradation. None of the materials so far investigated effectively protected the devices from reduction in their performance while exposed to air.
There are several items remaining to be addressed before organic transistors can be used to develop large arrays of multiplexed electrodes. First, the noise performance of the organic transistor amplifiers will be evaluated to insure sufficient signal to noise levels in the final system, while measuring neural signals of 1 mV amplitude or less. Initial noise measurements appear promising.
Second, the ability to pattern hundreds of organic transistors on a single flexible substrate will need to be developed. Prototypes of such devices have been fabricated, utilizing etching and deposition processes to create vertical integration access (VIA) holes to connect individual transistors using multiple metal layers.
Finally, a new encapsulation system will need to be developed to fully protect the transistors from exposure to air and biological fluids. New fabrication procedures and encapsulation materials are being evaluated for their long term reliability.
The devices described in this paper have been shown to work as amplifiers with adequate gain and bandwidth required for clinical and research neural sensor applications. While additional advancements are necessary, the potential advantages of organic μECoG electrode arrays motivate continued research to bring them to fruition.
Acknowledgments
This work was supported in part by the National Institutes of Health Grants NINDS RO1-NS041811, NINDS R01 NS 48598, the Dr. Michel and Mrs. Anna Mirowski Discovery Fund for Epilepsy Research, and the National Science Foundation (NSF MRSEC program DMR-0520020 and NSF SUNFEST program EEC-075474) J.V. was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award 2T32HL007954 from the NIH-NHLBI.
Contributor Information
Hank Bink, Email: binkh@seas.upenn.edu, Bioengineering Department, University of Pennsylvania, Philadelphia, PA 19104 USA.
Yuming Lai, Email: yumingl@seas.upenn.edu, Electrical and Systems Engineering Department, University of Pennsylvania.
Sangameshwar R. Saudari, Materials Science and Engineering Department, University of Pennsylvania
Brian Helfer, Electrical and Computer Engineering Department, University of Connecticut, Storrs, CT 06269.
Jonathan Viventi, Bioengineering Department, University of Pennsylvania, Philadelphia, PA 19104 USA.
Jan Van der Spiegel, Electrical and Systems Engineering Department, University of Pennsylvania.
Brian Litt, Email: littb@mail.med.upenn.edu, Departments of Bioengineering and Neurology, University of Pennsylvania.
Cherie Kagan, Email: kagan@seas.upenn.edu, Departments of Electrical and Systems Engineering, Materials Science and Engineering, and Chemistry, University of Pennsylvania.
References
- 1.Campbell PK, Jones KE, Huber RJ, Horch KW, Normann Ra. A silicon-based, three-dimensional neural interface: manufacturing processes for an intracortical electrode array. IEEE transactions on bio-medical engineering. 1991 Aug;38:758–68. doi: 10.1109/10.83588. [DOI] [PubMed] [Google Scholar]
- 2.Ryu SI, Shenoy KV. Human cortical prostheses: lost in translation? Neurosurgical focus. 2009;27:E5. doi: 10.3171/2009.4.FOCUS0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith RW, Humphrey DR. Long-term gliosis around chronically implanted platinum electrodes in the Rhesus macaque motor cortex. Neuroscience letters. 2006;406:81–6. doi: 10.1016/j.neulet.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. Journal of neuroscience methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Chao ZC, Nagasaka Y, Fujii N. Long-term asynchronous decoding of arm motion using electrocorticographic signals in monkeys. Frontiers in neuroengineering. 2010 Jan;3:3. doi: 10.3389/fneng.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeager JD, Phillips DJ, Rector DM, Bahr DF. Characterization of flexible ECoG electrode arrays for chronic recording in awake rats. Journal of neuroscience methods. 2008;173:279–85. doi: 10.1016/j.jneumeth.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leuthardt EC, Gaona C, Sharma M, Szrama N, Roland J, Freudenberg Z, Solis J, Breshears J, Schalk G. Using the electrocorticographic speech network to control a brain–computer interface in humans. Journal of Neural Engineering. 2011 Jun;8:036004. doi: 10.1088/1741-2560/8/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunner P, Ritaccio AL, Emrich JF, Bischof H, Schalk G. Rapid Communication with a ‘P300’ Matrix Speller Using Electrocorticographic Signals (ECoG) Frontiers in neuroscience. 2011 Jan;5:5. doi: 10.3389/fnins.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball T, Nawrot M, Pistohl T, Aertsen A, Schulze-Bonhage A, Mehring C. Towards an implantable brain-machine interface based on epicortical field potentials. Biomed Tech(Berlin) 2004;38:756–759. [Google Scholar]
- 10.Kellis SS, House Pa, Thomson KE, Brown R, Greger B. Human neocortical electrical activity recorded on nonpenetrating microwire arrays: applicability for neuroprostheses. Neurosurgical focus. 2009;27:E9. doi: 10.3171/2009.4.FOCUS0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellis S, Miller K, Thomson K, Brown R, House P, Greger B. Decoding spoken words using local field potentials recorded from the cortical surface. Journal of neural engineering. 2010 Sep;7:056007. doi: 10.1088/1741-2560/7/5/056007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, Litt B, Van Gompel J, Worrell Ga. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain: a journal of neurology. 2010 Oct;133:2789–97. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D-H, Viventi J, Amsden JJ, Xiao J, Vigeland L, Kim Y-S, Blanco Ja, Panilaitis B, Frechette ES, Contreras D, Kaplan DL, Omenetto FG, Huang Y, Hwang K-C, Zakin MR, Litt B, Rogers Ja. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nature materials. 2010 Apr;:1–8. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epilepsy & Neurosurgery Product Guide. Ad-Tech Medical Instrument Corporation; 2008. [Google Scholar]
- 15.Viventi J, Kim D-H, Moss JD, Kim Y-S, Blanco Ja, Annetta N, Hicks a, Xiao J, Huang Y, Callans DJ, Rogers Ja, Litt B. A Conformal, Bio-Interfaced Class of Silicon Electronics for Mapping Cardiac Electrophysiology. Science Translational Medicine. 2010;2:24ra22–24ra22. doi: 10.1126/scitranslmed.3000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klauk H, Halik M, Zschieschang U, Schmid G, Radlik W, Weber W. High-mobility polymer gate dielectric pentacene thin film transistors. Journal of Applied Physics. 2002;92:5259. [Google Scholar]
- 17.Afzali A, Dimitrakopoulos CD, Breen TL. High-performance, solution-processed organic thin film transistors from a novel pentacene precursor. Journal of the American Chemical Society. 2002 Jul;124:8812–3. doi: 10.1021/ja0266621. [DOI] [PubMed] [Google Scholar]
- 18.Saudari SR, Lin YJ, Lai Y, Kagan CR. Device configurations for ambipolar transport in flexible, pentacene transistors. Advanced materials (Deerfield Beach, Fla) 2010 Nov;22:5063–8. doi: 10.1002/adma.201001853. [DOI] [PubMed] [Google Scholar]
- 19.Saudari SR, Frail PR, Kagan CR. Ambipolar transport in solution-deposited pentacene transistors enhanced by molecular engineering of device contacts. Applied Physics Letters. 2009;95:023301. [Google Scholar]
- 20.Worrell Ga, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain: a journal of neurology. 2008;131:928–37. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]