Abstract
Background aims
Interest in natural killer (NK) cell-based immunotherapy has resurged since new protocols for the purification and expansion of large numbers of clinical-grade cells have become available.
Methods
We have successfully adapted a previously described NK expansion method that uses K562 cells expressing interleukin (IL)-15 and 4-1 BB Ligand (BBL) (K562-mb15-41BBL) to grow NK cells in novel gas-permeable static cell culture flasks (G-Rex).
Results
Using this system we produced up to 19 × 109 functional NK cells from unseparated apheresis products, starting with 15 × 107 CD3− CD56+ NK cells, within 8–10 days of culture. The G-Rex yielded a higher fold expansion of NK cells than conventional gas-permeable bags and required no cell manipulation or feeding during the culture period. We also showed that K562-mb15-41BBL cells up-regulated surface HLA class I antigen expression upon stimulation with the supernatants from NK cultures and stimulated alloreactive CD8+ T cells within the NK cultures. However, these CD3+ T cells could be removed successfully using the CliniMACS system. We describe our optimized NK cell cryopreservation method and show that the NK cells are viable and functional even after 12 months of cryopreservation.
Conclusions
We have successfully developed a static culture protocol for large-scale expansion of NK cells in the gas permeable G-Rex system under good manufacturing practice (GMP) conditions. This strategy is currently being used to produce NK cells for cancer immunotherapy.
Keywords: cell-based therapy, ex vivo expansion, gas-permeable static cell culture flasks (G-Rex), immunotherapy, natural killer cells
Introduction
Natural killer (NK) cells are cytotoxic lymphocytes of the innate immune system that have direct and indirect roles in controlling intracellular pathogens. NK cells also play a major role in tumor immunosurveillance, as NK activity has been inversely correlated with cancer incidence and outcome (1–4) and NK infiltration of tumors is associated with better prognosis of gastric (5), colorectal (6) and lung carcinomas (7,8). Both primary NK cells derived from apheresis products and a pure NK cell line (irradiated NK-92) have been used as immunotherapy for blood and solid malignancies (9–11). These studies have shown that NK cell infusions are well-tolerated, do not cause graft-versus-hostdisease (GvHD) or autoimmunity, and are associated with complete remission in poor-prognosis patients (9,10).
A major hurdle in NK cell clinical trials has been obtaining large numbers of NK cells with high purity and potency. Several protocols have been developed for ex vivo NK cell expansion using a range of cytokines, such as interleukin (IL)-2, IL-12 and IL-15, and feeder cells, including B-lymphoblastoid cell lines and monocytes (12–16). Recently, a novel method of NK cell expansion using HLA-negative K562 cells genetically modified to express membrane-bound IL-15 and 4-1 BB Ligand (BBL), which specifically activate NK cells and promote their proliferation and survival, was reported (17,18). This strategy induced a median 21.6-fold expansion of NK cells in small-scale and 90.5-fold expansion in large-scale 7-day cultures (18). Despite progress made in ex vivo expansion of NK cells from peripheral blood precursors, manufacturing large numbers of pure NK cells for clinical trials requiring high infusion doses remains challenging. As a Center for Production Assistance for Cellular Therapies (PACT), NHLBI, we were charged with the manufacture of NK cells for the treatment of multiple myeloma (MM) for investigators at the University of Arkansas for Medical Sciences (Little Rock, AR, USA). The clinical protocol for this trial required up to 5 × 107 NK cells/kg and a CD3 depletion step (for allogeneic products), therefore we had to validate the manufacture of up to 10 × 109 total cells. These numbers would require cultures in more than 40 200-mL gas-permeable tissue culture bags with frequent feeding. We had recently evaluated gas-permeable cell culture devices (G-Rex) for the expansion of T cells and tumor cell lines, in which gas exchange across the base of the culture allows increased volumes of medium per unit area, increases the rate of cell expansion, decreases cell death and minimizes cell manipulation. We therefore evaluated NK cell expansion in the G-Rex and compared the process with that in the bags. The G-Rex supported more than 100-fold NK cell expansion within 8–10 days of culture without medium exchange. These cells had an activated NK cell phenotype and killed tumor cell targets, and retained viability and recovery after cryopreservation over a 12-month period.
Methods
Cells
Peripheral blood mononuclear cells (PBMC) were purified on Ficoll gradients from leukopacks (Gulf Coast Blood Center, Houston, TX) or apheresis products from consenting healthy volunteers and patients at the University of Arkansas for Medical Sciences. K562-mb15-41BBL was obtained from St Jude Children's Research Hospital (Memphis, TN, USA) (17,18). A master cell bank of K562-mbIL15-41BBL feeder cells was manufactured and characterized as a part of a PACT project in the good manufacturing practice (GMP) facility of the Center for Cell and Gene Therapy (CAGT), Baylor College of Medicine (Houston, TX, USA). These cells express memrane-bound IL-15 and 4-1BBL as well as green fluorescent protein (GFP) (see Supplementary Figure 1 to be found online at http://www.informahealthcare.com/doi/abs/10.3109/14653249.2012.700767). HLA class I was induced on the surface of K562 and K562-mbIL15-41BBL cells with 10 ng/mL tumor necrosis factor (TNF)-α (R&D Systems, Minneapolis, MN, USA) and 100 ng/mL interferon (IFN)-γ (R&D Systems) for 3 days.
Ex vivo expansion of NK cells in gas-permeable cell culture devices (G-Rex)
After calculating the frequency of CD56+ CD3− NK cells in PBMC, they were seeded into a G-Rex (Wilson–Wolf Manufacturing, New Brighton, MN, USA) at 2–8 × 104 CD56+ CD3− NK cells/cm2. K562-mb15-41BBL cells were irradiated with 100 Gy in a Cs-137 irradiator and seeded at a 10:1 ratio of K562-mb15-41BBL to NK cells in Stem Cell Growth Medium (SCGM) and HBSS for Hanks' Balanced Salt Solution (CellGenix USA, Antioch, IL, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, ThermoScientific, Logan, UT, USA) and 10 U/mL IL-2 (Chiron Corporation, Emeryville, CA, USA). Cells were co-cultured for 8–10 days in a G-Rex100 (100-cm2 gas-permeable surface) or G-Rex10 (10-cm2 surface) in 400 or 40 mL medium, respectively. The only manipulation was the assessment of glucose levels with an Accu-Chek glucometer (Roche Diagnostics, Indianapolis, IN, USA) by withdrawing one drop of culture medium on days 4, 6, 8 and 10 of culture.
Ex vivo expansion of NK cells in gas-permeable bags
PBMC containing 2 × 106 CD56+ CD3− NK cells were seeded into 197-mL VueLife culture bags (CellGenix USA) in 40 mL SCGM medium containing 10% FBS and 10 U/mL IL-2. Irradiated K562-mb15-41BBL cells were seeded at a 10:1 ratio with NK cells as described above. Bags were partitioned with clamps at the beginning of culture to keep the medium as a 1-cm thick layer. Cells were fed every other day with 40 mL fresh medium with cytokine, to a maximum of 200 mL medium/bag.
CD3+ T-cell depletion using CliniMACS
CD3+ T cells were depleted from the expanded NK cells after magnetic labeling with a CliniMACS CD3 reagent (CellGenix USA) and an automated 2.1-depletion program on the CliniMACS instrument (CellGenix USA), according to the manufacturer's instructions.
Analysis of K562-mb15-41BBL cell proliferation after irradiation
Proliferation of K562-mb15-41BBL cells was analyzed using a Click-iT EdU Alexa Fluor 647 flow cytometry assay kit (Invitrogen, Eugene, OR, USA). Irradiated and non-irradiated positive control cells (1 × 106) were incubated for 16–24 h with 10 μm 5-ethynyl-2′-deoxyuridine (EdU). Cells were harvested, fixed and permeabilized with Click-iT™ fixative and Triton X-100-based reagents, respectively. EdU was detected with a Click-iT reaction cocktail containing Alexa Fluor 647 azide, according to manufacturer's protocol. Cells were analyzed by flow cytometry using an Allophycocyanin (APC) detecting laser.
Cytotoxicity assay
NK cell activity was analyzed in a standard 4–6 h chromium51-release assay. Briefly, target (K562 or U266) cells were labeled with 0.1 μCi NaCrO4 (MP BioMedicals, LLC, Solon, OH, USA) for 1 h. Target cells were washed three times and incubated with NK cells in triplicate at different effector to target (E:T) ratios in SCGM medium with 10% FBS. Spontaneous and maximum release were determined by incubating target cells without effectors in medium alone or in 1% Triton X-100, respectively. Supernatants were analyzed using a Packard gamma counter and the percentage of specific lysis was calculated % lysis = (experimental release − spontaneous release) × 100 ÷ (maximum release − spontaneous release). HLA class I/II blocking was performed with 94 μg/well anti-HLA-A,-B,-C and 77 μg/well anti-HLA-DP, -DQ, -DR antibodies (DakoCytomation Denmark A/S, Glostrup, Denmark) at an E:T of 20:1.
Stability of expanded NK cells
Expanded NK cells were cryopreserved at 2 × 107 cells/mL in cryomedium containing 12.5% human albumin Flexbumin The United States Pharmacopeial Convention (USP) (Baxter Healthcare Corp., Westlake Village, CA, USA) and 10% dimethyl sulfoxide (DMSO; Bioniche Pharma USA LLC, Lake Forest, IL, USA) using a Cryomed 7454 programmable freezing system. Cells were recovered from storage in vapor-phase liquid nitrogen at different time-points and were analyzed for viability by trypan blue and/or 7-Aminoactinomycin D (7-AAD) incorporation flow cytometry analysis and K562 cytotoxicity assay.
Statistical analysis
Data are presented as mean ± SEM. Data were analyzed using the unpaired Student's t-test to determine the statistical significance (P < 0.05) of differences when comparing two treatment groups in all assays. A one-way anova followed by Bonferroni's multiple comparison test was used to compare multiple treatment groups. Data were analyzed using GraphPad Prism version 5.0 software (GraphPad Software Inc., La Jolla, CA, USA).
Results
Optimization of NK cell expansion in the G-Rex
The Wilson–Wolf (G-Rex) devices support the growth of many suspension cells with minimal manipulation (19,20). To determine whether NK cells could also be expanded to large numbers, we first sought the optimal cell NK seeding density allowing us to grow these cells in G-Rex100 flasks containing 400 mL medium (Figure 1). The frequency of CD56+ CD3− NK cells in PBMC was determined by flow cytometry (Mean 8.8 ± 2.5%, n = 8; see Supplementary Figure 2 to be found online at http://www.informahealthcare.com/doi/abs/10.3109/14653249.2012.700767), therefore, the total number of PBMC seeded differed between donors. PBMC were co-cultured with 100-Gy irradiated K562-mbIL15-41BBL cells at a 1:10 ratio of CD56+ CD3− NK to K562-mb15-41BBL cells for 8 days at three seeding densities, i.e. 2 × 106, 4 × 106 and 8 × 106 NK cells/G-Rex (from 2 × 104 to 8 × 104 NK cells/cm2). Glucose was measured daily from days 5–8 of culture and approximately three-quarters of the culture medium was exchanged if glucose dropped below 100 mg/dL. The mean fold NK expansion was 209 (range 38–338), 175 (range 39–255) and 94 (range 48–126) in cultures from five different donors seeded with 2 × 106, 4 × 106 and 8 × 106 NK, respectively (Figure 1A, B and Table I). However, the highest final number of expanded NK cells was seen in G-Rexes seeded with 8 × 106 NK (7.5 ± 1.1 × 108/G-Rex100, n = 5) and 4 × 106 NK (7.1 ± 2.0 × 108 cells, n = 5). The numbers of NK cells expanded from 4 × 106 and 8 × 106 cells were significantly (P < 0.001) different compared with those expanded from 2 × 106 NK (4.2 ± 1.4 × 108/G-Rex100, n = 5). The glucose concentration dropped below 100 mg/dL only in cultures seeded with the highest cell numbers (Figure 1C). NK cultures initiated with 2 × 106 and 4 × 106 cells did not require feeding. As high numbers of NK cells could be expanded in G-Rexes seeded with 4 × 106 NK cells without additional feeding for the duration of the culture, we selected this seeding density for the clinical protocol.
Figure 1.
Ex vivo expansion of peripheral blood NK cells. (A) G-Rex100s were seeded with PBMC containing 2 × 106, 4 × 106 or 8 × 106 NK cells with irradiated K562-mbIL15-41BBL feeders at a 1:10 ratio of NK cells to K562-mb15-41BBL cells. Cells were counted on days 6, 7 and 8 of culture. Aliquots of cells from each G-Rex100 were analyzed by flow cytometry using anti-CD56 and anti-CD3 monoclonal antibodies, and the numbers of CD56+ CD3− NK cells (solid black symbols) and CD3+ CD56−T cells (open gray symbols) were plotted. *P < 0.001 difference in NK cell numbers between 4 × 106 and 2 × 106 and 8 × 106 and 2 × 106 groups (n = 5). (B) Expansion of CD56+ CD3− NK cell on days 6, 7 and 8 of culture. (C) Glucose levels were analyzed on days 5–8 of culture. Media were changed when glucose levels dropped below 100 mg/dL. (D) Increasing frequency of CD56+ CD3− NK cells over 8 days of culture. (E) Expression of NK-specific receptors on day 8. (F) Cytotoxicity of day 8-expanded NK cells against U266 MM, Raji Burkitt's lymphoma and K562 erythroleukemia cells. Result from five different donors are shown.
Table I.
Ex vivo expansion of CD56+ CD3− NK cells (n = 5) in G-Rex100 flasks on day 8 of culture.
| NK cell numbers at the start of culture (day 0) | Average total cell number, × 106 (range, × 106) | Frequency of CD56+ CD3− NK, % (range, %) | Average number of NK cells, × 106 (range, × 106) | Fold of NK expansion (range) |
|---|---|---|---|---|
| 2 × 106 | 420 (140–968) | 61 (54–70) | 418 (76–676) | 208 (38–338) |
| 4 × 106 | 720 (260–1420) | 66 (60–71) | 718 (155–1108) | 175 (39–255) |
| 8 × 106 | 931 (520–1392) | 75 (73–79) | 751 (385–1009) | 94 (48–126) |
We also tested whether different doses of IL-2 would affect NK cultures in G-Rexes. Although the frequency of CD56+ CD3− NK cells was not affected by 100 and 1000 U/mL IL-2, the mean number of cells was higher for those doses compared with NK grown with 10 U/mL (see Supplementary Figure 3 to be found online at http://www.informahealthcare.com/doi/abs/10.3109/14653249.2012.700767). However, this difference was not statistically significant (P > 0.5) and we continued to use 10 U/mL, which was provided in the original protocol.
The purity of the ex vivo-expanded CD56+ CD3− cells was 70 ± 11% (range 54–91%, n = 10, regardless of starting cell concentration) on day 8 of culture (Figure 1D). After expansion with feeder cells, NK cells up-regulated the expression of activating receptors NKp44 (CD336), NKp30 (CD337) and NKG2D (Figure 1D and Supplementary Figure 4 to be found online at http://www.informahealthcare.com/doi/abs/10.3109/14653249.2012.700767). Furthermore, these expanded NK cells showed cytotoxic activity to different cancer cell lines, such as K562, Raji Burkitt's lymphoma and U266 multiple myeloma cells (Figure 1F). The cytotoxicity of NK cells expanded using this method to primary MM cells was also demonstrated by Garg et al. (21), providing an additional proof of principle for MM therapy with NK cells. These results demonstrated that activated and potent CD56+ CD3− NK cells could be expanded efficiently during 8 days of culture in gas-permeable cell culture devices.
Comparison of ex vivo expansion of NK cells in the G-Rex and gas-permeable bags
In the original protocol, NK cells were expanded in bags for the production of up to 5 × 109 NK cells for infusion. To compare G-Rex and bag-based protocols, we seeded PBMC containing 2 × 106 NK with 2 × 107 K562-mbIL15-41BBL cells in a G-Rex100 in 400 mL medium and incubated the cells for 10 days. We also seeded PBMC containing 2 × 106 NK cells with 2 × 107 K562-mbIL15-41BBL cells in a 197-mL VueLife AC culture bag in 40 mL medium (bags were partitioned with clamps to preserve a 1-cm thickness of medium for equal gas exchange). According to the original protocol, cells in bags required feeding with 40 mL fresh medium and cytokine every other day, i.e. days 2, 4, 6 and 8 of culture, resulting in 200 mL total volume/bag by day 8 (Figure 2A). On days 4, 6, 8 and 10, cells in both vessels were counted and analyzed by flow cytometry for the frequency of CD56+ CD3− NK cells. There was no difference in NK cell numbers for the first 4 days of culture; however, in our hands, starting from day 6 there were significantly (P = 0.0003) more cells in G-Rexes compared with bags. By day 10, the mean total NK number in G-Rexes was 8.8 × 108 ± 0.6 × 108 cells (range 8.2−9.3 × 108, n = 3) and in bags 4.5 × 108 ± 1.8 × 108 cells (range 2.5−6.1 × 108, n = 3), with 442 ± 29- and 227 ± 91-fold expansion, respectively (Figure 2B, C). Therefore, for trials requiring large cell NK numbers, culturing cells the G-Rex system (a) results in higher fold expansion and (b) requires no interim cell manipulation, resulting in (c) a reduced possibility of contamination. For example, the expansion of 18 × 109 NK cells would require 20 G-Rex100s needing no medium exchange over 10 days, or 40 197-mL bags needing four medium additions during this culture period (Figure 2A). An added advantage of the G-Rex is that, at the end of culture, the majority of the medium could be easily aspirated (leaving cells resting on the bottom of the G-Rexes), resulting in a smaller harvest volume (c. 1000 mL from 20 G-Rex flasks) compared with a harvest of c. 8000 mL suspension cells from 40 bags.
Figure 2.
G-Rex yields higher numbers of NK cells and requires fewer culture manipulations than cultures in bags. (A) Schematic view of NK cell expansion in bags and G-Rexes. Less cell processing is required to grow a similar number of cells in G-Rexes. (B) NK cells from three different donors were seeded in the 197-mL bags and G-Rex100s. Bags were seeded with PBMC containing 2 × 106 NK cells and 2 × 107 K562-mb15-41BBL cells in a 40-mL starting volume and cultures required feedings with 40 mL fresh media and cytokine every other day, i.e. days 2, 4, 6 and 8, resulting in 200 mL of total volume by day 8. G-Rex100s were seeded with PBMC containing 2 × 106 NK cells and 2 × 107 K562-mbIL15-41BBL in 400 mL medium and were not subsequently fed. Cells were counted and analyzed by flow cytometry for percentage CD56+ CD3− cells (n = 3). (C) Fold expansion of CD56+ CD3− cells in G-Rexes and bags (n = 3). *P < 0.5.
Generation of large-scale clinical-grade NK cell products
The clinical trial of NK cells for the treatment of patients with myeloma requires doses of 3 × 107−5 × 107 NK cells/kg. For patients receiving allogeneic NK cells, T-cell depletion is required to prevent GvHD. Assuming a 50% loss of total cells during the T-cell depletion and washes, we anticipated requiring up to 10 × 109 NK cells for the final processing. We performed two validation runs using apheresis products from healthy donors, and we manufactured four patient-specific products from two patients with MM and two haplo-identical donors. Aphereses were performed at the University of Arkansas, then cryopreserved cells were shipped to Baylor in liquid nitrogen dry shippers. Thawed cells were washed, and the PBMC were recovered using a Ficoll gradient and seeded into G-Rex100s with irradiated K562-mbIL15-41BBL cells at a 1:10 ratio of K562-mb15-41BBL to CD56+ CD3− NK cells. Cells were expanded for 8–9 days, harvested and cryopreserved. The mean NK cell expansion after 8–9 days of culture in G-Rexes was c. 93-fold (range 26–128, n = 6), generating NK products of 8.5 × 109 ± 1.9 × 109 CD56+ CD3− NK cells (Table II) from 9 × 107 NK seeded on day 0 of culture. Although the fold expansion of patient cells was modest compared with cells from healthy donors, patients' cells contained a higher frequency of CD56+ CD3− NK cells (86 ± 3%, n = 2) and sufficient cell numbers were obtained to formulate infusion doses of 4.3 × 109 cells (5 × 107 cells/kg) for one patient and 2.5 × 109 cells (3.4 × 107 cells/kg) for another patient. Cells were analyzed by flow cytometry for purity as shown in Table III.
Table II.
Large-scale ex vivo expansion of NK cells in G-Rex vessels.
| Validation 1, healthy donor | Validation 2, healthy donor | Clinical product, MM patient | Clinical product, healthy haplo-identical donor | |
|---|---|---|---|---|
| Seeded NK cells | 9 × 107 | 9 × 107 | 9 × 107 | 15 × 107 |
| Day of harvest | 8 | 8 | 9 | 8 |
| Total number of cells harvested | 20 × 109 | 13 × 109 | 7 × 109 | 24 × 109 |
| % CD56+ CD3− | 52.2 | 69.3 | 88.3 | 84.8 |
| Number of expanded NK cells | 10 × 109 | 9.1 × 109 | 6.3 × 109 | 19.2 × 109 |
| Fold NK cell expansion | 111 | 101 | 70 | 128 |
Table III.
Characteristics of large-scale clinical-grade NK cell products expanded in G-Rex vessels.
| Validation 1, healthy donor | Validation 2, healthy donor | Clinical product, MM patient | Clinical product, healthy haplo-identical donor | |
|---|---|---|---|---|
| % viable cells (7-AAD−) | 72.4 | 86.4 | 82.9 | 90.8 |
| % CD3+ CD56− pre-T-cell depletion | 34.1 | 18.7 | 3.5 | 8.1 |
| % CD3+ CD56− post-T-cell depletion | 1 | 0.04 | n/a | 0.7 |
| % CD19+ | 0.56 | 0.56 | 1.98 | 1.24 |
| % CD14+ | 0.09 | 0.05 | 0.24 | 0.05 |
We validated the CliniMACS depletion of CD3+ T cells for two large-scale products containing high frequencies of CD3+ T cells. Depletion from 34% to 1% and from 19% to 0.04% CD3+ CD56− T cells in validation runs 1 and 2, respectively, was achieved (Table III), which met the release criterion of less than 2 × 105 CD3+ CD56− T cells/kg. The release criteria for Therapeutic Cell-Natural Killer (TC-NK) products are summarized in Table IV.
Table IV.
Release criteria for the TC-NK products.
| Test | Method | Criteria | Results available within 7 days after cryopreservation |
|---|---|---|---|
| USP sterility1 | Immersion USP 21 CFR 610.12 bacterial and fungal sterility test | Negative after 14 days | No |
| Sterility1 | Gram stain | No organism seen | Yes |
| Sterility1 | BACTEC for aerobic, anaerobic bacteria and fungus | No growth | Yes (preliminary) |
| Bacteria, fungus | |||
| Sterility1 | Endosafe PTS (LAL) | < 5.0 EU/mL | Yes |
| Endotoxin | |||
| Mycoplasma2 | Polymerase chain reaction (Takara) | Negative | Yes |
| Purity3 | Flow cytometry | < 0.1% GFP+ K562-mbl5-41BB-L feeder cells | Yes |
| Purity3 (only for allogeneic) | Flow cytometry | < 2 × 105 CD3+ CD56− cells/kg | Yes |
| Purity3 (only for autologous) | Flow cytometry | > 50% CD56+ CD3− cells | Yes |
| Purity3 (only for allogeneic) | Flow cytometry | > 70% CD56+ CD3− cells | Yes |
| Identity3 | HLA class I (A, B) genotyping | Identical to NK donor | Yes |
| Potency3 | Cytotoxic activity to K562 cells | > 20% lysis at a 20:1 NK:K562 ratio | Yes |
| Other3 | Flow cytometry | > 70% viable | Yes |
| Viability |
USP, The United States Pharmacopeial Convention; CFR , Code of Federal Regulations; LAL, Limulus Amebocyte Lysate; PTS, Prtable testing system.
Final product cells in cryopreservation medium.
Supernatant from end of culture cells.
End of production cells.
Evaluation of proliferation of irradiated K562-mb15-41BBL feeder cells and their frequency in NK products
Although genetically modified K562-mb15-41BBL cells receive 100 Gy irradiation and are highly susceptible to killing by proliferating NK cells in culture, we were required to ensure that the feeder cells in clinical products had no proliferative potential. Significant numbers of K562-mb15-41BBL cells (c. 60–80%; n = 5) cultured alone appeared to be viable, as measured by trypan blue incorporation 5–7 days after irradiation. The initial protocols using K562-mb15-41BBL cells for NK expansion (NKEXP and NKCD19; clinicaltrials.gov numbers NCT00640796 http://www.clinicaltrials.gov/ct2/results?term=+NCT00640796 and NCT00995137 http://www.clinicaltrials.gov/ct2/results?term=NCT00995137 accessed on July 24th, 2012, respectively) used the Click-iT proliferation assay to evaluate incorporation of EdU into DNA during cell replication. We applied the same method to our cultures. Irradiated and non-irradiated K562-mb15-41BBL cells were kept in culture without PBMC for 4–7 days before the assay. After pulsing with EdU, the cells were fixed, permeabilized and EdU detected in a covalent reaction between an azide containing Alexa Fluor 647 dye and an alkyne group of EdU. As shown in Figure 3A, irradiated cells did not proliferate after overnight pulsing with EdU, while more than 75% of non-irradiated cells (positive control) proliferated. We are currently using this assay as an in-process release test for each NK product.
Figure 3.
Proliferation and frequency of the K562-mb15-41BBL feeder cells in the final NK products. (A) Proliferation of K562-mbIL15-41BBL cells was analyzed in a Click-iT EdU Alexa Fluor 647 flow cytometry assay. Irradiated and non-irradiated positive control cells (1 × 106) were incubated for 1 h or overnight (16–24 h) with 10 mm EdU. EdU was detected with a Click-iT reaction cocktail containing Alexa Fluor 647 azide. The experiments were performed at least three times. (B) K562-mb15-41BBL cell gating in the final NK cell product was established by gating K562-mb15-41BBL cells (GFP+) alone and spiking 1000 and 5000 of those cells into 106 cells of the final NK product. The spiking experiments were performed three times and 12 NK products were analyzed. Representative results are shown.
The release criteria also demanded less than 0.1% of K562-mb15-41BBL cells in the final NK cell clinical products. K562-mb15-41BBL cells express GFP and should be identified easily by flow cytometry. However, because they are irradiated and killed by proliferating NK cells, it is difficult to identify such a low frequency of GFP-positive cells convincingly within the expanded NK cultures. To ensure that we could detect low numbers of K562-mb15-41BBL cells among large numbers of activated NK cells, we spiked 106 expanded NK cells with 1000 and 5000 K562-mb15-41BBL cells, which constituted 0.1% and 0.5% of the cells respectively. We first defined GFP+ K562-mb15-41BBL cells alone (Figure 3B, left panel). In the spiked population we could detect 0.15% and 0.5% K562-mbIL15-41BBL cells, reflecting the added numbers. The frequency of GFP+ cells in NK products (Figure 3B) was 0.01–0.07% (n = 4), which satisfied the release criterion of < 0.1%. These data demonstrated that K562-mb15-41BBL cells did not proliferate after irradiation and that we could detect very low frequencies of these cells efficiently in clinical NK products.
Characterization of CD3+ T cells in the expanded NK cell product
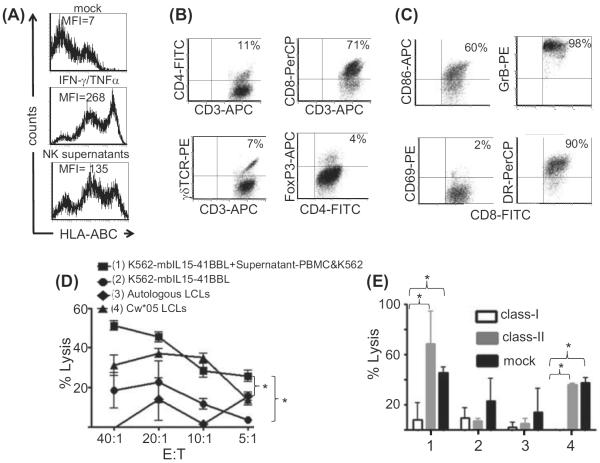
Moderate proliferation of T cells was detected during the NK cell expansion (Figure 1A). Previously it has been reported that HLA class IC molecules could be up-regulated on K562 cells stimulated with IFN-γ (22–25). We also found that K562 cells up-regulated HLA class I as well as HLA-DR expression upon stimulation with CD3/28-activated T cells or their supernatants. Figure 4A shows that K562-mb15-41BBL cells up-regulated HLA class I antigens upon stimulation with both IFN-γ and TNF-α, and in response to supernatants from NK cultures. Therefore, we were concerned that the activated T cells in the cultures might have alloreactive potential. Interestingly, we found that CD8+ T cells with an activated phenotype dominated the T-cell component (45–90% of all CD3+ T cells; n = 3) of the NK cultures and expressed high levels of granzyme B, CD86 and HLA-DR (Figure 4B, C). As we were unable to detect the HLA-A11 or HLA-A31 of K562 using monoclonal antibodies specific for those alleles, and previous publications suggested that only HLA class IC antigens were up-regulated, we tested the alloreactivity of CD8+ T cells from NK cultures prepared from an HLA class I-mismatched donor (A*02,03; B*07,41; C*07,17) to K562-mb15-41BBL cells (A*11,31; B*18,40; C*03,05), alone or pre-cultured with NK supernatants, and to the lymphoblastoid cell line (LCL) from donors matching only by HLA-Cw*05, which is shared by K562. The CD8+ T cells from NK cultures (PBMC co-cultured with irradiated K562-mb15-41BBL cells) were first enriched by two rounds of positive selection with CD8 monoclonal antibody-coated magnetic beads and subsequent flow sorting for CD8+ CD56− cells. This selection allowed us to deplete the NK and NK-like T cells. Interestingly, we found that CD8+ T cells purified from NK cultures could recognize and kill K562-mb15-41BBL cells stimulated with the supernatant from PBMC co-cultured with irradiated K562-mb15-41BBL cells (supernatant-PBMC&K562) (Figure 4D), but not unstimulated K562-mbIL15-41BBL. In addition, these T cells killed LCL sharing Cw*05 with K562-mbIL15-41BBL, indicating that at least some of these CD8+ cells were reactive to Cw*05 molecules. This cytotoxic activity was blocked by HLA class I antibodies (Figure 4E). Additional studies would be necessary to determine whether this activity is restricted by HLA Cw*05; however, these findings justified the CD3-depletion of allogeneic donor NK cell products.
Figure 4.
Alloreactive CD8+ T cells with an activated phenotype dominated the T-cell component of NK cultures. (A) HLA class I expression on K562-mb15-41BBL was induced with IFN-γ/TNF-α and NK culture supernatants. MFI, mean fluorescent intensity. (B) NK cell cultures were gated on CD3+ cells and analyzed for expression of CD4, CD8, γθ-T cell receptor (TCR) and intracellular Foxp3. (C) CD8+ T cells were stained for the surface expression of CD69, HLA-DR and CD86, and intracellular expression of granzyme B (GrB). The percentage of double-positive cells is depicted. The results are representative of three independent experiments. (D) Alloreactivity of CD8+ CD56− T cells purified by magnetic bead separation and flow sorting from NK cultures of the donor A*02,03; B*07,41; C*07,17 (class I mismatched to K562-mb15-41BBL cells). The cytotoxic specificity of the CD8+ T cells was analyzed in a 6-h chromium51 release assay using (1) K562-mbIL15-41BBL cells stimulated with supernatants from PBMC cultured with K562-mb15-41BBL cells (Sup-PMBC-K562), (2) K562-mb15-41BBL cells, (3) the NK cell donor (autologous) LCL and (4) HLA Cw*05-matched LCL (n = 3). (E) A cytotoxicity blocking assay (n = 3) with anti-HLA-ABC (clone W6/32) and HLA-DP, -DQ, -DR (clone CR3/43) antibodies was performed at an E:T ratio of 20:1. Targets as described in (D). *P < 0.5.
Stability of cryopreserved TC-NK products
Cryopreserved, expanded NK cells were shipped from Baylor College of Medicine GMP laboratory for infusion at the clinical site at the University of Arkansas for Medical Sciences. The NK cells were cryopreserved at 2 × 107 cells/mL medium containing 10%v DMSO, 40%v 1 × Hanks' Balanced Salt Solution (HBSS) and 50%v 25% Human Serum Albumin (HSA) (Flexbumin; Baxter Healthcare Corp.) and stored in Cryostore bags (OriGen Biomedical, Austin, TX) in a liquid nitrogen vapor storage tank. We determined whether cryopreservation and storage at −150°C affected NK cell viability and potency. The product was considered stable if, after thawing, it met key release criteria, i.e. a greater than 70% viability and greater than 20% killing of K562 at an E:T ratio of 20:1. We performed the potency assays immediately after thawing NK cells or after resting them overnight in complete SCGM medium with 10 U/mL IL-2. Immediately after recovery, NK cells were on average 91% viable (range 85–94%, n = 5) (Figure 5A); however, they failed to lyse K562 cells unless rested overnight (Figure 5B). After 3 months of cryopreservation, the mean NK cell viability was 90 ± 7% (n = 3), mean recovery 83 ± 3% (n = 3) and mean K562 lysis 79 ± 5% (n = 3) (Table V). At this time, we had 12 months of stability data for only two NK products (Table V), which were 94 ± 2% viable and lysed 81 ± 6% of K562 cells after 12 months of cryopreservation. However, we found that cell recovery immediately after thawing was donor-dependent, ranging from 51–95% in six different products within the first 12 months of cryopreservation. As the clinical trial progresses, additional cryopreserved NK products will be studied for stability and data will be published in the future.
Figure 5.
Stability of cryopreserved TC-NK products. (A) Cryopreserved NK cells were thawed in a 37°C waterbath and washed in pre-warmed 1 × phosphate-buffered saline (PBS). Live and dead cells were evaluated by flow cytometry using 7-AAD. (B) NK cells from three donors (n = 3) were analyzed in a cytotoxicity assay using K562 cells as targets. Fresh or cryopreserved NK cells were used as effectors. Frozen NK cells were used immediately after thawing or were rested overnight in SCGM medium supplemented with 10% FBS and 10 IU/mL IL-2. *P < 0.5.
Table V.
Stability ofTC-NK products.
| Viability (%) | Recovery (%) | Potency K562 lysis at a 20:1 E:T (%) | |
|---|---|---|---|
| Product 1 | |||
| Fresh | 91 | NA | 92 |
| 1 month | 95 | 95 | 43 |
| 6 months | 85 | 81 | 61 |
| Product 2 | |||
| Fresh | 84 | NA | 80 |
| 3 months | 82 | 80 | 75 |
| Product 3 | |||
| Fresh | 96.6 | NA | 65 |
| 1 month | 89 | 70 | 73 |
| Product 4 | |||
| Fresh | 85 | NA | 90 |
| 3 months | 70.6 | 85 | 75 |
| 12 months | 75 | 58 | 85 |
| Product 5 | |||
| Fresh | 93 | NA | 65 |
| 1 month | 94 | 85 | 93 |
| 6 months | 91 | 85 | 84 |
| Product 6 | |||
| Fresh | 85 | NA | 90 |
| 3 months | 92 | 85 | 87 |
| 12 months | 92 | 51 | 77 |
NA, not applicable.
Discussion
We report an efficient and reproducible cell culture protocol for ex vivo expansion of activated NK cells in gas-permeable cell culture devices (G-Rex). This protocol is based on a previously described method of ex vivo NK generation from peripheral blood cells stimulated with genetically modified K562-mb15-41BBL cells, expressing membrane-bound IL-15 and 4-1BBL (17,18). The original protocol used gas-permeable bags for growing NK cells and frequent medium supplementation during culture. Here we show that expansion of NK cells in the G-Rex produces an equivalent rate of NK cell expansion but requires no culture medium supplementation, and because the majority of the medium can be aspirated without disturbing the cells at the end of culture, minimal medium reduction is required. We could generate up to 19 × 109 NK cells from less than 40% of each apheresis product. These cell numbers could support multiple infusions of high (5–10 × 107 cells/kg) doses of cells. In agreement with a previous protocol (18), we found that NK cells generated in G-Rexes express typical NK cell activation markers, are more than 50% pure and display high cytotoxic activity to K562 chronic erythroid leukemia and U266 MM cells.
Although this method involves a K562-mb15-41BBL supportive feeder cell line, these cells are undetectable after 7 days of NK expansion. Moreover, this method allows a robust large-scale expansion of NK cells within a short time period (less than 10 days), allowing rapid treatment of cancer patients with progressive disease. This aggressive timeline is not possible for current cytokine-based methods, which require more than 3 weeks to generate similar numbers of NK cells (26,27). K562-mb15-41BBL cells express GFP, therefore they can be detected easily by flow cytometry in clinical NK products. In addition, we have validated a flow cytometry-based Click-iT assay for showing that irradiated K562-mbIL15-41BBL cells do not proliferate. While GFP is useful for confirming the elimination of K562 feeder cells, there may be concern that cells expressing GFP could induce an immune response to the transgene. However, since the K562 cells are undetectable at the time of harvest, they are unlikely to induce an immune response in the patient, and if GFP-specific T cells were induced during the culture they should have little or no negative consequence for the patient. A second concern might be the risk of contamination with replication-competent retroviral vector expressing the 4-1BBL and GFP. A master cell bank from which the K562-mb15-41BBL cells were derived tested negative for replication competent retrovirus (RCR). In addition, however, alternative methods (e.g. electroporation) (28) for transduction of feeder cells should be explored to provide safer clinical products.
A variety of protocols for the manufacture of NK cells has been described. Because NK cells constitute a small percentage of peripheral blood, a number of studies has focused on positive or negative selection of CD56+ NK cells from apheresis products or peripheral blood, using immunomagnetic separation followed by ex vivo culture for periods ranging from overnight to 3 weeks, in the presence of IL-2 or other cytokines (10,29–32). The NK products obtained by these methods were 20–50% pure (33). Other protocols expand NK cells from PBMC without prior enrichment, using cytokines (27,34,35) and/or feeder cells (18,32,36). Although cell-free methods may potentially be safer from a regulatory standpoint, they require prolonged culture periods (up to 3–6 weeks) to grow large numbers of cells (31,32,34,35), potentially inducing a more differentiated product. Moreover, some of these protocols use high concentrations (500 IU/mL) of IL-2 and anti-CD3 antibody (for activation of autologous T cells to help the NK expansion), and therefore produce relatively high numbers of undesirable CD3+ T cells (27). Using K562 cells expressing 4-1BBL and membrane-bound IL-15, we could induce up to 200-fold expansion of activated CD56+ CD3− NK cells within 10 days of culture. Although there was some contamination with T cells (less than 35%), these cells could be removed by magnetic depletion. In recently published work, the NK cells expanded by this method were able to inhibit human MM tumor growth in NOD/SCID/IL-2Rynull mice (21), indicating the anti-tumor potency of these cells. Additional in vivo studies are necessary to compare the clinical efficacy of NK cells generated using different protocols, including expanded and non-expanded NK cells. This will help to understand whether size, survival and trafficking can affect the potency of differently generated NK.
Current protocols for ex vivo NK cell expansion describe culturing in different vessels, such as small-volume flasks (37), medium-volume gas-permeable bags (30,32,38) and large-volume bioreactors, such as the 20-L WAVE (26,27). In gas-permeable bags the volume of medium is restricted by gas exchange and the shape of the bag. This limits the supply of nutrients, which are rapidly consumed by proliferating NK cells. Cell expansion is further inhibited by waste accumulation and acidic pH. Therefore frequent (every other day) feeding is required in order to achieve sufficient cells for infusion.
We have adapted the NK cell expansion procedure to the G-Rex system, in which cells grow on a gas-permeable silicon membrane, permitting efficient gas exchange across the base of the culture. This allows a height of medium above the membrane that is limited only by the structure of the G-Rex (4 cm). This compares with an ideal height of 0.3 cm above a plastic surface of regular tissue culture plastic and about 1 cm in a bag. Thus for cells seeded at 0.5 × 106/cm2, the volume of medium per 106 cells is 8 mL in the G-Rex, 0.6 mL in a 24-well plate and 2 mL in a bag. With the G-Rex, this eliminates the need for medium exchange, and cell manipulation is reduced to setting up the culture on day 0 and NK cell harvest between days 8 and 10. Furthermore, because the majority of the medium can be aspirated from the G-Rex without disturbing the cells, the harvest volume for clinical production is c. 1 L from 20 G-Rexes, compared with more than 7 L from 40 bags. This reduces the time for harvesting and eliminates the need for complex volume reducers or cell harvesters as used in the original protocol, a significant advance in large-scale NK cell expansion as frequent cell manipulations are time consuming, expensive, reduce the reproducibility of cell production and increase the risk of contamination. Although growing cells in G-Rexes is an open system procedure, high cell yields and a significant reduction in manipulations during culture make this system applicable for the production of clinical-grade cellular products in GMP facilities. Furthermore, this system could be readily adapted to a closed system.
Although our procedure reproducibly expanded NK cells from all donors, the rate of NK expansion varied between donors and therefore it was difficult to predict the number of seeded cells (vessels) necessary to achieve the required number of cells. Cell cultures in G-Rexes are scalable (Lapteva et al unpublished data), therefore one can approximate the number of cells grown in a larger G-Rex100 vessel from a small experiment with a sample of apheresis cells expanded in G-Rex10. For example, we expanded 31 × 106 ± 10 × 106 CD56+ CD3− NK cells in a G-Rex10 and 322.1 × 106 ± 128 × 106 NK in a G-Rex 100 from the same three donors, resulting in an average of 64- and 62-fold expansion respectively. Thus a small pilot expansion can be used to predict the number of starting cells needed to seed to achieve a given final dose.
Large numbers of NK cells may be critical for the treatment of large patients with multiple high doses, particularly if T-cell depletion is required for allogeneic products. In this study we have demonstrated that static cultures of NK cells expand well in the G-Rex system. Once G-Rexes with a larger surface area become available, this procedure will be further simplified.
Although CD3+ T cells are not expected to proliferate in cultures with K562-mbIL15-41BBL cells, we found 5–35% CD3+ T cells in the final NK products, indicating that CD3+ T cells proliferate in some cultures. Further analysis of these T cells demonstrated a preponderance of CD8+ T cells that expressed high levels of the activation markers CD86 and HLA-DR and the cytotoxic marker granzyme B. It has been shown previously that K562 cells up-regulate HLA class IC molecules upon stimulation with IFN-γ and TNF-α (22–25). In this study, we show for the first time that K562-mbIL15-41BBL cells up-regulate HLA class I expression upon stimulation with supernatants of ex vivo-expanded NK cells. Moreover, isolated CD8+ T cells from NK cultures of class I-mismatched donors recognized and killed K562-mbIL15-41BBL cells in which HLA class I molecules had been up-regulated, as well as LCL HLA matched at the HLA-C locus with K562. As killing could be inhibited by antibodies to HLA class I, these cells are probably alloreactive T cells. Additional studies are necessary to determine the exact K562 class I antigens for the alloreactive CD8+ T cells. Because CD3+ T cells are depleted from the final product to levels below 2 × 105 CD3+ T cells/kg, the risk to patients associated with alloreactivity to K562 class I antigens should be minimized.
The development of new improved methods for the production of large numbers of pure NK cells has injected new interest into clinical trials with adoptive NK cell infusions. As many of the protocols require several weeks of expansion, the coordination of patient readiness and the end of NK cell culture is crucial if expanded cells are to be infused fresh. However, the clinical course of cancer patients is unpredictable, hence there is a significant interest in methods of cryopreservation and the stability of cryopreserved NK cells. We have shown that our method of NK cryopreservation keeps cells stable for 12 months, because we can recover efficiently on average 90% viable and potent NK cells. However, viability and recovery were donor dependent and larger stability studies on clinical products of cryopreserved NK cells are required.
In conclusion, we have successfully established static NK cell cultures in the gas-permeable G-Rex system under GMP conditions in order to use these cells for adoptive immunotherapy. The safety and therapeutic potency of these NK cells is under evaluation in patients with relapsed MM at the University of Arkansas for Medical Sciences.
Supplementary Material
Acknowledgments
This work was supported in part by NIH-NHLBI-N01 HB37163, NHLBI-1U54 HL081007 and NIH-NCI PO1 CA94234. We would like to thank Oumar Diouf, Deborah Lyon, Jeannette Bloom and Huimin Zhang and all the GMP staff of CAGT for the technical support in enabling this work.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplementary material available online Supplementary Figures 1–4.
References
- 1.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 2.Piroozmand A, Hassan ZM. Evaluation of natural killer cell activity in pre and post treated breast cancer patients. J Cancer Res Ther. 2010;6:478–81. doi: 10.4103/0973-1482.77110. [DOI] [PubMed] [Google Scholar]
- 3.Dewan MZ, Takada M, Terunuma H, Deng X, Ahmed S, Yamamoto N, et al. Natural killer activity of peripheral-blood mononuclear cells in breast cancer patients. Biomed Pharmacother. 2009;63:703–6. doi: 10.1016/j.biopha.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Kondo E, Koda K, Takiguchi N, Oda K, Seike K, Ishizuka M, et al. Preoperative natural killer cell activity as a prognostic factor for distant metastasis following surgery for colon cancer. Dig Surg. 2003;20:445–51. doi: 10.1159/000072714. [DOI] [PubMed] [Google Scholar]
- 5.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Xiangming C, Iwashige H, et al. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett. 2000;159:103–8. doi: 10.1016/s0304-3835(00)00542-5. [DOI] [PubMed] [Google Scholar]
- 6.Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, et al. Tumor infiltration by FcgammaRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer. 2011;128:2663–72. doi: 10.1002/ijc.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Shibli K, Al-Saad S, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic value of intraepithelial and stromal innate immune system cells in non-small cell lung carcinoma. Histopathology. 2009;55:301–12. doi: 10.1111/j.1365-2559.2009.03379.x. [DOI] [PubMed] [Google Scholar]
- 8.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–8. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 9.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–94. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 10.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 11.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–32. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 12.Naume B, Gately M, Espevik T. A comparative study of IL-12 (cytotoxic lymphocyte maturation factor)-, IL-2-, and IL-7-induced effects on immunomagnetically purified CD56+ NK cells. J Immunol. 1992;148:2429–36. [PubMed] [Google Scholar]
- 13.Carson WE, Fehniger TA, Haldar S, Eckhert K, Lindemann MJ, Lai CF, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–43. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson MJ, Cameron C, Lazo S, Cochran KJ, Voss SD, Ritz J. Costimulation of human natural killer cell proliferation: role of accessory cytokines and cell contact-dependent signals. Nat Immun. 1996;15:213–26. [PubMed] [Google Scholar]
- 15.Perussia B, Ramoni C, Anegon I, Cuturi MC, Faust J, Trinchieri G. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat Immun Cell Growth Regul. 1987;6:171–88. [PubMed] [Google Scholar]
- 16.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–9. [PubMed] [Google Scholar]
- 17.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–83. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–7. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010;33:305–15. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapteva N, Vera JF. Optimization manufacture of virus- and tumor-specific T cells. Stem Cells Int. 2011:434392. doi: 10.4061/2011/434392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg TK, Szmania SM, Khan JA, Hoering A, Malbrough PA, Moreno-Bost A, et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica. 2012 doi: 10.3324/haematol.2011.056747. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garson D, Dokhelar MC, Wakasugi H, Mishal Z, Tursz T. HLA class-I and class-II antigen expression by human leukemic K562 cells and by Burkitt-K562 hybrids: modulation by differentiation inducers and interferon. Exp Hematol. 1985;13:885–90. [PubMed] [Google Scholar]
- 23.Chen E, Karr RW, Frost JP, Gonwa TA, Ginder GD. Gamma interferon and 5-azacytidine cause transcriptional elevation of class I major histocompatibility complex gene expression in K562 leukemia cells in the absence of differentiation. Mol Cell Biol. 1986;6:1698–705. doi: 10.1128/mcb.6.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland J, Mannoni P, Rosa F, Huyat D, Turner AR, Fellous M. Induction of the expression of HLA class I antigens on K562 by interferons and sodium butyrate. Hum Immunol. 1985;12:65–73. doi: 10.1016/0198-8859(85)90344-1. [DOI] [PubMed] [Google Scholar]
- 25.Takemura Y, Koide Y, Kawabata M, Kitajima S, Ohba C, Suzuki H, et al. Synergistic enhancement of class I major histocompatibility complex antigen expression in K562 cells induced by recombinant human interferon-gamma and tumor necrosis factor in combination. Exp Hematol. 1989;17:795–9. [PubMed] [Google Scholar]
- 26.Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte, et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One. 2011;6:e20740. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutlu T, Stellan B, Gilljam M, Quezada HC, Nahi H, Gahrton G, et al. Clinical-grade, large-scale, feeder-free expansion of highly active human natural killer cells for adoptive immunotherapy using an automated bioreactor. Cytotherapy. 2010;12:1044–55. doi: 10.3109/14653249.2010.504770. [DOI] [PubMed] [Google Scholar]
- 28.Shimasaki N, Fujisaki H, Cho D, Masselli M, Lockey T, Eldridge P, et al. A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies. Cytotherapy. 2012;14:830–40. doi: 10.3109/14653249.2012.671519. [DOI] [PubMed] [Google Scholar]
- 29.Klingemann HG, Martinson J. Ex vivo expansion of natural killer cells for clinical applications. Cytotherapy. 2004;6:15–22. doi: 10.1080/14653240310004548. [DOI] [PubMed] [Google Scholar]
- 30.Koehl U, Esser R, Zimmermann S, Tonn T, Kotchetkov R, Bartling T, et al. Ex vivo expansion of highly purified NK cells for immunotherapy after haploidentical stem cell transplantation in children. Klin Padiatr. 2005;217:345–50. doi: 10.1055/s-2005-872520. [DOI] [PubMed] [Google Scholar]
- 31.Decot V, Voillard L, Latger-Cannard V, Aissi-Rothe L, Perrier P, Stoltz JF, et al. Natural-killer cell amplification for adoptive leukemia relapse immunotherapy: comparison of three cytokines, IL-2, IL-15, or IL-7 and impact on NKG2D, KIR2DL1, and KIR2DL2 expression. Exp Hematol. 2010;38:351–62. doi: 10.1016/j.exphem.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Siegler U, Meyer-Monard S, Jorger S, Stern M, Tichelli A, Gratwohl A, et al. Good manufacturing practice-compliant cell sorting and large-scale expansion of single KIR-positive alloreactive human natural killer cells for multiple infusions to leukemia patients. Cytotherapy. 2010;12:750–63. doi: 10.3109/14653241003786155. [DOI] [PubMed] [Google Scholar]
- 33.McKenna DH, Jr, Sumstad D, Bostrom N, Kadidlo DM, Fautsch S, McNearney S, et al. Good manufacturing practice production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–8. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 34.Carlens S, Gilljam M, Chambers BJ, Aschan J, Guven H, Ljunggren HG, et al. A new method for in vitro expansion of cytotoxic human CD3− CD56+ natural killer cells. Hum Immunol. 2001;62:1092–8. doi: 10.1016/s0198-8859(01)00313-5. [DOI] [PubMed] [Google Scholar]
- 35.Suck G, Oei VY, Linn YC, Ho SH, Chu S, Choong A, et al. Interleukin-15 supports generation of highly potent clinical-grade natural killer cells in long-term cultures for targeting hematological malignancies. Exp Hematol. 2011;39:904–14. doi: 10.1016/j.exphem.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Somanchi SS, Senyukov VV, Denman CJ, Lee DA. Expansion, purification, and functional assessment of human peripheral blood NK cells. J Vis Exp. 2011 doi: 10.3791/2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alici E, Sutlu T, Bjorkstrand B, Gilljam M, Stellan B, Nahi H, et al. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111:3155–62. doi: 10.1182/blood-2007-09-110312. [DOI] [PubMed] [Google Scholar]
- 38.Lee DA, Verneris MR, Campana D. Acquisition, preparation, and functional assessment of human NK cells for adoptive immunotherapy. Methods Mol Biol. 2010;651:61–77. doi: 10.1007/978-1-60761-786-0_4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.