Abstract
Chorioamnionitis is a potentially life threatening infection of the fetal membranes, commonly caused by ascending bacteria from the vagina and cervix. In our case, a healthy nullipara with a term pregnancy presented clinical signs of infection after induced labour with an intracervical balloon. Thick green and foul smelling amniotic fluid was observed and culture showed massive growth of Aerococcus christensenii, a facultative anaerob species found in the human vagina, previously only rarely alleged to cause invasive infection. Additional testing with 16S rRNA gene analysis also identified the presence of Gemella asaccharolytica, Snethia sanguinegens, Parvimonas micra and Streptobacillus moniliformis. The patient was treated with cefuroxime and metronidazole and recovered quickly. The newborn showed no signs of infection. This case points at the possible role of these pathogens in female genital tract infections. The case also underlines the importance of the combination of culture and culture independent diagnostic approaches to reveal possible polymicrobial natures of selected infections, in this case chorioamnionitis.
Keywords: Aerococcus christensenii, gemella assacharolytica, parvimonas micra, polymicrobial chorioamnionitis, pregnancy, snethia sanguinegens, streptobacillus moniliformis
INTRODUCTION
Chorioamnionitis is an infection of the fetal membranes, thought to affect as many as 10 % of all labouring women [1] and it is a major challenge with regard to secure the mother as well as the foetus from life threatening complications. In term pregnancies it is thought to be caused primarily by ascending infections from the vagina and cervix, causing an inflammatory response in the decidua followed by a chorionitis [2].
In most healthy women, one or two species of Lactobacillus dominates the vaginal flora [3]. Yet, in up to one third of healthy women, the Lactobacillus species lack in appreciable numbers and may be replaced by several other species, e.g. Atopobium, Megasphaera and Leptotrichia species [3]. Aerococcus christensenii has been isolated from the human vagina too [4]. A. christensenii, Gemella asaccharolytica and Snethia (Leptotrichia) sanguinegens [5, 6] are relatively newly established taxons and have only rarely to very rarely been alleged to cause invasive infections. During labour, increased vaginal secretions are common, creating a favourable medium for growth of bacteria, which can ascend and stimulate maternal and foetal inflammatory responses with the release of cytokines, prostaglandins and endotoxins [1]. Applicating molecular laboratory methods may contribute considerably in diagnostic outlining of these organisms [7].
In this case, we describe a healthy woman with a singleton term pregnancy, whom after iatrogenic initiation of labour developed high fever, vomiting and tachycardia. Routine culture methodology, 16S rRNA gene analysis and amended interpretation of mixed chromatograms each contributed importantly to the detection and final understanding of the polymicrobial infectious episode. These revealed to be A. christensenii, G. asaccharolytica, S. sanguinegens/Leptotrichia amnionii, Parvimonas micra and Streptobacillus moniliformis.
Case story
A 33-year old healthy nullipara with an intrauterine singleton normal pregnancy of 42 weeks and 0 days’ gestational age was admitted to the maternity ward for inducing labour due to gravida prolongata.
An intracervical balloon was placed for ripening the cervix. Fourteen hours later, artificial rupture of membranes was performed, but amniotic fluid was never surely identified. Furthermore, accelerated stimulation with synthetic oxytocin was initiated. The foetus was constantly monitored with cardiotocography (CTG).
The patient began to feel unwell, having nausea and vertigo, twelve hours after artificial rupture of membranes. The CTG was normal (Appendix Fig. 1). The oxytocin stimulation was paused and the patient rested for the night. During the next day, the patient got increasingly ill, with high fever (39.2 °C), shivering, vomiting and tachycardia. The CTG also showed fetal tachycardia (FHR 160-170) and several episodes of bradycardia lasting between one and one and a half minute (Appendix Fig. 2). A fetal scalp-pH was 7, 34 (7, 25-7, 35).
Fig. (1).
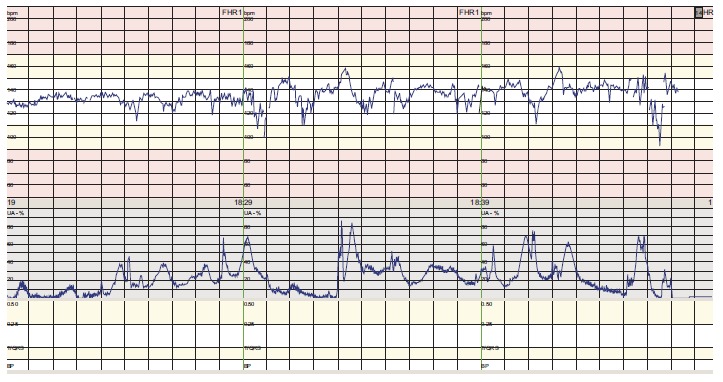
Normal CTG (cardiotocography).
Fig. (2).
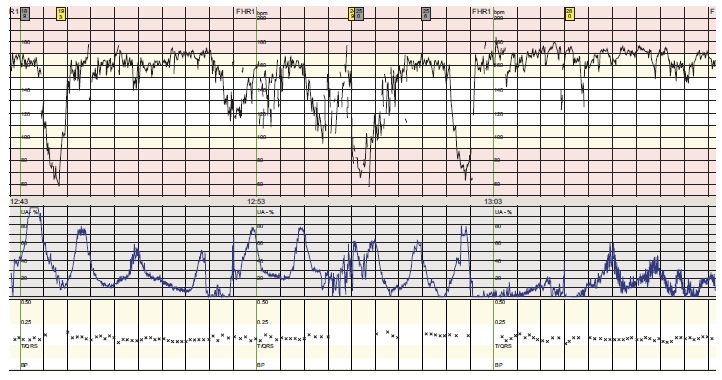
CTG from our case showing fetal tachycardia and complicated variable decelerations.
The patient had 3g intravenous benzylpenicillin, due to prolonged prelabour rupture of membranes (PROM). Thin, green amniotic fluid flowed from the vagina twenty-nine hours after artificial rupture of membranes. Because of high maternal temperature, fetal tachycardia combined with decelerations on the CTG (suggesting fetal distress), green amniotic fluid and poor progress of labour, an acute caesarean section was performed with finding of bad smelling, thick, green amniotic fluid. The newborn cried spontaneously with Apgar score 10/1, 10/5.
At surgery, a swab of the amniotic fluid was submitted for microbiological analysis.
After the caesarean section, the patient was treated with intravenous cefuroxime and metronidazole for three days and peroral amoxicillin for another five days. The patient recovered rapidly. The newborn showed no signs of infection and sepsis. Both mother and newborn were discharged wellbeing.
Microbiology
The Stuart charcoal swab with amniotic fluid taken at caesarian section was plated on 5% Danish horse blood agar for incubation in ambient air supplemented with 5% CO2 at 35°C. Furthermore on blue agar (optimized for growth of gram-negative rods, especially Enterobacteriaceae), tellurite agar, and on β-glucuronidase test agar (for demonstration of β-glucuronidase activity) for incubation in ambient air at 35°C, plus on anaerobic agar for anaerobic incubation at 37°C (Concept 400 chamber from Ruskinn Technology Ltd; gas mixture: 80% nitrogen, 10% hydrogen, 10% CO2/pure nitrogen).
All plates including Mueller Hinton plates were from SSI Diagnostica, Statens Serum Institut, Hillerød, Denmark. A. christensenii grew massively together with two other bacterial colonies, which could not be cultured further in preparation for identification. A. christensenii was identified by MALDI-TOF MS (Compass 1.4, Version 3.4, Build 3.4.76.0 by Bruker Daltonics) with a score value of 2.09, four identical hits and a distance to next taxon match of > 0.7. No anaerobic growth was noticed. In order to confirm the finding, partial 16S rRNA gene analysis was performed using the Select NA™ assay by Molzym (http://www. molzym.com/). By application of the Gram-positive primer set, a mixed chromatogram was found. By applicating the Gram-negative primer set, S. sanguinegens (sequence identity: 387 of 392 base pair) was identified in addition to a mixed chromatogram.
The mixed chromatograms were examined using the Isentio RipSeq website (http:// www.isentio.com/) confirming the presence of A. christensenii, but additionally detecting G. asaccharolytica and also revealing the presence of S. sanguinegens, P. micra and S. moniliformis.
A. christensenii and G. asaacharolytica are facultative anaerobic species, S. Sanguinegens/L. amnionii and P. micra are anaerobic gram negative rods and gram positive cocci, respectively. S. moniliformis is a fastidious facultatively anaerob gram negative rod.
By Oxoid disc susceptibility testing on Mueller Hinton 5% blood agar with NAD and incubated in ambient air supplemented with 5% CO2 at 35°C, the A. christensenii strain was found sensitive to penicillin.
DISCUSSION
Prompt action was taken in this severe chorioamnionitis episode, which surely influenced the positive outcome regarding both mother and newborn. Despite benzylpenicillin treatment, the patient developed high fever and illness twenty-four hours after artificial rupture of membranes and CTG indicated fetal distress. In addition, green and foul-smelling amniotic fluid was observed together with badly smelling pus at caesarean section, which is characteristic for infections involving anaerobic bacteria [8]. The patient recovered quickly after intravenously administering of cefuroxime and metronidazole.
Nulliparity, obesity, genetic factors and the time interval from premature rupture of membranes (PROM) to birth are factors that influence the risk of an ascending bacterial infection [9]. An intracervical balloon can theoretically increase the risk of infection, although a recent study showed no significantly higher risk of chorioamnionitis after placement of an intracervical balloon when adjusted for nulliparity [9]. The number of sterile vaginal explorations in term labour has not been found to be an independent risk factor for intrapartum fever [10].
Chorioamnionitis is often polymicrobiol. A variety of bacteria, including miscellaneous anaerobes, Streptococcus agalactiae (Group B haemolytic streptococci), Gardnerella vaginalis, Mycoplasma and Ureaplasma, Escherichia coli, S. sanguinegens and Leptotrichia species may be identified by culture and/or culture-independent methods, such as 16S rRNA gene analysis [11, 12].
A. christensenii has formerly only rarely been described. It was primarily isolated from the human vagina [4] and has recently been found in subacute bacterial endocarditis [13]. To our knowledge, this is the first case to describe A. christensenii in chorioamnionitis. Hence, our finding corresponds well with the previous observations by Collins and colleagues [4].
As described by DiGiulio, we also found S. sanguinegens and Leptotrichia species only by PCR and not by culture [11]. These pathogens have previously been described in infections in pregnancy and postpartum women [5, 14]. They appear more commonly in amnionitic fluid after the introduction of molecular methods [11].
The molecular analysis also revealed P. micra (formerly Peptostreptococcus micros). This anaerobic coccus is part of the normal human oral and gastrointestinal flora, primarily causing infections in the oral cavity [15]. It has been speculated that the microbe is part of the vaginal flora and that it could play a role in obstetric infections paralleling its role in the development of periodontitis [15]. Our finding supports this hypothesis.
Furthermore, the species G. asaccharolytica was identified by the analysis. To our knowledge this species has been described only once before by Ulger-Toprak et al. [6] in three patients with an arm wound, an infection following a cut finger and from a labial abscess, respectively.
Finally, we identified S. moniliformis from the amniotic fluid by molecular analysis. This bacterium is naturally occurring in the nasopharynx of wild and laboratory rats and is acquired through the bite or scratch of a rodent or by ingestion of contaminated water or food. It is the etiologic agent of rat-bite fever or in the absence of a rat, Haverhill fever. The disease is characterized by irregular fever with flu-like symptoms and a rash [16]. Amnionitis and abscesses associated with the female genital tract have been described [16, 17]. In contrast to the patient described by Faro et al., our patient had high fever and was unwell. There was no known history of a rat bite.
In our case, it was only possible to do susceptibility testing on the A. christensenii strain as the other present species were identified by non-culture methodology. However, the published literature supports a relevant antibiotic coverage by the antibiotics given to our case consistent with the quick recovery of mother and child [5, 18-20].
In conclusion, we present a case of chorioamnionitis consisting of five different microorganisms, where only A. christensenii could be identified by culture. Because of molecular diagnostic approaches, the polymicrobial nature of this infection was revealed. Albeit rare to very rare, all the bacteria identified have earlier been associated with infection or habitation in the female genital tract and this case underlines and stresses the possible role of these pathogens in female genital tract infections.
ACKNOWLEDGEMENTS
None declared.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Hastings-Tolsma M., Bernard R., Brody M.G., Hensley J., Koschoreck K., Patterson E. Chorioamnionitis: prevention and management. MCN Am. J. Matern. Child Nurs. 2013;38(4):206–212. doi: 10.1097/NMC.0b013e3182836bb7. [DOI] [PubMed] [Google Scholar]
- 2.Riggs J.W., Blanco J.D. Pathophysiology, diagnosis, and management of intraamniotic infection. Semin. Perinatol. 1998;22(4):251–259. doi: 10.1016/S0146-0005(98)80013-X. [DOI] [PubMed] [Google Scholar]
- 3.Lamont R.F., Sobel J.D., Akins R.A., Hassan S.S., Chaiworapongsa T., Kusanovic J.P., Romero R. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG. 2011;118(5):533–549. doi: 10.1111/j.1471-0528.2010.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins M.D., Jovita M.R., Hutson R.A., Ohlén M., Falsen E. Aerococcus christensenii sp. nov., from the human vagina. Int. J. Syst. Bacteriol. 1999;49(Pt 3):1125–1128. doi: 10.1099/00207713-49-3-1125. [DOI] [PubMed] [Google Scholar]
- 5.De Martino S.J., Mahoudeau I., Brettes J.P., Piemont Y., Monteil H., Jaulhac B. Peripartum bacteremias due to Leptotrichia amnionii and Sneathia sanguinegens, rare causes of fever during and after delivery. J. Clin. Microbiol. 2004;42(12):5940–5943. doi: 10.1128/JCM.42.12.5940-5943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulger-Toprak N., Summanen P.H., Liu C., Rowlinson M.C., Finegold S.M. Gemella asaccharolytica sp. nov., isolated from human clinical specimens. Int. J. Syst. Evol. Microbiol. 2010;60(Pt 5):1023–1026. doi: 10.1099/ijs.0.001966-0. [DOI] [PubMed] [Google Scholar]
- 7.Jensen K.H., Dargis R., Christensen J.J., Kemp M. Ribosomal PCR and DNA sequencing for detection and identification of bacteria: experience from 6 years of routine analyses of patient samples. APMIS. 2014;122(3):248–255. doi: 10.1111/apm.12139. [DOI] [PubMed] [Google Scholar]
- 8.Morita M., Wang H.L. Association between oral malodor and adult periodontitis: a review. J. Clin. Periodontol. 2001;28(9):813–819. doi: 10.1034/j.1600-051x.2001.028009813.x. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera I.B., Quinones J.N., Durie D.E., et al. Intracervical balloon placement and the risk of chorioamnionitis in term rupture of membranes. Obstet. Gynecol. 2014;123(Suppl. 1):43S. doi: 10.1097/01.AOG.0000447324.05518.6e. [DOI] [Google Scholar]
- 10.Cahill A.G., Duffy C.R., Odibo A.O., Roehl K.A., Zhao Q., Macones G.A. Number of cervical examinations and risk of intrapartum maternal fever. Obstet. Gynecol. 2012;119(6):1096–1101. doi: 10.1097/AOG.0b013e318256ce3f. [DOI] [PubMed] [Google Scholar]
- 11.DiGiulio D.B. Diversity of microbes in amniotic fluid. Semin. Fetal Neonatal Med. 2012;17(1):2–11. doi: 10.1016/j.siny.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Wang X., Buhimschi C.S., Temoin S., Bhandari V., Han Y.W., Buhimschi I.A. Comparative microbial analysis of paired amniotic fluid and cord blood from pregnancies complicated by preterm birth and early-onset neonatal sepsis. PLoS One. 2013;8(2):e56131. doi: 10.1371/journal.pone.0056131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jose A., Cunha B.A., Klein N.C., Schoch P.E. Aerococcus christensenii native aortic valve subacute bacterial endocarditis (SBE) presenting as culture negative endocarditis (CNE) mimicking marantic endocarditis. Heart Lung. 2014;43(2):161–163. doi: 10.1016/j.hrtlng.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Boennelycke M., Christensen J.J., Arpi M., Krause S. Leptotrichia amnionii found in septic abortion in Denmark. Scand. J. Infect. Dis. 2007;39(4):382–383. doi: 10.1080/00365540601053022. [DOI] [PubMed] [Google Scholar]
- 15.Murdoch D.A. Gram-positive anaerobic cocci. Clin. Microbiol. Rev. 1998;11(1):81–120. doi: 10.1128/cmr.11.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pins M.R., Holden J.M., Yang J.M., Madoff S., Ferraro M.J. Isolation of presumptive Streptobacillus moniliformis from abscesses associated with the female genital tract. Clin. Infect. Dis. 1996;22(3):471–476. doi: 10.1093/clinids/22.3.471. [DOI] [PubMed] [Google Scholar]
- 17.Faro S., Walker C., Pierson R.L. Amnionitis with intact amniotic membranes involving Streptobacillus moniliformis. Obstet. Gynecol. 1980;55(3) Suppl.:9S–11. doi: 10.1097/00006250-198003001-00003. [DOI] [PubMed] [Google Scholar]
- 18.Christensen J.J., Korner B., Casals J.B., Pringler N. Aerococcus-like organisms: use of antibiograms for diagnostic and taxonomic purposes. J. Antimicrob. Chemother. 1996;38(2):253–258. doi: 10.1093/jac/38.2.253. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen M. Aerococci and aerococcal infections. J. Infect. 2013;66(6):467–474. doi: 10.1016/j.jinf.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Uemura H., Hayakawa K., Shimada K., et al. Parvimonas micra as a causative organism of spondylodiscitis: a report of two cases and a literature review. Vol. 23. Int J Infect Dis IJID Off Publ Int Soc Infect Dis; 2014. pp. 53–55. [DOI] [PubMed] [Google Scholar]


