Abstract
Background:
Ophiopogonis Radix is a famous traditional Chinese medicine. It is necessary to establish a suitable quality control methods of Ophiopogonis Radix.
Objective:
To investigate the quality control methods of Ophiopogonis Radix by high-performance liquid chromatography (HPLC) coupled with evaporative light scattering detector (ELSD).
Materials and Methods:
A rapid and simple method, HPLC coupled with ELSD, was applied to determinate ruscogenin in 35 batches of Ophiopogenis Radix samples. Orthogonal tests and single factor explorations were used to optimize the extraction condition of ruscogenin. The content of ruscogenin in different origin was further analyzed by hierarchical clustering analysis (HCA).
Results:
The ruscogenin was successfully determined by HPLC-ELSD with a two-phase solvent system composed of methanol-water (88:12) at a flow rate 1.0 ml/min, column temperature maintained at 25°C, detector draft tube temperature at 42.2°C, nebulizer gas flow rate at 1.4 L/min, and the gain at 8. The result showed the good linearity of ruscogenin in the range of 40.20–804.00 μg/ml (R2 = 0.9996). Average of recovery was 101.3% (relative standard deviation = 1.59%). A significant difference of ruscogenin content was shown among 35 batches of Ophiopogenis Radix from different origin, varied from 0.0035% to 0.0240%. HCA based on the content of ruscogenin indicated that Ophiopogonis Radix in different origin was mainly divided into two clusters.
Conclusion:
This simple, rapid, low-cost, and reliable HPLC-ELSD method could be suitable for measurement of ruscogenin content rations and quality control of Ophiopogonis Radix.
SUMMARY
Ophiopogonis Radix is an important Traditional Chinese Medicine (TCM) to treat and prevent cardiovascular diseases and acute or chronic inflammation for thousands of years. Steroidal saponins were known as the dominant active components for their significant cardiovascular activity, and the most steroid sapogenin of them is ruscogenin. Therefore, ruscogenin was chosen as the marker component for evaluating the quality of Ophiopongonis Radix. This study focused on establishing a stable, low-cost, simple and practical method of HPLC-ELSD to determine the ruscogenin content, and 35 batches of samples of Ophiopogonis Radix were determined. Meanwhile, these results were analyzed by hierarchical clustering analysis and the methodology validation was based on USP34-NF-29 <1225>. Results showed that this analysis method was simple and stable, which would provide an important reference to establish the quality control methodology for other herb preparations and formulas containing Ophiopogonis Radix.
Keywords: Hierarchical clustering analysis, high-performance liquid chromatography-evaporative light scattering detector, Ophiopogonis Radix, ruscogenin
INTRODUCTION
Ophiopogonis Radix, a popular traditional Chinese medicine (TCM), was used to treat and prevent cardiovascular diseases and acute or chronic inflammation for thousands of years,[1,2] consisting of the dried tuberous root of Ophiopogon japonicus (L.f.) Ker-Gawl (known as Maidong).[3] O. japonicus is an evergreen perennial widely distributed in South China, especially in Zhejiang Province (Zhe Maidong in China) and Sichuan Province (Chuan Maidong in China).[2,4]
On the basis of previous phytochemical studies, steroidal saponins, homoisoflavonoids, and saccharides have been reported as the major components in O. japonicas.[5,6,7,8,9] In these components, steroidal saponins were known as the dominant active components for their significant cardiovascular activity,[1] and the most steroid sapogenin of them is ruscogenin [Figure 1].[10] Ruscogenin was used to treat acute and chronic flammatory diseases[11,12] and cardiovascular diseases such as arrhythmia, angina, and thrombosis.[13,14,15] To ensure their clinical efficacy, quality control of O. japonicus is of great importance. Given this, ruscogenin was chosen as the marker component for evaluating the quality of Ophiopogonis Radix.
Figure 1.
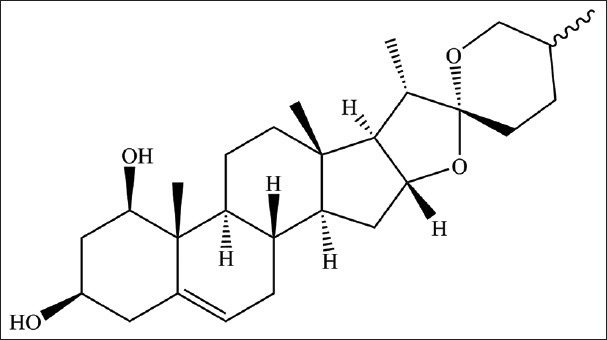
The structure of ruscogenin
Ruscogenin along with its glycoside saponins are difficult to be detected by UV detector due to the lack of UV chromophore. ELSD is a universal and nonspecific mass detector based on the detection of solute molecules by light scattering after nebulization and evaporation of the mobile phase.[16,17] Therefore, high-performance liquid chromatography-evaporative light scattering detector (HPLC-ELSD), a practical and low-cost method, serves as a complementary tool for the detection of steroid sapogenin.
In the past years, it is a challenge for the quality control of Ophiopogonis Radix because of the lower content of individual steroidal saponin and homoisiflavonoid. Although they may be detected by mass spectrometry (MS),[6] there are no enough equipments in every laboratory because of immense expense. ELSD could overcome this problem. Several studies have been conducted on the determination of Ophiopogonin D or Ophiopogonin D’ by ELSD,[18,19] total saponins by UV-VIS spectrophotometer,[8,20,21] homoisoflavonoids by HPLC,[22] diosgenin, and ruscogenin by nonaqueous capillary electrophoresis,[23] and fingerprint analysis have been used in some investigations.[17,24] However, until now few studies[25] focus on determination of ruscogenin in Ophiopogonis Radix by HPLC-ELSD.
In the present study, the total ruscogenin after hydrolysis was determined by HPLC-ELSD, suitable extraction conditions of ruscogenin were systematically optimized, the content in different origin was analyzed and the quality of Ophiopogonis Radix was evaluated combining hierarchical clustering analysis (HCA).
MATERIALS AND METHODS
Plant materials
Thirty-five batches of Ophiopogenis Radix were collected from different provinces in China, including Jiangsu, Anhui, Guizhou, Yunnan, Zhejiang, Guangxi, Sichuan, Chongqing, Jiangxi, and Hunan. All samples were identified by Prof. Bo-Yang Yu, the voucher specimen (Number: 20111008) was deposited in the Herbarium of Department of Traditional Chinese Medicine of China Pharmaceutical University. Standard of ruscogenin was prepared in our laboratory and identified with purity no <98%.
Chemicals
HPLC-grade methanol was purchased from Yuwang Group (Shandong, China); ultrapure water was prepared by Milli-Qsystem (Milford, MA, USA); other chemicals and solvents were analytical grade. Sulfuric acid was bought from Nanjing Chemical Reagent Co., Ltd. (Jiangsu, China); sodium hydroxide was bought from Xilong Reagent Co., Ltd. (Guangdong, China); diethyl ether was bought from Nanjing Chemical Reagent Co., Ltd. (Jiangsu, China).
Instruments
Agilent 1260 HPLC System (Agilent Technologies, Palo Alto, CA, USA) comprises a quaternary solvent delivery system, an on-line degasser, an auto-sampler, a column temperature controller, and an analytical workstation (Agilent LC B.04.03 ChemStation) coupled with detector Alltech 3300 ELSD (Alltech, USA).
Chromatographic conditions
The analytical column was a Merck RP-C18 (4.6 mm × 250 mm, 5 μm) with C18 guard column. The mobile system consisted of methanol-water (88:12) at a flow rate of 1.0 ml/min. Column temperature was maintained at 25°C. The draft tube temperature was at 42.2°C, the nebulizer gas flow rate was at 1.4 L/min, the gain was at 8. The solvent was filtered through a 0.22 μm filter and degassed. The sample injection volume was 10 μl. The total acquisition time was 25 min.
Reference standard solution preparation
Standard stock solutions with known concentrations were obtained by dissolving accurately weighted ruscogenin in methanol.
Optimization of extraction condition
As, the extraction of ruscogenin was affected by many factors, so orthogonal designs were taken to optimize the extraction condition, including optimization of acid hydrolysis conditions and extraction of ruscogenin.
Optimization of acid hydrolysis conditions
According to related literatures and previous preliminary experiments,[25] orthogonal design 1 was applied to select the acid hydrolysis conditions of ruscogenin in Ophiopogonis Radix. 2.0 g Ophiopogonis Radix was transferred to a suitable flask, and 25 ml sulfuric acid with different concentration was respectively added to hydrolysis for different time in water bath with different temperature according to factors [Table 1]. Then the extracting solution was adjusted to neutral with 16% NaOH and filtered. The residue was collected and dried at 60°C and then soxhlet extracted with 70 ml petroleum ether for 6 h. Petroleum ether layer was collected and evaporated to dryness. Then the residue was transferred to a 2 ml volumetric flask and diluted to 2 ml with methanol. The obtained solution was passed through a membrane filter (0.45 μm), and the first part of the filtrate was discarded, then the successive filtrate was collected as the sample solution. Ten microliters sample solution and standard solution were injected into the chromatography, in which the chromatograms were recorded, and the content of rescogenin was calculated in Ophiopogonis Radix coupled with visual analysis and variance analysis. The results of the orthogonal test and extreme difference analysis were presented in Table 2. All the analysis of variance were performed by the statistical software SPSS version 19.0 (SPSS Inc., Chicago, Illinois, USA) and the result was listed in Table 3.
Table 1.
Orthogonal factors and levels (acid hydrolysis)
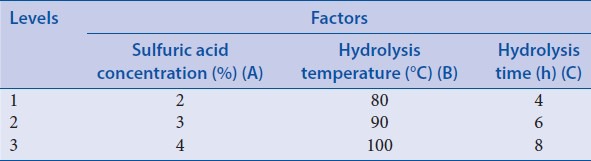
Table 2.
L9 (34) results of orthogonal design 1
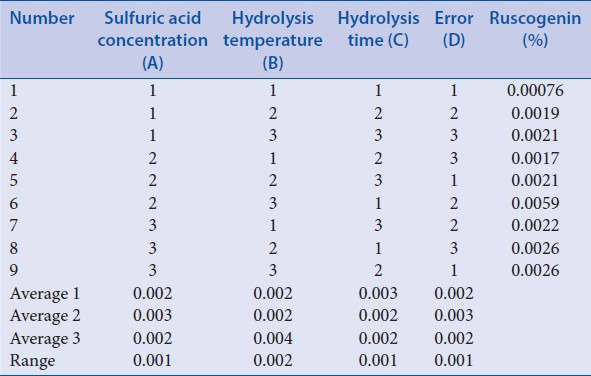
Table 3.
Analysis of variance
Optimization of extraction of ruscogenin
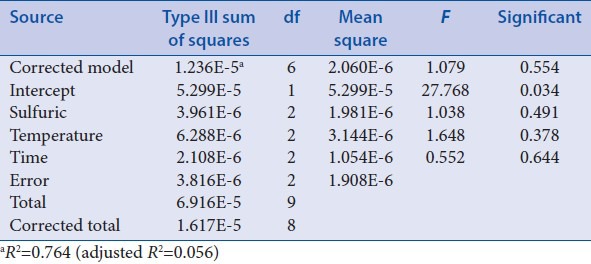
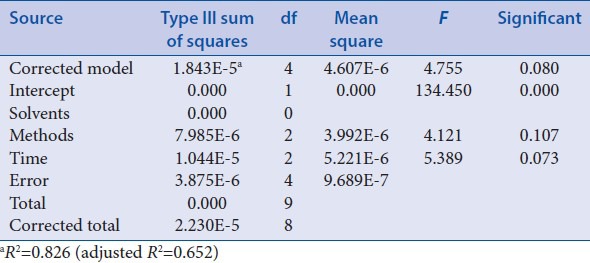
Orthogonal design 2 was applied to select the best extraction condition of ruscogenin. During this procedure, the steps before being extracted with petroleum ether were similar to “Optimization of acid hydrolysis conditions,” and just the acid hydrolysis was performed using certain factors with 25 ml 3% H2SO4 and being refluxed in boiling water bath for 6 h. After being adjusted to neutral with 16% NaOH and filtered, residue was collected and dried at 60°C and then extracted with 70 ml different solvents, different methods, and different time as factors [Table 4]. The rest steps were same as the corresponding process of “Optimization of acid hydrolysis conditions.” The results of orthogonal test 2 and extreme difference analysis were presented in Table 5. The analysis of variance [Table 6] was performed by statistical software SPSS version 19.0 (SPSS Inc.).
Table 4.
Factors and levels
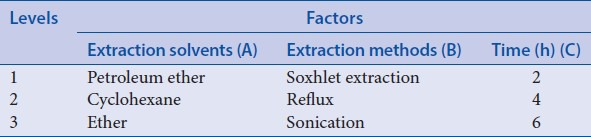
Table 5.
L9 (34) results of orthogonal design 2
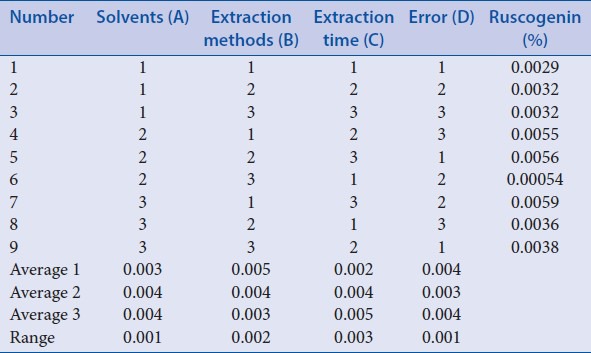
Table 6.
Analysis of variance
Single factor experiment
To select better extraction method, the different hydrolysis time was investigated based on orthogonal design 1, compared in these experiments (4 h, 6 h) as shown in Table 7, and the different extraction solvents (cyclohexane, ether) were analyzed on the basis of orthogonal experiment 2 [Table 8].
Table 7.
Results of different hydrolysis time (n=3)

Table 8.
Results of different extraction solvents (n=3)

Sample solutions preparation
Sample solutions preparation were based on above optimized conditions as following: About 2.0 g Ophiopogons Radix, powdered to pass through a 24 mesh sieve and accurately weighed, was transferred to a 100 ml glass-stoppered conical flask. Twenty-five milliliters 3% H2SO4 was accurately added to reflux in boiling water bath for 6 h. Extracting solution was adjusted to neutral with 16% NaOH and then filtered. The collected residue, dried at 60°C, was soxhlet extracted with 70 ml ether for 6 h. Ether layer was collected, and evaporated to dryness and the residual were transferred to a 2 ml volumetric flask and diluted to 2 ml with methanol, which passed through a membrane filter (0.45 μm porosity), discarding the first part of the filtrate and collecting the successive filtrate as the sample solution. Ten microliters sample solution and standard solution were injected into the chromatography as “Chromatographic conditions,” in which the chromatograms were recorded, and the content of ruscogenin of Ophiopogonis Radix was calculated. Thirty-five batches of Ophiopogonis Radix samples were extracted with this same method.
Statistical analysis
Statistics was analyzed with SPSS version 19.0 statistical software (SPSS, USA).
RESULTS
Optimization of extraction condition
The results [Table 2] showed that hydrolysis temperature has a notable influence on the acid hydrolysis of saponins from O. japonicus. Besides, visual analysis [Table 2] stated that the influence to the mean extraction yields of the ruscogenin decreases in the order: B > A ≥ C according to the R values, and the best combination of test factors was A2B3C1. However, based on analysis of variance [Table 3], A, B, and C have no significant difference. Therefore, to ensure the repeatability and stability of the method, further experiments as “Investigation of hydrolysis time” were applied to optimize the hydrolysis time.
Optimization of extraction of ruscogenin
Extraction time have a notable influence on the extraction of ruscogenin [Table 5]. Besides, visual analysis [Table 5] stated that the influence to the mean extraction yields of the ruscogenin decreases in the order: C > B > A according to the R values, and the best combination of test factors was A2B1C3 or A3B1C3. However, based on analysis of variance [Table 6], A, B and C have no significant difference. Therefore, to select the best extraction method and ensure the stability, further experiments were performed to compare A2B1C3 with A3B1C3 as “Investigation of extraction solvents.”
Single factor experiment
Investigation of hydrolysis time
The results [Table 7] showed that 6 h was appropriate for hydrolysis because its relative standard deviation (RSD) was not more than 2.0%. So, the optimal conditions were determined: 25 ml, 3% sulfuric acid concentration, refluxed in the boiling water bath for 6 h.
Investigation of extraction solvents
Solution ether was appropriate [Table 8] because of its RSD < 2.0% as well as relatively high content of ruscogenin, so ether was chosen for further study.
Determination of ruscogenin in Ophiopogonis Radix
Method validation of ruscogenin: Precision, linearity, accuracy, specificity, stability, detection limit, quantitation limit, and ruggedness.
The method was validated through investigation on the precision, linearity, accuracy, specificity, stability, and ruggedness.
Precision (repeatability and intermediate precision)
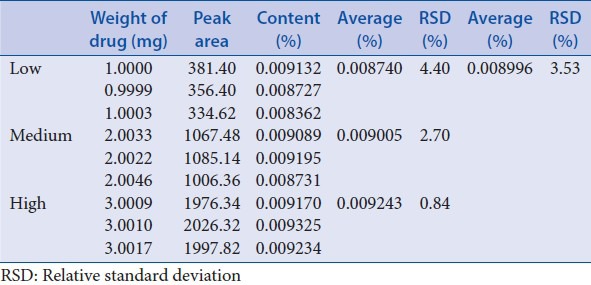
Repeatability was assessed with 9 sample solutions of low, medium, and high levels (1.0, 2.0 and 3.0 g respectively), each with three triplicates. The content of ruscogenin was calculated with the external reference method [Table 9]. The RSDs of inner-day variation of three levels were in the range from 0.84% to 4.40% for the content of ruscogenin, and average RSD was 3.53%.
Table 9.
Repeatability
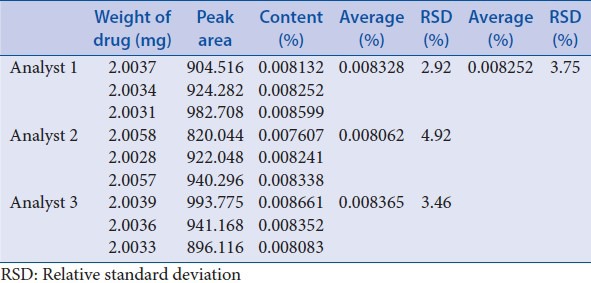
Intermediate precision was performed through investigation of 3 different days, 3 different analysts and 3 different columns, each with 3 triplicates [Tables 10–12]. These results showed good precision.
Table 10.
Intermediate precision-different days
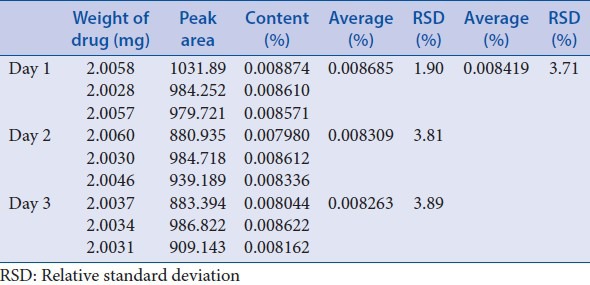
Table 12.
Intermediate precision-different column
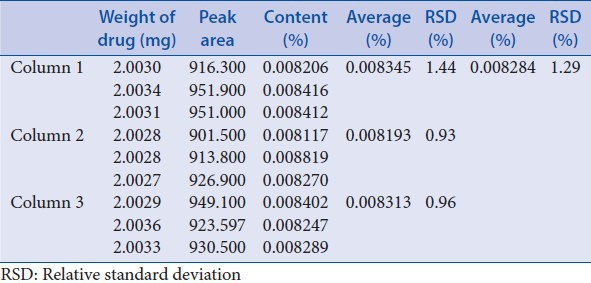
Table 11.
Intermediate precision-different analysts
Linearity and range
Good linearity was shown with correlation 0.9996 [Table 13].
Table 13.
Linearity and range

Accuracy
Accuracy was calculated as the percentage of recovery by the assay of the known added amount of ruscogenin in the sample.
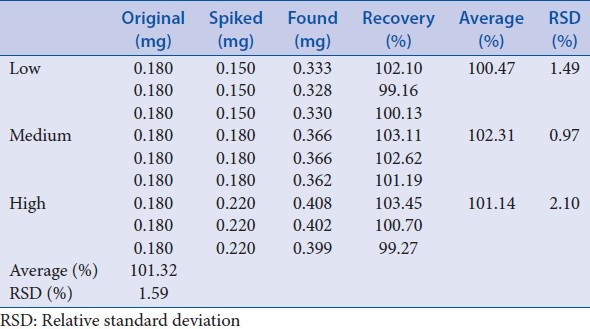
Three different concentrations (low, medium, and high) of reference standard were spiked to 2.0 g of Ophiopogonis Radix, all in triplicates. Average recovery was 101.32% [Table 14].
Table 14.
Recovery test
Specificity
The integration peak in the chromatogram of the sample solution was corresponding in time to the peak in the chromatogram of standard solution. No such peak of that retention time appeared in the chromatogram of blank solvent [Figure 2].
Figure 2.
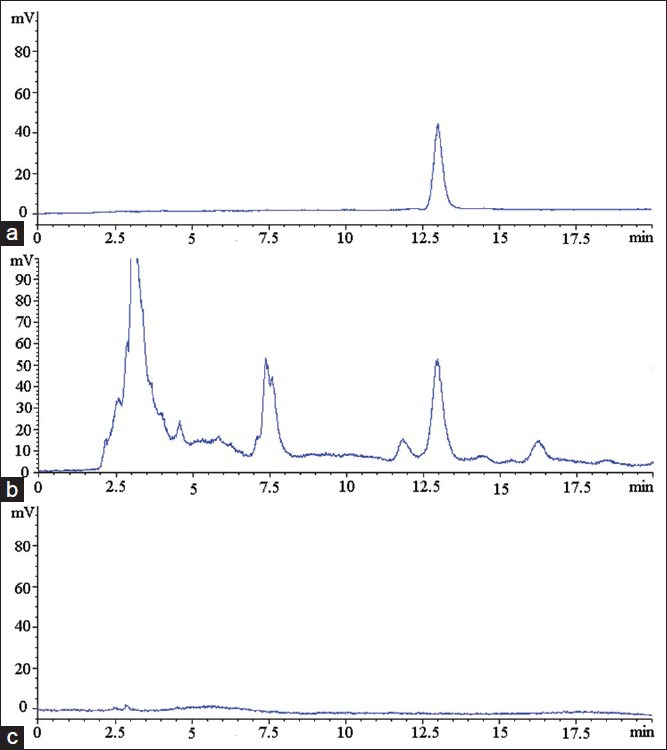
Specificity, (a) Standard solution; (b) Sample solution; (c) Blank solution
Stability
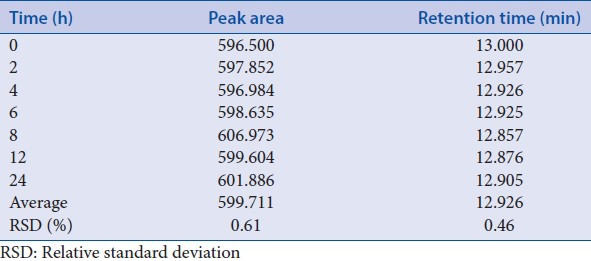
The stability of sample solution was investigated by comparing the peak areas and retention time of ruscogenin in the chromatograph of the same sample solution, after stored at room temperature for different time (0, 2, 4, 6, 8, 12, 24 h). Stability was evaluated by calculating the RSD of peak area and retention time. The RSD were 0.61% and 0.46%, respectively [Table 15]. The analyses were found to be stable within 24 h in the sample solution.
Table 15.
Stability
Detection limit
According to USP34 NF 29 chapter <1225>, detection limit was 0.0804 μg/ml and when the concentration was 0.0804 μg/ml, signal to noise ratios were about 3:1.
Quantitation limit
According to USP34 NF 29 chapter <1225>, when the concentration was 0.1608 μg/ml, signal to noise ratios were about 10:1. Quantitation limit was 0.1608 μg/ml.
Ruggedness
The ruggedness of the established method was evaluated by examining its stability with small variations of procedural parameters, including pH of mobile phase, ratio of components in mobile phase, ELSD detector, different columns, flow rate, injection volume, and column temperature.
Results of ruggedness indicated that the drift tube temperature of ELSD should be not lower than 42.2°C; the pH of mobile phase, ratio of components in mobile phase, flow rate, injection volume, and column temperature can be slightly adjusted for system suitability.
Content of ruscogenin in 35 batches of Ophiopogonis Radix
Sample solutions of 35 batches of Ophiopogonis Radix were determined using the validated chromatographic method for ruscogenin. Its content varied from 0.0035% to 0.0240% [Table 16, Figures 3 and 4].
Table 16.
Content of 35 batches of opiopogons radix (n=2)
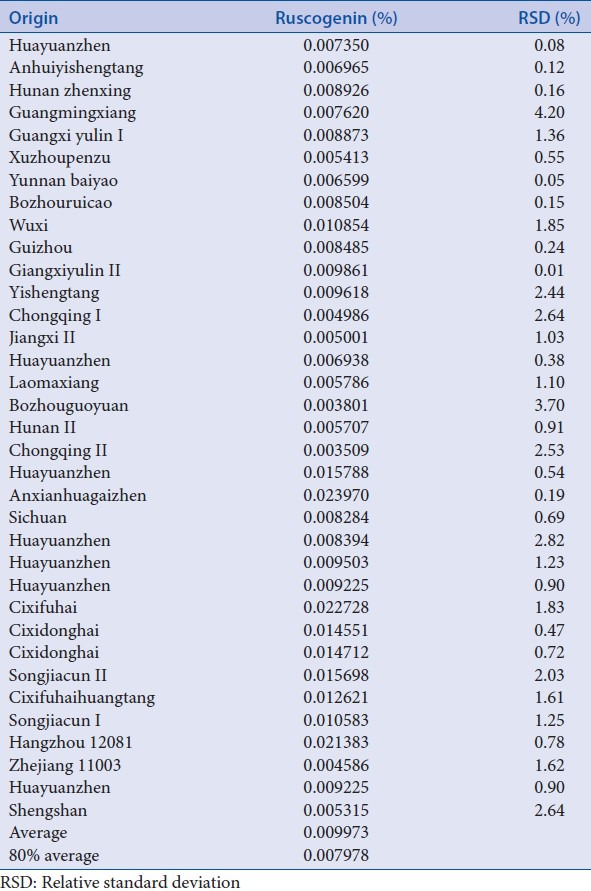
Figure 3.
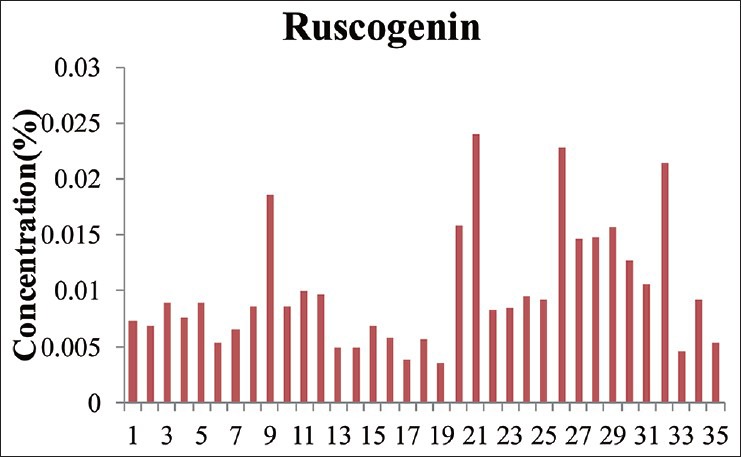
The content of ruscogenin in 35 batches of samples
Figure 4.
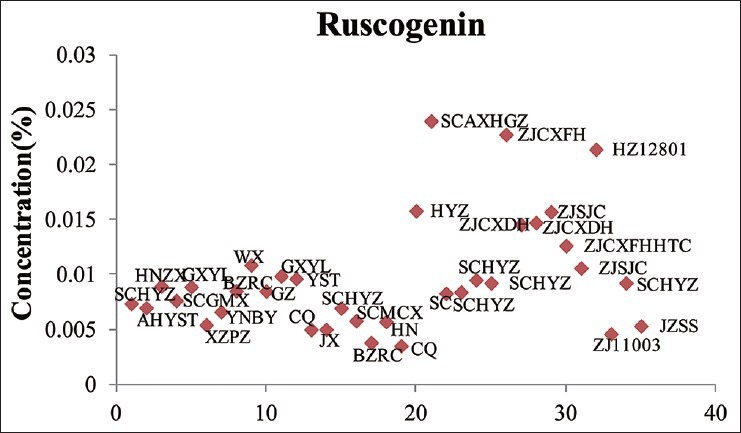
Content of ruscogenin in Ophiopogonis Radix in different origin
Hierarchical clustering analysis
In order to assess the variation of Ophiopogonis Radix in different origin, HCA was performed based on the content of rescogenin from HPLC-ELSD profiles. Between groups linkage method was performed, and Squared Euclidean distance was selected as a measurement. In HCA dendrogram, the shorter distance between two samples indicated their higher similarity and the samples clustered into the same group were the most similar ones.[26] The results [Figure 5] demonstrated that the 35 batches of samples were obviously divided into two main clusters according to their contents. Sample 1–19 were belonging to cluster-I, which were mainly commercial Ophiopogonis Radix (Chuan Maidong) although collected from different province, whereas sample 20–35 were belonging to cluster-II, which were mainly collected from Zhejiang Province and partly from cultivation base in Sichuan Province.
Figure 5.
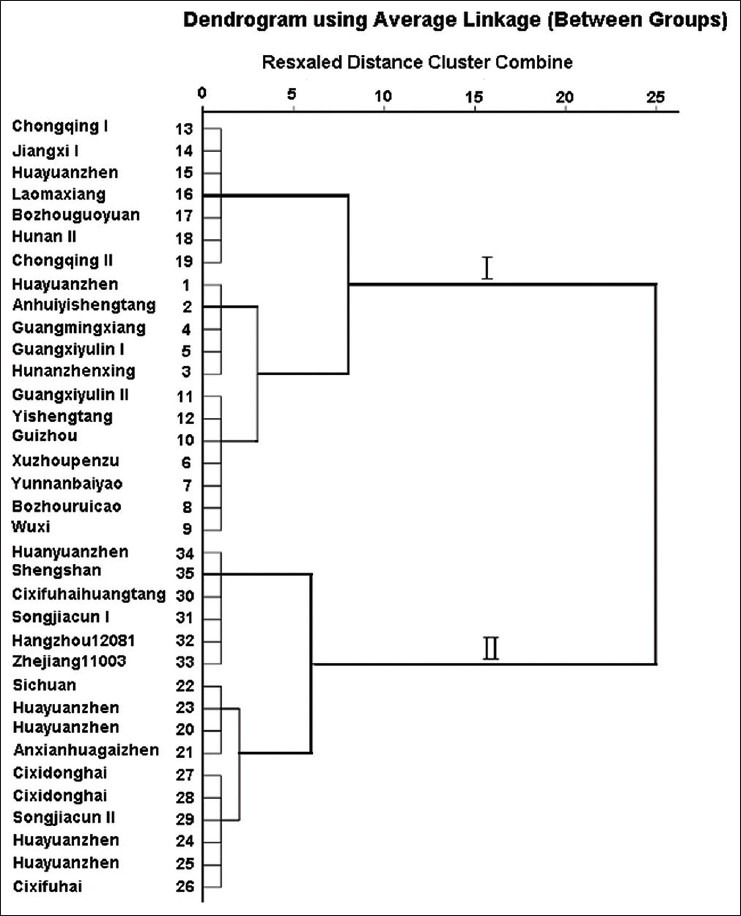
Dendrograms of hierarchical cluster analysis for the 35 tested samples of Ophiopogonis Radix
DISCUSSION
Optimization of high-performance liquid chromatography-evaporative light scattering detector condition
HPLC, a simple, stable, and durable analysis techniques, is widely applied to assess the types and content of chemical compositions in TCMs. HPLC can be equipped with multiple detectors, such as a UV detector, ELSD, fluorescence detector and MS, of which the UV detector is most frequently used.[27] Given that ruscogenin lack of UV chromophore, HPLC coupled with ELSD was applied to our research. In order to achieve rapid and stable analysis of ruscogenin in Ophiopogonis Radix, ELSD different nebulizer-gas flow rate (1.3, 1.4, 1.5, 1.6 L/min), and drift tube temperature (39.2°C, 40.6°C, 42.2°C, and 45°C) were compared according to the value of signal-to-noise ratio. These results indicated that the value of signal-to-noise ratio was better when the nebulizer-gas flow rate was at 1.4 L/min and drift tube temperature was at 42.2°C. Therefore, they were chosen for the analysis.
To obtain the chromatograms with the good separation, different chromatographic condition, including mobile phase system (methanol- and acetonitrile-water), pH of mobile phase (water, 0.02% aqueous solution of formic acid, 0.10% aqueous solution of formic acid, and 0.3% aqueous solution of triethylamine), methanol ratio (85%, 88%, and 90%), flow rate (1.0, 1.1, and 1.2 ml/min), column temperature (15°C, 25°C, and 30°C), and injection volume (5, 10, 15 and 20 μl) were compared and analyzed. As a result, based on system suitability parameters (tailing factor, resolution, and number of theoretical plates), methanol-water (88–12), flow rate 1.0 ml/min, column temperature 25°C, and injection volume 10 μl were finally chosen.
Optimization of sample preparation
In order to obtain better extraction condition, variables involved in the procedure such as hydrolysis and extraction method (extraction firstly, then hydrolysis) and different acid (hydrochloric acid, sulfuric acid) were optimized in preliminary experiment. About 2.0 g Ophiopogonis Radix, powdered to pass through a 24 mesh sieve, accurately weighed, was soxhlet extracted with 70 ml methanol for 4 h once, then solution was collected, evaporated to dryness, and residue was hydrolyzed with 25 ml 3% H2SO4 in water bath for 4 h, and 16% NaOH was added to adjust neutral. Then the hydrolysate was extracted with 50 ml petroleum ether for 3 times, and petroleum ether layer was collected and evaporated to dryness. The rest steps were similar with corresponding programs in “Sample solutions preparation.” This result suggested that more impurity was included in a sample solution with this method, compared with the procedure of hydrolsis firstly, then extraction as “Optimization of extraction condition” of the methods section. Moreover, the preliminary experiment also investigated the hydrolysis of different acid (2.22% hydrochloric acid, 3% sulfuric acid), the other steps was same as corresponding “Optimization of extraction condition” of methods section. The result indicated that content of rescogenin with 3% sulfuric acid was more stable (the RSD was <2%, while more than 10% with hydrochloric acid, n = 3). Thus, sulfuric acid was chosen for further experiments. Based on these preliminary experiments, orthogonal tests, and single factor explorations were designed in a present experiment to select the best extraction condition.
Validation of methodology
According to USP34-NF-29 chapter <1225>, precision (repeatability and intermediate precision), linearity, accuracy, specificity, stability, detection limit, quantitation limit, and ruggedness were investigated. Average RSD of repeatability was 3.53%; average RSD of intermediate precision-different days, different analysts and different column were 3.71%, 3.75%, and 1.29%, respectively. The calibration curves exhibited good linearity (R2 = 0.9996) in the range of 40.20–804.00 g/ml. Average of recovery was 101.3% (RSD = 1.59%, n = 9). Stability RSD of the area and retention time was 0.61% and 0.46%. The detection limit was 0.0804 μg/ml and quantitation limit was 0.1608 μg/ml. Results of ruggedness indicated that the drift tube temperature of ELSD detector (Alltech ELSD 3300) should be not lower than 42.2°C. The pH of mobile phase, the ratio of components in the mobile phase, flow rate, injection volume, and column temperature can be slightly adjusted for system suitability. These indicated that the analysis method was simple, feasible, and stable.
Hierarchical clustering analysis
Based on the content of ruscogenin in Ophiopogonis Radix, HCA was performed to analyze results of 35 batches of samples (Ophiopogonis Radix). These results illustrated that samples could be appropriately divided into two main clusters related to their contents and the difference of content in samples was significant in a different origin. It is well known that types and quantities of chemical components in medicinal plants are probably considerably affected on collection at different times and from different localities.[26,28] This reason is related to different environments, soil texture condition, growing time, harvesting time, and cultivated techniques. Therefore, in order to control the quality of Ophiopogonis Radix, it is necessary to establish a stable and simple analysis method. In the present study, a practical and the low-cost technique could evaluate the quality through systematically optimization of extraction condition, chromatographic condition, and method validation.
CONCLUSION
In the present work, an impersonal, valid, low-cost and the stable analysis method was developed and applied to the quality control of the Ophiopogonis Radix through systematically optimization of extraction condition, chromatographic condition and method validation. Sample solutions of 35 batches of Ophiopogonis Radix were determined using the validated chromatographic method for ruscogenin. The content of ruscogenin varied from 0.0035% to 0.0240%. Our results also showed that HPLC-ELSD enjoyed the practical advantages of simple analysis procedure and reduced quantity of cost consumption for the quality control of Ophiopogonis Radix. Therefore, the method developed in this study would provide an important reference to establish the quality control method for other herb preparations and formulas containing Ophiopogonis Radix.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Bo-yang Yu
Bo-yang Yu, Jiangsu Key Laboratory of TCM Evaluation and Translational Research, Department of Complex Prescription of TCM, China Pharmaceutical University, Nanjing 211198; State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing 211198, PR China.

Jin Qi
Jin Qi, Jiangsu Key Laboratory of TCM Evaluation and Translational Research, Department of Complex Prescription of TCM, China Pharmaceutical University, Nanjing 211198, PR China.
Acknowledgements
The authors would like to thank the United States Pharmacopeia Convention and National Natural Science Foundation of China (No. 81274004 and No. 81473317) for technical and financial support, respectively.
REFERENCES
- 1.Zhou YF, Qi J, Zhu DN, Yu BY. Homoisoflavonoids from Ophiopogon japonicus and its oxygen free radicals (OFRs) scavenging effects. Chin J Nat Med. 2008;6:201–4. [Google Scholar]
- 2.Li N, Zhang JY, Zeng KW, Zhang L, Che YY, Tu PF. Anti-inflammatory homoisoflavonoids from the tuberous roots of Ophiopogon japonicus. Fitoterapia. 2012;83:1042–5. doi: 10.1016/j.fitote.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Shanghai: Shanghai Science and Technology Publishers; 1999. China SAoTCMotPsRo. Herbal of China; p. 122. [Google Scholar]
- 4.Qi J, Xu D, Zhou YF, Qin MJ, Yu BY. New features on the fragmentation patterns of homoisoflavonoids in Ophiopogon japonicus by high-performance liquid chromatography/diode-array detection/electrospray ionization with multi-stage tandem mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:2193–206. doi: 10.1002/rcm.4608. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Zhang L, Zeng KW, Zhou Y, Zhang JY, Che YY, et al. Cytotoxic steroidal saponins from Ophiopogon japonicus. Steroids. 2013;78:1–7. doi: 10.1016/j.steroids.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Xie T, Liang Y, Hao H, A J, Xie L, Gong P, et al. Rapid identification of ophiopogonins and ophiopogonones in Ophiopogon japonicus extract with a practical technique of mass defect filtering based on high resolution mass spectrometry. J Chromatogr A. 2012;1227:234–44. doi: 10.1016/j.chroma.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, Kang LP, Yu HS, Liu YX, Zhao Y, Xiong CQ, et al. Steroidal saponins from the tuber of Ophiopogon japonicus. Steroids. 2012;77:1298–305. doi: 10.1016/j.steroids.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Zhou YH, Wu XR, Xu DS, Feng Y, Li JJ. Purification of total saponins of Ophiopogons Radix by macroporous resin. Chin Tradit Herb Drugs. 2003;34:1089–91. [Google Scholar]
- 9.Wagner H, Bauer R, Melchart D, Xiao PG, Staudinger A. Radix Ophiopogonis – Maidong. In: Wagner H, Bauer R, Melchart D, Xiao PG, Staudinger A, editors. Chromatographic Fingerprint Analysis of Herbal Medicines. Springer Vienna: Springer Wien NewYork; 2011. pp. 819–30. [Google Scholar]
- 10.Liu N, Wen X, Liu J, Liang M, Zeng H, Lin Y, et al. Determination of ruscogenin in crude Chinese medicines and biological samples by immunoassay. Anal Bioanal Chem. 2006;386:1727–33. doi: 10.1007/s00216-006-0767-9. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Chen T, Yu B, Xu Q. Ruscogenin glycoside (Lm-3) isolated from Liriope muscari inhibits lymphocyte adhesion to extracellular matrix. J Pharm Pharmacol. 2002;54:959–65. doi: 10.1211/002235702760089081. [DOI] [PubMed] [Google Scholar]
- 12.Wu F, Cao J, Jiang J, Yu B, Xu Q. Ruscogenin glycoside (Lm-3) isolated from Liriope muscari improves liver injury by dysfunctioning liver-infiltrating lymphocytes. J Pharm Pharmacol. 2001;53:681–8. doi: 10.1211/0022357011775802. [DOI] [PubMed] [Google Scholar]
- 13.Bi LQ, Zhu R, Kong H, Wu SL, Li N, Zuo XR, et al. Ruscogenin attenuates monocrotaline-induced pulmonary hypertension in rats. Int Immunopharmacol. 2013;16:7–16. doi: 10.1016/j.intimp.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Xiao PG. Vol. 1. Beijing: Chinese Industry Press; 2002. Modern Chinese Materia; pp. 71–81. [Google Scholar]
- 15.Sun Q, Chen L, Gao M, Jiang W, Shao F, Li J, et al. Ruscogenin inhibits lipopolysaccharide-induced acute lung injury in mice: Involvement of tissue factor, inducible NO synthase and nuclear factor (NF)-kappaB. Int Immunopharmacol. 2012;12:88–93. doi: 10.1016/j.intimp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Li FJ, Zhang HY, Li Y, Yu YJ, Chen YL, Xie MF, et al. Simultaneous identification and quantification of dextran 20 and sucrose in lyophilized thrombin powder by size exclusion chromatography with ELSD. Chromatographia. 2012;75:187–91. [Google Scholar]
- 17.Li N, Che YY, Zhang L, Zhang JY, Zhou Y, Jiang Y, et al. Fingerprint analysis of Ophiopogonis Radix by HPLC-UV-ELSD coupled with chemometrics methods. J Chin Pharm Sci. 2013;22:55–63. [Google Scholar]
- 18.Jia C, Ye ZL, Jiang XJ, Zhou DZ, Li DK. Quantitative determination of Ophiopogonin D and Ophiopogonin D’ from Ophiopongon japonicus by ELSD-HPLC. Res Pract Chin Med. 2012;26:79–81. [Google Scholar]
- 19.Yu JP, Ma YG, Shao JF, Zhu M. Quantitative determination of Ophiopongon D in Zhe Ophiopongon japonicus (Thunb.) Ker-Gawl. and Chuan Ophiopongon japonicus (Thunb.) Ker-Gawl. by HPLC-ELSD. Tradit Chin Drug Res Clin Pharmacol. 2002;13:253–5. [Google Scholar]
- 20.2010th ed. Vol. 1. Beijing: People's Medical Publishing House; 2010. P.R.China TSPCo. Pharmacopoeia of the People's Republic of China; p. 144. [Google Scholar]
- 21.Tang XQ, Cao YX, Yu BY. Study on the quality control of Ophiopogons Radix. China J Chin Mater Med. 1999;24:390–3. [Google Scholar]
- 22.Zeng P, Zhou H, Zheng Y, Xu X, Fu S. Simultaneous determination of three homoisoflavonoids in Ophiopogon japonicus by HPLC. Zhongguo Zhong Yao Za Zhi. 2012;37:71–4. [PubMed] [Google Scholar]
- 23.Huang BM, Yao CW, Bian QQ, Wang ZG, Mo JY. Determination of diosgenin and ruscogenin in Radix Ophiopogonis by nonaqueous capillary electrophoresis. Yao Xue Xue Bao. 2011;46:443–6. [PubMed] [Google Scholar]
- 24.Liu L, Lu Y, Shao Q, Cheng YY, Qu HB. Binary chromatographic fingerprinting for quality evaluation of Radix Ophiopogonis by high-performance liquid chromatography coupled with ultraviolet and evaporative light-scattering detectors. J Sep Sci. 2007;30:2628–37. doi: 10.1002/jssc.200700214. [DOI] [PubMed] [Google Scholar]
- 25.Tang XQ, Yu BY, Xu DR, Tang Y, Xu LS. Determination of sapogenins in Radix Ophiopogonis by HPLC-ELSD. J China Pharm Univ. 2001;32:270–2. [Google Scholar]
- 26.Liang X, Ma M, Su W. Fingerprint analysis of Hibiscus mutabilis L. leaves based on ultra performance liquid chromatography with photodiode array detector combined with similarity analysis and hierarchical clustering analysis methods. Pharmacogn Mag. 2013;9:238–43. doi: 10.4103/0973-1296.113277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang XM, Jin Y, Wang YP, Jin GW, Fu Q, Xiao YS. Qualitative and quantitative analysis in quality control of traditional Chinese medicines. J Chromatogr A. 2009;1216:2033–44. doi: 10.1016/j.chroma.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 28.Kong WJ, Zhao YL, Xiao XH, Jin C, Li ZL. Quantitative and chemical fingerprint analysis for quality control of rhizoma Coptidischinensis based on UPLC-PAD combined with chemometrics methods. Phytomedicine. 2009;16:950–9. doi: 10.1016/j.phymed.2009.03.016. [DOI] [PubMed] [Google Scholar]


