Abstract
Background:
Ginseng is Chinese traditional herbal medicine, and the ginsenoside Rg3 is the main bioactive ingredient for the anti-tumor effect. However, there is no study on pharmacokinetics (PKs) of ginsenoside Rg3 and its main metabolite after oral ginsenoside Rg3 in tumor-bearing plasma. The aim of this study was to investigate the PK profiles of ginsenoside Rg3 and ginsenoside Rh2 after oral administration of pure ginsenoside Rg3 were administered, and compare the difference of the PK profiles between normal and Walker 256 tumor-bearing rats.
Materials and Methods:
The concentrations of two ginsenosides in plasma were determined by using a simple and rapid high-performance liquid chromatography. All the rats were divided randomly into two groups (Walker 256 tumor-bearing and normal groups). Each group received oral administration of 50 mg/kg ginsenoside Rg3.
Results:
The results showed that ginsenoside Rh2, possibly as a glycosylation hydrolysis product of ginsenoside Rg3, were found in plasma after oral administration of ginsenoside Rg3 to rats. Ginsenoside Rg3 had shown better absorption than ginsenoside Rh2, whether the oral administration of ginsenoside Rg3, normal rats showed better absorption than tumor-bearing rats.
Discussion and Conclusion:
The PKs properties of the ginsenoside Rg3 and ginsenoside Rh2 differed between tumor-bearing rats and normal rats, including area under the plasma level/time curve and concentration maximum (P < 0.05).
SUMMARY
Ginsenoside Rh2 was found in plasma after oral administration of ginsenoside Rg3 to rats
HPLC could be used to determine simultaneously, the concentration of ginsenoside Rg3 and ginsenoside Rh2 in rat plasma after oral administration of ginsenoside Rg3
Normal rats showed better absorption than tumor-bearing rats after oral administration of ginsenoside Rg3.0.
Keywords: Ginsenoside Rg3, ginsenoside Rh2, high-performance liquid chromatography, pharmacokinetic
INTRODUCTION
Ginseng is Chinese traditional herbal medicine; ginsenosides are the main bioactive ingredients. At present, there are many monomer components had been separated.[1] The ginsenoside Rg3 is the saponin from Ginseng, its structure belongs to protopanaxadiol type saponin in monomer, the molecular formula is C42H72O13, and its relative molecular mass is 784.[2] In various isolated saponins, the anti-tumor effect of Rg3 was the most significant and had been widely used in clinical treatment.[3,4] The ginsenoside Rg3 is the main active ingredient in Shenyi capsule, the first anti-tumor Chinese medicine, in China. Up to now, there have been many reports about ginsenoside Rg3 in vitro and in vivo pharmacokinetic (PK) studies.[5,6,7] It was reported that ginsenoside Rg3 may be prodrug,[8,9,10] and it can be hydrolysated ginsenoside Rh2 and ginsenoside Rh2 played an important role in anti-cancer action.[11,12,13] However, there is no page about detecting ginsenoside Rh2 in vivo after oral ginsenosides Rg3, and also no study on PKs of ginsenoside Rg3 in tumor-bearing plasma. Therefore, the purpose of this study was to develop a sensitive, simple, and accurate high-performance liquid chromatography (HPLC) method to simultaneously determine the concentration of ginsenoside Rg3 and ginsenoside Rh2 in normal and tumor-bearing rat plasma and to investigate, and compare the PK parameters of ginsenoside Rg3 and ginsenoside Rh2 after oral administration of ginsenoside Rg3.
MATERIALS AND METHODS
Materials and chemicals
Pure ginsenoside Rg3 was obtained from Prof. Fu Li (Dalian Fusheng Natural Drug Development Co., Ltd). The purities of ginsenoside Rg3 was determined to be up to 98% by HPLC. Ginsenoside Rh2 (>98%) and the internal standard (IS) ginsenoside Rb1 (>98%) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The solvents (HPLC grade) used for chromatographic analysis were purchased from Fisher Company Inc., USA. Deionized water was prepared in a Mill-Q academic water purification system (Millipore, Bedford, MA, USA). All the other reagents were of analytical grade and provided by Kermel Chemical Co., (Tianjin, China).
Apparatus and chromatographic conditions
The concentrations of two ginsenosides in plasma were assayed using reverse-phase HPLC (Agilent 1200 series) equipped with a variable wavelength ultraviolet (UV) detector and pump (Agilent model G1314A VWD). The separation was accomplished on a Welch Ultimate AQ-C18 column (150 mm × 4.6 mm, 5 μm particle size). The mobile phase was composed of acetonitrile (A): Water (B) (0 → 5 min, 35:65; 5 → 10 min, 60:40; 10 → 20 min, 60:40; v/v) at a flow rate of 1.0 mL/min with gradient elution. The column temperature was 30°C. The detector was set at 203 nm. The injection volume was 20 μL. The chromatographic run time for each analysis was 35.0 min.
Animals
Male Wistar rats, weighing 200–250 g, were obtained from Liao Ning Chang Sheng Biotechnology Co., Ltd., (Benxi, China). Animal welfare and experimental procedures were strictly in accordance with the Guide for the Care and Use of Laboratory Animals (US National Research Council, 1996) and the related ethics regulations of Liaoning University of Traditional Chinese Medicine. Rats were housed in an air-conditioned animal quarter at a temperature of 22°C ± 2°C and a relative humidity of 50% ± 2%. All animals received food and water ad libitum. The animals were acclimatized to the facilities for 5 days and then fasted with free access to water for 24 h prior to each experiment.
Animal model
Walker 256 carcinosarcoma cells were purchased from Beijing AnBona Science and Technology Co., Ltd., (Beijing, China). Tumor cells for the establishment of the experimental animal model were obtained from ascitic fluid in Wistar rats, after two cycles of 7 days cell passage by intraperitoneal injection of 107 Walker 256 carcinoma cells. When cell harvesting dilute the tumor cells suspension to 107 cells/mL with sterile saline.
After 3 days of acclimatization in metabolic cages, rats were randomly divided into two groups: Model group (n = 6, labeled M01−M06) and control group (n = 6, labeled C01−C06). The rats in model group were established by subcutaneous injection of a 200 μL suspension of Walker 256 carcinosarcoma cells (1 × 107 cells/mL) into the right armpit while the control group was injected with an equal volume of sterile saline.
Preparation of stocks, calibration samples, and quality control samples
The stock solutions were prepared by dissolving 2.73 mg of ginsenoside Rg3, 5.52 mg of ginsenoside Rh2, and 5.27 mg IS in 10 mL methanol, respectively. A series of mixture standard working solutions with concentrations of 5.46, 6.83, 13.7, 27.3, 54.6, 81.9, 109 μg/mL for ginsenoside Rg3 and 0.552, 1.10, 2.21, 4.17, 5.70, 8.28, 11.4 μg/mL for ginsenoside Rh2, were obtained by diluting the mixture of the stock standard solutions with methanol. The IS working solution was prepared by diluting the IS stock solution with methanol. All solutions were stored at 4°C.
Sample preparation
Plasma samples (200 μL) were spiked with 50 μL IS, and the mixtures were extracted with 1000 μL acetonitrile by vortex mixing for 3 min. After centrifugation at 4000 × g for 5 min, the solution was transferred to a polypropylene tube and dried under nitrogen gas at room temperature. The plasma residue was reconstituted in 50 μL of methanol, respectively. The injection volume was 20 μL for analysis.
Method validation
The validation has been performed according to the Food and Drug Administration guidelines.
Linearity and quantification
The method was fully validated for its specificity, linearity, lower limits of detection (LLOD), lower limits of quantification (LLOQ), accuracy, and precision. The LLOD was determined during the evaluation of linear range of the calibration curve and is defined as the lowest concentration level resulting in a signal-to-noise ratio of 3:1. The LLOQ was determined as the lowest concentration of the analyte in rat plasma and tissue that could be quantified with an inter-assay relative standard deviation (%RSD) lower than 20% and with accuracy rates between 80% and 120%.
Accuracy and precision
The precision and accuracy of the method was evaluated by analyzing quality control (QC) samples with different concentrations. The intra-day variability was determined by assaying five replicates on the same day, and the inter-day variability was determined by assaying five replicates on three consecutive days. Precision was defined as the coefficient of variation expressed as a percentage. The accuracy of these samples was determined by comparing the calculated concentration obtained from the calibration curve with the known concentration.
Extraction recovery
Extraction recoveries from rat plasma were determined at three concentrations by comparing the peak areas extracted from rat plasma with those of the same quantities added to methanol.
Stability
Stability of ginsenoside Rg3 and ginsenoside Rh2 in rat plasma was assessed with QC samples (n = 3) stored at −20°C for 30 days. Freeze-thaw stability of ginsenoside Rg3 and ginsenoside Rh2 in rat plasma was investigated with QC samples (n = 3) subjected to three freeze/thaw cycles.
Pharmacokinetic study
The normal Wistar rats (n = 6) and Walker 256 tumor-bearing rats (n = 6) were assigned to receive a ginsenoside Rg3 solution by oral administration at the dose of 50 mg/kg of ginsenoside Rg3, respectively. Serial blood samples (0.4 mL) were obtained via the rats’ orbital vein at 1, 1.5, 2, 3, 4, 6, 8, and 12 h after administration and were collected into heparinized centrifuge tubes. The blood samples were immediately centrifuged at 667 × g for 10 min at room temperature. The plasma samples were analyzed by the previously described methods.
Statistical analysis
The content of ginsenoside Rg3 and ginsenoside Rh2 in plasma at different times were evaluated by means of linear regression analysis. All data were calculated using Microsoft Excel 2003 (Microsoft). The relevant PK parameters were calculated using the computer program DAS 2.0 (Chinese Society of Mathematical Pharmacology, Beijing, China) from the Chinese Pharmaceutical Association.
RESULTS AND DISCUSSION
High-performance liquid chromatography assay
The selectivity of the method was evaluated by analyzing blank plasma samples prior to administration. The chromatograms of the plasma and organs are shown in Figure 1. Ginsenoside Rb1 (IS), ginsenoside Rg3, and ginsenoside Rh2 were well separated at 7.064, 13.250, and 18.822 min, respectively with no endogenous interference.
Figure 1.
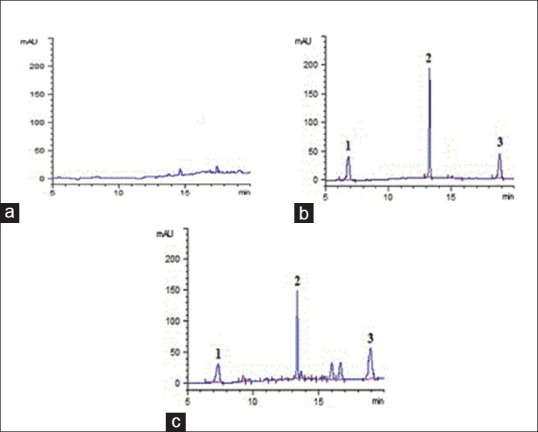
Chromatograms of rat plasma samples: (a) Blank plasma; (b) blank plasma spiked with internal standard, ginsenoside Rg3 and ginsenoside Rh2; (c) plasma sample obtained 2 h after oral administration of ginsenoside Rg3 at a dose of 50 mg/kg to rat; (internal standard, tR = 7.064 min; ginsenoside Rg3, tR = 13.250 min;;ginsenoside Rh2, tR = 18.822 min)
The linear calibration curves were obtained in the given concentration range of ginsenoside Rg3 or ginsenoside Rh2 in plasma samples, respectively. The standard curves were fitted to a first-degree polynomial, Y = aX + b, where Y was the peak area of ginsenoside Rg3/IS or ginsenoside Rh2/IS, a and b were constants, and X was the concentration (μg/mL) of ginsenoside Rg3 or ginsenoside Rh2. Calibration curves were found to be linear over the calibration range of 5.46–109 μg/mL for ginsenoside Rg3 and 0.552–11.4 μg/mL for ginsenoside Rh2 in rat plasma. All curves have correlation coefficients of >0.99. The LLOQ of ginsenoside Rg3 and ginsenoside Rh2 were 5.46 and 0.552 μg/mL for plasma with the RSD <20%.
The RSD for the intra-day (repeatability) and inter-day precision ranged from 1.13% to 10.7%, for QC standards. The percentage of extraction recoveries of ginsenoside Rg3 and ginsenoside Rh2 for plasma were between 75.6% and 91.1%, respectively. These data indicated that the sample preparation method were satisfied and resulted in no appreciable matrix effect for ginsenoside Rg3, ginsenoside Rh2 and IS.
The stability tests were designed by taking into account the anticipated conditions that real samples may experience. The RSD of the stability studies were 5.75–12.8%, respectively.
Pharmacokinetics of ginsenoside Rg3 and ginsenoside Rh2 after oral administration of ginsenoside Rg3 to rats
The method presented here was successfully used to quantify the ginsenoside Rg3 and ginsenoside Rh2 in rat plasma after oral administration of ginsenoside Rg3. The concentration-time profiles of the ginsenoside Rg3 and ginsenoside Rh2 are shown in Figure 2. According to the F-test and the Akaike's information criterion, a two-compartment PK model fitted best the plasma data of the ginsenoside Rg3 and ginsenoside Rh2. The calculated PK parameters are listed in Table 1.
Figure 2.
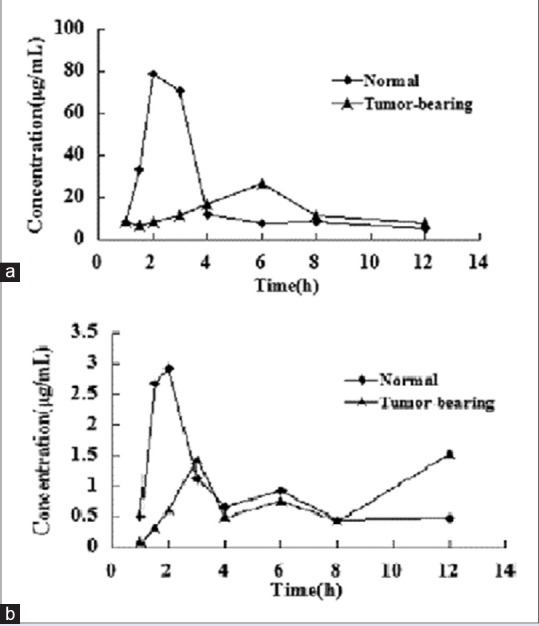
The mean plasma concentration-time curves of ginsenoside Rg3 and ginsenoside Rh2 after oral administration of ginsenoside Rg3 to normal rat and tumor-bearing at different doses. (a) ginsenoside Rg3 and (b) ginsenoside Rh2
Table 1.
Pharmacokinetic parameters for ginsenoside Rg3 and gingsenoside Rh2 in normal rats and tumor-bearing rats (mean±SD, n=6) after a single oral administration of ginsenoside Rg3
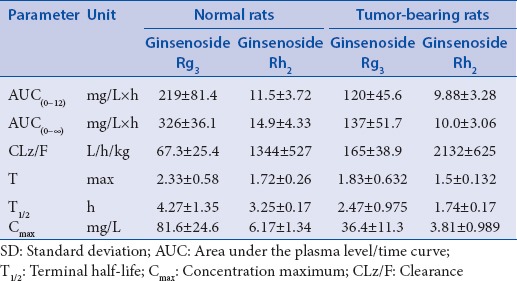
The noncompartmental model was applied to the PK evaluation of ginsenoside Rh2 against the original compound ginsenoside Rg3. Ginsenoside Rg3 exhibited a rapid and poor absorption phase followed by a sharp but lasting disappearance of ginsenoside Rh2. The concentration peak values of ginsenoside Rg3 were much higher than ginsenoside Rh2 indicating that ginsenoside Rg3 should also be a major compound in vivo. The data suggested that ginsenoside Rg3 were a major compound for pharmacological effects because there were significant differences in the area under the plasma level/time curve (AUC[0-t]) between ginsenoside Rg3 and ginsenoside Rh2.
After oral administration of Rg3, the AUC values, terminal half-life, and concentration maximum of ginsenosides Rg3 and ginsenoside Rh2 in normal rats were higher than those in the tumor-bearing rats. The result showed that the absorption of Rg3 in tumor-bearing was lower than in normal rats, but the clearance is higher than normal mice. It may be that the tumor changes rats’ body environment, which affect the absorption and metabolism of drug. Therefore, the dosage needs to be adjusted appropriately, according to the practical applications and achieve the desired therapeutic effect.
CONCLUSION
Our study is the first evaluation of the plasma PKs of ginsenoside Rg3, as well as its metabolite, ginsenoside Rh2. The ginsenoside Rg3 and ginsenoside Rh2 have been quantified by HPLC-UV. The validated method was simple, fast, reproducible, and suitable for the research of ginsenoside Rg3 and ginsenoside Rh2 in rat plasma with ginsenoside Rb1 as the IS. The assay utilized an acetonitrile extraction method and a reversed-phase separation with sufficient selectivity and sensitivity. The evaluation of the PKs of ginsenoside Rg3 and ginsenoside Rh2 will help further the understanding of their pharmacological activity and clinical use. We need to take caution when extrapolating PK and exposure data from healthy animals to diseased animals in designing pharmacological studies.
Financial support and sponsorship
We thank the elitist funding of innovation in Science and Technology of Liaoning Provincial Department of Education.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank the elitist funding of innovation in Science and Technology of Liaoning Provincial Department of Education.
REFERENCES
- 1.Dou D, Chen Y, Ren J, Pei YM, Chen YJ. Oeotillone-type ginsenoside from leaves of Panax ginseng. J Chin Pharm Sci. 2002;11:119–21. [Google Scholar]
- 2.Iza M, Wall MM, Heimberg RG, Rodebaugh TL, Schneier FR, Liu SM, et al. Latent structure of social fears and social anxiety disorders. Psychol Med. 2014;44:361–70. doi: 10.1017/S0033291713000408. [DOI] [PubMed] [Google Scholar]
- 3.Cheong JH, Kim H, Hong MJ, Yang MH, Kim JW, Yoo H, et al. Stereoisomer-specific anticancer activities of ginsenoside Rg3 and Rh2 in HepG2 cells: Disparity in cytotoxicity and autophagy-inducing effects due to 20(S)-epimers. Biol Pharm Bull. 2015;38:102–8. doi: 10.1248/bpb.b14-00603. [DOI] [PubMed] [Google Scholar]
- 4.Kim JW, Jung SY, Kwon YH, Lee JH, Lee YM, Lee BY, et al. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol Ther. 2012;13:504–15. doi: 10.4161/cbt.19599. [DOI] [PubMed] [Google Scholar]
- 5.Bae SH, Park JB, Zheng YF, Jang MJ, Kim SO, Kim JY, et al. Pharmacokinetics and tissue distribution of ginsenoside Rh2 and Rg3 epimers after oral administration of BST204, a purified ginseng dry extract, in rats. Xenobiotica. 2014;44:1099–107. doi: 10.3109/00498254.2014.929192. [DOI] [PubMed] [Google Scholar]
- 6.Cai ZW, Qian TX, Wong R. Liquid chromatography-electrospray ionization mass spectrometry for metabolism and pharmacokinetic studies of ginsenoside Rg3. Anal Chim Acta Incl Cumulative Indexes. 2003;492:283–94. [Google Scholar]
- 7.Wang ZZ, Yao JH, Su CY. Studies on pharmacokinetics of 20(R)-ginsenoside Rg3 in rats. Asian J Drug Metab Pharmacokinet. 2003;3:327–30. [Google Scholar]
- 8.Xie HT, Wang GJ, Sun JG, Tucker I, Zhao XC, Xie YY, et al. High performance liquid chromatographic-mass spectrometric determination of ginsenoside Rg3 and its metabolites in rat plasma using solid-phase extraction for pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;818:167–73. doi: 10.1016/j.jchromb.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Gu Y, Wang GJ, Sun JG, Jia YW, Xie HT, Wang W. Quantitative determination of ginsenoside Rh2 in rat biosamples by liquid chromatography electrospray ionization mass spectrometry. Anal Bioanal Chem. 2006;386:2043–53. doi: 10.1007/s00216-006-0857-8. [DOI] [PubMed] [Google Scholar]
- 10.Gu Y, Wang GJ, Sun JG. Development of a development of a sensitive LC-ESI-MS assay for 20(R)-ginsenoside Rh2 and its pharmacokinetic application in dogs: A case for the influence of micronization on traditional Chinese medicine. Int J Mass Spectrom. 2006;52:11–9. [Google Scholar]
- 11.Kikuchi Y, Sasa H, Kita T, Hirata J, Tode T, Nagata I. Inhibition of human ovarian cancer cell proliferation in vitro by ginsenoside Rh2 and adjuvant effects to cisplatin in vivo. Anticancer Drugs. 1991;2:63–7. doi: 10.1097/00001813-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Tang XP, Tang GD, Fang CY, Liang ZH, Zhang LY. Effects of ginsenoside Rh2 on growth and migration of pancreatic cancer cells. World J Gastroenterol. 2013;19:1582–92. doi: 10.3748/wjg.v19.i10.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi S, Oh JY, Kim SJ. Ginsenoside Rh2 induces Bcl-2 family proteins-mediated apoptosis in vitro and in xenografts in vivo models. J Cell Biochem. 2011;112:330–40. doi: 10.1002/jcb.22932. [DOI] [PubMed] [Google Scholar]


