Abstract
Background:
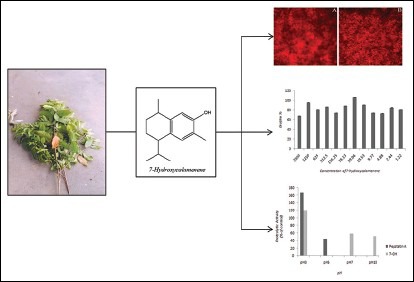
The 7-hydroxycalamenenene-rich essential oil (EO) obtained from the leaves of Croton cajucara (red morphotype) have been described as active against bacteria, protozoa, and fungi species. In this work, we aimed to evaluate the effectiveness of 7-hydroxycalamenenene against Candida albicans and nonalbicans species.
Materials and Methods:
C. cajucara EO was obtained by hydrodistillation and its major compound, 7-hydroxycalamenene, was purified using preparative column chromatography. The anti-candidal activity was investigated by minimum inhibitory concentration (MIC) and secreted aspartic proteases (SAP) and biofilm inhibition assays.
Results:
7-hydroxycalamenene (98% purity) displayed anti-candidal activity against all Candida species tested. Higher activity was observed against Candida dubliniensis, Candida parapsilosis and Candida albicans, showing MIC values ranging from 39.06 μg/ml to 78.12 μg/ml. The purified 7-hydroxycalamenene was able to inhibit 58% of C. albicans ATCC 36801 SAP activity at MIC concentration (pH 7.0). However, 7-hydroxycalamenene demonstrated poor inhibitory activity on C. albicans ATCC 10231 biofilm formation even at the highest concentration tested (2500 μg/ml).
Conclusion:
The bioactive potential of 7-hydroxycalamenene against planktonic Candida spp. further supports its use for the development of antimicrobials with anti-candidal activity.
SUMMARY
Croton cajucara Benth. essential oil provides high amounts of 7-hydroxycalamenene
7-Hydroxycalameneneisolated from C. cajucarais active against Candida spp
7-Hydroxycalameneneinhibits C. albicans aspartic protease activity
7-Hydroxycalamenene was not active against C. albicans biofilm formation.
Figure.
Keywords: 7-hydroxycalamenene, anti-candidal activity, Croton cajucara, essential oil, secreted aspartic proteases
INTRODUCTION
Candida species are currently the fourth-leading cause of hospital- acquired bloodstream infections, reaching a mortality rate of up to 35–40% for systemic or disseminated infections. Systemic mycoses can occur in patients with severely impaired immune systems, people with organ or bone marrow transplants, cancer patients undergoing chemotherapy or in intensive care unit patients, as well as both neonates and the elderly.[1] Moreover, the pattern of candidal species causing infection impacts in the management of this disease. For example, Candida glabrata tends to have higher minimum inhibitory concentrations (MIC) values to the currently used antifungals, particularly the azoles.[2]
Secreted aspartic proteases (SAP) have been described as a major virulence factor in Candida spp. infections. SAP could contribute to host tissue adhesion and invasion by degrading or distorting host cell surface structures and intercellular substances, or by destroying cells and molecules of the host immune system to avoid or resist antimicrobial attack.[3] Several researches have been proposing that SAP could be an interesting target for new anti-candidal candidates from synthetic[4,5,6] or natural[7,8] origins.
Croton cajucara Benth. (Euphorbiaceae) has been a very important traditional medicinal plant in Brazil.[9,10] Two morphotypes of C. cajucara are known, white “sacaca” and red “sacaca,” mainly identified by young leaf color and steams.[3] In general, essential oils (EO) from the white morphotype are rich in linalool, while those from red morphotype are rich in 7-hydroxycalamenene, although some exceptions have been registered.[11,12] The linalool-rich EO from the leaves of C. cajucara has been shown to be very toxic for Leishmania amazonensis and Candida albicans.[13,14] Actually, our group demonstrated that 7-hydroxycalamenene-rich EO from C. cajucara was effective against L. chagasi,[15] Mycobacterium spp., Staphylococcus aureus MRSA, Mucor polymorphosporus, and Rhizopus oryzae.[16]
7-Hydroxycalamenene is a hydroxylated sesquiterpene whose molecular weight is 218. It has been identified in Eremophila drummondii (Scrophulariaceae), Ulmus glabra (Ulmaceae), Ganoderma applanatum (Ganodermataceae), cotton leaves inoculated with Xanthomonas campestris pv. malvacearum (Malvaceae; Xanthomonadaceae), Syzygium cumini (Myrtaceae), Bazzania trilobata (Lepidoziaceae), and Tilia europaea (Tiliaceae). It shows good activity against Botrytis cinerea, Cladosporium cucumerinum, Phytophthora infestans, Pyricularia oryzae, and Septoria tritici.[17,18,19,20,21,22,23] Our group also demonstrated the promising activity of 7-hydroxycalamenene against some zygomycetes.[24] Thus, the objective of this work was to evaluate the anti-candidal activity of 7-hydroxycalamenene isolated from C. cajucara EO.
MATERIALS AND METHODS
Chemicals
All solvents used were spectroscopic grade from Tedia (Fairfield, OH, USA). Reagents were from Sigma-Aldrich (St Louis, MO, USA). Column chromatographic products were obtained from Merck (Darmstadt, Germany).
Plant material
The individual of red morphotype of C. cajucara was kept in the germplasm bank. The leaves were collected between 8 h and 9 h. A voucher specimen was deposited at EMBRAPA Amazon Occidental Herbarium (registry IAN 165013).
Essential oil extraction, analysis, and 7-hydroxycalamenene purification
Extraction was performed as previously described.[4] The EO was analyzed in an Agilent (Palo Alto, CA, USA) 6890N gas chromatograph fitted with a 5% phenyl –95% methyl silicone (HP-5, 30 m × 0.32 mm × 0.25 mm) fused silica capillary column. The analytical procedures were conducted in accordance to the protocol described by Vandendool and Kratz.[25] Identification of the EO components was based on computer search using Wiley sixth Edition library of mass spectral data and by comparison of their calculated linear retention index with literature data.[26] 7-Hydroxycalamenene was also identified by injection of the authentic standard.
The standard was prepared after isolation of the 7-hydroxycalamenene as described by Pereira et al.[12] The purity of the material was over 98%.
Microorganisms
C. albicans (ATCC 10231), C. albicans serotype A (ATCC 36801), C. albicans serotype B (ATCC 36802), Candida dubliniensis (clinical isolated), Candida parapsilosis (ATCC 22019), Candida famata (clinical isolated), Candida guilliermondii (ATCC 6260), Candida tropicalis (clinical isolated), Candida krusei (clinical isolated), and Candida glabrata (ATCC 9003) were used for the anti-candidal tests. The yeasts were stored in specific culture media slanted tubes at 4°C. Prior to use, the microorganisms were grown in Sabouraud agar for 24 h at 37°C.
Inhibitory concentration assays
The in vitro susceptibility was determined by the MIC determination method. The MICs of 7-hydroxycalamenene were determined by two-fold serial dilution as described by Clinical and Laboratory Standards Institute M-27.[27] The microbicidal/microbiostatic concentrations were determined according to Khan et al.[28] sub-culturing the test dilutions onto a specific fresh solid media and incubated further for 24 h. The experiments were made in triplicate. All experiments were repeated at least 3 times.
Influence of 7-hydroxycalamenene on Candida albicans biofilm formation
C. albicans (ATCC10231) was grown as a biofilm in a 96-well microtiter plate as reported previously[29] with some modifications. Briefly, C. albicans was grown in yeast-nitrogen base broth (YNB) supplemented with 50 mM glucose pH 7.0 at 37°C overnight. After incubation, the cells were resuspended at a density of 1 × 107 cells/ml in YNB. Microtiter plate previously coated with 100 μl of fetal bovine serum for 30 min and washed once with phosphate buffer saline (PBS) 0.01M pH 7.2 was incubated with 100 μl of cell suspension for 90 min at 37°C. Nonadherent cells were removed by washing twice with PBS, and 100 μl of different concentrations of 7-hydroxycalamenene (two-fold serial dilution) diluted in YNB medium supplemented with 50 mM glucose were added to the wells. The plate was incubated for up to 24 h at 37°C. The exopolysaccharide matrix was evaluated as previously described.[30,31]
Secreted aspartic protease inhibition
C. albicans serotype A was grown in YEPD broth for 24 h at 25°C to optimize the SAP production.[22] Cells were removed by centrifugation, and the supernatant was concentrated 25 times in an ultrafiltration cell (Amicon Corp.). The quantitative proteolytic activity was made according to Palmeira et al.[32] with some modifications. Briefly, 20 μl of concentrated supernatant, 20 μl of bovine serum albumin (BSA) (0.1 mg/ml) and 60 μl of buffer solutions (pH 1.0–11.0) was added to a 96-well microtiter plate and incubated for 1 h at 37°C. After incubation, it was added 100 μl of Coomassie solution (0.025% Coomassie brilliant blue G-250, 11.75% ethanol, and 21.25% phosphoric acid). A control, where the substrate was added just after the reactions were stopped, was used as blank. After 10 min to allow dye binding, the plate was read on a Molecular Devices Thermomax microplate reader at an absorbance of 630 nm. One unit of enzyme activity was defined as the amount of enzyme that caused an increase of 0.001 in absorbance unit, under standard assay conditions. Alternatively, the concentrated supernatant was preincubated for 20 min at 37°C in the presence or absence of pepstatin A (10 mM) and 7-hydroxycalamenene (39.06 μg/ml). In this last case, the results were expressed as relative percentage of activity. Besides, the reaction mixtures were applied on sodium dodecyl sulfate polyacrylamide gel electrophoresis to demonstrate the fragments correspondent to the BSA hydrolysis; thus, the gels were stained with Coomassie blue R-250 solution.
RESULTS AND DISCUSSION
The average EO yield obtained was 0.65% (dry weight). The compounds present in the EO from C. cajucara used are shown in Table 1. The percentage of 7-hydroxycalamenene on the EO was about 35.4%.
Table 1.
Main components from C. cajucara essential oil
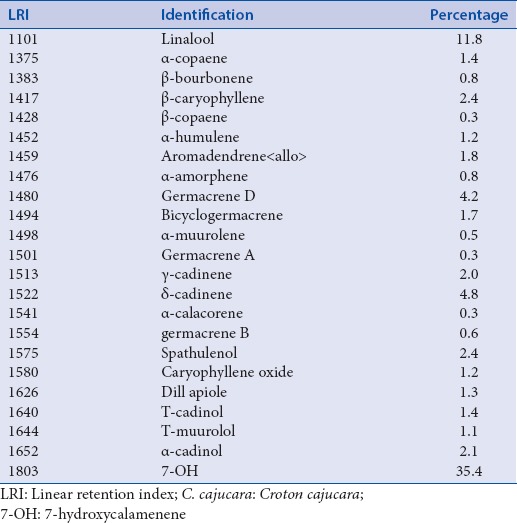
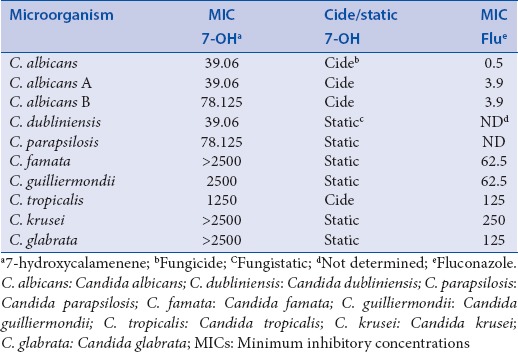
In previous work, our group evaluated the antioxidant and antimicrobial activities of 7-hydroxycalamenene-rich EO from C. cajucara. In that study, we demonstrated that 7-hydroxycalamenene was the main bioactive component of the EO.[16] Here, we report for the 1st time the anti-candidal activity of purified 7-hydroxycalamenene. The results of MIC assay using this substance can be observed in Table 2. Best results were observed against C. albicans, C. albicans serotype A, C. dubliniensis (MIC values at 39.06 μg/ml), but also against C. albicans serotype B (MIC value at 78.125 μg/ml). It is usually considered that strong activity is for MIC values between 50 and 500 μg/ml, moderate activity values between 600 μg/ml and 1500 μg/ml and above 1500 μg/ml as a weak activity.[33] According to this classification it could be stated that 7-hydroxycalamenene presents high activity against C. albicans, C. albicans serotype A and C. dubliniensis, moderate activity against C. albicans serotype B, C. parapsilosis and C. tropicalis, and weak activity to C. famata, C. guilliermondii, C. krusei and C. glabrata, which shows that it is a promising antifungal substance mainly against C. albicans. Previously, we showed that the best MIC of 7-hydroxycalamenene-rich EO from C. cajucara for C. albicans was about 0.038 μg/ml. The strong activity presented by EO may be explained by its chemical composition. In fact, the EO has other components that may act synergistically in inhibiting the microorganisms.[15]
Table 2.
MICs values (in μg/ml) of 7-OH
Biofilm formation represents the most common mode of growth and environment resistance of some microorganisms. In fact, the ability of Candida spp. to form biofilms allows them to resist drugs and also to resist host immunological response during the course of infection. Raut et al.[34] reported that eight more active phenylpropanoids of plant origin were able to significantly inhibit biofilm formation by C. albicans at concentrations ranging from 125 μg/ml to 512 μg/ml. Here, we evaluated the effects of 7-hydroxycalamenene on C. albicans biofilm formation. Our results revealed that 7-hydroxycalamenene did not affect biofilm formation at MIC value (39.06 μg/ml), but only at the concentration of 2500 μg/ml (27% of inhibition) [Figures 1 and 2]. These results corroborate the literature data which describes that the microorganisms within biofilms display increased resistance to antimicrobials in comparison to their planktonic counterparts. This phenomenon is generally associated with poor antimicrobial penetration (due to the presence of an extracellular matrix), activation of adaptive stress responses (including the production of persisted cells and induction of quorum sensing mechanisms), and physiological heterogeneity within the biofilm population.[35,36] It is worth mentioning that IC50 and MIC values for biofilm inhibition have been described as 5–8 and 30–2000 times higher than for planktonic cells, respectively.[37]
Figure 1.
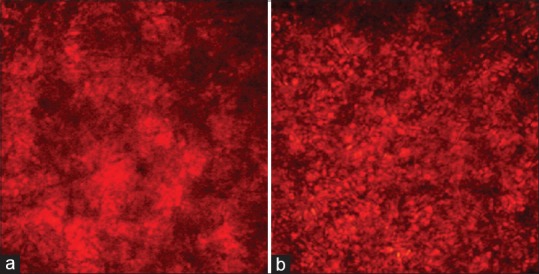
Effect of 7-hydroxycalamenene against Candida albicans biofilm. The plates were incubated at 37°C for 24 h. (a) Biofilm control, (b) Biofilm treated with 2500 µg/ml of 7-hydroxycalamenene. The images were taken at ×40 using an inverted microscope
Figure 2.
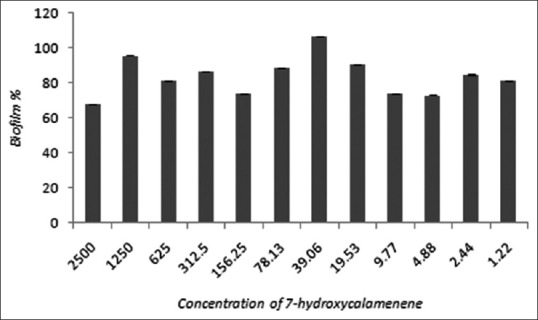
Percentage of biofilm of Candida albicans (ATCC 10231) at different concentrations of 7-hydroxycalamenene
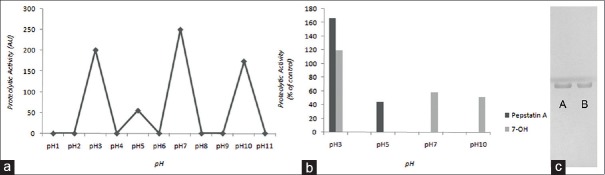
In order to evaluate the inhibitory effect of 7-hydroxycalamenene on SAP, we first establish the optimal pH condition for the enzymatic assay. The supernatant of YEPD culture C. albicans serotype A was collected and concentrated 25 times. Then, peptidase activity was detected under different pHs conditions. The highest hydrolysis level of BSA was detected at pH 7.0 [Figure 3a]. Through the inhibition of pepstatin A [Figures 3b and 3c] our results suggest that the supernatant obtained was rich in SAP. These results are in accordance to those reported by White and Agabian.[38] Therefore, the concentrated supernatant was incubated in the presence of 39.06 μg/ml (MIC) of 7-hydroxycalamenene at pH 7.0. After the incubation period, we observed a reduction of 58%in the SAP activity. There are few works in literature describing the inhibitory activity of plant extracts or their isolated bioactive components on SAP from Candida spp., making it a promising study field. Li et al.[39] described SAP inhibitory activity of phenolic compounds isolated from Miconia myriantha. Mattucinol-7-O- (4’ ‘, 6’ ‘-O-[S]-hexahydroxydiphenoyl)-beta-D-glucopyranoside and galic acid displayed the best activities at IC50 values of 8.4 and 10.5 μM, respectively. SAP from C. albicans were also inhibit by two new xanthones, 1,3,5,7-tetrahydroxy-8-isoprenylxanthone and 1,3,5-trihydroxy-8-isoprenylxanthone, but also by 3-geranyl-2,46-trihydroxybenzophenone and betulinic acid obtained from Tovomita krukovii. The IC50 of these compounds were 15, 25, 40 and 6.5 μg/ml, respectively.[7] The same research group described SAP inhibitory activity of apigenin-4’- O-(2‘‘,6‘‘- di-O-p-coumaroyl) -β-d-glucopyranoside and 3β, 14α, 15α, 21β-tetrahydroxyserratane-24-oic acid, both isolated from an ethanol extract of Lycopodium cernuum, at IC50 values of 20 and 8.5 μg/mL, respectively.[8] It is interesting to note that among the compounds described above only 3-geranyl-2,46-trihydroxybenzophenone and 7-hydroxycalamene were also cytotoxic for the microorganism.
Figure 3.
(a) Effect of pH on extracellular proteolytic activity of Candida albicans serotype A. (b) Effect of Pepstatin A and 7-hydroxycalamenene on the secreted peptidase activity from Candida albicans serotype A. (c) Sodium dodecyl sulfate polyacrylamide gel electrophoresis: The system is at pH 5.0 where A is the bovine serum albumin control and B is the reaction with 7-hydroxycalamenene
CONCLUSION
This study provides biological evidence that 7-hydroxycalamenene, the major compound in C. cajucara (red morphotype) EO, is active against Candida spp. Our results demonstrate that despite its poor inhibitory activity on Candida biofilm formation, 7-hydroxycalamenene displayed strong activity against the planktonic cells. In addition, this substance was also able to inhibit SAP activity, an important Candida virulence factor. These results further support the potential use of 7-hydroxycalamenene in the combat against Candida spp.
Financial support and sponsorship
The Brazilian agencies Rio de Janeiro State Research Foundation (FAPERJ), Coordination for Improvement of Higher Education Personnel (CAPES), and National Council for Scientific and Technological Development (CNPq) for the financial support.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tscherner M, Schwarzmüller T, Kuchler K. Pathogenesis and anti-fungal drug resistance of the human fungal pathogen Candida glabrata. Pharmaceuticals. 2011;4:169–86. [Google Scholar]
- 2.Ghannoum MA. Candida: A causative agent of an emerging infection. J Investig Dermatol Symp Proc. 2001;6:188–96. doi: 10.1046/j.0022-202x.2001.00047.x. [DOI] [PubMed] [Google Scholar]
- 3.Mardegan RD, Foglio MA, Gonçalves RB, Höfling JF. Candida albicans proteinases. Braz J Oral Sci. 2006;5:944–52. [Google Scholar]
- 4.Cadicamo CD, Mortier J, Wolber G, Hell M, Heinrich IE, Michel D, et al. Design, synthesis, inhibition studies, and molecular modeling of pepstatin analogues addressing different secreted aspartic proteinases of Candida albicans. Biochem Pharmacol. 2013;85:881–7. doi: 10.1016/j.bcp.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Santos AL, Braga-Silva LA. Aspartic protease inhibitors: Effective drugs against the human fungal pathogen Candida albicans. Mini Rev Med Chem. 2013;13:155–62. [PubMed] [Google Scholar]
- 6.Bein M, Schaller M, Korting HC. The secreted aspartic proteinases as a new target in the therapy of candidiasis. Curr Drug Targets. 2002;3:351–7. doi: 10.2174/1389450023347542. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, ElSohly HN, Jacob MR, Pasco DS, Walker LA, Clark AM. Natural products inhibiting Candida albicans secreted aspartic proteases from Tovomita krukovii. Planta Med. 2002;68:49–54. doi: 10.1055/s-2002-20049. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, ElSohly HN, Jacob MR, Pasco DS, Walker LA, Clark AM. Natural products inhibiting Candida albicans secreted aspartic proteases from Lycopodium cernuum. J Nat Prod. 2002;65:979–85. doi: 10.1021/np0200616. [DOI] [PubMed] [Google Scholar]
- 9.Hiruma-Lima CA, Gracioso JS, Bighetti EJ, Grassi-Kassisse DM, Nunes DS, Brito AR. Effect of essential oil obtained from Croton cajucara Benth. on gastric ulcer healing and protective factors of the gastric mucosa. Phytomedicine. 2002;9:523–9. doi: 10.1078/09447110260573155. [DOI] [PubMed] [Google Scholar]
- 10.Perazzo FF, Carvalho JC, Rodrigues M, Morais EK, Maciel MA. Comparative anti-inflammatory and antinociceptive effects of terpenoids and an aqueous extract obtained from Croton cajucara Benth. Rev Bras Farmacognosia. 2007;17:521–8. [Google Scholar]
- 11.Chaves FC, Bizzo HR, Angelo PM, Xavier JJ, Sá-Sobrinho AF. Rendimento e composição química do óleo essencial de folhas de dois morfotipos de sacaca (Croton cajucara Benth) Rev Bras Planta Med. 2006;8:117–9. [Google Scholar]
- 12.Pereira AQ, Chaves FC, Pinto SC, Leitão SG, Bizzo HR. Isolation and identification of cis-7-hydroxycalamenene from the essential oil of Croton cajucara Benth. J Essent Oil Res. 2011;23:20–3. [Google Scholar]
- 13.do Socorro S Rosa Mdo S, Mendonça-Filho RR, Bizzo HR, de Almeida Rodrigues I, Soares RM, Souto-Padrón T, et al. Antileishmanial activity of a linalool-rich essential oil from Croton cajucara. Antimicrob Agents Chemother. 2003;47:1895–901. doi: 10.1128/AAC.47.6.1895-1901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alviano WS, Mendonça-Filho RR, Alviano DS, Bizzo HR, Souto-Padrón T, Rodrigues ML, et al. Antimicrobial activity of Croton cajucara Benth linalool-rich essential oil on artificial biofilms and planktonic microorganisms. Oral Microbiol Immunol. 2005;20:101–5. doi: 10.1111/j.1399-302X.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues IA, Azevedo MM, Chaves FC, Bizzo HR, Corte-Real S, Alviano DS, et al. In vitro cytocidal effects of the essential oil from Croton cajucara (red sacaca) and its major constituent 7- hydroxycalamenene against Leishmania chagasi. BMC Complement Altern Med. 2013;13:249. doi: 10.1186/1472-6882-13-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azevedo MM, Chaves FC, Almeida CA, Bizzo HR, Duarte RS, Campos-Takaki GM, et al. Antioxidant and antimicrobial activities of 7-hydroxy-calamenene-rich essential oils from Croton cajucara Benth. Molecules. 2013;18:1128–37. doi: 10.3390/molecules18011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croft KD, Ghisalberti EL, Hocart CH, Jefferies PR, Raston CL, White AH. Absolute configuration of the (+)-calamenenes: Crystal structure of 7-hydroxycalamenene. Chem Soc Perkin Trans. 1978;1:1267–70. [Google Scholar]
- 18.Davila-Huerta G, Hamada H, Davis GD, Stipanovic RD, Adams CM, Essenberg M. Cadinane-type sesquiterpenes induced in Gossypium cotyledons by bacterial inoculation. Phytochemistry. 1995;39:531–6. [Google Scholar]
- 19.Grayer RJ, Harborne JB. A survey of antifungal compounds from higher plants, 1982-1993. Phytochemistry. 1994;37:19–42. [Google Scholar]
- 20.Melcher E, Jüngel P, Möllendorf B, Schmitt U. Identification of a hydroxy substituted calamenene – A sesquiterpene associated with wound reactions in non-infected xylem of Tilia spp. Phytochemistry. 2003;62:707–13. doi: 10.1016/s0031-9422(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 21.Paterson RR. Ganoderma – A therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001. doi: 10.1016/j.phytochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Scher JM, Speakman JB, Zapp J, Becker H. Bioactivity guided isolation of antifungal compounds from the liverwort Bazzania trilobata (L.) S.F. Gray. Phytochemistry. 2004;65:2583–8. doi: 10.1016/j.phytochem.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Sikder MA, Kaisar MA, Rahman MS, Hasan CM, Al-Rehaily AJ, Rashid MA. Secondary metabolites from seed extracts of Syzygium cumini (L.) J Phys Sci. 2012;23:83–7. [Google Scholar]
- 24.Azevedo MM, Almeida CA, Bizzo HR, Chaves FC, Alviano DS, Alviano CS. Anti-zygomycetes activity of 7-hydroxycalamenene isolated from Croton cajucara. Planta Med. 2011;77:1449. doi: 10.1055/s-0034-1368441. [DOI] [PubMed] [Google Scholar]
- 25.Vandendool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr. 1963;11:463–71. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 26.Adams RP. IL: Allured Publishing Corporation; 2007. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. [Google Scholar]
- 27.Wayne, PA: Clinical and Laboratory Standards Institute; 2005. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution anti-fungal Susceptibility Testing of Yeasts. Approved Standard m27-a2. [Google Scholar]
- 28.Khan R, Islam B, Akram M, Shakil S, Ahmad A, Ali SM, et al. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules. 2009;14:586–97. doi: 10.3390/molecules14020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Silva AC, Lopes PM, de Azevedo MM, Costa DC, Alviano CS, Alviano DS. Biological activities of α-pinene and ß-pinene enantiomers. Molecules. 2012;17:6305–16. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G, Vellucci VF, Kyc S, Hostetter MK. Simvastatin inhibits Candida albicans biofilm in vitro. Pediatr Res. 2009;66:600–4. doi: 10.1203/PDR.0b013e3181bd5bf8. [DOI] [PubMed] [Google Scholar]
- 31.Singh R, Shivaprakash MR, Chakrabarti A. Biofilm formation by zygomycetes: Quantification, structure and matrix composition. Microbiology. 2011;157(Pt 9):2611–8. doi: 10.1099/mic.0.048504-0. [DOI] [PubMed] [Google Scholar]
- 32.Palmeira VF, Kneipp LF, Rozental S, Alviano CS, Santos AL. Beneficial effects of HIV peptidase inhibitors on Fonsecaea pedrosoi: Promising compounds to arrest key fungal biological processes and virulence. PLoS One. 2008;3:e3382. doi: 10.1371/journal.pone.0003382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sartoratto A, Machado AL, Delarmelina C, Figueira GM, Duarte CT, Rehder VL. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz J Microbiol. 2004;35:275–80. [Google Scholar]
- 34.Raut JS, Shinde RB, Chauhan NM, Karuppayil SM. Phenylpropanoids of plant origin as inhibitors of biofilm formation by Candida albicans. J Microbiol Biotechnol. 2014;24:1216–25. doi: 10.4014/jmb.1402.02056. [DOI] [PubMed] [Google Scholar]
- 35.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR, 3rd, et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8:e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–6. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 38.White TC, Agabian N. Candida albicans secreted aspartyl proteinases: Isoenzyme pattern is determined by cell type, and levels are determined by environmental factors. J Bacteriol. 1995;177:5215–21. doi: 10.1128/jb.177.18.5215-5221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li XC, Jacob MR, Pasco DS, ElSohly HN, Nimrod AC, Walker LA, et al. Phenolic compounds from Miconia myriantha inhibiting Candida aspartic proteases. J Nat Prod. 2001;64:1282–5. doi: 10.1021/np010172p. [DOI] [PubMed] [Google Scholar]