Abstract
Background:
Caffeine and L-theanine are pharmacologically important constituents of tea, especially due to their effects on the central nervous system. The effects of these two compounds are opposite: While caffeine is a well-known stimulant, theanine has a relaxing effect. Tea processing may influence the caffeine and theanine content of tea leaves.
Objective:
The aim of our work was to quantify these constituents from a set of commercial products to reveal the possible correlations of caffeine and theanine content and processing methods.
Materials and Methods:
Theanine and caffeine contents of 37 commercial white, green, oolong, black, and pu-erh tea samples were quantified by high-performance liquid chromatography-diode array detector.
Results:
The mean L-theanine content of white, green, oolong, and black teas were 6.26, 6.56, 6.09, and 5.13 mg/g, respectively. The same values for caffeine content were 16.79, 16.28, 19.31, and 17.73 mg/g.
Conclusion:
Though the effect of processing on theanine content was evident, quantification for these analytes does not seem to be a good criterion to discriminate the different types of tea. Caffeine content provided no information on the effect of processing, and the theanine content of the samples was rather variable, independently from the type of the tea. The quantitative analysis of caffeine and theanine is essential to assess the stimulating effect of the tea, however, for chemical profiling further secondary metabolites have to be determined.
SUMMARY
Thirty-seven commercial white, green, oolong, black, and pu-erh tea samples were analyzed for caffeine and theanine content
While the caffeine content was similar, the theanine contents of black teas were slightly lower and practically zero in pu-erh
The great variability of these two compound within the tea categories allows no discrimination of tea types based solely on theanine and caffeine quantification
Contrary to the previous data, the way of processing has no determining effect on theanine content.
Figure.
Abbreviations used: CZE: Capillary zone electrophoresis, DAD: Diode array detector, EEG: Electroencephalography, GC: Gas chromatography, HPLC: High-performance liquid chromatography, IR: Infrared spectroscopy, MEKC: Micellar electrokinetic capillary chromatography, MS: Mass spectrometry, RP: Reversed phase, RSD: Relative standard deviation, SD: Standard deviation, TLC: Tile liquid chromatography, UV: Ultraviolet
Keywords: Caffeine, Camellia sinensis, high-performance liquid chromatography-diode array detector, quantitative analysis tea, theanine
INTRODUCTION
Although the importance of tea (Camellia sinensis L.) as a medicinal plant is increasing, this plant is above all a raw material of the food industry. Pharmacological research focuses mainly on the polyphenols of tea due to their antioxidant, anticancer properties,[1] nevertheless, tea drinkers experience the immediate effect of two other compounds: Both caffeine and theanine exert their activities on the central nervous system. The purine alkaloid caffeine, the most widely applied stimulating agent, acts through adenosine receptors,[2] whereas the mechanism of action of the anxiolytic, relaxing effect of the amino acid L-theanine is not fully discovered yet.[3] However, there is an experimental evidence that L-theanine counteracts the stimulatory effect of caffeine. In rats, theanine administered after caffeine dosing blunted the stimulant effect of caffeine.[4] L-theanine facilitates the generation of alpha waves in the brain, which are believed to be associated with a relaxed yet alert mental state.[5] This effect has been confirmed in clinical studies as well.[6] Though there are only a few human data on the interaction of these two compounds, it is obvious that the ratio of theanine and caffeine determines the degree of stimulant effect of tea drinks. Moreover, together with caffeine, L-theanine has a synergistic positive effect on attention as demonstrated in human electroencephalography studies,[7] and in a behavioral study.[8]
The dried leaves of tea shrub contain 1–2% theanine as free amino acid and up to 7–9% methylxanthines, with caffeine as the quantitatively (3–6%) and pharmacologically most significant compounds.[9] According to the processing of the raw leaves, the main types of tea are green, white, oolong, black, and pu-erh teas. Green and white teas are subject to minimal oxidation, whereas oolong and black teas are allowed to partially and extensively oxidize as a result of fermentation, respectively, with the highest grade of oxidization in case of pu-erh. The extent of fermentation has been found to be determinant of the concentration of L-theanine, with more theanine contained in unfermented teas and less in fermented teas.[10] If steaming is avoided during the processing of green tea, the final product is remarkably rich in theanine (>2% theanine).[11] Nevertheless, other factors also influence the composition of the final product, since some green teas are almost devoid of theanine, and there are black teas with remarkable theanine content.[10] However, the presence of caffeine in the tea counteracts the relaxing effect. The amount of caffeine in different tea varieties is unaffected by the various processing methods.[12] Apart from the composition of the raw material, the way the tea is prepared by the consumer (grade of comminution of tea leaves, extraction time, temperature, drug extract ratio, stirring, and addition of milk) has also major influence on the concentration of theanine and caffeine in the tea drink.[10]
Despite the practical relevance of the quantity of caffeine and theanine in tea leaves, there are only a few papers dealing with the simultaneous quantification of these substances from tea drinks or dry tea leaves. In case of caffeine, a plethora of methods, including gravimetric, spectrophotometric, gas chromatographic (GC), high performance liquid chromatography coupled with UV detection (HPLC-UV), HPLC-mass spectrometry (HPLC-MS), chemometric, infrared spectroscopic (IR), capillary electrophoretic and electroanalytical protocols are available for quantification.[13] For theanine, the most frequently applied methods are tile liquid chromatography (TLC)-densitometry,[14,15] HPLC-UV or coupled with diode array detector (DAD)[16] and capillary electrophoresis. In case of reversed-phase (RP)-HPLC, the chromatographic separation and/or detection of theanine can be improved by derivatization with o-phthalaldehyde,[17] dimethylaminoazobenzene sulfonyl chloride[18] or as 2,4-dinitrophenyltheanine derivative[11] or by the application of fluorescence detector.[17] HPLC-MS[19] and capillary electrophoresis methods have also been published.[20]
For the simultaneous determination of the two substances, capillary zone electrophoresis,[21] micellar electrokinetic capillary chromatographic methods,[22] HPLC method using C18[23] and amide-C16 column,[16] and a multidimensional HPLC-DAD-MS quantification[24] are available in literature.
From a practical point of view, the caffeine and theanine content of the tea infusions belong to the most important data characterizing a tea. Though there are such results for theanine from an extensive study (Keenan 2011), there are no detailed data on the caffeine and theanine content of infusions of different tea types using standardized sample preparation methods. Here we report the results of a comprehensive analytical study on the caffeine and theanine contents of infusions of 34 commercial samples using HPLC-DAD on a C-18 column.
MATERIALS AND METHODS
Chemicals and reagents
L-theanine (>99%) and caffeine (>99%) – used as calibration standards – was purchased from Sigma to Aldrich (Steinheim, Germany) and Phoenix Pharma cPlc. (Budapest, Hungary), respectively. Acetonitrile used for chromatographic analysis (LiChrosolv® HPLC grade) was obtained from Merck (Darmstadt, Germany). Millipore Direct-Q UV3 clarifier (Molsheim, France) was used to produce purified water for HPLC measurements.
Sample preparation and calibration standards
The analyzed tea samples (34) were commercially available green, black, white, oolong, and pu-erh teas. Samples 1–25, 32, 33, and 38 were purchased in a tea shop (Latin Negyed), samples 26–31 in a hypermarket (Metro) in Szeged, Hungary and samples 34–37 in a tea shop in Kaohsiung, Taiwan. Extraction of L-theanine and caffeine was carried out with 100 ml 80°C water for 3 min from 1 g sample. Dissolution of these two analytes was analyzed similarly, but applying longer extraction time (up to 90 min).
A stock standard solution of L-theanine was prepared in 1.3870 μg/μl concentration, and the standard solutions were made in the concentration range of 0.0277–0.5548 μg/μl by diluting the stock standard solution. The standard solutions were prepared in water and stored at 4°C. The calibration range was 0.277–5.548 μg L-theanine/injection. The calibration standard was injected in triplicate at six concentration levels.
A stock standard solution of caffeine was prepared in 0.2600 μg/μl concentration, and the standard solution was made in the concentration of 0.0260 μg/μl by diluting the stock standard solution. The standard solutions were prepared in methanol and stored at 4°C. The calibration range was 0.130–5.200 μg caffeine/injection. The calibration standard was injected in triplicate at six volume levels.
High-performance liquid chromatography apparatus and measurement conditions
HPLC analysis was carried out on a Waters 600 system (Waters Corporation, Milford, USA), equipped with a 2998 photodiode array detector, on-line degasser and autosampler using an RP Kinetex XB-C18 (2.6 μm, 100Å, 100 mm × 4.6 mm) column (Phenomenex, Torrance, USA). With a column temperature of 40°C gradient elution was applied which was as follows: Starting with 100% of mobile phase A (water + 0.05% phosphoric acid) with a flow rate of 0.5 ml/min which was held for 6 min, then the flow rate was changed in 1 min to 0.8 ml/min. Then the composition of the mobile phase was changed in 18 min to 95% A and 5% B (acetonitrile), then in 15 min to 80% A and 20% B and held up to 10 min. At last, it was returned to the initial composition in 1 min and the column was equilibrated for 9 min with 0.8 ml/min flow rate. The flow rate was decreased to 0.5 ml/min in 1 min. L-theanine and caffeine were monitored at 210 nm and 273 nm, respectively. Data acquisition and evaluation were performed using Waters Empower Pro software (Milford, MA, USA).
RESULTS AND DISCUSSION
Quantification method
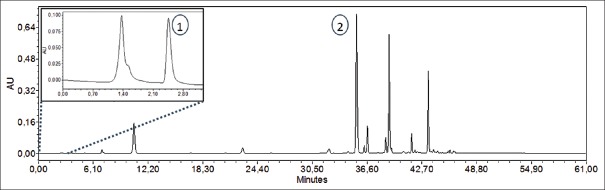
Caffeine and L-theanine were identified by the comparison their retention times and UV spectra with analytical standards. L-theanine was detected at 2.5 min; caffeine was eluted with a retention time of 35.0 min [Figure 1]. Table 1 shows the slope (S), the intercept (I), their standard deviation (SD), and the correlation coefficient (R2) for the calibration curves. The relationship between area and concentration was linear within the studied concentration range. The method applied by us allows reliable determination of these compounds.
Figure 1.
High-performance liquid chromatography chromatogram of a green tea sample with the peaks of L-theanine (1, 210 nm) and caffeine (2, 273 nm)
Table 1.
Statistics of calibration curves for L-theanine and caffeine

Dissolution test
The amount of time required for the total extraction of L-theanine and caffeine from a green tea sample (China Yunnan FOP) was established [Figure 2–3]. According to our results, about 20 min is required for the total extraction of the studied components. Though an extraction time of 3 min does not allow maximal extraction of theanine and caffeine (90 min extraction results in ×2 and ×1.5 higher analyte concentration in the extract), for detailed analysis sample preparation similar to the traditional green tea brewing method was applied to mimic the extraction efficacy of tea preparation.
Figure 2.
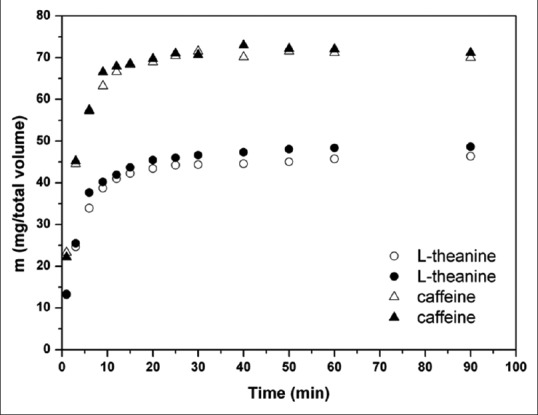
Dissolution test for L-theanine and caffeine (two replicates)
Figure 3.
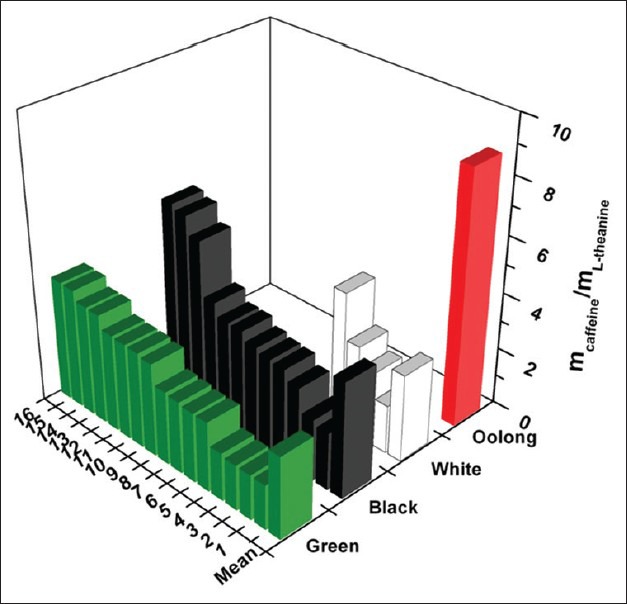
L-theanine and caffeine content of the examined tea samples
L-theanine and caffeine content of the examined tea samples
Living tea shoots contain many enzymes. The postmortem activity of these enzymes influences the chemical composition of the processed tea. The general steps of tea processing are as follows: Withering of the leaves, rolling, fermenting, and firing. White tea is subjected only to firing, green tea is produced by withering and firing, in case of oolong these two steps are followed by rolling, and the processing of black teas is the most complex (withering, rolling, fermentation, and firing). Withering leads to loss of water, rolling causes the disruption of cell walls and cell membranes (and activates several enzymes), and fermentation catalyzes complex chemical reactions in the plant material. Firing deactivates the enzymes of the plant thereby preventing the transformation of compounds.[25] The concentration of caffeine is not significantly altered by processing, although it may increase during withering and may decrease during the firing process of tea manufacturing. Caffeine in the plant is the product of nucleic acid metabolism and as this breakdown continues also during the withering stage, the caffeine content increases.[26] The level of free amino acids, including L-theanine, may increase during processing because of the increase in the activity of proteolytic enzymes. Hydrolysis is augmented by high temperature and moist environment.[25] However, withering and drying results in a significant decrease of L-theanine content.[27]
In case of white tea, the average L-theanine content was 6.26 mg/g, with a mean caffeine concentration of 16.79 mg/g (caffeine/theanine ratio: 3.07). [Table 2]. Black tea samples contained a lower amount of theanine (5.13 mg/g) with slightly higher caffeine concentration (17.77 mg/g), therefore, the caffeine/theanine ratio was higher (4.07). Green tea contained the highest amount of theanine (6.56 mg/g) and since the caffeine content was comparable with the values of the previous samples (16.28 mg/g in average), the caffeine/theanine ratio was remarkably lower (2.79). The average theanine concentration of oolong samples was 6.09 mg/g with a mean caffeine level of 19.31 mg/g (caffeine/theanine ratio 4.20). In the pu-erh tea, no theanine was detected. The SD of the results in case of the green, white, and black tea samples was remarkably high (2.45–2.79 for theanine and 1.50–3.95 for caffeine). However, in case of oolong samples the extremely high variation of results (0.90–12.37 mg/g theanine and 10.96–39.71 mg caffeine) hampers the assessment of these values. Although the interaction of caffeine and L-theanine has not been studied so far in detail in case of human application, based on the available scientific data, it can be stated that samples with lower caffeine/theanine ratio have less pronounced stimulating effect.
Table 2.
L-theanine and caffeine content of the examined tea samples
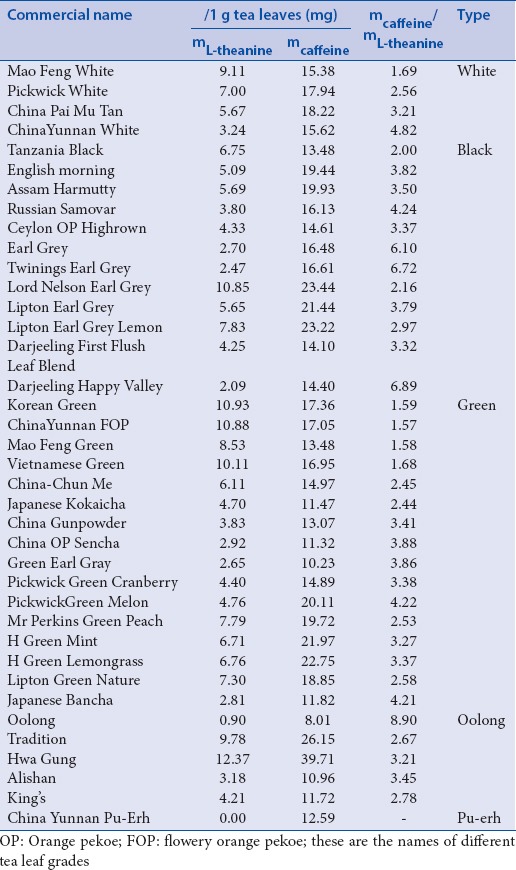
The analysis of 17 tea samples carried out by Ekborg-Ott et al., revealed that the average theanine content of black teas was 1.40%, as compared to 1.42% in green teas, and 1.16% in oolong teas. The widest range of theanine content (0.60–1.72%) was detected in oolong samples. One of the black tea samples contained the highest amount of theanine (2.38%).[28] The results of a comprehensive analytical study points out the high variability of theanine content within the different tea types (3.97–14.47 mg/g for oolong, 0.89–17.27 mg/g for black, 3.07–21.18 mg/g for green and 2.23–14.21 mg/g for white tea). In pu-erh, the concentration of this amino acid was rather low (0.07–0.26 mg/g).[29] Our results are in accordance with these findings, however, the theanine contents were significantly lower than those measured by Ekborg-Ott et al.[28] This can be explained by the application of different extraction methods. Although the variation of theanine content among the different tea types was high, the effect of processing is reflected in the measured values (highest theanine content in green and white tea, followed by oolong and black, and no theanine in pu-erh). Interestingly, one oolong sample contained extremely high amount of caffeine (HWA Gung, 39.71 mg/g) and in this tea category one sample was almost void of theanine (Oolong, 0.9 mg/g). However, the caffeine content of the whole sample set was more uniform than the theanine concentration (relative SD% 33.04 vs. 49.50). This reflects the difference of the stability of these two compounds in the biological matrix of tea leaves during processing. The high variability of the values within each category and highlights the major influence of the processing conditions (time and temperature of withering, rolling, fermentation, and drying).[27] Based on the analysis of caffeine and theanine content, it is almost impossible to categorize teas to the groups of white, green, oolong, and black major tea groups. The only exception is pu-erh tea, which is characterized by its negligibly low theanine content.
CONCLUSION
HPLC-DAD analysis of 34 tea samples for their L-theanine and caffeine content revealed large variation in the quantities of these compounds in the samples. Though the effect of processing on theanine content is evident, quantification for these analytes does not seem to be a good criterion to discriminate the different types of tea. The quantitative analysis of caffeine and theanine is essential to assess the stimulating effect of the tea, however, for chemical profiling further secondary metabolites have to be determined.
Financial support and sponsorship
This research was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4. A/2-11-1-2012-0001 “National Excellence Program.”
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Dezső Csupor
Dezső Csupor, assistant professor at the Department of Pharmacognosy, University of Szeged, Hungary, is the leader of a research group dealing with phytochemical analysis of medicinal plant products. His scientific interest includes pharmacological analysis of natural products as well. Dezső Csupor is a member of the scientific committe of ESCOP and was working for 6 years for the European Medicines Agency as an expert of medicinal plants.
REFERENCES
- 1.Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–33. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992;17:139–70. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 3.L-theanine Monograph. Altern Med Rev. 2005;10:136–8. [PubMed] [Google Scholar]
- 4.Kakuda T, Nozawa A, Unno T, Okamura N, Okai O. Inhibiting effects of theanine on caffeine stimulation evaluated by EEG in the rat. Biosci Biotechnol Biochem. 2000;64:287–93. doi: 10.1271/bbb.64.287. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi K, Nagato Y, Aoi N, Juneja LR, Kim M, Yamamoto T, et al. Effects of L-theanine on the release of α-brain waves in human volunteers. Nippon Nôeikagaku Kaishi. 2002;72:153–7. [Google Scholar]
- 6.Cooper R, Morré DJ, Morré DM. Medicinal benefits of green tea: Part I. Review of noncancer health benefits. J Altern Complement Med. 2005;11:521–8. doi: 10.1089/acm.2005.11.521. [DOI] [PubMed] [Google Scholar]
- 7.Kelly SP, Gomez-Ramirez M, Montesi JL, Foxe JJ. L-theanine and caffeine in combination affect human cognition as evidenced by oscillatory alpha-band activity and attention task performance. J Nutr. 2008;138:1572S–7S. doi: 10.1093/jn/138.8.1572S. [DOI] [PubMed] [Google Scholar]
- 8.Owen GN, Parnell H, De Bruin EA, Rycroft JA. The combined effects of L-theanine and caffeine on cognitive performance and mood. Nutr Neurosci. 2008;11:193–8. doi: 10.1179/147683008X301513. [DOI] [PubMed] [Google Scholar]
- 9.Juneja LR, Chu DC, Okubo T, Nagato Y, Yokogoshi H. L-theanine – A unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci Technol. 1999;10:199–204. [Google Scholar]
- 10.Keenan EK, Finnie MD, Jones PS, Rogers PJ, Priestley CM. How much theanine in a cup of tea?. Effects of tea type and method of preparation. Food Chem. 2011;125:588–94. [Google Scholar]
- 11.Friedman M, Levin CE, Choi SH, Lee SU, Kozukue N. Changes in the composition of raw tea leaves from the Korean Yabukida plant during high-temperature processing to pan-fried Kamairi-cha green tea. J Food Sci. 2009;74:C406–12. doi: 10.1111/j.1750-3841.2009.01185.x. [DOI] [PubMed] [Google Scholar]
- 12.Mohanpuria P, Kumar V, Yadav SK. Tea caffeine: Metabolism, functions, and reduction strategies. Food Sci Biotechnol. 2010;19:275–87. [Google Scholar]
- 13.De Mariza CA, Moreira RF. Cafeína: Revisão sobre métodos de análise. Quim Nova. 2007;30:99–105. [Google Scholar]
- 14.Feldheim W, Yongvanit P, Cummings PH. Investigation of the presence and significance of theanine in the tea plant. J Sci Food Agric. 1986;37:527–34. [Google Scholar]
- 15.Csupor D, Boros K, Hunyadi A, Veres K, Hohmann J. Validation of a densitometric method for the determination of theanine in tea extracts using CP atlas software. J Planar Chromatogr Mod TLC. 2012;25:571–4. [Google Scholar]
- 16.Peng L, Song X, Shi X, Li J, Ye C. An improved HPLC method for simultaneous determination of phenolic compounds, purine alkaloids and theanine in Camellia species. J Food Compost Anal. 2008;21:559–63. [Google Scholar]
- 17.Ying Y, Ho JW, Chen ZY, Wang J. Analysis of theanine in tea leaves by HPLC with fluorescence detection. J Liq Chromatogr Relat Technol. 2005;28:727–37. [Google Scholar]
- 18.Syu KY, Lin CL, Huang HC, Lin JK. Determination of theanine, GABA, and other amino acids in green, oolong, black, and Pu-erh teas with dabsylation and high-performance liquid chromatography. J Agric Food Chem. 2008;56:7637–43. doi: 10.1021/jf801795m. [DOI] [PubMed] [Google Scholar]
- 19.Desai MJ, Armstrong DW. Analysis of derivatized and underivatized theanine enantiomers by high-performance liquid chromatography/atmospheric pressure ionization-mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:251–6. doi: 10.1002/rcm.1319. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh MM, Chen SM. Determination of amino acids in tea leaves and beverages using capillary electrophoresis with light-emitting diode-induced fluorescence detection. Talanta. 2007;73:326–31. doi: 10.1016/j.talanta.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 21.Horie H, Mukai T, Kohata K. Simultaneous determination of qualitatively important components in green tea infusions using capillary electrophoresis. J Chromatogr A. 1997;758:332–5. [Google Scholar]
- 22.Aucamp JP, Hara Y, Apostolides Z. Simultaneous analysis of tea catechins, caffeine, gallic acid, theanine and ascorbic acid by micellar electrokinetic capillary chromatography. J Chromatogr A. 2000;876:235–42. doi: 10.1016/s0021-9673(00)00145-x. [DOI] [PubMed] [Google Scholar]
- 23.Song R, Kelman D, Johns KL, Wright AD. Correlation between leaf age, shade levels, and characteristic beneficial natural constituents of tea (Camellia sinensis) grown in Hawaii. Food Chem. 2012;133:707–14. [Google Scholar]
- 24.Zhu X, Chen B, Ma M, Luo X, Zhang F, Yao S, et al. Simultaneous analysis of theanine, chlorogenic acid, purine alkaloids and catechins in tea samples with the help of multi-dimension information of on-line high performance liquid chromatography/electrospray-mass spectrometry. J Pharm Biomed Anal. 2004;34:695–704. doi: 10.1016/s0731-7085(03)00605-8. [DOI] [PubMed] [Google Scholar]
- 25.Xu N, Chen ZM. Green tea, black tea and semi-fermented tea. In: Zhen YS, editor. Tea – Bioactivity and Therapeutic Potential. London and New York: Taylor and Francis; 2002. pp. 35–56. [Google Scholar]
- 26.Tomlins KI, Mashingaidze A. Influence of withering, including leaf handling, on the manufacturing and quality of black teas – A review. Food Chem. 1997;60:573–80. [Google Scholar]
- 27.Sari F, Velioglu YS. Changes in theanine and caffeine contents of black tea with different rolling methods and processing stages. Eur Food Res Technol. 2013;237:229–36. [Google Scholar]
- 28.Ekborg-Ott KH, Taylor A, Armstrong DW. Varietal differences in the total and enantiomeric composition of theanine in tea. J Agric Food Chem. 1997;45:353–63. [Google Scholar]
- 29.Horanni R, Engelhardt UH. Determination of amino acids in white, green, black, oolong, pu-erh teas and tea products. J Food Compost Anal. 2013;31:94–100. [Google Scholar]