Abstract
Background:
In traditional Chinese medicine, Atractylodis rhizoma is the dried rhizome of Atractylodes lancea (Thunb.) DC. or Atractylodes chinensis (DC.) Koidz. After being processed, the dryness of A. rhizoma decreased, and the function of tonifying spleen increased. Therefore, the processed A. rhizoma is the best choice of clinical application. As the main active components, polyethylene alkynes exhibits various desirable pharmacological effects including anti-inflammatory, anti-bacterial and anti-arrhythmia activity. However, there is no report on the pharmacokinetic comparisons of atractylodin, one of polyethylene alkynes, in bio-samples after oral administration of crude and processed A. rhizoma until now. The in vivo study of active components of A. rhizoma would be necessary and helpful for clinical application and clarification of processing principle.
Objective:
To compare the pharmacokinetic parameters of atractylodin after oral administration of crude and processed A. rhizoma, and clarify the processing principle of A. rhizoma.
Materials and Methods:
Plasma concentrations of atractylodin in rats were determined by reversed-phase high-performance liquid chromatogram and the main pharmacokinetic parameters were estimated with Drug and Statistics 2.0 Software Package (Chinese Pharmacological Society, Shanghai, China).
Results:
The AUC0−t, AUC0→∞, Tmax, and Cmax of processed A. rhizoma were increased significantly (P < 0.05) compared with that in crude A. rhizoma after using Student's t-test.
Conclusions:
Processing A. rhizoma with wheat bran by stir-frying can promote and accelerate the absorption of atractylodin.
SUMMARY
In this paper, a RP-HPLC method with UV detection for quantification of atractylodin (a main active component in Atractylodis Rhizoma) in rat plasma has been developed and applied to a preliminary pharmacokinetic study of atractylodin after oral administration of crude and processed Atractylodis Rhizoma respectively. The result indicates that processing Atractylodis Rhizoma with wheat-bran can promote and accelerate the absorption of atractylodin.
Keywords: Atractylodin, Atractylodis rhizoma, crude and processed, pharmacokinetics, polyethylene alkynes
INTRODUCTION
In China, Atractylodis rhizoma, the dried rhizome of Atractylodes lancea (Thunb.) DC. or Atractylodes chinensis (DC.) Koidz., is widely used for the treatment of rheumatic diseases, digestive disorders, mild diarrhea, and influenza.[1] In clinic, A. rhizoma is often processed by stir-frying with wheat bran with the aim of reducing its dryness and increasing the function of tonifying spleen.[2,3] In order to clarify the influence of processing on pharmacological properties of A. rhizoma, an investigation was carried out to compare the pharmacokinetics of typical constituent after oral administration of crude A. rhizoma and processed ones. A simple, rapid and sensitive high-performance liquid chromatogram (HPLC) with ultraviolet (UV) detection was developed and validated for the determination of atractylodin in rat plasma. Atractylodin is one of polyethylene alkyne components.[4,5] Some literature reported HPLC method for determination its content in A. rhizoma.[6,7,8] There is no report on a pharmacokinetic study of atractylodin after oral administration of crude and processed A. rhizoma in animals or in human until now. The in vivo study of atractylodin, an active components of A. rhizoma, would be necessary and helpful for further clinical application and explanation the processing mechanism.
MATERIALS AND METHODS
Materials and reagents
A. rhizoma was identified by Professor LiFeng (Liaoning University of TCM) according to the standards of Chinese Pharmacopoeia 2010. The processed A. rhizoma comes from the same batch A. rhizoma and were stir-fried with wheat bran. The herb was stored in a cool and dry place. Atractylodin (purity, 98%) was supplied by National Institute for Food and Drug Control (Beijing, China). The IS called physcion (purity, 98%) was supplied by the national institute for food and drug control (Beijing, China). The chemical structures of atractylodin and IS are shown in Figure 1. HPLC grade acetonitrile was purchased from Fisher Scientific Company (New Jersey, USA), pure water was supplied by Wahaha Company (Hangzhou, China). Analytical grade ethanol and chloroform were from Baierdi Company (Beijing, China).
Figure 1.
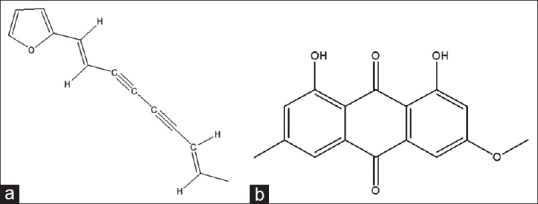
Chemical structures of (a) atractylodin and (b) physcion
Liquid chromatographic condition
The liquid chromatographic (LC) system consisted of an LC-10AD pump (Shimadzu, Kyoto, Japan) with a 20 μL loop (Cotata, CA, USA) and an SPD-10 A ultraviolet-visible detector (Shimadzu, Kyoto, Japan). A LC-10AD workstation was used for data acquisition. A Diamonsil C18 analytical column (250 mm × 4.6 mm; 5 μm) from Dikma Technologies (Dalian, China) was used. The mobile phase consisted of acetonitrile and water (76:24, v/v), and it was delivered with the flow rate of 1 mL/min. The detection wavelength was set at 340 nm. All the measurements were performed at 25°C, and the sample injection volume was 20 μL.
Preparation of Atractylodes rhizoma ethanol extract
A. rhizoma (50 g) was crushed into powder and soaked into 600 mL of 95% ethanol for 24 h and then percolated at 2 mL/min. Ethanol was evaporated to near dryness under reduced pressure to get the residue. Distilled water was added to the residue and then vortexed. The final concentration of A. rhizoma solution was 2 g/mL.[9] The sample was stored in dry and dark place before use.
Animals
All the studies on animals were in accordance with the Guidelines for the Care and Use of Laboratory Animals. Healthy Sprague-Dawley rats (250 ± 20 g) were purchased from The Medical University of Benxi (Benxi, China) and acclimated in the laboratory for 1-week before to the experiments. The rats were maintained in an air-conditioned animal quarter at a temperature of 22°C ± 2°C and a relative humidity of 50% ± 10%. Rats for oral ingestion were fasted for 12 h with free access to water. Rats were oral administration crude and processed A. rhizoma at a single dose of 40 g/kg (The concentration of the extracted sample solution was 2 g/mL), respectively.
In vivo pharmacokinetic study of atractylodin
Drug administration and blood sampling
For plasma samples, 18 rats were randomly assigned to three groups for pharmacokinetic investigation (n = 6/group). The blood sample (0.3 mL) was collected at 0, 0.17, 0.33, 0.5, 1, 2, 3, 4, 6, 8, and 12 h. All samples were immediately transferred into heparinized tubes and centrifuged for 5 min at 10,000 rpm. The supernatant was stored at −20°C and dark place until analysis.
Preparation of plasma sample
For plasma samples, the 200 μL of rat plasma was mixed with 20 μL IS. After the protein was precipitated with 1000 μL of acetonitrile in 1.5 mL polypropylene tube by vortexing for 2 min, the sample was centrifuged at 10,000 rpm for 5 min. The supernatant was transferred into a 5.0 mL tube and was added with 1000 μL of chloroform, extract and the under organic phase was transferred to another tube and evaporated to dryness at 40°C with nitrogen. The resulting extract was dissolved in 50 μL of methanol, and vortex mixed for 2 min. After centrifugation at 10,000 rpm for 5 min, 20 μL supernatant was injected for analysis.[10] For each time, 20 μL was injected into HPLC system. Then, the concentration of atractylodin was determined.
Method validation
Specificity
The selectivity of the method was demonstrated by comparing chromatograms of blank plasma samples (without IS) obtained from rats, plasma samples spiked with the analytes and IS, and plasma samples after an oral dose. All blank plasma samples were prepared and analyzed to ensure the absence of interfering peaks.
Calibration cure
The standard curve in the plasma of atractylodin was linear in the range from 0.029 to 5.800 μg/mL.
Recovery and stability
The extraction recoveries of atractylodin were determined at the low, medium, and high level of quality control (QC) samples. Recoveries were calculated by comparing the observed peak area ratios in bio-samples to those nonprocessed standard solutions at the same.
The stability of atractylodin in plasma was determined under different storage or handling conditions. Short-term stability was assessed by analyzing QC samples kept at room temperature for 8 h. Freeze-thaw stability was evaluated at three consecutive freeze-thaw cycles. Long-term stability was studied by assaying samples following a period of 10-day of storage at −20°C.
Precision
The precision of the method were assessed by determination of quality control samples (n = 5) on three different validation days. To determine intra-day precision, the assays were carried out on standard solutions of the analyte at different times during the same day. Inter-day precision was determined by assaying the standard solutions of the analyte over three consecutive days.
Data analyses
Data were processed by noncompartmental method using Drug and Statistics 2.0 Software Package (Chinese Pharmacological Society, Shanghai, China).
RESULTS
Method validation
Specificity
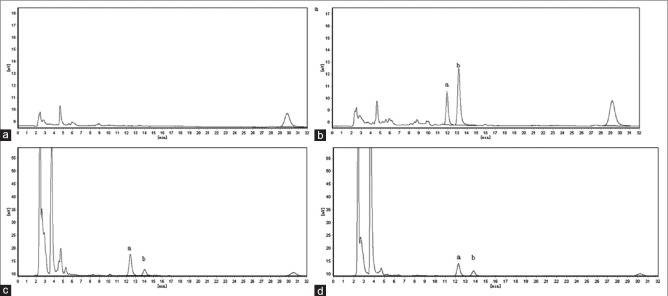
Figure 2 shows that no interference peaks from endogenous constituents were detected.
Figure 2.
Chromatograms of blank plasma (a); blank plasma spiked with atractylodin 20 µL and IS 20 µL (b); rat plasma sample (1-h) after oral administration of crude A. rhizoma 40 g/kg (c); rat plasma sample (1-h) after oral administration of processed A. rhizoma 40 g/kg (d)
Linearity of calibration curve
The calibration curves were linear in the determined concentration ranges. And the detection limited for analyses in the range 0.029–5.800 μg/ml for atractylodin with a correlation coefficient at 0.9932. The mean standard curves were typically described by the equations: Y =1.292X + 0.033 for atractylodin.
Recovery and stability
The recoveries of atractylodin at each quality control level 2.900, 0.580, 0.029 μg/mL were 83.39% ± 6.97%, 80.92% ± 7.17%, and 81.71% ± 4.82%, respectively. The extraction recoveries determined for atractylodin were consistent and reproducible. Stability of analysis showed no significant sample loss over 12 h at room temperature, three freeze-thaw cycles, and 10 days storage condition. The RE of three conditions was within ± 15%.
Precision and accuracy
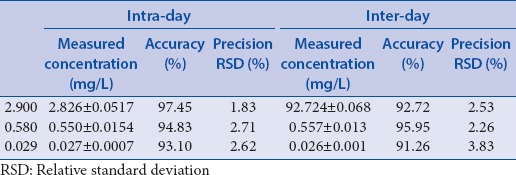
The precision data for atractylodin was given in Table 1.
Table 1.
The precision data of atractylodin
Pharmacokinetics study
Plasma concentrations of atractylodin were determined after oral administration of crude and processed A. rhizoma. The pharmacokinetic parameters were given in Table 2. As shown in Figures 3 and 4, Table 2, there were significant differences of Cmax, Tmax, AUC0 → t, AUC0→∞ and CL.
Table 2.
The pharmacokinetic parameters of atractylodin of crude and processed Atractylodis rhizoma at a dose of 40 g/kg to rats, respectively
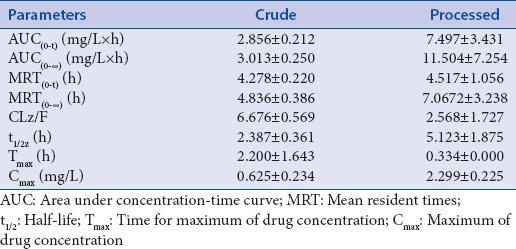
Figure 3.
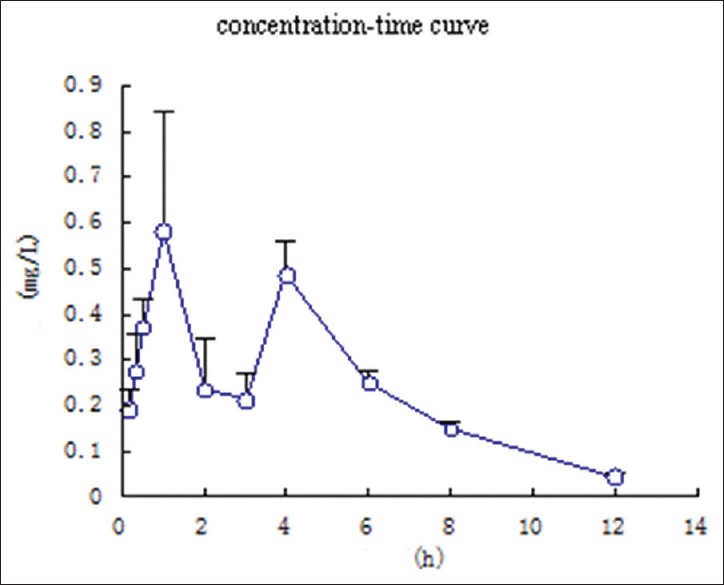
Mean plasma concentration-time curve of atractylodin after oral administration of crude Atractylodis rhizoma (40 g/kg) to rats. (Mean ± standard deviation, n = 6)
Figure 4.
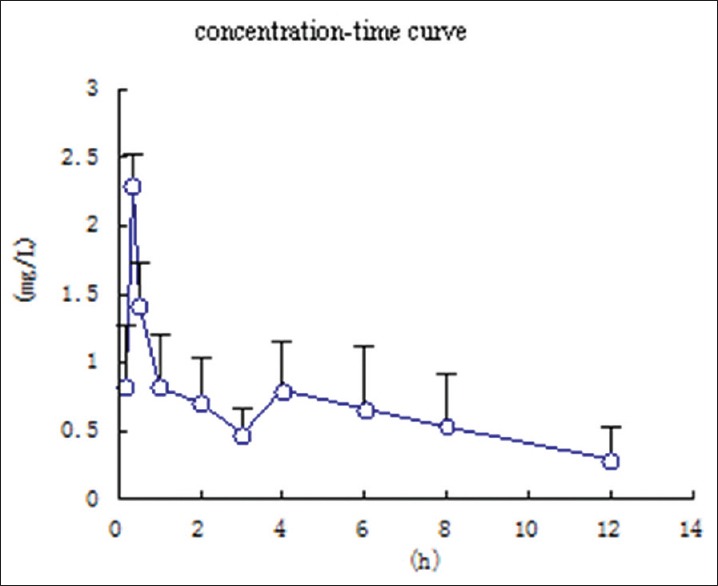
Mean plasma concentration-time curve of atractylodin after oral administration of processed Atractylodis rhizoma (40 g/kg) to rats. (Mean ± standard deviation, n = 6)
DISCUSSION
The assay was applied to a preliminary pharmacokinetic experiment in the rat after oral administration of 40 g/kg crude and processed A. rhizoma to rats, respectively. Mean concentration-time curves were shown in Figures 3 and 4. The pharmacokinetic parameters were shown in Table 2.
A significant result of this study was found that atractylodin showed double peaks after oral administration, which demonstrated that a hepatoenteral circulation may exist. For after oral crude A. rhizoma the absorption peaks in rat plasma at 1.0 h and 4.0 h, respectively. The Cmax was 0.625 ± 0.234 mg/L. And for after oral processed A. rhizoma the absorption peaks at 0.34 h and 4.0 h, respectively. The Cmax is 2.299 ± 0.225 mg/L. So, the time of absorption peak was 0.67 h in advanced and the concentration of rat plasma was increased after processing. The value of Tmax and T1/2 indicated that the atractylodin was rapidly distributed but slowly eliminated. The reason for this result also requires further study.
CONCLUSION
A simple, specific and rapid reversed phase-HPLC method with UV detection for quantification of atractylodin in rat plasma has been developed for the first time. It has been successfully applied to a preliminary pharmacokinetic study of atractylodin after oral administration of 40 g/kg crude and processed A. rhizoma respectively. The data have a significant difference (P < 0.05). The result indicates that processing A. rhizoma with wheat bran by stir-frying can promote and accelerate the absorption of atractylodin.
Financial support and sponsorship
This project was supported by grants from the National Natural Science Foundation of China (No. 81202919) and the Special Scientific Research for Traditional Chinese Medicine of State Administration of Traditional Chinese Medicine of China (No. 20110700712). Fund project: The state administration of traditional Chinese medicine of traditional Chinese Medicine Industry Special Scientific Research projects (20110700712); the National Natural Science fund project (81202919).
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Qian Cai
Qian Cai, Professor of Medicine, Liaoning University of Traditional Chinese Medicine, Dalian, China.
Acknowledgement
This project was supported by grants from the National Natural Science Foundation of China (No. 81202919) and the Special Scientific Research for Traditional Chinese Medicine of State Administration of Traditional Chinese Medicine of China (No. 20110700712).
REFERENCES
- 1.Beijing, China: Chinese Medicine Science and Technology Publishing House; 2010. Chinese Pharmacopoeia Committee. Pharmacopeia of People's Republic of China. [Google Scholar]
- 2.Pu SS, Pu ZX, Yuan ST. Study on processing historical evolution of Atractylodes lancea. Chin Pharm J. 2000;35:87–91. [Google Scholar]
- 3.Liu YJ, Xu LY, Yuan ST. Study on processing technology of stir-frying Atractylodes with wheat bran. Chin J Hosp Pharm. 2009;29:1267–8. [Google Scholar]
- 4.Resch M, Steigel A, Chen ZL, Bauer R. 5-Lipoxygenase and cyclooxygenase-1 inhibitory active compounds from Atractylodes lancea. J Nat Prod. 1998;61:347–50. doi: 10.1021/np970430b. [DOI] [PubMed] [Google Scholar]
- 5.Resch M, Heilmann J, Steigel A, Bauer R. Further phenols and polyacetylenes from the rhizomes of Atractylodes lancea and their anti-inflammatory activity. Planta Med. 2001;67:437–42. doi: 10.1055/s-2001-15817. [DOI] [PubMed] [Google Scholar]
- 6.Fang CW, Fan M, Liu SJ, Pan LB. Simultaneous determination of three acetylenic polyethylene components in Atractylodes lancea. Chin Med Mater. 2010;33:932–4. [Google Scholar]
- 7.Liu YQ, Jia TZ, Cai Q. Determination of three components in stir-frying Atractylodes rhizoma with and without bran by HPLC. Chin Tradit Pat Med. 2013;35:131–5. [Google Scholar]
- 8.Chen Y, Chou G, Wang Z. Simultaneous determination of polyacetylene components in Cangzhu by reversed-phase high performance liquid chromatography. Se Pu. 2007;25:84–7. [PubMed] [Google Scholar]
- 9.Zhang YS, Wang ZM, Zhu JJ, Chen B, Li YQ. Determination of atractylodin in rat plasma by HPLC-UV method and its application to a pharmacokinetic study. J Liq Chromatogr Relat Technol. 2012;35:778–87. [Google Scholar]
- 10.Liu DF, Zhang L, Chen T. Pharmacokinetics Study on Paeoniflorin in Silli San. China J Exp Tradit Med Formulae. 2005;11:36–8. [Google Scholar]