Abstract
Background
The aim of this study was to determine the expression of apoptotic factors Bax, Bcl-2, and Caspase-3 in lens epithelial cells (LECs) from cataracts secondary to pars plana vitrectomy with silicone oil (SO) tamponade. We also investigated the impact of SO emulsification on the expression of apoptotic factors.
Material/Methods
Anterior capsulotomy specimens of 20 eyes in 20 patients with cataract secondary to SO tamponade (Group 2), were collected. Another 20 eyes of 20 patients with age-related cataract (Group 1) were recruited as controls. The anterior capsule specimens were obtained from the patients during cataract surgery, frozen and later analyzed with respect to immunohistochemical stains of Bax, Bcl-2, and Caspase-3 using a confocal microscope.
Results
Age, sex, and laterality did not show any difference between the 2 groups. There was a greater increase in Bax and Caspase-3 expression in LEC in Group 2 than in Group 1 (PBax<0.0001, PCaspase-3<0.0001). The Bcl-2 expression decreased in Group 2, although the difference was not significant (P=0.616). The changes of apoptosis factors are not associated to SO emulsification (PBax=0.354, PBcl-2=0.728, PCaspase-3=0.939).
Conclusions
The caspase-3-dependent apoptosis of LECs increased in complicated cataract patients with SO endotamponade. The Bax played a critical role in regulating apoptosis of LECs in vitrectomized eyes with SO tamponade. The SO emulsification had no significant impact on the expression of apoptosis factors.
MeSH Keywords: Apoptosis Regulatory Proteins, Cataract, Epithelial Cells
Background
Vitrectomy, which is the surgical removal of vitreous, has become a standard treatment for various vitreoretinal diseases. Vitrectomy surgery can significantly increase intraocular oxygen tension, and the abnormal exposure of the crystalline lens to high oxygen levels may lead to nuclear cataract formation [1]. Silicone oil (SO) is frequently used in vitrectomy. Vitrectomy with SO tamponade can produce a transient posterior subcapsular cataract postoperatively, which is resolved and followed by accelerated nuclear opacification [2]. Both direct contact by SO [3] and obstruction of normal metabolic exchange at the silicone-lens interface [4] can result in cataract formation. Accordingly, the cataract is one of the most common complications of pars plana vitrectomy with SO endotamponade, which is mainly caused by oxidative stress [3]. The occurrence or progression of a cataract can deteriorate visual acuity again after vitreoretinal surgery for phakic eyes and the incidence is about 60–98% within 2 years [5,6].
Lens epithelial cells (LECs) are the most active parts of the lens metabolism and their apoptosis is a common cellular basis for noncongenital cataract development in human and animals [7,8]. The relationship between oxidative stress and apoptosis has been widely studied [9,10]. Oxidative stress induced by H2O2 can cause cell viability decrease and apoptosis in human LECs [11]. Currently, there is no clear evidence of cataractogenesis after vitrectomy with SO endotamponade.
The aim of our study was to investigate the expression of apoptotic factors (Bax, Bcl-2, and Caspase-3) and to determine the effect of SO emulsification on them in the LECs of vitrectomized eyes with SO tamponade.
Material and Methods
A prospective study of patients who visited our medical center for phacoemulsification combined with SO removal from January to July 2013 was conducted. Twenty consecutive patients were included. Previous vitreoretinal surgery with SO instillation was performed for the rhegmatogenous retinal detachment (RRD) with proliferative vitreoretinopathy. Patients who had uveitis or intraocular hypertension after vitreoretinal surgery or who had coexisting ocular diseases were excluded from this study. A control group comprised 20 consecutive cases of age-related cataract (ARC) without history of systemic or other ocular diseases. The cataract grade was 2 to 3 according to LOCS III classification [12]. Demographic and clinical characteristics were collected from all case records.
In RRD cases, the patients underwent vitreoretinal surgery with 23-gauge standard sclerotomies, followed by injections with silicone oil (Oxane 5700; Bausch & Lomb, Rochester, NY, USA).
SO tamponade was performed to ensure intraocular pressure of about 20 mmHg when the procedure was finished, which ranged from 4 ml to 5 ml. SO removal and cataract extraction by phacoemulsification with intraocular lens implantation were accomplished in the same operation. Visible particles of SO observed via gonioscopy and slit-lamp biomicroscopy were regarded as SO emulsification. In ARC cases, the patients underwent cataract extraction and intraocular lens implantation. All the surgeries were uneventful and were performed by the same surgeon (DH Lou).
The anterior capsule epithelium specimens were obtained, immediately fixed in 4% paraformaldehyde (PFA), and stored at 4°C in a refrigerator. The PFA-fixed anterior capsules were washed with 0.1% Tween and phosphate-buffered saline (PBS) for 5 min 4 times. They were transfected with 0.5% TritonX100, followed by washing again with 0.1% Tween 20 and PBS. After that, they were blocked with Block Solution (PBS, 10% serum) for 25 min. Each anterior capsular was split into 2 pieces. One piece was incubated overnight with the primary antibodies (Bax and Bcl-2, Abcam) (1:100 dilution) and the other piece was incubated overnight with the primary antibody (Caspase-3, Abcam) (1:100 dilution) at 4°C. The capsules were incubated with their appropriate secondary antibody (Abcam) (1:100 dilution) at room temperature for 1.5 h. After they were washed with 0.1% Tween 20 and PBS for 15 min, they were stained with Hoechst33342 (Life technologies Corp.) to allow visualization of the nuclei. The capsules were mounted on glass slides and analyzed with a confocal microscope (Nikon A1).
The number of immunopositive cells was counted in 4 representative fields. An eyepiece-calibrated grid with ×10 magnification and an objective calibrated grid with ×60 magnification were used. At this magnification, the total number of cells in 1 microscopic field was counted and the percentage of positively stained cells was determined (positive cells/total cells ×100%). The average of the 4 fields was calculated for Bax, Bcl-2, and Caspase-3, and then analyzed for scoring. The scoring system was based on a scale of 0 to 4+ as follows [13]: 4+=very high (75% to 100% positive cells); 3+=high (50% to 75% positive cells); 2+=moderate (25% to 50% positive cells); 1+=low (<25% positive cells); 0=negative (0 positive cells).
All analyses were performed using the Statistical Package for Social Sciences (SPSS) for windows, Version 19.0. The Pearson’s chi-squared test, Mann-Whitney U test, and independent-samples t test were used for statistical analysis. A P value of less than 0.05 was considered statistically significant. This study was approved by the hospital Ethics Committee and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. No patient information was extracted except for research purposes.
Results
There were 13 females (65%) and 7 males (35%) whose average age was 61.60±6.81 (SD) years (range, 50 to 78 years) in the cataract secondary to SO tamponade group. The left eye was affected in 13 cases and the right eye was affected in 7 cases. The duration of SO endotamponade ranged from 12 to 52 weeks (22.95±8.90 weeks). The ARC group consisted of 11 females (55%) and 9 males (45%) whose average age was 65.15±6.64 (SD) years (range, 56 to 76 years). The left eye was affected in 9 cases and the right eye was affected in 11 cases. There was no significant difference in age, sex and laterality between the 2 studied groups (P>.05) (Table 1).
Table 1.
Characteristics of the patients in this study.
| Variables | Group 1 | Group 2 | P value |
|---|---|---|---|
| Age (mean years ±SD) | 65.15±6.64 | 61.60±6.81 | 0.112 |
| Gender (M/F) | 9/11 | 7/13 | 0.519 |
| Laterality (R/L) | 7/13 | 11/9 | 0.204 |
Group 1 – age-related cataract; Group 2 – cataract with SO endotamponade; SO – silicone oil.
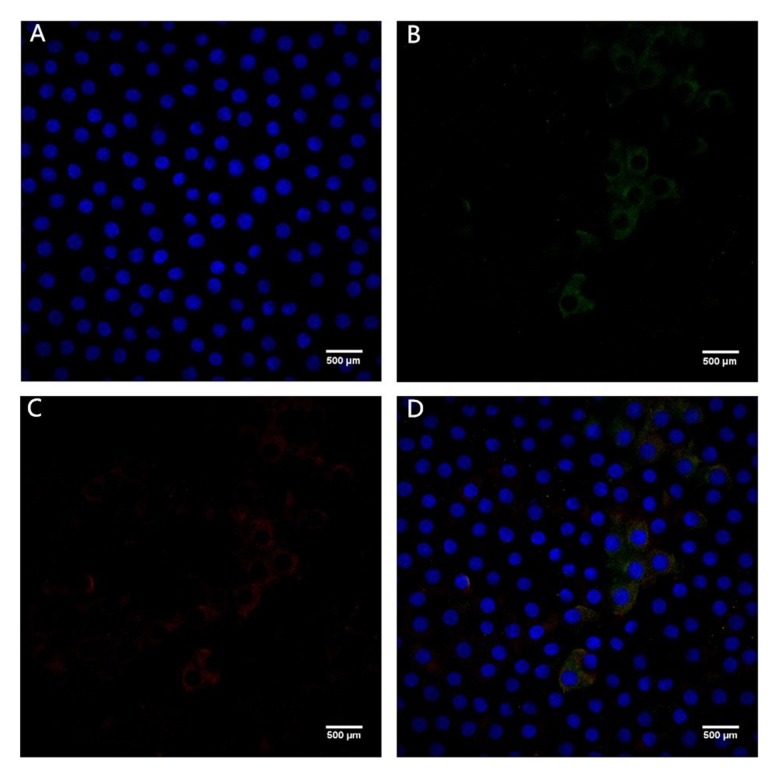
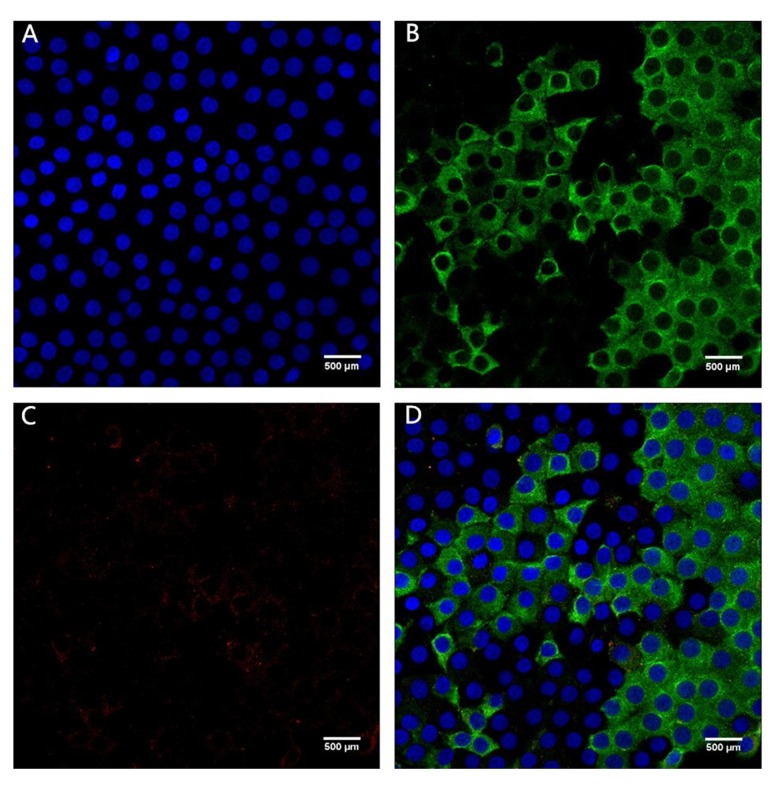
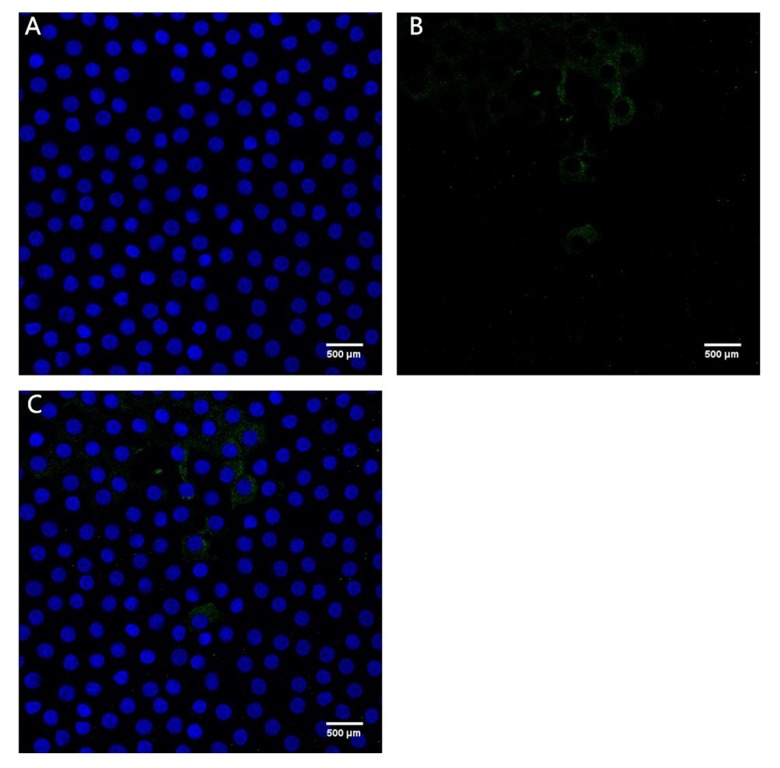
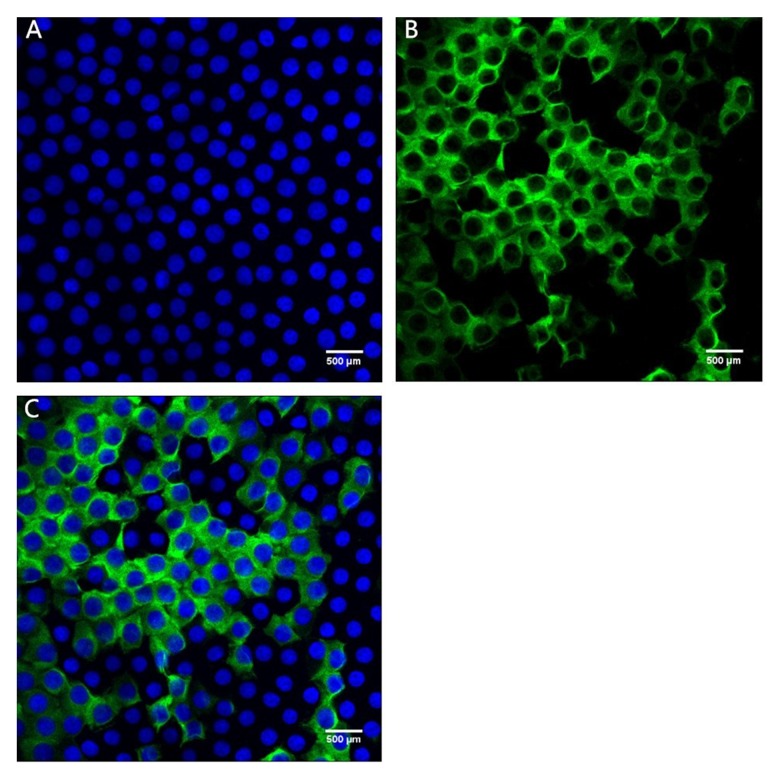
Figures 1 and 2 show confocal immunofluorescent images of stained Bax and Bcl-2 in LECs of the 2 groups. Bax was weakly stained (1+) in the ARC group and strongly stained (3+) in the cataract with SO endotamponade group. Bcl-2 was weakly stained (1+) in both groups. Figures 3 and 4 show confocal immunofluorescent images of stained Caspase-3 in LECs of the 2 groups. Caspase-3 was weakly stained (1+) in the ARC group and strongly stained (3+) in the cataract with SO endotamponade group.
Figure 1.
Confocal immunofluorescent image of LECs in Group 1 (age-related cataract group). (A) Blue nuclei visualized using Hoechst stain. (B) Bax is weakly stained (1+) (green). (C) Bcl-2 is weakly stained (1+) (red). (D) Merged image shows weak (1+) Bax and Bcl-2 expression (original magnification ×600).
Figure 2.
Confocal immunofluorescent image of LECs in Group 2 (cataract with SO endotamponade group). (A) Blue nuclei visualized using Hoechst stain. (B) Bax is strongly stained (3+) (green). (C) Bcl-2 is weakly stained (1+) (red). (D) Merged image shows strong (3+) Bax and weak (1+) Bcl-2 expression (original magnification ×600).
Figure 3.
Confocal immunofluorescent image of LECs in Group 1 (age-related cataract group). (A) Blue nuclei visualized using Hoechst stain. (B) Caspase-3 is weakly stained (1+) (green). (C) Merged image shows weak (1+) Caspase-3 expression (original magnification ×600).
Figure 4.
Confocal immunofluorescent image of LECs in Group 2 (cataract with SO endotamponade group). (A) Blue nuclei visualized using Hoechst stain. (B) Caspase-3 is strongly stained (3+) (green). (C) Merged image shows strong (3+) Caspase-3 expression (original magnification ×600).
Table 2 shows the immunohistochemical scoring of Bax, Bcl-2, and Caspase-3 in the ARC group and the cataract with SO endotamponade group (Bax, 0: 0 vs. 0, 1+: 9 vs. 0, 2+: 10 vs. 5, 3+: 1 vs. 10, 4+: 0 vs. 5; Bcl-2, 0: 0 vs. 2, 1+: 17 vs. 17, 2+: 3 vs. 1, 3+: 0 vs. 0, 4+: 0 vs. 0; Caspase-3, 0: 0 vs. 0, 1+: 13 vs. 0, 2+: 7 vs. 7, 3+: 0 vs. 9, 4+: 0 vs. 4). As compared to ARC, there was a statistically significant increase of Bax and Caspase-3 expression in the cataract with SO endotamponade group (PBax<0.001; PCaspase-3<0.001) (Table 3). There was no significant difference of Bcl-2 expression between the 2 groups (P>.05) (Table 3).
Table 2.
Immunohistochemical scoring for the apoptotic factors Bax, Bcl-2 and Caspase-3 in LECs from two groups.
| Score | 0 | 1+ | 2+ | 3+ | 4+ | |
|---|---|---|---|---|---|---|
| Bax | Group 1 (n) | – | 9 | 10 | 1 | – |
| Group 2 (n) | – | – | 5 | 10 | 5 | |
| Bcl-2 | Group 1 (n) | – | 17 | 3 | – | – |
| Group 2 (n) | 2 | 17 | 1 | – | – | |
| Caspase-3 | Group 1 (n) | – | 13 | 7 | – | – |
| Group 2 (n) | – | – | 7 | 9 | 4 |
Group 1 – age-related cataract; Group 2 – cataract with SO endotamponade; SO – silicone oil.
Table 3.
Immunohistochemical analysis for the apoptotic factors Bax, Bcl-2 and Caspase-3 in LECs from two groups.
| Factor | Mean positive cells (%) ±SD | P Value | |
|---|---|---|---|
| Group 1 | Group 2 | ||
| Bcl-2 | 11.80±9.17 | 10.05±7.88 | 0.616 |
| Bax | 25.35±11.91 | 63.30±16.92 | <0.0001 |
| Caspase-3 | 21.15±10.49 | 58.10±19.90 | <0.0001 |
Group 1 – age-related cataract; Group 2 – cataract with SO endotamponade; SO – silicone oil.
To further clarify the effect of clinical characteristics on apoptosis factors in LECs, we analyzed the differences in Bax, Bcl-2, and Caspase-3 distribution between the patients with emulsified SO and those without emulsified SO (Table 4). Eight patients had intraocular SO emulsification in the cataract with SO endotamponade group. The distribution of apoptotic factors was: Bax, 0: 0 vs. 0, 1+: 0 vs. 0, 2+: 4 vs. 1, 3+: 2 vs. 8, 4+: 2 vs. 3; Bcl-2, 0: 1 vs. 1, 1+: 7 vs. 10, 2+: 0 vs. 1, 3+: 0 vs. 0, 4+: 0 vs. 0 and Caspase-3, 0: 0 vs. 0, 1+: 0 vs. 0, 2+: 3 vs. 4, 3+: 4 vs. 5, 4+: 1 vs. 3. However, no significant difference in the expression of the 3 apoptosis factors (Bax, Bcl-2, and Caspase-3) was found between patients with emulsified SO and those without emulsified SO (P>.05) (Table 5).
Table 4.
Emulsification of SO and immunohistochemical scoring for Bcl-2, Bax and Caspase-3 in LECs from patients with and without emulsified SO.
| Score | 0 | 1+ | 2+ | 3+ | 4+ | |
|---|---|---|---|---|---|---|
| Bax | With emulsified SO(n) | – | – | 4 | 2 | 2 |
| Without emulsified SO (n) | – | – | 1 | 8 | 3 | |
| Bcl-2 | With emulsified SO(n) | 1 | 7 | – | – | – |
| Without emulsified SO (n) | 1 | 10 | 1 | – | – | |
| Caspase-3 | With emulsified SO(n) | – | – | 3 | 4 | 1 |
| Without emulsified SO (n) | – | – | 4 | 5 | 3 |
SO – silicone oil.
Table 5.
Immunohistochemical analysis for the apoptotic factors Bax, Bcl-2 and Caspase-3 in LECs from patients with and without emulsified SO.
| Factor | Mean positive cells (%) ±SD | P Value | |
|---|---|---|---|
| With emulsified SO | Without emulsified SO | ||
| Bcl-2 | 11.25±9.19 | 9.25±7.20 | 0.728 |
| Bax | 58.88±22.35 | 66.25±12.37 | 0.354 |
| Caspase-3 | 58.75±20.43 | 57.67±20.46 | 0.939 |
SO – silicone oil.
Discussion
The complicated cataract is the most common complication after vitrectomy, especially with intraocular tamponades. This kind of cataract not only leads to decreased vision, but also impairs the fundus observation. Injecting silicone oil into the human eye is likely to cause cataract formation [14]. It was reported that cataracts developed in all phakic eyes with silicone oil tamponade after vitrectomy within 6 months [15].
LEC apoptosis, which is a common cellular basis for noncongenital cataract development [7], can be induced by many factors, such as oxidative stress and hyperglycemia [16–19]. The increased apoptosis of hLECs and intracellular reactive oxygen species (ROS) were observed when the hLECs were incubated with high galactose [20]. Boyun Kim et al. [21] reported that the mean percentage of Bax-immunopositive cells was significantly higher in patients with DM than in those without DM, indicating that the apoptosis of LECs was higher in cataract patients with DM.
Vitrectomy surgery can markedly increase intraocular oxygen tension for prolonged periods after surgery, and this exposes the crystalline lens to abnormally high oxygen [22]. Studies have found that the oxidation of lens protein during vitrectomy was one of the reasons for nuclear cataract after vitrectomy [23], whereas adding antioxidants into the perfusion fluid during vitrectomy could inhibit the abnormal lens epithelial cell growth and lens fiber formation and thus prevent the cataractogenesis [24]. Our study shows that the expression of Bax and caspase-3 is significantly higher in cataracts secondary to SO tamponade than in age-related cataracts. To the best of our knowledge, this is the first study to describe the Bax/Bcl-2 and caspase-3 distribution of in vivo hLECs in vitrectomized eyes with SO tamponade.
The Bcl-2 family comprises antiapoptotic proteins (e.g., Bcl-2, Bcl-XL, and Mcl-1) and proapoptotic proteins (e.g., Bax, Bak, and Bad) [25]. Bax/Bcl-2 are 2 important pro- and anti-apoptotic proteins that influence cell fate. Overexpressed Bax counters the death repressor activity of Bcl-2 and accelerates apoptotic death induced by cytokine deprivation [26]. During apoptosis, Bax is activated and translocated from the cytosol into mitochondria, which can be better inhibited by R21A [27]. Our study demonstrated that the expression of Bax increased significantly in complicated cataracts secondary to SO tamponade. Hence, inhibition of Bax overexpression might be used to prevent this type of cataract formation.
Caspases are important signs of apoptosis and caspase-3 is a major downstream effector caspase in the apoptotic process of LECs [28,29]. Andersson et al. [29] proposed that posterior subcapsular cataracts result from defective differentiation, in which caspase-3-dependent apoptosis may play an important role. In our studies, the protein levels of caspase-3 were elevated after vitrectomy with silicone oil tamponade, suggesting that caspase-3 is involved in apoptosis of LECs after par plana vitrectomy.
Emulsification is a clinically significant complication of using SO tamponade [30].To the best of our knowledge, there have been no studies on the effect of SO emulsification on complicated cataracts; therefore, we cannot ascertain whether complicated cataracts are related to SO emulsification in vitrectomized eyes or to SO tamponade. One study revealed that emulsified silicone may potentially enhance opacification of residual anterior capsule following pars plana vitrectomy by silicone oil deposition and subsequent activation of LECs [31]. However, the present study is the first to show no significant difference in the expression of 3 apoptosis factors (Bax, Bcl-2, and Caspase-3) between patients with emulsified SO and those without emulsified SO.
Although we found no difference in apoptosis factors expression in LECs between the 2 groups, several known complications of emulsified SO, such as keratopathy [32], glaucoma [33] and retina toxicity [34], have been reported. According to the literature [35], the duration of SO tamponade remains the most significant risk factor for SO emulsification. The mean silicone oil removal time in our cases (22.95±8.90 weeks) was longer than that of a previous study (4.4±1.1 months) [36]. Three patients lost to follow-up with longer duration (52, 35, and 30 weeks) prolonged SO removal time in our cases. Follow-up visits and timely treatment were hampered by poverty and lack of health awareness in these 3 patients, who were all low-income people. More extensive education for patients who undergo vitreoretinal surgery with SO tamponade is necessary, especially in developing countries.
Our results may have been affected by the fact that this was a non-randomized, small case series in patients with advanced age. Tang et al. [37] found that activation of the unfolded protein response-induced ROS production suppressed the antioxidant status and triggered the apoptotic pathway, ultimately leading to the formation of age-related cataracts. We found that Bcl-2 was expressed weakly in all these cataract cases. It is possible that advanced age was a limitation of our study, but fortunately there was no significant difference in age between the 2 studied groups (P>.05). In our study, particles of silicone oil were observed via gonioscopy and slit-lamp biomicroscopy by the same ophthalmologist. The Coulter counter can detect emulsified SO droplets below 2 μm size, which are difficult see in slit-lamp biomicroscopy [38]. And meanwhile, the study also found a correlation between the number of droplets between 7 and 30 μm and the number of droplets less than 2 μm in diameter [38]. We speculate that the large visible droplets reflected the extent of SO emulsification. Hence, we believe that these limitations did not significantly affect the major findings of this study.
In summary, the expression of Bax and Caspase-3 was remarkably higher in cataracts secondary to SO tamponade vs. ARC. These results suggest that caspase-3 dependent apoptosis contributes to complicated cataracts with SO tamponade after vitrectomy, which is regulated by Bax/bal-2.
Conclusions
Although the exact pathogenesis in vitrectomized eyes with SO tamponade is unclear, our results raised the possibility that use of antioxidant would be beneficial for preventing cataractogenesis. Further studies will be required to explore the abnormal signaling in LECs and to investigate the potential antiapoptotic targets for the development of complicated cataracts in vitrectomized eye with SO tamponade.
Footnotes
Conflict of interest
No conflicting relationship exists for any author.
Source of support: Health and Family planning Comission of Zhejiang Province, P.R. China. Grant No. 2016138988 and No. 201475932
References
- 1.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: A possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139:302–10. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Hsuan JD, Brown NA, Bron AJ, et al. Posterior subcapsular and nuclear cataract after vitrectomy. J Cataract Refract Surg. 2001;27:437–44. doi: 10.1016/s0886-3350(00)00585-x. [DOI] [PubMed] [Google Scholar]
- 3.Milazzo S. Pathogenesis of cataract after vitrectomy. J Fr Ophtalmol. 2014;37:243–44. doi: 10.1016/j.jfo.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Leaver PK, Grey RH, Garner A. Silicone oil injection in the treatment of massive preretinal retraction. II. Late complications in 93 eyes. Br J Ophthalmol. 1979;63:361–67. doi: 10.1136/bjo.63.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson JT, Glaser BM, Sjaarda RN, Murphy RP. Progression of nuclear sclerosis and long-termvisual results of vitrectomy with transforming growth factor beta-2 for macular holes. Am J Ophthalmol. 1995;119:48–54. doi: 10.1016/s0002-9394(14)73812-7. [DOI] [PubMed] [Google Scholar]
- 6.Abu El-Asrar AM, Al-Kwikbi HF, Kangave D. Prognostic factors after primary vitrectomy and perfluorocarbon liquids for bullous rhegmatogenous retinal detachment. Eur J Ophthalmol. 2009;19:107–17. doi: 10.1177/112067210901900116. [DOI] [PubMed] [Google Scholar]
- 7.Li WC, Kuszak JR, Dunn K, et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995;130:169–81. doi: 10.1083/jcb.130.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Kim CS, Sohn E, et al. Lens epithelial cell apoptosis initiates diabetic cataractogenesis in the Zucker diabetic fatty rat. Graefes Arch Clin Exp Ophthalmol. 2010;248:811–18. doi: 10.1007/s00417-010-1313-1. [DOI] [PubMed] [Google Scholar]
- 9.Manuele MG, Barreiro Arcos ML, Davicino R, et al. Limonene exerts antiproliferative effects and increases nitric oxide levels on a lymphoma cell line by dual mechanism of the ERK pathway: Relationship with oxidative stress. Cancer Invest. 2010;28:135–45. doi: 10.3109/07357900903179583. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhary SC, Siddiqui MS, Athar M, Alam MS. D-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum Exp Toxicol. 2012;31:798–811. doi: 10.1177/0960327111434948. [DOI] [PubMed] [Google Scholar]
- 11.Zhou YF, Guo B, Ye MJ, et al. Protective effect of rutin against H2O2-induced oxidative stress and apoptosis in human lens epithelial cells. Curr Eye Res. 2015;17:1–10. doi: 10.3109/02713683.2015.1082186. [DOI] [PubMed] [Google Scholar]
- 12.Chylack LT, Jr, Wolfe JK, Singer DM, et al. for the Longitudinal Study of Cataract Study Group. The Lens Opacities Classification System III. Arch Ophthalmol. 1993;111:831–36. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 13.Vorkauf M, Duncker G, Nölle B, Sterry W. Adhesion molecules in normal human conjunctiva; an immunohistological study using monoclonal antibodies. Graefes Arch Clin Exp Ophthalmol. 1993;231:323–30. doi: 10.1007/BF00919028. [DOI] [PubMed] [Google Scholar]
- 14.Riedel KG, Gabel VP, Neubauer L, et al. Intravitreal silicone oil injection: complications and treatment of 415 consecutive patients. Graefes Arch Clin Exp Ophthalmol. 1990;228:19–23. doi: 10.1007/BF02764284. [DOI] [PubMed] [Google Scholar]
- 15.Federman JL, Schubert HD. Complications associated with the use of silicone oil in 150 eyes after retina-vitreous surgery. Ophthalmology. 1988;95:870–76. doi: 10.1016/s0161-6420(88)33080-0. [DOI] [PubMed] [Google Scholar]
- 16.Li WC, Spector A. Lens epithelial cell apoptosis is an early event in the development of UVB-induced cataract. Free Radic Biol Med. 1996;20:301–11. doi: 10.1016/0891-5849(96)02050-3. [DOI] [PubMed] [Google Scholar]
- 17.Harocopos GJ, Alvares KM, Kolker AE, Beebe DC. Human age-related cataract and lens epithelial cell death. Invest Ophthalmol Vis Sci. 1998;39:2696–706. [PubMed] [Google Scholar]
- 18.Li WC, Kuszak JR, Wang GM, et al. Calcimycin-induced lens epithelial cell apoptosis contributes to cataract formation. Exp Eye Res. 1995;61:91–98. doi: 10.1016/s0014-4835(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 19.Spector A, Wang GM, Wang RR, et al. A brief photochemically induced oxidative insult causes irreversible lens damage and cataract. II. Mechanism of action. Exp Eye Res. 1995;60:483–93. doi: 10.1016/s0014-4835(05)80063-6. [DOI] [PubMed] [Google Scholar]
- 20.Ou Y, Geng P, Liao GY, et al. Intracellular GSH and ROS levels may be related to galactose-mediated human lens epithelial cell apoptosis: Role of recombinant hirudin variant III. Chem Biol Interact. 2009;179:103–9. doi: 10.1016/j.cbi.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Kim B, Kim SY, Chung SK. Changes in apoptosis factors in lens epithelial cells of cataract patients with diabetes mellitus. J Cataract Refract Surg. 2012;38:1376–81. doi: 10.1016/j.jcrs.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Petermeier K, Szurman P, Bartz-Schmidt UK, Gekeler F. Pathophysiology of cataract formation after vitrectomy. Klin Monbl Augenheilkd. 2010;227:175–80. doi: 10.1055/s-0029-1245271. [DOI] [PubMed] [Google Scholar]
- 23.Ogura Y, Takanashi T, Ishigooka H, Ogino N. Quantitative analysis of lens changes after vitrectomy by fluorophotometry. Am J Ophthalmol. 1991;111:179–83. doi: 10.1016/s0002-9394(14)72256-1. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh S, Bishayee K, Paul A, et al. Homeopathic mother tincture of Phytolacca decandra induces apoptosis in skin melanoma cells by activating caspase-mediated signaling via reactive oxygen species elevation. J Integr Med. 2013;11:116–24. doi: 10.3736/jintegrmed2013014. [DOI] [PubMed] [Google Scholar]
- 25.Vander Heiden MG, Chandel NS, Williamson EK, et al. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–37. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 26.Pasupuleti N, Matsuyama S, Voss O, et al. The anti-apoptotic function of human αA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2010;1:e31. doi: 10.1038/cddis.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo D, Bi H, Wu Q, et al. Zinc oxide nanoparticles induce rat retinal ganglion cell damage through bcl-2, caspase-9 and caspase-12 pathways. J Nanosci Nanotechnol. 2013;13:3769–77. doi: 10.1166/jnn.2013.7169. [DOI] [PubMed] [Google Scholar]
- 28.Li DW, Xiang H, Mao YW, et al. Caspase-3 is actively involved in okadaic acid-induced lens epithelial cell apoptosis. Exp Cell Res. 2001;266:279–91. doi: 10.1006/excr.2001.5223. [DOI] [PubMed] [Google Scholar]
- 29.Andersson M, Honarvar A, Sjöstrand J, et al. Decreased caspase-3 activity in human lens epithelium from posterior subcapsular cataracts. Exp Eye Res. 2003;76:175–82. doi: 10.1016/s0014-4835(02)00283-x. [DOI] [PubMed] [Google Scholar]
- 30.Miller JB, Papakostas TD, Vavvas DG. Complications of emulsified silicone oil after retinal detachment repair. Semin Ophthalmol. 2014;29:312–18. doi: 10.3109/08820538.2014.962181. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto T, Saika S, Yamanaka A, et al. Deposition of silicone oil droplets in the residual anterior lens capsule after vitrectomy and lensectomy in rabbits. Br J Ophthalmol. 2004;88:703–7. doi: 10.1136/bjo.2003.021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iyer G, Srinivasan B, Gupta J, et al. Boston keratoprosthesis for keratopathy in eyes with retained silicone oil: A new indication. Cornea. 2011;30:1083–87. doi: 10.1097/ICO.0b013e318213a8b5. [DOI] [PubMed] [Google Scholar]
- 33.Al-Jazzaf AM, Netland PA, Charles S. Incidence and management of elevated intraocular pressure after silicone oil injection. J Glaucoma. 2005;14:40–46. doi: 10.1097/01.ijg.0000145811.62095.fa. [DOI] [PubMed] [Google Scholar]
- 34.Mrejen S, Sato T, Fisher Y, Spaide RF. Intraretinal and intra-optic nerve head silicone oil vacuoles using adaptive optics. Ophthalmic Surg Lasers Imaging Retina. 2014;45:71–73. doi: 10.3928/23258160-20131220-11. [DOI] [PubMed] [Google Scholar]
- 35.Toklu Y, Cakmak HB, Ergun SB, et al. Time course of silicone oil emulsification. Retina. 2012;32:2039–44. doi: 10.1097/IAE.0b013e3182561f98. [DOI] [PubMed] [Google Scholar]
- 36.Citirik M, Sargon MF, Has S, Bilgin S. Alterations of the anterior lens capsule in vitrectomized eyes with silicone oil tamponade. Ophthalmic Surg Lasers Imaging. 2012;43:388–94. doi: 10.3928/15428877-20120531-02. [DOI] [PubMed] [Google Scholar]
- 37.Tang HZ, Yang LM. Activation of the unfolded protein response in aged human lenses. Mol Med Rep. 2015;12:389–93. doi: 10.3892/mmr.2015.3417. [DOI] [PubMed] [Google Scholar]
- 38.Chan YK, Cheung N, Chan WS, Wong D. Quantifying silicone oil emulsification in patients: Are we only seeing the tip of the iceberg? Graefes Arch Clin Exp Ophthalmol. 2015;253:1671–75. doi: 10.1007/s00417-014-2866-1. [DOI] [PubMed] [Google Scholar]