Abstract
Objectives
To determine whether defining diurnal periods by self-report, fixed-time or actigraphy produce different estimates of nighttime and daytime ambulatory blood pressure (ABP).
Methods
Over a median of 28 days, 330 participants completed two 24-hour ABP and actigraphy monitoring periods with sleep diaries. Fixed nighttime and daytime periods were defined as 00:00–06:00 and 10:00–20:00, respectively. Using the first ABP period, within-individual differences for mean nighttime and daytime ABP and Kappa statistics for nighttime and daytime hypertension (systolic/diastolic ABP ≥120/70 mmHg and ≥135/85 mmHg, respectively) were estimated comparing self-report, fixed-time or actigraphy for defining diurnal periods. Reproducibility of ABP was also estimated.
Results
Within-individual mean differences in nighttime systolic ABP were small, suggesting little bias, when comparing the three approaches used to define diurnal periods. The distribution of differences, represented by 95% confidence intervals (CI), in nighttime systolic and diastolic ABP and daytime systolic and diastolic ABP was narrowest for self-report versus actigraphy. For example, mean differences (95% CI) in nighttime systolic ABP for self-report versus fixed-time was −0.53 (−6.61,+5.56) mmHg, self-report versus actigraphy was 0.91 (−3.61,+5.43) mmHg and fixed-time versus actigraphy was 1.43 (−5.59,+8.46) mmHg. Agreement for nighttime and daytime hypertension was highest for self-report versus actigraphy: Kappa statistic (95% CI)=0.91 (0.86,0.96) and 1.00 (0.98,1.00), respectively. The reproducibility of mean ABP and hypertension categories was similar using each approach.
Conclusion
Given the high agreement with actigraphy, these data support using self-report to define diurnal periods on ABP monitoring. Further, the use of fixed-time periods may be a reasonable alternative approach.
Keywords: ambulatory blood pressure, sleep, diurnal periods, daytime, nighttime, clinic blood pressure, blood pressure
Introduction
Ambulatory blood pressure monitoring (ABPM) measures out-of-clinic blood pressure (BP), typically over 24 hours. Using ABPM, several diurnal ambulatory BP (ABP) parameters can be derived including mean nighttime and daytime ABP (i.e., the mean of ABP readings during the nighttime and daytime periods, respectively) and BP non-dipping status (i.e., ABP ratio >0.90 between the nighttime and daytime periods).[1] Higher mean nighttime ABP, daytime ABP and BP non-dipping status have been associated with increased risk for target-organ damage, cardiovascular disease (CVD) and all-cause mortality, independent of clinic blood pressure (CBP).[1–3]
Nighttime and daytime periods on ABPM can be defined using several approaches: self-reported, fixed-time, or less commonly, actigraphy.[4–6]Few hypertension guidelines, position papers or scientific statements that recommend ABPM describe how best to assess the nighttime and daytime periods in clinical practice.[7–15] The guidelines, position papers and scientific statements that provide recommendations endorse self-report and fixed-time period approaches but there are limited empirical evidence to support their use.[1, 16–18] Whereas self-report is subject to recall bias, fixed-time periods may have limited ecological validity since the time when people go to sleep and awaken may differ between individuals and also within the same individual on separate days.[1, 6] Also, fixed-time periods may omit the typical sleep-awake transition periods[6, 19] and important BP fluctuations during the sleep-awake transition periods, including the morning BP surge which has been associated with an increased risk for CVD events in some studies.[1] Actigraphy requires a device to be worn for 24 hours and it may be difficult to employ in clinical practice.
Mean nighttime and daytime ABP and the degree of nighttime BP dipping may differ depending on which approach is used to define the daytime and nighttime periods. The current study evaluated (1) whether utilizing self-reported, fixed-time and actigraphy approaches to define the nighttime and daytime periods produce different estimates of diurnal ABP parameters, and (2) whether the reproducibility of diurnal ABP parameters differs by the approach used.
Methods
Study population
The Improving the Detection of Hypertension (IDH) study is a community-based study that enrolled adult participants primarily from the communities comprising upper Manhattan in New York City. Participants were excluded if they had any of the following: screening systolic CBP ≥160 mmHg or diastolic CBP ≥105 mmHg; evidence of secondary hypertension; were taking antihypertensive or other medications known to affect BP (i.e. steroids, tricyclic antidepressants, etc.); history of overt CVD, chronic kidney disease, liver disease, adrenal disease, thyroid disease, rheumatologic disease, hematologic disease, and cancer or dementia; were pregnant; or had a history of organ transplantation.
Between March 2011 and October 2013, 375 participants were enrolled in the IDH study. Participants who did not have complete data on CBP, ≥80% of the ABPM readings during the two planned ABPM periods, and complete sleep and awake times derived from self-report and actigraphy were excluded. After these exclusions, the current analysis included 330 participants. The Institutional Review Board at Columbia University approved the current study and this statistical analysis was approved by the Institutional Review Board at the Univesity of Alabama at Birmingham. All participants provided written informed consent.
Overview of study procedures
Sociodemographic and CVD risk factor information were collected using self-administered questionnaires and through two clinical exam visits (i.e., anthropometric measurements, bio-specimen assays). The median (25th–75th percentile) time between the two visits was 28 (23–36) days. Following each visit, participants began 24-hour ABPM and actigraphy (ABPM periods 1 and 2).
Sociodemographic and CVD risk factors
Highest level of education completed was categorized as less than versus at least a high school/general education diploma. Annual household income was categorized as less than or greater than or equal to $30,000 USD. Participants were categorized as being current smokers or non-smokers based on self-report. Alcohol use was defined as non-drinker (no weekly alcohol consumption), moderate drinker (1–14 and 1–7 alcoholic beverages per week for men and women, respectively) or heavy drinker (>14 and >7 alcoholic beverages per week for men and women, respectively). Body mass index (BMI) was calculated as weight in kilograms (kg) divided by height in meters squared. BMI was categorized as normal (≥18 and <25 kg/m2), overweight (≥25 and <30 kg/m2) and obese (≥30 kg/m2). Diabetes was defined as a self-reported prior physician diagnosis. An enzymatic colorimetric assay was used for determining fasting total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol concentrations. The Friedewald equation was used to calculate low-density lipoprotein (LDL) cholesterol.[20] Fasting glucose and hemoglobin A1c were also measured. High-sensitivity C-reactive protein (hs-CRP) was measured by particle-enhanced immunonephelometry. Urinary albumin and creatinine were measured, and the urinary albumin-to-creatinine ratio was calculated.
CBP measurement
At each of the two clinic visits, BP was measured three times by trained technicians following a standardized protocol using a mercury sphygmomanometer (Baum, Copiague, NY), appropriately sized BP-cuff and stethoscope. Prior to the first CBP measurement, participants sat for five minutes with both feet on the floor. There was at least one minute between BP measurements. Mean CBP at each visit was calculated by averaging the three systolic and diastolic measurements, separately. Clinic hypertension was defined as mean systolic CBP ≥140 mmHg or diastolic CBP ≥90 mmHg.
ABPM
ABPM and actigraphy were simultaneously performed over a 24 hour period. Each participant was fitted with an appropriate-sized ABPM arm cuff (Spacelabs Model 90207, Snoqualmie, WA). ABP was recorded every 30 minutes. The editing criteria used to exclude readings were systolic ABP <70mmHg or >260mmHg and/or diastolic ABP <40mmHg or >150mmHg. A valid 24-hour ABPM recording was defined as having ≥80% of the total planned ABPM readings. Mean daytime and nighttime systolic and diastolic ABP and the nighttime BP dipping were calculated using each approach for defining the nighttime and daytime periods (described below).
Nighttime hypertension was defined as mean nighttime systolic ABP ≥120 mmHg or mean nighttime diastolic ABP ≥70 mmHg.[1, 21] Daytime hypertension was defined as mean daytime systolic ABP ≥135 mmHg or mean daytime diastolic ABP ≥85 mmHg.[1, 22] Nighttime BP dipping was calculated as the ratio of mean nighttime to mean daytime systolic ABP. Non-dipping status was defined as nighttime BP dipping >0.90.[1] The magnitude of dipping status was also categorized into four groups based on nighttime BP dipping: extreme dipping (≤0.80), normal dipping (>0.80 to ≤0.90), non-dipping (>0.90 to ≤1.00) and reverse dipping (>1.00).[1]
Nighttime and daytime periods
Nighttime and daytime periods were determined using three approaches: self-report using a sleep diary, fixed-time periods, and wrist actigraphy. Upon completion of each 24-hour ABPM period, participants completed a sleep diary of the times they went to sleep and awoke during the 24-hour ABPM period. Fixed-time periods were defined by the International Database of ABP in relation to Cardiovascular Outcome (IDACO) criteria for Europeans (nighttime: 00:00 to 06:00 [6 hours], daytime: 10:00 to 20:00 [10 hours]).[6, 19] Additionally, sleep and awake periods were estimated from a wrist actigraphy device (ActiWatch, Phillips Respironics, Murrayville, PA) worn on the non-dominant wrist using ActiWatch processing software.[23]
Statistical analysis
Characteristics were calculated as percentages or means and standard deviations. Using data collected during ABPM period 1, the mean (95% CI) and absolute value (95% CI) of the total nighttime period (in minutes) and pairwise within-individual differences, self-reported versus fixed-time, self-reported versus actigraphy, and fixed-time versus actigraphy, were plotted. The distribution of the clock time for the start of the nighttime and daytime periods, separately, were also plotted. Pairwise systematic differences of nighttime systolic and diastolic ABP were assessed using Bland-Altman plots. On a Bland-Altman plot, the average of the differences in measurements between each method corresponds to the average bias. The 95% CI provides a measure of the spread of the within-individual differences. Pearson linear correlation coefficients were estimated for ABP comparing each pairwise approach for defining the diurnal periods. The prevalence of nighttime hypertension, daytime hypertension, non-dipping status, and dipping status categories was calculated for each approach. Overall percent agreement and the Kappa statistic were calculated for pairwise comparisons for defining diurnal periods. The Kappa statistic provides an estimate of concordance after accounting for the expected agreement by chance alone.[24] Agreement was classified into the following levels: poor (−1.00 to 0.00), slight (>0.00 to 0.20), fair (>0.20 to 0.40), moderate (>0.40 to 0.60), substantial (>0.60 to 0.80), and almost perfect (>0.80 to 1.00).[24] Additionally, estimates of the prevalence and agreement were calculated using a more stringent definition requiring the presence of nighttime hypertension, daytime hypertension, non-dipping status and magnitude of dipping status categories during both ABPM periods 1 and 2.
Next, the reproducibility of the duration of the nighttime period, mean nighttime and daytime systolic and diastolic BP, and hypertension categories between ABPM periods 1 and 2 were estimated for each approach to defining the diurnal period using the intra-class correlation coefficient (95% CI) for continuous measures and the Kappa statistic (95% CI) for categorical measures. Paired t-tests and McNemar’s Chi Square Test of paired proportions were used to evaluate differences in mean values and the prevalence of hypertension categories between ABPM periods 1 and 2 for each approach (self-report, fixed and actigraphy). Analyses were conducted using Stata/IC 13.1 (Stata Corporation, College Station, Texas).
Results
Participant characteristics
Table 1 shows the characteristics of the study population. Participants’ mean age was 40.5 (SD 13.0) years. Also, 41.8% of the population was male and 20.6% and 64.5% of participants were black and Hispanic, respectively.
Table 1.
Characteristics of participants in the Improving the Detection of Hypertension Study.
| Number of participants | (n = 330) |
|---|---|
| Age | 40.5 ± 13.0 |
| Race/ethnicity | |
| Black | 20.6% |
| Hispanic | 64.5% |
| Male | 41.8% |
| Education less than high school | 11.8% |
| Annual income less than $30,000 USD | 47.9% |
| Body mass index (kg/m2) | 27.5 ± 5.0 |
| Normal (<25) | 33.4% |
| Overweight (≥25 and < 30) | 42.7% |
| Obese (≥30) | 23.9% |
| Total cholesterol (mg/dL) | 182.3 ± 38.0 |
| Low-density-lipoprotein cholesterol (mg/dL) | 109.8 ± 33.3 |
| C-reactive protein (mg/L) | 1.36 (0.55 – 3.25) |
| Family history of hypertension | 11.8% |
| Diabetes | 4.5% |
| Current cigarette smoking | 8.8% |
| Alcohol consumption* | |
| None | 43.3% |
| Moderate | 51.5% |
| Heavy | 5.2% |
Table numbers are percentages or mean ± standard deviation, except C-reactive protein which is median (25th – 75th percentile).
ABPM: ambulatory blood pressure monitoring. USD: US dollars.
Alcohol use defined as: non-drinker (no weekly alcohol consumption), moderate drinker (1–14 and 1–7 alcoholic beverages per week for men and women, respectively) or heavy (>14 and >7 alcoholic beverages per week for men and women, respectively).
Duration of the diurnal periods
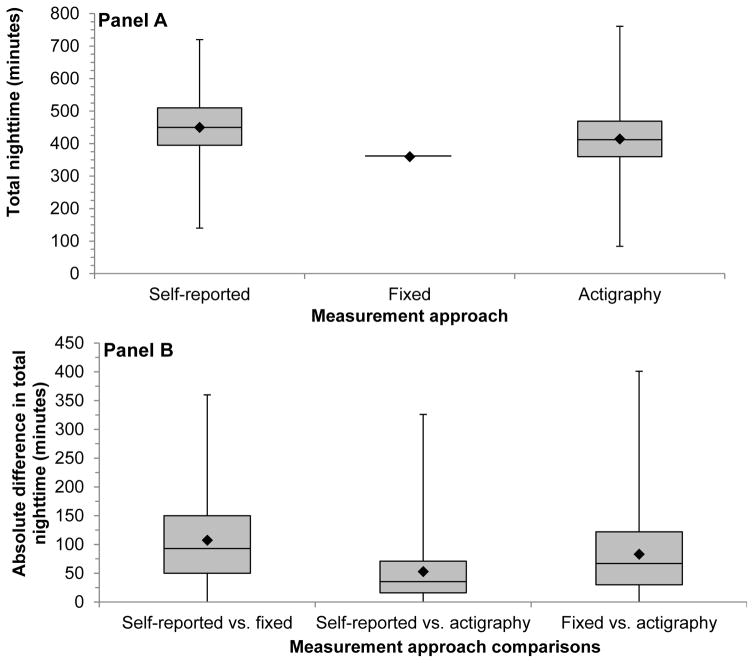
The median (25th–75th percentiles) total duration of the nighttime period for participants using the self-reported and actigraphy approaches was 450 (395–510) minutes and 412 (360–468) minutes, respectively (Figure 1, Panel A). All participants had 360 minutes of sleep using fixed-time. The median (25th–75th percentiles) absolute difference of the nighttime period duration for self-report versus fixed-time approach was 93 (50–150) minutes, self-report versus actigraphy approach was 36 (16–71) minutes, and fixed-time versus actigraphy approach was 67 (30–122) minutes (Figure 1, Panel B). The median total duration of the daytime period (25th–75th percentiles) using the self-report, fixed-time, and actigraphy approaches, and the median (25th–75th percentiles) absolute difference between the three approaches are shown in Supplemental Figure 1 (Panels A and B).
Figure 1.
Box and whisker plots of the total time during the nighttime period (Panel A) and the absolute value of the within-individual differences in time during the nighttime period (Panel B) defined by self-reported, fixed-time* and actigraphy during ambulatory blood pressure monitoring period 1 (n = 330).
*Using the definition of the International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO) for Europeans (nighttime: 0000 to 0600).
The median value is represented by the solid line inside the shaded box.
The shaded region below the median value represents the values between the median and 25th percentile.
The shaded region above the median value represents the values between the median and 75th percentile.
The whiskers represent the minimum and maximum values.
The diamond (◆) represents the mean value.
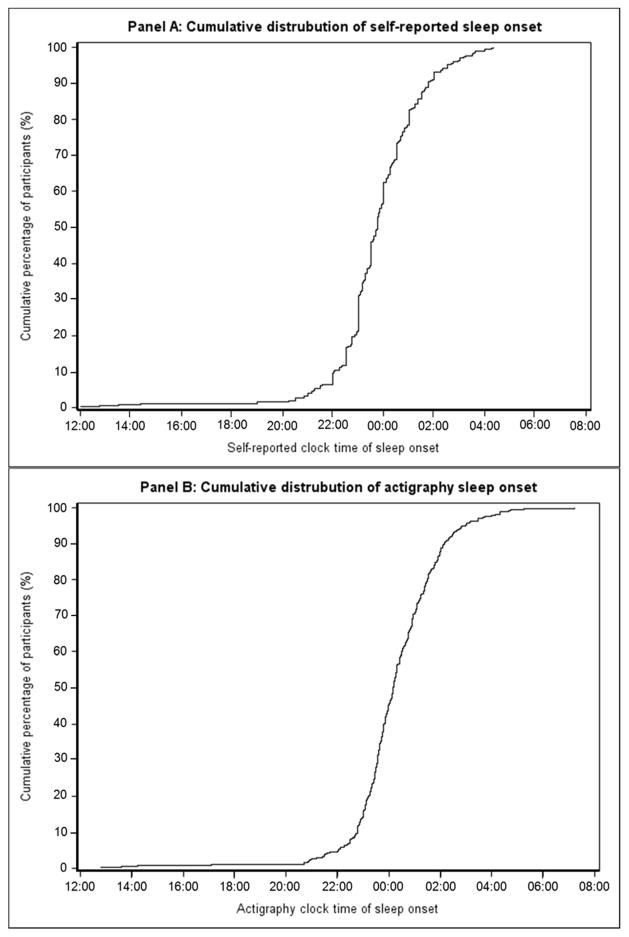
Figure 2 shows the cumulative distribution of clock times for the start of the nighttime period assessed by self-report (Panel A) and actigraphy (Panel B). The cumulative distribution of clock times for the start of the daytime period are shown in Supplemental Figure 2 (Panels A and B). The percentage of participants who had a clock time for sleep onset after 00:00 AM was 43.3% by self-report and 55.2% by actigraphy. The percentage of participants who had a clock time for awakening after 06:00 AM was 87.3% and 78.5% for self-reported and actigraphy approaches, respectively, and after 10:00 AM was 5.5% and 7.3%, respectively.
Figure 2.
Cumulative distribution of the clock time for start of the nighttime period by self-report (Panel A) and actigraphy (Panel B) on ambulatory blood pressure monitoring period 1.
The earliest and latest sleep onset clock times were 12:00 and 04:20 for self-report (median: 23:45) and 12:46 and 07:12 (median: 00:10) using actigraphy, respectively.
Nighttime ABP
During ABPM period 1, the mean (95% CI) nighttime systolic ABP was 110 (109–111) mmHg for self-report, 111 (109–112) mmHg for fixed-time and 109 (108–111) mmHg for the actigraphy approach. The mean (95% CI) nighttime diastolic ABP was 65 (64–65) mmHg for self-report, 65 (64–66) mmHg for fixed-time and 64 (63–65) mmHg for the actigraphy approach.
For nighttime systolic ABP, the Pearson linear correlation coefficients (95% CI) comparing self-report versus fixed-time, self-report versus actigraphy, and fixed-time versus actigraphy were 0.97 (0.96–0.97), 0.98 (0.98–0.98) and 0.95 (0.94–0.96) and for nighttime diastolic ABP were 0.96 (0.95–0.97), 0.97 (0.96–0.97) and 0.93 (0.92–0.95), respectively.
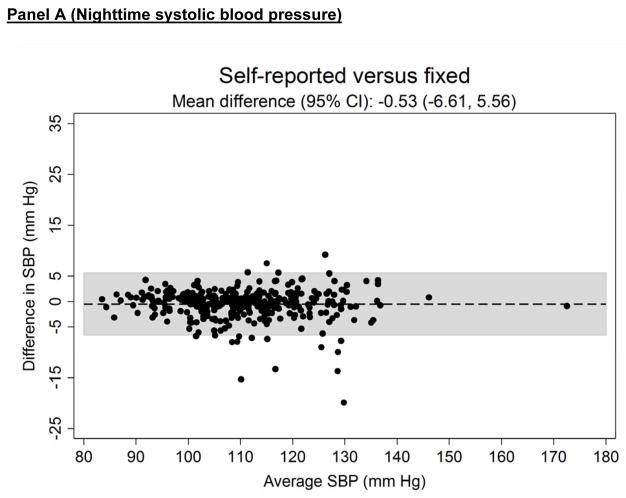
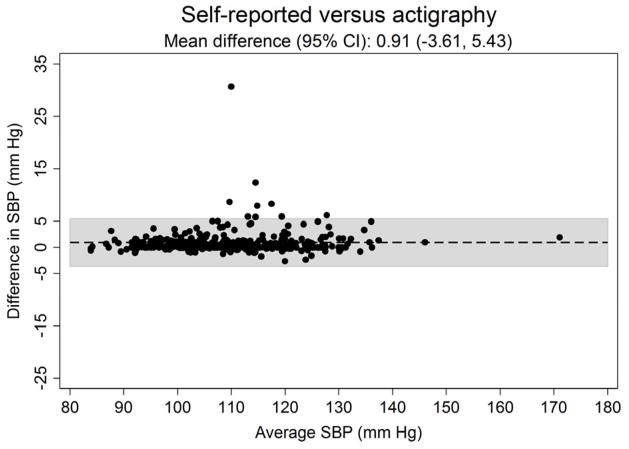
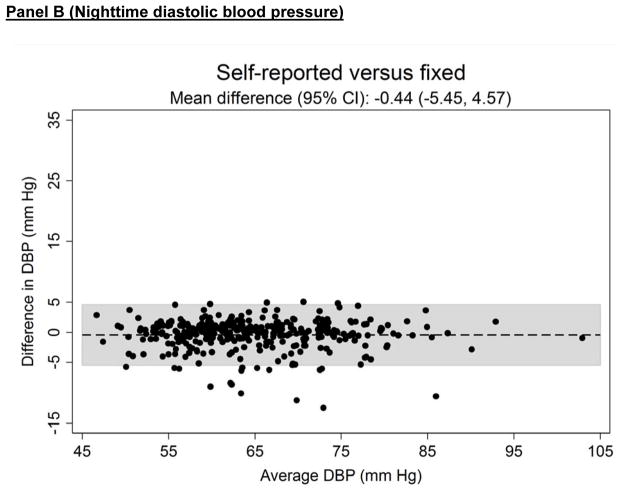
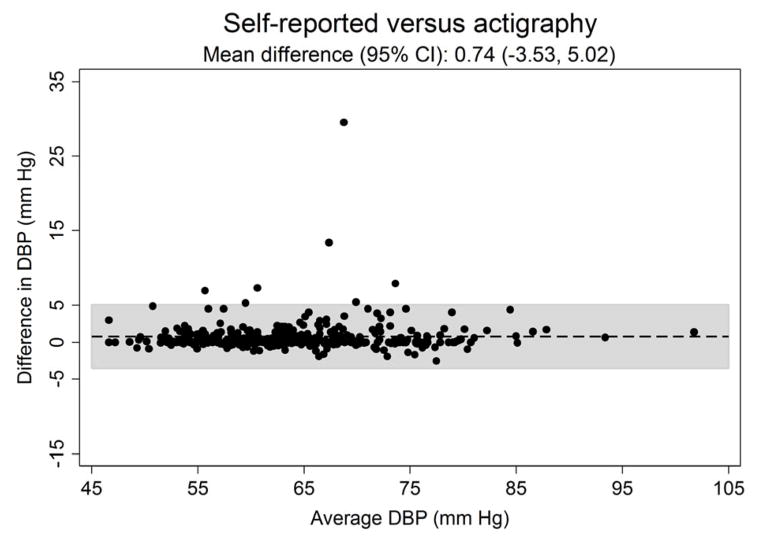
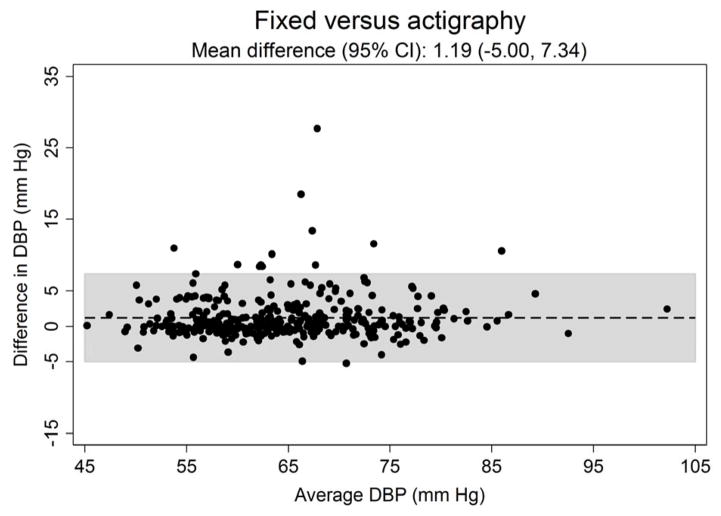
Figure 3 shows the systematic differences in nighttime systolic (Panel A) and diastolic ABP (Panel B) between the three approaches for defining diurnal periods. The mean difference (95% CI) in systolic ABP was −0.53 (−6.61 to 5.56) mmHg for self-reported versus fixed-time, 0.91 (−3.61 to 5.43) mmHg for self-reported versus actigraphy and 1.43 (−5.59 to 8.46) mmHg for fixed-time versus actigraphy. The mean differences (95% CI) in diastolic ABP comparing self-reported versus fixed-time, self-reported versus actigraphy and fixed-time versus actigrpahy was −0.44 (−5.45 to 4.57), 0.74 (−3.53 to 5.02) and 1.19 (−5.00 to 7.34) mmHg, respectively.
Figure 3.
Bland-Altman plots comparing the systematic differences of nighttime systolic (Panel A) and diastolic (Panel B) blood pressure defined by self-reported, fixed-time* and actigraphy-derived times from ambulatory blood pressure monitoring period 1 (n = 330).
*Using the definition of the International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO) for Europeans (nighttime: 0000 to 0600).
SBP: systolic blood pressure.
DBP: diastolic blood pressure.
CI: confidence interval.
Daytime ABP
During ABPM period 1, the mean (95% CI) daytime systolic ABP was 124 (123–126) mmHg for self-report, 125 (124–126) mmHg for fixed-time and 124 (123–125) mmHg for the actigraphy approach. The mean (95% CI) daytime diastolic ABP was 78 (77–79) mmHg for self-report, 79 (78–79) mmHg for fixed-time and 78 (77–78) mmHg for the actigraphy approach. The Pearson linear correlation coefficients (95% CI) for daytime systolic ABP were 0.98 (0.97–0.98), 1.00 (0.99–1.00), and 0.97 (0.97–0.98) comparing self-report versus fixed-time, self-report versus actigraphy, and fixed-time versus actigraphy, respectively. The Pearson linear correlation coefficients (95% CIs) was >0.95 for all pairwise comparisons of estimating daytime diastolic ABP. Supplemental Figure 3 shows the systematic differences in daytime systolic (Panel A) and diastolic (Panel B) ABP between the three approaches for defining diurnal periods.
Prevalence of hypertension categories at ABPM period 1
At ABPM period 1, clinic hypertension was present in 12.5% of participants. The prevalence and agreement of nighttime hypertension, daytime hypertension, non-dipping status, and magnitude of dipping status categories are shown in Table 2 for the self-report, fixed-time, and actigraphy-based approaches. The highest agreement for nighttime hypertension, daytime hypertension, non-dipping status, and magnitude of dipping status categories was between self-reported versus actigraphy (Table 2). The prevalence and agreement for nighttime hypertension, daytime hypertension, and non-dipping status, comparing each of the three approaches were similar when defining hypertension based on its presence during both ABPM periods 1 and 2 (Supplemental Table 1).
Table 2.
Prevalence, Kappa statistic and overall agreement for classification of daytime hypertension, nighttime hypertension, non-dipping status, and magnitude of dipping blood pressure categories using times derived from self-report, fixed-time periods† and actigraphy from ambulatory blood pressure monitoring period 1 (n = 330).
| Overall (%) | No | No | Yes | Yes | Kappa Statistic (95% confidence interval) | Overall percent agreement (95% confidence interval) | |
|---|---|---|---|---|---|---|---|
| No (%) | Yes (%) | No (%) | Yes (%) | ||||
|
| |||||||
| Daytime hypertension | |||||||
|
| |||||||
| Self-reported | 17.9 | 80.0 | 2.1 | 1.2 | 16.7 | 0.89 (0.82 – 0.95) | 0.97 (0.95 – 0.99) |
| Fixed-time | 18.8 | ||||||
|
| |||||||
| Self-reported | 17.9 | 82.1 | 0.0 | 0.0 | 17.9 | 1.00 (0.98 – 1.00) | 1.00 (0.99 – 1.00) |
| Actigraphy | 17.9 | ||||||
|
| |||||||
| Fixed-time | 18.8 | 80.0 | 1.2 | 2.1 | 16.7 | 0.89 (0.82 – 0.95) | 0.97 (0.95 – 0.99) |
| Actigraphy | 17.9 | ||||||
|
| |||||||
| Nighttime hypertension | |||||||
|
| |||||||
| Self-reported | 30.0 | 65.8 | 4.2 | 2.1 | 27.9 | 0.85 (0.79 – 0.91) | 0.94 (0.91 – 0.96) |
| Fixed-time | 32.1 | ||||||
|
| |||||||
| Self-reported | 30.0** | 69.7 | 0.3 | 3.3 | 26.7 | 0.91 (0.86 – 0.96) | 0.96 (0.94 – 0.98) |
| Actigraphy | 27.0 | ||||||
|
| |||||||
| Fixed-time | 32.1** | 67.3 | 0.6 | 5.8 | 26.4 | 0.85 (0.79 – 0.91) | 0.94 (0.91 – 0.96) |
| Actigraphy | 27.0 | ||||||
|
| |||||||
| Non-dipping status (2-groups) | |||||||
|
| |||||||
| Self-reported | 37.6 | 57.3 | 5.2 | 5.8 | 31.8 | 0.72 (0.64 – 0.91) | 0.89 (0.86 – 0.92) |
| Fixed-time | 37.0 | ||||||
|
| |||||||
| Self-reported | 37.6* | 60.6 | 1.8 | 5.2 | 32.4 | 0.85 (0.79 – 0.91) | 0.93 (0.90 – 0.96) |
| Actigraphy | 34.2 | ||||||
|
| |||||||
| Fixed-time | 37.0 | 57.9 | 5.2 | 7.9 | 29.1 | 0.77 (0.69 – 0.84) | 0.87 (0.83 – 0.91) |
| Actigraphy | 34.2 | ||||||
|
| |||||||
| Magnitude of dipping status (4-groups) | |||||||
|
| |||||||
| Extreme dipping | |||||||
|
| |||||||
| Self-reported | 5.8** | 89.1 | 5.2 | 1.5 | 4.2 | 0.53 (0.35 – 0.70) | 0.93 (0.91 – 0.96) |
| Fixed-time | 9.4 | ||||||
|
| |||||||
| Self-reported | 5.8 | 93.9 | 0.3 | 0.3 | 5.5 | 0.94 (0.87 – 1.00) | 0.99 (0.99 – 1.00) |
| Actigraphy | 5.8 | ||||||
|
| |||||||
| Fixed-time | 9.4** | 89.1 | 1.5 | 5.2 | 4.2 | 0.53 (0.35 – 0.70) | 0.93 (0.91 – 0.96) |
| Actigraphy | 5.8 | ||||||
|
| |||||||
| Dipping | |||||||
|
| |||||||
| Self-reported | 56.7 | 36.1 | 7.3 | 10.3 | 46.4 | 0.65 (0.56 – 0.73) | 0.82 (0.78 – 0.87) |
| Fixed-time | 53.6 | ||||||
|
| |||||||
| Self-reported | 56.7* | 38.2 | 5.2 | 1.8 | 54.9 | 0.86 (0.80 – 0.91) | 0.93 (0.90 – 0.96) |
| Actigraphy | 60.0 | ||||||
|
| |||||||
| Fixed-time | 53.6** | 33.6 | 12.7 | 6.4 | 47.3 | 0.61 (0.53 – 0.70) | 0.81 (0.77 – 0.85) |
| Actigraphy | 60.6 | ||||||
|
| |||||||
| Non-dipping | |||||||
|
| |||||||
| Self-reported | 34.6 | 60.0 | 5.5 | 8.5 | 26.1 | 0.69 (0.60 – 0.77) | 0.86 (0.82 – 0.90) |
| Fixed-time | 31.5 | ||||||
|
| |||||||
| Self-reported | 34.6 | 63.0 | 2.4 | 5.2 | 29.4 | 0.83 (0.77 – 0.89) | 0.92 (0.90 – 0.95) |
| Actigraphy | 31.8 | ||||||
|
| |||||||
| Fixed-time | 31.5 | 61.5 | 7.0 | 6.7 | 24.9 | 0.68 (0.60 – 0.77) | 0.86 (0.83 – 0.90) |
| Actigraphy | 31.8 | ||||||
|
| |||||||
| Reverse dipping | |||||||
|
| |||||||
| Self-reported | 3.0* | 93.3 | 3.6 | 1.2 | 1.8 | 0.41 (0.17 – 0.64) | 0.95 (0.93 – 0.97) |
| Fixed-time | 5.5 | ||||||
|
| |||||||
| Self-reported | 3.0 | 96.7 | 0.3 | 0.9 | 2.1 | 0.77 (0.55 – 0.99) | 0.99 (0.98 – 1.00) |
| Actigraphy | 2.4 | ||||||
|
| |||||||
| Fixed-time | 5.5** | 93.9 | 0.6 | 3.6 | 1.8 | 0.44 (0.20 – 0.68) | 0.96 (0.94 – 0.98) |
| Actigraphy | 2.4 | ||||||
Daytime hypertension is defined as daytime systolic or diastolic blood pressure ≥135 or ≥85 mmHg at ambulatory blood pressure monitoring period 1.
Nighttime hypertension is defined as a nighttime systolic or diastolic blood pressure ≥120 or ≥70 mmHg at ambulatory blood pressure monitoring periods 1.
Non-dipping status defined as night-day BP ratio is >0.90 at ambulatory blood pressure monitoring periods 1.
Magnitude of dipping defined as 4 categories (must be present at ambulatory blood pressure monitoring periods 1: extreme dipper (mean night-day BP ratio ≤0.80), dipper (mean night-day BP ratio >0.80 and ≤0.90), non-dipper (mean night-day BP ratio >0.90 and ≤1.00), and reverse dipper (mean night-day BP ratio >1.00).
Indicates p-value ≤0.05 using McNemar’s chi-square test for paired proportions.
Indicates p-value ≤0.01 using McNemar’s chi-square test for paired proportions.
Using the definition of the International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO) for Europeans (nighttime: 00:00 to 06:00; daytime: 10:00 to 20:00).
Reproducibility of ABP parameters between ABPM periods 1 and 2
The intra-class correlation coefficients (95% CI) for systolic CBP was 0.71 (0.66–0.76) and 0.63 (0.56–0.69) for diastolic CBP across ABPM periods 1 and 2. The kappa statistic (95% CI) for clinic hypertension across the two ABPM periods was 0.47 (0.32–0.62). Using self-report, fixed-time and actigraphy for defining the diurnal periods, the intra-class correlation coefficients were substantial to almost perfect for nighttime and daytime systolic ABP and nighttime and daytime diastolic ABP, and fair to moderate for nighttime BP dipping (Table 3). The Kappa statistic for the agreement across ABPM periods 1 and 2 was moderate to substantial for daytime hypertension and substantial for nighttime hypertension, fair to moderate for non-dipping status and fair for magnitude of dipping status using each of the three approaches.
Table 3.
Reproducibility of total sleep time, mean ambulatory blood pressure, nighttime blood pressure dipping, and ambulatory blood pressure categories between ambulatory blood pressure monitoring periods 1 and 2 using times derived from self-report, fixed-time periods† and actigraphy (analysis of 330 paired ABPM assessments in 330 participants).
| Comparing ambulatory blood pressure monitoring period 1 and 2 | |||
|---|---|---|---|
| Self-reported | Fixed-time | Actigraphy | |
| Number of participants | (n=330) | (n=330) | (n=330) |
| Intra-class correlation coefficient (95% confidence interval)‡ | |||
| Total sleep time | 0.46 (0.37 – 0.54) | – | 0.29 (0.20 – 0.39)* |
| Mean ambulatory systolic blood pressure | |||
| Daytime | 0.79 (0.75 – 0.83) | 0.76 (0.72 – 0.81) | 0.81 (0.77 – 0.85) |
| Nighttime | 0.77 (0.72 – 0.81) | 0.75 (0.70 – 0.79) | 0.77 (0.72 – 0.81) |
| Mean ambulatory diastolic blood pressure | |||
| Daytime | 0.79 (0.75 – 0.83)* | 0.76 (0.72 – 0.81) | 0.81 (0.77 – 0.85)* |
| Nighttime | 0.77 (0.72 – 0.81) | 0.76 (0.71 – 0.80) | 0.78 (0.73 – 0.82) |
| Nighttime blood pressure dipping | 0.43 (0.34 – 0.58)** | 0.43 (0.35 – 0.52) | 0.44 (0.35 – 0.52)** |
| Kappa statistic (95% confidence interval)‡ | |||
| Daytime hypertension | 0.65 (0.54 – 0.76) | 0.54 (0.43 – 0.65) | 0.63 (0.52 – 0.74) |
| Nighttime hypertension | 0.64 (0.54 – 0.73) | 0.61 (0.52 – 0.70) | 0.62 (0.53 – 0.72) |
| Non-dipping status (2-groups) | 0.40 (0.30 – 0.50) | 0.36 (0.26 – 0.47) | 0.36 (0.25 – 0.46) |
| Magnitude of dipping status (4-groups)†† | 0.32 (0.24 – 0.40) | 0.30 (0.22 – 0.38) | 0.28 (0.20 – 0.37) |
Daytime hypertension is defined as daytime systolic ≥135 mmHg or diastolic blood pressure ≥85 mmHg (yes/no).
Nighttime hypertension is defined as a nighttime systolic ≥120 mmHg or diastolic blood pressure ≥70 mmHg (yes/no).
Non-dipping status defined as a dichotomous outcome (yes/no; non-dipping status if mean night-day BP ratio is >0.90).
Magnitude of dipping defined as 4 categories: extreme dipper (mean night-day BP ratio ≤0.80), dipper (mean night-day BP ratio >0.80 and ≤0.90), non-dipper (mean night-day BP ratio >0.90 and ≤1.00), and reverse dipper (mean night-day BP ratio >1.00).
Indicates p-value ≤0.05 using McNemar’s chi-square test for paired proportions or paired t-test as appropriate.
Indicates p-value ≤0.01 using McNemar’s chi-square test for paired proportions or paired t-test as appropriate.
Using the definition of the International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO) for Europeans (nighttime: 00:00 to 06:00; daytime: 10:00 to 20:00).
Intra-class correlation coefficient or Cohen’s Kappa statistic Test of agreement for continuous or categorical variables, respectively.
Weighted Kappa for categorical variables with greater than 2 levels.[19]
Discussion
Using self-reported, fixed-time or actigraphy approaches to define the nighttime and daytime periods on ABPM may lead to different estimates of ABP. There are scarce empirical data on diurnal ABP parameters and reproducibility of ABP parameters using the three approaches studied in the same population. In the current analysis, the systematic biases in nighttime and daytime systolic and diastolic ABP were small when diurnal periods were defined by self-report, fixed-time or actigraphy. Further, the prevalence of nighttime hypertension, daytime hypertension, and BP non-dipping status were similar regardless of the approach used to define diurnal periods. However, there were large individual-level differences in systolic and diastolic nighttime and daytime ABP between approaches as demonstrated by wide 95% CIs. The narrowest to widest 95% CIs for nighttime systolic and diastolic ABP were for the comparison between self-report versus actigraphy, followed by self-report versus fixed-time, and fixed-time versus actigraphy. Also, self-report versus actigraphy had consistently higher agreement for hypertension status compared with self-report or actigraphy with fixed time periods. Finally, using the two ABPM periods conducted approximately 28 days apart, the reproducibility of mean ABP, nighttime BP dipping, and nighttime and daytime hypertension was similar for the three approaches used to define diurnal periods.
Nighttime ABP and non-dipping BP status have been associated with increased risk for CVD outcomes and mortality, independent of mean daytime ABP.[25, 26] In a prior systematic review, each 10-mmHg higher nighttime ABP was associated with a higher incidence of CVD events and mortality.[25] Additionally, individuals with non-dipping status were more likely to have CVD events and die compared with their counterparts with nighttime BP dipping.[25] Of the 16 studies identified by the systematic review, the nighttime and daytime periods were defined using self-report diaries in 6 studies, and a fixed-time approach in 10 studies.[25] Although actigraphy has been used in prior ABPM studies, scarce data exist on the associations of nighttime ABP parameters with outcomes using this method to define the nighttime period.[27, 28] In the current study, the mean differences in nighttime and daytime ABP were small for all comparisons between approaches. Further, the prevalence of nighttime hypertension, daytime hypertension, and non-dipping status were similar regardless of the approach used to define the nighttime and daytime periods. Therefore, from a population-level, each of the three approaches (self-report, fixed-time, actigraphy) are satisfactory in estimating nighttime and daytime ABP and nighttime and daytime hypertension, and no approach is likely to be superior to use when estimating the association between ABP and CVD events.
The 2013 European Society of Hypertension (ESH) position paper on ABPM recommended that the daytime and nighttime periods be defined using self-report, but stated fixed-time periods may also be used.[1] The use of validated measurement devices (e.g., actigraphy) to determine daytime and nighttime periods was not mentioned in the ESH position paper.[1] Using each of the three approaches for defining diurnal periods (self-report, fixed-time, actigraphy) has limitations. The internal validity of self-report is compromised by recall bias. Defining diurnal periods by self-report diaries requires adherence of the individual to accurately report sleep and wake times, whereas actigraphy requires the individual to wear the device for 24 hours simultaneously with the ABPM. Also, actigraphy may be difficult to employ in clinical practice due to its relatively high purchase cost and the required training needed to download and analyze the recorded data. Ecological validity is limited for fixed-time periods since nighttime and daytime periods may differ inter-individually and also intra-individually across time.[1, 6] Also, fixed-time periods may omit important BP fluctuations (e.g., morning BP surge) during the sleep-awake transition period that is associated with CVD risk.[1]
In the current study, the self-report and actigraphy approaches had the closest individual-level values in nighttime and daytime ABP and nighttime and daytime hypertension, whereas the fixed-time approach had less agreement with either self-report or actigraphy. In a prior retrospective study of 95 clinic patients, the within-individual estimates of nighttime BP were similar for the self-report versus fixed-time (01:00 to 07:00 [6 hours]) approaches.[29] Comparing self-report, fixed-time and actigraphy was not possible since diurnal periods were not objectively assessed.[29] The contrasting results for self-report versus fixed-time between the current and prior study may be attributed to differences in the sample sizes and populations (i.e., population-based not taking antihypertensive medications and clinic-based taking antihypertensive medications, respectively). Also, the prior study estimated the mean ABP by averaging the mean hourly ABP levels, not by using each BP measurement recorded during the diurnal period as in the current study. The results herein suggest it may be reasonable to primarily use self-report in clinical practice to define the nighttime and daytime periods due to its ease in implementation over actigraphy. If self-report is unreliable for a particular individual, a fixed-time approach may be appropriate with the caveat that estimates of ABP for this individual may differ from estimates obtained by self-report.
The reproducibility of ABP parameters is also important to consider when choosing a method in determining the daytime and nighttime periods.[30] Eissa analyzed ABP data among 62 participants aged 6 to 71 years old comparing sleep and awake periods defined by actigraphy and self-report.[30] There were no differences in sleep or awake ABP or in daytime and nighttime hypertension status across the sample for actigraphy versus self-report. However, 32% and 23% of participants were reclassified as having a nighttime BP non-dipping or dipping pattern, respectively, when using the self-report rather than actigraphy approach.[30]. As scarce data exist on comparisons between all three approaches (fixed-time, self-report, actigraphy) to estimate ABP parameters, our study provides essential information to the published literature. The results of the current study indicate the reproducibility across time is similar across each of the three assessed approaches.
There are several strengths of the current study. The IDH Study is one of the largest studies of ABP in which data are available at two visits to compare self-report, fixed-time and actigraphy approaches for determining the diurnal periods for estimating ABP parameters. Also, ABPM and the self-report and actigraphy approaches used to estimate the nighttime period were conducted following a standardized protocol in all participants. There are also several possible limitations of the current study. The results may have limited generalizability since the study cohort was relatively young, few participants had clinic hypertension, diabetes, chronic kidney disease and no participants were taking antihypertensive medication. Additionally, the study participants were from a single, large urban community and may not represent populations living outside of a large city who may have different sleep patterns.
In conclusion, there are scarce empirical data comparing self-reported, fixed-time and actigraphy approaches for estimating ABP during the nighttime and daytime periods on ABPM. The results reported herein indicate that using these three approaches to define diurnal periods on ABPM provide similar population estimates of nighttime and daytime ABP and nighttime and daytime hypertension. For most individuals, the differences in nighttime and daytime ABP and nighttime and daytime hypertension were smallest when comparing self-report versus actigraphy. The current study provides evidence that self-report, which is more easily implemented in clinical practice than actigraphy, can be used to define diurnal periods on ABPM. Further, the fixed-time approach may be a reasonable alternative when self-report data is not available.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by P01-HL047540 (PI: JE Schwartz), R01-HL098604 (PI: AJ Viera), and K24-HL125704 (PI: D Shimbo) from the National Heart, Lung, and Blood Institute at the National Institutes of Health (NIH). The research was also supported by two NIH Diversity Supplements R01-HL116470-02S1 (KM Diaz) and HL117323-02S2 (M Abdalla). The contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations
- ABPM
ambulatory blood pressure monitoring
- BP
blood pressure
- ABP
ambulatory blood pressure
- CVD
cardiovascular disease
- CBP
clinic blood pressure
- IDH
Improving the Detection of Hypertension Study
- CI
confidence interval
- USD
United States Dollar
- BMI
body mass index
- HDL
high-density-lipoprotein cholesterol
- LDL
low-density-lipoprotein cholesterol
- hs-CRP
high-sensitivity C-reactive protein
- ESH
European Society of Hypertension
Footnotes
A portion of the current results were presented at the American Society of Hypertension on May 16, 2015.
Study conception and design: JNBIII, PM, DS; Acquisition, analysis or interpretation of data: JNBIII, JES, DS; Statistical analysis: JNBIII, PM, MA, JES, DS; Drafting of the manuscript: JNBIII, DS; Critical revision of the manuscript: JNBIII, PM, MA, KMD, AV, KR, JES, DS. JNBIII and DS had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: JNBIII, PM, MA, KMD, AB, KR, JES, DS: None.
References
- 1.O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European society of hypertension position paper on ambulatory blood pressure monitoring. Journal of hypertension. 2013;31(9):1731–68. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 2.Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51(1):55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- 3.Shimbo D, Kuruvilla S, Haas D, Pickering TG, Schwartz JE, Gerin W. Preventing misdiagnosis of ambulatory hypertension: algorithm using office and home blood pressures. Journal of hypertension. 2009;27(9):1775–83. doi: 10.1097/HJH.0b013e32832db8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Staessen JA, Lu L, Li LH, Wang GL, Wang JG. Is isolated nocturnal hypertension a novel clinical entity? Findings from a Chinese population study. Hypertension. 2007;50(2):333–9. doi: 10.1161/HYPERTENSIONAHA.107.087767. [DOI] [PubMed] [Google Scholar]
- 5.Owens P, Lyons S, O’Brien E. Ambulatory blood pressure in the hypertensive population: patterns and prevalence of hypertensive subforms. Journal of hypertension. 1998;16(12 Pt 1):1735–43. doi: 10.1097/00004872-199816120-00005. [DOI] [PubMed] [Google Scholar]
- 6.Xu T, Zhang YQ, Tan XR. The dilemma of nocturnal blood pressure. Journal of clinical hypertension. 2012;14(11):787–91. doi: 10.1111/jch.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 8.Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. Journal of the American College of Cardiology. 2011;57(20):2037–114. doi: 10.1016/j.jacc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Krause T, Lovibond K, Caulfield M, McCormack T, Williams B Guideline Development G. Management of hypertension: summary of NICE guidance. Bmj. 2011;343:d4891. doi: 10.1136/bmj.d4891. [DOI] [PubMed] [Google Scholar]
- 10.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA: the journal of the American Medical Association. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Bauman MA, Coleman King SM, Fonarow GC, Lawrence W, Williams KA, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Journal of the American College of Cardiology. 2014;63(12):1230–8. doi: 10.1016/j.jacc.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. Journal of hypertension. 2014;32(1):3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 13.(USPSTF) USPSTF. Final Recommendation Statement. [Accessed February 3, 2015];Blood Pressure in Adults (Hypertension): Screening. 2014 [Google Scholar]
- 14.American Diabetes A. Standards of medical care in diabetes--2014. Diabetes care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 15.KBPW G. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney international. 2012;2(suppl):337–414. [Google Scholar]
- 16.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Journal of hypertension. 2013;31(7):1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 17.Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. Journal of hypertension. 2014;32(7):1359–66. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 18.Dasgupta K, Quinn RR, Zarnke KB, Rabi DM, Ravani P, Daskalopoulou SS, et al. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. The Canadian journal of cardiology. 2014;30(5):485–501. doi: 10.1016/j.cjca.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370(9594):1219–29. doi: 10.1016/S0140-6736(07)61538-4. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 21.Fan HQ, Li Y, Thijs L, Hansen TW, Boggia J, Kikuya M, et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. Journal of hypertension. 2010;28(10):2036–45. doi: 10.1097/HJH.0b013e32833b49fe. [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 23.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep medicine reviews. 2002;6(2):113–24. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 25.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57(1):3–10. doi: 10.1161/HYPERTENSIONAHA.109.133900. [DOI] [PubMed] [Google Scholar]
- 26.Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertension research: official journal of the Japanese Society of Hypertension. 2012;35(7):695–701. doi: 10.1038/hr.2012.26. [DOI] [PubMed] [Google Scholar]
- 27.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34(6):1270–6. doi: 10.2337/dc11-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. Journal of the American College of Cardiology. 2011;58(11):1165–73. doi: 10.1016/j.jacc.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 29.Fagard R, Brguljan J, Thijs L, Staessen J. Prediction of the actual awake and asleep blood pressures by various methods of 24 h pressure analysis. Journal of hypertension. 1996;14(5):557–63. doi: 10.1097/00004872-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Eissa MA, Poffenbarger T, Portman RJ. Comparison of the actigraph versus patients’ diary information in defining circadian time periods for analyzing ambulatory blood pressure monitoring data. Blood pressure monitoring. 2001;6(1):21–5. doi: 10.1097/00126097-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychological bulletin. 1968;70(4):213–20. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.