Abstract
Background
Establishing minimally clinically important difference (MCID) for patient-reported outcomes questionnaires is essential in outcomes research to evaluate patients’ perspective of treatment effectiveness. We aim to determine (MCID) after carpal tunnel release in diabetic and non-diabetic patients using the Boston Carpal Tunnel Questionnaire (BCTQ).
Methods
We prospectively evaluated 114 patients (87 non-diabetic, 27 diabetic) undergoing carpal tunnel release. In addition to standard history and physical examination, we obtained preoperative electrodiagnostic studies to confirm Carpal Tunnel Syndrome (CTS). The BCTQ was administered before and after the surgery at 3 and 6 months. Patients were asked about their level of satisfaction at the final follow-up period. We applied the receiver operating characteristic (ROC) curve approach to determine the MCID of symptom and function severity scales of the questionnaire. We used patient satisfaction as the reference standard to compare against the standardized change in scores after surgery for the 2 groups.
Results
For both diabetic and non-diabetic patients, symptom and function severity scales showed large effect size of >0.8 at 3 and 6 months after the surgery. At 6 months after surgery to be satisfied, diabetic patients required an MCID of 1.55 and 2.05 points for symptom and function scales, whereas non-diabetic patients required 1.45 and 1.6 points, respectively.
Conclusion
Diabetic patients needed a greater improvement in BCTQ score to be satisfied on functional and symptom severity scales than non-diabetic patients. Overall diabetic patients had less improvement in BCTQ final scores compared to non-diabetics.
Keywords: Boston carpal tunnel questionnaire, carpal tunnel syndrome, diabetes, minimal clinically important difference, patient-reported evaluation
Introduction
Carpal tunnel syndrome is the most common entrapment neuropathy with a reported prevalence of 2% to 4% in the general population.1,2 Diabetes mellitus is a known risk factor for developing carpal tunnel syndrome (CTS) with a prevalence approaching 15%.3–5 Despite its high prevalence in the diabetic population, studies reporting outcomes of surgical treatment in this patient group are limited.6–10 Outcomes based on recovery of muscle wasting and sensory loss did not appear to be favorable for diabetic patients.6 However, these physical examination findings may not reflect patients’ self-reported outcomes such as satisfaction. Patient-reported outcome measures are necessary in the evaluation of surgical interventions to appropriately reflect patient’s opinion and overall satisfaction.11
Boston Carpal Tunnel Questionnaire (BCTQ) is a validated outcomes instrument for CTS.12–16 The interpretation of change in scores can be challenging owing to its quantitative nature. Minimal clinically important difference (MCID) is useful in “judging the magnitude of the benefit when making inferences about the percentage of patients improved by a therapeutic intervention”.17 Basically, MCID constitutes a bridge between a quantitative and a qualitative variable. To calculate MCID, an external reference needs to be established to define the important difference that occurred as the result of treatment.
The aim of this comparative prospective study is to assess the patient perspective of clinical outcome after carpal tunnel release in diabetic and non-diabetic patients. We hypothesize that diabetic patients will be satisfied after carpal tunnel surgery, although this group requires a greater improvement in score because of the chronic nerve dysfunction to achieve the same level of satisfaction compared to non-diabetic patients.
PATIENTS AND METHODS
We examined data from a prospective cohort of CTS patients who underwent carpal tunnel release. Institutional review board approved this study that was conducted between January 2007 and August 2011. All patients with signs and symptoms consistent with CTS were sent for confirmatory electrodiagnostic test (EDX). Patients with positive EDX testing for mild CTS received a steroid injection and night splinting. Patients diagnosed with moderate or severe CTS underwent surgery, which form the cohort for this study. A single surgeon performed all the operations using a mini-open carpal tunnel release technique.18 All patients received the same postoperative instructions and care. The diagnosis of CTS and diabetes mellitus (DM) were made according to the criteria established by American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM), and American Diabetes Association, respectively.19,20
Inclusion criteria for this study included patients between the age of 18 and 80 meeting the EDX criteria for CTS. Exclusion criteria included patients with cervical radiculopathy, inflammatory joint disease, renal failure, thyroid disorders, previous wrist fracture on the affected side, long-term exposure to vibrating tools, and pregnancy.
The study subjects included 87 non-diabetic patients (58 female and 29 male patients) and 27 diabetics (21 female and 6 male patients). Mean age of non-diabetic group was 46.7 ± 11 years, and diabetic group was 50.9 ±11 years. Information regarding occupational status, past medical and surgical history, psychiatric history, smoking, alcohol, and drug use were collected from all participants. In addition to a standard history and physical examination, we administered the BCTQ at baseline (preoperative) and at postoperative evaluations (3 months and 6 months after surgery). The BCTQ consists of two components, symptom severity scale (SSS) with 11 questions and functional status scale (FSS) with 8 questions. Each question is scored on a five point scale (range 1–5) depending on the degree of impairment. The individual scores are summed and divided by the number of questions to give a final score for each scale, range 1–5, with a higher score indicating a worse condition. In addition to BCTQ, we also asked patients to grade their satisfaction as satisfied, not-satisfied, or not sure. The operating surgeon was blinded to the results of the BCTQ in this study.
Statistical analysis
We used statistical software to perform the data analysis. We calculated the change in scores of SSS and FSS for each patient in both groups at two time points (3 months and 6 months after surgery) from preoperative scores. Standard deviation of baseline scores for all patients was calculated. The changes in scores were then divided by pooled standard deviation to obtain the effect size. We used these scores to compare with patient satisfaction reported as ‘yes’, ’no’ or ‘not sure’ by the study participants.
We used receiver operating characteristics (ROC) curve analysis approach to determine MCID of SSS and FSS of BCTQ. We used patient satisfaction as the reference standard to compare against the standardized change in scores after surgery. ROC curve is a plotted graph of all the sensitivity and 1-specificity pairs over the entire range of each patient-reported outcome. Sensitivity or the true positive fraction is plotted on the y-axis and 1-specificity or the false positive fraction is plotted on x-axis.21 The optimal cutoff point for the ROC curve with maximum sensitivity and specificity or the point at the upper left corner with sensitivity close to 1 and 1-specificity close to zero represents MCID.21–23 Area under the curve (AUC) of the ROC plot is a widely used measure to depict the discriminative ability of the continuous variable (SSS and FSS) by the dichotomous variable, satisfaction.21 We created descriptive statistics of the study participants. In addition, we performed the paired sample t-tests to assess the differences between baseline function and symptoms in both patient groups. We also evaluated the effect of age of the participants on the amount of change in BCTQ scores required to provide a clinically important difference in both diabetic and non-diabetic patients.
RESULTS
Table 1 presents the demographic characteristics of 114 patients (27 diabetics and 87 non-diabetics) with 140 carpal tunnel surgeries performed. A few patients with bilateral involvement had surgery performed on both hands but at different time points; our analysis included the results from these patients because there was time gap between the procedures and patients were asked about their satisfaction levels at each of the time points. The following counts represent the number of patients who underwent the surgery. Females represented majority of patients in both the diabetic and non-diabetic groups, 78% and 67% respectively. The difference in the proportion of gender is statistically significant between two groups. (P<0.001) Patients were mostly right hand dominant and a greater proportion of them were satisfied with surgical procedure (diabetics-65%, non-diabetics-86%). Table 2 shows the change in BCTQ scores during the study period, 3 and 6 months after surgery. The mean changes in scores after 6 months were higher than those after 3 months for both scales in two groups.
Table 1.
Characteristics of study participants (n=114)
| Diabetics | Non-diabetics | |
|---|---|---|
| Mean Age (range), y | 50.9 (33–73) | 46.7 (22–72) |
| Gender | ||
| Male (%) | 6(22) | 29(33) |
| Female (%) | 21(78) | 58(67) |
| Employed/unemployed/disabled/retired/unknown | 3/7/12/1/14 | 29/34/12/5/7 |
| Dominant Hand (right/left/unknown) | 19/2/6 | 62/5/20 |
| Smoking status (yes/no/unknown) | 11/2/14 | 26/18/43 |
| Satisfaction (yes/no) | 21/11* | 93/15* |
n- Number of patients, y- Years,
Number of carpal tunnel surgeries
Table 2.
FSS and SSS of BCTQ before and after carpal tunnel surgery
| Measure | Pre-op Mean (SD) |
3rd. month Mean (SD) |
6th. month Mean (SD) |
Change score after 3 months Mean (SD) |
Change score after 6 months Mean (SD) |
|
|---|---|---|---|---|---|---|
| Diabetics | SSS | 3.5(0.7) | 2.1(0.7) | 1.8(0.9) | 1.3(0.8) | 1.6(0.8) |
| FSS | 3.5(0.6) | 2.1(0.7) | 1.8(0.8) | 1.3(0.6) | 1.6(0.6) | |
| Non-diabetics | SSS | 3.5(0.7) | 1.9(0.7) | 1.5(0.7) | 1.5(0.9) | 2.0(0.9) |
| FSS | 3.5(0.6) | 2.0(0.7) | 1.5(0.7) | 1.5(0.8) | 2.0(0.9) |
BCTQ- Boston Carpal Tunnel Questionnaire, FSS- Functional Status Scale, SSS- Symptom Severity Scale, SD- Standard deviation
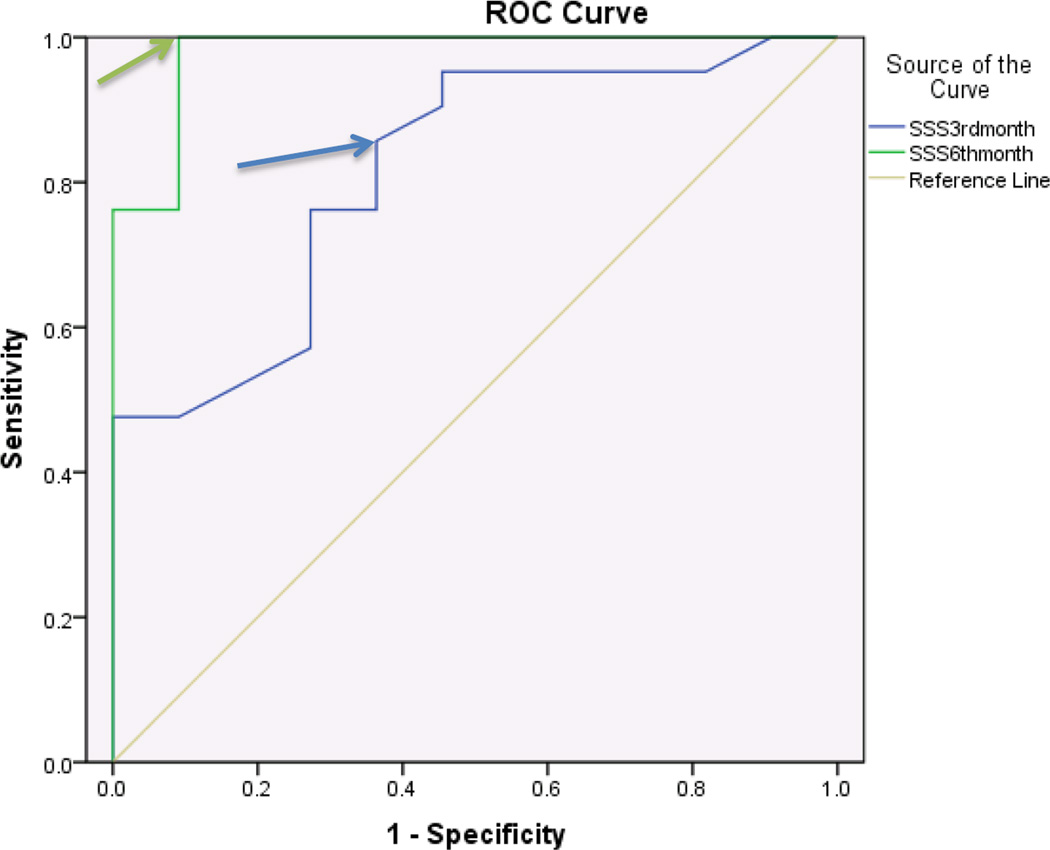
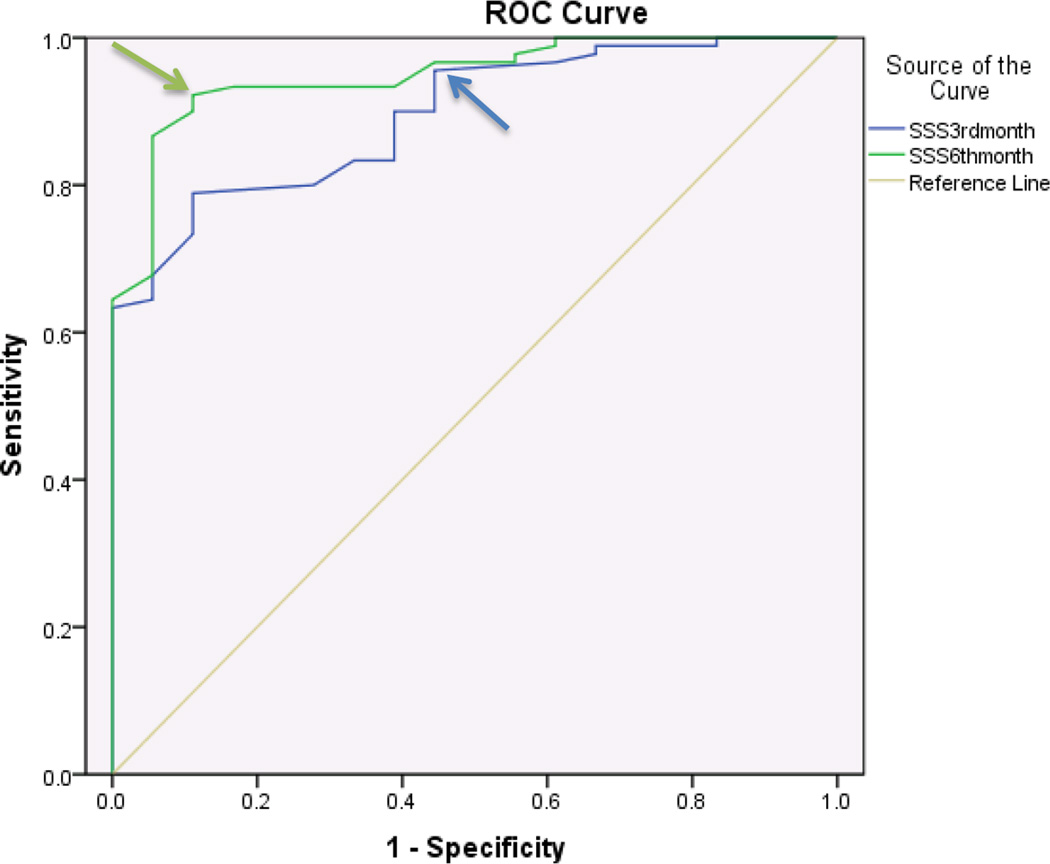
ROC curves were stratified for SSS and FSS and further stratified for 3 months and 6 months. In diabetics, the SSS component showed discriminatory ability with regards to patient satisfaction at both 3 and 6 months after surgery with an area under the curve (AUC) of .81 and .97 respectively. An MCID of 1.45 points at 3 months and 1.55 points at 6 months were identified for SSS (Figure 1, Table 3). The FSS component of BCTQ at 6 months showed greater discriminatory ability with regards to patient satisfaction than at 3 months with an AUC of .78 and .63 respectively. An MCID of 1.95 points at 3 months and 2.05 points at 6 months were identified for FSS (Figure 2, Table 3).
Figure 1.
Receiver operating characteristic curve for BCTQ-Symptom severity scale and patient satisfaction 3 months and 6 months after surgery in Diabetics (n=32 surgeries)
Table 3.
Summary Results of BCTQ at 3 and 6 months
| Measure | Sensitivity | 1-Specificity | AUC | MCID | |
|---|---|---|---|---|---|
| Diabetics (3 months) | SSS | .85 | .36 | .81 | 1.45 |
| FSS | .71 | .54 | .63 | 1.95 | |
| Non-diabetics (3 months) | SSS | .95 | .44 | .89 | 0.8 |
| FSS | .88 | .44 | .88 | 1.25 | |
| Diabetics (6 months) | SSS | 1.0 | .91 | .97 | 1.55 |
| FSS | .85 | .36 | .78 | 2.05 | |
| Non-diabetics (6 months) | SSS | .93 | .16 | .94 | 1.6 |
| FSS | .97 | .5 | .97 | 1.45 |
BCTQ- Boston Carpal Tunnel Questionnaire, AUC- Area Under the Curve, MCID- Minimal Clinically important Difference, SSS- Symptom Severity Scale, FSS- Function Severity Scale
Figure 2.
Receiver operating characteristic curve for BCTQ-functional status scale and patient satisfaction 3 months and 6 months after surgery in Diabetics (n=32 surgeries)
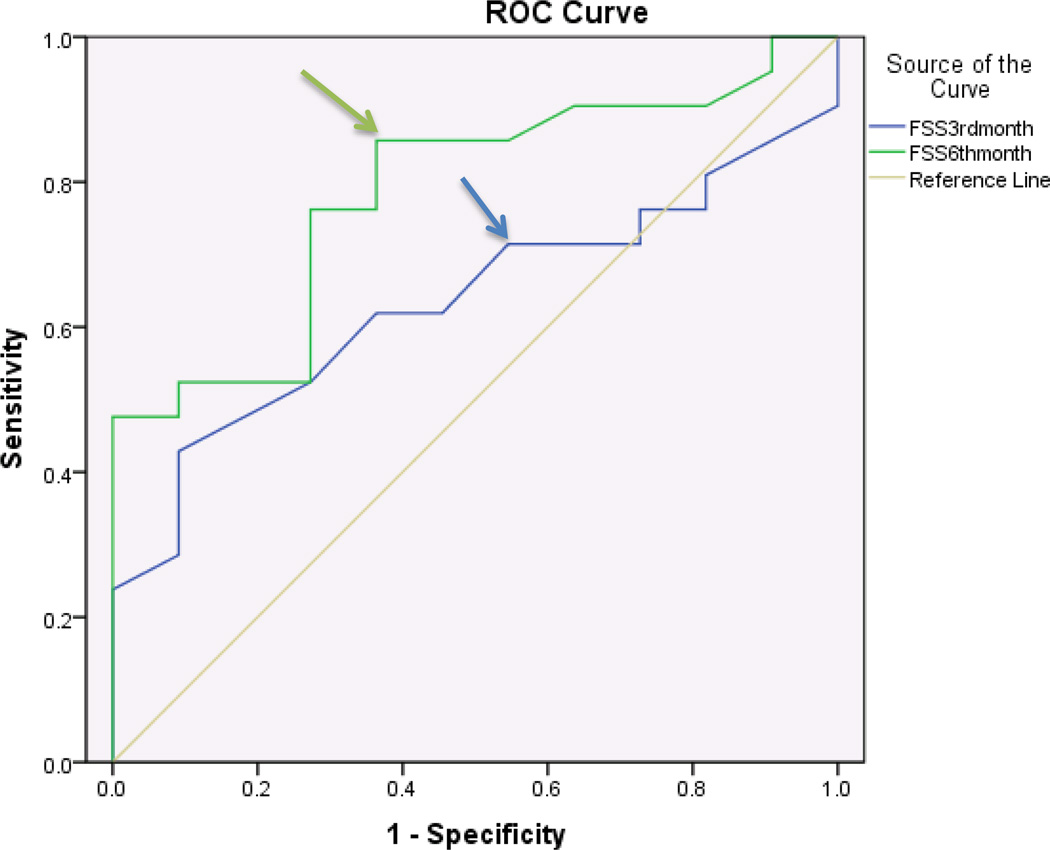
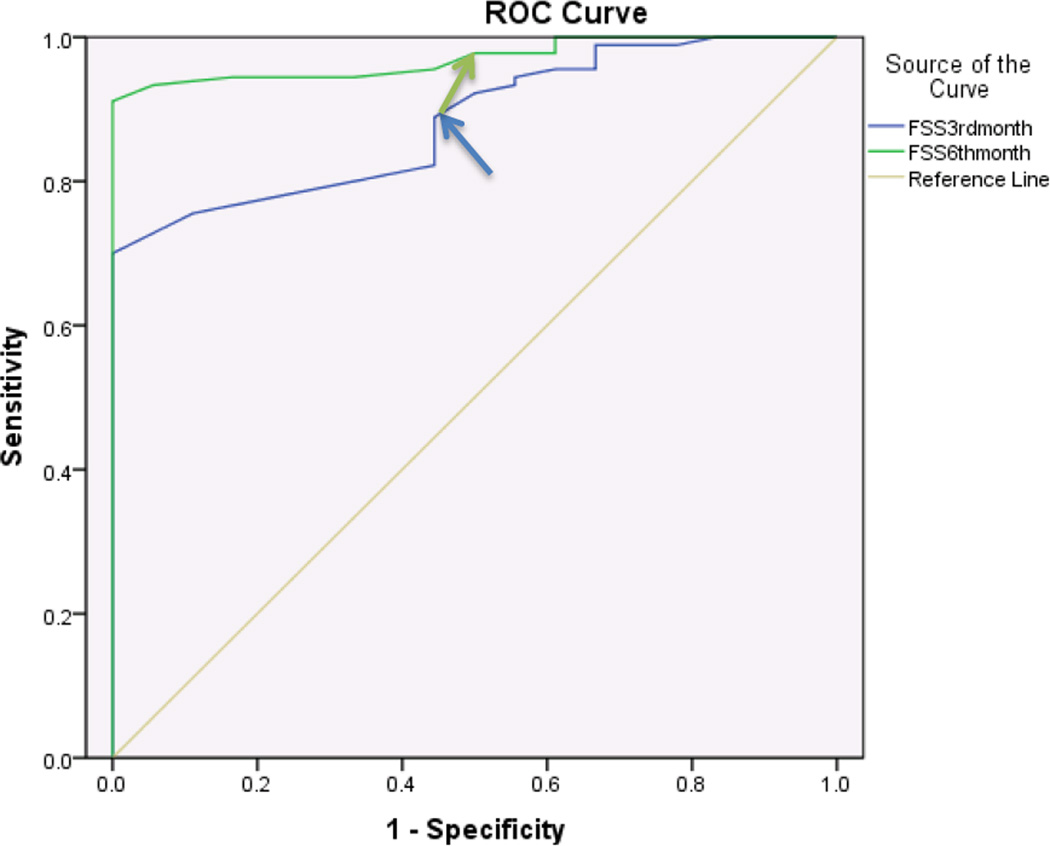
For non-diabetics, the SSS component showed discriminatory ability with regards to patient satisfaction at both 3 and 6 months after surgery with an AUC of .89 and .94 respectively. An MCID of 0.8 points and 1.6 points at 3 months and 6 months respectively were identified for SSS (Figure 3, Table 3). The FSS component of BCTQ showed discriminatory ability with regards to patient satisfaction at 3 and 6 months with an AUC’s of .88 and .97 respectively. An MCID of 1.25 points at 3 months and 1.45 points at 6 months were identified for FSS (Figure 4, Table 3). In addition, we performed a paired t-test analysis to compare means of change in scores in both groups for FSS and SSS. The mean change in the scores of SSS between 3rd and 6th month in diabetics and non-diabetics, change scores of FSS between 3rd and 6th month in diabetics and non-diabetics were statistically significant with p values <.001, <.001, 0.03 and <.001 respectively. Age of the study participants did not have a statistically significant influence on the change in BCTQ scores required for a clinically important difference. P values were 0.8, 0.5 in diabetics, and 0.8, 0.2 in non-diabetics for SSS and FSS at 6 months respectively. (Significance level is set at p=.05).
Figure 3.
Receiver operating characteristic curve for BCTQ-Symptom severity scale and patient satisfaction 3 months and 6 months after surgery in Non-Diabetics (n=108 surgeries)
Figure 4.
Receiver operating characteristic curve for BCTQ-functional status scale and patient satisfaction 3 months and 6 months after surgery in Non-Diabetics (n=108 surgeries)
Discussion
This study indicates a high level of satisfaction among diabetic patients following carpal tunnel release. Both functional and sensory BCTQ scales showed significant change in scores after surgery. Diabetic patients, however, required a higher MCID value to have the same level of satisfaction, both on the sensory as well as function scales, compared to non-diabetic patients. This perceptional difference may stem from a number of factors including autonomic dysfunction, peripheral neuropathy, limited joint mobility and other related conditions having a negative impact on hand function and sensation.5,24 In addition, the required MCID did not change with the age of the participants in diabetic and non-diabetic groups in this study.
Diabetes is suggested to be associated with poorer outcomes after CTR.25–27 This may seem reasonable, given the high incidence of sensorimotor neuropathy in diabetic patients. The evidence to support worse outcomes after CTR suffers from limited sample size, lack of a control group, and inclusion of a group without a clear diagnosis of carpal tunnel syndrome.26,27 On the contrary, recent reports support carpal tunnel surgery in diabetics. Thomsen and Dahlin reported over 95% patient satisfaction among diabetic patients.10 Mondelli and others also achieved a similar result by reporting significant improvement and satisfaction after the carpal tunnel release in diabetic patients.6–8,28,29 Because of a lack of high-grade evidence, both the American Academy of Orthopaedic Surgery and the American Academy of Neurology do not make any specific recommendations with regards to the surgical treatment (one way or another) of carpal tunnel syndrome on diabetic patients.9,30
Our results indicate that the surgery for diabetics is satisfactory in 68% of the patients. Although this may be lower than what Dahlin and others reported, it shows a beneficial effect and supports the idea of offering surgery, providing with a level 2 evidence. The difference in satisfaction rate likely stems from the duration and severity of diabetes between the two groups.
In this study, we used an anchor-based approach and explicitly defined the “minimal importance.” Although this gives a limited distribution (as satisfied, not satisfied or not sure), this approach is more clinically oriented compared to distribution-based approaches, which may produce estimates that are of no clinical relevance. Anchor-based approach also directly reflects the point of view of patients, which we consider as the standard for judging important changes. Two previous studies assessed responsiveness of the BCTQ based only on patients reporting greater satisfaction with the result of the surgery.31,32 Others reported responsiveness indices separately for each BCTQ scale.12–16,31,32 In previous studies, the effect sizes for the FSS ranged from 0.48 at 6 weeks to 1.44 at 27 weeks after surgery, and for the SSS it ranged from 1.13 at 13.5 weeks to 2.33 at 27 weeks after surgery. Effect sizes and standard response means for the SSS were higher than for the FSS, however both scales yielded moderate (>0.5) to large (>0.8) responsiveness indices supporting the notion that both scales are sensitive to clinical change in patients undergoing surgical interventions. Our results also supported these findings.
The strengths of our study include prospective design, operations performed by a single surgeon and the use of patient-reported outcome questionnaire avoiding bias. Its limitations include the smaller sample size for diabetic patients, and we did not factor in the presence of diabetic neuropathy. Therefore, our results may not be generalized to all diabetic patients.
This study provides further details of the scale properties of the BCTQ on diabetic and non-diabetic patients. A single value representing the minimal change for non-diabetics may not be used to interpret the outcome of diabetic patients, neither at an individual level nor to determine the sample size for diabetic patients. Findings of this study can be used to calculate sample size for future trials, or to determine whether a diabetic patient has improved to a clinically significant level.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K24 AR053120 (to Dr. Kevin C. Chung). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Papanicolaou GD, McCabe SJ, Firrell J. The prevalence and characteristics of nerve compression symptoms in the general population. J Hand Surg Am. 2001;26:460–466. doi: 10.1053/jhsu.2001.24972. [DOI] [PubMed] [Google Scholar]
- 2.Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 3.Becker J, Nora DB, Gomes I, et al. An evaluation of gender, obesity, age and diabetes mellitus as risk factors for carpal tunnel syndrome. Clin Neurophysiol. 2002;113:1429–1434. doi: 10.1016/s1388-2457(02)00201-8. [DOI] [PubMed] [Google Scholar]
- 4.Singh R, Gamble G, Cundy T. Lifetime risk of symptomatic carpal tunnel syndrome in Type 1 diabetes. Diabet Med. 2005;22:625–630. doi: 10.1111/j.1464-5491.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 5.Chammas M, Bousquet P, Renard E, Poirier JL, Jaffiol C, Allieu Y. Dupuytren's disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus. J Hand Surg Am. 1995;20:109–114. doi: 10.1016/S0363-5023(05)80068-1. [DOI] [PubMed] [Google Scholar]
- 6.Mondelli M, Padua L, Reale F, Signorini AM, Romano C. Outcome of surgical release among diabetics with carpal tunnel syndrome. Arch Phys Med Rehabil. 2004;85:7–13. doi: 10.1016/s0003-9993(03)00770-6. [DOI] [PubMed] [Google Scholar]
- 7.Ozkul Y, Sabuncu T, Kocabey Y, Nazligul Y. Outcomes of carpal tunnel release in diabetic and non-diabetic patients. Acta Neurol Scand. 2002;106:168–172. doi: 10.1034/j.1600-0404.2002.01320.x. [DOI] [PubMed] [Google Scholar]
- 8.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27:1458–1486. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry V, Stevens JC, Kincaid J, So YT. Practice Advisory: utility of surgical decompression for treatment of diabetic neuropathy: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2006;66:1805–1808. doi: 10.1212/01.wnl.0000219631.89207.a9. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen NO, Cederlund R, Rosen I, Bjork J, Dahlin LB. Clinical outcomes of surgical release among diabetic patients with carpal tunnel syndrome: prospective follow-up with matched controls. J Hand Surg Am. 2009;34:1177–1187. doi: 10.1016/j.jhsa.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Pynset P, Fairbank JCT, Carr AJ. Outcome Measures in Orthopaedics. Oxford: Butterwoth-Heinemann; 1993. [Google Scholar]
- 12.Levine DW, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg. 1993;75A:1585–1615. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Amadio PC, Silverstein MD, Ilstrup DM, Schleck CD, Jensen LM. Outcome assessment for carpal tunnel surgery: the relative responsiveness of generic, arthritis-specific, disease-specific, and physical examination measures. J Hand Surg Am. 1996;21:338–346. doi: 10.1016/S0363-5023(96)80340-6. [DOI] [PubMed] [Google Scholar]
- 14.Atroshi I, Johnsson R, Sprinchorn A. Self-administered outcome instrument in carpal tunnel syndrome. Reliability, validity and responsiveness evaluated in 102 patients. Acta Orthop Scand. 1998;69:82–88. doi: 10.3109/17453679809002363. [DOI] [PubMed] [Google Scholar]
- 15.Gay RE, Amadio PC, Johnson JC. Comparative responsiveness of the disabilities of the arm, shoulder, and hand, the carpal tunnel questionnaire, and the SF-36 to clinical change after carpal tunnel release. J Hand Surg Am. 2003;28:250–254. doi: 10.1053/jhsu.2003.50043. [DOI] [PubMed] [Google Scholar]
- 16.Greenslade JR, Mehta RL, Belward P, Warwick DJ. Dash and Boston questionnaire assessment of carpal tunnel syndrome outcome: what is the responsiveness of an outcome questionnaire? J Hand Surg Br. 2004;29:159–164. doi: 10.1016/j.jhsb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Beaton DE, Bombardier C, Katz JN, Wright JG. A taxonomy for responsiveness. J Clin Epidemiol. 2001;54:1204–1217. doi: 10.1016/s0895-4356(01)00407-3. [DOI] [PubMed] [Google Scholar]
- 18.Klein RD, Kotsis SV, Chung KC. Open carpal tunnel release using a 1-centimeter incision: technique and outcomes for 104 patients. Plast Reconstr Surg. 2003;111:1616–1622. doi: 10.1097/01.PRS.0000057970.87632.7e. [DOI] [PubMed] [Google Scholar]
- 19.Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve. 2002;25:918–922. doi: 10.1002/mus.10185. [DOI] [PubMed] [Google Scholar]
- 20.Association AD. Standards of medical care in diabetes-2007. Diabetes Care. 2007;30(Suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 21.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 22.Skolasky RL, Albert TJ, Maggard AM, Riley LH., 3rd Minimum clinically important differences in the Cervical Spine Outcomes Questionnaire: results from a national multicenter study of patients treated with anterior cervical decompression and arthrodesis. J Bone Joint Surg Am. 2011;93:1294–1300. doi: 10.2106/JBJS.J.01136. [DOI] [PubMed] [Google Scholar]
- 23.Shauver MJ, Chung KC. The minimal clinically important difference of the Michigan hand outcomes questionnaire. J Hand Surg Am. 2009;34:509–514. doi: 10.1016/j.jhsa.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771–775. doi: 10.1016/j.jhsa.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 25.Turner A, Kimble F, Gulyas K, Ball J. Can the outcome of open carpal tunnel release be predicted?: a review of the literature. ANZ J Surg. 2010;80:50–54. doi: 10.1111/j.1445-2197.2009.05175.x. [DOI] [PubMed] [Google Scholar]
- 26.al-Qattan MM, Manktelow RT, Bowen CV. Outcome of carpal tunnel release in diabetic patients. J Hand Surg Br. 1994;19:626–629. doi: 10.1016/0266-7681(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 27.Haupt WF, Wintzer G, Schop A, Lottgen J, Pawlik G. Long-term results of carpal tunnel decompression. Assessment of 60 cases. J Hand Surg Br. 1993;18:471–474. doi: 10.1016/0266-7681(93)90149-a. [DOI] [PubMed] [Google Scholar]
- 28.Perkins BA, Olaleye D, Bril V. Carpal tunnel syndrome in patients with diabetic polyneuropathy. Diabetes Care. 2002;25:565–569. doi: 10.2337/diacare.25.3.565. [DOI] [PubMed] [Google Scholar]
- 29.Pagnanelli DM, Barrer SJ. Outcome of carpal tunnel release surgery in patients with diabetes. Neurosurg Focus. 1997;3:e9. doi: 10.3171/foc.1997.3.4.10. [DOI] [PubMed] [Google Scholar]
- 30.Keith MW, Masear V, Amadio PC, et al. Treatment of carpal tunnel syndrome. J Am Acad Orthop Surg. 17:397–405. doi: 10.5435/00124635-200906000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Bessette L, Sangha O, Kuntz KM, et al. Comparative responsiveness of generic versus disease-specific and weighted versus unweighted health status measures in carpal tunnel syndrome. Med Care. 1998;36:491–502. doi: 10.1097/00005650-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Katz JN, Gelberman RH, Wright EA, Lew RA, Liang MH. Responsiveness of self-reported and objective measures of disease severity in carpal tunnel syndrome. Med Care. 1994;32:1127–1133. doi: 10.1097/00005650-199411000-00005. [DOI] [PubMed] [Google Scholar]