Abstract
Unconventional myosins are a superfamily of actin-based motors implicated in diverse cellular processes. In recent years, much progress has been made in describing their biophysical properties, and headway has been made into analyzing their cellular functions. Here, we focus on the principles that guide in vivo motor function and targeting to specific cellular locations. Rather than describe each motor comprehensively, we outline the major themes that emerge from research across the superfamily and use specific examples to illustrate each. In presenting the data in this format, we seek to identify open questions in each field as well as to point out commonalities between them. To advance our understanding of myosins’ roles in vivo, clearly we must identify their cellular cargoes and the protein complexes that regulate motor attachment to fully appreciate their functions on the cellular and developmental levels.
Keywords: motor protein, cargo, trafficking, actin, cytoskeleton
INTRODUCTION TO UNCONVENTIONAL MYOSINS
The highly dynamic actin and microtubule cytoskeletons, with their dozens of associated motors and other accessory proteins, provide the cell with structure and organization. These tracks, along which molecular motors travel, are polarized, with so-called (+) ends and (−) ends, and the (+) ends of both types of tracks are directed toward the plasma membrane (Ross et al. 2008a). Although highly organized, the cytoskeleton changes upon cues from a wide variety of sources, such as entry into mitosis or in response to external signals, and motor proteins can mediate many such changes (Goode et al. 2000).
Much effort has advanced our understanding of the structure and biophysical properties of the molecular motors themselves and the tracks along which they move. Kinesins, dyneins, and myosins couple the chemical energy of ATP hydrolysis to precise mechanical motions, hence their classification as motor proteins (Goode et al. 2000). This review focuses on actin-based myosin motors. Actin is a highly conserved protein that assembles into filaments with monomers arranged in a left-handed, single-start helix. This configuration yields two protofilaments that twist around each other in a right-handed fashion. The helical repeat of each protofilament is 72 nm, which gives the filament a pseudorepeat of 36 nm (Howard 1997).
Myosins are defined by a characteristic ~80-kDa catalytic domain, the head, that contains the actin- and nucleotide-binding sites. Following the catalytic domain is a neck region consisting of an α-helical strand stabilized by the binding of one or more calmodulin or calmodulin-like light chain subunits. The sequence of the tail, the C-terminal region, is the most divergent among different myosin classes. It serves purposes such as mediating the association of myosins with each other, anchoring myosins for movement relative to actin filaments, and specifying binding to different cargoes (Foth et al. 2006).
The myosin motor superfamily contains more than 20 distinct classes, and in humans, as many as 40 myosin genes may be expressed (Foth et al. 2006). Different myosins serve different functions and are built accordingly. Conventional myosin II, for example, found in muscle and in the contractile ring of nonmuscle cells, forms bipolar thick filaments that pull actin filaments together to produce contractions, moving towards the (+) end of actin filaments (Howard 1997). In contrast, the unconventional myosin motors, on which this review is focused, do not form bipolar thick filaments. All those characterized move toward the (+) end of actin filaments except for myosin VI, which moves in the opposite direction (Wells et al. 1999).
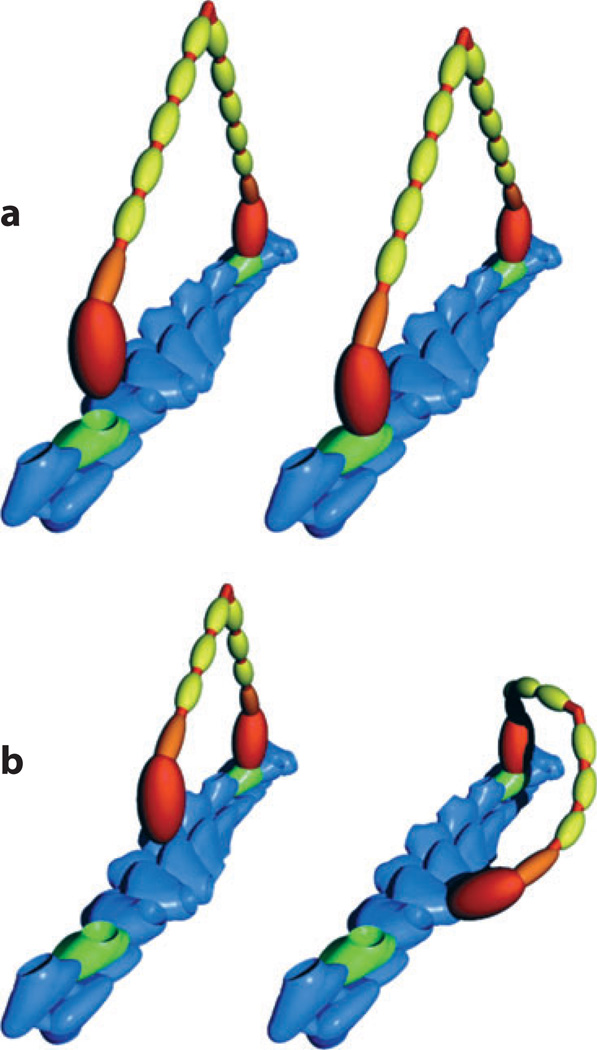
The ATPase activity of myosin is coupled to the binding and release of actin such that the motor is able to do mechanical work; with each cycle of ATP hydrolysis, the motor takes one step. Directed motion depends on the neck region, where the light chain-associated lever arm amplifies smaller conformational changes that occur during the catalytic cycle. The length of the lever arm is significantly different among the classes of myosin motors, which gives rise to different step sizes. The long myosin V lever arm, for example, allows this motor to extend from one helical ridge of an actin filament to the next ridge, 36 nm away (Figure 1a; Howard 1997).
Figure 1.
Myosin movement along actin filaments (blue). (a) A myosin V with a long lever arm bound by six light chains (orange and yellow) reaches a binding site on the same face of the filament (green), 36 nm away. (b) A myosin V with an artificially short lever arm, or any myosin with a shorter step size, must twist around the filament to find a binding site. From Purcell et al. (2002).
Another difference among the different myosin motors is the amount of time the molecule spends tightly bound to an actin filament during each ATP hydrolysis cycle. Each head of myosin II, for example, spends only a few milliseconds, or ~5% of its ATPase cycle time, tightly bound, whereas each head of the unconventional myosins V and VI spends more than 50% of its ATPase cycle time in a strongly bound state. The higher value accounts for the processivity of myosins that take many sequential steps along a single filament (Howard 1997).
Thus, each type of molecular motor takes a step of particular length that propels the motor in a specific direction along its polarized track. A wealth of information is now available regarding the detailed mechanisms by which motors transduce the chemical energy of ATP hydrolysis into mechanical movement, and many insights have been obtained that pertain to fundamental issues of protein structure and function. The reader is referred to excellent reviews for in-depth descriptions of the mechanism of energy transduction by actin-based and microtubule-based motors alike (Gennerich & Vale 2009; Howard 1997; Kardon & Vale 2009; O’Connell et al. 2007; Spudich & Sivaramakrishnan 2010; Sweeney & Houdusse 2010a,b; Vale & Milligan 2000).
In contrast, we know much less about how molecular motors associate with their cargoes and deliver them within a cell. Indeed, there is a general paucity of biochemical information about the nature of the cargoes themselves. The proteins that link the cargoes to their respective motors and the regulation of those interactions are also largely unknown, and we do not understand much about how the movement of traffic is regulated. Some studies suggest that there may be an array of protein complexes, and any one complex may consist of multiple motor types and all of their associated regulatory elements. There is much to be learned in this important area of cell biology, and we highlight what is known to help identify what remains to be done. We have not attempted to be comprehensive in our description of the literature on these research areas but rather have chosen selected examples (see Table 1) to illustrate approaches in use and directions for future research. We refer the reader to related reviews (Akhmanova & Hammer 2010; Bahler 2000; Goldstein 2001a,b; Hirokawa et al. 2009; Kamal & Goldstein 2002; Kim & Flavell 2008; Krendel & Mooseker 2005; Lin et al. 2005a; Nambiar et al. 2010; Sellers 2000; Soldati & Meissner 2004; Sousa & Cheney 2005; Sparkes 2010; Sweeney & Houdusse 2007; Titus 2009; Trybus 2008; Woolner & Bement 2009) for examples that are not covered here.
Table 1.
Examples of function, targeting, and regulation of unconventional myosins, organized by myosin class
| Name | Class | Species | Example illustrated |
|---|---|---|---|
| Myo1a | I | Vertebrates | Regulation of membrane tension in intestinal brush border cells |
| Myo1b | I | Rat | Differential expression patterns of splice isoforms that behave differently under load |
| Myo1c | I | Vertebrates | Adaptation in hair-cell stereocilia |
| Myo1c | I | Mouse | Ca2+-dependent regulation of cargo binding in adipocytes upon insulin stimulation |
| Myo1f | I | Mouse | Migration versus adhesion of neutrophils |
| Myo1g | I | Mammals | Pleckstrin homology domain-mediated binding to phosphoinositides that results in tight association with plasma membranes |
| Myosin I | I | Yeast | Stimulation of actin nucleation/polymerization |
| Myosin I | I | Yeast | Inactivation by tropomyosin-associated filaments |
| Myosin I | I |
Acanthamoeba, Dictyostelium, yeast |
Phosphorylation at TEDS rule site required for myosin function |
| Myo3A | III | Mammals | Autophosphorylation of motor domain affects motor function and localization |
| Myo2p | V | Yeast | Degradation of cargo-binding protein Vac17p provides possible mechanism for cargo switching |
| Myo4p | V | Yeast | Transport of mRNAs to bud tip |
| Myosin V | V | Yeast | Tropomyosin-dependent effect on motor’s ATPase rates and processivity |
| Myosin V | V | Mouse, Xenopus | Tethers melanosomes to cortical actin cytoskeleton |
| Myosin V | V | Vertebrates | Cell cycle– or signaling-dependent phosphorylation of tail domain regulates motor activity |
| Myosin V | V | Mammals | Interaction with the melanophilin adaptor-Rab27a complex is required for melanosome transport |
| Myosin V | V | Mammals, Drosophila |
Concentration of Ca2+ affects processive movement, which might influence function in fly photoreceptors |
| Myosin Va | V | Mammals | Disruption of interaction with SNARE complex by Ca2+ regulates signaling |
| Myosin Va | V | Mammals | Differentially spliced exons provide different binding sites for cargo-binding proteins |
| Myosin VI | VI | Drosophila | Actin organization in spermatid individualization |
| Myosin VI | VI | Mammals, Drosophila |
Potential cargo-mediated dimerization |
| Myosin VI | VI | Mammals | Different splice isoforms show different localization patterns and may play different roles in endocytosis |
| Myosin VIIa | VII | Mammals | Ca2+ disrupts inhibited state in which head and tail interact with each other |
| Myosin VII | VII | Mammals | Formation of a complex with MyRIP and Rab27a is required for targeting in retinal pigment epithelium |
| Myosin VIIa | VII | Mammals | FERM domain in tail recruits myosin to a large complex involved in cell adhesion |
| Myosin X | X | Mammals | Preferential movement on bundled actin, such as in filopodia |
| Myosin XI-K, −1, -2, -B, and -I |
XI | Flowering plants | Family of myosins that play overlapping roles in vesicle trafficking and organelle motility |
| MyoA | XIV | Apicomplexan parasites |
Cell movement (gliding motility) and infectivity |
| MyoA | XIV | Toxoplasma | Effect of lipid composition on localization of myosin complex in membrane |
| Myo18a | XVIII | Human | Organization and structure of Golgi complex |
| Myo19 | XIX | Mammals | Transport of mitochondria in neurons |
INFERRED FUNCTIONS
A given cell can have as many as 100 different types of motor proteins performing the wide variety of functions that are crucial for its organization. Each motor likely is involved in multiple functions, and each function likely depends on multiple types of motors. In this section, we describe some of the specific functions that have been ascribed to unconventional myosins. The names of specific myosins are capitalized and begin with “Myo,” whereas the class is designated as a roman numeral preceded by “myosin.” We also use the term, for example, “type V myosin” to refer to the class.
Transport
Processive motors are well suited for the task of transporting cargo along cytoskeletal tracks, but there are only a few clear examples of unconventional myosins playing that role in cells. One of the best studied is the transport of mRNA by a type V myosin in Saccharomyces cerevisiae, Myo4p. Polarized actin cables in yeast provide a route for the transport of macromolecules from the mother cell to the tip of the bud, and Myo4p transports nearly 30 mRNAs there (reviewed in Gonsalvez et al. 2005). One of these mRNAs, encoding the transcription factor ASH1, is required for the inhibition of mating type switching and localizes exclusively to the daughter cell (reviewed in Muller et al. 2007). Bertrand et al. (1998) observed Myo4p moving fluorescently labeled ASH1 mRNA toward the bud tip in live cells at a rate of 200– 400 nm s−1. Subsequent work has shown that Myo4p is a monomeric and nonprocessive motor that constitutively associates with an adaptor protein, She3p (Dunn et al. 2007). The protein She2p, which binds to structural elements in the ASH1 mRNA, recruits Myo4p-She3p to form a ribonucleoprotein (RNP) complex that transports the mRNA to the bud tip (Long et al. 2000). Thus, despite its nonprocessive activity in isolation, ensembles of multiple Myo4p motors are able to move ASH1 mRNA molecules across large distances in cells. This is likely accomplished by tetramers of She2p recruiting multiple motors into each transport particle (Chung & Takizawa 2010).
Molecular motors are responsible for the regulated transport and immobilization of mitochondria within cells (reviewed in Titus 2009 and others). A recently characterized unconventional myosin, Myo19 (type XIX), associates tightly with mitochondria in a manner dependent on its short tail domain. When the tail region of Myo19 was overexpressed in a neuronal cell line, run lengths of mitochondria in neurites decreased significantly, by approximately 40%. Overexpression of full-length Myo19 in a human epithelial cell line increased mitochondrial run lengths beyond their typically short, saltatory movements (Quintero et al. 2009). Although much remains to be learned about its recruitment to and coordination at mitochondrial membranes, Myo19 is at least partially responsible for transporting mitochondria in mammalian neurons. Interestingly, the Drosophila genome lacks this class of motor, and the transport of mitochondria in Drosophila axons is microtubule-motor based. Furthermore, myosin V and myosin VI seem to play an important role in opposing this transport by serving as anchors or tethers (Pathak et al. 2010), which is another function ascribed to unconventional myosins and discussed in more detail below.
Anchoring/Tethering
Melanosome trafficking has proved an advantageous system to study both transport and tethering by molecular motors. Made by melanocytes, these membrane-bound organelles contain pigment granules and are transported to the tips of dendritic processes for transfer to keratinocytes. Mice lacking myosin V are known as dilute because their coat color is lighter than that of wild-type mice owing to defects in melanosome transport (reviewed in Tuxworth & Titus 2000). Melanosomes are uniformly distributed in the bulk of the cytoplasm of wild-type melanocytes, with a concentration of the granules at the tips of dendrites. In contrast, melanosomes from dilute mice, while normal in number and morphology, have a distinct perinuclear distribution (Figure 2a; Provance et al. 1996), which leads to the hypothesis that myosin V is involved in their transport to the tips of dendrites or their tethering there.
Figure 2.
Myosin V tethers melanosomes to the cortical actin cytoskeleton. (a) Melanosomes are distributed throughout the cytoplasm in wild-type melanocytes (left) but cluster around the nucleus in melanocytes from dilute (myosin V–null) mice (right). Adapted with permission from Wu et al. (2001). (b) The interaction between myosin V and melanosomes is regulated by cargo (a complex of melanophilin and Rab27a in the membrane), Ca2+, and phosphorylation. Myosin V can interrupt fast, long-range transport of melanosomes by microtubule motors to anchor the organelles in the cell periphery in preparation for release.
Wu et al. (1998) elegantly demonstrated that the long-range, bidirectional transport of melanosomes is actually driven by microtubule motors and that myosin V is responsible only for short intermittent steps, primarily in the distal actin-rich region of the dendrites. The hypothesis that myosin V plays a tethering role was supported by the demonstration that overexpression of the dominant-negative myosin V tail in cells increased rapid movement of melanosomes toward the center of the cell (Levi et al. 2006). Recent work has shed some light on the mechanism of tethering by myosin V; single-particle tracking of melanosomes from Xenopus melanophores indicated that the motor transports the organelles along actin filaments with steps nearly the same size as the pseudorepeat of the actin filament (Levi et al. 2006). Transport occurred for short distances only before the motor fell off the filament, diffused passively, and then reattached to a nearby, randomly oriented filament (Brunstein et al. 2009). Thus, myosin V interrupts otherwise longer, microtubule-based movements within the cell (Figure 2b). This mechanism, most prominent in the dense actin network of the cell cortex, presumably prevents the microtubule motors attached to the same melanosomes from rapidly transporting the organelles back to the center.
Another system for studying tethering by molecular motors is Myo18a (type XVIII) function in the organization of the Golgi complex in HeLa cells, where it likely anchors the membranous organelle to actin filaments. Myo18a interacts directly with Golgi phosphoprotein 3 (GOLPH3), a lipid-binding, Golgi complex– localized protein; depletion of either protein leads to smaller, dilated Golgi stacks and reduced protein secretion, phenotypes similar to those caused by treatment with the actin-disrupting drug latrunculin. These data suggest a role for the Myo18a-GOLPH3 complex in linking actin to the Golgi complex and therefore in the stretching of the membrane to form functional, flattened cisternae (Dippold et al. 2009). Although it is not clear exactly how the required tensile forces are generated at the Golgi membrane, this process likely involves at least one other myosin (type VI; Warner et al. 2003) and a host of other factors; sensing tension is the next function we discuss for unconventional myosins.
Tension Sensing
Plasma membrane tension is thought to control cellular processes that involve membrane structure and dynamics. In intestinal brush border cells, where particularly high levels of membrane-cytoskeleton adhesion are needed to maintain and stabilize microvilli, Myo1a (type I) directly contributes to membrane tension and enables the apical membrane to resist deformation (Nambiar et al. 2009). Members of the myosin I family are typically divided into two subclasses on the basis of differences in their catalytic motor domains. Class 1 domains include most of the protozoan isoforms and Myo1e, a long-tailed myosin I. These are thought to be well suited to function in regions of the cell with high concentrations of cross-linked actin filaments and could participate in rapid sliding events (such as pseudopod extension). The Class 2 catalytic domains, which are found in the majority of vertebrate type I myosins, have slower ATPase rates and could have structural roles or participate in maintaining tension at cell membranes (Barylko et al. 2005, De La Cruz & Ostap 2004, El Mezgueldi et al. 2002). However, the categories are probably not that straightforward, as Myo1e was the most effective of all the isoforms tested at increasing the tether force in optical trap experiments (Nambiar et al. 2009). Thus, as all myosin I isoforms tested are apparently capable of sensing tension and responding to small loads by increasing their actin-attachment lifetime (Laakso et al. 2008), they may carry out all of their cellular transport roles either directly or indirectly through regulating membrane tension.
Myo1c (type I) is one of the many myosins essential for hearing, and mutations in its sequence cause deafness in humans (Zadro et al. 2009). The protein localizes to stereocilia, the actin-based projections that mediate responsiveness to sound in inner ear hair cells. Upon mechanical deformation of stereocilia, ion channels at their tips open to allow influx of Ca2+ and downstream signaling to the nerves underneath. With a prolonged stimulus, these channels close so that responsiveness to new stimuli is maintained, a process known as adaptation (reviewed in Gillespie & Muller 2009). Myo1c, which is essential for adaptation (Holt et al. 2002, Stauffer et al. 2005), is located at sites on taller stereocilia where they attach to their shorter neighbors (Garcia et al. 1998) and is in a complex with the adhesion proteins that make up those links (Siemens et al. 2004). Myo1c is therefore poised to sense the increased tension upon deformation and provide adaptation by slipping down the taller stereocilium, releasing the tension and allowing channel closure. Or, its function in adaptation may be through the pool of Myo1c located near the channels at the shorter tips (Garcia et al. 1998), where the Ca2+ influx may influence its activity (Gillespie & Muller 2009). Biophysical studies examining the combined effect of tension and Ca2+ on the motor properties will help in distinguishing between these models.
Actin Organization
In addition to carrying out motor functions on actin filaments, myosins can influence the organization of actin itself. Myosin I affects the distribution and structure of actin filaments in both budding and fission yeast (Goodson et al. 1996, Lee et al. 2000), where it associates and shares functions with orthologs of WASP (Wiskott-Aldrich syndrome protein) and WIP (WASP-interacting protein) (Carnahan & Gould 2003, Evangelista et al. 2000, Geli et al. 2000, Goodson et al. 1996, Lee et al. 2000, Sirotkin et al. 2005). WASP has a well-documented role in actin filament nucleation through activation of the Arp2/3 complex (reviewed in Campellone & Welch 2010). The combination of a yeast myosin I tail and WIP can stimulate actin polymerization via Arp2/3 in vitro (Lechler et al. 2001, Sirotkin et al. 2005), and this complex is redundant with (and may also contain) WASP in vivo (Anderson et al. 1998, Carnahan & Gould 2003, Evangelista et al. 2000, Geli et al. 2000, Lechler et al. 2000, Lee et al. 2000). These data suggest a model in which a myosin I tail can affect actin dynamics while bound to filaments though the head domain, thus enabling specific regulation of polymerization at given sites in the cell.
Myosin VI also functions in actin organization, and this role is well documented in Drosophila spermatogenesis. After undergoing several rounds of nuclear division without cell division, spermatids are separated by the individualization complex (IC), which contains several types of actin structures that propel its movement (Noguchi et al. 2008). Myosin VI is essential for the sustained movement of the IC, and its absence leads to male sterility in flies (Hicks et al. 1999). The presences of myosin VI and Arp3 at the leading edge of the actin cone are dependent on each other, suggesting that they may associate (Noguchi et al. 2008, Rogat & Miller 2002). Although the exact mechanism of myosin VI’s involvement in actin structure is not clear, it likely involves the motor activity (Noguchi et al. 2006) and may be different from that of myosin I. Further work in vitro may help clarify how Drosophila myosin VI influences filament organization.
Cell Motility/Adhesion
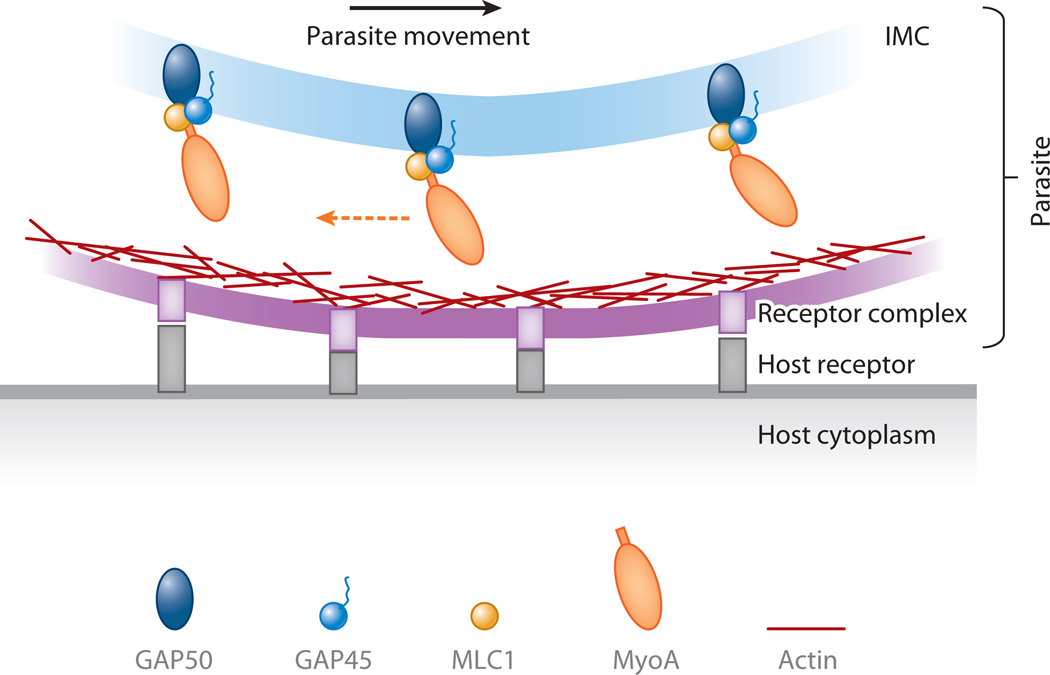
Unconventional myosins have been proposed to generate the force for cell motility, as type XIV myosins do in apicomplexan parasites. Toxoplasma, Plasmodium, and other organisms in this phylum are intracellular parasites that cause devastating diseases. During the part of their life cycle in which they enter into and spread between host cells, they exhibit what is known as gliding motility (Soldati & Meissner 2004). Gliding motility, and therefore infectivity, critically depends on their myosins of the novel class XIV, such as MyoA in Toxoplasma gondii (Meissner et al. 2002). MyoA is situated in the inner membrane complex just underneath the plasma membrane (Gaskins et al. 2004), to which several proteins anchor actin (Soldati & Meissner 2004). The actin complex is also linked to the extracellular proteins that bind host cell receptors such that when MyoA walks along the actin filaments, the parasite can generate traction along the host cells and produce the force needed for forward movement (Figure 3; Soldati & Meissner 2004). Either the myosin or the actin must be oriented for directed movement to occur; this could be accomplished via immobilization of MyoA in the membrane (see below) or by actin organization (Wetzel et al. 2003). MyoA has a small step size (~5 nm) and a very fast velocity (~5 µm s−1) (Herm-Gotz et al. 2002); the combination of its physical properties and biological function begets its comparison with muscle myosin and sheds light on how unicellular organisms use an unconventional myosin for cell movement.
Figure 3.
The unconventional myosin MyoA generates force for cell motility. MyoA is anchored to the inner membrane complex (IMC) via its myosin light chain 1 (MLC1) and gliding-associated proteins (GAPs), and actin is connected to the receptors that bind host molecules. As the myosin strokes (dashed arrow), the force is utilized for movement.
Unconventional myosins also play indirect roles in cell motility. One of the two long-tailed isoforms of the myosin I family, Myo1f, is expressed mainly in immune cells. Neutrophils from the Myo1f-null mouse have no defects in phagocytosis or pathogen killing, but they exhibit increased adhesion and a decreased ability to migrate. The null mice are thus more susceptible to Listeria, as the neutrophils fail to mobilize and protect against infection in the absence of Myo1f. The defect in cell motility in this system is a result of increased exocytosis of integrin-containing granules, which Myo1f normally inhibits at the cell cortex to regulate the adhesion of neutrophils to the surface (Kim et al. 2006).
REGULATION OF FUNCTION
The functions described above represent some of what myosins accomplish on a cellular level. Considering how many myosins are present in a cell, mechanisms must be in place to ensure they function only at the appropriate times and in the correct compartments. Here, we discuss how their functions are regulated by highlighting well-studied systems as examples of what may be broadly used patterns.
Phosphorylation
A consensus phosphorylation site, known as the TEDS (Thr, Glu, Asp, Ser) rule site, in the head domain of many myosins influences their activities. The kinases responsible for phosphorylation of Acanthamoeba and Dictyostelium myosin I at a TEDS site were identified some time ago, and similar kinases are thought to act on yeast type I myosins. Mutation of the TEDS site to create a nonphosphorylatable residue has functional consequences in the organisms studied. Myosin I with a Thr/Ser to Ala mutation is generally unable to substitute for wild type, whereas myosin I with a change to Glu or Asp is usually able to rescue a null mutation at least partially (reviewed in Barylko et al. 2000). Cdc42 and binding to lipids appear to regulate the kinases (Barylko et al. 2000; Lechler et al. 2000, 2001); further characterization of the enzymes responsible for myosin I phosphorylation should reveal essential information about this mode of regulation.
Myo3a (type III) contains an active serine/threonine kinase domain and autophosphorylates a site in its motor domain in a concentration-dependent, intermolecular event. Phosphorylation of that site inhibits the motor’s ATPase activity and decreases its affinity for actin, and a kinase-dead mutant of Myo3a (K50R) is significantly enriched at the tips of filopodia in transfected mammalian cells. This implies that in regions of cells where concentrations of Myo3a are high, phosphorylation negatively regulates motor function and could result in the diffusion or transport of Myo3a away from the tips of actin bundles, thereby providing a mechanism for regulating the number of these protrusions in cells (Quintero et al. 2010).
Calcium-Dependent Activation
Intracellular Ca2+ concentration can also influence myosin function. Similar to myosin VII, mammalian myosin V has been shown by electron microscopy and analytical ultracentrifugation to fold into a more compact, inactive structure in the absence of Ca2+ (Wang et al. 2004). As the concentration of Ca2+ rises, the motor domain extends away from the globular tail domain (Figure 2b), and ATPase activity resumes. However, in the presence of very high Ca2+ concentrations, the calmodulin light chains dissociate from the heavy chain, and the motor no longer moves processively (Lu et al. 2006). This raises the question of how myosin V activity is regulated in cells: Is binding of cargo to the globular tail domain sufficient to activate the motor, and can high localized Ca2+ concentrations terminate processive runs and stop the transport of cargo when appropriate?
The Drosophila eye is a case in which such regulation seems to occur. In fly photoreceptors, the intracellular Ca2+ concentration rises significantly upon exposure to bright light, and small pigment granules migrate toward the rhabdomere, the light-sensitive organelle consisting of tens of thousands of densely packed microvilli. These pigment granules serve a function analogous to the pupil: When the granules are packed against the base of the rhabdomere, the amount of light transmitted decreases, and reflection from the eye increases (Stavega & Kuiper 1977). In myosin V loss-of-function fly eyes, the pigment granules fail to migrate, and thus these pseudopupils do not “close,” even after prolonged exposure to bright light. The level of endogenous calmodulin likely needs to be high enough such that even in the presence of light-induced increased Ca2+ levels, the motility of myosin V is preserved. In a mutant expressing less than 10% of the wild-type calmodulin level, myosin V fails to localize normally, and pigment granule transport is abolished (Satoh et al. 2008).
Intra- and Intermolecular Associations
Myosins also may regulate their functions through intramolecular interactions. Best described for myosin Va (Trybus 2008) and also noted for a type I myosin (Stoffler & Bahler 1998), the folding of the tail domain to interact with the head and inhibit ATPase activity may be a general mechanism of myosin regulation. Myosin VIIa, for example, was also found recently to possess a folded, inhibited state involving at least one subdomain in the tail. Ca2+ disrupts the head-tail interaction, as is the case for myosin Va, which suggests a possible mechanism for intracellular activation (Umeki et al. 2009, Yang et al. 2009). As myosin VIIa is essential for hearing, a process known to involve calcium signaling (reviewed in Gillespie & Muller 2009), this mode of regulation may indeed be functionally significant.
There has been much discussion on whether unconventional myosins are predominantly monomers or dimers, as their oligomeric state has important implications for their abilities to sense tension and/or move processively. When analyzed in vitro, many myosins are artificially dimerized to facilitate single-molecule analysis, and there have been far fewer investigations into this issue in vivo. For myosin VI, however, this issue is particularly relevant. Full-length myosin VI purified using baculovirus expression (Lister et al. 2004) or isolated from Drosophila embryos is a monomer (Noguchi et al. 2009), and several groups have suggested a cargo-mediated dimerization model. A truncated myosin VI and cargo have been crystallized in the dimeric state (Yu et al. 2009), and other in vitro data provide evidence for this model (Park et al. 2006, Phichith et al. 2009). The clearest in vivo support comes from ARPE-19 cells, a retinal pigment epithelial cell line in which the motor is known to have a role in vesicle trafficking (Aschenbrenner et al. 2003, 2004; Park et al. 2006; Phichith et al. 2009). Fluorescence anisotropy measurements indicate that the myosin VI cargo-binding domain is monomeric in the cytosol and self-associates on membranous cargo (Altman et al. 2007), likely by binding to dimeric proteins on the cargo’s periphery. Analogous experiments in other cell types and with other myosin tails will help in determining how universal this model is.
Isoform Specificity of Function
Regulation of the mRNA splicing of unconventional myosins into different isoforms can also influence their functions in cells, as is the case for the tension sensing of myosin Ib. Rat myosin Ib has at least three isoforms that vary in the neck region, creating combinations of four to six light chain–binding domains, and that are differentially expressed in a variety of tissues (Ruppert et al. 1993). The proteins have similar kinetic properties, and differences in their velocities can be attributed to the variable lever arm lengths (Lin et al. 2005b). Their behavior under load distinguishes the isoforms, as their distinct responses to force lead to different attachment times in an optical trap (Laakso et al. 2010), which implies that they have different functions in their respective environments and are adapted to respond to the levels of force that they each encounter.
Conversely, gene duplication events have resulted in several similar myosins performing overlapping but slightly specialized roles (Peremyslov et al. 2010). This is seen most prominently in plants, which have the most rapid known organelle transport powered by actin and myosins. Flowering plants such as Arabidopsis possess 13 myosins in the class XI family, which are structurally related to mammalian type V myosins. The absence of any one of these genes does not cause major developmental defects. Rather, knocking out paralogous pairs is necessary to observe stunted growth phenotypes. Inactivation of pairs of closely related myosins also results in a significant reduction in the motility of Golgi stacks, peroxisomes, and mitochondria in leaf epidermal cells (Prokhnevsky et al. 2008). These results are difficult to interpret and imply that each of the myosin XI family members contributes to intracellular trafficking to different degrees. Further research is needed to clarify the mechanism of specialization of type XI myosins, be it through association with different cargoes, heterodimerization, or differential expression patterns.
Actin Structure and Binding Proteins
The organization of actin filaments can also influence myosin function, a phenomenon perhaps best exemplified by the preference of myosin X to walk along bundled actin. Myosin X localizes to and moves along filopodia, thin cellular extensions that contain bundled actin (Berg & Cheney 2002), and participates in their formation (Bohil et al. 2006, Tokuo et al. 2007). Its penchant for moving on actin bundles, such as those created by the protein fascin, may be due to a smaller step size that would force the molecule to twist around a single filament to find the next binding site (Figure 1b; Ricca & Rock 2010, Sun et al. 2010). In regions of bundled actin, myosin X could switch between filaments without twisting, allowing it to move much farther in a single run (Nagy et al. 2008). Although recent data indicate that the step size and preference for bundled actin may depend on construct design (Ricca & Rock 2010, Sun et al. 2010), a model in which enhanced processivity of myosin X in filopodia facilitates its specific transport functions is intriguing, and further work may address these discrepancies.
The influence of actin structure on myosin activity has also been noted in fission yeast, in which two actin-binding proteins seem to exert opposite effects on two different classes of myosins. Tropomyosin is best known for its role in the regulation of the interaction between muscle myosin and actin in response to Ca2+ (reviewed in Stossel et al. 1985). In budding yeast, tropomyosin is involved in the formation of long actin cables that are necessary for the polarized movement of cellular contents (Bretscher 2003) such as Myo2p–associated secretory vesicles (Pruyne et al. 1998). The type V myosins in fission yeast are also influenced by tropomyosin, the presence of which increases their actin-activated ATPase rates and processivity. It has the opposite effect, however, on type I myosin, which is essentially inactive on tropomyosin-associated filaments (Clayton et al. 2010). The addition of fimbrin, an actin-bundling protein (Stossel et al. 1985), can relieve this inhibition, and these data support a model in which locally distinct actin structures can regulate myosin distribution and activity (Clayton et al. 2010).
TARGETING TO SUBCELLULAR COMPARTMENTS
How does a single myosin carry out many cellular roles? Beyond the regulatory mechanisms described above, most of which suggest distinct on and off states, myosins must be both active and on the correct compartment to carry out their functions. In analyzing how motors link to their cargoes, several themes emerge that are exemplified by the myosins described in this section.
Myosin-Adaptor-GTPase Complexes
Perhaps the best-characterized motor-cargo complex consists of myosin V, melanophilin, and the small GTPase Rab27a. The complex is required for the proper distribution of melanosomes at the actin-rich dendritic tips of melanocytes (Figure 2b). Mice lacking any one, two, or all three of the proteins display identical defects in melanosome distribution (Seabra & Coudrier 2004). In vitro reconstitution experiments demonstrated that with GTP-bound Rab27a, the complex is sufficient to transport cargo along actin filaments, although additional proteins are likely to be required for regulating the transport of melanosomes in vivo (Wu et al. 2006).
In the retinal pigment epithelium, melanosomes are similarly transported from the perinuclear region of cells to apical processes upon exposure to light. The tethering and motility of melanosomes in these cells also require Rab27a but involve a different adaptor and myosin motor. Mice lacking myosin VIIa have defects in retinal function, and melanosomes in their pigment epithelial cells are abnormally distributed. A yeast two-hybrid screen of a human retinal complementary DNA (cDNA) library using the myosin VIIa tail as bait found a novel adaptor named MyRIP (myosin VIIA- and Rab-interacting protein); this protein contains a domain orthologous to other Rab effectors and binds specifically to active, GTP-bound Rab27a (El-Amraoui et al. 2002). Further work demonstrated that the three proteins form a complex in vivo and that all three are necessary for normal tethering of melanosomes on the actin cytoskeleton (Lopes et al. 2007). The association of an adaptor protein with an active GTPase (Rab27a in melanosome membranes) to form a receptor complex that recruits an unconventional myosin seems to be a recurring pattern for targeting motors to their appropriate subcellular compartments (Akhmanova & Hammer 2010).
Myosins as Part of Larger Complexes
There are also examples in which a myosin binds to one or more members of a large complex that localize it to a specific subcellular compartment. In mammalian cell lines, myosin VIIa coimmunoprecipitates with a complex consisting of E-cadherin, β-catenin, α-catenin, and vezatin and was shown to interact directly with vezatin through the FERM domain [Band 4.1 (F), Ezrin (E), Radixin (R), and Moesin (M)] in its tail (Kussel-Andermann et al. 2000). This complex, which recruits myosin VIIa to the membrane sites of cell-cell contacts, is thought to be involved in the actin dynamics necessary for forming cell-cell adhesions. Interestingly, Listeria exploits this adhesion complex when it infects cells that do not normally undergo phagocytosis, such as intestinal epithelial cells (reviewed in Ireton 2007). In this case, the myosin is required for the E-cadherin-dependent entry of Listeria and is recruited to the complex through its direct interaction with vezatin (Sousa et al. 2004). At the site of entry, the motor is thought to provide the force necessary for internalization of the bacterium.
Targeting by Lipids and Membrane Microdomains
Despite extensive efforts, few cargo-binding proteins have been found for myosin I family members. Instead, type I myosins may interact primarily with lipids, as their tails contain a positively charged domain that binds membranes with high affinity (reviewed in Barylko et al. 2005). All short-tailed myosin I isoforms appear to contain pleckstrin homology (PH)-like domains, which suggests a widespread mechanism for their targeting. The binding of myosin Ic to phosphoinositides, for example, is dependent on a few basic residues in its putative PH domain (Hokanson et al. 2006). Similarly, myosin Ig was shown to be a tightly associated component of detergent-resistant membrane fractions prepared from bovine neutrophil plasma membranes. It has therefore been speculated to play a role with the actin cytoskeleton in the organization and lateral movement of signaling domains in the plasma membrane (Nebl et al. 2002). The specific lipids in these membrane domains that interact directly with the myosin Ig tail in that case are still unknown.
As previously mentioned, type XIV myosins in apicomplexan parasites are responsible for their gliding motility and therefore for entry into host cells. The generation of force by actomyosin in this system is critically dependent on the immobilization of its components in membranes. For Toxoplasma MyoA, lipid composition is known to influence its stability in the inner membrane complex (IMC), which is enriched in cholesterol. The tail of MyoA binds myosin light chain 1 (MLC1) (Herm-Gotz et al. 2002). These two proteins associate with gliding-associated protein (GAP45), whose Plasmodium ortholog is acylated and predicted to be peripherally membrane associated, and GAP50, an integral membrane protein (Figure 3; Gaskins et al. 2004, Rees-Channer et al. 2006). GAP45 and GAP50 require detergent extraction for release from the IMC, and the cholesterol-sequestering drug mβCD also releases this complex, which indicates that this lipid may be responsible for the near-complete immobility of GAP50 in the membrane (Johnson et al. 2007). If GAP50 is indeed located in a cholesterol-rich membrane microdomain, the lipid composition may directly influence its mobility and consequently the location of the MyoA motility complex.
There are few examples in which the connection between a myosin, its adaptor, and membranous cargo has been described in full. Although several other binding partners have been identified for individual myosins, how they link the motors to cargoes is largely unknown. As the targeting of an active myosin to a specific location in the cell is absolutely critical to its function, much work remains to be done to identify the proteins in complexes with myosins and the direct contacts between them. We and others have begun to address this problem (Finan et al. 2011), and advances in mass spectrometry and high-resolution microscopy should yield progress in achieving a more thorough understanding of cargo composition.
REGULATION OF TARGETING/LOCALIZATION
With many potential binding partners for myosins in a cell, the targeting of myosins to any single structure also must be regulated. Several parameters have been demonstrated to influence myosin localization and cargo association. One important method is likely through the cell type-specific expression of a given myosin and its cargoes; other potential mechanisms are presented below.
Posttranslational Modifications
In Xenopus melanophores, myosin V, kinesin-II, and dynein all cooperate to transport melanosomes along microtubules and actin filaments. This transport is inhibited during mitosis, when myosin V dissociates from the surface of melanosomes as a result of phosphorylation of a serine residue in its globular tail domain (Figure 2b). A phos-phomimetic mutant of myosin V was unable to bind to melanosomes, and substitution of the acceptor serine with alanine prevented its dissociation from melanosomes even after incubation with mitotic extracts. Furthermore, in mitotic extracts treated with inhibitors of calcium/calmodulin-dependent protein kinase II (CaMKII), wild-type myosin V no longer dissociated from melanosomes, which provided evidence that CaMKII activity is involved. As the consensus phosphorylation site is conserved in all three isoforms of vertebrate myosin V, and the two proteins often colocalize in cells, this mode of negative regulation likely is widely used in vivo (Karcher et al. 2001). Interestingly, phosphorylation of the same serine residue also has been reported to positively regulate the motor’s activity in another system. The insulin-activated kinase Akt/PKB (protein kinase B) phosphorylates myosin Va in adipocytes. When myosin Va is phosphorylated, it has an increased affinity for both actin filaments and glucose transporter type 4 (GLUT4)-containing vesicles, which allows GLUT4 transport through the cortical actin network to the plasma membrane for its rapid exocytosis in response to insulin (Yoshizaki et al. 2007).
Phosphorylation is likely involved in both the function and localization of unconventional myosins in cells. Although other posttranslational modifications have not been linked conclusively to myosins, further work should reveal their involvement as influencing factors, as suggested by the potential for farnesylation of Dictyostelium Myo1K (Dieckmann et al. 2010) and the apparent regulation of the Drosophila orphan myosin Dachs by a predicted palmitoyltransferase (Matakatsu & Blair 2008). In addition, the modification of cargoes could affect their binding to myosins and thus the targeting of the motor, as seems to be the case for the interaction of yeast myosin V with a vacuolar protein upon phosphorylation of the adaptor (Peng & Weisman 2008).
Calcium-Dependent Cargo Binding
Ca2+, in addition to its known role in releasing the inhibitory head-tail interactions of myosins, can also affect their targeting to specific locations. The interaction of myosin Va with syntaxin-1a, a member of the soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptor (SNARE) complex responsible for the fusion of synaptic vesicles with the plasma membrane, is also regulated by Ca2+. The light chains of myosin Va and other myosins are known to release from the heavy chain upon an increase in Ca2+ concentration. In the case of secretory granule exocytosis in nerve cells, light chain release from myosin Va permits the motor to interact with the plasma membrane-bound syntaxin-1a, which potentially allows the myosin to hold synaptic vesicles just underneath the membrane. When the Ca2+ concentration increases even further, the myosin Vasyntaxin interaction is disrupted, and the SNARE is free to induce vesicle fusion, which implicates myosin Va in poising vesicles for exocytosis (Watanabe et al. 2005). Myosin Va was previously known to function in secretory granule trafficking, and the elucidation of its regulated interaction with a SNARE provides an elegant mechanism for its importance in neuroendocrine signaling (Eichler et al. 2006).
Myo1c (type I) in mouse adipocytes provides another example in which Ca2+ likely regulates a motor’s localization and association with cargo. In these cells, Myo1c plays a role in the trafficking of GLUT4-containing vesicles to the plasma membrane upon insulin stimulation. The small GTPase RalA, which when bound to GTP associates with GLUT4-containing vesicles, also associates with Myo1c in a nucleotide-independent manner. Interestingly, the interaction between Myo1c and RalA requires the motor’s IQ domains and the Ca2+-bound light chain calmodulin (Chen et al. 2007). This implies that two conditions need to be met for vesicle association and trafficking to occur, and that calmodulin and Ca2+ play a key role in the recognition of cargo by the motor during insulin-stimulated uptake of glucose.
Degradation/Turnover
If myosin functions depend on their cargo binding, how do they switch between cargoes to carry out multiple roles in a single cell type? In the case of the yeast type V myosin Myo2p, this switching is thought to occur via the degradation of a cargo-binding protein. Myo2p has multiple roles in S. cerevisiae, two of which are movement of vacuoles to the daughter and delivery of membrane to the ingressing cytokinetic furrow. The Vac17p protein that links Myo2p to the vacuolar protein Vac8p is degraded in the bud, which allows release of the vacuole from the myosin once it has reached its destination. The myosin is then free to move back toward the furrow and interact with other adaptors; indeed, prevention of Vac17p turnover results in vacuolar mislocalization to the site of cytokinesis (Tang et al. 2003). This regulated turnover of a myosin receptor may be a more general mechanism for cargo switching, and examination of cargo-binding protein sequences may reveal the presence of degradation signals such as the PEST amino acid motif found in Vac17p.
Isoform Specificity for Cargo
Mammalian myosin Va has several differentially spliced exons, two of which seem to mediate binding of distinct cargoes. All reported forms have the exons A, C, and E. In the brain, exon B is present but F is not, whereas in melanocytes, transcripts contain exon F but not exon B (Seperack et al. 1995). The addition of exon B creates a binding site for the dynein light chain LC8 in the coiled-coil region (Hodi et al. 2006, Wagner et al. 2006), while exon F is part of the binding domain for melanophilin (Fukuda & Kuroda 2004, Wu et al. 2002). Whereas melanophilin is well characterized as the adaptor linking this motor to Rab27 and melanosomes, the role of a myosin V-LC8 complex is less clear. Given that some Griscelli syndrome patients have only a pigmentation phenotype resulting from the lack of exon F or a mutation in melanophilin (Menasche et al. 2003), loss of exon B and its connections to cargoes could be responsible for the neurological problems that occur in more severe cases. Discovery of these cargoes and how they cooperate with myosin V in neurological development is essential to understanding how this myosin functions in vivo.
Mammalian cells also express a variety of isoforms of myosin VI, and their differential expression has been suggested to regulate the motor’s roles in endocytosis. An isoform expressing a large (~32 amino acid) insert in the tail region is specifically expressed in polarized cells with apical microvilli and might play a role in enhancing the targeting of myosin VI to clathrin-coated structures in these cells (Buss et al. 2001). All isoforms of myosin VI, however, localize to the uncoated vesicles that serve as intermediates between coated vesicles and early endosomes (Dance et al. 2004). Thus, the hypothesis that cell type–specific expression of splice isoforms and their specific adaptor proteins influence myosin VI’s involvement in either early or late steps in the endocytic pathway needs further exploration (reviewed in Hasson 2003).
COORDINATED FUNCTION OF MULTIPLE MOTORS IN VIVO
Studies on the motor function of unconventional myosins in the past decade have focused primarily on the interaction of single myosins (monomers or dimers) with single actin filaments, and they have shed light on the motors’ directionality, processivity, and response to load. To translate these findings into the functions of myosins in vivo, however, the number and types of motors in particular cargo complexes need to be understood. The former has begun to be addressed for myosin V, which is thought to be present in many copies on a single organelle (Miller & Sheetz 2000). Recent advances have revealed important information linking the biophysical behaviors and in vivo mechanics of this motor.
The organization of actin filaments into a variety of structures in cells, from parallel filament bundles to dense dendritic networks, adds another level of complexity beyond the work in most in vitro experiments (Mooseker & Tilney 1975). As it steps along actin, a processive myosin is presented with a choice at the intersection of two actin filaments in an actin meshwork: continue to step along the same actin filament or switch to a different filament. In one experiment, Snider et al. (2004) tracked the movement of myosin V-associated melanosomes in live cells and compared the movement patterns with simulations of myosin V-driven transport on an actin meshwork. They found that the characteristic frequency of switching between actin filaments can greatly impact the transport pattern and therefore the steady-state localization of myosin V cargo. Another study examining the movement of single, labeled molecules of myosin V negotiating the intersection of two actin filaments showed that although filament intersections can pose a physical barrier to a stepping motor, myosin V was able to either step across or switch filaments at a branch point (Ali et al. 2007). Additionally, the interaction between myosin V and the microtubule-based motor dynein has been examined at actin-microtubule intersections. The switch between actin and microtubule tracks in an in vitro system was found to be an outcome of the tug-of-war between myosin V and dynein; the relative motor numbers dictated the outcome (Schroeder et al. 2010). Some recent work has focused on other myosins as well. For example, lamellipodia in fish keratocytes were used as a model of the dense actin cortex in which coordinated movement of multiple myosin VI molecules was observed. Surprisingly, both monomeric and dimeric forms of myosin VI were equally effective at bringing about long-distance (>10 µm) transport on this dense actin mesh-work (Sivaramakrishnan & Spudich 2009).
Given that unconventional myosins associate with a variety of subcellular structures, it is not surprising that more than one type of unconventional myosin is bound to the same cargo. For instance, myosin VI and Myo18a are known to localize to the Golgi complex and are essential for maintenance of its morphology (Dippold et al. 2009, Sahlender et al. 2005). Myosin VI is the only myosin known to move toward the minus end of actin filaments, which raises the intriguing possibility that myosin VI and Myo18a tug on the Golgi membrane in opposing directions to maintain Golgi complex structure. Alternatively, myosin VI and Myo18a could perform two completely unrelated functions at the Golgi complex, both of which are involved in maintenance of its morphology. Similarly, myosin V and VI are both involved in the secretory pathway, though the extent to which they overlap in membrane trafficking is unclear (Eichler et al. 2006, Hasson 2003). The interactions between different unconventional myosins on the same cargo is an emerging field of research and will be influenced by similar studies of the interactions between the microtubule-based motors kinesin and dynein (Holzbaur & Goldman 2010, Ross et al. 2008b). Overall, the in vitro assays developed to study function need to be tailored to individual myosins to capture the relative subcellular organization of actin and myosin. A missing component of multiple motor studies thus far is the full composition of protein complexes that target myosins to specific subcellular compartments. Reconstituting these regulated interactions with the binding partners that influence myosin localization and function for in vitro assays is one important future direction of unconventional myosin research.
CONCLUSIONS AND PERSPECTIVES
Although much research is being conducted on the roles of the myosin family of molecular motors in cells, we are at the tip of an iceberg regarding our understanding of this important area of biology. Nearly all aspects of cell and developmental biology involve one or more myosins, often working in conjunction with microtubule-based motors. Potentially hundreds of accessory proteins interact directly or indirectly with motors to carry out the multitude of tasks involved in cellular organization, and it is imperative to identify and characterize them. Some recent progress has occurred in this area (Akhmanova & Hammer 2010), and similar approaches need to be applied to all motors. Only then will we begin to see the overlaps and connections between them, which will enable long-range studies to reveal the structure/function relationships of each.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Akhmanova A, Hammer JA., III Linking molecular motors to membrane cargo. Curr. Opin. Cell Biol. 2010;22:479–487. doi: 10.1016/j.ceb.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MY, Krementsova EB, Kennedy GG, Mahaffy R, Pollard TD, et al. Myosin Va maneuvers through actin intersections and diffuses along microtubules. Proc. Natl. Acad. Sci. USA. 2007;104:4332–4336. doi: 10.1073/pnas.0611471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D, Goswami D, Hasson T, Spudich JA, Mayor S. Precise positioning of myosin VI on endocytic vesicles in vivo. PLoS Biol. 2007;5:e210. doi: 10.1371/journal.pbio.0050210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BL, Boldogh I, Evangelista M, Boone C, Greene LA, Pon LA. The Src homology domain 3 (SH3) of a yeast type I myosin, Myo5p, binds to verprolin and is required for targeting to sites of actin polarization. J. Cell Biol. 1998;141:1357–1370. doi: 10.1083/jcb.141.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner L, Lee T, Hasson T. Myo6 facilitates the translocation of endocytic vesicles from cell peripheries. Mol. Biol. Cell. 2003;14:2728–2743. doi: 10.1091/mbc.E02-11-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner L, Naccache SN, Hasson T. Uncoated endocytic vesicles require the unconventional myosin, Myo6, for rapid transport through actin barriers. Mol. Biol. Cell. 2004;15:2253–2263. doi: 10.1091/mbc.E04-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler M. Are class III and class IX myosins motorized signalling molecules? Biochim. Biophys. Acta. 2000;1496:52–59. doi: 10.1016/s0167-4889(00)00008-2. [DOI] [PubMed] [Google Scholar]
- Barylko B, Binns DD, Albanesi JP. Regulation of the enzymatic and motor activities of myosin I. Biochim. Biophys. Acta. 2000;1496:23–35. doi: 10.1016/s0167-4889(00)00006-9. [DOI] [PubMed] [Google Scholar]
- Barylko B, Jung G, Albanesi JP. Structure, function, and regulation of myosin 1C. Acta Biochim. Pol. 2005;52:373–380. [PubMed] [Google Scholar]
- Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat. Cell Biol. 2002;4:246–250. doi: 10.1038/ncb762. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Mol. Cell. 1998;2:437–445. doi: 10.1016/s1097-2765(00)80143-4. [DOI] [PubMed] [Google Scholar]
- Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc. Natl. Acad. Sci. USA. 2006;103:12411–12416. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Polarized growth and organelle segregation in yeast: the tracks, motors, and receptors. J. Cell Biol. 2003;160:811–816. doi: 10.1083/jcb.200301035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein M, Bruno L, Desposito M, Levi V. Anomalous dynamics of melanosomes driven by myosin-V in Xenopus laevis melanophores. Biophys. J. 2009;97:1548–1557. doi: 10.1016/j.bpj.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F, Arden SD, Lindsay M, Luzio JP, Kendrick-Jones J. Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO J. 2001;20:3676–3684. doi: 10.1093/emboj/20.14.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan RH, Gould KL. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J. Cell Biol. 2003;162:851–862. doi: 10.1083/jcb.200305012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev. Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Chung S, Takizawa PA. Multiple Myo4 motors enhance ASH1 mRNA transport in Saccharomyces cerevisiae . J. Cell Biol. 2010;189:755–767. doi: 10.1083/jcb.200912011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JE, Sammons MR, Stark BC, Hodges AR, Lord M. Differential regulation of unconventional fission yeast myosins via the actin track. Curr. Biol. 2010;20:1423–1431. doi: 10.1016/j.cub.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Dance AL, Miller M, Seragaki S, Aryal P, White B, et al. Regulation of myosin-VI targeting to endocytic compartments. Traffic. 2004;5:798–813. doi: 10.1111/j.1600-0854.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- De La Cruz EM, Ostap EM. Relating biochemistry and function in the myosin superfamily. Curr. Opin. Cell Biol. 2004;16:61–67. doi: 10.1016/j.ceb.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Dieckmann R, von Heyden Y, Kistler C, Gopaldass N, Hausherr S, et al. A myosin IK-Abp1-PakB circuit acts as a switch to regulate phagocytosis efficiency. Mol. Biol. Cell. 2010;21:1505–1518. doi: 10.1091/mbc.E09-06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippold HC, Ng MM, Farber-Katz SE, Lee SK, Kerr ML, et al. GOLPH3 bridges phosphatidylinositol-4-phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009;139:337–351. doi: 10.1016/j.cell.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Sakamoto T, Hong MS, Sellers JR, Takizawa PA. Myo4p is a monomeric myosin with motility uniquely adapted to transport mRNA. J. Cell Biol. 2007;178:1193–1206. doi: 10.1083/jcb.200707080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler TW, Kogel T, Bukoreshtliev NV, Gerdes HH. The role of myosin Va in secretory granule trafficking and exocytosis. Biochem. Soc. Trans. 2006;34:671–674. doi: 10.1042/BST0340671. [DOI] [PubMed] [Google Scholar]
- El Mezgueldi M, Tang N, Rosenfeld SS, Ostap EM. The kinetic mechanism of Myo1e (human myosin-IC) J. Biol. Chem. 2002;277:21514–21521. doi: 10.1074/jbc.M200713200. [DOI] [PubMed] [Google Scholar]
- El-Amraoui A, Schonn JS, Kussel-Andermann P, Blanchard S, Desnos C, et al. MyRIP, a novel Rab effector, enables myosin VIIa recruitment to retinal melanosomes. EMBO Rep. 2002;3:463–470. doi: 10.1093/embo-reports/kvf090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M, Klebl BM, Tong AH, Webb BA, Leeuw T, et al. A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol. 2000;148:353–362. doi: 10.1083/jcb.148.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan D, Hartman MA, Spudich JA. Proteomics approach to study the functions of Drosophila myosin VI through identification of multiple cargo-binding proteins. Proc. Natl. Acad. Sci. USA. 2011;108:5566–5571. doi: 10.1073/pnas.1101415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foth BJ, Goedecke MC, Soldati D. New insights into myosin evolution and classification. Proc. Natl. Acad. Sci. USA. 2006;103:3681–3686. doi: 10.1073/pnas.0506307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Kuroda TS. Missense mutations in the globular tail of myosin-Va in dilute mice partially impair binding of Slac2-a/melanophilin. J. Cell Sci. 2004;117:583–591. doi: 10.1242/jcs.00891. [DOI] [PubMed] [Google Scholar]
- Garcia JA, Yee AG, Gillespie PG, Corey DP. Localization of myosin-Iβ near both ends of tip links in frog saccular hair cells. J. Neurosci. 1998;18:8637–8647. doi: 10.1523/JNEUROSCI.18-21-08637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins E, Gilk S, DeVore N, Mann T, Ward G, Beckers C. Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J. Cell Biol. 2004;165:383–393. doi: 10.1083/jcb.200311137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli MI, Lombardi R, Schmelzl B, Riezman H. An intact SH3 domain is required for myosin I-induced actin polymerization. EMBO J. 2000;19:4281–4291. doi: 10.1093/emboj/19.16.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennerich A, Vale RD. Walking the walk: how kinesin and dynein coordinate their steps. Curr. Opin. Cell Biol. 2009;21:59–67. doi: 10.1016/j.ceb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Muller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LS. Kinesin molecular motors: transport pathways, receptors, and human disease. Proc. Natl. Acad. Sci. USA. 2001a;98:6999–7003. doi: 10.1073/pnas.111145298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LS. Molecular motors: from one motor many tails to one motor many tales. Trends Cell Biol. 2001b;11:477–482. doi: 10.1016/s0962-8924(01)02143-2. [DOI] [PubMed] [Google Scholar]
- Gonsalvez GB, Urbinati CR, Long RM. RNA localization in yeast: moving towards a mechanism. Biol. Cell. 2005;97:75–86. doi: 10.1042/BC20040066. [DOI] [PubMed] [Google Scholar]
- Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr. Opin. Cell Biol. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Goodson HV, Anderson BL, Warrick HM, Pon LA, Spudich JA. Synthetic lethality screen identifies a novel yeast myosin I gene (MYO5): Myosin I proteins are required for polarization of the actin cytoskeleton. J. Cell Biol. 1996;133:1277–1291. doi: 10.1083/jcb.133.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T. Myosin VI: two distinct roles in endocytosis. J. Cell Sci. 2003;116:3453–3461. doi: 10.1242/jcs.00669. [DOI] [PubMed] [Google Scholar]
- Herm-Gotz A, Weiss S, Stratmann R, Fujita-Becker S, Ruff C, et al. Toxoplasma gondii myosin A and its light chain: a fast, single-headed, plus-end-directed motor. EMBO J. 2002;21:2149–2158. doi: 10.1093/emboj/21.9.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JL, Deng WM, Rogat AD, Miller KG, Bownes M. Class VI unconventional myosin is required for spermatogenesis in Drosophila. Mol. Biol. Cell. 1999;10:4341–4353. doi: 10.1091/mbc.10.12.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- Hodi Z, Nemeth AL, Radnai L, Hetenyi C, Schlett K, et al. Alternatively spliced exon B of myosin Va is essential for binding the tail-associated light chain shared by dynein. Biochemistry. 2006;45:12582–12595. doi: 10.1021/bi060991e. [DOI] [PubMed] [Google Scholar]
- Hokanson DE, Laakso JM, Lin T, Sept D, Ostap EM. Myo1c binds phosphoinositides through a putative pleckstrin homology domain. Mol. Biol. Cell. 2006;17:4856–4865. doi: 10.1091/mbc.E06-05-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JR, Gillespie SK, Provance DW, Shah K, Shokat KM, et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell. 2002;108:371–381. doi: 10.1016/s0092-8674(02)00629-3. [DOI] [PubMed] [Google Scholar]
- Holzbaur EL, Goldman YE. Coordination of molecular motors: from in vitro assays to intracellular dynamics. Curr. Opin. Cell Biol. 2010;22:4–13. doi: 10.1016/j.ceb.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. Molecular motors: structural adaptations to cellular functions. Nature. 1997;389:561–567. doi: 10.1038/39247. [DOI] [PubMed] [Google Scholar]
- Ireton K. Entry of the bacterial pathogen Listeria monocytogenes into mammalian cells. Cell Microbiol. 2007;9:1365–1375. doi: 10.1111/j.1462-5822.2007.00933.x. [DOI] [PubMed] [Google Scholar]
- Johnson TM, Rajfur Z, Jacobson K, Beckers CJ. Immobilization of the type XIV myosin complex in Toxoplasma gondii. Mol. Biol. Cell. 2007;18:3039–3046. doi: 10.1091/mbc.E07-01-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Goldstein LS. Principles of cargo attachment to cytoplasmic motor proteins. Curr. Opin. Cell Biol. 2002;14:63–68. doi: 10.1016/s0955-0674(01)00295-2. [DOI] [PubMed] [Google Scholar]
- Karcher RL, Roland JT, Zappacosta F, Huddleston MJ, Annan RS, et al. Cell cycle regulation of myosin-V by calcium/calmodulin-dependent protein kinase II. Science. 2001;293:1317–1320. doi: 10.1126/science.1061086. [DOI] [PubMed] [Google Scholar]
- Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat. Rev. Mol. Cell Biol. 2009;10:854–865. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SV, Flavell RA. Myosin I: from yeast to human. Cell. Mol. Life Sci. 2008;65:2128–2137. doi: 10.1007/s00018-008-7435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SV, Mehal WZ, Dong X, Heinrich V, Pypaert M, et al. Modulation of cell adhesion and motility in the immune system by Myo1f. Science. 2006;314:136–139. doi: 10.1126/science.1131920. [DOI] [PubMed] [Google Scholar]
- Krendel M, Mooseker MS. Myosins: tails (and heads) of functional diversity. Physiology (Bethesda) 2005;20:239–251. doi: 10.1152/physiol.00014.2005. [DOI] [PubMed] [Google Scholar]
- Kussel-Andermann P, El-Amraoui A, Safieddine S, Nouaille S, Perfettini I, et al. Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin-catenins complex. EMBO J. 2000;19:6020–6029. doi: 10.1093/emboj/19.22.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso JM, Lewis JH, Shuman H, Ostap EM. Myosin I can act as a molecular force sensor. Science. 2008;321:133–133. doi: 10.1126/science.1159419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso JM, Lewis JH, Shuman H, Ostap EM. Control of myosin-I force sensing by alternative splicing. Proc. Natl. Acad. Sci. USA. 2010;107:698–702. doi: 10.1073/pnas.0911426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Jonsdottir GA, Klee SK, Pellman D, Li R. A two-tiered mechanism by which Cdc42 controls the localization and activation of an Arp2/3-activating motor complex in yeast. J. Cell Biol. 2001;155:261–270. doi: 10.1083/jcb.200104094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Shevchenko A, Li R. Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J. Cell Biol. 2000;148:363–373. doi: 10.1083/jcb.148.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WL, Bezanilla M, Pollard TD. Fission yeast myosin-I, Myo1p, stimulates actin assembly by Arp2/3 complex and shares functions with WASp. J. Cell Biol. 2000;151:789–800. doi: 10.1083/jcb.151.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi V, Gelfand VI, Serpinskaya AS, Gratton E. Melanosomes transported by myosin-V in Xenopus melanophores perform slow 35 nm steps. Biophys. J. 2006;90:L07–L09. doi: 10.1529/biophysj.105.075556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Schneider ME, Kachar B. When size matters: the dynamic regulation of stereocilia lengths. Curr. Opin. Cell Biol. 2005a;17:55–61. doi: 10.1016/j.ceb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Lin T, Tang N, Ostap EM. Biochemical and motile properties of Myo1b splice isoforms. J. Biol. Chem. 2005b;280:41562–41567. doi: 10.1074/jbc.M508653200. [DOI] [PubMed] [Google Scholar]
- Lister I, Schmitz S, Walker M, Trinick J, Buss F, et al. A monomeric myosin VI with a large working stroke. EMBO J. 2004;23:1729–1738. doi: 10.1038/sj.emboj.7600180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long RM, Gu W, Lorimer E, Singer RH, Chartrand P. She2p is a novel RNA-binding protein that recruits the Myo4p–She3p complex to ASH1 mRNA. EMBO J. 2000;19:6592–6601. doi: 10.1093/emboj/19.23.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes VS, Ramalho JS, Owen DM, Karl MO, Strauss O, et al. The ternary Rab27a–Myrip-Myosin VIIa complex regulates melanosome motility in the retinal pigment epithelium. Traffic. 2007;8:486–499. doi: 10.1111/j.1600-0854.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Krementsova EB, Trybus KM. Regulation of myosin V processivity by calcium at the single molecule level. J. Biol. Chem. 2006;281:31987–31994. doi: 10.1074/jbc.M605181200. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Curr. Biol. 2008;18:1390–1395. doi: 10.1016/j.cub.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–840. doi: 10.1126/science.1074553. [DOI] [PubMed] [Google Scholar]
- Menasche G, Ho CH, Sanal O, Feldmann J, Tezcan I, et al. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1) J. Clin. Investig. 2003;112:450–456. doi: 10.1172/JCI18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Characterization of myosin V binding to brain vesicles. J. Biol. Chem. 2000;275:2598–606. doi: 10.1074/jbc.275.4.2598. [DOI] [PubMed] [Google Scholar]
- Mooseker MS, Tilney LG. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J. Cell Biol. 1975;67:725–743. doi: 10.1083/jcb.67.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Heuck A, Niessing D. Directional mRNA transport in eukaryotes: lessons from yeast. Cell. Mol. Life Sci. 2007;64:171–180. doi: 10.1007/s00018-006-6286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy S, Ricca BL, Norstrom MF, Courson DS, Brawley CM, et al. A myosin motor that selects bundled actin for motility. Proc. Natl. Acad. Sci. USA. 2008;105:9616–9620. doi: 10.1073/pnas.0802592105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar R, McConnell RE, Tyska MJ. Control of cell membrane tension by myosin-I. Proc. Natl. Acad. Sci. USA. 2009;106:11972–11977. doi: 10.1073/pnas.0901641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar R, McConnell RE, Tyska MJ. Myosin motor function: the ins and outs of actin-based membrane protrusions. Cell. Mol. Life Sci. 2010;67:1239–1254. doi: 10.1007/s00018-009-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW, Luna EJ. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J. Biol. Chem. 2002;277:43399–43409. doi: 10.1074/jbc.M205386200. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Frank DJ, Isaji M, Miller KG. Coiled-coil-mediated dimerization is not required for myosin VI to stabilize actin during spermatid individualization in Drosophila melanogaster. Mol. Biol. Cell. 2009;20:358–367. doi: 10.1091/mbc.E08-07-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Lenartowska M, Miller KG. Myosin VI stabilizes an actin network during Drosophila spermatid individualization. Mol. Biol. Cell. 2006;17:2559–2571. doi: 10.1091/mbc.E06-01-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Lenartowska M, Rogat AD, Frank DJ, Miller KG. Proper cellular reorganization during Drosophila spermatid individualization depends on actin structures composed of two domains, bundles and meshwork, that are differentially regulated and have different functions. Mol. Biol. Cell. 2008;19:2363–2372. doi: 10.1091/mbc.E07-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell CB, Tyska MJ, Mooseker MS. Myosin at work: motor adaptations for a variety of cellular functions. Biochim. Biophys. Acta. 2007;1773:615–630. doi: 10.1016/j.bbamcr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Park H, Ramamurthy B, Travaglia M, Safer D, Chen LQ, et al. Full-length myosin VI dimerizes and moves processively along actin filaments upon monomer clustering. Mol. Cell. 2006;21:331–336. doi: 10.1016/j.molcel.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J. Neurosci. 2010;30:8984–8992. doi: 10.1523/JNEUROSCI.1621-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Weisman LS. The cyclin-dependent kinase Cdk1 directly regulates vacuole inheritance. Dev. Cell. 2008;15:478–485. doi: 10.1016/j.devcel.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV, Prokhnevsky AI, Dolja VV. Class XI myosins are required for development, cell expansion, and F-actin organization in Arabidopsis. Plant Cell. 2010;22:1883–1897. doi: 10.1105/tpc.110.076315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phichith D, Travaglia M, Yang Z, Liu X, Zong AB, et al. Cargo binding induces dimerization of myosin VI. Proc. Natl. Acad. Sci. USA. 2009;106:17320–17324. doi: 10.1073/pnas.0909748106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhnevsky AI, Peremyslov VV, Dolja VV. Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation, and organelle motility. Proc. Natl. Acad. Sci. USA. 2008;105:19744–19749. doi: 10.1073/pnas.0810730105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provance DW, Jr, Wei M, Ipe V, Mercer JA. Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution. Proc. Natl. Acad. Sci. USA. 1996;93:14554–14558. doi: 10.1073/pnas.93.25.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne DW, Schott DH, Bretscher A. Tropomyosin-containing actin cables direct the Myo2p–dependent polarized delivery of secretory vesicles in budding yeast. J. Cell. Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Purcell TJ, Morris C, Spudich JA, Sweeney HL. Role of the lever arm in the processive stepping of myosin V. Proc. Natl. Acad. Sci. USA. 2002;99:14159–14164. doi: 10.1073/pnas.182539599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero OA, DiVito MM, Adikes RC, Kortan MB, Case LB, et al. Human Myo19 is a novel myosin that associates with mitochondria. Curr. Biol. 2009;19:2008–2013. doi: 10.1016/j.cub.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero OA, Moore JE, Unrath WC, Manor U, Salles FT, et al. Intermolecular autophosphorylation regulates myosin IIIA activity and localization in parallel actin bundles. J. Biol. Chem. 2010;285:35770–35782. doi: 10.1074/jbc.M110.144360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees-Channer RR, Martin SR, Green JL, Bowyer PW, Grainger M, et al. Dual acylation of the 45 kDa gliding-associated protein (GAP45) in Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 2006;149:113–116. doi: 10.1016/j.molbiopara.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Ricca BL, Rock RS. The stepping pattern of myosin X is adapted for processive motility on bundled actin. Biophys. J. 2010;99:1818–1826. doi: 10.1016/j.bpj.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogat AD, Miller KG. A role for myosin VI in actin dynamics at sites of membrane remodeling during Drosophila spermatogenesis. J. Cell Sci. 2002;115:4855–4865. doi: 10.1242/jcs.00149. [DOI] [PubMed] [Google Scholar]
- Ross JL, Ali MY, Warshaw DM. Cargo transport: molecular motors navigate a complex cytoskeleton. Curr. Opin. Cell Biol. 2008a;20:41–47. doi: 10.1016/j.ceb.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Shuman H, Holzbaur EL, Goldman YE. Kinesin and dynein-dynactin at intersecting microtubules: motor density affects dynein function. Biophys. J. 2008b;94:3115–3125. doi: 10.1529/biophysj.107.120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert C, Kroschewski R, Bahler M. Identification, characterization and cloning of myr1, a mammalian myosin-I. J. Cell Biol. 1993;120:1393–1403. doi: 10.1083/jcb.120.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlender DA, Roberts RC, Arden SD, Spudich G, Taylor MJ, et al. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J. Cell Biol. 2005;169:285–295. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh AK, Li BX, Xia H, Ready DF. Calcium-activated Myosin V closes the Drosophila pupil. Curr. Biol. 2008;18:951–955. doi: 10.1016/j.cub.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder HW, III, Mitchell C, Shuman H, Holzbaur EL, Goldman YE. Motor number controls cargo switching at actin-microtubule intersections in vitro. Curr. Biol. 2010;20:687–696. doi: 10.1016/j.cub.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra MC, Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic. 2004;5:393–399. doi: 10.1111/j.1398-9219.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- Sellers JR. Myosins: a diverse superfamily. Biochim. Biophys. Acta. 2000;1496:3–22. doi: 10.1016/s0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- Seperack PK, Mercer JA, Strobel MC, Copeland NG, Jenkins NA. Retroviral sequences located within an intron of the dilute gene alter dilute expression in a tissue-specific manner. EMBO J. 1995;14:2326–2332. doi: 10.1002/j.1460-2075.1995.tb07227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, et al. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 2004;428:950–955. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- Sirotkin V, Beltzner CC, Marchand JB, Pollard TD. Interactions of WASp, myosin-I, and verprolin with Arp2/3 complex during actin patch assembly in fission yeast. J. Cell Biol. 2005;170:637–648. doi: 10.1083/jcb.200502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Spudich JA. Coupled myosin VI motors facilitate unidirectional movement on an F-actin network. J. Cell Biol. 2009;187:53–60. doi: 10.1083/jcb.200906133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider J, Lin F, Zahedi N, Rodionov V, Yu CC, Gross SP. Intracellular actin-based transport: How far you go depends on how often you switch. Proc. Natl. Acad. Sci. USA. 2004;101:13204–13209. doi: 10.1073/pnas.0403092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati D, Meissner M. Toxoplasma as a novel system for motility. Curr. Opin. Cell Biol. 2004;16:32–40. doi: 10.1016/j.ceb.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Sousa AD, Cheney RE. Myosin-X: a molecular motor at the cell’s fingertips. Trends Cell Biol. 2005;15:533–539. doi: 10.1016/j.tcb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Sousa S, Cabanes D, El-Amraoui A, Petit C, Lecuit M, Cossart P. Unconventional myosin VIIa and vezatin, two proteins crucial for Listeria entry into epithelial cells. J. Cell Sci. 2004;117:2121–2130. doi: 10.1242/jcs.01066. [DOI] [PubMed] [Google Scholar]
- Sparkes IA. Motoring around the plant cell: insights from plant myosins. Biochem. Soc. Trans. 2010;38:833–838. doi: 10.1042/BST0380833. [DOI] [PubMed] [Google Scholar]
- Spudich JA, Sivaramakrishnan S. Myosin VI: an innovative motor that challenged the swinging lever arm hypothesis. Nat. Rev. Mol. Cell Biol. 2010;11:128–137. doi: 10.1038/nrm2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer EA, Scarborough JD, Hirono M, Miller ED, Shah K, et al. Fast adaptation in vestibular hair cells requires myosin-1c activity. Neuron. 2005;47:541–553. doi: 10.1016/j.neuron.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavega DG, Kuiper JW. Insect pupil mechanisms. I. On the pigment migration in the retinula cells of Hymenoptera (suborder Apocrita) J. Comp. Physiol. 1977;113:55–72. [Google Scholar]
- Stoffler HE, Bahler M. The ATPase activity of Myr3, a rat myosin I, is allosterically inhibited by its own tail domain and by Ca2+ binding to its light chain calmodulin. J. Biol. Chem. 1998;273:14605–14611. doi: 10.1074/jbc.273.23.14605. [DOI] [PubMed] [Google Scholar]
- Stossel TP, Chaponnier C, Ezzell RM, Hartwig JH, Janmey PA, et al. Nonmuscle actin-binding proteins. Annu. Rev. Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Sun Y, Sato O, Ruhnow F, Arsenault ME, Ikebe M, Goldman YE. Single-molecule stepping and structural dynamics of myosin X. Nat. Struct. Mol. Biol. 2010;17:485–491. doi: 10.1038/nsmb.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Houdusse A. What can myosin VI do in cells? Curr. Opin. Cell Biol. 2007;19:57–66. doi: 10.1016/j.ceb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Houdusse A. Myosin VI rewrites the rules for myosin motors. Cell. 2010a;141:573–582. doi: 10.1016/j.cell.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Houdusse A. Structural and functional insights into the myosin motor mechanism. Annu. Rev. Biophys. 2010b;39:539–557. doi: 10.1146/annurev.biophys.050708.133751. [DOI] [PubMed] [Google Scholar]
- Tang F, Kauffman EJ, Novak JL, Nau JJ, Catlett NL, Weisman LS. Regulated degradation of a class V myosin receptor directs movement of the yeast vacuole. Nature. 2003;422:87–92. doi: 10.1038/nature01453. [DOI] [PubMed] [Google Scholar]
- Titus MA. Motors: unleashing mitochondria. Curr. Biol. 2009;19:R1076–R1078. doi: 10.1016/j.cub.2009.10.053. [DOI] [PubMed] [Google Scholar]
- Tokuo H, Mabuchi K, Ikebe M. The motor activity of myosin-X promotes actin fiber convergence at the cell periphery to initiate filopodia formation. J. Cell Biol. 2007;179:229–238. doi: 10.1083/jcb.200703178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybus KM. Myosin V from head to tail. Cell. Mol. Life Sci. 2008;65:1378–1389. doi: 10.1007/s00018-008-7507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxworth RI, Titus MA. Unconventional myosins: anchors in the membrane traffic relay. Traffic. 2000;1:11–18. doi: 10.1034/j.1600-0854.2000.010103.x. [DOI] [PubMed] [Google Scholar]
- Umeki N, Jung HS, Watanabe S, Sakai T, Li XD, et al. The tail binds to the head-neck domain, inhibiting ATPase activity of myosin VIIA. Proc. Natl. Acad. Sci. USA. 2009;106:8483–8488. doi: 10.1073/pnas.0812930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288:88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- Wagner W, Fodor E, Ginsburg A, Hammer JA., III The binding of DYNLL2 to myosin Va requires alternatively spliced exon B and stabilizes a portion of the myosin’s coiled-coil domain. Biochemistry. 2006;45:11564–11577. doi: 10.1021/bi061142u. [DOI] [PubMed] [Google Scholar]
- Wang F, Thirumurugan K, Stafford WF, Hammer JA, III, Knight PJ, Sellers JR. Regulated conformation of myosin V. J. Biol. Chem. 2004;279:2333–2336. doi: 10.1074/jbc.C300488200. [DOI] [PubMed] [Google Scholar]
- Warner CL, Stewart A, Luzio JP, Steel KP, Libby RT, et al. Loss of myosin VI reduces secretion and the size of the Golgi in fibroblasts from Snell’s waltzer mice. EMBO J. 2003;22:569–579. doi: 10.1093/emboj/cdg055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Nomura K, Ohyama A, Ishikawa R, Komiya Y, et al. Myosin-Va regulates exocytosis through the submicromolar Ca2+-dependent binding of syntaxin-1A. Mol. Biol. Cell. 2005;16:4519–4530. doi: 10.1091/mbc.E05-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, et al. Myosin VI is an actin-based motor that moves backwards. Nature. 1999;401:505–508. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- Wetzel DM, Hakansson S, Hu K, Roos D, Sibley LD. Actin filament polymerization regulates gliding motility by apicomplexan parasites. Mol. Biol. Cell. 2003;14:396–406. doi: 10.1091/mbc.E02-08-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolner S, Bement WM. Unconventional myosins acting unconventionally. Trends Cell Biol. 2009;19:245–252. doi: 10.1016/j.tcb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Bowers B, Rao K, Wei Q, Hammer JA., III Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J. Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Rao K, Bowers MB, Copeland NG, Jenkins NA, Hammer JA., III Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J. Cell Sci. 2001;114:1091–1100. doi: 10.1242/jcs.114.6.1091. [DOI] [PubMed] [Google Scholar]
- Wu X, Sakamoto T, Zhang F, Sellers JR, Hammer JA., III In vitro reconstitution of a transport complex containing Rab27a, melanophilin and myosin Va. FEBS Lett. 2006;580:5863–5868. doi: 10.1016/j.febslet.2006.09.047. [DOI] [PubMed] [Google Scholar]
- Wu XS, Rao K, Zhang H, Wang F, Sellers JR, et al. Identification of an organelle receptor for myosin-Va. Nat. Cell Biol. 2002;4:271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- Yang Y, Baboolal TG, Siththanandan V, Chen M, Walker ML, et al. A FERM domain autoregulates Drosophila myosin 7a activity. Proc. Natl. Acad. Sci. USA. 2009;106:4189–4194. doi: 10.1073/pnas.0808682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Imamura T, Babendure JL, Lu JC, Sonoda N, Olefsky JM. Myosin 5a is an insulin-stimulated Akt2 (protein kinase Bβ) substrate modulating GLUT4 vesicle translocation. Mol. Cell Biol. 2007;27:5172–5183. doi: 10.1128/MCB.02298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Feng W, Wei Z, Miyanoiri Y, Wen W, et al. Myosin VI undergoes cargo-mediated dimerization. Cell. 2009;138:537–548. doi: 10.1016/j.cell.2009.05.030. [DOI] [PubMed] [Google Scholar]
- Zadro C, Alemanno MS, Bellacchio E, Ficarella R, Donaudy F, et al. Are MYO1C and MYO1F associated with hearing loss? Biochim. Biophys. Acta. 2009;1792:27–32. doi: 10.1016/j.bbadis.2008.10.017. [DOI] [PubMed] [Google Scholar]