Abstract
A region in the first intron of a metallothionein-encoding gene of the sea urchin Strongylocentrotus purpuratus (SpMTA gene) regulates its 5' promoter activity. Within this region is a 290-bp cassette of six sequence motifs that are present in other genes in this species and posited to operate as regulatory elements. The cassette, present at high multiplicity in the genome, was used to screen genomic DNA clones. Of these, six diverse individuals were partially sequenced and found to have segments 94% identical to the 290-bp cassette in the SpMTA gene. Their next 80 bp diverged from the SpMTA sequence but were highly identical among the six non-SpMTA clones and contained an additional regulatory motif. These diverse clones thus contained 370-bp cassettes of an overall 94% sequence identity and an apparent content of seven regulatory elements. The regulatory cassettes were transposon-like, insofar as the termini of the highly identical regions consisted of 24- to 25-bp inverted repeats, bracketed by 6- to 9-bp direct repeats in the divergent regions. In addition to being in transcripts of the SpMTA intron, the cassette was found in other sea urchin embryo poly(A)+ RNAs, in eggs and embryos, and enriched in pluteus ectoderm. The cassette sequence was present in moderate abundance in transcripts in both sense and antisense orientation. We report the presence of a transposon-like cassette of regulatory elements that is also represented in RNA, which potentially could function differently from previously described transposons.
Full text
PDF

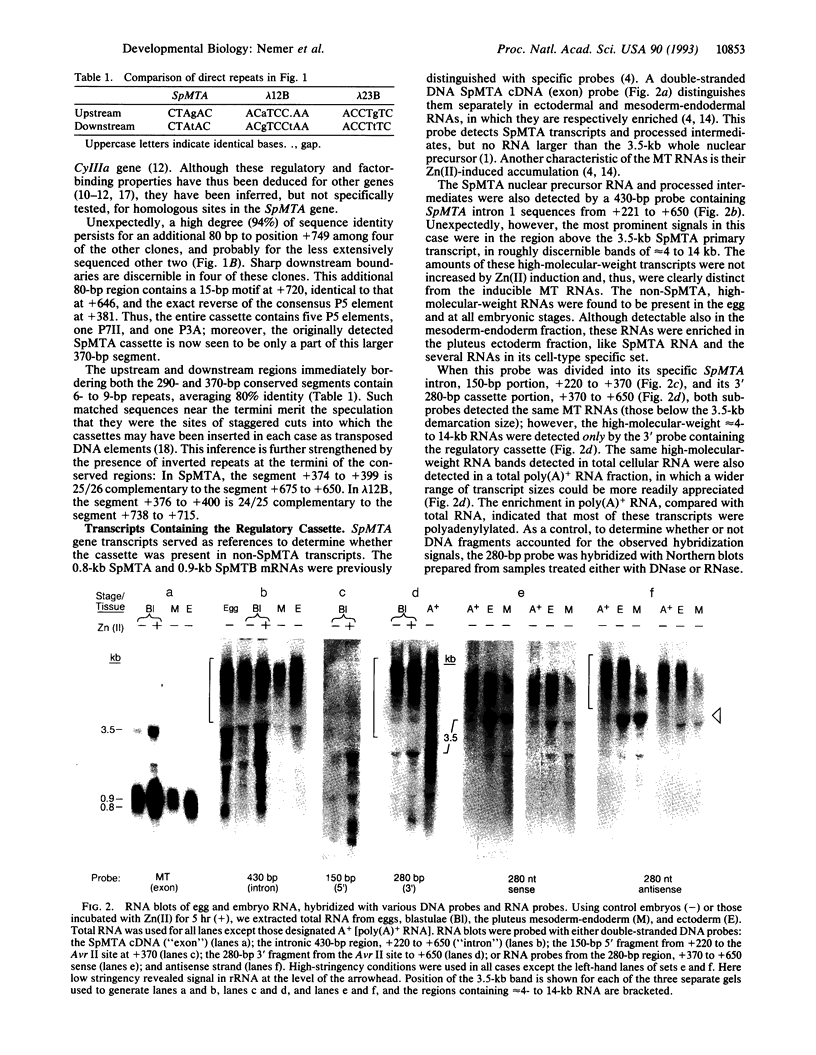


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bai G., Stuebing E. W., Parker H. R., Harlow P., Nemer M. Combinatorial regulation by promoter and intron 1 regions of the metallothionein SpMTA gene in the sea urchin embryo. Mol Cell Biol. 1993 Feb;13(2):993–1001. doi: 10.1128/mcb.13.2.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Repetitive and non-repetitive DNA sequences and a speculation on the origins of evolutionary novelty. Q Rev Biol. 1971 Jun;46(2):111–138. doi: 10.1086/406830. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Hög C., Teplow D. B., Cutting A. E., Zeller R. W., Britten R. J., Davidson E. H. Gene regulatory factors of the sea urchin embryo. I. Purification by affinity chromatography and cloning of P3A2, a novel DNA-binding protein. Development. 1991 May;112(1):335–350. doi: 10.1242/dev.112.1.335. [DOI] [PubMed] [Google Scholar]
- Cox K. H., Angerer L. M., Lee J. J., Davidson E. H., Angerer R. C. Cell lineage-specific programs of expression of multiple actin genes during sea urchin embryogenesis. J Mol Biol. 1986 Mar 20;188(2):159–172. doi: 10.1016/0022-2836(86)90301-3. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombroski B. A., Mathias S. L., Nanthakumar E., Scott A. F., Kazazian H. H., Jr Isolation of an active human transposable element. Science. 1991 Dec 20;254(5039):1805–1808. doi: 10.1126/science.1662412. [DOI] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks R. R., Anderson R., Moore J. G., Hough-Evans B. R., Britten R. J., Davidson E. H. Competitive titration in living sea urchin embryos of regulatory factors required for expression of the CyIIIa actin gene. Development. 1990 Sep;110(1):31–40. doi: 10.1242/dev.110.1.31. [DOI] [PubMed] [Google Scholar]
- Gan L., Zhang W., Klein W. H. Repetitive DNA sequences linked to the sea urchin spec genes contain transcriptional enhancer-like elements. Dev Biol. 1990 May;139(1):186–196. doi: 10.1016/0012-1606(90)90287-s. [DOI] [PubMed] [Google Scholar]
- Harlow P., Watkins E., Thornton R. D., Nemer M. Structure of an ectodermally expressed sea urchin metallothionein gene and characterization of its metal-responsive region. Mol Cell Biol. 1989 Dec;9(12):5445–5455. doi: 10.1128/mcb.9.12.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough-Evans B. R., Franks R. R., Zeller R. W., Britten R. J., Davidson E. H. Negative spatial regulation of the lineage specific CyIIIa actin gene in the sea urchin embryo. Development. 1990 Sep;110(1):41–50. doi: 10.1242/dev.110.1.41. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Wong C., Youssoufian H., Scott A. F., Phillips D. G., Antonarakis S. E. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature. 1988 Mar 10;332(6160):164–166. doi: 10.1038/332164a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Liebermann D., Hoffman-Liebermann B., Weinthal J., Childs G., Maxson R., Mauron A., Cohen S. N., Kedes L. An unusual transposon with long terminal inverted repeats in the sea urchin Strongylocentrotus purpuratus. Nature. 1983 Nov 24;306(5941):342–347. doi: 10.1038/306342a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemer M., Thornton R. D., Stuebing E. W., Harlow P. Structure, spatial, and temporal expression of two sea urchin metallothionein genes, SpMTB1 and SpMTA. J Biol Chem. 1991 Apr 5;266(10):6586–6593. [PubMed] [Google Scholar]
- Nemer M., Travaglini E. C., Rondinelli E., D'Alonzo J. Developmental regulation, induction, and embryonic tissue specificity of sea urchin metallothionein gene expression. Dev Biol. 1984 Apr;102(2):471–482. doi: 10.1016/0012-1606(84)90212-4. [DOI] [PubMed] [Google Scholar]
- Nisson P. E., Hickey R. J., Boshar M. F., Crain W. R., Jr Identification of a repeated sequence in the genome of the sea urchin which is transcribed by RNA polymerase III and contains the features of a retroposon. Nucleic Acids Res. 1988 Feb 25;16(4):1431–1452. doi: 10.1093/nar/16.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posakony J. W., Flytzanis C. N., Britten R. J., Davidson E. H. Interspersed sequence organization and developmental representation of cloned poly(A) RNAs from sea urchin eggs. J Mol Biol. 1983 Jun 25;167(2):361–389. doi: 10.1016/s0022-2836(83)80340-4. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Blau H. M. Genetic complementation reveals a novel regulatory role for 3' untranslated regions in growth and differentiation. Cell. 1993 Mar 26;72(6):903–917. doi: 10.1016/0092-8674(93)90579-f. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Haile D. J., Harford J. B., Klausner R. D. The iron-responsive element binding protein: a method for the affinity purification of a regulatory RNA-binding protein. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5768–5772. doi: 10.1073/pnas.86.15.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson L. C., Wiebauer K., Snow C. M., Meisler M. H. Retroviral and pseudogene insertion sites reveal the lineage of human salivary and pancreatic amylase genes from a single gene during primate evolution. Mol Cell Biol. 1990 Jun;10(6):2513–2520. doi: 10.1128/mcb.10.6.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller R. H., Costantini F. D., Kozlowski M. R., Britten R. J., Davidson E. H. Specific representation of cloned repetitive DNA sequences in sea urchin RNAs. Cell. 1978 Sep;15(1):189–203. doi: 10.1016/0092-8674(78)90094-6. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Davidson E. H., Britten R. J. Retroviral-like element in a marine invertebrate. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8401–8404. doi: 10.1073/pnas.88.19.8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenhagen J. B., Robins D. M. An ancient provirus has imposed androgen regulation on the adjacent mouse sex-limited protein gene. Cell. 1988 Oct 21;55(2):247–254. doi: 10.1016/0092-8674(88)90047-5. [DOI] [PubMed] [Google Scholar]
- Theunissen O., Rudt F., Guddat U., Mentzel H., Pieler T. RNA and DNA binding zinc fingers in Xenopus TFIIIA. Cell. 1992 Nov 13;71(4):679–690. doi: 10.1016/0092-8674(92)90601-8. [DOI] [PubMed] [Google Scholar]
- Thiebaud P., Goodstein M., Calzone F. J., Thézé N., Britten R. J., Davidson E. H. Intersecting batteries of differentially expressed genes in the early sea urchin embryo. Genes Dev. 1990 Nov;4(11):1999–2010. doi: 10.1101/gad.4.11.1999. [DOI] [PubMed] [Google Scholar]
- Walden W. E., Daniels-McQueen S., Brown P. H., Gaffield L., Russell D. A., Bielser D., Bailey L. C., Thach R. E. Translational repression in eukaryotes: partial purification and characterization of a repressor of ferritin mRNA translation. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9503–9507. doi: 10.1073/pnas.85.24.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. B., Sutcliffe J. G. Primate brain-specific cytoplasmic transcript of the Alu repeat family. Mol Cell Biol. 1987 Sep;7(9):3324–3327. doi: 10.1128/mcb.7.9.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Nemer M. Metallothionein genes MTa and MTb expressed under distinct quantitative and tissue-specific regulation in sea urchin embryos. Mol Cell Biol. 1987 Jan;7(1):48–58. doi: 10.1128/mcb.7.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]