Trial results are used to support licensure, inform cost-effectiveness analyses, and guide clinical decision making. We found the majority of coinfected patients were not included in clinical trials of direct-acting antivirals, raising concerns about the generalizability of these trial results.
Keywords: HIV–hepatitis C coinfection, direct-acting antivirals, generalizability, people who inject drugs, clinical trials
Abstract
Background. Direct-acting antivirals (DAAs) against hepatitis C virus (HCV) have been described as revolutionary. However, it remains uncertain how effective these drugs will be for individuals coinfected with human immunodeficiency virus (HIV)–HCV. Bridging this gap between efficacy and effectiveness requires a focus on the generalizability of clinical trials.
Methods. Generalizability of DAA trials was assessed by applying the eligibility criteria from 5 efficacy trials: NCT01479868, PHOTON-1 (NCT01667731), TURQUOISE-I (NCT01939197), ION-4 (NCT02073656), and ALLY-2 (NCT02032888) that evaluated simeprevir; sofosbuvir; ombitasvir, paritaprevir/ritonavir/dasabuvir; sofosbuvir/ledipasvir; and daclatasvir/sofosbuvir, respectively, to the Canadian Coinfection Cohort, representing approximately 23% of the total coinfected population in care in Canada.
Results. Of 874 active participants, 70% had chronic HCV, of whom 410, 26, 94, and 11 had genotypes 1, 2, 3, and 4, respectively. After applying trial eligibility criteria, only 5.9% (24/410) would have been eligible for enrollment in the simeprevir trial, 9.8% (52/530) in PHOTON-1, 6.3% (26/410) in TURQUOISE-I, and 8.1% (34/421) in ION-4. The ALLY-2 study was more inclusive; 43% (233/541) of the cohort would have been eligible. The most exclusive eligibility criteria across all trials with the exception of ALLY-2 were restriction to specific antiretroviral therapies (63%–79%) and active illicit drug use (53%–55%).
Conclusions. DAA trial results may have limited generalizability, since the majority of coinfected individuals were not eligible to participate. Exclusions appeared to be related to improving treatment outcomes by not including those at higher risk of poor adherence and reinfection—individuals for whom real-world data are urgently needed.
(See the Editorial Commentaries by Martinello and Dore on pages 927–8.)
Worldwide, approximately 5 million people are coinfected with human immunodeficiency virus (HIV) and hepatitis C (HCV) [1]. Coinfected people are heterogeneous, have complex medical needs, and are often socially disenfranchised. Injection drug use is responsible for the majority of both incident and prevalent cases in most developed countries. Despite effective HIV suppression and immune restoration, liver disease remains the leading cause of death in HIV–HCV coinfected individuals [2–4]. To reduce the clinical and healthcare burden of advanced liver disease, coinfected individuals need to be treated and cured of HCV [5–7]. Unfortunately, fewer than 10% of coinfected individuals have ever been treated [8, 9].
The development of direct-acting antivirals (DAAs) for HCV has been rightfully described as revolutionary. Based on compelling clinical trial results, multiple DAAs, including simeprevir; sofosbuvir; ledipasvir; ombitasvir; paritaprevir/ritonavir/dasabuvir (3D); and daclatasvir have been approved by licensing authorities globally [10–16]. Clinical trial results show that DAAs are well tolerated, more conveniently dosed, and highly efficacious compared with earlier interferon-based HCV therapies. Among interferon-free DAA trials, with as little as 12 to 24 weeks of treatment, sustained virologic response (SVR) rates ranged from 91% to 97% across genotypes and fibrosis stages in coinfected individuals, representing a remarkable advance compared with previous therapies [17–19].
Trials evaluating these new agents have so far included relatively small numbers of participants (subgroups ranging from 6 to 160) and have applied very strict eligibility criteria, likely excluding a substantial segment of the coinfected population. Substance abuse, comorbid medical and psychiatric conditions, advanced liver disease, and drug–drug interactions with antiretrovirals are common and among some of the primary factors that may influence access to treatment and outcomes in the real world [20, 21]. This raises the question: If a large proportion of coinfected patients are excluded from participating in clinical trials, how generalizable are DAA trials for people living with HIV–HCV coinfection?
METHODS
To evaluate the generalizability of DAA trials, we examined the eligibility criteria of trial protocols. We performed a review of all published phase 3 trials that evaluated second-generation DAAs in individuals with HIV–HCV coinfection as of November 2015 by searching PubMed and clinical trial registries (clinicaltrials.gov). The DAAs that were identified included simeprevir, sofosbuvir, ledipasvir, grazoprevir/elbasvir, ombitasvir, paritaprevir/ritonavir/dasabuvir, faldaprevir, and daclatasvir. We further restricted trials to those where trial protocols were available. The following trials that met our inclusion criteria were analyzed: NCT01479868; the PHOTON-1 trial (NCT01667731); the TURQUOISE-I trial (NCT01939197); the ION-4 trial (NCT02073656); and the ALLY-2 trial (NCT02032888) that evaluated simeprevir; sofosbuvir; ombitasvir, paritaprevir/ritonavir/dasabuvir (3D); sofosbuvir/ledipasvir; and daclatasvir/sofosbuvir, respectively [17–19, 22, 23]. Specific eligibility criteria used to assess the generalizability of each trial are listed in Table 1. Supplementary Table 1 summarizes permitted combination antiretroviral therapy (cART) regimens by each trial. Supplementary Table 2 provides trial-specific definitions of active drug use. We then used the Canadian Coinfection Cohort (CCC) as a representative population to evaluate the percentage of current cohort participants that would be eligible to participate in these trials.
Table 1.
Selection of Exclusion Criteria—Each Exclusivea
| Criteria | Trial-Specific Exclusion Criteria |
Simeprevir Trial (GT1) N = 410 No. (%) |
PHOTON-1 Trial (GT 1, 2, or 3) N = 530 No. (%) |
TURQUOISE-I Trial (GT 1): N = 410 No. (%) |
ION-4 Trial (GT 1 or 4) N = 421 No. (%) |
ALLY-2 Trial (GT 1, 2, 3, or 4) N = 541 No. (%) |
|---|---|---|---|---|---|---|
| Combined antiretroviral therapy Regimensb–f | Supplementary Table 1 | 291 (71) | 336 (63) | 301 (73) | 334 (79) | 44 (8) |
| Active illicit drug use (excluding marijuana) | Supplementary Table 2 | 221 (54) | 294 (55) | 221 (54) | 223 (53) | NA |
| CD4 T-cell count (cells/mm3) | <300b | |||||
| <200c,d,f | ||||||
| <100e | 77 (19) | 57 (11) | 39 (10) | 12 (3) | 47 (9) | |
| Human immunodeficiency virus RNA (copies/mL) | >50b,c,e,f | |||||
| >40d | 70 (17) | 82 (15) | 73 (18) | 71 (17) | 80 (15) | |
| Active psychiatric disorderg | NA | NA | NA | NA | 65 (12) | |
| Neutrophils (cells/mm3) | <1.5b | |||||
| <1.2d | ||||||
| <0.75f | 35 (9) | NA | 10 (2) | NA | 2(<1) | |
| Albumin (g/dL) | <3.3b | |||||
| <3.0c,e,f | ||||||
| <2.8d | 53 (11) | 25 (4) | 12 (3) | 19 (5) | 22 (4) | |
| Hemoglobin (g/dL) | <110 (female) or <120 (male)b–e | |||||
| <100f | 44 (11) | 47 (9) | 35 (9) | 36 (9) | 9 (2) | |
| Platelets (cells/mm3) | <90,000b | |||||
| <60,000c,d | ||||||
| <50,000e,f | 33 (8) | NA | 8 (2) | 7 (2) | 8 (2) | |
| Decompensated liver diseaseh | 30 (7) | 38 (7) | 30 (7) | 31 (7) | 39 (7) | |
| AIDS illnessi | 14 (3) | 16 (3) | 14 (3) | 14 (3) | 21 (4) | |
| Hypertension (mmHg) | Systolic blood pressure ≥160 or diastolic blood pressure ≥100 | NA | NA | NA | NA | 14 (3) |
| Coinfection with hepatitis B | HBsAg positive | 13 (3) | 17 (3) | 13 (3) | 14 (3) | 18 (3) |
| Serum creatinine (mg/dL) or Cockcroft-Gault equation (mL/min) | <1.5b | |||||
| <60 mL/minc–e | ||||||
| <50 mL/minf | 9 (2) | 56 (11) | 40 (10) | 43 (10) | 41(8) | |
| Age (y) | <18c,e,f | |||||
| <18 and >70b,d | 5 (1) | 3 (<1) | 5 (1) | 2 (<1) | 3(<1) | |
| Body mass index (kg/m2) | <18c,e | |||||
| ≤18 and>38d | ||||||
| ≤18 and>35f | NA | 22 (4) | 20 (5) | 20 (5) | 40 (7) | |
| Bilirubin (mg/dL) | >3b–e | |||||
| > 2f | 3 (<1) | 3 (<1) | 3 (<1) | 3 (<1) | 3 (<1) | |
| International normalized ratio | >1.5 | 4 (<1) | 4 (<1) | 4 (<1) | 5 (<1) | NA |
| Alpha-fetoprotein (ng/mL) | <50b | |||||
| <100d,f | 6 (1) | NA | 4(<1) | NA | 4(<1) | |
| Aspartate Aminotransferase (U/L) | <10× ULNb,c,e | |||||
| <7× ULNd | 3 (<1) | 5 (<1) | 5 (1) | 3 (<1) | NA | |
| Alanine Aminotransferase (U/L) | <10× ULNb,c,e,f | |||||
| <7× ULNd | 1 (<1) | 1 (<1) | 7 (2) | 1 (<1) | 1 (<1) |
Abbreviations: NA, not applicable; ULN, upper limit of normal.
a The n (%) of the cohort population excluded based on each of the individual criteria.
b Simeprevir trial allowed: raltegravir, efavirenz, and rilpivirine.
c PHOTON-1 trial (sofosbuvir) allowed: tenofovir/emtricitabine with atazanavir/ritonavir, darunavir/ritonavir, efavirenz, raltegravir, or rilpivirine.
d TURQUOISE-I trial (ombitasvir, paritaprevir/ritonavir/dasabuvir) allowed: tenofovir/emtricitabine with atazanavir/ritonavir, darunavir/ritonavir, raltegravir.
e ION-4 trial (ledipasvir/sofosbuvir) allowed: tenofovir/emtricitabine with efavirenz, raltegravir, or ripilvirine.
f ALLY-2 trial (daclatasvir/sofosbuvir) only excluded unboosted protease inhibitors and cobicistat.
g Active severe psychiatric disorders include, but are not limited to, schizophrenia, psychosis, bipolar disorder, posttraumatic stress disorder, mania, and similar.
h Decompensated liver disease includes, but is not limited to, radiologic evidence of a history or presence of ascites, bleeding varices, or hepatic encephalopathy.
i Presence of AIDS-defining opportunistic infections.
As of 1 April 2015, the CCC had enrolled 1423 HIV–HCV coinfected patients from 18 Canadian centers that provide care to HIV-infected persons. Details on the CCC have been published elsewhere [24]. Briefly, participating centers include large urban tertiary care and community-based hospitals, private clinics, and street outreach programs in the attempt to capture a representative population in care. After obtaining informed consent, sociodemographic, behavioral, and medical data were prospectively collected via self-administered questionnaires/chart review and blood sampled every 6 months. Research involving this cohort was approved by all of the institutional ethics boards of the participating centers.
Of the 1423 cohort participants, we excluded those who died (n = 184), withdrew from the study (n = 107), and were lost to follow-up (defined as not completing a questionnaire within 18 months of the database closure; n = 258). Of the 874 remaining participants, 615 (70%) had evidence of chronic HCV infection (HCV RNA positive, based on each center's standard of care). We further subdivided the cohort into those who had a documented HCV genotype that reflected the trial populations. A total of 410 coinfected individuals were infected with genotype 1, 26 with genotype 2, 94 with genotype 3, and 11 with genotype 4. Participants with missing genotypes (n = 74) were excluded from the analysis. The simeprevir and TURQUOISE-I trials evaluated patients infected with genotype 1 only. PHOTON-1 evaluated patients with genotypes 1, 2, or 3. The ION-4 trial evaluated those with genotypes 1 or 4, and the ALLY-2 study was open to coinfected patients with genotypes 1, 2, 3, 4, 5, or 6.
RESULTS
The diverse demographic, clinical, and risk profiles of active CCC participants overall and subdivided by trial target populations according to eligible genotypes are presented in Table 2. Eighty percent of cohort participants had a history of injection drug use (IDU) and 31% had been using injection drugs at their last cohort visit. Poverty and history of incarceration were very common. Despite these factors, 87% of the cohort received cART and the majority maintained HIV viral suppression with high CD4 cell counts. The cART regimens that were used were diverse; an equal proportion received tenofovir and abacavir-based backbones and the majority use boosted protease inhibitors or efavirenz—drugs with potential for drug–drug interactions with some DAAs. The median duration of HCV infection was more than 20 years; 15% had evidence of advanced fibrosis based on an aspartate-to-platelet ratio index (APRI) score of >1.5, and 13% had a diagnosis of cirrhosis (clinically verified).
Table 2.
Characteristics of the Canadian Coinfection Cohort Participants at Last Visit and According to Specific Trial Target Populations
| Characteristic | Total Active Patients (N = 874) | Simeprevir and TURQUOISE-I Genotype 1 (N = 410) | PHOTON-1 Genotype 1, 2, or 3 (N = 530) GT1 = 410 GT2 = 26 GT3 = 94) |
ION-4 Genotype 1 or 4 (N = 421) GT1 = 410 GT4 = 11 |
ALLY-2 Genotype 1, 2, 3, 4, 5, 6 (N = 541) GT1 = 410 GT2 = 26 GT3 = 94 GT4 = 11 |
|---|---|---|---|---|---|
| Age, median (IQR), y | 49 (43, 55) | 47 (42, 52) | 49 (43, 55) | 49 (44, 54) | 49 (43, 55) |
| Female, no. (%) | 244 (28) | 102 (25) | 147 (28) | 108 (26) | 153 (28) |
| Aboriginal, no. (%) | 171 (20) | 81 (20) | 113 (21) | 81 (19) | 113 (21) |
| Gross annual income <$18 000 CAN,a no. (%) | 634 (73) | 311 (76) | 403 (76) | 317 (75) | 412 (76) |
| History of incarceration, no. (%) | 489 (56) | 234 (57) | 308 (58) | 236 (56) | 310 (57) |
| Current psychiatric diagnosis, no. (%) | 80 (9) | 55 (13) | 65 (12) | 55 (13) | 65 (12) |
| Currently living in shelter or homeless, no. (%) | 73 (8) | 43 (10) | 47 (9) | 43 (10) | 47 (9) |
| History of IDU, no. (%) | 703 (80) | 336 (82) | 438 (82) | 336 (80) | 438 (81) |
| Current IDU,b no. (%) | 259 (30) | 130 (32) | 68 (32) | 130 (31) | 168 (31) |
| Current alcohol use, no. (%) | 497 (57) | 213 (52) | 278 (52) | 220 (53) | 285 (53) |
| Current alcohol abuse,c no. (%) | 132 (15) | 61 (15) | 81 (15) | 62 (15) | 82 (15) |
| Time since HIV diagnosis, median (IQR), (y) | 15.8 (9.6, 21.4) | 15.8 (8.7, 21.5) | 15.7 (8.5, 21.2) | 15.7 (8.8, 21.5) | 15.7 (8.5, 21.3) |
| Undetectable HIV RNA, no. (%) | 680 (78) | 292 (71) | 388 (73) | 301 (72) | 397 (73) |
| CD4 T-cell count, median (IQR), (cells/mm3) | 500 (332, 690) | 490 (300, 674) | 480 (298, 670) | 490 (300, 680) | 480 (300, 675) |
| Currently cART naive, no. (%) | 23 (3) | 13 (3) | 17 (3) | 13 (3) | 17 (3) |
| On cART, no. (%) | 752 (86) | 356 (87) | 455 (86) | 366 (87) | 465 (86) |
| NRTI backbone, no. (%) | |||||
| Tenofovir/emtricitabine | 318 (36) | 147 (36) | 191 (36) | 153 (36) | 197 (36) |
| Abacavir/lamivudine, % | 317 (36) | 142 (35) | 186 (35) | 146 (35) | 190 (35) |
| NNRTI based, no. (%) | |||||
| Efavirenz | 127 (15) | 54 (14) | 67 (13) | 57 (14) | 70 (13) |
| Nevirapine | 20 (2) | 11 (3) | 11 (2) | 12 (3) | 12 (2) |
| Rilpivirine | 22 (3) | 19 (5) | 23 (5) | 19 (5) | 23 (3) |
| Etravirine | 36 (4) | 18 (4) | 25 (5) | 18 (4) | 25 (5) |
| Protease inhibitors/Ritonavir, no. (%) | |||||
| Atazanavir | 164 (19) | 75 (18) | 100 (19) | 76 (18) | 101 (19) |
| Lopinavir | 76 (9) | 30 (7) | 42 (8) | 32 (8) | 44 (8) |
| Darunavir | 159 (18) | 79 (19) | 103 (19) | 81 (20) | 105 (19) |
| Integrase inhibitors | |||||
| Raltegravir | 190 (22) | 90 (22) | 117 (22) | 93 (22) | 120 (22) |
| Dolutegravir | 27 (3) | 17 (4) | 17 (3) | 17 (4) | 17 (3) |
| Elvitegravir | 43 (5) | 19 (5) | 23 (4) | 19 (5) | 23 (4) |
| Duration of HCV infection, median (IQR), y | 21.7 (13.7, 30.0) | 21.4 (13.0, 29.1) | 21.3 (13.2, 29.3) | 21.0 (13.0, 29.0) | 21.0 (13.0, 29.0) |
| Prior HCV treatment experience, no. (%) | 334 (38) | 113 (28) | 148 (28) | 119 (28) | 152 (28) |
| Current AST to platelet ratio index (APRI) >1.5, no. (%) | 130 (15) | 78 (19) | 109 (21) | 81 (19) | 112 (21) |
| History of cirrhosis (clinical diagnosis), no. (%) | 115 (13) | 64 (16) | 78 (15) | 66 (16) | 80 (15) |
| History of end stage liver disease diagnosis,d no. (%) | 129 (15) | 74 (18) | 89 (17) | 76 (18) | 91 (17) |
Active patients (n = 874) includes all active cohort participants. Undetectable HIV RNA (RNA < 50 copies/mL). Trials restricted participation to specific genotypes; therefore, the cohort is subdivided into those genotypes. The simeprevir and TURQUOISE-I trials evaluated patients infected with genotype 1. The PHOTON-1 trial evaluated patients with genotypes 1, 2, or 3. The ION-4 trial evaluated those with genotypes 1 or 4, and the ALLY-2 study was open to coinfected patients with genotypes 1, 2, 3, 4, 5, or 6.
Abbreviations: AST, aspartate aminotransferase; cART, combined antiretroviral therapy; GT, genotype; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; IQR, interquartile range; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor.
a Single person poverty is considered an annual income of <$18 421/yr CAN.
b Current IDU is defined as use of any injection drugs within 6 months of last cohort visit (self reported).
c Current alcohol abuse is defined as drinking more than 2 units of alcohol on a “typical day” within 6 months of last cohort visit (self reported).
d Includes ascites, bleeding esophageal varices, portal hypertension, hepatocellular carcinoma, spontaneous bacterial peritonitis.
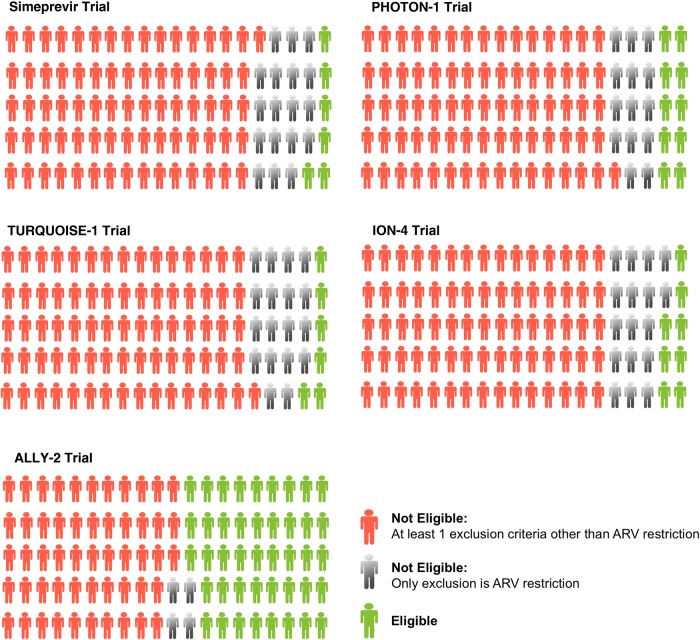
After applying all trial eligibility criteria to the CCC participants, only 5.9% (24/410) would have been eligible to be screened for the simeprevir trial, 9.8% (52/530) for the PHOTON-1 trial, 6.3% (26/410) for the TURQUOISE-I trial, and 8.1% (34/421) for the ION-4 trial. The ALLY-2 trial stood out as being far more inclusive, with 43% (233/541) of the cohort eligible for screening. Table 1 details the exclusive criteria that led to noneligibility for each trial. The most common reasons for noneligibility in all trials except the ALLY-2 trial were restriction to specific antiretroviral therapies that resulted in the exclusion of 63%–79% of the cohort, followed by active drug use (excluding marijuana), which excluded 53%–55% of the cohort. Figure 1 illustrates that even if antiretroviral eligibility criteria were not considered (eg, assuming patients could be safely switched to other regimens compatible with DAAs under study), 74%–77% of the cohort would still have been excluded, primarily due to active drug use for 4 of the 5 trials. Among all trials, as many as 1 in 6 participants would have been excluded because of either detectable HIV RNA (15%–18%) and/or not meeting minimal CD4 count requirements (3%–19%). Criteria related to safety concerns, specifically clinical cutoffs for anemia and renal and liver function resulted in relatively few exclusions. Despite the enhanced ease and tolerability of all oral interferon-free DAAs, eligibility into these trials was just as exclusive as the trial with pegylated-interferon and ribavirin, with the notable exception of the ALLY-2 trial.
Figure 1.
Green figures represent the number of Canadian Coinfection Cohort participants who would be eligible to be screened in NCT01479868 (trial evaluating simeprevir); PHOTON-1: NCT01667731 (trial evaluating sofosbuvir); TURQUOISE-I: NCT01939197 (trial evaluating ombitasvir, paritaprevir/ritonavir/dasabuvir [3D]); ION-4: NCT02073656 (trial evaluating ledipasvir/sofosbuvir); and ALLY-2: NCT02032888 (trial evaluating daclatasvir/sofosbuvir). Gray figures represent participants whose only exclusion was specific antiretroviral (ARV) therapies. Red figures represent participants not eligible regardless of ARV restriction.
DISCUSSION
When clinical trials are internally valid, they are considered the gold standard for estimating treatment effects. Trial results are used to support licensure, inform health authorities in conducting cost-effectiveness analyses, and guide clinical decision making. However, to make these inferences to the wider population, trials also need to be externally valid. Here, we illustrate that the majority of HIV–HCV-coinfected patients in clinical care would not be included in recent clinical trials that evaluated HCV therapy. Therefore, DAA trial results may have limited generalizability.
In the last 5 years we have witnessed SVR rates previously unimaginable, especially in hard-to-treat coinfected patients. However, it is important to evaluate how trial efficacy translates to real-world effectiveness, which is driven by factors such as adherence, loss to follow-up, and comorbidities [25]. Real-world data on the effectiveness of DAAs in HCV-monoinfected populations have been, on average, 5%–15% lower than what was reported in phase 3 trials [26, 27]. It is unclear what proportion of these lower SVR rates are explained by patients not adhering to their medications or being lost to follow-up as opposed to poorer efficacy. In one real-world analysis of interferon-free therapies in 151 HCV-monoinfected patients, the authors reported SVR rates of 88%; 7% relapsed and 4% were lost to post-treatment follow-up and could not be assessed for SVR [28]. With the widespread use of DAAs by increasingly marginalized populations, higher failure rates than those seen in clinical trials could translate to hundreds of thousands of treatment failures with limited future treatment options.
Fundamentally, trial participants are different; they include highly motivated people who may receive compensation and extensive support from trial staff, including for adherence. Such extensive programs are not feasible in most real-world healthcare settings, although they might serve as an effective model of care. Regardless of eligibility criteria, selection into clinical trials is not random. Sites select patients who are more likely to comply with strict trial procedures. Additionally, we observed that trial populations were, on average, “healthier” than the cohort population. This was evident by comparing baseline CD4 cell counts of trial participants to those in the CCC. Regardless of minimum cutoffs, trial participants had higher CD4 cell counts (between 31 and 139 cells/mL higher) than the average CCC participant [17–19, 22, 23].
While restriction into clinical trials for the purposes of protecting the safety of participants is legitimate, we found that the majority of exclusionary criteria were not related to safety but appear to be aimed at maximizing treatment response rates. In particular, exclusion of active drug users may have been overly conservative as studies have shown they can achieve comparable SVRs as those not injecting drugs in well-supported settings [29]. Reinfection and interactions between illicit drugs and DAAs, however, remain a concern for the active drug-using population. However, this should not prevent the inclusion of this important subgroup of individuals, especially when the eradication of HCV in developed countries is contingent on expanding treatment to active drug users. On the contrary, more data on the effectiveness of DAAs in this population are urgently needed in order to support scaling-up treatment strategies. The eligibility criteria for the ALLY-2 study appeared to be far more inclusive with respect to permitting enrollment of stable people who use drugs, illustrating that it is possible to conduct studies that are more reflective of the target population. Despite these broader criteria, there was actually no evidence that any drug users were included in the study. It will be important for future studies to report on the number of active drug users enrolled. Finally, given the prohibitive cost of treatments, restricting trials to ideal populations may also have profound effects on policy decisions, as evidenced by the state-level Medicaid restrictions of sofosbuvir where the majority of US states require abstinence from drugs and alcohol despite international guidelines stating the opposite [30].
For coinfected patients, HCV treatment is further complicated by potential drug–drug interactions between cART and DAAs [31]. While some drug–drug interactions are well documented and exclusion of individuals taking these medications is justifiable, others have either not been studied or have no basis for restriction [31]. Even if it were feasible to switch HIV regimens, the majority of the CCC participants would remain ineligible primarily due to active drug use and HIV viral load/CD4 cell count cutoffs or advanced liver disease. Even though the ALLY-2 trial permitted the majority of cART regimens and stable drug users, 57% of the cohort would still have been excluded from participating in this trial. This is particularly alarming given that the CCC comprises individuals who are able to access care and maintain cART successfully.
To evaluate generalizability, we assumed the CCC is a representative population. The CCC is open to all HIV-positive patients with evidence of HCV infection followed at participating sites without restriction and is estimated to include 23% of the total coinfected population in care in Canada. Since participants have access to universal healthcare, insurance does not restrict those who can attend clinics. Although other socioeconomic determinants may affect access to care, this does not appear to be the case as cohort participants did have very high rates of substance abuse and poverty. Representativeness of this cohort can likely be extended to individuals with health insurance in the United States and in certain European countries where the prevalence of active illicit drug use is similar to that in Canada.
We focused on eligibility criteria listed in trial protocols. Additional factors such as overall willingness and motivation to participate in clinical trials were not assessed and may further reduce the proportion of coinfected trial participants. Other clinical criteria such as evidence of malignancies or other significant illnesses, electrocardiographic abnormalities, clinical cutoffs for HCV RNA, and glycosylated hemoglobin involve data that have not been routinely collected as part of the CCC and therefore they were not assessed. Moreover, historically HCV trial protocols in coinfection have restricted participation into clinical trials based on the presence of HIV resistance. This was only an exclusionary criterion for the TURQUOISE-I trial (“past virologic failure to more than 1 HIV-1 ART regimen and specifically darunavir resistance”). Additionally, documentation on previous HCV treatment failures and clinical definitions of what constituted cirrhotic vs noncirrhotic patients could also have further excluded trial participation. Taken together, our estimate of trial eligibility is likely to be conservative.
We restricted our analysis to phase 3 trials. Populations from the PHOTON-2 trial, which evaluated sofosbuvir (NCT01783678), and the C-EDGE Co-infection trial, which evaluated grazoprevir/elbasvir (NCT02105662), were not included in this analysis because trial protocols were not published. Based solely on the limited eligibility criteria available from published papers and publically from clinical trial registries, we would estimate that only 12.6% of cohort participants would have been eligible to be screened for the PHOTON-2 trial and 10.2% for the C-EDGE trial. Similar to the other trials, the most exclusive eligibility criterion was restriction to specific antiretroviral regimens, excluding 63% of the CCC from PHOTON-2 and 80% from the C-EDGE trials [11, 32]. Thus, for the coinfected population, drug–drug interactions will remain a limiting factor for those who cannot be safely switched to alternative regimens.
HCV is the first chronic viral disease that can be cured. However, many paradoxes exist. DAAs are the most expensive antivirals ever to be developed on a per pill basis, costing between $54 000 and $122 000 per treatment course in Canada. Globally, HCV disproportionately affects the poorest and most disenfranchised populations. Clinical trials have demonstrated very high efficacy in people who do not reflect target populations and in ideal trial settings. Despite breakthroughs in HCV treatments, many psychosocial disadvantages still require intervention in order to increase treatment uptake and obtain successful outcomes. Unless mandated to do so, the pharmaceutical industry has little incentive to evaluate DAAs in representative populations. Even when restriction is more inclusive, there is still no guarantee of enrolling representative populations. Therefore, observational study designs that estimate unbiased treatment effects in the coinfected population will be essential to determine how effective these therapies will be in the real world [33]. This work illustrates the need to evaluate the external validity of all marketed pharmaceuticals in order to determine whether trial populations represent target populations. If generalizability is found to be limited, then targeted phase 4 studies need to be considered.
The advent of DAAs, especially interferon-free regimens, has given hope that the burden of liver disease can be reduced among HIV–HCV-coinfected individuals and that HCV can ultimately be eliminated. It remains to be seen how effective these therapies will be for the average patient who urgently requires them.
Supplementary Material
Notes
Acknowledgements. The CCC investigators (CTN222) include Drs Jeff Cohen, Windsor Regional Hospital Metropolitan Campus, Ontario; Brian Conway, Vancouver Infectious Diseases Research and Care Centre, British Columbia; Curtis Cooper, the Ottawa Hospital Research Institute, Ontario; Pierre Côté, Clinique du Quartier Latin, Montréal, Québec; Joseph Cox, Montréal General Hospital, Quebec; John Gill, Southern Alberta HIV Clinic, Calgary; Shariq Haider, McMaster University, Hamilton, Ontario; Marianne Harris, St. Paul's Hospital, Vancouver, British Columbia; David Haase, Capital District Health Authority, Halifax, Nova Scotia; Mark Hull, British Columbia Centre for Excellence in HIV/AIDS, Vancouver; Julio Montaner, St. Paul's Hospital, Vancouver, British Columbia; Erica Moodie, McGill University, Montreal, Quebec; Neora Pick, Oak Tree Clinic, Children's and Women's Health Centre of British Columbia, University of British Columbia, Vancouver; Anita Rachlis, Sunnybrook & Women's College Health Sciences Centre, Toronto, Ontario; Danielle Rouleau, Centre Hospitalier de l′Université de Montréal, Quebec; Roger Sandre, Haven Program, Sudbury, Ontario; Joseph Mark Tyndall, Department of Medicine, Infectious Diseases Division, University of Ottawa, Ontario; Marie-Louise Vachon, Centre Hospitalier Universitaire de Québec, Québec City; Sharon Walmsley, University Health Network, Toronto, Ontario; and David Wong, University Health Network, Toronto, Ontario.
Author contributions. As the corresponding author, M. B. K. had full access to all data from the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: S. S. and M. B. K. Acquisition, analysis, and/or interpretation of data: S. S., M. B. K., C. C., J. C., M. J. G., M. H., V. M.-L., N. P., E. C. S., and S. L. W. Drafting of the manuscript: S. S. and M. B. K. Critical revision of the manuscript for important intellectual content: S. S., M. B. K., C. C., J. C., M. J. G., M. H., V. M.-L., N. P., K. R.-K., E. C. S., and S. L. W. Statistical analysis: S. S. Obtained funding: M. B. K. Study supervision: M. B. K. and E. C. S.
Financial support. This study was funded by the Fonds de recherche du Québec–Santé (FRQ-S); Réseau SIDA/maladies infectieuses, the Canadian Institutes of Health Research (CIHR MOP-79529); and the CIHR Canadian HIV Trials Network (CTN222). S. S. is supported by a doctorate award from the National CIHR Research Training Program in Hepatitis C/Canadian Network on Hepatitis C. M. B. K. is supported by the “Chercheurs Nationaux” career award from the FRQ-S.
Potential conflicts of interest. There was no pharmaceutical industry support to conduct this study. M. B. K. has received research support from Janssen, Bristol-Myers Squibb, ViiV, Merck, and Gilead; honoraria for lectures from Gilead, Schering-Plough, Janssen ViiV Healthcare, and Merck; consulting fees from ViiV Healthcare, Bristol-Myers Squibb, Gilead, Merck, and AbbVie. S. W. has received grants, consulting fees, lecture fees, nonfinancial support, and fees for the development of educational presentations from Merck, ViiV Healthcare, GlaxoSmithKline, Pfizer, Gilead, AbbVie, Bristol-Myers Squibb, and Janssen. M. J. G. has received personal fees for being a member of the national advisory boards of AbbVie, Gilead, Merck, Janssen, and ViiV Healthcare. M. H. has served as a consultant for Merck, Vertex Pharmaceuticals, Pfizer, ViiV Healthcare, Ortho-Jansen, Gilead, and Bristol-Myers Squibb; has received grants from the National Institute on Drug Abuse; and has received payment from Merck and Ortho-Janssen for lectures. J. C. has received grants from ViiV Healthcare and Gilead and personal fees from Bristol-Myers Squibb, Merck, and Gilead. C. C. has received personal fees and research grants from Gilead, Merck, and AbbVie. V. M.-L. has received research grants from Gilead and personal consultant fees from Gilead and AbbVie. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the Canadian Co-Infection Cohort Study, Jeff Cohen, Brian Conway, Curtis Cooper, Pierre Côté, Joseph Cox, John Gill, Shariq Haider, Marianne Harris, David Haase, Mark Hull, Julio Montaner, Erica Moodie, Neora Pick, Anita Rachlis, Danielle Rouleau, Roger Sandre, Joseph Mark Tyndall, Marie-Louise Vachon, Sharon Walmsley, and David Wong
References
- 1.Soriano V, Barreiro P, Sherman KE. The changing epidemiology of liver disease in HIV patients. AIDS Rev 2013; 15:25–31. [PubMed] [Google Scholar]
- 2.Weber R, Sabin CA, Friis-Moller N et al. . Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166:1632–41. [DOI] [PubMed] [Google Scholar]
- 3.Salmon-Ceron D, Rosenthal E, Lewden C et al. . Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: the French national mortality 2005 study. J Hepatol 2009; 50:736–45. [DOI] [PubMed] [Google Scholar]
- 4.Klein MB, Rollet KC, Saeed S et al. . HIV and hepatitis C virus in Canada: challenges and opportunities for reducing preventable morbidity and mortality. HIV Med 2013; 14:10–20. [DOI] [PubMed] [Google Scholar]
- 5.Labarga P, Soriano V, Vispo ME et al. . Hepatotoxicity of antiretroviral drugs is reduced after successful treatment of chronic hepatitis C in HIV-infected patients. J Infect Dis 2007; 196:670–6. [DOI] [PubMed] [Google Scholar]
- 6.Torriani FJ, Rodriguez-Torres M, Rockstroh JK et al. . Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004; 351:438–50. [DOI] [PubMed] [Google Scholar]
- 7.Limketkai BN, Mehta SH, Sutcliffe CG et al. . Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA 2012; 308:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas DL. The challenge of hepatitis C in the HIV-infected person. Annu Rev Med 2008; 59:473–85. [DOI] [PubMed] [Google Scholar]
- 9.Young J, Potter M, Cox J et al. . Variation between Canadian centres in the uptake of treatment for hepatitis C by patients coinfected with HIV: a prospective cohort study. CMAJ Open 2013; 1:E106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson IM, Dore GJ, Foster GR et al. . Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2014; 384:403–13. [DOI] [PubMed] [Google Scholar]
- 11.Molina JM, Orkin C, Iser DM et al. . Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet 2015; 385:1098–106. [DOI] [PubMed] [Google Scholar]
- 12.Afdhal N, Zeuzem S, Kwo P et al. . Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–98. [DOI] [PubMed] [Google Scholar]
- 13.Lawitz E, Mangia A, Wyles D et al. . Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–87. [DOI] [PubMed] [Google Scholar]
- 14.Poordad F, Hezode C, Trinh R et al. . ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014; 370:1973–82. [DOI] [PubMed] [Google Scholar]
- 15.Ferenci P, Bernstein D, Lalezari J et al. . ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 2014; 370:1983–92. [DOI] [PubMed] [Google Scholar]
- 16.Manns M, Pol S, Jacobson IM et al. . All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet 2014; 384:1597–605. [DOI] [PubMed] [Google Scholar]
- 17.Sulkowski MS, Eron JJ, Wyles D et al. . Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA 2015; 313:1223–31. [DOI] [PubMed] [Google Scholar]
- 18.Naggie S, Cooper C, Saag M et al. . Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyles DL, Ruane PJ, Sulkowski MS et al. . Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373:714–25. [DOI] [PubMed] [Google Scholar]
- 20.Soriano V, Labarga P, Vispo E, Fernandez-Montero JV, Barreiro P. Treatment of hepatitis C in patients infected with human immunodeficiency virus in the direct-acting antiviral era. Infect Dis Clin North Am 2012; 26:931–48. [DOI] [PubMed] [Google Scholar]
- 21.Davies A, Singh KP, Shubber Z et al. . Treatment outcomes of treatment-naive hepatitis C patients co-infected with HIV: a systematic review and meta-analysis of observational cohorts. PloS One 2013; 8:e55373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sulkowski MS, Naggie S, Lalezari J et al. . Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA 2014; 312:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dieterich D, Rockstroh JK, Orkin C et al. . Simeprevir (TMC435) with pegylated interferon/ribavirin in patients coinfected with HCV genotype 1 and HIV-1: a phase 3 study. Clin Infect Dis 2014; 59:1579–87. [DOI] [PubMed] [Google Scholar]
- 24.Klein MB, Saeed S, Yang H et al. . Cohort profile: the Canadian HIV-hepatitis C co-infection cohort study. Int J Epidemiol 2010; 39:1162–9. [DOI] [PubMed] [Google Scholar]
- 25.Mathes T, Antoine SL, Pieper D. Factors influencing adherence in hepatitis-C infected patients: a systematic review. BMC Infect Dis 2014; 14:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen D, O'Leary J, Pockros P, Sherman K. Safety and efficacy of sofosbuvir-containing regimens for hepatitis C: real-world experience in a diverse, longitudinal observational cohort (abstract 45). In: AASLD 2014/10/03 ed Vol. 60 Suppl 1 Boston, Massachusetts: Hepatology, 2014:197a–695. [Google Scholar]
- 27.Dieterich D, Bacon B, Flamm S, Kowdley K. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population (abstract 46). In: AASLD 2014/10/03 ed Vol. 60 Suppl 1 Boston, Massachusetts: Hepatology, 2014:197a–695. [Google Scholar]
- 28.Brown RS Jr., O'Leary JG, Reddy KR et al. . Interferon-free therapy for genotype 1 hepatitis C in liver transplant recipients: real world experience from HCV-TARGET. Liver Transpl 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis 2009; 49:561–73. [DOI] [PubMed] [Google Scholar]
- 30.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163:215–23. [DOI] [PubMed] [Google Scholar]
- 31.Chen TY, Jain MK. Treatment of hepatitis C in HIV-infected patients: moving towards an era of all oral regimens. AIDS Patient Care STDS 2015; 29:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockstroh JK, Nelson M, Katlama C et al. . Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV 2015; 2:e319–27. [DOI] [PubMed] [Google Scholar]
- 33.D'Agostino RB Jr., D'Agostino RB Sr. Estimating treatment effects using observational data. JAMA 2007; 297:314–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.