This randomized trial compared peripheral and central fat changes 96 weeks after a first-line regimen containing darunavir/ritonavir, atazanavir/ritonavir, or raltegravir. Similar and significant increases were seen in all arms. Pre-treatment HIV-1 RNA level was the strongest predictor of fat gains.
Keywords: lipodystrophy; lipoatrophy, visceral fat, limb fat, body composition
Abstract
Background. Fat gain after antiretroviral therapy (ART) occurs, and its association with protease inhibitors (PIs) is unclear.
Methods. Peripheral and central fat depots and lean mass were measured using standardized and centrally read abdominal CT scans and whole-body dual-energy absorptiometry scans over a 96-week period in human immunodeficiency virus (HIV)–infected treatment-naive participants. The patients were randomized to tenofovir-emtricitabine (TDF/FTC) plus atazanavir-ritonavir (ATV/r), darunavir-ritonavir (DRV/r), or raltegravir (RAL) in ACTG A5260s, a substudy of A5257. Within arm changes were assessed with signed-rank tests. The 96-week percentage changes in fat and lean mass in the 2 PI arms were not different, thus the PI arms were combined and compared to the RAL arm. Associations between baseline biomarkers and changes in body composition were assessed. All analyses used linear regression models.
Results. 328 patients were randomized (90% male, 44% white non-Hispanic). The median age was 36 years, HIV-1 RNA 4.6 log10 copies/mL, and CD4 349 cells/μL. Overall, at week 96, increases in limb fat (13.4%), subcutaneous (19.9%) and visceral abdominal fat (25.8%), trunk fat (18%), and lean mass (1.8%) were apparent (P < .001 for changes within each arm). Changes for all fat and lean outcomes were not different between the PI arms or between the RAL and the combined PI arms. Higher baseline HIV-1 RNA levels were associated with greater gains in peripheral and central fat.
Conclusions. In treatment-naive participants initiating ART with TDF/FTC, no differences in lean mass and regional fat were found with RAL when compared with ATV/r or DRV/r over 96 weeks.
Clinical Trials Registration. NCT00811954 and NCT00851799.
Gains in fat and lean body mass (LBM) occur commonly after the initiation of treatment for chronic illnesses including human immunodeficiency virus (HIV). Lipodystrophy is a term used to describe alterations in body fat associated with HIV treatment. These fat alterations could be in the form of isolated lipoatrophy (or fat loss in face, buttocks, or limbs), isolated lipohypertrophy (or accumulation of fat in abdomen, dorsocervical region, or breasts), or a combination of both. These fat changes are clinically important as they can predispose to cardiovascular disease (CVD) and could have devastating consequences on self-image and adherence to antiretroviral therapy (ART) [1–3]. Lipoatrophy has been linked to the use of thymidine nucleoside reverse transcriptase inhibitors (NRTIs) and the resultant mitochondrial toxicity [4, 5], and avoidance of thymidine NRTIs can prevent most cases of clinically visible lipoatrophy [6, 7]. While lipohypertrophy was initially attributed to the use of protease inhibitors (PIs) [8], eliminating PIs has not reversed lipohypertrophy [9, 10]. Moreover, avoidance of PIs in first-line therapy was still associated with gains in central fat [7].
There are limited data on the effect of integrase inhibitors on body composition. A small study reported smaller gains in trunk fat after 96 weeks of raltegravir (RAL) compared with efavirenz (EFV) [11]. Our objective in this study was to describe the changes in body composition over 96 weeks after initiating tenofovir/emtricitabine (TDF/FTC) plus atazanavir/ritonavir (ATV/r), darunavir/ritonavir (DRV/r), or RAL in HIV-positive individuals naive to treatment. Also, we explored associations between baseline factors and changes in body composition.
METHODS
A5260s was a substudy of AIDS Clinical Trials Group (ACTG) A5257 in which HIV-infected ART-naive persons aged ≥18 years with HIV-1 RNA ≥1000 copies/mL were randomized in an open-label fashion to receive TDF/FTC plus ATV/r, DRV/r, or RAL. A5257 participants without known CVD or diabetes mellitus, uncontrolled thyroid disease, or use of lipid-lowering medications were eligible to enroll in A5260s. Randomization was stratified by screening HIV-1 RNA level (>100 000 or ≤100 000 copies/mL) and Framingham 10-year CVD risk score (<6% risk or ≥6% risk). A5260s primary objective was to compare cardiovascular markers between those initiating the randomized regimens [12]. A secondary objective of assessing changes in immune activation markers was also published [13]. Another secondary objective was to examine the effects of A5260s regimens on body composition. The parent study and substudy were approved by the institutional review boards at participating institutions, and participants provided written informed consent.
Body Composition Measures
Substudy evaluations occurred at baseline and week 96. Fat distribution was measured using whole-body dual-energy absorptiometry (DXA) in anteroposterior view using the same scanner on the same participant throughout the study. The whole-body DXA scan was used to quantify total limb fat (the sum of upper and lower extremity fat), trunk fat, and LBM. Single-slice computed tomography (CT) scan at the L4–L5 level was used to quantify visceral adipose tissue (VAT), subcutaneous abdominal tissue (SAT), and total adipose tissue (TAT). Scans were standardized and centrally read by blinded personnel at the Body Composition Analysis Center at Tufts University (Boston, Massachusetts; DXA) and LA Biomed (Torrance, California; CT).
Laboratory Assessment
Blood samples (fasting for ≥8 hours) were collected. Plasma biomarkers were measured at the University of Vermont (Burlington) Laboratory for Clinical Biochemistry Research on batched plasma samples that were stored at −70°C. Tests included high-sensitivity C-reactive protein by nephelometry, D-dimer with immunoturbidometric methods, and soluble interleukin-2 receptor (sIL-2R), soluble cluster of differentiation 14 (sCD14), soluble cluster of differentiation 163 (sCD163), IL-6, and the adipokines leptin and adiponectin using enzyme linked immunosorbent assay.
Monocytes and T cells were phenotyped by flow cytometry as previously described [13]. The following 3 monocyte subsets were each quantified as a percentage of overall monocyte population: inflammatory: CD14+ CD16+, patrolling: CD14dimCD16+, and classic: CD14+ CD16-. T-cell activation was quantified as the percentage of CD4+ or CD8+ cells that expressed both CD38 and human leukocyte antigen - antigen D related (HLA-DR); T-cell exhaustion was quantified as the percentage of CD4+ or CD8+ expressing PD1+; and T-cell senescence was quantified as the percentage of CD4+ or CD8+ expressing CD28-CD57+.
Statistical Analyses
The primary objective of these analyses was to determine the extent to which body composition changed in the first 96 weeks after initiating the randomized treatments and to compare these changes between groups. Linear regression, with adjustment for stratification factors, was used to compare changes in body composition between arms. These comparisons modeled treatment as a 3-level factor and used reverse Helmert contrasts. ATV/r was first compared with DRV/r at a 2.5% significance level. If not significant, the PI/r arms were pooled and compared with RAL; otherwise, pairwise comparisons were performed. All treatment comparisons were assessed with a type I error rate of 2.5% and 97.5% confidence intervals; all other comparisons were at a 5% alpha level. Within each treatment arm, changes in body composition were assessed with Wilcoxon signed rank tests, and Spearman correlations were used to assess associations between body composition endpoints. Associations between baseline factors and changes in body composition were evaluated with linear regression using univariate and adjusted models. Adjustments included baseline age, body mass index (BMI), HIV-RNA, CD4 count, sex, and race/ethnicity. Analyses were conducted as intent-to-treat (ITT); an as-treated (AT) secondary analysis showed results similar to those for the ITT analysis (data not shown). Extreme fat changes, defined as standardized residual (z-score) of > 4 or <−4, were excluded from all linear regression models; that excluded 8 participants.
RESULTS
Baseline Characteristics and Disposition
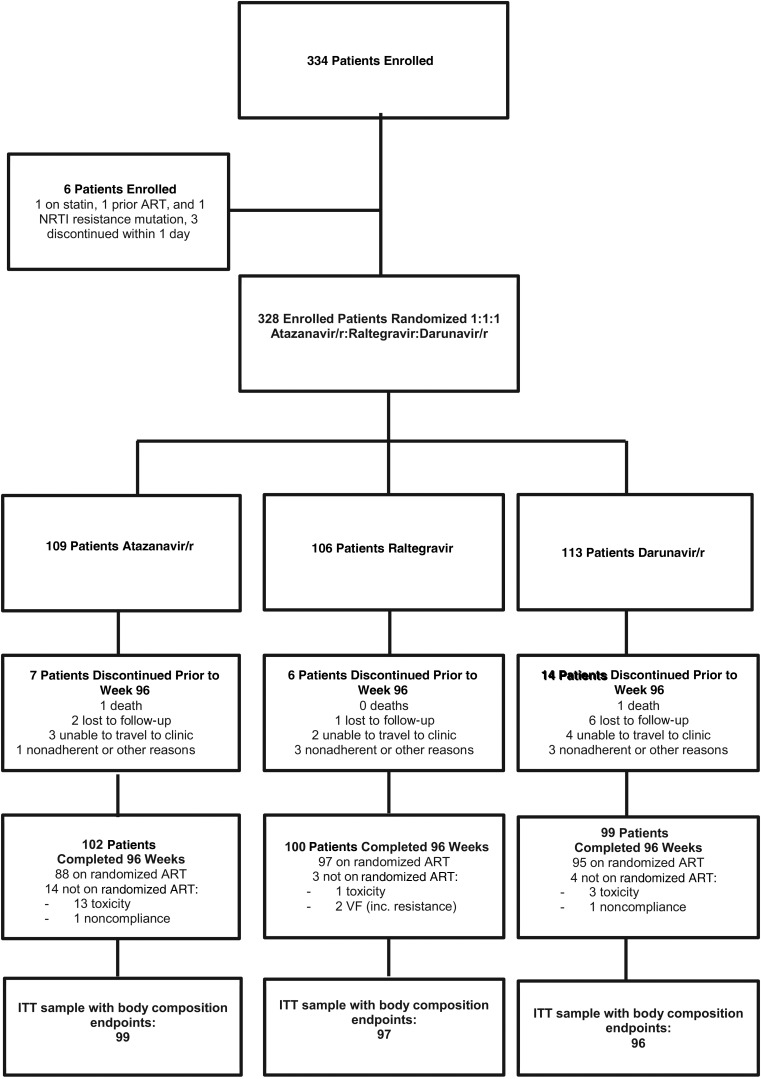
A total of 334 participants entered A5260s from 26 ACTG sites in the United States from June 2009 to April 2011 (Table 1). Of these, 3 did not meet the eligibility criteria (1 not ART naive, 1 with NRTI mutations, 1 on statin) and 3 discontinued follow-up within 1 day of enrollment, leaving 328 in the included analysis (Figure 1). Overall, 90% were male, 44% white non-Hispanic, and 20% Hispanic. The median age was 36 years; CD4 was 349 cells/µL; HIV-1 RNA was 4.6 log10 copies/mL; BMI was 25 kg/m2; limb fat was 7.6 kg; and VAT was 72.9 cm2. Overall 25% had HIV-1 RNA ≥100 000 copies/mL at screening (high HIV-RNA stratum). Over the 96-week follow-up period, 25 (8%) prematurely discontinued the substudy, 2 died, and 20 (7%) changed treatment regimens (Figure 1). Among the premature study discontinuation, 2 participants discontinued due to body composition concerns: 1 to “lipoatrophy” and another to “weight change, likely due to ritonavir.”
Table 1.
Baseline Characteristics
| Characteristic | Atazanavir-Ritonavir (n = 109) | Raltegravir (n = 106) | Darunavir-Ritonavir (n = 113) |
|---|---|---|---|
| Age (y) | 37 (31,45) | 36 (27,44) | 35 (27,46) |
| Sex | 91% M, 9% F | 89% M, 11% F | 89% M, 11% F |
| Race/ethnicity (%) | |||
| White | 49 | 41 | 42 |
| Black | 31 | 32 | 33 |
| Hispanic | 18 | 19 | 22 |
| Current smoking | 40 | 37 | 36 |
| CD4+ cell count (cells/mm³) | 350 (211, 461) | 343 (207, 461) | 355(207, 461) |
| HIV-1 RNA (log10 copies/mL) | 4.62 (4.05, 5.10) | 4.52 (4.13, 5.08) | 4.52 (3.95, 4.95) |
| HIV-1 RNA (%) | |||
| <100 000 copies/mL | 71 | 69 | 76 |
| ≥100 000 copies/mL | 29 | 31 | 24 |
| Weight (kg) | 80 (69,88) | 77 (66,89) | 77(67,83) |
| Body mass index (kg/m2) | 26 (23, 29) | 24 (22,28) | 24 (22, 27) |
| Limb fat | 8.3 (6.0, 10.5) | 7.3 (5.0, 11.0) | 7.3 (4.9, 10.0) |
| Visceral adipose tissue | 78.2 (42.6, 111.3) | 75.3 (41.6, 110.1) | 59.8 (32.8, 99.2) |
| Trunk fat | 9.7 (6.5, 13.1) | 8.8 (5.6, 13.3) | 8.5 (5.0, 11.9) |
| Subcutaneous adipose tissue | 212.8 (133.0, 310.6) | 201.6 (124.5, 291.5) | 176.9 (104.3, 266.4) |
| Lean body mass | 55.7 (49.4, 62.0) | 54.2 (49.1, 60.5) | 55.2 (48.3, 61.1) |
| Physical activity (%) | |||
| Low | 16 | 22 | 17 |
| Moderate | 84 | 78 | 83 |
Abbreviation: HIV, human immunodeficiency virus.
Figure 1.
Subject disposition. Abbreviations: ART, antiretroviral therapy; ITT, intent-to-treat; NRTI, nucleoside reverse transcriptase inhibitor; VF, virologic failure.
Changes in Body Mass Index
At week 96, BMI increased in all arms by 3.8%–4.7% from baseline (all P < .001), with no differences between arms (P ≥ .65).
Changes in Central Fat Depots
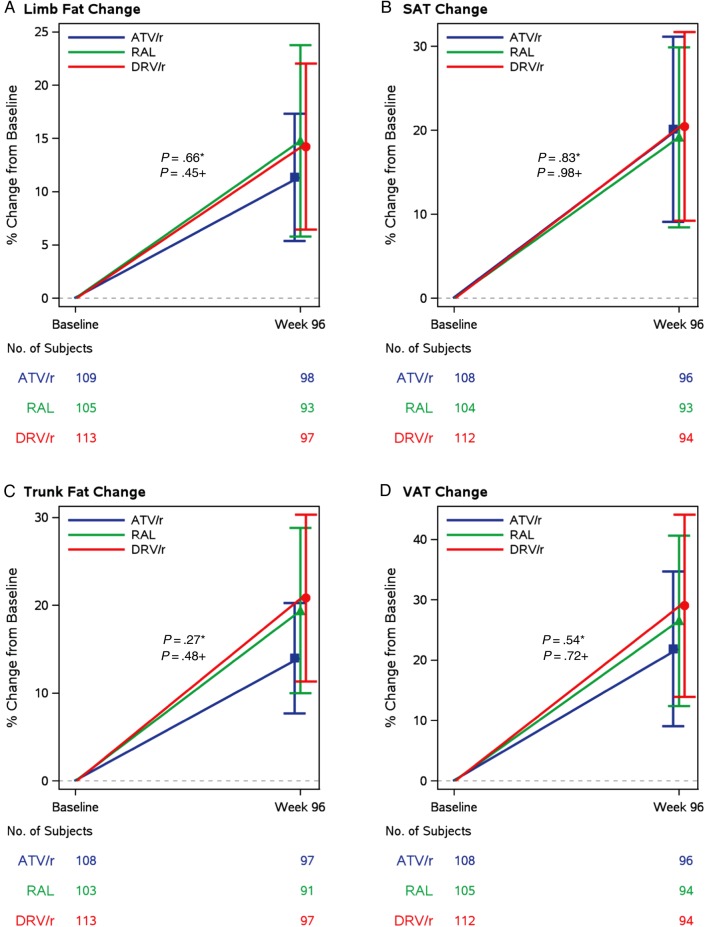
At week 96, the mean (standard deviation [SD]) percent change in trunk fat was 18.0% (36.3%); mean increase of 14% on ATV/r, 19.4% on RAL, and 20.8% on DRV/r; all P < .001, and not different between the 2 PI arms (P = .27) or between the RAL and the combined PI arms (P = .48) (Figure 2). Changes in VAT mirrored changes in trunk fat, with an overall mean increase of 25.8% (SD 59.8%) by week 96. All 3 regimens were associated with similar increases in VAT (21.9%, ATV/r; 26.5%, RAL; and 29%, DRV/r); all P < .001, and not different between arms (P ≥ .54). Changes in trunk fat were strongly correlated with changes in VAT (r = 0.72, P < .001). Both measures reflected changes in central fat depots, although trunk fat is reflective of combined subcutaneous and visceral fat depots. No changes in the VAT:TAT ratio were seen within any of the arms (P ≥ .68).
Figure 2.
Changes in body composition parameters in peripheral fat depots: (A) limb fat measured by dual-energy absorptiometry (DXA) scan and (B) subcutaneous adipose tissue measured by computed tomography (CT) scan of abdomen. Changes in body composition parameters in central fat depots: (C) trunk fat measured by DXA scan and (D) visceral adipose tissues measured by CT scan of abdomen. *, ATV/r vs DRV/r and +, PI/r vs RAL. Abbreviations: ATV/r, atazanavir-ritonavir; DRV/r, darunavir-ritonavir; PI, protease inhibitor; RAL, raltegravir; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
The subgroup of participants with the most VAT gains during the study (>Q3, or 43% VAT change) was equally distributed across treatment regimens (P > .36). There were no differences in baseline characteristics, including age and gender, between the 71 participants and the rest of the A5260s population, except for lower CD4 and BMI, higher HIV-1 RNA, and lower marijuana use.
Changes in Peripheral Fat Depots
As shown in Table 2 and Figure 2, at week 96, the mean (SD) percent change in limb fat was 13.4% (32.8%); a mean increase of 11.4% on ATV/r, 14.8% on RAL, and 14.2% on DRV/r; all P < .001, but changes were not different between the 2 PIs (P = .66) or between RAL and the combined PI arm (P = .45). Changes in SAT mirrored changes in limb fat, with an overall mean increase of 19.9% (SD, 47.4%) by week 96. All regimens were associated with similar increases in SAT: 20.1% on ATV/r, 19.2% on RAL, and 20.5% on DRV/r; all P < .001, and not different between arms (P ≥ .83). Changes in limb fat were strongly associated with changes in SAT (r = 0.80, P < .001), both reflecting changes in subcutaneous fat depots.
Table 2.
Changes in Measures of Body Composition
| Body Composition Measure | Total | Atazanavir-Ritonavir | Raltegravir | Darunavir-Ritonavir |
|---|---|---|---|---|
| Percent Change in Body Composition Measures Overall and by Treatment Arm | ||||
| Limb fat | ||||
| Mean (SD) | 13.4 (32.8) | 11.4 (26) | 14.8 (38) | 14.2 (33.7) |
| P value | <.001 | <.001 | <.001 | <.001 |
| SAT | ||||
| Mean (SD) | 19.9 (46.7) | 20.1 (47.4) | 19.2 (45.3) | 20.5 (47.9) |
| P value | <.001 | <.001 | <.001 | <.001 |
| Trunk fat | ||||
| Mean (SD) | 18 (36.3) | 14 (27.1) | 19.4 (39.4) | 20.8 (41.1) |
| P value | <.001 | <.001 | <.001 | <.001 |
| VAT | ||||
| Mean (SD) | 25.8 (59.8) | 21.9 (55.1) | 26.5 (60.1) | 29 (64.2) |
| P value | <.001 | <.001 | <.001 | <.001 |
| Lean mass | ||||
| Mean (SD) | 1.8 (6.1) | 2 (5.8) | 2 (6) | 1.2 (6.4) |
| P value | <.001 | <.001 | <.001 | <.001 |
| atazanavir/ritonavir vs darunavir/ritonavir | protease inhibitor/ritonavir vs raltegravir | |||
| Treatment Group Comparisons of Body Composition Measure Changes | ||||
| Limb fat | ||||
| Mean (CI) | −1.98 (−12.22, 8.27) | −3.02 (−12.04, 6.00) | ||
| P value | .66 | .45 | ||
| SAT | ||||
| Mean (CI) | 1.45 (−13.52, 16.42) | 0.18 (−12.86, 13.22) | ||
| P value | .83 | .98 | ||
| Trunk fat | ||||
| Mean (CI) | −5.51 (−16.74, 5.72) | −3.10 (−13.04, 6.83) | ||
| P value | .27 | .48 | ||
| VAT | ||||
| Mean (CI) | −5.17 (−24.24, 13.91) | −2.65 (−19.23, 13.93) | ||
| P value | .54 | .72 | ||
| Lean mass | ||||
| Mean (CI) | 1.5 (−.24, 3.25) | −0.62 (−2.15, .90) | ||
| P value | .05 | .36 | ||
Abbreviations: CI, confidence interval; SAT, subcutaneous adipose tissue; SD, standard deviation; VAT, visceral adipose tissue.
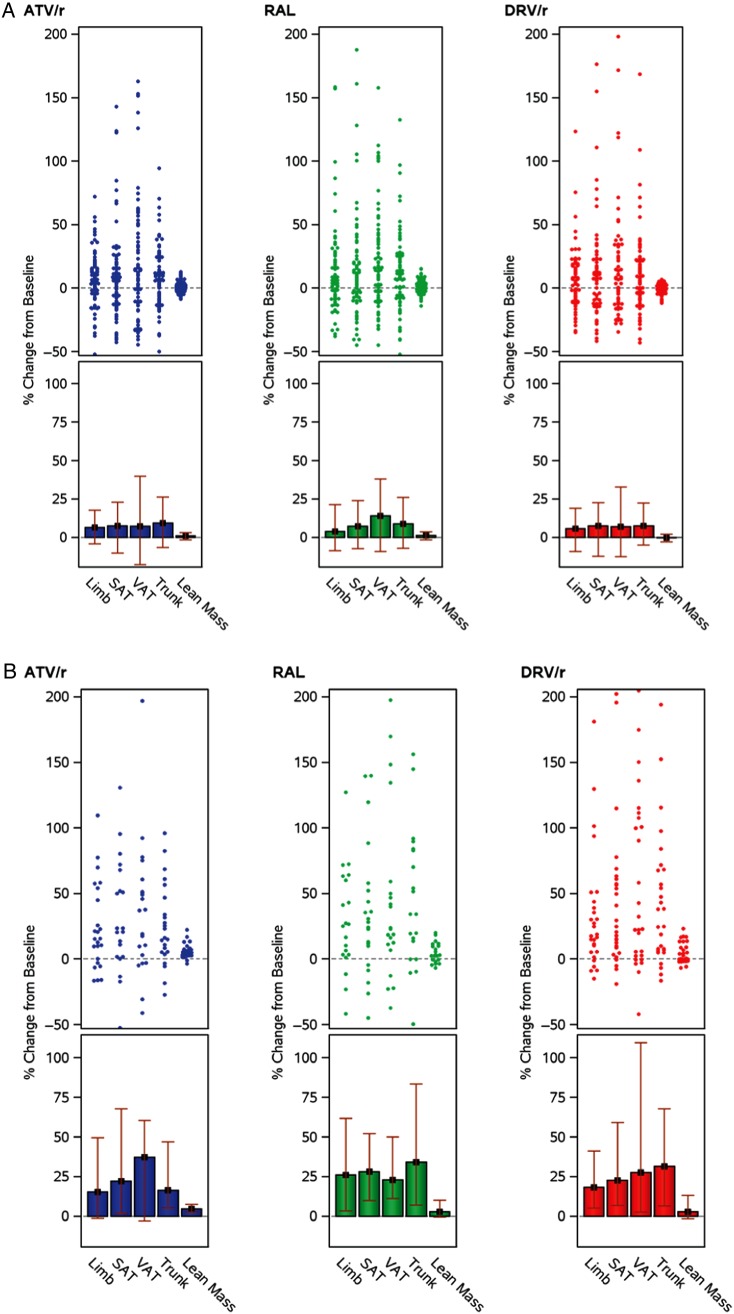
A subgroup of 54 (16%) participants (17%, ATV/r; 19%, RAL; 18%, DRV/r) lost >10% of their baseline limb fat, and 22 (7%) participants (7%, ATV/r; 8%, RAL; and 6%, DRV/r) lost >20%, without differences between treatment regimens (P > .88). There were no differences in baseline characteristics, including age, gender, BMI, limb fat, CD4 count, and HIV-1 RNA, between these 54 participants who lost >10% and the rest of the A5260s population, except for a larger proportion of participants who smoked marijuana (48% vs 33%; P = .022). The mean decrease in limb fat in this subgroup was 22%. Fat loss was not limited to peripheral compartments, as this subgroup also lost proportional amounts of VAT(−18%) and trunk fat (−20%), without a change in the VAT:TAT ratio. LBM and BMI also decreased in this subgroup by a mean of 1% and 6%, respectively. The 54 participants had an increase in CD4 (+269 cells/mm3) similar to that of the entire study population, and all 54 had HIV-1 RNA <50 copies/mL at week 96.
Changes in Lean Body Mass
At week 96, the overall mean (SD) percent change in LBM was 1.8% (6.1%); a mean increase of 2% on ATV/r and 2% on RAL (P < .001). However, in the DRV/r arm, LBM did not significantly increase by week 96 (1.2%; P = .28). Some evidence of greater gains in LBM was apparent with ATV/r compared with DRV/r (P = .05) in adjusted models. When the pooled PI arms were compared with RAL, there were no apparent differences for LBM (P = .36). Changes in LBM moderately correlated with changes in body fat measures (r = 0.34–0.37; P < .001).
Association Between Baseline Factors and Changes in Body Composition
Linear regression analyses assessed the association of baseline factors with changes in limb fat, VAT, and LBM at week 96 (Table 3). In multivariable analyses, higher baseline log10 HIV-1 RNA was associated with increases in limb fat, VAT, and LBM (all P < .01). Being in the high HIV-1 RNA stratum was associated with a 2- to 3-fold increase in the amounts of fat gain in all fat depots (Figure 3). Lower baseline CD4 was not associated with changes in any fat depots after adjustment. In addition, lower baseline leptin and higher adiponectin were associated with greater gains in VAT (marginally associated with adiponectin) and limb fat, and higher IL-6 levels were associated with greater gains in limb fat. For baseline associations with changes in LBM, greater IL-6 and D-dimer and lower CD4 count were independently associated with greater LBM gains (Table 3).
Table 3.
Baseline Associations With Percent Change in Body Composition Parameters
| Variable | Limb Fat |
Visceral Adipose Tissue |
Lean Mass |
|||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | |
| Age (y) | 0.14 (−.21, .48) | .44 | 0.26 (−.39, .91) | .44 | 0.04 (−.02, .10) | .20 |
| Physical activity (low/moderate) | −4.39 (−14.28, 5.50) | .38 | −15.16 (−32.87, 2.54) | .09 | −1.64 (−3.33, .04) | .06 |
| CD4 count (100 cells/mm³) | −1.6 (−3.84, .63) | .16 | −1.5 (−5.64, 2.63) | .48 | −0.86 (−1.23, −.48) | <.001 |
| HIV-1 RNA (log10 copies/mL) | 9.13 (3.24, 15.03) | .002 | 14.55 (3.52, 25.57) | .010 | 1.34 (.35, 2.32) | .008 |
| Adiponectin (ng/mL)a | 4.78 (.45, 9.12) | .031 | 7.38 (−.72, 15.48) | .07 | −0.06 (−.79, .66) | .87 |
| Leptin (pg/mL)a | −21.31 (−32.69, −9.93) | <.001 | −26.44 (−47.85, −5.04) | .015 | 0.77 (−1.18, 2.72) | .44 |
| C-reactive protein (μg/mL)a | 1.9 (−5.50, 9.31) | .61 | 0.42 (−13.37, 14.20) | .95 | 0.42 (−.80, 1.65) | .50 |
| IL-6 (pg/mL)a | 15.12 (2.34, 27.89) | .020 | 11.07 (−13.23, 35.37) | .37 | 2.71 (.60, 4.83) | .012 |
| D-dimer (μg/mL)a | 5.82 (−2.16, 13.79) | .15 | −2.29 (−16.70, 12.12) | .76 | 2 (.68, 3.31) | .003 |
| Soluble CD14 (ng/mL)a | 29.18 (−12.28, 70.65) | .17 | 18.38 (−61.05, 97.82) | .65 | 3.92 (−3.06, 10.91) | .27 |
| Soluble CD163 (ng/mL)a | 12.89 (−5.53, 31.31) | .17 | 9 (−25.51, 43.50) | .61 | −0.32 (−3.36, 2.72) | .84 |
| Soluble IL-2R (pg/mL)a | 3.99 (−16.87, 24.85) | .71 | 29.23 (−9.18, 67.63) | .14 | −2.87 (−6.34, .60) | .10 |
| % CD4+: CD28-CD57+ | −1.57 (−3.86, .72) | .18 | −1.17 (−5.37, 3.02) | .58 | 0.1 (−.27, .48) | .58 |
| % CD8+: CD28-CD57+ | −0.34 (−2.17, 1.49) | .71 | 0.94 (−2.35, 4.24) | .57 | −0.01 (−.30, .29) | .97 |
| % CD4+: CD28-CD57 + PD1+ ² | 33.67 (5.19, 62.15) | .020 | 36.31 (−15.11, 87.73) | .17 | 5.71 (1.06, 10.37) | .016 |
| % CD8+: CD28-CD57 + PD1+ ² | 24.39 (−8.28, 57.07) | .14 | 1.64 (−55.92, 59.19) | .96 | −4.32 (−9.62, .97) | .11 |
| % CD4+: CD38 + HLA-DR | 0.84 (−.67, 2.36) | .28 | 1.35 (−1.39, 4.09) | .33 | 0.23 (−.02, .48) | .08 |
| % CD8+: CD38 + HLA-DR | −0.02 (−1.51, 1.46) | .97 | −0.78 (−3.43, 1.87) | .56 | −0.14 (−.38, .10) | .25 |
| % MNC: CD14 + CD16+ | −1.17 (−3.24, .91) | .27 | 0.8 (−3.09, 4.68) | .69 | −0.16 (−.49, .17) | .34 |
| % MNC: CD14(hi)CD16(hi) | −2.63 (−10.09, 4.82) | .49 | −0.25 (−13.53, 13.03) | .97 | −0.11 (−1.31, 1.10) | .86 |
| % MNC: CD14(low)CD16(hi) | −0.09 (−1.29, 1.12) | .89 | −1.38 (−3.52, .76) | .21 | −0.02 (−.21, .18) | .85 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HLA-DR, human leukocyte antigen - antigen D related; IL, interleukin; MNC, mononuclear cells.
a Estimates are presented as per 0.3 log10 units, which is equivalent to a 2-fold difference.
Figure 3.
Effect of baseline human immunodeficiency virus type 1 (HIV-1) RNA levels on changes in body composition. (A) A low viral load stratum (HIV-1 RNA <100 000 copies/mL at screening) and (B) a high viral load stratum (HIV-1 RNA ≥100 000 copies/mL at screening). Abbreviations: ATV/r, atazanavir-ritonavir; DRV/r, darunavir-ritonavir; RAL, raltegravir; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
No associations were apparent between baseline markers of monocyte activation (sCD14 and sCD163) or proportion of proinflammatory monocytes and changes in any body composition measures. In unadjusted models, greater CD4+ activation (%CD4+ CD38+ HLA-DR) was associated with gains in all body composition endpoints. However, in adjusted models, no significant associations were apparent. Higher markers of CD4+ cell senescence and exhaustion (%CD4+ CD28-CD57+ PD1+) were associated with greater gains in limb fat and LBM and marginally associated with VAT changes.
DISCUSSION
For the first time in a large randomized ART-initiation trial, we showed that central and peripheral fat changes were not different after 96 weeks of treatment with 2 boosted PIs or with the integrase inhibitor RAL. Overall, the fat gains in the peripheral compartments were in keeping with prior studies of thymidine-NRTI–free regimens [6, 7]. A key finding was that the gains in central fat, including VAT, were not different with RAL compared with the 2 PIs. The VAT:TAT ratio remained unchanged and BMI significantly increased, suggesting that the fat gained was generalized and proportionally distributed in the visceral and subcutaneous compartments, regardless of the regimen used.
Obesity is prevalent in the general population and in people living with HIV. Our study showed an overall increase in BMI after 96 weeks of ART in a mildly overweight population. The increase in BMI after ART was perceived as a favorable consequence of virological control, or “return-to-health,” at a time when HIV-infected patients started therapy late in the disease course with low CD4 count and BMI. However, in the current era of universal ART for HIV infection, it is likely that this increase in BMI surpasses what is expected from return-to-health and may have unfavorable consequences on health.
Earlier studies had linked gains in central fat to the use of earlier generation of PIs [8, 14]. However, these earlier studies were not randomized and, as such, the link between PI use and “lipodystrophy” could have been affected by the lack of clear definition of lipodystrophy, lumping lipoatrophy and lipohypertrophy together, or a channeling bias where the sickest participants (with higher HIV-1 RNA) were placed on PI-based regimens. Several randomized studies have assessed fat changes on ART in the era of thymidine NRTIs, but the focus was on the overwhelming effect of lipoatrophy that dominated the effect of ART on body composition [6, 15]. Although imaging of VAT was not included in these studies, changes in DXA-measured trunk fat were described without differential effect of PI-based regimens. For instance, Dubé et al [15] showed a 16.5% increase in trunk fat after 64 weeks of EFV-based regimens vs 8.1% with the PI nelfinavir (P = .01). Similarly, in ACTG 5142, the randomization to PI (LPV/r) led to similar gains of trunk fat (on average 27% increase at week 96) than randomization to the nonnucleoside reverse transcriptase inhibitor EFV [6].
We expected that RAL might lead to more favorable effects on body composition when compared with the PI arms since in vitro studies have shown minimal to no effect of RAL on adipogenesis [16, 17]. To date, a single study of ART-naive individuals starting a Integrase Strand Transfer inhibitors (INSTI)-based regimen with RAL had reported on changes in body composition. The study included a convenience sample of 75 participants who enrolled in the large STARTMRK study and were randomized to RAL or EFV, with a backbone of TDF/FTC [18]. By week 96, trunk and limb fat increased by 30% and 10%, respectively, with EFV and by 17% and 14%, respectively, with RAL. There are no data yet on the effect of other INSTI on body composition.
Another important observation is the lack of differences in the changes in fat depots between the 2 contemporary PIs used in this study, ATV/r and DRV/r. While our study may have been underpowered to detect small differences, it is notable that the median changes across groups are nearly identical. Two prior randomized studies compared the fat changes after initiation of treatment with these 2 PIs. In a 48-week study (n = 65), Aberg et al found that, similar to our study, SAT increased by approximately 25% [19], but with more attenuated VAT changes. Martinez et al reported that when combined with TDF/FTC, ATV/r was associated with greater increases in peripheral fat than DRV/r, although with similar central fat gains at 96 weeks [20]. The discrepancies in the peripheral fat changes may be due to lower baseline BMI and limb fat in the Martinez study or due to the fact that our study adjusted for stratification factors, including HIV-1 RNA strata, with HIV-1 RNA being the main predictor of fat gains in our study.
It is interesting that although the majority of participants in this study experienced gains in limb fat, a subset (16%, similarly distributed across arms) experienced ≥10% loss of limb fat and 7% experienced ≥20% loss. These participants also lost central fat and overall weight. Except for higher marijuana use, the baseline characteristics, including BMI, of this subgroup were similar to those of the entire study population. Moreover, this subset had excellent virologic response and a gain in CD4 similar to that of the entire study population. Dietary assessments were not collected in this study, thus changes in food intake could have accounted for the fat and weight loss in this subset. There were no apparent differences in self-reported physical activity (data not shown). Regardless of the cause, these losses of limb fat are likely subclinical, as studies have shown the need for approximately 30% loss of limb fat before lipoatrophy becomes noticeable [21]. Nonetheless, a subgroup may still experience fat loss in the contemporary era, and a contribution of mild mitochondrial toxicity cannot be ruled out, as we have recently shown [22]. Genetic or non-ART factors may have predisposed this subset to fat loss and mitochondrial toxicity [23–25].
It may be time to redirect the study of fat changes in HIV away from the prior drug toxicity focus and toward evaluation of the interplay between the virus and host immune response. We found that higher pre-ART viral load was a major determinant of fat gains, regardless of the regimen type. This is consistent with a study that reported that HIV disease severity is associated with an increased prevalence of lipodystrophy [26]. The reason for this association is unclear and may be due to the persistent HIV-infected macrophages in adipose tissue, which could enhance local inflammation and cause expansion of fat tissues. It could be postulated that even with effective ART, the size of the reservoir of fat macrophages is likely related to the severity of the initial infection and may be resistant to treatment with antiretrovirals [27]. This expansion of fat related to infiltrating macrophages is similar to what has been described with other inflammatory diseases [28, 29]. There was a positive correlation between baseline IL-6 and gains in limb fat and LBM, suggesting a heightened inflammatory state that is responsive to ART and ultimately leads to greater gains.
Our study has some limitations, including the lack of collection of dietary habits; the lack of assessment of facial lipoatrophy, which is not captured by DXA/CT; and the lack of adjustment for multiple comparisons. The lack of available body composition measures from the period prior to HIV seroconversion makes it difficult to discern how much of the fat gain constitutes a desirable return-to-health phenomenon rather than a pathological effect. Additionally, longer follow-up would better characterize the trajectory of fat changes. Finally, the long-term consequences of these initial fat gains, including on CVD and diabetes, need to be elucidated.
Notes
Financial support. This research was supported by grants from the National Institutes of Health (grant numbers HL095132, HL095126, AI069501, AI068636, AI068634, AI69471, and AI56933.
Potential conflicts of interest. G. A. M. has served as a consultant for BMS, Pfizer, ICON, Merck, Gilead, and GSK/ViiV and has received research grants from BMS, GSK, and Gilead. M. P. D. has served as a consultant for Gilead and Astra Zeneca and receives research funding from Gilead, ViiV, BMS, and Merck. J. H. S. served on a Data Safety Monitoring Board for Lilly and was the principal investigator of a core ultrasound research grant to the University of Wisconsin from Gilead. T. T. B. has served as a consultant for BMS, GSK, Merck, Abbott, Gilead, ViiV Healthcare, Theratechnologies, and EMD-Serono and has received research funding from Merck and GSK. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Duran S, Saves M, Spire B et al. Failure to maintain long-term adherence to highly active antiretroviral therapy: the role of lipodystrophy. AIDS 2001; 15:2441–4. [DOI] [PubMed] [Google Scholar]
- 2.Mutimura E, Stewart A, Crowther NJ. Assessment of quality of life in HAART-treated HIV-positive subjects with body fat redistribution in Rwanda. AIDS Res Ther 2007; 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plankey M, Bacchetti P, Jin C et al. Self-perception of body fat changes and HAART adherence in the Women's Interagency HIV Study. AIDS Behav 2009; 13:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McComsey GA, Walker UA. Role of mitochondria in HIV lipoatrophy: insight into pathogenesis and potential therapies. Mitochondrion 2004; 4:111–8. [DOI] [PubMed] [Google Scholar]
- 5.McComsey GA, Libutti DE, O'Riordan M et al. Mitochondrial RNA and DNA alterations in HIV lipoatrophy are linked to antiretroviral therapy and not to HIV infection. Antivir Ther 2008; 13:715–22. [PMC free article] [PubMed] [Google Scholar]
- 6.Haubrich RH, Riddler SA, DiRienzo AG et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS 2009; 23:1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McComsey GA, Kitch D, Sax PE et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis 2011; 53:185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez E, Mocroft A, Garcia-Viejo MA et al. Risk of lipodystrophy in HIV-1-infected patients treated with protease inhibitors: a prospective cohort study. Lancet 2001; 357:592–8. [DOI] [PubMed] [Google Scholar]
- 9.Curran A, Martinez E, Saumoy M et al. Body composition changes after switching from protease inhibitors to raltegravir: SPIRAL-LIP substudy. AIDS 2012; 26:475–81. [DOI] [PubMed] [Google Scholar]
- 10.Lake JE, McComsey GA, Hulgan TM et al. A randomized trial of raltegravir replacement for protease inhibitor or non-nucleoside reverse transcriptase inhibitor in HIV-infected women with lipohypertrophy. AIDS Patient Care STDS 2012; 26:532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockstroh JK, Lennox JL, Dejesus E et al. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis 2011; 53:807–16. [DOI] [PubMed] [Google Scholar]
- 12.Stein JH, Ribaudo H, Hodis H et al. A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. AIDS 2015; 29:1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelesidis T, Tran TT, Stein JH et al. Changes in inflammation and immune activation with atazanavir-, raltegravir-, darunavir-based initial antiviral therapy: ACTG 5260s. Clin Infect Dis 2015; 61:651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet 1998; 351:871–5. [DOI] [PubMed] [Google Scholar]
- 15.Dubé MP, Parker RA, Tebas P et al. Glucose metabolism, lipid, and body fat changes in antiretroviral-naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS 2005; 19:1807–18. [DOI] [PubMed] [Google Scholar]
- 16.Minami R, Yamamoto M, Takahama S, Ando H, Miyamura T, Suematsu E. Comparison of the influence of four classes of HIV antiretrovirals on adipogenic differentiation: the minimal effect of raltegravir and atazanavir. J Infect Chemother 2011; 17:183–8. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Matute P, Perez-Martinez L, Blanco JR, Oteo JA. Neutral actions of raltegravir on adipogenesis, glucose metabolism and lipolysis in 3T3-L1 adipocytes. Curr HIV Res 2011; 9:174–9. [DOI] [PubMed] [Google Scholar]
- 18.Lennox JL, Dejesus E, Berger DS et al. Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 2010; 55:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aberg JA, Tebas P, Overton ET et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retroviruses 2012; 28:1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez E, Gonzalez-Cordon A, Ferrer E et al. Differential body composition effects of protease inhibitors recommended for initial treatment of HIV infection: a randomized clinical trial. Clin Infect Dis 2015; 60:811–20. [DOI] [PubMed] [Google Scholar]
- 21.Podzamczer D, Ferrer E, Martinez E et al. How much fat loss is needed for lipoatrophy to become clinically evident? AIDS Res Hum Retroviruses 2009; 25:563–7. [DOI] [PubMed] [Google Scholar]
- 22.McComsey GA, Daar ES, O'Riordan M et al. Changes in fat mitochondrial DNA and function in subjects randomized to abacavir-lamivudine or tenofovir DF-emtricitabine with atazanavir-ritonavir or efavirenz: AIDS Clinical Trials Group study A5224s, substudy of A5202. J Infect Dis 2013; 207:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wangsomboonsiri W, Mahasirimongkol S, Chantarangsu S et al. Association between HLA-B*4001 and lipodystrophy among HIV-infected patients from Thailand who received a stavudine-containing antiretroviral regimen. Clin Infect Dis 2010; 50:597–604. [DOI] [PubMed] [Google Scholar]
- 24.Zanone Poma B, Riva A, Nasi M et al. Genetic polymorphisms differently influencing the emergence of atrophy and fat accumulation in HIV-related lipodystrophy. AIDS 2008; 22:1769–78. [DOI] [PubMed] [Google Scholar]
- 25.Hendrickson SL, Kingsley LA, Ruiz-Pesini E et al. Mitochondrial DNA haplogroups influence lipoatrophy after highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2009; 51:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson DL, Knox T, Spiegelman D, Skinner S, Gorbach S, Wanke C. Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis 2005; 40:1837–45. [DOI] [PubMed] [Google Scholar]
- 27.Damouche A, Lazure T, Avettand-Fenoel V et al. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog 2015; 11:e1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation—visceral obesity and creeping fat. Front Immunol 2014; 5:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drouet M, Dubuquoy L, Desreumaux P, Bertin B. Visceral fat and gut inflammation. Nutrition 2012; 28:113–7. [DOI] [PubMed] [Google Scholar]