Abstract
Blood vessel growth from preexisting vessels (angiogenesis) underlies many severe diseases including major blinding retinal diseases such as retinopathy of prematurity (ROP) and aged macular degeneration (AMD). This observation has driven development of antibody inhibitors that block a central factor in AMD, named vascular endothelial growth factor (VEGF), from binding to its receptors VEGFR-1 and VEGFR-2. However, some patients are insensitive to current anti-VEGF drugs or develop resistance, and the required repeated intravitreal injection of these large molecules is costly and clinically problematic. Here, we have evaluated a small cyclic retro-inverted peptidomimetic, D(Cys-Leu-Pro-Arg-Cys), abbreviated as D(CLPRC), and hereafter named Vasotide, that inhibits retinal angiogenesis by binding selectively to the VEGF receptors, VEGFR-1 and Neuropilin-1 (NRP-1). Delivery of Vasotide in eye drops or via intraperitoneal injection in a laser-induced monkey model of human wet AMD, a mouse genetic knockout model of the AMD subtype called retinal angiomatous proliferation (RAP), and a mouse oxygen-induced model of retinopathy of prematurity (ROP) markedly decreased retinal angiogenesis in all three animal models. This prototype drug candidate is a promising new dual receptor inhibitor of the VEGF ligand with potential for translation into safer, less invasive applications to combat pathological angiogenesis in retinal disorders.
Introduction
Pathological angiogenesis is centrally involved in common and severe retinal diseases that affect the very young (Retinopathy of Prematurity, ROP), adults (Diabetic Retinopathy, DR) and the elderly (Aged Macular Degeneration, AMD) (1). Estimates indicate that 14 million persons are blind or nearly blind because of AMD (http://www.who.int/blindness/causes/priority/en/), with “wet” AMD causing ~90% of AMD cases with severe vision loss (2). The prevalence is increasing as the average lifespan continues to rise (3).
Vascular Endothelial Growth Factor (VEGF) is a key component in normal and pathological vascular growth. The VEGF family consists of five VEGF isoforms A, B, C, D, E, and Placental Growth Factor (PlGF) (4) that bind to one or more VEGF receptors (VEGFR) (5). Each of the receptors is membrane-bound, with an external component that dimerizes upon binding of VEGF ligand, thereby activating the intracellular tyrosine kinase component. Several retinal cell types synthesize VEGF, and even more cell types participate in blood vessel formation (6, 7). With respect to vascular endothelial cells, the most studied of the five ligands is VEGF-A, which binds mainly to VEGFR-2 and stimulates endothelial cell proliferation and migration (8, 9). VEGFR-1 signaling is less well-defined, but is known to bind to three VEGF ligands: VEGF-A, VEGF-B, and PlGF. Another VEGF receptor, Neuropilin-1 (NRP-1), binds to several VEGF isoforms including PIGF, and, sometimes simultaneously with VEGFR-1 or VEGFR-2, influence endothelial cell migration, sprouting, and tubular branching during normal development as well as in pathological angiogenesis.
The U.S. Food & Drug Administration (FDA) has approved for human use three anti-VEGF drugs that reduce binding of VEGF to specific receptors or perhaps act through additional mechanisms. The first of these was pegaptanib (Macugen) (10), followed by the therapeutic anti-VEGF antibody fragment, ranibizumab (Lucentis), and then the recombinant fusion protein aflibercept (Eyelea) that serves as a “VEGF trap” decoy. Together these drugs, along with the off-label anti-VEGF antibody bevacizumab (Avastin), have revolutionized the treatment of wet AMD in patients (11–13). Ranibizumab and bevacizumab are thought to affect mainly VEGF-A and reduce the binding of this ligand, especially to the VEGFR-2 receptor (14). Although these therapeutic drugs are relatively effective for treating AMD and related eye diseases, not all patients respond to them and many develop decreased responsiveness during treatment (15). Additional problems include the repeated intravitreal injections that require skilled professional execution, are costly, and have rare but serious side-effects such as ocular pain, infection, or hemorrhage (16). Therefore, agents that block angiogenesis through additional mechanisms and, in particular, drugs that would be effective when administered by simpler and safer routes are a highly desirable as-yet unmet need in ophthalmology and some other branches of medicine.
Our previous work utilized a subtractive bacteriophage display-library screening strategy to identify the peptide CPQPRPLC as a selective ligand to VEGFR-1 and NRP-1 (17). Subsequent NMR analyses showed arginine-proline-leucine (Arg-Pro-Leu; RPL) to be a minimal necessary and sufficient tripeptide motif for dual ligand-binding to these two receptors (18). We then showed that synthetic cyclic retro-inverted peptidomimetic derivatives of CPQPRPLC had enhanced stability in vivo and were stronger ligands for VEGFR-1 and NRP-1 than the original sequence. Moreover, in pilot experiments, these degradation-resistant small molecules administered topically inhibited eye angiogenesis in young mice with ROP features (19) and accumulated within the vitreous fluid in direct contact with the inner (vitreal) retinal surface, where tufts of new blood vessels are forming, a hallmark of murine, primate, and human eye diseases with an angiogenesis component (19–22). The likely reason that blood vessel tufts grow from the retinal surface inward into the vitreous fluid in ROP mice instead of repeating the normal vessel growth outward into the ganglion cell and inner nuclear and plexiform (synaptic) layers is that in this disorder semaphorin 3A (SEMA3A) is secreted by retinal ganglion cells and forms a repulsive barrier to angiogenesis within the retina (23) so that the pathological vessels instead grow mainly inward as tufts projecting into the vitreous chamber.
The peptide motif RPL and its derivatives, LPR and D(Cys-Leu-Pro-Arg-Cys) prototype, are the only known exogenous reagents that target both NRP-1 and VEGFR-1 pathways (17–19). The VEGF family member PlGF, which is expressed in pathological retinas and several other diseased organs, also binds to NRP-1 and VEGFR-1, and is thought to stimulate angiogenesis by different mechanisms than VEGF-A (22). This inhibitory mechanism via dual receptors, along with the potential for less invasive eye drop administration -- illustrated by the preliminary ROP results in neonatal mice (19) -- clearly distinguishes our drug prototype from the intravitreal therapies thought to affect predominantly binding to VEGFR-2. This led us to evaluate the preclinical therapeutic potential of Vasotide in three independent murine and non-human primate models of retinal diseases with angiogenic pathogenesis.
Mouse oxygen-related ROP, also known as oxygen-induced retinopathy (OIR), is the most widely studied model of a retinal disease featuring pathological angiogenesis (17, 19–22, 25, 26). This model involves placing nursing seven-day-old (P7) mice with their mothers in a 75% oxygen (O2) chamber until P12, which suppresses the still-ongoing normal retinal vascular development by destroying many endothelial and astrocytic cells, so that several retinal areas become totally avascular (25, 26). When the mice are returned to room air (21% O2), the relative hypoxia over the subsequent 5–7 days promotes excess VEGF production and leads to a pathological increase in retinal angiogenesis (20, 21, 25, 26). Tufts of new blood vessels characteristic of the ROP disease form on the inner surface of the retina (20, 21, 25, 26). This mimics the development of human ROP, which is common in premature infants due to exposure to high O2 to support immature lung function (27, 28).
In normal human gestation, retinal development occurs mainly in the second and third trimesters, reaching completion shortly before or a few days after birth (25, 29, 30). At 16 weeks of gestation the retina has not yet acquired any blood vessels. Astrocytes migrate in from the optic nerve head, coat the inner surface of the retina and secrete VEGF and additional factors that stimulate development of a vascular network spreading from the optic nerve head, beginning at about 20 weeks, to radiate peripherally over the entire inner surface (optic nerve fiber layer) of the retina, reaching its nasal edge by 36 weeks and its temporal edge by about 40 weeks. During this second half of gestation, vascular branches penetrate outward radially through the ganglion cell and inner nuclear layers and spread horizontally through the two plexiform layers, but do not penetrate into the photoreceptor cell zone.
Similar developmental events occur in normal mice during the first two postnatal weeks. In a pilot ROP study, we had administered the non-cyclic tripeptide Dleucine- Dproline- Darginine, D(LPR) intraperitoneally (i.p.) daily during the first seven days of return to room air, or as eye drops three times per day in the same time period (19). Another small non-cyclic retroinverted peptidomimetic, Dalanine-Dproline- Dalanine, D(Ala-Pro-Ala), differs from D(LPR) in not binding to the VEGFR-1 and NRP-1 receptors (18) and served as a negative control. A reduction in pathological angiogenesis occurred with either i.p. or eye drop D(LPR) treatment, essentially equivalent to i.p. bevacizumab (19).
Results
Vasotide reduces vascular tuft formation in ROP mice
In this study, instead of repeating tests with D(LPR) we used the corresponding cyclic effective Vasotide and control D(CAPAC) peptides. Given the role of increased VEGF binding in the abnormal neurovascular formation in mouse and human ROP (9, 21), we then carefully analyzed retinal morphology and pathophysiology in Vasotide-treated and control mice and found that they had similar actions to the linear tripeptides, D(LPR) and D(APA) (19).
We found the extent of vascularization and the areas of vascular obliteration and tuft formation (IB4 staining) to be stable from P17 to P23 (fig. S1), slightly longer than the P20 described for the same ROP model technique by many other investigators (20, 25, 31). We examined eyes at P19 for consistency between the current Vasotide study and our previous D(LPR) experiments (19), yielding results, according to all published studies, equivalent to the more usual termination date of P17 (6, 20). The tissue component stained with IB4 was confirmed to be blood vessels by co-staining with anti-CD31, which is selective in retina for endothelial cells. Macrophages, which are also involved in ROP progression, were detected in the vascular area by co-staining with anti-CD68. Upon treatment with Vasotide administered either i.p. or by eye drops in the room air phase on days P12-P18, there was no significant difference in retinal avascular areas at P19 compared to the control (Fig. 1A to D). However, there was a significant decrease in vascular tufts projecting from the retina into the eye’s vitreous chamber with the higher Vasotide dosage administered i.p. (Fig. 1 E to G) or by eye drops (Fig. 1 H to J) (p<0.05).
Fig. 1. Effects of Vasotide on the vasculature and tuft formation in ROP mice.
(A) Vasculature at P19 in whole-mount retinas from normal mice (left upper and lower panels) or mice with OIR. Mice were intraperitoneally injected daily on P12 to P18 with phosphate-buffered saline (PBS) (OIR + PBS), D(CAPAC) (OIR + CAPAC) 40 mg/kg, or Vasotide (OIR + Vasotide) 40 mg/kg. Blood vessels were stained with a red fluorescent dye (IB4); red staining is adjusted to white in all panels. Yellow areas in the lower row are sites of vascular obliteration. (B) Quantification of obliterated vascular areas as in (A), Vasotide low dose 8 mg/kg (OIR + Vasotide low) or Vasotide high dose 40 mg/kg (OIR + Vasotide high). (C and D) Vascular obliteration area at P19 after eye drop treatment with Systane vehicle, or control peptide D(CAPAC) or Vasotide daily from P12 to P18. Quantification of obliterated areas as in (B). Values in (B) and (D): mean ± SD, n = 6. (E) Normal vasculature and abnormal vascular tufts in whole-mount retinas of P19 normal mice intraperitoneally injected daily with PBS, and OIR mice injected with PBS, control D(CAPAC) peptide, or therapeutic Vasotide peptide. IB4 staining is adjusted to white, and tufts are shown in red in the bottom row. (F) Enlarged images of vasculature and tufts, as in (E). (G) Quantitation of tuft areas in wild-type (WT) mice given eye drops containing PBS or in OIR mice similarly treated or given control D(CAPAC) peptide or Vasotide peptide at the low or high dose. Mean ± SD, n = 5 to 8; P > 0.05 in all group comparisons, calculated with analysis of variance (ANOVA) and the c2 test. (H) Tufts in whole-mount retinas of P19 OIR mice treated topically with eye drops as in (C). IB4-stained vessels adjusted to white in upper row, tufts shown in red in lower row. (I) Enlarged images of tufts as in (H). (J) Quantitation of tuft areas in OIR mice given eye drops containing Systane alone or Systane with control D(CAPAC) peptide or Systane with Vasotide. Mean ± SD, n = 5 to 8; P < 0.05, statistical methods as in (G).
We followed these experiments with a morphometric analysis of tuft formation in the inner retina of P19 mice by horizontal confocal scanning. Treatment of O2-induced retinopathy in young mice with Vasotide reduced the number and projection of vascular tufts through the inner retinal surface and into the vitreous chamber, and led to clear differences in the attributes of blood vessel network patterning and vascular density within the inner retina, compared with control D(CAPAC)-treated mice (Fig. 2A to C). Serial confocal images captured at 4 µm intervals through the outermost part of the vitreous chamber and about 36% of the innermost part of the retina revealed that Vasotide decreased the total vasculature density almost to normal levels and reduced extension of the vascular zone into the vitreous chamber (Fig. 2D to F). As illustrated in Fig. 2F, in the normal mouse retina, vessels occupy the granule cell layer (GCL), inner plexiform layet (IPL), and inner nuclear layer (INL), to the inner edge of the outer plexiform layer (OPL). OIR+D(CAPAC) retinas showed increased but disordered vasculature through these layers, extending slightly outward through the OPL and more extensively inward into the vitreous with extensive tufts. Vasculature was reduced in the vasotide-treated retinas, which appeared more similar to normal retinas than to the D(CAPAC)-treated retinas (Fig. 2D)..
Fig. 2. Vascular tufts in OIR mice extend from the retina into the vitreous.
(A) IB4-stained branched vessels and tufts in horizontal retinal scans in normal mice and mice with oxygen-induced retinopathy (OIR) treated with D(CAPAC) control peptide or Vasotide. (A’) Scans rotated 90°, showing tufts above dashed lines, innermost retina below. (B) Magnified cryostat sections with IB4-stained vessels in red and DAPI counterstained nuclei in blue at P19. Pathological tuft formation is shown above the dashed lines, and reduced vessel formation within the inner retina is shown below the dashed lines. GCL, ganglion cell layer; IPL, inner plexiform layer. (C) Paraffin sections show tuft formation above dashed lines and retinal layers below dashed lines. INL, inner nuclear layer; ONL, outer nuclear layer. (C’) Inner retina and adjacent vitreous, enlarged. (D) Horizontal confocal images through the outermost vitreous region and innermost ~36% of the retina at P19 in the same three categories of mice. Vitreal images have negative numbers (lowest number closest to retinal inner edge), intraretinal images are labeled progressively outward to a depth of 72 µm. (E) Quantitation of percent blood vessel area at 4 µm intervals summed through the full retina on a relative scale in each mouse type. (F) Diagram of the vasculature in different regions of the retina. ROS, rod (and cone) outer segments; CV, choroidal vessels.
Vasotide reduced angiogenesis in a laser-induced monkey model of AMD
To determine whether Vasotide could also be therapeutically beneficial in other retinopathies that may involve different angiogenic mechanisms and in larger animals, we next utilized laser-induced choroidal neovascularization in Old World monkeys, which is a standard wet AMD animal model (32). During angiogenesis, growth of new vascular branches from existing blood vessels is usually accompanied by fluid leakage because of an abnormally increased microvascular permeability. In this monkey model, laser treatment generates lesions that disrupt Bruch’s membrane leading to growth of new blood vessels from the choroid across the retinal pigment epithelium and into the subretinal space, thus contributing to the formation of destructive choroidal neovascular (CNV) complexes whose size and leakage are readily quantified by ophthalmic imaging procedures (32).
Eight male adult African Green monkeys were studied (Table S1). Six monkeys were treated with eye drops containing Vasotide in a standard GelTears vehicle (6 mg in 50 µl, see Materials and Methods), and two control monkeys (4 eyes) received only the vehicle (GelTears 50 µl). Dosage was administered to each eye twice each day from day 1 through day 5 post-laser photocoagulation, then once daily on days 6 through 21. In both groups, minor clouding of the inferior cornea not impairing fundoscopy and/or image analyses was observed secondary to low pH of the topical formulation.
The prototype Vasotide administered by eye drops led at day 29 to a significantly lower incidence of combined grade III and IV lesions, which represent clinically relevant CNV, compared to those treated with vehicle alone (Fisher’s exact test, P < 0.0001) (Table S3). Vasotide completely prevented development of grade III and IV retinal lesions (Fig. 3A, Fig. S2, and Tables S2, S3), an outcome comparable to that achieved with bevacizumab (32) or ranibizumab (22) in this primate model. CNV development at day 29 was also analyzed with ImageJ densitometric analysis (32) on non-enhanced 6-minute digital fluorescein angiograms to provide comparison of late phase relative fluorescence intensity in lesions from Vasotide-treated or vehicle-treated eyes. The methods for statistical analysis of fluorescein signal intensity in laser spots are described in the Materials and Methods. Fluorescein signal intensity in laser spots was compared between groups by a non-parametric statistical analysis (Table S3). Vasotide-treated monkey eyes had significantly lower late phase fluorescein signal intensity than vehicle-treated control eyes (Chi-square test = 7.63, P = 0.0057). The enlarged images in Fig. S2 allow clearer visual recognition of the differences in the responses to the laser lesions with or without Vasotide treatment. CNV complex formation was quantified in each laser photocoagulation site by area analysis of cross-sectional optical coherence tomography (OCT) images (Fig. 3B). Mean maximal CNV complex areas were significantly lower in eyes treated with Vasotide than in vehicle-treated eyes (Fig. 3F; see also Student’s t-test on Box-Cox transformed data, P < 0.05; Table S3).
Fig. 3. Fluorescein angiograms, OCT images, and histopathology of choroidal neovessels in monkey retinas.
(A) Representative 6-minute fluorescein angiograms (FA) from eye drop-treated monkeys containing vehicle (left) or Vasotide (right), at 29 days post laser-induced photocoagulation. (B) OCT images from monkeys given eye drops: vehicle alone (left) or Vasotide (right) at 29 days after the laser-induced lesion. Yellow arrows indicate CNV complex boundaries for lesion area calculation. Scale markers: 200 µm. (C) Cryostat cross-sectional photomicrographs of the retinal pigment epithelium and rod/cone outer segment areas stained as follows: (Left) top row endothelial cells (IB4, red), middle row macrophages (F4/80, green), lower row for cell nuclei (DAPI, blue); (Right) leukocytes (GR1, red), macrophages (F4/80, green), and cell nuclei (DAPI, blue). (D) Photomicrographs of immunostained whole-mount monkey eyecups in the angiogenesis area at higher magnification. (E) H&E-stained monkey retinas at low (upper row) and high (lower row) magnifications showing eosin red-stained vacuolated fibroblast layer outside of the choroid in the upper row. Red boxes indicate macular region; dashed ovals indicate the RPE and ROS zones 29 days after laser-induced lesioning. (F) Endothelial cell and inflammatory cell numbers in peptide-treated compared to vehicle-treated eyes. Values: mean ± SD, n = 4. (G) Diagrams showing distribution of blood vessels (red) in monkey retina. Colored dots indicate severe cellular infiltrate. H&E, Hematoxylin & Eosin; NFL, Nerve Fiber Layer; GCL, Ganglion Cell Layer; IPL, Inner Plexiform Layer; INL, Inner Nuclear Layer; OPL, Outer Plexiform Layer; ONL, Outer Nuclear Layer; ROS, Rod (and Cone) Outer Segments; RPE, Retinal Pigment Epithelium; CV, Choroidal Vessels.
Detailed histological analysis at day 34 revealed that treatment with Vasotide dramatically reduced pathological features such as tissue infiltration with inflammatory macrophages and granulocytes (Fig. 3 C and D), and both CNV and fibroplasia as shown by swelling of the macular region and severe cellular infiltrate in the retinal pigment epithelium (RPE) and rod outer segment (ROS) zone, compared to the vehicle control (Fig. 3E). Quantitative analysis confirmed that all of these pathological features were significantly reduced in Vasotide-treated animals (Fig. 3F) (p<0.01). Imaging data were collected at the end of week 4 (day 29) post-laser photocoagulation. This was the time point at which peak development of CNV complex formation was detected in the previous characterization of this monkey model (32) and is illustrated in (Fig. 3B).
Vascular morphology becomes abnormal postnatally in the vldlr knockout mouse model of retinal angiogenesis proliferation
As a third test case, we measured the effect of i.p. injections of Vasotide in a mouse model of the human AMD subtype Retinal Angiogenesis Proliferation (RAP) (33, 34). This model was generated by knockout (KO) of the Very Low Density Lipoprotein Receptor (vldlr) gene. The retinas of vldlr-null mice (as found in the 10–15% of human AMD patients who have the RAP form of the disease) grow new blood vessels into the normally avascular photoreceptor zone (Fig. 4A, Fig. S4), which is outward from the normally vascular inner layers of the retina (35–38). Mouse vldlr mRNA was undetectable in vldlr-null retinas and was reduced in vldlr-heterozygote retinas (fig. S3), as described previously (37).
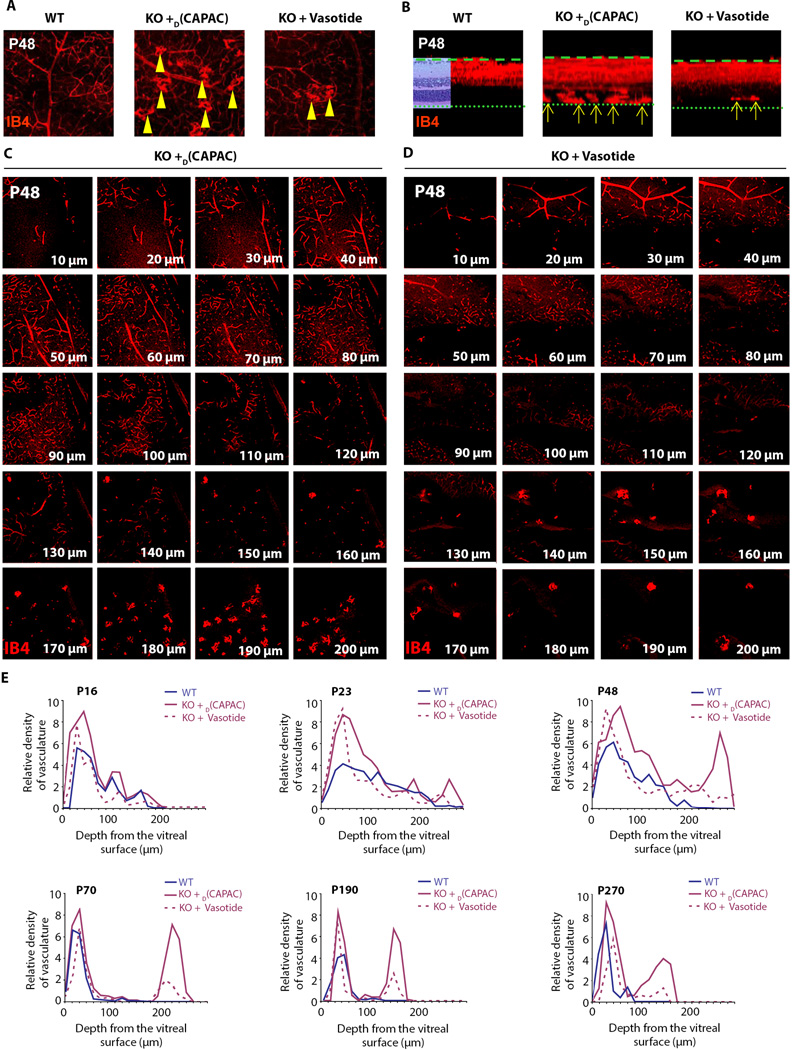
Fig. 4. Histopathologyof vldlr-nullmouse retinas treated with control peptide or Vasotide.
(A) As indicated by the 3D block of retinal tissue (left), the row of images to its right shows the assembled 21 confocal planes through each mouse retina in cross-sectional aspect. Arrows indicate new blood vessels already entering the normally avascular outer retina at these early ages in untreated vldlr-null mice. (B) Blood vessel concentrations at P8 (left image pair) and P12 (right image pair), prior to treatment with Vasotide. Wildtype (WT, left) and vldlr-null (KO, right) retinal fields in the confocal sections 20 µm from the vitreal surface, photographed with a 20X microscope objective. Yellow arrowheads point to vascular tufts. (C) Quantification of vessel concentration in each of the 21 confocal sections through the full 200 µm retinal thickness imaged with a 10X microscope objective at P8 and P12. (D) Comparison of H&E-stained retinal sections from wildtype (WT) mice and vldlr-null (KO) mice treated with control peptide D(CAPAC) or Vasotide at P23, P48, and P270 treatment ages. Yellow arrows indicate pathological vascular growth through the photoreceptor cell layers. (E) Diagrams show vascular differences in the retinas ofWT mice and vldlr-null (KO)mice treatedwith control D(CAPAC) peptide or Vasotide. NFL, nerve fiber layer; CV, choroidal vessels.
To analyze retinal vascular morphology, blood vessels were stained fluorescent-red with Alexa Fluor 568 conjugated-isolectin IB4, as in the two models described above. In the wild-type retina at P8, blood vessels had already distributed through their normal mature territory by growing from their initial entry site at the optic nerve head to positions among the ganglion cell axons at the vitreal surface and downward through the narrow ganglion cell layer (GCL), the much wider inner plexiform (synaptic) layer (IPL), and the inner nuclear layer (INL) (25, 29, 30, 39). In the vldlr-null retinas, additional blood vessels, most of them oriented vertically, had already grown from the INL across the narrow outer plexiform (synaptic) layer (OPL) and entered the outer nuclear layer (ONL), which contains the photoreceptor cell nuclei and surrounding cytoplasm (Fig. 4A, P8 vldlr null, white arrows). Such blood vessels have not previously been described as early as P8 in the vldlr-null mouse retina. In addition, blood vessels in vldlr-null retinas also proliferated excessively within the already vascularized inner half of the vldlr-null retina relative to the wildtype retina (Fig. 4B, C).
At P8 and P12 many newly forming vessels normally branch off from large and small blood vessels near the inner retinal surface and cluster in tight balls (tufts). These tufts were observed in larger numbers in the vldlr-null retina compared to the wildtype retina at this early age (Fig. 4B, arrowheads). By P12, the vascular growth in the vldlr-null retina had further advanced into the ONL (Fig. 4A, arrows), as described previously (34, 37, 38). Confocal images at 10 µm intervals through the full retinal thickness in vldlr-null and wildtype retinas at P8 and P12 are shown in Fig. S4. Our data confirm that pathological angiogenesis in vldlr-null mice begins much earlier in virtually all regions of the retina than is typical in human RAP, for which this mouse is otherwise a suitable disease model (33, 34).
Vasotide administered i.p. reduced angiogenesis in the mouse vldlr model of RAP
We injected into vldlr-null mice either (1) control D(CAPAC), or (2) the targeted Vasotide peptide once daily i.p. at P12–18, P48–54, P108–114, and P208–214. Retinas of wildtype mice, control-treated vldlr-null mice and Vasotide-treated vldlr-null mice were systematically examined and characterized at nine different postnatal ages (Fig. 5). Hematoxylin and eosin (H&E)-stained serial histological sections (Fig. 4D) showed more severe pathological vascular growth through the photoreceptor cell layers in many but not all sections from retina of the control-treated vldlr-null mice. More severe photoreceptor cell damage was evident in the D(CAPAC)-treated vldlr-null retinas compared to the Vasotide-treated vldlr-null retinas. All retinal sections from the Vasotide-treated vldlr-null mice lacked vessels penetrating through the photoreceptor cell layer toward and through the pigment epithelial layer. Further analysis of the distribution of blood vessels in the untreated wildtype and vldlr-null retinas revealed that the control vldlr-null retinas showed not only pathological growth of blood vessels through the photoreceptor cell layers and across the retinal pigment epithelium (RPE) to reach and fuse with some of the choroidal vessels (CVs), but also moderately disorganized anastamotic networks of blood vessels in the retinal inner layers, rather than a normal vascular pattern in this area (Fig. 4A at P12, and diagrammed in Fig. 4E).
Fig. 5. Blood vessel growth in the outer retina of vldlr-null mice treated with control peptide or Vasotide at P16 to P270.
(A) Tuft formation in the pooled outer retinal areas of WT mice or vldlr-null (KO) mice treated with control D(CAPAC) peptide or Vasotide was visualized by confocal microscopy with a 20Å~ microscope objective. Scale bar, 25 mm. (B) Graphs of tuft number per retinal field (n = 6) and single tuft sizes (n = 20) showing consistently decreased numbers and sizes of tufts in Vasotide-treated compared to control D(CAPAC)-treated vldlr-null (KO) retinas. *P < 0.05, at each age, using ANOVA.
Quantitatively, in confocal sections at 10 µm intervals, the percentage of the sampled retinal area occupied by blood vessels in the outer half of the retina (120–200 µm from the vitreal surface) was 0% for wildtype retinas versus 19.5% for the control vldlr-null retinas; in the inner half of the retina (0–110 µm from the vitreal surface) it was 25.3% in the wildtype retinas versus 65.5% in the vldlr-null retinas. Table S4 lists the percentage of the area occupied by blood vessels in wildtype, D(CAPAC)-treated vldlr-null, and Vasotide-treated vldlr-null retinas at each of the nine ages, and documents the increase in D(CAPAC)-treated compared to wildtype and Vasotide-treated vldlr-null retinas.
The ratio of percent area occupied by blood vessels in control D(CAPAC)-treated vldlr-null mice versus that in wildtype animals was about 2:1 (209.9% ± 36.1%, Mean ± S.D. for all nine ages (P16-P270); Table S5, right column). The ratio of percent area occupied by blood vessels in the set of confocal sections (0–200 µm, the full thickness of whole mount retinas) in Vasotide-treated versus control D(CAPAC)-treated retinas was about 0.5:1 (53.5% ± 8.0%, Mean ± S.D.; Table S5, left column). In the retinal area of greatest interest, the normally avascular photoreceptor cell zone, (confocal sections 120–200 µm from the vitreal surface, containing the OPL and the ONL), the ratio of blood vessels in Vasotide-treated versus control D(CAPAC) -treated vldlr-null retinas was decreased even further to about 0.35:1 (38.4% ± 14.9%, Mean ± S.D.; Table S5, middle column).
Pooled confocal images of the outer half of the mouse retinas revealed that pathological vessels were reduced by Vasotide treatment in the photoreceptor cell zone from P16 onward as evidenced by the reduced numbers of vascular clusters (tufts) typical of pathological angiogenesis (Fig. 5A). The decrease in number and size of tufts per sampled age in Vasotide-treated compared to D(CAPAC)-treated retinas was significant (P < 0.05) at all ages (Fig. 5B). Tuft concentration peaked at about P48, whereas tuft size progressively increased to P270. At P190 and P270, the ONL was extensively disrupted by blood vessel growth into this layer and perhaps across the RPE layer (Fig. 4D and diagrammed in Fig. 4E), as has been described in human RAP, with its final pathology similar to that in the more common “wet” AMD.
At P48, vascular tufts were absent in the wildtype retinas and were more numerous in the D(CAPAC)-treated compared to the Vasotide-treated vldlr-null retinas (Fig. 6A, arrowheads). In cross-sectional view of the full series of confocal images, there was a dramatic increase in vessels (arrows) in the outer half of the D(CAPAC)-treated vldlr-null retinas (along the full length of the photoreceptor cells, from their synapses in the OPL, though the ONL, to the outer segments, as recognizable by comparison with the H&E-stained inset in the wildtype specimen), compared with the fewer vessels in these layers in the Vasotide-treated vldlr-null retinas (Fig. 6B, arrows). The full confocal set of optical sections at 10 µm intervals through the 200 µm retinal thickness at P48 for D(CAPAC)-treated (Fig. 6C) versus Vasotide-treated (Fig. 6D) vldlr-null retinas show that Vasotide treatment reduced angiogenesis in the outer half of the retina compared to the D(CAPAC)-treated control.
Fig. 6. Confocal image series at 10-mmintervals through the full retinal thickness at P48 in WT and vldlr-nullmice.
(A, B) Isolectin IB4-stained vasculature of whole-mount retinas viewed through the entire 200 µm thickness (microscope objective 10X, 21 superimposed confocal slices) of P48 wildtype (WT), D(CAPAC)-treated control and Vasotide-treated vldlr-null (KO) mice. In A, these 3D vascular images are viewed from the retinal inner surface (“z” direction) and in B, in a cross-sectional view (“y” direction). Formation of abnormal vascular tufts are indicated by yellow arrowheads in A and yellow arrows in B. In the wildtype mouse retina, a representative H&E-stained retinal image has been superimposed to show the relationship between retinal layers and the vasculature. (C) A full set of vascular images of vldlr-null mouse retina treated with control D(CAPAC) peptide. (D) Comparable images from a vldlr-null mouse retina treated with Vasotide. (E) Graphs of blood vessel concentrations at six treatment ages.
The normalized percentage of the sampled retinas in all 21 confocal fields occupied by blood vessels in wildtype mice, D(CAPAC)-treated vldlr-null mice, and Vasotide-treated vldlr-null mice at six different ages (Fig. 6E) indicated that even as early as the fifth day of the initial phase of treatment, angiogenesis in the inner retina was slightly decreased by Vasotide treatment. At all subsequent ages, the enhanced angiogenesis in the outer (normally avascular) half of the retina in D(CAPAC)-treated vldlr-null mice was reduced by Vasotide treatment, as illustrated by the representative images for P120 (Fig. S4). At the oldest ages studied, P190 and P270, the outermost 70 µm of the retina had sustained sufficient damage from the ongoing disease that blood vessels could not be quantified reliably, but even in these retinas angiogenesis at and around the 100 µm depth was markedly increased in the D(CAPAC)-treated vldlr-null retinas compared to the Vasotide-treated vldlr-null retinas (Fig. 6E). Statistical data at each age sampled are listed in Table S5.
Discussion
We have shown that the VEGF receptor-targeted prototype peptidomimetic Vasotide can reduce pathological angiogenesis in two preclinical murine models and one non-human primate model of human retinal diseases: the mouse ROP model, the vldlr knockout mouse model of retinal angiogenesis proliferation (RAP), and the laser-induced monkey model of wet AMD. Each disease type displays both common and unique angiogenic features. This suggests that, like the currently FDA-approved anti-VEGF therapeutics, Vasotide may be valuable for a range of human retinal diseases involving angiogenesis. Indeed, our results with Vasotide are roughly similar to the therapeutic response to bevacizumab previously reported in eyes of animals of the same primate colony comparably lesioned by laser-induced photocoagulation (22, 32). More elaborate pharmacokinetic/therapeutic analysis of Vasotide is required to identify additional characteristics such as duration of efficacy, safety, convenience and cost (1).
To our knowledge, the Vasotide peptide prototype is the only known exogenous drug candidate that binds effectively to two important VEGF receptors, NRP-1 and VEGFR-1. In contrast, the clinically available anti-VEGFA therapeutics mainly bind to VEGF-A and inhibit binding of this ligand to its major receptors, or serve as alternative receptors competitively interfering with binding of VEGF ligands to VEGFR-2 or other receptors. Thus, Vasotide may be useful in patients who are either unresponsive to the currently used agents or become resistant during repeated intraocular injections. In addition, although NRP-1 and VEGFR-1 have been less intensively studied than VEGFR-2, evidence suggests that they may regulate angiogenesis via different mechanisms of action. Also supporting this conclusion is that another VEGF ligand, PlGF, expressed in pathological retinas, also binds to NRP-1 and VEGFR-1 (24) and stimulates angiogenesis in part by promoting downstream phosphorylation of Akt and ERK (40), which does not occur when vascular endothelial cells are stimulated with VEGF-A. Thus, it is likely that Vasotide, like PlGF, does not affect VEGF-A-stimulated endothelial cell activation and proliferation through VEGFR-2, but rather exerts its antiangiogenic effects mainly through alternative pathways specifically involving both VEGFR-1 and NRP-1 (19).
At least some postnatal angiogenic actions of NRP-1 appear to be mediated by the binding of ligands other than VEGF (37). Moreover, bone marrow-derived myeloid cells that are increased in the ROP retina release Wnt proteins, which reduce branching during blood vessel development (42), apparently by specifically inducing VEGFR-1 (43). Given that Vasotide inhibits NRP-1, it may also regulate immunological mechanisms at play in the hyper-vascularized retina (44). These data, along with our own results suggesting a decrease in inflammatory infiltrates in the treated monkey retinas, widen the potential mechanisms of action of Vasotide, strengthening its potential value for topical treatment of angiogenic retinal diseases.
Another disease mechanism relevant to pathological angiogenesis that Vasotide may affect is oxidative stress. The targeting of VEGFR-1 by Vasotide may play a greater role in the angiogenic mechanism than the targeting of VEGFR-2 by VEGF-A (45) if the onset of angiogenesis is indeed a consequence of a hypoxic state. Cellular responses to hypoxia in retina and other organs are regulated by HIF-1α and HIF-2α. Hypoxia initiates cleavage of filamin A, a large cytoskeletal actin-binding protein, to form a C-terminal fragment that interacts with HIF-1α by as-yet unknown mechanisms to induce angiogenesis (46). This action may not be entirely independent of VEGF, as transcriptional activation of HIF-1 in myeloid cells can promote angiogenesis via VEGF (47). Although this has not yet been demonstrated unequivocally in retinal vasculature, it is likely that the myeloid cell reduction we observed in Vasotide-treated monkey retinas is a factor in the preservation of retinal vasculature and photoreceptor cells. Interestingly, retinal hypoxia affecting particularly the metabolically active, mitochondria-rich photoreceptor cell inner segments, has been suggested to be the primary event in several retinal diseases, including diabetic retinopathy (48). Thus, Vasotide might be affecting hypoxia-based mechanisms that precede the angiogenic abnormalities in AMD, a disease mechanism we have commented on previously (21, 49).
Our results also indicate that Vasotide may be a valuable alternative to the present therapeutic agents that are injected intravitreally because the peptidomimetic’s administration routes are far simpler and less likely to damage the retina iatrogenically. Several early attempts to develop eye drop delivery for retinal disorders were unsuccessful because of membrane barriers and drug-destructive mechanisms at the eye surface (50). Also, the normal intraocular fluid flow is from vitreous to aqueous, so that compounds delivered into the anterior xhamber of the eye show fairly limited diffusion to the retina. A small molecule given topically, however, can effectively reach the posterior ocular tissues via the conjunctiva, with passage subsequently through sclera, choroid, retinal pigment epithelium, and retina (51–53). Indeed, topical application of a cyclic arginine-glycine-aspartic acid (Arg-Gly-Asp; RGD) peptide, which targets integrin α5-enriched blood vessels in the CNV zone, has previously shown efficacy in reducing neovascularization in the ROP mouse model (54). The data presented here also show successful delivery of a therapeutic peptide via this route. Our earlier data demonstrated transfer of the linear D(LPR) peptide from eye drops into the vitreous chamber of the eye (fig. S2A to C in ref. 19), so that this therapeutic agent directly contacted the inner surface of the retina, where the angiogenic tufts of neovasculature characteristic of the ROP disease are formed (20, 21, 25, 26). Eye drop drug therapy has already been used successfully in the ROP mouse model and has been extended to rats and to laser-injured monkeys with AMD-like choroid/retina neovascular lesions (22). Finally, one could speculate that this delivery route may be augmented in the future by taking advantage of endogenous carrier systems, such as harnessing the high transferrin receptor expression on conjunctival cells by applying nanoparticles that are surface-coated with transferrin (55, 56), or perhaps bacteriophage nanoparticles engineered to carry both Vasotide and our recently described iron-mimic peptide motif (57).
Another hypothesis is that Vasotide may have additional promise if administered in combination with other agents. In addition to possible combination therapy with the current intravitreal agents that affect binding mainly of VEGF-A to VEGFR-2, consideration is merited for combination therapy with agents acting by other molecular mechanisms that affect pathological angiogenesis. This might include Imatinib (Gleevec), an FDA-approved anti-leukemia inhibitor of the tyrosine kinase ABL1, which forms complexes with NRP-1, probably through the mediation of binding of NRP-1 to selected integrins, with resultant downstream actions that are VEGF-independent leading to reduction of pathological angiogenesis in the ROP mouse model (58). Additional agents of interest (59) include angiopoietin-2, a Tie2 receptor agonist, expressed at the inner retinal surface where pathological vascular tufts are forming in ROP mice (60). Also a possibility is pazopanib, an FDA-approved oral therapeutic for advanced renal cell carcinoma that inhibits several VEGFRs, with in vivo tests focused mainly on VEGFR-2 although pazopanib also inhibits two Platelet-derived Growth Factors and KIT thus reducing angiogenesis; the related but somewhat more soluble GW771806 can be administered in eye drops (61). We have previously described that retinal vessels formed by angiogenesis in the ROP mouse model also express on their vascular cells αvβ3 and αvβ5 integrins, aminopeptidase A, and aminopeptidase N receptors that can selectively bind to circulating bacteriophage-carried peptide ligands including a proapoptotic peptide that kills vascular cells (62).
As discussed above, immune mechanisms also are at play in eye diseases featuring pathological angiogenesis and vascular leakage, with interesting recent data reporting that the cytokine IL-18, delivered subcutaneously (s.c.) to mice two weeks before laser-induced CNV, reduced lesion volume without disturbing retinal function, and was even more effective when delivered s.c. in combination with intravitreal injection of an anti-VEGF drug (63). Constructive reports are beginning to appear on control of glycolytic mechanisms that provide sufficient energy to endothelial cells to initiate and regulate angiogenic sprouting (64), and could be combined with Vasotide therapy. The most active glycolytic enzyme in endothelial cells is 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), which converts fructose-6-phosphate to fructose-2,6 biphosphate, an activator of phosphofructokinase-1. Inhibition of PFKFB3 by a small molecule abbreviated as 3PO (60) reduced glycolytic flux sufficiently to deactivate angiogenesis, but not enough to kill the cells. VEGF is known to activate endothelial “tip cells” (located at the tips of sprouting vessels) that steer vessel growth toward and into hypo-oxygenated areas. Notch suppresses tip cells but activates proliferation and linear migration of endothelial cells further down the vessel stalk (“stalk cells”) to lengthen a growing vessel segment. The relation between PFKFB3 inhibition and VEGF’s actions on VEGFR-2 or possibly VEGFR-1 regarding influence on tip cell function are under study, but it is already clear that 3PO inhibition of PFKFB3 can block angiogenesis not only in animal models of psoriasis and colitis, but also in the key mouse retinal models of ROP and wet AMD (65). Yet another prospect for combination therapy is Vasotide plus pertinent glycosphingolipids, particularly lactosylceramide (66, 67).
ROP and wet AMD are widely recognized to be human diseases of considerable complexity, and the animal models are few in number and do not ideally match their human counterparts. These limitations of the models apply even more to models of Diabetic Retinopathy, the third in this major class of vision-impairing diseases that feature pathological angiogenesis as an important causal factor. Our studies thus far have not addressed several issues of great importance regarding disease mechanisms and potential therapies. We have not yet established Vasotide’s ocular pharmacological properties with respect to its routes and rates of entry into ocular tissues and blood, its half-life in each relevant site, optimal dosage, and possible side effects, all of which require thorough analysis although they only can be approximated in animal studies. What we have established is that the Vasotide peptidomimetic is therapeutically effective when delivered in eye drop or i.p. routes in two murine and one non-human primate angiogenic retinal disease models (). Another issue of great interest is whether our peptide or other agents either prevent or correct the loss of vessels during the hyper-oxygenation phase of the ROP disease. Offsetting that deficit, as pointed out by other investigators, would eliminate the need for subsequent correction of hypoxia or treatment of its deleterious effects, which are related to the increase in VEGF and changes in other genes and metabolites that can cause pathological angiogenesis. We did not investigate this key issue, having administered our peptide only in the subsequent hypoxic phase as a direct block to angiogenesis itself, but sirtuin and probably some of its downstream metabolites may contribute to this highly desirable objective in the hyper-oxygenation phase (68, 69).
In summary, we have demonstrated that the prototype Vasotide peptidomimetic is a promising anti-angiogenic drug candidate when delivered in three different preclinical murine and primate retinal disease models, and may have translational potential that complements existing anti-VEGF therapeutics. Exogenous Vasotide has a unique mechanism of action among anti-angiogenesis agents by competing with the binding of endogenous ligands to VEGFR-1 and NRP-1, and (particularly relevant for human retinopathies) it likely can be administered more conveniently, safely, and cost-effectively to patients in the form of eye drops than the intravitreal drugs in current use.
Materials and Methods
Study Design
The primary objective of the experimental design was to test by quantitative histopathology whether eye drop treatment with a new unusually small cyclic peptidomimetic would inhibit the pathological hypervascularization induced by VEGF in retinas of three independent models of major human diseases that reduce vision, two models in mice and one in non-human primates,
Reagents
The peptidomimetics Vasotide and D(CAPAC) were synthesized and purified by HPLC to >95% purity by Polypeptide Laboratories (Torrance, CA). Retinal blood vessels were stained in mouse retinal whole mounts with Alexa Fluor 568 conjugated isolectin IB4 (Invitrogen, Carlsbad, CA) and whole mount tissue transparency was increased with Ioversol Injection 68% (Mallinckrodt, St. Louis, MO), an organic iodine agent used mainly in radiological imaging. For detection of inflammation, immunohistochemical reactions were carried out on 4% paraformadehyde immersion-fixed monkey retinas with anti-F4/80 polyclonal antibody (Chemicon International, Burlingame, CA) as a macrophage marker, and anti-GR1 monoclonal antibody (Thermo Scientific, Waltham, MA) as a granulocyte marker. Anti-CD31 polyclonal antibody was purchased from ABcam (Cambridge, MA), and anti-CD68 monoclonal antibody from Life Technologies (Woburn, MA). Secondary fluorescent antibodies in monkey and mouse specimens were conjugated with Cy3 or FITC (Chemicon, Billerica, MA).
Monkey model of laser-induced CNV
Eight male African green monkeys (C. sabaeus) ranging in weight from 3.78 to 6.14 kg and estimated by weight and dentition to be between 4- and 6--years-old were anesthetized with intramuscular injection of ketamine (8 mg/kg) and xylazine (1.6 mg/kg). Supplemental anesthesia was administered as needed. Prior to laser photocoagulation and drug administration, baseline ocular assessments were performed under ketamine/xylazine sedation on each animal to confirm health of animals and their eyes and adequacy of achievable mydriasis. Animals were equally distributed into treatment groups and randomized by weight (Table S1). The laser treatment consisted of placing six laser spots were concentrically spaced approximately 1.0–1.5 disc diameters from the fovea at the anatomic periphery of the macula, within the temporal vascular arcades of both eyes for all eight animals (Fig. S1). As previously optimized (32), laser spots were induced with an Iridex Oculight TX 532 nm laser at laser energy of 900 mW, pulse duration of 100 ms, and spot size of 50 µm. The laser was mounted on a slit lamp with a slit lamp adapter and the laser beam directed onto the retina with a Volk Centralis Direct ANF+ 0.9X laser lens of 10 mm contact diameter (Volk Optical, Mentor, OH). Saline was used as the coupling agent between lens and cornea. Laser treatment reliably produced acute vapor bubbles in the retinas, typically associated with Bruch’s membrane rupture. Further details on minor complications occurring with the laser treatment are described in the Supplementary Materials. Imaging data were collected at the end of week 4 (day 29) post-laser photocoagulation, the time point at which peak development of CNV complex formation was detected in prior characterization of the model (20).
Monkeys were used according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Permission for bilateral treatments and all aspects of the animal studies was obtained from the primate facility (St. Kitts Biomedical Research Foundation, St. Kitts, West Indies) Animal Care and Use Committee.
Eye drop administration of drugs to monkeys
A topical formulation of the Vasotide peptide was administered at a concentration of 120 mg/ml. Following laser photocoagulation on day 1, the test article, Blink Gel Tears (Abbott Medical Optics, Abbott Park, IL), referred to in this text as GelTears, was administered topically by pipette onto both right and left eyes (50 µl) twice daily for 5 days, then once daily for 16 days. The vehicle alone (50 µl) was delivered in an identical fashion. Treatment regimen was as defined in Table S1. Monkeys were dosed while in a supine position.
Mouse retinopathy of prematurity (oxygen-induced retinopathy, OIR) model
We used the widely accepted, readily reproduced model developed by Smith and her colleagues (20), on C57BL/6J mice obtained from The Jackson Laboratory (Bar Harbor, ME). On P7, cages with mouse pups and their mothers were placed in an airtight incubator exposing the mice to an atmosphere of 75% ± 3% O2 (hyperoxia) for 5 days. Incubator temperature was maintained at 23 ± 2°C, and O2 was measured three times per day with an oxygen analyzer. On P12, the mice were returned to room air, and drug treatment was begun. Additional mice of the same age and inbred strain were kept in room air as normal controls. Treatment procedures included: (1) i.p. injection, daily for 7 days, from P12-P18, 8 µg peptide/g body weight (designated as low dose), or 40 µg/g (designated as high dose) in PBS as vehicle; or (2) eye topical administration, 3 times per day, for 7 days, from P12-P18, 200 µg/ 2 µl drop of Systane. The mouse studies were approved and supervised by the Harvard Medical School Institutional Animal Care and Use Committee.
Mouse vldlr-null (knockout) model of the human Retinal Angiomatous Proliferation (RAP) disorder
Breeding pairs of mutant mice with targeted deletion of the vldlr gene (B6;129S7-Vldlrtm1Her/J; vldlr KO) (The Jackson Laboratory, Bar Harbor, ME) were maintained and bred in standardized conditions. Age-matched C57BL/6J mice were used as normal wildtype controls. Vldlr KO mice were randomly divided into three groups: vldlr KO without treatment, vldlr KO given control peptide D(CAPAC), and vldlr KO given the therapeutic peptide Vasotide. We injected intraperitoneally (i.p.) 20 µg peptide/µl PBS, 40 µg/g body weight, daily at P12-P18, P48-P54, P108-P114 and P208-P214. Mice were maintained according to the Association for Research in Vision and Ophthalmology statement on animal usage in ophthalmic research. The study was approved by Harvard Medical School Institutional Animal Care and Use Committee (HMS IACUC).
The PCR genotyping procedures are described in the Supplementary Materials.
Mouse retinal whole-mount preparation and retinal vasculature visualization
Mice were deeply anesthetized with intraperitoneal tribromoethanol (0.2 ml/10 g body weight) and then were perfused through the left ventricle with 4% paraformaldehyde. Both eyes were removed and further fixed by immersion in 4% paraformaldehyde at 4°C.
For staining of entire retina or eyecup, we removed the cornea, iris, and lens and then carefully dissected the retina free. We washed the isolated retina or full eyecup with PBS for 30 min at room temperature, and then stained with Alexa Fluor 568 conjugated isolectin IB4 (10 µg/ml) in 1% Triton X100-PBS at 4°C for 24–48 h. We rinsed each retina 3X in PBS, stained with IB4, mounted in Loversal Injection 68% to increase tissue transparency, and overlaid the tissue with a coverslip. IB4 selectively and strongly binds with terminal α-D-galactosyl residues in endothelial and selected perivascular cells (31, 72).
For immunostaining, we blocked whole mount retina or eyecup with 20% fetal bovine serum and 20% normal goat serum in 1% Triton X100-PBS at room temperature for one hour and then incubated with primary antibody against markers for macrophage (F4/80 or CD68), granulocytes( GR1) or endothelial cells CD31 at 4°C for 48 h. After washing with PBS, tissues were stained overnight with secondary Cy3 or FITC antibody at 4°C.
For whole-mount preparation, IB4-stained retina and eyecup were rinsed three times in PBS, radially cut from the edge of the retina or eyecup to the equator, flat-mounted in Loversal Injection 68% (Mallinckrodt, St. Louis, MO) to increase transparency, and protected with a coverslip. For retinas, the ganglion cell layer was facing up; for eyecups, the retinal pigment epithelium (RPE) was facing up. Images were captured with a Nikon Eclipse 800 fluorescence microscope equipped with a Micromax CCD camera (Princeton Instruments, Trenton, NJ) and MetaVue software (Molecular Devices, Downingtown, PA), or with a confocal microscope (Axiovert 100M; Carl Zeiss, New York, NY). We obtained image stacks spanning the entire 200 µm retinal thickness and three-dimensional images with Zeiss LSM image analysis software. Quantitative analysis of retinal neovascularization is described in the Supplementary Materials.
Statistics
In monkey eyes, graded (I-IV) scoring of lesions was analyzed with Fisher’s exact test where incidence of both grade III and IV lesions was assigned ‘Yes’ and any other grading assigned ‘No’. Validation of the laser-induced CNV model has demonstrated that grade III or IV lesion scores represent clinically significant CNV. Mean OCT-derived CNV complex area was analyzed with Student’s t test on Box-Cox-transformed data. We used a non-parametric test to analyze mean fluorescein intensities based on ImageJ background corrections. All statistics were performed with the analysis software JMP9 (SAS Institute, Cary, NC), P values < 0.05 considered as statistically significant.
We performed each mouse experiment at least three times and used representative data per treatment and age in the calculations. Comparisons of immunofluorescence and histological results between groups were performed with ANOVA, and the χ2 test for enumeration data. The percentage of the total sampled retinal area occupied by red-fluorescent blood vessels was calculated with ImageJ on the individual 21 images at 10 µm intervals through each mouse retina. Values were expressed as mean ± SD, and differences were considered significant at P < 0.05 or very significant at P < 0.01.
Supplementary Material
Accessible Summary.
A suggested new treatment for blinding retinal diseases
This paper describes a novel small drug candidate named Vasotide™, made of engineered amino acids, that blocks abnormal overgrowth of blood vessels in the eye’s retina, leading to loss of vision. A distinctive feature of this drug is its delivery in simple eye drops. Vasotide uniquely prevents a blood vessel growth-promoting molecule called VEGF from attaching to two different receptor molecule types on endothelial cells, which line the inside of blood vessels, thereby inhibiting the pathology in animal models of major retinal diseases called aged macular degeneration, retinopathy of prematurity, and possibly the untested but similarly caused diabetic retinopathy.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (NIH R01EY019459) and by AMP Pharm. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute, the NIH, or AMP Pharm. AMP Pharm had no role in study design, data collection and analysis, or manuscript preparation.
Footnotes
Author Contributions: J.L. performed the mouse experiments and processed mouse and primate retina whole mounts for microscopic analysis. M.L. and W.H. performed the primate experiments. R.L.S., J.L., M.L., W.H., G.F.M. R.J.G., and M.C.-V. provided reagents or technical expertise. R.L.S., W.A., and R.P. conceived and supervised the study, analyzed data, and wrote the manuscript with editorial input from J.L., M.L., W.H. and M.C.-V.
Competing Interests: G.F.M., R.P., W.A. and R.L.S. are founders and equity-holders in AMP Pharm and G.F.M. is an employee of AMP Pharm. M.C.-V., R.J.G., R.P., and W.A. are inventors on related U.S. and international filed patents and are entitled to standard Royalties if licensing or commercialization occurs. The University of New Mexico Comprehensive Cancer Center currently manages these arrangements in accordance with its established institutional conflict of interest policy. US and International Patent applications for “VEGFR-1/NRP-1 Targeting Peptides” include: US application #12/672,647, European application #08797529.8, Hong Kong application #12104981.9, Australia application #2008296733 (granted), China application #200880110564.3, Russia application #2010108499 (allowed), Japan application #2010–520333 (issued), Canada application #2695960, Brazil application #PI0815131.8
References
- 1.Bek T. Regional morphology and pathophysiology of retinal vascular disease. Prog. Retin. Eye Res. 2013;36:247–259. doi: 10.1016/j.preteyeres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Congdon N The Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS The Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Djordjevic S, Driscoll PC. Targeting VEGF signalling via the neuropilin co-receptor. Drug Discov. Today. 2013;18:447–455. doi: 10.1016/j.drudis.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012:2. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Löfqvist C, Hellström A, Smith LEH. The mouse retina as an angiogenesis model. Invest. Ophthalmol. Vis. Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geudens I, Gerhardt H. Coordinating cell behavior during blood vessel formation. Devel. 2011;138:4569–4583. doi: 10.1242/dev.062323. [DOI] [PubMed] [Google Scholar]
- 8.Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor A in intraocular vascular disease. Ophthalmol. 2013;120:106–114. doi: 10.1016/j.ophtha.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 9.Cavallaro G, Filippi L, Bagnoli P, La Marca G, Cristofori G, Raffaeli G, Padrini L, Araimo G, Fumagalli M, Groppo M, Dal Monte M, Osnaghi S, Fiorini P, Mosca F. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol. 2014;92:2–20. doi: 10.1111/aos.12049. [DOI] [PubMed] [Google Scholar]
- 10.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M. D.R. Guyer. Pegaptanib for neovascular age-related macular degeneration. New England J. Med. 2004;251:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 11.Munk MR, Kiss C, Huf W, Sulzbacher F, Roberts P, Mittermüller TJ, Sacu S, Simader C, Schmidt-Erfurth U. One year follow-up of functional recovery in neovascular AMD during monthly anti-VEGF treatment. Am. J. Ophthalmol. 2013;156:633–643. doi: 10.1016/j.ajo.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt-Erfurth U, Kaiser PK, Korobelnik J-F, Brown DM, Chong V, Nguyen QD, Ho AC, Ogura Y, Simader C, Jaffe GJ, Slakter JS, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Sowade O, Zeitz O, Norenberg C, Sandbrink R, Heier JS. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six week results of the VIEW studies. Ophthalmol. 2014;121:193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Broadhead GK, Hong T, Chang AA. Treating the untreatable patient: current options for the management of treatment-resistant neovascular age-related macular degeneration. Acta Ophthalmol. 2014;92:713–723. doi: 10.1111/aos.12463. [DOI] [PubMed] [Google Scholar]
- 14.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat. Rev. Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 15.Jaffe GJ, Martin DF, Toth CA, Daniel E, Maguire MG, Ying G-S, Grunwald JE, Huang J. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmol. 2013;120:1860–1870. doi: 10.1016/j.ophtha.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Englander RJM, Chen TC, Paschalis EI, Miller JW, Kim IK. Intravitreal injections at the Massachusetts Eye and Ear Infirmary: analysis of treatment indications and postinjection endophthalmitis rates. Br. J. Ophthalmol. 2013;97:460–465. doi: 10.1136/bjophthalmol-2012-302435. [DOI] [PubMed] [Google Scholar]
- 17.Giordano RJ, Cardó-Vila M, Lahdenranta J, Pasqualini R, Arap W. Biopanning and rapid analysis of selective interactive ligands. Nat. Med. 2001;7:1249–1253. doi: 10.1038/nm1101-1249. [DOI] [PubMed] [Google Scholar]
- 18.Giordano RJ, Anobom CD, Cardó-Vila M, Kalil J, Valente AP, Pasqualini R, Almeida FC, Arap W. Structural basis for the interaction of a vascular endothelial growth factor mimic peptide motif and its corresponding receptors. Chem. Biol. 2005;12:1075–1083. doi: 10.1016/j.chembiol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Giordano RJ, Cardó-Vila M, Salameh A, Anobom CD, Zeitlin BD, Hawke DH, Valente AP, Almeida FC, Nör JE, Sidman RL, Pasqualini R, Arap W. From combinatorial peptide selection to drug prototype (I): Targeting the vascular endothelial growth factor receptor pathway. Proc. Natl. Acad. Sci. USA. 2010;107:5112–5117. doi: 10.1073/pnas.0915141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith LEH, Wesolowski E, McLellan SK, Kostyk A, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 21.Lahdenranta J, Pasqualini R, Schlingemann RO, Hagedorn M, Bucana CD, Stallcup WB, Sidman RL, Arap W. An anti-angiogenic state in mice and humans with retinal photoreceptor cell degeneration. Proc. Natl. Acad. Sci. USA. 2001;98:10368–10373. doi: 10.1073/pnas.181329198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cloutier F, Lawrence M, Goody R, Lamoureux S, Al-Mahmood S, Colin S, Ferry A, Conduzorgues J-P, Hadri A, Cursiefen C, Udaondo P, Viaud E, Thorin E, Chemtob S. Antiangiogenic activity of aganirsen in nonhuman primate and rodent models of retinal neovascular disease after topical administration. Invest. Ophthalmol. Vis. Sci. 2012;53:1195–1203. doi: 10.1167/iovs.11-9064. [DOI] [PubMed] [Google Scholar]
- 23.Joyal J-S, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, Shao Z, Polosa A, Zhu T, Hamel D, Djavari M, Kunik D, Honoré J-C, Picard E, Zabeida A, Varma DR, Hickson G, Mancini J, Klagsbrun M, Costantino S, Beauséjour C, Lachapelle P, Smith LEH, Chemtob S, Sapieha P. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood. 2011;117:6024–6035. doi: 10.1182/blood-2010-10-311589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat. Rev. Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 25.Smith LE, Hard AL, Hellström A. The biology of retinopathy of prematurity: how knowledge of pathogenesis guides treatment. Clin. Perinatol. 2013;40:201–214. doi: 10.1016/j.clp.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bucher F, Stahl A, Agostini HT, Martin G. Hyperoxia causes reduced density of retinal astrocytes in the central avascular zone in the mouse model of oxygen-induced retinopathy. Mol. Cell. Neurosci. 2013;56:225–233. doi: 10.1016/j.mcn.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Austeng D, Källen KBM, Hellström A, Tornqvist K, Holmström GE. Natural history of Retinopathy of Prematurity in infants born before 27 weeks’ gestation in Sweden. Arch. Ophthalmol. 2010;128:1289–1294. doi: 10.1001/archophthalmol.2010.234. [DOI] [PubMed] [Google Scholar]
- 28.Maitre NL, Ballard RA, Ellenberg JH, Davis SD, Greenberg JM, Hamvas A, Pryhuber GS. PROP. Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J. Perinatol. 2015;35:313–321. doi: 10.1038/jp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.K M, Asano P, Dray B. Retinopathy of prematurity. Disease-a-Month. 2014;60:282–291. doi: 10.1016/j.disamonth.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Fruttiger M. Development of the retinal vasculature. Angiogen. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 31.Connor KP, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LEH. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goody RJ, Hu W, Shafiee A, Struharik M, Bartels S, López FJ, Lawrence MS. Optimization of laser-induced choroidal neovascularization in African green monkeys. Exp. Eye Res. 2011;92:464–472. doi: 10.1016/j.exer.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Yannuzzi LA, Negrāo S, Iida T, Carvalho C, Rodriuez-Coleman H, Slakter J, Freund KB, Sorenson J, Orlock D, Borodoker N. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21:416–434. doi: 10.1097/00006982-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Marticorena J, Di Leva V, Cennamo GL, de Crecchio G. Retinal angiomatous proliferatio. n. Curr. Drug Targets (Bentham Science) 2011;12:199–205. doi: 10.2174/138945011794182683. [DOI] [PubMed] [Google Scholar]
- 35.Heckenlively JR, Hawes NL, Friedlander M, Nusinowitz S, Hurd R, Davisson M, Chang B. Mouse model of subretinal neovascularization with choroidal anastomosis. Retina. 2003;23:518–522. doi: 10.1097/00006982-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Li C, Huang Z, Kingsley R, Zhou X, Li F, Parke DW, II, Cao W. Biochemical alterations in the retinas of very low-density lipoprotein receptor knockout mice. An animal model of Retinal Angiomatous Proliferation. Arch. Ophthalmol. 2007;125:795–803. doi: 10.1001/archopht.125.6.795. [DOI] [PubMed] [Google Scholar]
- 37.Hu W, Jiang A, Liang J, Meng H, Chang B, Gao H, Qiao X. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model’s Retinal Angiomatous Proliferation. Invest. Ophthalmol. Vis. Sci. 2008;49:407–415. doi: 10.1167/iovs.07-0870. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Hu Y, Moiseyev G, Zhou KK, Chen D, Ma J. Photoreceptor degeneration and retinal inflammation induced by very low-density lipoprotein receptor deficiency. Microvasc. Res. 2009;28:119–127. doi: 10.1016/j.mvr.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanks JC, Johnson LV. Vascular atrophy in the retinal degenerative rd mouse. J. Comp. Neurol. 1986;254:543–553. doi: 10.1002/cne.902540407. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, Gu Q, Xu X. Inhibition of ocular neovascularization by a novel peptide derived from human placenta growth factor-1. Acta Ophthalmol. 2012;90:e512–e523. doi: 10.1111/j.1755-3768.2012.02476.x. [DOI] [PubMed] [Google Scholar]
- 41.Fantin A, Herzog B, Mahmoud M, Yamaji M, Plein A, Denti L, Ruhrberg C, Zachary I ,Neuropilin 1 (NRP1) hypomorphism combined with defective VEGF-A binding reveals novel roles for NRP1 in developmental and pathological angiogenesis. Devel. 2014;141:556–562. doi: 10.1242/dev.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, Sapieha P, Hua J, Hatton CJ, Juan AM, Aderman CM, Willett KL, Guerin KI, Mammoto A, Campbell M, Smith LEH. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation. 2011;124:1871–1881. doi: 10.1161/CIRCULATIONAHA.111.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stefater JA, III, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, Wills-Karp M, Pollard JW, Yamaguchi T, Ferrara N, Gerhardt H, Lang RA. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–516. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kikumanogoh A, Kikutani H. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol. 2013;13:802–814. doi: 10.1038/nri3545. [DOI] [PubMed] [Google Scholar]
- 45.Shih S-C, Ju M, Liu N, Smith LE. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. J. Clin. Invest. 2003;112:50–57. doi: 10.1172/JCI17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng X, Zhou A-X, Rouhi P, Uramoto H, Borén J, Cao Y, Pereira T, Akyürek LM, Poellinger L. Hypoxia-induced and calpain-dependent cleavage of filamin A regulates the hypoxic response. Proc. Natl. Acad. Sci. USA. 2014;111:2560–2565. doi: 10.1073/pnas.1320815111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahn G-O, Seita J, Hong B-J, Kim Y-E, Bok S, Lee C-J, Kim KS, Lee JC, Leeper NJ, Cooke JP, Kim HJ, Kim IH, Weissman IL, Brown JM. Transcriptional activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells promotes angiogenesis through VEGF and S100A8. Proc. Natl. Acad. Sci. USA. 2014;111:2698–2703. doi: 10.1073/pnas.1320243111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dowling JE. Concluding Remarks. Lasker Foundation and International Retinal Research Foundation Report on DR. 2012:83–87. http://www.laskerfoundation.org/programs/images/irrf_12.pdf.
- 49.Arden GB, Sidman RL, Arap W, Schlingemann RO. Spare the rod and spoil the eye. Br. J. Ophthalmol. 2005;89:764–769. doi: 10.1136/bjo.2004.062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maurice DM. Drug delivery to the posterior segment from drops. Surv. Ophthalmol. 2002;47(Suppl. 1):S41–S52. doi: 10.1016/s0039-6257(02)00326-0. [DOI] [PubMed] [Google Scholar]
- 51.Furrer E, Berdugo M, Stella C, Behar-Cohen F, Gurny R, Feige U, Lichtlen P, Urech DM. Pharmacokinetics posterior segment biodistribution of ESBA105, an anti-TNF-α single-chain antibody upon topical administration to the rabbit eye. Invest. Ophthalmol. Vis. Sci. 2009;50:771–778. doi: 10.1167/iovs.08-2370. [DOI] [PubMed] [Google Scholar]
- 52.Ottiger M, Thiel MA, Feige U, Lichtlen P, Urech DM. Efficient intraocular penetration of topical anti-TNF-α single-chain antibody (ESBA105) to anterior and posterior segment without penetration enhancer. Invest. Ophthalmol. Vis. Sci. 2009;50:779–786. doi: 10.1167/iovs.08-2372. [DOI] [PubMed] [Google Scholar]
- 53.Thrimawithana TR, Young S, Bunt CR, Green C, Alany RG. Drug delivery to the posterior segment of the eye. Drug Disc. Today. 2011;16:270–277. doi: 10.1016/j.drudis.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Riecke B, Chavakis E, Bretzel RG, Linn T, Preissner KT, Brownlee M, Hammes H-P. Topical application of integrin antagonists inhibits proliferative retinopathy. Horm. Metab. Res. 2001;33:307–11. doi: 10.1055/s-2001-15279. [DOI] [PubMed] [Google Scholar]
- 55.Kompella UB, Sundaram S, Raghava S, Escobar ER. Luteinizing hormone-releasing hormone agonist and transferrin functionalizations enhance nanoparticle delivery in a novel bovine ex vivo eye model. Mol. Vis. 2006;12:1185–1198. [PubMed] [Google Scholar]
- 56.Kompella UB, Amrite AC, Ravi RP, Durazo SA. Nanomedicines for back of the eye drug delivery gene delivery and imaging. Prog. Retin. Eye Res. 2013;36:172–198. doi: 10.1016/j.preteyeres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Staquicini FI, Ozawa MG, Moya CA, Driessen WHP, Barbu EM, Nishimori H, Soghomonyan S, Flores LG, 2nd, Liang X, Paolillo V, Alauddin MM, Basilion JP, Furnari FB, Bogler O, Lang FF, Aldape KD, Fuller GN, Höök M, Gelovani JG, Sidman RL, Cavenee WK, Pasqualini R, Arap W. Systemic combinatorial peptide selection yields a non-canonical iron-mimicry mechanism for targeting tumors in a mouse model of human glioblastoma. J. Clin. Invest. 2011;121:161–173. doi: 10.1172/JCI44798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raimondi C, Fantin A, Lampropoulou A, Denti L, Chikh A, Ruhrberg C. Imatinib inhibits VEGF-independent angiogenesis by targeting neuropilin 1-dependent ABL1 activation in endothelial cells. J. Exp. Med. 2014;211:1167–1183. doi: 10.1084/jem.20132330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campochiaro PA. Ocular neovascularization. J. Mol. Med. 2013;91:311–321. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. Angiopoietin-2 plays an important role in retinal angiogenesis. J. Cell Physiol. 2002;192:182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 61.Robbie SJ, von Leithner PL, Ju M, Lange CA, King AG, Adamson P, Lee D, Sychterz C, Coffey P, Ng Y-S, Bainbridge JW, Shima DT. Assessing a novel depot delivery strategy for noninvasive administration of VEGF/PDGF RTK inhibitors for ocular neovascular disease. Invest. Ophthalmol. Vis. Sci. 2013;54:1490–1500. doi: 10.1167/iovs.12-10169. [DOI] [PubMed] [Google Scholar]
- 62.Lahdenranta L, Sidman RL, Pasqualini R, Arap W. Treatment of hypoxia-induced retinopathy with targeted proapoptotic peptidomimetic in a mouse model of disease. FASEBJ. 2007;21:3272–3278. doi: 10.1096/fj.07-8273com. [DOI] [PubMed] [Google Scholar]
- 63.Doyle SL, Ozaki E, Brennan K, Humphries MM, Mulfaul K, Keaney J, Kenna PF, Maminishkis A, Kiang A-S, Saunders SP, Hams E, Lavelle EC, Gardiner C, Fallon PG, Adamson P, Humphries P, Campbell M. IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Sci. Transl. Med. 2014;6:230ra44. doi: 10.1126/scitranslmed.3007616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rivera LB, Bergers G. Targeting vascular sprouts. Manipulating metabolism could control angiogenesis. Science. 2014;344:1449–1450. doi: 10.1126/science.1257071. [DOI] [PubMed] [Google Scholar]
- 65.Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquière B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, Bifari F, Wang X, Blanco R, Tembuyser B, Cornelissen I, Bouche A, Vinckier S, Diaz-Moralli S, Gerhardt H, Telang S, Cascante M, Chesney J, Dewerchin M, Carmeliet P. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014;19:37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Chatterjee S, Pandey A. The Yin and Yang of lactosylceramide metabolism: implications in cell function. Biochim. Biophys. Acta - General Subjects. 2008;1780:370–382. doi: 10.1016/j.bbagen.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Kolmakova A, Rajesh M, Zang D, Pili R, Chatterjee S. VEGF recruits lactosylceramide to induce endothelial cell adhesion molecule expression and angiogenesis in vitro and in vivo. Glycoconj. J. 2009;26:547–558. doi: 10.1007/s10719-008-9206-9. [DOI] [PubMed] [Google Scholar]
- 68.Huang H, Van de Veire S, Dalal M, Parlier R, Semba RD, Carmeliet P, Vinores SA. Reduced retinal neovascularization vascular permeability and apoptosis in ischemic retinopathy in the absence of propyl hydroxylase-1 due to the prevention of hyperoxia-induced vascular obliteration. Invest. Ophthalmol. Vis. Sci. 2011;52:7565–7573. doi: 10.1167/iovs.11-8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J, Michan S, Juan AM, Hurst CG, Hatton CJ, Pei DT, Joyal J-S, Evans LP, Cui Z, Stahl A, Sapieha P, Sinclair DA, Smith LEH. Neuronal sirtuin1 mediates retinal vascular regeneration in oxygen-induced ischemic retinopathy. Angiogen. 2013;16:985–992. doi: 10.1007/s10456-013-9374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Aspects Med. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frykman PK, Brown MS, Tamamoto T, Goldstein JL, Herz J. Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA. 1995;92:8453–8457. doi: 10.1073/pnas.92.18.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peters BP, Goldstein IJ. The use of fluorescein-conjugated Bandeiraea simplicifolia B4-isolectin as a histochemical reagent for the detection of α-D-galactopyranosyl groups. Their occurrence in basement membranes. Exp. Cell Res. 1979;120:321–334. doi: 10.1016/0014-4827(79)90392-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.