Abstract
Objective
Carboplatin is a common chemotherapy agent with potential ototoxic side effects that is used to treat a variety of pediatric cancers, including retinoblastoma. Retinoblastoma is a malignant tumor of the retina that is usually diagnosed in young children. Distortion-product otoacoustic emission tests offer an effective method of monitoring for ototoxicity in young children. This study was designed to compare measurements of distortion-product otoacoustic emissions obtained before and after several courses of carboplatin chemotherapy in order to examine if (a) mean distortion-product otoacoustic emission levels were significantly different; and (b) if criterion reductions in distortion-product otoacoustic emission levels were observed in individual children.
Methods
A prospective repeated measures study. Ten children with a median age of 7.6 months (range, 3–72 months) diagnosed with unilateral or bilateral retinoblastoma were examined. Distortion-product otoacoustic emissions were acquired from both ears of the children with 65/55 dB SPL primary tones (f2= 793–7996 Hz) and a frequency resolution of 3 points/octave. Distortion-product otoacoustic emission levels in dB SPL were measured before chemotherapy treatment (baseline measurement) and after 3–4 courses of chemotherapy (interim measurement). Comparisons were made between baseline and interim distortion-product otoacoustic emission levels (collapsed across ears). Evidence of ototoxicity was based on criterion reductions (≥ 6 dB) in distortion-product otoacoustic emission levels.
Results
Significant differences between baseline and interim mean distortion-product otoacoustic emission levels were only observed at f2=7996 Hz. Four children exhibited criterion reductions in distortion-product otoacoustic emission levels.
Conclusions
Mean distortion-product otoacoustic emission levels at most frequencies were not changed following 3–4 courses of carboplatin chemotherapy in children with retinoblastoma. However, on an individual basis, children receiving higher doses of carboplatin exhibited criterion reductions in distortion-product otoacoustic emission level at several frequencies. These findings suggest that higher doses of carboplatin affect outer hair cell function, and distortion-product otoacoustic emission tests can provide useful information when monitoring children at risk of developing carboplatin ototoxicity.
Keywords: Carboplatin, Child, Cochlea, Hearing, Retinoblastoma
1. Introduction
Retinoblastoma is the most commonly encountered, malignant intraocular tumor in children [1]. It has a mean age-adjusted incidence in the United States of 11.8 per million children [2]. Retinoblastoma develops from immature cells within the retina and displays a high mitotic rate, replacing the retina and other intraocular structures with cancerous tissue [3]. The vast majority of children with retinoblastoma are diagnosed before three years of age, with cases of bilateral retinoblastoma usually recognized at an earlier age than cases of unilateral retinoblastoma [2,3] Traditionally, the treatment options for retinoblastoma included enucleation (removal of the affected eye or eyes) and external beam radiation therapy. Due to efforts to preserve vision and avoid disfigurement from radiation, a more conservative approach with systematic chemotherapy is often recommended in children meeting the criteria for this form of treatment [4]. Carboplatin (cis-diammine [1,1-cyclobutanedicarboxylate]-platinum [II]), a second-generation platinum compound with known potential ototoxic side effects, is often included in multi-drug chemotherapy regimens designed to treat retinoblastoma [1,5]. Carboplatin is generally recognized to produce less ototoxicity in children than its analog, cisplatin [6,7,8]. However, the range of the incidence of carboplatin ototoxicity varies across studies. Macdonald et al. [9] found that 50% of children in their study had a sensorineural hearing loss in the 4000–12,000 Hz range following treatment with carboplatin. They found that hearing losses could occur after the first dose of carboplatin, and that hearing losses could progress with subsequent doses. Simon et al. [10] reported that 40% of children treated with high-dose carboplatin developed a hearing impairment and Knight et al. [7] found that 38% of children treated with carboplatin developed hearing loss. In contrast, Stern and Bunin [11] found that ototoxic complications from carboplatin chemotherapy were rare and mild in severity. Other studies have also reported a lack of ototoxic side effects in children receiving carboplatin [12,13]. Factors that may potentiate carboplatin ototoxicity include prior exposure to cisplatin or other ototoxic medications and high dosages of carboplatin [7,10,14]. Younger children may also be at increased risk compared with older children of developing ototoxicity from platinum compounds [15].
The pathophysiology of carboplatin ototoxicity is not completely understood, but evidence from animal models suggests dose-dependent and species-specific effects of carboplatin. In chinchillas, low doses of carboplatin cause progressive inner hair cell (IHC) and spiral ganglion neuron (SGN) loss from the apex to the base of the cochlea, and outer hair cells (OHCs) are largely unaffected [16,17]. At higher doses of carboplatin, extensive loss of IHCs is exhibited across all cochlear turns and loss of OHCs is exhibited most prominently in the basal turn [17,18]. Studies of carboplatin administration in guinea pigs reveal that primarily OHCs are destroyed [19]. Given the potential for damage to cochlear structures, monitoring for carboplatin ototoxicity in pediatric cancer patients is a priority. Early detection of potential ototoxicity from carboplatin can lead to clinical decisions to lower drug dosage to preserve residual hearing or can lead to enrollment in early intervention programs in cases where the dosage cannot be modified. In addition, children with retinoblastoma can have visual impairment in one or both eyes, making it crucial to recognize if carboplatin is potentially causing an additional sensory handicap.
Monitoring cochlear function in pediatric cancer patients is challenging for the clinician. Behavioral hearing tests in young children can be problematic, as it may be difficult to condition the children to participate in the test and their responses can be hard to interpret. Distortion-product otoacoustic emission (DPOAE) tests are an effective alternative to behavioral hearing tests in the early detection of ototoxicity in children receiving chemotherapy drugs known to damage OHCs, such as cisplatin [8,13,20]. Carboplatin has been shown to damage OHCs and reduce the amplitude of DPOAEs in an animal model [17]. However, relatively few clinical studies have examined the efficacy of DPOAE tests as a monitoring tool in humans receiving carboplatin chemotherapy, particularly in children diagnosed with retinoblastoma. Smits et al. [21] utilized several audiometric techniques (including DPOAEs) in evaluating 21 children with retinoblastoma receiving carboplatin. They concluded that there were no signs of ototoxicity in the sample of children they studied, although no details concerning what constituted a change in DPOAE level were provided. Given the fact that hearing loss is evident in some children with retinoblastoma years after the completion of carboplatin chemotherapy [22], it may be important to identify children at risk of developing progressive hearing loss for monitoring purposes. Platinum compounds, including carboplatin, initially damage the basal region of the cochlea where high frequencies are coded before they begin to affect cochlear function at apical regions that code low frequencies. Therefore the ability of a monitoring test (such as DPOAEs) to detect high-frequency cochlear pathology is an important consideration for any ototoxicity monitoring protocol. Implentation of a criterion that defines what constitutes a change in DPOAE level due to potential ototoxicity can be used to identify incipient OHC damage in children while they are in treatment. Children exhibiting criterion-based changes in DPOAE levels may also be targeted for long-term audiometric testing. The aims of this pilot study conducted in children diagnosed with retinoblastoma were twofold: a) on a group basis, to examine if DPOAE levels acquired during tests completed before and after several courses of carboplatin were significantly different, b) on an individual basis, to examine if criterion reductions in DPOAE levels were observed in the children while they were in treatment.
2. Methods
2.1 Subjects
The subjects were children undergoing treatment for retinoblastoma at St. Jude Children’s Research Hospital as part of a frontline protocol. Initially, fifteen children were scheduled for DPOAE monitoring tests, but these tests could not be completed in three children due to scheduling issues. Two additional children did not receive DPOAE monitoring tests because of known active cases of otitis media. The final sample was comprised of 10 children (five females, five males). There were four cases of unilateral retinoblastoma and six cases of bilateral retinoblastoma in the final sample. The ages (rounded to the nearest month) of the children at the onset of treatment ranged from 3 to 72 months. Prior to enrollment in the study, the parents/legal guardians of the children signed informed consent forms approved by the Institutional Review Boards at The University of Memphis and St. Jude Children’s Research Hospital. The demographic characteristics of the children are listed in Table 1.
Table 1.
Subject characteristics.
| Subject | Age at Onset of Treatment (months) | Gender | Type of Retinoblastoma | Number of Carboplatin Courses | Cumulative Dose of Carboplatin (mg/m2) | Ototoxic Change in DPOAE? |
|---|---|---|---|---|---|---|
| S01 | 7 | Female | Bilateral | 3 | 1200 | No |
| S02 | 8 | Male | Unilateral | 4 | 1888 | Yes |
| S03 | 9 | Female | Unilateral | 4 | 2210 | No |
| S04 | 10 | Female | Bilateral | 4 | 2004 | Yes |
| S05 | 3 | Male | Bilateral | 4 | 1606 | No |
| S06 | 5 | Female | Bilateral | 4 | 1290 | No |
| S07 | 72 | Male | Unilateral | 3 | 1851 | Yes |
| S08 | 10 | Female | Bilateral | 3 | 1477 | No |
| S09 | 6 | Male | Bilateral | 3 | 1312 | Yes |
| S10 | 7 | Male | Unilateral | 4 | 1768 | No |
2.2 Drug Dosage Schedule
Carboplatin was delivered via intravenous (i.v.) administration at 3–4 week intervals. Six children received four courses of carboplatin and four children received three courses of carboplatin. The cumulative dose of carboplatin received by each child ranged from 1200–2210 mg/m2 (see Table 1). The children also received vincristine and topotecan or etoposide either concurrently with or previous to the carboplatin treatments. Although the children received multiple chemotherapy drugs, only carboplatin has well-documented ototoxic side effects. The main side effect of vincristine is neurotoxicity, and when given as a single chemotherapy agent, it is not thought to alter measurements of DPOAEs in children [23]. Primary side effects associated with topotecan and etoposide include myelosuppression, nausea, vomiting, and alopecia.
2.3 Ototoxicity Monitoring
Audiological tests were conducted at St. Jude Children’s Research Hospital. The tests were conducted by experienced pediatric audiologists. DPOAE tests in children < 12 months of age at the onset of treatment were conducted in a surgical suite. The older child was tested in a double-walled sound-treated booth. All of the children completed the monitoring protocol during a baseline evaluation and an interim evaluation. For nine of the 10 children, the baseline evaluation took place prior to chemotherapy treatment. In the remaining child, the baseline evaluation was conducted one week after the child received one course of carboplatin. The interim evaluation in all children was conducted following the course of carboplatin at the midpoint of the child’s chemotherapy regimen. During the baseline and interim evaluations, eight children were sedated during testing. One child was tested during natural sleep, and the remaining child (the oldest) was tested after completing pure-tone audiometry. In this child, hearing thresholds were obtained at the baseline and interim tests using a standard clinical procedure with a diagnostic audiometer. Middle-ear function was assessed in both ears of each child using tympanometers capable of obtaining measurements with 226- or 1000-Hz probe tones. Tympanometry results suggested normal middle-ear function bilaterally in the children at the time of the baseline and interim evaluations. This was indicated by the presence of single-peaked 226- and/or 1000-Hz tympanograms with a maximum admittance near 0 deca Pascals [24]. Measurements of DPOAEs were obtained from both right and left ears of each child with an otoacoustic emission analyzer (Otodynamics, ILO 296) interfaced with a laptop computer. The ILO probe was calibrated using the 1-cc cavity provided by the manufacturer prior to each baseline and interim test. The primary-tone stimuli for 2f1-f2 DPOAE measurements were generated by the ILO v6 software program at targeted sound pressure levels (SPL) of 65 dB SPL (L1) and 55 dB SPL (L2). In the case of one subject (S10), the L2 level was lowered to 45 dB SPL at the baseline and interim tests due to a technical error. The primary tones were stepped across an f2 frequency range of 793–7996 Hz at a fixed f2: f1 ratio of 1.22 and with a resolution of 3 points/octave. In order to assess the adequacy of the probe fit in the ear canal for the purposes of meeting targeted primary-tone levels, a checkfit procedure preceded actual measurements of DPOAEs. This procedure measures the ear-canal sound pressure at a distance from the tympanic membrane. It is recognized that in adults, setting of the primary-tone level may be influenced by standing waves, particularly at higher frequencies [25]. The checkfit procedure utilized in this study was limited in accounting for the influence of standing waves. When the checkfit procedure was completed, distortion-product grams (DP-grams) were obtained by plotting the levels (in dB SPL) of the 2f1-f2 DPOAEs as a function of f2 frequency. Estimates of the noise floor were made by determining the mean level of five frequency bins above and below the 2f1-f2 DPOAE frequency bin. No automated stopping rules were utilized, due to time constraints in many of the children who were under sedation at the time of the test. Instead, the tests were manually terminated once the DPOAE levels were determined to be stabilized through visual inspection of the DP-gram. In order to be counted as a valid response, the level of the DPOAE was required to exceed the mean level of the noise floor + 2 standard deviations by ≥ 6 dB at each f2 frequency. A minimum signal-to-noise ratio (SNR) of 6 dB is recommended when DPOAEs are used to monitor cochlear status over time [26].
2.4 Data Analysis
The data were analyzed using the Statistical Analysis Software (SAS) version 9.2. A significance level of 0.05 was adopted for all statistical tests, while multiple tests were adjusted using the Bonferroni correction. The DPOAE levels were averaged across right and left ears at each f2 frequency in children exhibiting valid DPOAEs in both ears, resulting in mean DPOAE levels. Wilcoxon signed-rank tests showed there was no significant difference in DPOAE levels between right and left ears. Therefore, the DPOAE levels were collapsed across ears in order to avoid increasing type I error due to lack of independence between ears according to the recommendation of Coren and Hakstian [27]. Wilcoxon signed-rank tests were conducted on mean DPOAE levels measured at the baseline and interim evaluations in order to determine if any significant differences were registered between these conditions. Since DPOAE levels can be altered due to changes in the level of primary-tone stimulation alone, it is important to recognize if primary-tone levels in dB SPL were similar at the baseline and interim evaluations. Mean primary-tone levels (L1 & L2) estimated in the ear canal at the baseline and interim evaluations were also evaluated with Wilcoxon signed-rank tests. Another factor that may influence DPOAE levels is the noise floor. In order to determine the similarity of noise floor levels (in dB SPL) at the baseline and interim evaluations, Wilcoxon signed-rank tests were conducted on mean noise floor levels.
In addition to mean data, potential ototoxic changes on an individual basis were examined by applying a criterion used in current practice at St. Jude Children’s Research Hospital. In comparing between baseline and interim tests, the criterion for ototoxic change was defined as a ≥ 6 dB decrease in DPOAE level at two or more adjacent f2 frequencies in a given ear where decreases in DPOAE levels were calculated by subtracting the interim DPOAE levels from the baseline DPOAE levels for each f2 frequency. Currently, there is no universal agreement on the criterion for ototoxic change using OAE tests. Sockalingam et al. [28] calculated minimal changes in DPOAE level that exceeded the limits of test-retest reliability at individual f2 frequencies in children aged 5–12 years. Based on their data, the average minimal change exceeding the limits of normal variation at four f2 frequencies between 2530–7029 Hz was 6.47 dB. This is reasonably close to the minimal value for ototoxic change selected in this investigation. The number of ears and children with both ears for which baseline DPOAEs met or exceeded the SNR criterion at each individual f2 frequency are listed in Table 2. As can be seen, the SNR criterion was met in limited numbers of children at the f2 frequencies of 793 Hz–2515 Hz. However, the criterion was met in the majority (>75%) of children at the f2 frequencies of 3174 Hz, 4004 Hz, 5042 Hz, 6348 Hz, and 7996 Hz. Wilcoxon signed-rank tests were conducted on mean DPOAE levels measured at the baseline and interim conditions at the 5 highest f2 frequencies (3174–7996 Hz). The Bonferroni correction was applied to adjust the level of significance for the number of tests conducted (0.05/5= 0.01).
Table 2.
The number of ears and children with both ears meeting the + 6 dB or greater DPOAE signal-to-noise criterion at the baseline evaluation for each f2 frequency.
| f2 Frequency (Hz) | 793 | 1001 | 1257 | 1587 | 2002 | 2515 | 3174 | 4004 | 5042 | 6348 | 7996 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ears | 3/20 | 3/20 | 3/20 | 11/20 | 14/20 | 15/20 | 17/20 | 18/20 | 19/20 | 16/20 | 18/20 |
| Children | 1/10 | 1/10 | 1/10 | 5/10 | 6/10 | 6/10 | 8/10 | 8/10 | 9/10 | 8/10 | 9/10 |
3. Results
The first aim of this study was to examine if mean DPOAE levels acquired before and after several courses of carboplatin chemotherapy were significantly different. The mean ± standard error of DPOAE levels at the baseline and interim evaluations are plotted as a function of f2 frequency in Figure 1. At the f2 frequency of 7996 Hz, DPOAE levels were reduced at the interim test compared to the baseline test. This observation was confirmed through statistical testing, as the results of the Wilcoxon signed-rank tests revealed that mean DPOAE levels at the baseline and interim evaluations were significantly different only at the f2 frequency of 7996 Hz (p=0.004) for 9 children with valid responses in both ears. Wilcoxon signed-rank tests were also conducted separately on mean L1 and L2 primary-tone levels at corresponding f2 frequencies from 3174–7996 Hz evaluated at the baseline and interim tests. There were no significant differences between L1 levels at the baseline and interim tests or between L2 levels at the baseline and interim tests. The mean noise floor levels at f2 frequencies from 3174–7996 Hz were not significantly different at the baseline and interim tests.
Figure 1.
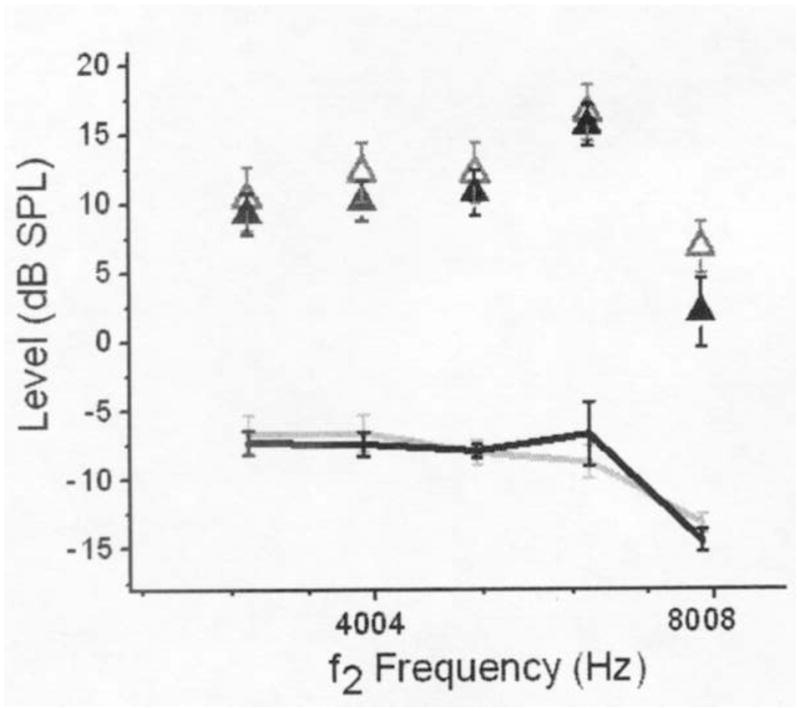
Mean DPOAE and noise levels (collapsed across ears) at the baseline and interim evaluations plotted as a function of f2 frequency. Only data from children with valid DPOAEs in both ears were included in mean calculations. Mean DPOAE levels are depicted with open (baseline) and filled (interim) triangles. Mean noise levels are depicted with light (baseline) and dark (interim) lines. Error bars represent ± 1 standard error of the mean.
The second aim of the study was to examine if criterion reductions in DPOAE levels were observed in individual children. Figures 2 and 3 illustrate decreases in DPOAE level across f2 frequency for left and right ears in each child. DPOAE level decreases ≥ 6 dB at two or more adjacent f2 frequencies in at least one ear were observed in four children (6/20 ears). Criterion DPOAE level decreases were observed in both ears of S02, the right ear of S04, both ears of S07, and the left ear of S09. At f2 frequencies where criterion changes in DPOAE level were observed in these children, the lowest DPOAE SNR was 12.3 dB. This suggested that DPOAE SNRs were sufficiently high so that the minimum criterion decrease of 6 dB could easily be detected at the f2 frequencies where the changes were observed. Three out of the four children identified with criterion changes in DPOAE level received cumulative dosages of carboplatin above 1800 mg/m2 at the time of the interim test (see Table 1). In one of these children (S07), behavioral hearing thresholds were obtained at the baseline and interim evaluations using a standard clinical procedure. These hearing thresholds are displayed in Table 3. As can be seen, hearing thresholds were at or better than 10 dB HL (dB re: normal hearing level) from 250–8000 Hz at the baseline test, and were at or better than 15 dB HL across the same frequency range at the interim test. On both occasions, the tympanograms from both ears of this subject were within normal limits. Figure 3 illustrates the decreases in DPOAE level seen in this subject. Decreases exceeding the 6 dB criterion were observed at adjacent f2 frequencies at or above 4004 Hz in both right and left ears. These findings suggest that reductions in DPOAE level have occurred prior to hearing loss being manifested on the audiogram in this child.
Figure 2.
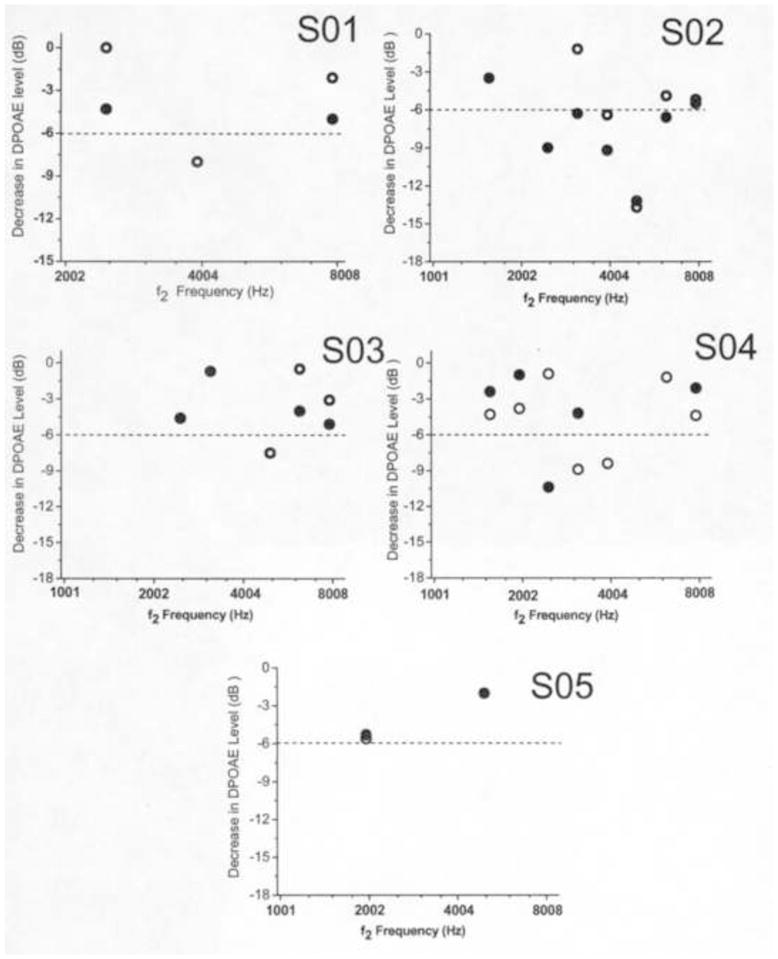
Scatterplot of DPOAE level decreases as a function of f2 frequency in subjects S01–S05. Data points representing DPOAEs not meeting the SNR criterion are not depicted. Open circles represent DPOAE data in right ears and closed circles represent DPOAE data in left ears. The dashed line represents the 6 dB criterion required for ototoxic change to be recognized.
Figure 3.
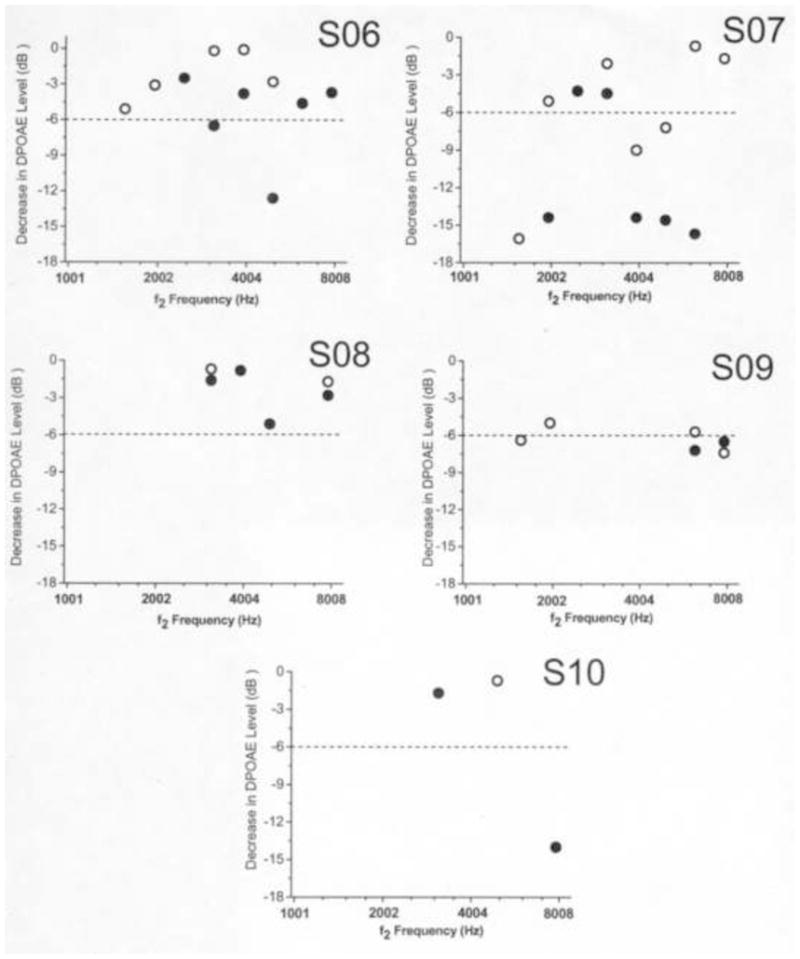
Scatterplot of DPOAE level decreases as a function of f2 frequency in subjects S06–S10. Data points representing DPOAEs not meeting the SNR criterion are not depicted. Open circles represent DPOAE data in right ears and closed circles represent DPOAE data in left ears. The dashed line represents the 6 dB criterion required for ototoxic change to be recognized.
Table 3.
Hearing thresholds in dB HL for subject S07 measured at the baseline evaluation and interim evaluation in the right and left ears.
| Frequency (Hz) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 250 | 500 | 1000 | 2000 | 3000 | 4000 | 6000 | 8000 |
| Right | 5 | 5 | 5 | 5 | 0 | 0 | 0 | 0 |
| Left | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Interim | 250 | 500 | 1000 | 2000 | 3000 | 4000 | 6000 | 8000 |
| Right | 15 | 10 | 15 | 15 | 5 | 0 | 0 | 0 |
| Left | 10 | 10 | 5 | 5 | 0 | 0 | 5 | 0 |
4. Discussion
The first aim of this pilot study was to examine if mean DPOAE levels obtained from the ears of children diagnosed with retinoblastoma during tests completed before and after several courses of carboplatin chemotherapy were significantly different. The results of Wilcoxon signed-rank tests indicated that mean DPOAE levels were significantly reduced only at the highest frequency tested (f2=7996 Hz). The change in DPOAE level seen at this frequency is not likely due to variation in primary-tone stimulation or noise floor levels at the baseline and interim tests, as no significant differences in these factors were observed between measurement periods. Another explanation for the significant decrease in DPOAE level at this frequency is that exposure to carboplatin resulted in a change in OHC function. It is well known that platinum compounds, such as carboplatin, initially affect OHC function in the basal turn of the cochlea [17,18]. When carboplatin induces hearing loss, it is usually manifested first at frequencies higher than 4000 Hz [9]. Since the children evaluated in this study received 3–4 courses of carboplatin, it is conceivable that initial changes in OHC function would be localized to higher f2 frequencies, resulting in a reduction of DPOAE levels. Further exploration of this hypothesis would be aided by examining DPOAEs acquired with primary tones higher than 8000 Hz. Although there is a considerable research effort aimed at evaluating high-frequency DPOAE tests, most of these studies have been conducted in adults with normal hearing thresholds at the conventional audiometric frequencies [29,30]. Evaluation of high-frequency DPOAE tests in normal-hearing infants and young children and the creation of a normative database would facilitate the clinical application of this method, particularly in pediatric populations exposed to ototoxic agents.
The second aim of this study was to examine if criterion changes in DPOAE level would be observed in individual children while they were receiving chemotherapy. Ototoxic changes in DPOAE levels were observed in four out of the 10 children monitored. There were two cases of unilateral retinoblastoma and two cases of bilateral retinoblastoma among these children. Their ages were 6, 8, 10, and 72 months at the onset of treatment. There did not appear to be any systematic influence of type of retinoblastoma or age at onset of treatment on observation of criterion reductions in DPOAE level. However, three of these children (S02, S04, S07) received cumulative carboplatin dosages above 1800 mg/m2 at the time of the interim test. Therefore, these higher cumulative carboplatin dosages appeared to be related to children being identified with ototoxic changes in DPOAE levels. In the oldest child (S07), criterion reductions in DPOAE levels were observed in both ears, even though hearing thresholds remained within normal limits bilaterally at the time of the interim test. The selection of the criterion defining ototoxic change for DPOAEs used in this study was arbitrary. Other investigators have recommended different criteria that define significant changes in DPOAE level. For example, Beattie et al. [31] found that changes in DPOAE levels across measurement conditions needed to exceed 7 dB in the 1000–4000 Hz range in order to be considered statistically significant. Reavis et al. [32] proposed that a DPOAE reduction of 4 dB or greater at adjacent f2 frequencies constituted an ototoxic change. However, both of these studies were conducted in adults, and the criterion selected for this study was more consistent with the limits exceeding normal variation in children identified by Sockalingam et al. [28]. In addition, the finding that three children receiving high cumulative dosages of carboplatin were identified as having ototoxic change in DPOAE levels was in agreement with previous research in animal models. Hofstetter et al. [17] found OHC loss and large reductions in DPOAE levels in chinchillas were only observed for high doses of carboplatin.
4.1 Limitations of the Study
There is some controversy in the scientific literature concerning the risk of ototoxic hearing loss from carboplatin in pediatric populations. Previous research has indicated a low incidence of ototoxicity in children with retinoblastoma receiving carboplatin chemotherapy [22]. The purpose of this study was to determine if DPOAE tests could provide evidence of ototoxic change in cochlear (OHC) function. This information is clinically important, as shifts in DPOAE levels have been shown to precede hearing threshold shifts at conventional audiometric frequencies in children receiving cisplatin chemotherapy [8]. The tests described in this report describe clinical findings at the midpoint of each child’s chemotherapy regimen, corresponding to 3–4 courses of carboplatin and other chemotherapy drugs. We elected to report the findings at this juncture, in an effort to determine if changes in DPOAE tests could be registered early in the course of therapy. While DPOAE level reductions were observed in some children at some frequencies, it is unclear if the children suffered hearing threshold shifts since for most of the children, behavioral hearing tests were not completed at the baseline and interim evaluations. The children described in this report continue to be monitored at St. Jude Children’s Research Hospital as they complete their therapy and will also receive long term follow-up testing. Longitudinal studies are planned that will investigate the incidence of ototoxic hearing loss due to carboplatin in children with retinoblastoma receiving treatment at St. Jude. The findings of this study will be of specific interest, insofar as the children identified with ototoxic change in DPOAE levels will be followed to determine if they eventually develop hearing loss on the audiogram. Our results, indicating that DPOAE levels were reduced in some children following 3–4 courses of carboplatin, contrasted with the findings of a previous investigation. Smits et al. [21] reported no signs of ototoxicity based on an audiometric testing protocol including DPOAE tests in 21 children with retinoblastoma receiving carboplatin. This disparity may have resulted from differences in susceptibility to carboplatin in the populations examined. We utilized an ototoxic criterion based on changes in DPOAE levels. Previous work has shown that examination of DPOAE levels may be used to detect subtle impairments in cochlear function that may not be revealed as hearing loss on the behavioral audiogram [33, 34]. Therefore, changes in DPOAE levels seen following carboplatin chemotherapy in some children in this study may signify an early warning sign of incipient OHC damage.
Another contentious issue involves the best method of monitoring ototoxicity in children receiving carboplatin chemotherapy. The findings of this study need to be interpreted cautiously, given the small sample of children examined. Evidence from some animal models suggests that carboplatin-induced hearing loss results from damage to IHCs, and that OHC damage occurs only from high-dose carboplatin administration. Therefore, detection of carboplatin ototoxicity by DPOAEs may be limited to children receiving higher doses of carboplatin. In infants and younger children receiving lower doses of carboplatin, the auditory brainstem response (ABR) may be more appropriate for monitoring these cases. In older children, pure-tone audiometry can replace ABR tests. However, even in older children, DPOAE tests should be included in the monitoring protocol as they are more sensitive to the initial effects of platinum compounds than are conventional pure-tone tests [8,20] and DPOAE tests can evaluate cochlear function at high frequencies where cochlear damage is first manifested. The detection of the onset of potential ototoxicity in children with retinoblastoma is a priority, given the fact that many of these children are diagnosed at an early age and already have visual impairments. If the onset of ototoxicity is detected early, the dosage of carboplatin or other drugs may be modified to prevent further cochlear deterioration. In cases where the drug dosage cannot be modified, early intervention programs can be planned to facilitate speech and language development.
5. Conclusions
For the conditions examined in this study:
Comparison between baseline and interim evaluations revealed significant decreases in mean DPOAE levels only at the highest f2 frequency tested. This may suggest impairment in OHC function at high frequencies where carboplatin ototoxicity is usually initially manifested.
On an individual basis, 4/10 children were identified with criterion changes in DPOAE levels. Three of these children received high cumulative dosages of carboplatin (above 1800 mg/m2).
DPOAE tests may provide important information in children receiving high doses of carboplatin.
Acknowledgments
We wish to thank Tammy Free and Vicki Given for their contributions to this project. Portions of this report were presented at the American Auditory Society meeting, March 5–7, 2009 in Scottsdale, Arizona and at the MidWinter Research Meeting of the Association for Research in Otolaryngology, February 6–10, 2010 in Anaheim, California. This work was supported in part by grants CA 21765 and CA 23099 from the U.S. Public Health Service and by the American Lebanese Syrian Associated Charities (ALSAC). The sponsors had no role in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Statement
Shaum P. Bhagat: none
Johnnie K. Bass: none
Stephanie T. White: none
Ibrahim Qaddoumi: none
Matthew W. Wilson: none
Jianrong Wu: none
Carlos Rodriguez-Galindo: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim JW, Abramson DH, Dunkel IJ. Current management strategies for intraocular retinoblastoma. Drugs. 2007;67:2173–2185. doi: 10.2165/00003495-200767150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975–2004. Br J Opthalmol. 2009;93:21–23. doi: 10.1136/bjo.2008.138750. [DOI] [PubMed] [Google Scholar]
- 3.Chintagumpala M, Chevez-Barrios P, Paysse EA, Plon SE, Hurwitz R. Retinoblastoma: review of current management. Oncologist. 2007;12:1237–1246. doi: 10.1634/theoncologist.12-10-1237. [DOI] [PubMed] [Google Scholar]
- 4.Lin P, O’Brien JM. Frontiers in the management of retinoblastoma. Am J Opthalmol. 2009;148:192–198. doi: 10.1016/j.ajo.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Galindo C, Wilson MW, Haik BG, Merchant TE, Billups CA, Shah N, et al. Treatment of intraocular retinoblastoma with vincristine and carboplatin. J Clin Oncol. 2003;21:2019–2025. doi: 10.1200/JCO.2003.09.103. [DOI] [PubMed] [Google Scholar]
- 6.Bacha DM, Caparros-Sison B, Allen JA, Walker R, Tan CT. Phase I study of carboplatin (CBDCA) in children with cancer. Cancer Treat Rep. 1986;70:865–869. [PubMed] [Google Scholar]
- 7.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 8.Knight KR, Kraemer DF, Winter C, Neuwelt EA. Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25:1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald MR, Harrison RV, Wake M, Bliss B, Macdonald RE. Ototoxicity of carboplatin: comparing animal and clinical models at the Hospital for Sick Children. J Otolaryngol. 1994;23:151–159. [PubMed] [Google Scholar]
- 10.Simon T, Hero B, Dupuis W, Selle B, Berthold F. The incidence of hearing impairment after successful treatment of neuroblastoma. Klin Padiatr. 2002;214:149–152. doi: 10.1055/s-2002-33179. [DOI] [PubMed] [Google Scholar]
- 11.Stern JW, Bunin N. Prospective study of carboplatin-based chemotherapy for pediatric germ cell tumors. Med Pediatr Oncol. 2002;39:163–167. doi: 10.1002/mpo.10134. [DOI] [PubMed] [Google Scholar]
- 12.Bertolini P, Lasalle M, Mercier G, Raquin MA, Izzi G, Corradini N, et al. Platinum compound-related ototoxicity in children: long term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26:649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 13.Dhooge I, Dhooge C, Geukens S, De Clerck B, De Vel E, Vinck BM. Distortion product otoacoustic emissions: an objective technique for the screening of hearing loss in children treated with platin derivatives. Int J Audiol. 2006;45:337–343. doi: 10.1080/14992020600582117. [DOI] [PubMed] [Google Scholar]
- 14.Parsons SK, Neault MW, Lehmann LE, Brennan LL, Eickhoff CE, Kretschmar CS, et al. Severe ototoxicity following carboplatin-containing conditioning regimen for autologous marrow transplantation for neuroblastoma. Bone Marrow Transplant. 1998;22:669–674. doi: 10.1038/sj.bmt.1701391. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Takeno S, Harrison RV, Ibrahim D, Wake M, Mount RJ. Cochlear function after selective inner hair cell degeneration induced by carboplatin. Hear Res. 1994;75:93–102. doi: 10.1016/0378-5955(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 17.Hofstetter P, Ding D, Powers N, Salvi R. Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction of distortion product otoacoustic emission amplitude in chinchillas. Hear Res. 1997;112:199–215. doi: 10.1016/s0378-5955(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 18.Bauer CA, Brozoski TJ. Cochlear structure and function after round window application of ototoxins. Hear Res. 2005;201:121–131. doi: 10.1016/j.heares.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Saito T, Saito H, Saito K, Wakui S, Manabe Y, Tsuda G. Ototoxicity of carboplatin in guinea pigs. Auris, Nasus, Larynx. 1989;16:13–21. doi: 10.1016/s0385-8146(89)80003-3. [DOI] [PubMed] [Google Scholar]
- 20.Stavroulaki P, Apostolopoulos N, Segas J, Tsakanikos M, Adamopoulos G. Evoked otoacoustic emissions-an approach for monitoring cisplatin induced ototoxicity in children. Int J Pediatr Otorhinolaryngol. 2001;59:47–57. doi: 10.1016/s0165-5876(01)00455-4. [DOI] [PubMed] [Google Scholar]
- 21.Smits C, Swen SJ, Theo Goverts S, Moll AC, Imhof SK, Schouten-van Meeteren AY. Assessment of hearing in very young children receiving carboplatin for retinoblastoma. Eur J Cancer. 2006;42:492–500. doi: 10.1016/j.ejca.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Jehanne M, Lumbroso-Le Rouic L, Savignoni A, Aerts I, Mercier G, Bours D, et al. Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatr Blood Cancer. 2009;52:637–643. doi: 10.1002/pbc.21898. [DOI] [PubMed] [Google Scholar]
- 23.Riga M, Psarommatis I, Korres S, Lyra C, Papadeas E, Varvutsi M, et al. The effect of treatment with vincristine on transient evoked and distortion product otoacoustic emissions. Int J Pediatr Otorhinolaryngol. 2006;70:1003–1008. doi: 10.1016/j.ijporl.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Alaerts J, Luts H, Woulters J. Evaluation of middle ear function in young children: clinical guidelines for the use of 226- and 1,000-Hz tympanometry. Otol Neurotol. 2007;28:727–732. doi: 10.1097/mao.0b013e3180dca1e5. [DOI] [PubMed] [Google Scholar]
- 25.Siegel JH. Ear-canal standing waves and high-frequency sound calibration using otoacoustic emission probes. J Acoust Soc Am. 1994;95:2589–2597. [Google Scholar]
- 26.Wagner W, Heppelmann G, Vonthein R, Zenner HP. Test-retest repeatability of distortion product otoacoustic emissions. Ear Hear. 2008;29:378–391. doi: 10.1097/AUD.0b013e31816906e7. [DOI] [PubMed] [Google Scholar]
- 27.Coren A, Hakstian AR. Methodological implications of interaural correlation: count heads not ears. Percept Psychophys. 1990;48(3):291–294. doi: 10.3758/bf03211533. [DOI] [PubMed] [Google Scholar]
- 28.Sockalingam R, Choi JL, Choi D, Kei J. Test-retest reliability of distortion-product otoacoustic emissions in children with normal hearing: a preliminary study. Int J Audiol. 2007;46:351–354. doi: 10.1080/14992020701311168. [DOI] [PubMed] [Google Scholar]
- 29.Dreisbach LE, Siegel JH. Level dependence of distortion-product otoacoustic emissions measured at high frequencies in humans. J Acoust Soc Am. 2005;117:2980–2988. doi: 10.1121/1.1880792. [DOI] [PubMed] [Google Scholar]
- 30.Dreisbach LE, Long KM, Lees LE. Repeatability of high-frequency distortion-product emissions in normal-hearing adults. Ear Hear. 2006;27:466–479. doi: 10.1097/01.aud.0000233892.37803.1a. [DOI] [PubMed] [Google Scholar]
- 31.Beattie RC, Kenworthy OT, Luna CA. Immediate and short-term reliability of distortion-product otoacoustic emissions. Int J Audiol. 2003;42:348–354. doi: 10.3109/14992020309101328. [DOI] [PubMed] [Google Scholar]
- 32.Reavis KM, Phillips DS, Fausti SA, Gordon JS, Helt WJ, Wilmington D, et al. Factors affecting sensitivity of distortion-product otoacoustic emissions to ototoxic hearing loss. Ear Hear. 2008;29:875–893. doi: 10.1097/AUD.0b013e318181ad99. [DOI] [PubMed] [Google Scholar]
- 33.Bhagat SP, Davis AM. Modification of otoacoustic emissions following ear-level exposure to MP3 player music. Int J Audiol. 2008;47:751–760. doi: 10.1080/14992020802310879. [DOI] [PubMed] [Google Scholar]
- 34.Bhagat S. Analysis of distortion product otoacoustic emission spectra in normal-hearing adults. Am J Audiol. 2009;18:60–68. doi: 10.1044/1059-0889(2009/08-0025). [DOI] [PubMed] [Google Scholar]


