Abstract
Abstract After stage 1 palliation (S1P) with a Norwood operation, infants commonly experience growth failure during the initial interstage period. Growth failure during this high-risk period is associated with worse outcomes. This study evaluated the growth patterns of patients enrolled in the authors’ interstage home-monitoring program (HMP), which uses a multidisciplinary team approach to nutrition management. From 2000 to 2009, 148 infants were enrolled in the HMP after S1P. Families recorded daily weights during the interstage period and alerted the interstage monitoring team about protocol violations of nutritional goals. Interstage monitoring and inpatient data from the S1P hospitalization were reviewed to identify risk factors for poor growth. Growth outcomes were compared with published norms from the Centers for Disease Control. Interstage survival for patients in the HMP was 98 % (145/148). Growth velocity during the interstage period was 26 ± 8 g/day. The weight-for-age z-scores decreased from birth to discharge after S1P (−0.4 ± 0.9 to −1.3 ± 0.9; p < 0.001) but then increased during the interstage period to the time of S2P (−0.9 ± 1; p < 0.001). The factors associated with improved growth during the interstage period included male gender, greater birth weight, full oral feeding at S1P discharge, and a later birth era. After S1P, infants enrolled in an HMP experienced normal growth velocity during the interstage period. Daily observation of oxygen saturation, weight change, and enteral intake together with implementation of a multidisciplinary feeding protocol is associated with excellent interstage growth and survival.
Keywords: Congenital, Growth, Heart defects, Home-monitoring program, Interstage growth, Norwood operation
Growth failure in infants with congenital heart disease (CHD) is common, proving to be especially problematic among infants undergoing palliation for single-ventricle anatomy. Normal infant growth is an important marker of well-being, and for patients with CHD failure to thrive can be an early clinical sign of physiologic decompensation. Patients with single-ventricle anatomy and ductal-dependent blood flow undergo staged palliation with a series of operations including the initial Norwood operation, or stage 1 palliation (S1P), followed by bidirectional Glenn (BDG), or stage 2 palliation (S2P), at the age of 3 to 6 months and culminating with Fontan completion at the age of 2 to 4 years [4, 19, 30].
Despite improved overall survival, infants undergoing S1P continue to experience up to a 20 % rate of interstage mortality, defined as death after S1P discharge and before a planned S2P [15, 21, 26, 32]. Failure to thrive among single-ventricle patients has been associated with worse outcomes at both S2P and Fontan completion [1, 3, 9, 34]. Given the impact of the interstage period on overall outcomes and its importance in setting the stage for subsequent palliative surgeries, ensuring normal infant growth has become a priority of single-ventricle management during this critical period.
Despite the importance of growth failure in infants with CHD, few studies have demonstrated interventions that improve growth in this high-risk population. In addition, there is no consensus on standardized management of feeding and nutrition for patients with CHD, including infants undergoing staged palliation [20, 36]. Published reports describing individual institutional approaches to nutrition for this population are available, but none have demonstrated improved interstage growth outcomes in association with these feeding protocols [8, 13, 35].
After the introduction of a quality improvement initiative to reduce interstage mortality at Children’s Hospital of Wisconsin, we identified early infant growth failure as a modifiable risk factor [17]. We subsequently integrated a nutritional program into the interstage home-monitoring program (HMP) and have continued to reevaluate and refine our approach over time. In this report, we describe the interstage growth patterns of infants after S1P who were managed with standardized nutritional support as part of an HMP and investigate risk factors for poor growth.
Patients and Methods
Patients
The Children’s Hospital of Wisconsin Human Research Review Board approved a review of prospective perioperative and interstage databases for this observational study of interstage growth. Informed parental consent was obtained for all the study subjects. During the study period (October 2000 through December 2009), 194 patients underwent S1P, with 93 % (180/194) S1P survival (Fig. 1). Of those surviving S1P, 16 % (29/180) remained inpatient during the interstage period, and 82 % (148/180) were enrolled in the HMP at the time of S1P discharge. Three additional patients survived to S1P hospital discharge but moved out of state and were not enrolled in the HMP. The 29 patients who remained inpatient between S1P and S2P were excluded from this analysis. Of the 148 patients discharged to home after S1P, 145 (98 %) survived to S2P and were included in the analysis.
Fig. 1.

Patient population. HMP home monitoring program, S1P stage 1 palliation, S2P stage 2 palliation
HMP and Nutritional Support
Since October 2000, patients discharged home after S1P have been enrolled in an HMP designed to reduce interstage mortality [17, 18]. These patients have been required to consume a minimum of 110 kcal/kg/day and to demonstrate adequate weight gain in preparation for hospital discharge. If a patient is unable to meet these requirements with oral intake, a surgical gastrostomy tube is placed. Before discharge, parents are trained to obtain and record daily oxygen saturation, weight, and volume of enteral intake. Parents also are instructed to notify the HMP team for the following breaches in nutritional criteria: enteral intake of less than 100 ml/kg/day, weight loss exceeding 30 g in a day, and failure to gain at least 20 g over a 3-day period.
Failure to meet the criteria triggers evaluation by the multidisciplinary team. When a breach of HMP criteria is limited to a failure to gain weight without concurrent symptoms of progressive heart failure, anatomic problems, or intercurrent illness, adjustments in the nutritional plan are made. The HMP team performs routine nutritional assessments during interstage phone communications and at interstage clinic visits every 2 to 4 weeks.
Data Collection
Interstage growth velocity, anthropometric measures at all study time points (birth, hospital discharge after S1P, and admission for S2P), and risk factors for growth failure were collected. Demographic data included sex, gestational age, birth weight, and cardiac diagnosis. High-risk patients were defined as those having prematurity (gestational age <37 weeks), low birth weight (<2.5 kg) or the presence of a major extracardiac malformation or known genetic syndrome. Cardiac diagnoses were categorized as defined by the International Working Group for Mapping and Coding of Nomenclatures for Paediatric and Congenital Heart Disease, the Nomenclature Working Group [41]. In addition, the presence of aortic atresia and shunt type (modified Blalock-Taussig shunt vs right ventricle-to-pulmonary artery shunt) were recorded. Perioperative data included age at S1P, time to chest closure, ventilator days, need for extracorporeal support, and hospital length of stay. Additional hospital data included the need for gastrostomy tube placement and the presence of vocal cord paralysis demonstrated by direct laryngoscopy. Laryngoscopy was not performed routinely but rather when vocal cord dysfunction was suspected. Breach of weight gain and enteral intake criteria were collected. Daily interstage weights recorded by families and all weights obtained at clinic visits and during inpatient hospitalizations were recorded. Weight-for-age z-scores (WAZ) were calculated at birth, at S1P discharge, and at S2P using Centers of Disease Control (CDC) standards [31]. Interstage growth velocity was reported as grams per day and calculated as S2P weight - S1P discharge weight/age at S2P - age at S1P discharge. Growth failure was defined as WAZ less than −2 at S2P or an interstage growth velocity of less than 20 g/day.
Statistical Analysis
Data are presented as means ± standard deviations, medians with ranges, and frequencies as appropriate. Demographic, anatomic, and surgical variables were initially tested in a univariate fashion to assess their association with the following growth outcomes: weight gain (g/day) during the interstage period and WAZ at the time of S2P. Variables found to impact growth in the univariate analysis then were entered into a multivariable model. A random regression model was used for weight with a linear curve fitted, in which age was the independent variable and sex, need for a gastrostomy tube, high-risk status, and birth year were covariates. An unstructured variance covariance matrix was used. A general linear model was used in the multivariable analysis of each of the two growth outcomes. The independent variables were birth weight, sex, birth era, extracardiac malformations, length of hospital stay, gestational age, vocal cord paralysis, open chest days, need for a gastrostomy tube, and days of intubation. Stepwise selection was used. Statistical analyses were performed using SAS version 9.2 (Cary, NC, USA).
Results
Detailed perioperative and interstage growth data including 6,017 weight observations are available for the 145 patients included in this analysis. Patient and diagnostic characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Variable |
n = 145 n (%) |
|---|---|
| Male gender | 87 (60) |
| Birth weight (kg) | 3.2 ± 0.5 |
| Gestational age (weeks) | 38.3 ± 1.4 |
| High risk | 32 (22) |
| Extracardiac | 16 (11) |
| GA<37 weeks | 14 (10) |
| BW<2.5 kg | 8 (6) |
| HLHS | 122 (84) |
| Aortic atresia | 80 (55) |
| Shunt type (BTS) | 91 (63) |
| Age at S1P: days (range) | 6 (2–64) |
| Hospital LOS: days (range) | 29 (9–113) |
| Intubation days: n (range) | 6 (2–39) |
| Reoperation | 14 (10) |
| Shunt revision | 10 (7) |
| Days of open chest: n (range) | 3 (1–26) |
| ECMO postop | 9 (6) |
| Diaphragm paresis | 5 (3) |
| Vocal cord paresis | 44 (30) |
| Age at S2P: months (range) | 3.8 (1.6–9.1) |
GA gestational age, BW birth weight, HLHS hypoplastic left heart syndrome, BTS Blalock-Taussig shunt, S1P stage 1 palliation, LOS length of stay, ECMO extracorporeal membrane oxygenation, postop postoperatively, S2P stage 2 palliation
All the patients underwent an initial Norwood operation. From 2000 to 2003, the modified Blalock-Taussig shunt was uniformly used. The right ventricle-to-pulmonary artery conduit was first used at our institution in 2003. At S2P, the patients had a mean age of 3.8 months (range, 1.6–9.1 months) and a mean weight of 5.68 ± 0.7 kg. Survival to hospital discharge after S2P for this cohort was 100 %.
Growth Outcomes and Feeding Data
The mean WAZ was −0.4 ± 0.9 at birth and decreased to −1.3 ± 0.9 at S1P discharge. However, the WAZ showed an improvement of 0.4 during the interstage period, to −0.9 ± 1 at the time of S2P (Fig. 2). The interstage growth velocity of the entire cohort was 26 ± 8 g/day. Overall, 124 (86 %) of the 145 patients had a WAZ more positive −2 at S2P, and 110 (76 %) of the 145 patients achieved an interstage weight gain of more than 20 g/day. The growth pattern during the interstage period is described by the linear fit curve equation (weight = 3.06 + 0.02 × age) and parallels normal infant growth (Fig. 3a).
Fig. 2.
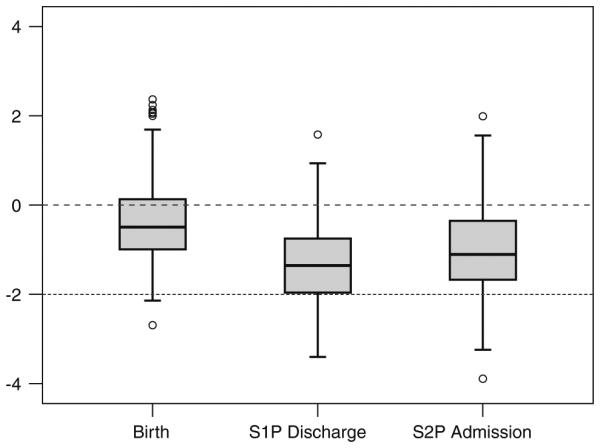
Weight-for-age z-score (WAZ) at birth, at discharge after S1P, and at S2P admission. Dashed line represents normal weight (WAZ = 0), and dotted line represents growth failure (WAZ = 2)
Fig. 3.
Interstage growth velocity. a Study cohort versus Centers for Disease Control (CDC) normal growth. b Patients with a gastrostomy tube (GT) versus no GT. c Birth era comparison. G-tube, gastrostomy tube
Further subgroup comparisons demonstrated greater growth velocity for the patients fed entirely orally at S1P discharge compared with those who had gastrostomy tubes (27.8 vs 21.8 g/day) (Fig. 3b) as well as improved growth in the more recent era, (26.5 vs 22.9 g/day) (Fig. 3c). The nutritional variables for this cohort are shown in Table 2. The majority of the patients required fortified breast milk or infant formulas to achieve feeding goals.
Table 2.
Feeding and interstage variables
| Variable |
n = 145 n (%) |
|---|---|
| GT placed | 41 (28) |
| Kcal/kg/day at D/C | 117 ± 14 |
| Caloric density (kcal/oz) | 26.6 ± 2.1 |
| Any HMP violation | 89 (61) |
| Interstage weight gain (g/day) | 26 ± 8 |
GT gastrostomy tube, D/C discharge, HMP home-monitoring program
During the study period, 89 (61 %) of the 145 patients experienced HMP protocol violations resulting in evaluation by the HMP team, with 46 (52 %) of 89 patients isolated to a feeding or growth problem resulting in nutritional intervention. These interventions included increasing the volume or caloric density of formula, modifying formula type, admission for acute weight loss or dehydration, or interval placement of either a gastrostomy tube or a nasogastric tube.
Risk Factors for Growth Failure
Table 3 lists candidate risk factors for poor interstage growth velocity and lower WAZ at S2P. Multivariable modeling identified only the presence of a gastrostomy tube as an independent risk factor for lower growth velocity, whereas lower birth weight, female gender, earlier birth era, and the presence of gastrostomy tubes were risk factors for lower WAZ at S2P.
Table 3.
Factors associated with interstage growth failure
| Category | Univariable |
Multivariable |
||
|---|---|---|---|---|
| (g/day) | WAZ at BDG | (g/day) | WAZ at BDG | |
| Patient characteristics | ||||
| Gender | 0.009 | 0.45 | 0.004 | |
| Birth weight | 0.14 | <0.001 | <0.0001 | |
| Birth era | 0.2 | 0.004 | <0.0001 | |
| GA | 0.6 | 0.2 | ||
| High risk | 0.16 | 0.1 | ||
| GA<37 weeks | 0.9 | 0.52 | ||
| BW<2.5 kg | 0.5 | 0.04 | ||
| Extracardiac | 0.04 | 0.02 | ||
| Anatomic charactersitics | ||||
| HLHS | 0.73 | 0.38 | ||
| Aortic atresia | 0.79 | 0.42 | ||
| Shunt type | 0.14 | 0.16 | ||
| Hospital and postoperative variables | ||||
| Age at surgery (in days) | 0.24 | 0.83 | ||
| Norwood LOS | <0.001 | 0.11 | ||
| Intubation days | 0.04 | 0.43 | ||
| Reoperation | 0.39 | 0.55 | ||
| Days of open chest | 0.04 | 0.06 | ||
| ECMO | 0.37 | 0.21 | ||
| Diaphragm paresis | 0.5 | 0.53 | ||
| Vocal cord paresis | 0.16 | 0.62 | ||
| GT placed | <0.001 | 0.03 | <0.0001 | 0.002 |
| Any interstage event | 0.9 | 0.22 | ||
GA gestational age, BW birth weight, HLHS hypoplastic left heart syndrome, LOS hospital length of stay, ECMO extracorporeal membrane oxygenation, GT gastrostomy tube
Discussion
This is the first report of a structured nutritional program resulting in normal infant growth in the single-ventricle population. Failure to thrive has been a common finding in previous studies of growth among single-ventricle infants, with worsening of WAZ during the early interstage period and then catch-up growth after S2P through the time of Fontan [2, 9, 10, 12, 23, 33, 34, 39, 42].
In contrast to previous studies, our data demonstrate that catch-up growth can begin in the early interstage period with targeted nutritional intervention. To achieve a normal daily weight gain of 20 to 30 g/day in this age group [11], the patients in our cohort consumed at S1P discharge an average of 117 ± 14 kcal/kg/day of fortified breast milk or infant formula that had an average caloric density of 26.6 kcal/oz, with 28 % requiring supplemental gastrostomy tube feeding.
Failure to thrive in infants with CHD results from inadequate energy intake, excess energy requirements, or both. Multiple factors are involved including an elevated metabolic rate associated with heart failure, critical illness, surgical stress, cyanosis, genetic syndromes, gastrointestinal complications, altered intestinal circulatory properties, and oral–motor dysfunction [24, 27, 29, 38].
To establish adequate growth, the most severely affected infants may require more than 150 kcal/kg/day [37]. The single-ventricle population is at particularly high risk for feeding problems and gastrointestinal complications. Altered gastrointestinal perfusion before and after S1P may contribute to a high incidence of feeding intolerance and continued risk of gastrointestinal complications [22]. After S1P, patients continue to experience cyanosis and volume overload, both of which contribute to heart failure and an imbalance in energy utilization. This population also may experience a variety of comorbidities, both congenital (genetic syndromes, aero-digestive abnormalities) and acquired (vocal cord paresis, gastroesophageal reflux), which may influence feeding and nutrition.
Adequate growth and nutritional status are particularly relevant for single-ventricle patients undergoing staged palliation and may be important for surgical risk stratification and long-term outcomes. In a heterogeneous cohort of 100 consecutive infants who underwent a bidirectional Glenn, 89 % of the patients showed failure to thrive by CDC criteria, and a lower WAZ at the time of surgery predicted a longer hospital stay as well as a trend toward higher rates of postoperative complications [1].
Similarly, in another series of 50 patients who had a median WAZ consistent with failure to thrive (less than −2), those with worse growth had evidence of a more complex postoperative course and more evidence of heart failure [23]. Although growth failure was uncommon in our series (86 % had a WAZ exceeding −2, and 76 % had a growth velocity exceeding 20 g/day), it is important to note that we did not assess the interaction of interstage growth with outcomes other than survival at S2P in this cohort.
Previously reported risk factors for lower WAZ at the time of S2P include lower birth weight, feeding difficulties, and older age at the time of S2P [1, 42]. The finding in the current cohort that gastrostomy tube placement relates to poor growth is likely a surrogate for underlying pathology including overall severity of illness, degree of underlying cardiopulmonary insufficiency, or the presence of gastrointestinal pathology. Notably, although 30 % of the patients in this cohort were found to have vocal cord paralysis, less than half these patients required placement of a gastrostomy tube. Investigation and management of vocal cord pathology included postoperative feeding and swallowing evaluation by dedicated speech pathologists for all the patients undergoing S1P, with laryngoscopy performed for those with an abnormal bedside evaluation. After a diagnosis of vocal cord pathology, multiple attempts were made to achieve safe and effective oral feeding before committing patients to gastrostomy. An alternate explanation for the finding of reduced growth in tube-fed patients may relate to management strategies. These patients often experience multiple periods of halted enteral nutrition around various studies and surgeries as well as multiple failed oral feeding trials before they are committed to a surgical enteral feeding tube. Additionally, in contrast to the patient feeding ad lib, patients who are wholly tube fed require active advancement of feed volume to maintain adequate caloric intake over time.
In our analysis, we dichotomized birth era into two periods: 2000–2004 versus 2005–2009. The finding of improved growth in the later era may reflect the confluence of multiple improvements as the quality initiative of the HMP has developed. Notably, during the most recent era, nutrition criteria were refined, an inpatient feeding protocol was developed, and a registered dietician was consistently involved in the multidisciplinary management of these patients during inpatient and outpatient visits.
Although patients with the right ventricle-to-pulmonary artery conduit have evidence of improved gastrointestinal perfusion, this has not translated into improved growth. This includes the current cohort, which showed no difference in growth between shunt types [22].
Nutritional interventions have been proved effective in various pediatric populations and can have an impact on long-term outcomes, including CHD [7, 35, 40]. In verylow-birth-weight babies, poor growth velocity during the initial inpatient stay has a significant impact on growth and neurodevelopmental outcome 18 to 22 months later [14]. However, when longer-term outcomes are measured, there is evidence that reestablishing a normal growth curve in high-risk patients results in improvement of this outcome gap [5, 16].
After S1P, postoperative feeding protocols have been associated with reduced time to achievement of goal calories, a reduced hospital stay, and a lower rate of necrotizing enterocolitis [8, 13]. In a study of patients with unrepaired CHD enrolled in an intensive multidisciplinary nutrition protocol, weight-for-height z-scores improved from −1.17 before intervention to –0.3 after intervention [6]. In a previous report of interstage outcomes, we found that weight gain after S1P can parallel normal infant growth but typically plateaus after 150 days despite standard nutrition management [18].
The aforementioned investigations highlight the importance of growth as a measure of physiologic vulnerability in infants with underlying heart failure and clarify the benefit of a standardized nutrition protocol for high-risk patients. Whereas intensive nutrition interventions may overcome growth failure due to inadequate intake or mildly increased energy requirements, for patients with significant heart failure, the salutary effects of nutritional intervention are likely to be limited. Therefore, patients who fail to thrive despite standard nutritional management in the HMP require more intense medical and hemodynamic evaluation and consideration for early cardiac intervention.
Although the focus of this study was the interstage period, we found that the early postoperative growth trend (S1P to S1P discharge) is consistent with the early growth pattern described in a number of previous studies, with near-normal birth weights but a significant drop in WAZ at the time of discharge after S1P [2, 12, 23, 28]. In the immediate postoperative period after S1P, patients may be hypermetabolic, with a negative caloric and protein balance [25]. A longer hospital stay after S1P generally is associated with poor outcomes and may be a surrogate for patient complexity. Single-ventricle patients with a longer hospital stay have a lower WAZ at S1P discharge [12, 23, 28], and existing nutrition intervention studies have demonstrated the ability of aggressively initiated enteral nutrition to shorten the hospital stay without increasing morbidity [8, 13]. Given the potential impact on long-term outcomes, further investigation of postoperative inpatient growth failure is indicated.
Conclusion
Infants enrolled in an interstage HMP after stage 1 palliation for single-ventricle heart disease experienced normal growth (26 ± 8 g/day) during the interstage period. The majority of the patients experienced catch-up growth during the interstage period, with a positive change in WAZ of ±0.4. Greater interstage growth was observed in patients who were male, had a higher birth weight, were fed exclusively by mouth, and were born in the more recent era. Our results demonstrate that normal infant growth velocity can be achieved in this high-risk population when intensive multidisciplinary nutritional intervention is used in the setting of an interstage HMP.
Contributor Information
David A. Hehir, Division of Pediatric Critical Care, Children’s Hospital of Wisconsin, Medical College of Wisconsin, 9000 West Wisconsin Avenue, Milwaukee, WI 53201, USA Division of Pediatric Cardiology, Children’s Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI, USA.
Nancy Rudd, Division of Pediatric Cardiology, Children’s Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI, USA.
Julie Slicker, Division of Clinical Nutrition, Children’s Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI, USA.
Kathleen A. Mussatto, Division of Pediatric Cardiology, Children’s Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI, USA
Pippa Simpson, Division of Quantitative Health Services, Children’s Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI, USA.
Shun-Hwa Li, Division of Pediatric Cardiology, Children’s Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Quantitative Health Services, Children’s Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI, USA.
Michele A. Frommelt, Division of Pediatric Cardiology, Children’s Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI, USA
James S. Tweddell, Division of Cardiothoracic Surgery, Children’s Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI, USA
Nancy S. Ghanayem, Division of Pediatric Critical Care, Children’s Hospital of Wisconsin, Medical College of Wisconsin, 9000 West Wisconsin Avenue, Milwaukee, WI 53201, USA
References
- 1.Anderson JB, Beekman RH, III, Border WL, Kalkwarf HJ, Khoury PR, Uzark K, et al. Lower weight-for-age z-score adversely affects hospital length of stay after the bidirectional Glenn procedure in 100 infants with a single ventricle. J Thorac Cardiovasc Surg. 2009;138:397–404. doi: 10.1016/j.jtcvs.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JB, Beekman RH, III, Eghtesady P, Kalkwarf HJ, Uzark K, Kehl JE, et al. Predictors of poor weight gain in infants with a single ventricle. J Pediatr. 2010;157:407–413. doi: 10.1016/j.jpeds.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JB, Kalkwarf HJ, Kehl JE, Eghtesady P, Marino BS. Low weight-for-age z-score and infection risk after the Fontan procedure. Ann Thorac Surg. 2011;91:1460–1466. doi: 10.1016/j.athoracsur.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Bartmus DA, Driscoll DJ, Offord KP, Humes RA, Mair DD, Schaff HV, et al. The modified Fontan operation for children less than 4 years old. J Am Coll Cardiol. 1990;15:429–435. doi: 10.1016/s0735-1097(10)80073-7. [DOI] [PubMed] [Google Scholar]
- 5.Belfort MB, Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Infant growth and child cognition at 3 years of age. Pediatrics. 2008;122:e689–e695. doi: 10.1542/peds.2008-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benzecry SG, Leite HP, Oliveira FC, Santana e Meneses JF, de Carvalho WB, Silva CM. Interdisciplinary approach improves nutritional status of children with heart diseases. Nutrition. 2008;24:669–674. doi: 10.1016/j.nut.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Bougle DI, Kahyat M, Duhamel AJF. Nutritional treatment of congenital heart disease. Arch Dis Child. 1986;61:799–801. doi: 10.1136/adc.61.8.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braudis NJ, Curley MA, Beaupre K, et al. Enteral feeding algorithm for infants with hypoplastic left heart syndrome poststage I palliation. Pediatr Crit Care Med. 2009;10:460–466. doi: 10.1097/PCC.0b013e318198b167. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MI, Bush DM, Ferry RJ, Jr, Spray TL, Moshang T, Jr, Wernovsky G, et al. Somatic growth failure after the Fontan operation. Cardiol Young. 2000;10:447–457. doi: 10.1017/s1047951100008118. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MS, Zak V, Atz AM, Printz BF, Pinto N, Lambert L, Pemberton V, Li JS, Margossian R, Dunbar-Masterson C, McCrindle BW. Anthropometric measures after Fontan procedure: implications for suboptimal functional outcome. Am Heart J. 2010;160:1092–1098. doi: 10.1016/j.ahj.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danner E, Joeckel R, Michalak S, Phillips S, Goday P. Weight velocity in infants and children. Nutr Clin Pract. 2009;24:76–79. doi: 10.1177/0884533608329663. [DOI] [PubMed] [Google Scholar]
- 12.Davis D, Davis S, Cotman K, Worley S, Londrico D, Kenny D, et al. Feeding difficulties and growth delay in children with hypoplastic left heart syndrome versus d-transposition of the great arteries. Pediatr Cardiol. 2008;29:328–333. doi: 10.1007/s00246-007-9027-9. [DOI] [PubMed] [Google Scholar]
- 13.del Castillo SL, McCulley ME, Khemani RG, et al. Reducing the incidence of necrotizing enterocolitis in neonates with hypoplastic left heart syndrome with the introduction of an enteral feed protocol. Pediatr Crit Care Med. 2010;11:373–377. doi: 10.1097/PCC.0b013e3181c01475. [DOI] [PubMed] [Google Scholar]
- 14.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole K. Growth outcomes of extremely low-birth-weight infants. Pediatrics. 2006;117:1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 15.Fenton KN, Siewers RD, Rebovich B, Pigula FA. Interim mortality in infants with systemic-to-pulmonary artery shunts. Ann Thorac Surg. 2003;76:152–156. doi: 10.1016/s0003-4975(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 16.Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009;123:e101–e109. doi: 10.1542/peds.2008-1352. [DOI] [PubMed] [Google Scholar]
- 17.Ghanayem NS, Hoffman GM, Mussatto KA, Cava JR, Frommelt PC, Rudd NA, Steltzer MM, Bevandic SM, Frisbee SS, Jaquiss RD, Litwin SB, Tweddell JS. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg. 2003;126:1367–1377. doi: 10.1016/s0022-5223(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 18.Ghanayem NS, Tweddell JS, Hoffman GM, Mussatto K, Jaquiss RD. Optimal timing of the second stage of palliation for hypoplastic left heart syndrome facilitated through home monitoring, and the results of early cavopulmonary anastomosis. Cardiol Young. 2006;16:61–66. doi: 10.1017/S1047951105002349. [DOI] [PubMed] [Google Scholar]
- 19.Glenn WW. Superior vena cava–pulmonary artery shunt. Ann Thorac Surg. 1989;47:62–64. doi: 10.1016/0003-4975(89)90238-5. [DOI] [PubMed] [Google Scholar]
- 20.Golbus JR, Wojcik BM, Charpie JR, Hirsch JC. Feeding complications in hypoplastic left heart syndrome after the Norwood procedure: a systematic review of the literature. Pediatr Cardiol. 2011;32:539–552. doi: 10.1007/s00246-011-9907-x. [DOI] [PubMed] [Google Scholar]
- 21.Hehir DA, Dominguez TE, Ballweg JA, Ravishankar C, Marino BS, Bird GL, et al. Risk factors for interstage death after stage 1 reconstruction of hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg. 2008;136:94–99. doi: 10.1016/j.jtcvs.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JN, Ansong AK, Li JS, Xu M, Gorentz J, Hehir DA, del Castillo SL, Lai WW, Uzark K, Pasquali SK. Celiac artery flow pattern in infants with single right ventricle following the Norwood procedure with a modified Blalock-Taussig or right ventricle to pulmonary artery shunt. Pediatr Cardiol. 2011;32:479–486. doi: 10.1007/s00246-011-9906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition. 2006;22:237–244. doi: 10.1016/j.nut.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Leite HP, Fisberg M, Novo NF, Nogueira EB, Ueda IK. Nutritional assessment and surgical risk markers in children submitted to cardiac surgery. Sao Paulo Med J. 1995;113:706–714. doi: 10.1590/s1516-31801995000100008. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Zhang G, Herridge J, Holtby H, Humpl T, Redington AN, et al. Energy expenditure and caloric and protein intake in infants following the Norwood procedure. Pediatr Crit Care Med. 2008;9:55–61. doi: 10.1097/01.PCC.0000298756.82286.23. [DOI] [PubMed] [Google Scholar]
- 26.Mahle WT, Spray TL, Gaynor JW, Clark BJ., III Unexpected death after reconstructive surgery for hypoplastic left heart syndrome. Ann Thorac Surg. 2001;71:61–65. doi: 10.1016/s0003-4975(00)02324-9. [DOI] [PubMed] [Google Scholar]
- 27.Medoff-Cooper B, Naim M, Torowicz D, Mott A. Feeding, growth, and nutrition in children with congenitally malformed hearts. Cardiol Young. 2010;20:149–153. doi: 10.1017/S1047951110001228. [DOI] [PubMed] [Google Scholar]
- 28.Medoff-Cooper B, Irving SY, Marino BS, Garcia-Espana JF, Ravishankar C, Bird GL, et al. Weight change in infants with a functionally univentricular heart: from surgical intervention to hospital discharge. Cardiol Young. 2010;12:1–9. doi: 10.1017/S104795111000154X. [DOI] [PubMed] [Google Scholar]
- 29.Menon G, Poskitt EM. Why does congenital heart disease cause failure to thrive? Arch Dis Child. 1985;60:1134–1139. doi: 10.1136/adc.60.12.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med. 1983;308:23–26. doi: 10.1056/NEJM198301063080106. [DOI] [PubMed] [Google Scholar]
- 31.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for disease control and prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 32.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ono M, Boethig D, Goerler H, Lange M, Westhoff-Bleck M, Breymann T. Somatic development long after the Fontan operation: factors influencing catch-up growth. J Thorac Cardiovasc Surg. 2007;134:1199–1206. doi: 10.1016/j.jtcvs.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Ovroutski S, Ewert P, exi-Meskishvili V, Stiller B, Nurnberg JH, Abdul-Khaliq H, et al. Comparison of somatic development and status of conduit after extracardiac Fontan operation in young and older children. Eur J Cardiothorac Surg. 2004;26:1073–1079. doi: 10.1016/j.ejcts.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Pillo-Blocka F, Adatia I, Sharieff W, McCrindle BW, Zlotkin S. Rapid advancement to more concentrated formula in infants after surgery for congenital heart disease reduces duration of hospital stay: a randomized clinical trial. J Pediatr. 2004;145:761–766. doi: 10.1016/j.jpeds.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 36.Schidlow DN, Anderson JB, Klitzner TS, Beekman RH, III, Jenkins KJ, Kugler JD, et al. Variation in interstage outpatient care after the Norwood procedure: a report from the Joint Council on Congenital Heart Disease National Quality Improvement Collaborative. Congenit Heart Dis. 2011;6:98–107. doi: 10.1111/j.1747-0803.2011.00509.x. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz SM, Gewitz MH, See CC, Berezin S, Glassman MS, Medow CM, et al. Enteral nutrition in infants with congenital heart disease and growth failure. Pediatrics. 1990;86:368–373. [PubMed] [Google Scholar]
- 38.Sondheimer JM, Hamilton JR. Intestinal function in infants with severe congenital heart disease. J Pediatr. 1978;92:572–578. doi: 10.1016/s0022-3476(78)80290-x. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan C, Jaquiss RD, Morrow WR, et al. Impact of staged palliation on somatic growth in patients with hypoplastic left heart syndrome. Congenit Heart Dis. 2010;5:546–551. doi: 10.1111/j.1747-0803.2010.00457.x. [DOI] [PubMed] [Google Scholar]
- 40.Stark LJ, Opipari-Arrigan L, Quittner AL, Bean J, Powers SW. The effects of an intensive behavior and nutrition intervention compared to standard of care on weight outcomes in CF. Pediatr Pulmonol. 2011;46:31–35. doi: 10.1002/ppul.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tchervenkov CI, Jacobs JP, Weinberg PM, Stellin G. The nomenclature, definition, and classification of hypoplastic left heart syndrome. Cardiol Young. 2006;16:339–368. doi: 10.1017/S1047951106000291. [DOI] [PubMed] [Google Scholar]
- 42.Vogt KN, Manlhiot C, Van Arsdell G, Russell JL, Mital S, McCrindle BW. Somatic growth in children with singleventricle physiology impact of physiologic state. J Am Coll Cardiol. 2007;50:1876–1883. doi: 10.1016/j.jacc.2007.07.050. [DOI] [PubMed] [Google Scholar]



