This article seeks to comprehensively review the state of the art in the endovascular management of lower extremity deep venous disease.
Abstract
The societal and individual burden caused by acute and chronic lower extremity venous disease is considerable. In the past several decades, minimally invasive endovascular interventions have been developed to reduce thrombus burden in the setting of acute deep venous thrombosis to prevent both short- and long-term morbidity and to recanalize chronically occluded or stenosed postthrombotic or nonthrombotic veins in symptomatic patients. This state-of-the-art review provides an overview of the techniques and challenges, rationale, patient selection criteria, complications, postinterventional care, and outcomes data for endovascular intervention in the setting of acute and chronic lower extremity deep venous disease.
Online supplemental material is available for this article.
© RSNA, 2015
Learning Objectives:
After reading the article and taking the test, the reader will be able to:
■ Identify risk factors for the development of the postthrombotic syndrome
■ Discuss the current state of endovascular intervention to treat acute deep vein thrombosis
■ Explain the endovascular options for the treatment of established postthrombotic syndrome
■ Discuss the endovascular options and imaging evaluation of nonthrombotic deep venous disease
Accreditation and Designation Statement
The RSNA is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The RSNA designates this journal-based SA-CME activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Disclosure Statement
The ACCME requires that the RSNA, as an accredited provider of CME, obtain signed disclosure statements from the authors, editors, and reviewers for this activity. For this journal-based CME activity, author disclosures are listed at the end of this article.
Introduction
Lower extremity deep venous disease is highly prevalent, economically burdensome, morbid, and debilitating. In selected situations, when associated with acute pulmonary embolus or limb-threatening venous ischemia, it can be fatal. This article reviews the interventional management of acute and chronic lower extremity deep venous disease. While many questions remain to be answered, substantial progress has been made in our understanding of the disease and how and when to intervene. Techniques have evolved to reduce bleeding and more efficiently remove acute thrombus. Endovascular recanalization in the setting of chronic venous disease has compared well against open surgery with much lower morbidity. Thus, this article seeks to comprehensively review the state of the art in the endovascular management of lower extremity deep venous disease.
The first section discusses acute deep venous thrombosis—its epidemiology, consequences beyond pulmonary embolus (ie, postthrombotic syndrome [PTS]), patient selection for catheter-directed intervention, and outcomes of prior and modern techniques. The second section describes both thrombotic and nonthrombotic chronic lower extremity venous disease, including imaging and clinical assessment, noninterventional management, endovascular techniques, postprocedure management, and outcomes data. Upper extremity deep venous disease and pulmonary embolus will not be addressed in this review.
Part 1: Acute Lower Extremity Deep Vein Thrombosis
Epidemiology
In 2008, echoing what has long been recognized by health care professionals, the U.S. Surgeon General issued a national Call to Action that recognized deep vein thrombosis (DVT) as a clear and present danger to public health (1). By numbers, DVT represents the third most common cardiovascular disease.
The most feared consequence of DVT is pulmonary embolism, given its significant case fatality rate. Therefore, treatment recommendations for DVT have historically been rooted in preventing pulmonary embolism by means of anticoagulant drugs (2). For most patient groups, initial therapy consists of a parenteral anticoagulant drug (unfractionated heparin, a low-molecular-weight heparin, or fonadaparinux) with subsequent transition to long-term oral vitamin K antagonist therapy for at least 3 months, with the duration of therapy dependent on the presence or absence of ongoing risk factors for recurrence. The preferred anticoagulant for patients with active cancer is low-molecular-weight heparin monotherapy for at least 3–6 months (3,4). In November 2012, rivaroxaban, an oral direct thrombin inhibitor, was approved by the U.S. Food and Drug Administration for the treatment of venous thromboembolism (VTE) and has gained traction due to its convenience and paucity of drug-drug interactions compared with warfarin. Its pharmacokinetics are also unaffected by diet. However, no validated antidote exists at present in the event of serious bleeding. This agent and others have shown comparable efficacy to warfarin in the prevention of recurrent VTE (5,6). Postmarketing surveillance will be essential to determine if these newer agents are as effective and safe as prior regimens.
Beyond Pulmonary Embolism: Postthrombotic Syndrome
Contemporary prospective studies suggest that the above concept of pulmonary embolism prevention alone needs to be modernized substantially. Despite the use of anticoagulant therapy, these studies indicate that PTS develops in approximately 40% of patients who experience a first episode of symptomatic lower extremity DVT (7). PTS is a chronic condition defined as a set of symptoms and signs that develop in a limb months to years after an acute DVT. These include daily limb pain and/or aching, fatigue, heaviness, and/or swelling that worsens with upright position and activity. In severely affected patients, limiting venous claudication, stasis dermatitis, subcutaneous fibrosis, and/or skin ulceration may develop. Studies have consistently shown that PTS impairs DVT patients’ quality of life, and a large prospective cohort study (the VETO [Venous Thrombosis Outcomes] study) found the presence and severity of PTS to be the leading predictors of patients’ health-related quality of life 2 years after a DVT episode (8,9). PTS has also been shown to lead to venous leg ulcers that are difficult to treat and that often recur. The direct medical costs of treating PTS and the indirect costs of the related work disability have been shown to result in substantial economic burden to the health care systems of several North American and European countries (10–13).
The pathogenesis of PTS is complex and poorly understood. Studies have demonstrated that an initial inflammatory response to thrombosis strongly influences thrombus resolution, organization, and subsequent vein wall injury (14–16). The ultimate result of this process on the composition of the adjacent vein wall appears to be an increase in thickness and reduced compliance, impaired valvular function, and other abnormalities. At a macroscopic level, the continued presence of thrombus within the deep venous system during the initial weeks after an acute DVT leads to PTS by at least two pathways. First, even with anticoagulant therapy, incomplete clearance of thrombus physically blocks venous blood flow (obstruction). Second, the inflammatory response to acute thrombosis directly damages the venous valves and alters the adjacent vein wall, leading to valvular reflux (17–21). Uninvolved distal deep veins and superficial collaterals may dilate and become incompetent as well. When reflux and/or obstruction is present, ambulatory venous hypertension develops and ultimately leads to edema, tissue hypoxia and injury, progressive calf pump dysfunction, subcutaneous fibrosis, and skin ulceration (22–25).
Risk Factors for Developing PTS after DVT
The predictors for the development of PTS are also poorly understood. The occurrence of recurrent ipsilateral DVT is associated with a two- to sixfold increased risk of PTS (7). Additionally, the quality of anticoagulant therapy delivered probably influences the later development of PTS. In one registry, a 2.5-fold increase in PTS was observed in patients whose international normalized ratio was nontherapeutic more than 50% of the time (26). Therefore, anticoagulation should be viewed as a key PTS prevention measure, but it is clear that despite anticoagulation many DVT patients will still develop PTS. Studies have identified relatively minor PTS risk increases in patients of advanced age, increased body mass index, and female sex.
The anatomic extent of DVT is an important predictor of a patient’s subsequent risk of developing PTS. Patients with proximal DVT have a higher incidence of PTS compared with patients with isolated calf DVT (7). More importantly, patients with “iliofemoral” DVT (defined as DVT involving the common femoral vein and/or iliac vein, with or without involvement of other veins as well), experience recurrent VTE twice as frequently as patients with less extensive proximal DVT, and have 2-year PTS rates that exceed 50% despite the use of anticoagulation therapy (7,27,28). These patients are also more likely to develop severe PTS manifestations such as disabling venous claudication and venous ulcers (29,30). The common femoral vein is frequently involved in cases of iliofemoral DVT, and many of these patients can be identified from the initial ultrasonography (US) that was performed to diagnose the DVT, although isolated iliac vein thrombus, which is unusual in the authors’ experience and in the literature, can potentially be missed with US. Hence, clinicians should consider iliofemoral DVT a high-risk condition for which particular attention to secondary VTE prevention and PTS prevention should be paid.
Noninterventional Methods to Prevent PTS
The study of adjunctive measures to prevent PTS has been very limited to our knowledge. In two open-label, single-center, randomized trials that did not utilize a placebo control, the daily use of 30–40 mm Hg, knee-high elastic compression stockings in patients with proximal DVT was observed to decrease the incidence of PTS (31,32). However, a subsequent much larger, placebo-controlled, double-blind, multicenter randomized controlled trial (the SOX trial) found no difference in PTS in patients using elastic compression stockings versus a sham stocking (33,34). Hence, while elastic compression stockings may help some patients with symptom control and are a low-risk intervention, it is most likely that they do not prevent the development of PTS.
Beyond Anticoagulation: The “Open Vein” Hypothesis and Lessons Learned from Systemic Thrombolysis for DVT
It has been hypothesized for many years that rapid thrombus elimination and restoration of unobstructed deep venous flow in patients with acute DVT may prevent late valvular reflux, venous obstruction, and PTS. Proof-of-concept support for this “open vein hypothesis” exists. In a secondary analysis of data from a randomized trial evaluating the use of compression therapy, Prandoni et al (21) found that 2-year PTS developed more frequently in proximal DVT patients who had residual venous thrombus or popliteal valvular reflux at 6-month follow-up. In 2005, Hull et al (35) performed a meta-analysis of 11 randomized DVT treatment trials and found a strong correlation between the amount of residual thrombus after a course of anticoagulant therapy and the subsequent incidence of recurrent VTE. As discussed above, recurrent VTE is associated with the development of PTS. Finally, small randomized trials have observed the use of contemporary surgical venous thrombectomy and systemic thrombolysis to be associated with reduced PTS rates compared with anticoagulation alone, but at the price of greater invasiveness and more complications, including major bleeding (36–42).
An important observation from systemic thrombolysis studies was the finding that significant clot lysis occurred much more frequently in patients with nonocclusive thrombi rather than occlusive thrombi, suggesting that the systemic administration route afforded inadequate access of the thrombolytic drug to its target sites within the thrombus (43). The use of intermittent injections of a plasminogen activator into nearby veins in the affected leg, with or without a tourniquet system to direct the drug into the deep veins (“flow-directed” thrombolysis), did not prove more effective (44,45). In contrast, the imaging-guided intrathrombus infusion of thrombolytic drugs into DVT has shown greater efficacy and safety, and this principle underlies the use of catheter-directed thrombolytic DVT therapy in current practice (46,47).
Image-guided Endovascular Thrombus Removal: Description and Evolution of Techniques
Catheter-directed thrombolysis.—Catheter-directed thrombolysis (CDT) refers to the infusion of a fibrinolytic drug directly into thrombus by means of a multi-sidehole catheter embedded in the thrombus (Table 1). This practice aims to deliver a higher local intrathrombus drug concentration (enhancing efficacy) with a reduced drug dose (enhancing safety). It was the first endovascular thrombolytic method applied to DVT patients (46,47). With this technique, US guidance is used to obtain access into the deep venous system of the affected limb; whenever possible, it is ideal to access an open flowing vein below the thrombosed venous segment. The popliteal vein is an excellent target if patent, owing to its size and the ability to compress it after sheath removal. The situation is more challenging if the popliteal vein is occluded. In such instances, access can be gained into the caudal aspect of the thrombosed popliteal vein, into a calf vein, or into the posterior tibial vein, although these latter two may be more technically challenging. Internal jugular venous access can also be utilized, although going against the direction of valves in the femoropopliteal veins can be problematic. Internal jugular access also requires long catheters and wires. A venogram is then obtained to define the extent of thrombus. The multi-sidehole catheter is embedded within the thrombus and attached to an infusion of a dilute solution of a thrombolytic drug. Although no lytic drug is currently approved by the U.S. Food and Drug Administration, those used in clinical practice include recombinant tissue plasminogen activator (0.01 mg/kg/h up to a maximum of 1.0 mg/h), reteplase (0.25–0.50 units/h), and tenecteplase (0.25 mg/h). No specific comparison has been made among these agents to suggest superiority or safety of one over the other, and lytic choice is based on operator and institutional preference. The infusion is typically continued for 6–24 hours, during which time the patient is carefully monitored for bleeding. A complete blood count, partial thromboplastin time, and fibrinogen level can be drawn every 6 hours, although absolute values should not be solely relied on to cease or continue the lytic infusion. Minor sentinel bleeds, pericatheter oozing, and epistaxis should prompt closer monitoring and, in conjunction with laboratory values, may require adjustment of the infusion (47). At the end of the infusion, repeat venography is performed, the catheter is repositioned to span the remaining thrombus, and the infusion is continued. Clot maceration with an angioplasty balloon is sometimes used to facilitate thrombolysis by increasing the surface area for thrombolysis. After thrombus removal, venography is used to evaluate for obstructive lesions. Once identified, these are treated with balloon angioplasty and/or stent placement (46,47). Stents are typically reserved for iliac obstructions, although extension into the common femoral vein is necessary if the obstruction includes it or the peripheral external iliac vein. While no stent has U.S. Food and Drug Administration approval for venous obstructions, longitudinally flexible, self-expandable bare stents are generally favored (Fig 1) because (a) they conform to tortuous veins, (b) they have sufficient hoop strength for most venous obstructions, and (c) they allow inflow from nonthrombosed tributaries. The routine use of inferior vena cava (IVC) filters during CDT is unnecessary; in a large prospective registry, symptomatic pulmonary embolism occurred in only 1.3% of individuals undergoing CDT (45).
Table 1.
Endovascular Techniques for Thrombus Removal
Note.—PCDT = pharmacomechanical catheter directed thrombolysis, PMT = percutaneous mechanical thrombectomy.
*PCDT is a combination of CDT and PMT.
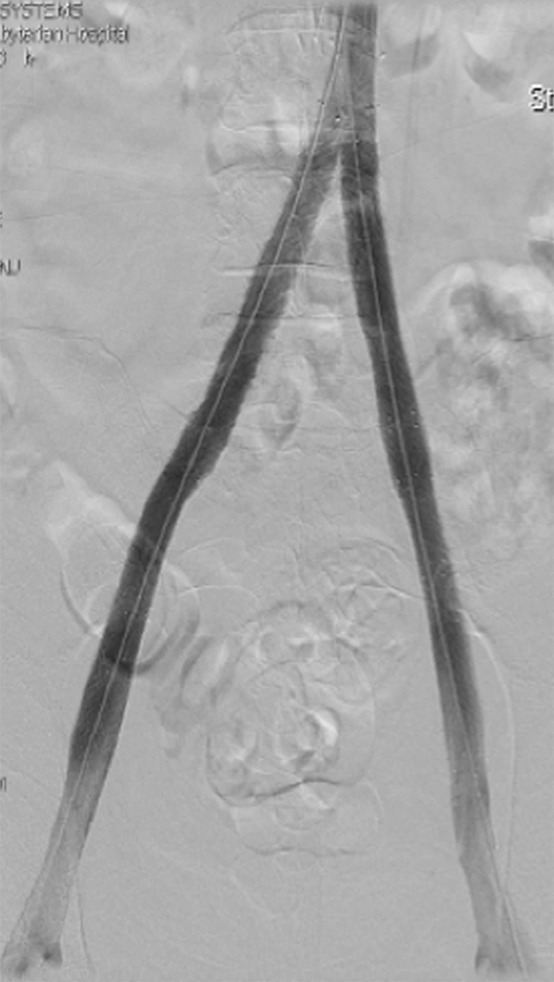
Figure 1a:
CDT and stent placement in a patient with progressive bilateral DVTs in spite of anticoagulation. (a) Left femoral venogram (patient prone) demonstrates extensive acute thrombus along the length of the vein. (b) Right iliac venogram demonstrates no filling of the iliac vein. (c) Fluoroscopic image depicts infusion catheters along the length of the left and right iliac thrombi. (d) Postinfusion left femoral venogram demonstrates excellent patency. (e) After stent placement, venogram of both iliacs demonstrates rapid flow through the stents (see also Figs E1a and E1b in this patient [online]).
Figure 1b:
CDT and stent placement in a patient with progressive bilateral DVTs in spite of anticoagulation. (a) Left femoral venogram (patient prone) demonstrates extensive acute thrombus along the length of the vein. (b) Right iliac venogram demonstrates no filling of the iliac vein. (c) Fluoroscopic image depicts infusion catheters along the length of the left and right iliac thrombi. (d) Postinfusion left femoral venogram demonstrates excellent patency. (e) After stent placement, venogram of both iliacs demonstrates rapid flow through the stents (see also Figs E1a and E1b in this patient [online]).
Figure 1c:
CDT and stent placement in a patient with progressive bilateral DVTs in spite of anticoagulation. (a) Left femoral venogram (patient prone) demonstrates extensive acute thrombus along the length of the vein. (b) Right iliac venogram demonstrates no filling of the iliac vein. (c) Fluoroscopic image depicts infusion catheters along the length of the left and right iliac thrombi. (d) Postinfusion left femoral venogram demonstrates excellent patency. (e) After stent placement, venogram of both iliacs demonstrates rapid flow through the stents (see also Figs E1a and E1b in this patient [online]).
Figure 1d:
CDT and stent placement in a patient with progressive bilateral DVTs in spite of anticoagulation. (a) Left femoral venogram (patient prone) demonstrates extensive acute thrombus along the length of the vein. (b) Right iliac venogram demonstrates no filling of the iliac vein. (c) Fluoroscopic image depicts infusion catheters along the length of the left and right iliac thrombi. (d) Postinfusion left femoral venogram demonstrates excellent patency. (e) After stent placement, venogram of both iliacs demonstrates rapid flow through the stents (see also Figs E1a and E1b in this patient [online]).
Figure 1e:
CDT and stent placement in a patient with progressive bilateral DVTs in spite of anticoagulation. (a) Left femoral venogram (patient prone) demonstrates extensive acute thrombus along the length of the vein. (b) Right iliac venogram demonstrates no filling of the iliac vein. (c) Fluoroscopic image depicts infusion catheters along the length of the left and right iliac thrombi. (d) Postinfusion left femoral venogram demonstrates excellent patency. (e) After stent placement, venogram of both iliacs demonstrates rapid flow through the stents (see also Figs E1a and E1b in this patient [online]).
Limitations of the original CDT technique include the long infusion times required to obtain complete lysis of extensive DVT (typically 1–3 days) and the health care resources used. In an early multicenter registry, major bleeding occurred in 11% of DVT patients treated with urokinase CDT infusions (45). In this registry, which included a relatively unselected patient population, intracranial bleeding was observed in 0.4% of patients. Symptomatic pulmonary embolism and fatal pulmonary embolism occurred in 1.3% and 0.2% of patients, respectively. In more recent experiences using infusions of recombinant tissue plasminogen activator at low doses (0.5–1.0 mg/h), major bleeding has occurred in only 2%–4% of patients (48–50). Reasons for this apparent difference may be improved patient selection, use of “subtherapeutic” heparin dosing during thrombolysis, and the routine use of US-guided venipuncture, which has limited access site bleeding due to inadvertent arterial puncture.
After successful lysis, patients should receive optimal medical management for their DVT, including full anticoagulation, if safe, to prevent recurrent thrombosis. As mentioned previously, routine use of compression stockings is controversial but can be used for symptomatic relief.
Subsequent CDT technologies have evolved to address the above limitations. One approach is the use of low-power ultrasound energy–equipped catheter to disperse the thrombolytic drug within the thrombus (EKOS, Bothell, Wash) (51) (Fig 2). Proponents cite theoretical advantages to this approach: (a) fast intrathrombus drug dispersion (and therefore faster thrombolysis using a lower drug dose), (b) valvular preservation because of better lysis of perivalvular thrombus, and (c) reduced venous wall and valvular trauma compared with mechanical thrombectomy devices. However, these potential advantages should be considered unproven until clinical studies verify improved outcomes (52).
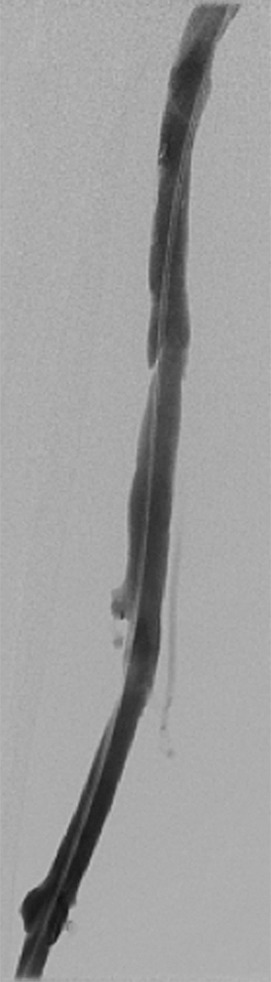
Figure 2:
Spot fluoroscopic image of US-assisted catheter infusion in a patient with common femoral and femoral deep venous thrombosis. Note the caudal aspect of an external iliac stent.
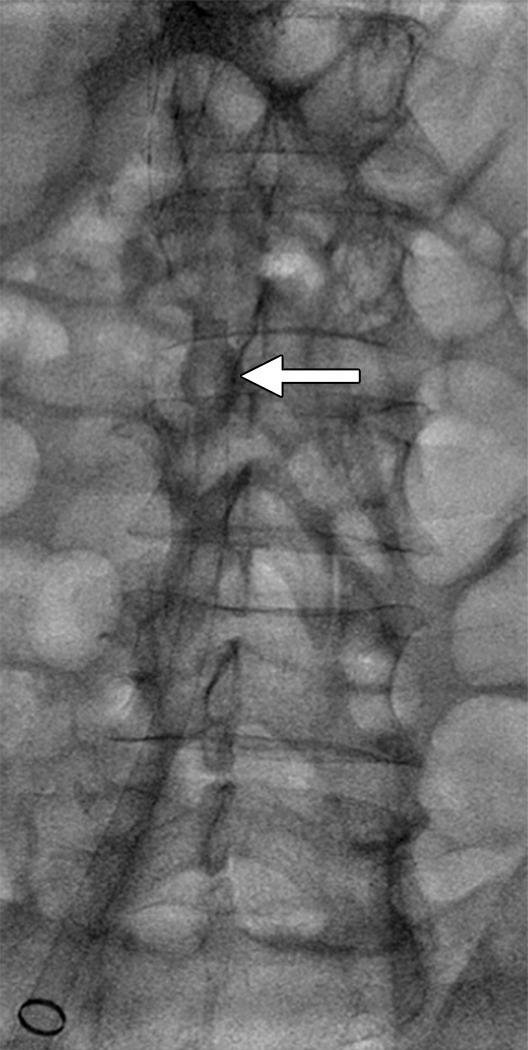
PMT without thrombolysis.—PMT devices macerate thrombus and/or remove thrombus fragments from the venous lumen. The use of PMT increases the surface area of residual thrombus and can create a central flow channel within an occluded vein, which together may improve the efficiency of endogenous thrombolysis. However, potential disadvantages of PMT methods include the increased on-table procedure time, the potential for embolizing thrombus with mechanical manipulation, and the theoretical potential for causing venous valve injury. Published experience with stand-alone PMT (ie, without concomitant infusion of a fibrinolytic drug) for DVT has been disappointing—with currently available devices, it does not appear to remove sufficient thrombus volumes to be clinically useful (53). A recently introduced device (AngioVac; Angiodynamics, Latham, NY) employs a recirculation circuit and a large bore (22-F) suction catheter to remove thrombus from large vessels such as the IVC (Fig 3).
Figure 3a:
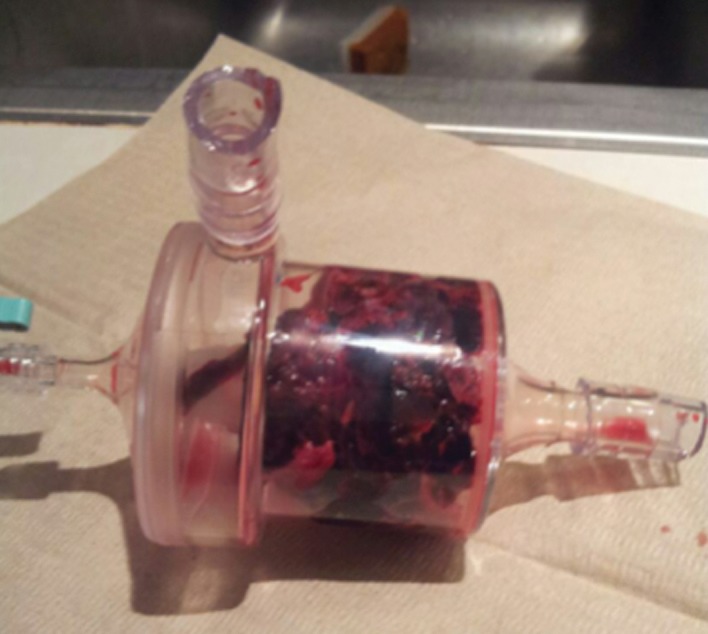
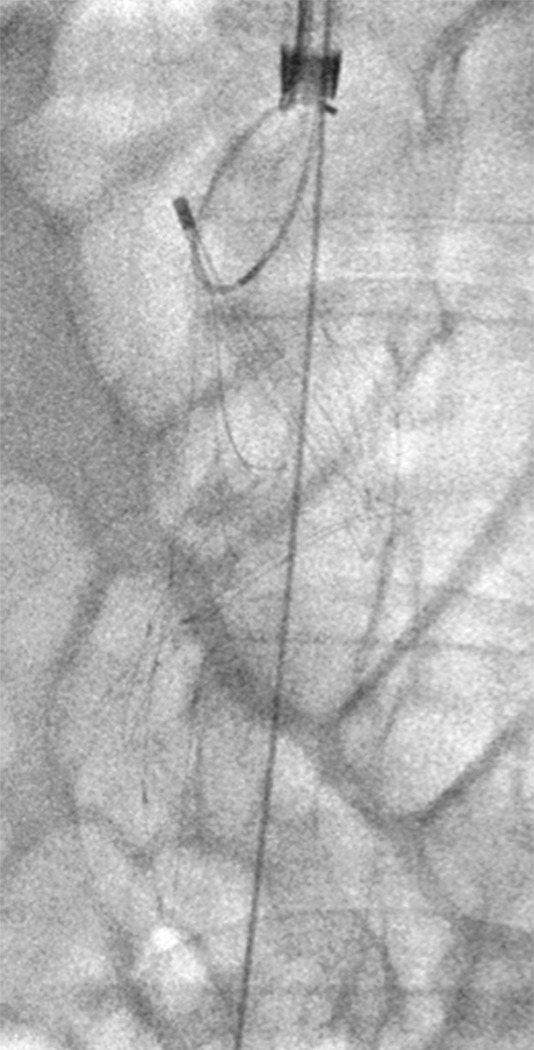
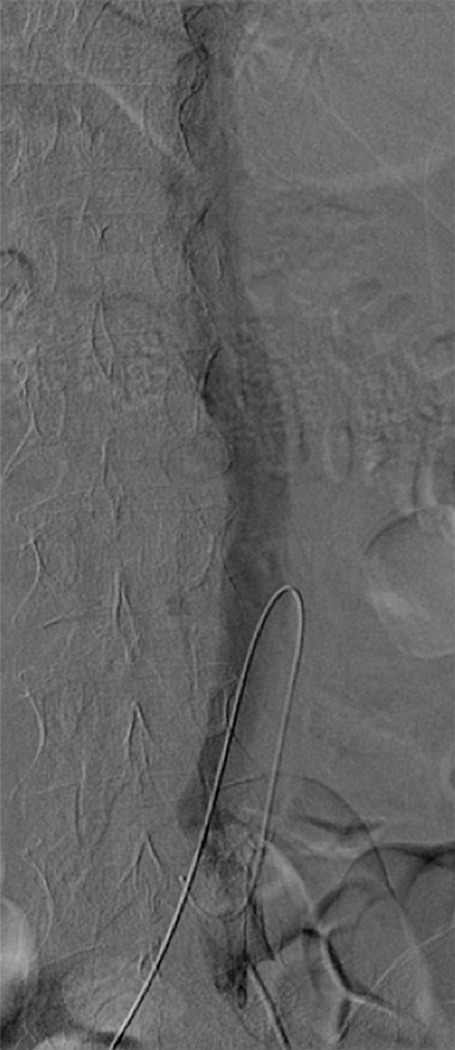
Caval thrombus treated with large-bore aspiration device. (a) IVC venogram demonstrates extensive caval thrombus and a malpositioned suprarenal IVC filter. (b) Fluoroscopic image depicts the suction/aspiration device (AngioVac; Angiodynamics) in the IVC. The arrow points to the balloon at the tip, which when inflated flares the tip. (c) Photograph of the recirculation filter shows bulky extracted thrombus. (d) Fluoroscopic image during filter retrieval shows a tip deflecting wire grasping the malpositioned filter. The tip of the wire has been snared, and the filter is subsequently pulled through the sheath. (e) IVC venogram obtained the next day after IVC filter removal demonstrates marked reduction in thrombus burden and free flow through the cava. No lytic drug was used during this case owing to a hemorrhagic stroke in this patient 3 weeks earlier.
Figure 3b:
Caval thrombus treated with large-bore aspiration device. (a) IVC venogram demonstrates extensive caval thrombus and a malpositioned suprarenal IVC filter. (b) Fluoroscopic image depicts the suction/aspiration device (AngioVac; Angiodynamics) in the IVC. The arrow points to the balloon at the tip, which when inflated flares the tip. (c) Photograph of the recirculation filter shows bulky extracted thrombus. (d) Fluoroscopic image during filter retrieval shows a tip deflecting wire grasping the malpositioned filter. The tip of the wire has been snared, and the filter is subsequently pulled through the sheath. (e) IVC venogram obtained the next day after IVC filter removal demonstrates marked reduction in thrombus burden and free flow through the cava. No lytic drug was used during this case owing to a hemorrhagic stroke in this patient 3 weeks earlier.
Figure 3c:
Caval thrombus treated with large-bore aspiration device. (a) IVC venogram demonstrates extensive caval thrombus and a malpositioned suprarenal IVC filter. (b) Fluoroscopic image depicts the suction/aspiration device (AngioVac; Angiodynamics) in the IVC. The arrow points to the balloon at the tip, which when inflated flares the tip. (c) Photograph of the recirculation filter shows bulky extracted thrombus. (d) Fluoroscopic image during filter retrieval shows a tip deflecting wire grasping the malpositioned filter. The tip of the wire has been snared, and the filter is subsequently pulled through the sheath. (e) IVC venogram obtained the next day after IVC filter removal demonstrates marked reduction in thrombus burden and free flow through the cava. No lytic drug was used during this case owing to a hemorrhagic stroke in this patient 3 weeks earlier.
Figure 3d:
Caval thrombus treated with large-bore aspiration device. (a) IVC venogram demonstrates extensive caval thrombus and a malpositioned suprarenal IVC filter. (b) Fluoroscopic image depicts the suction/aspiration device (AngioVac; Angiodynamics) in the IVC. The arrow points to the balloon at the tip, which when inflated flares the tip. (c) Photograph of the recirculation filter shows bulky extracted thrombus. (d) Fluoroscopic image during filter retrieval shows a tip deflecting wire grasping the malpositioned filter. The tip of the wire has been snared, and the filter is subsequently pulled through the sheath. (e) IVC venogram obtained the next day after IVC filter removal demonstrates marked reduction in thrombus burden and free flow through the cava. No lytic drug was used during this case owing to a hemorrhagic stroke in this patient 3 weeks earlier.
Figure 3e:
Caval thrombus treated with large-bore aspiration device. (a) IVC venogram demonstrates extensive caval thrombus and a malpositioned suprarenal IVC filter. (b) Fluoroscopic image depicts the suction/aspiration device (AngioVac; Angiodynamics) in the IVC. The arrow points to the balloon at the tip, which when inflated flares the tip. (c) Photograph of the recirculation filter shows bulky extracted thrombus. (d) Fluoroscopic image during filter retrieval shows a tip deflecting wire grasping the malpositioned filter. The tip of the wire has been snared, and the filter is subsequently pulled through the sheath. (e) IVC venogram obtained the next day after IVC filter removal demonstrates marked reduction in thrombus burden and free flow through the cava. No lytic drug was used during this case owing to a hemorrhagic stroke in this patient 3 weeks earlier.
PCDT.—PCDT, which is the combined use of CDT and PMT, has enhanced physicians’ ability to efficiently remove large thrombus volumes in patients with DVT. This combination therapy is predicated on the ideas that (a) PMT can increase the surface area of thrombus, accelerate pharmacologic thrombolysis, reduce the required drug dose and infusion duration, and thereby reduce bleeding complications, and (b) CDT can dissolve PMT-created thrombus fragments that might otherwise cause pulmonary embolism.
Physicians have used many different combinations of drugs and devices for DVT treatment, but no single technique has been established as superior, to our knowledge. Two general categories of PCDT techniques may be considered: “First-generation” PCDT methods involve the use of thrombectomy devices with traditional infusion CDT, to speed thrombolytic progress and reduce the needed drug dose. “Single-session” PCDT methods enable rapid intrathrombus dispersion of a thrombolytic drug bolus to enable complete on-table removal of thrombus in a single 1–3-hour procedure.
It should be noted that retrievable IVC filter insertion prior to PCDT has not been fully evaluated to our knowledge, so it is frequently at the discretion of the operator as to whether a filter is indicated. It is reasonable to insert a filter during PCDT if IVC thrombus is present, although every effort should be made to retrieve the filter when appropriate.
First-generation PCDT.—Two forms of first-generation PCDT have been used. “Infusion-first PCDT” refers to the use of an initial CDT infusion, with subsequent use of PMT (with either an aspirating or nonaspirating device) at follow-up sessions to macerate and/or remove residual thrombus. The other method, termed by some “buzz-lyse,” involves use of as aspirating device to first debulk the thrombus, followed by CDT infusion. In limited studies, first-generation PCDT has resulted in (a) initial treatment safety and clot removal efficacy at least comparable to traditional stand-alone CDT; (b) 40%–50% reductions in drug dose and treatment time compared with traditional stand-alone CDT; and (c) reduced hospital stays, intensive care unit utilization, and hospital costs (54).
Single-session PCDT.—Two PCDT techniques can enable single-session endovascular DVT therapy to be completed without the need for further drug infusions or monitoring in the intensive care unit. With the “powerpulse” technique, a rheolytic thrombectomy catheter (AngioJet; Bayer Healthcare) is first used to forcefully pulse-spray a bolus dose of the thrombolytic drug directly into the thrombus (55,56). The drug is allowed to dwell within the thrombus for 15–30 minutes, after which the AngioJet catheter is used to aspirate the residual thrombus. With “isolated thrombolysis,” inflation of catheter-mounted balloons on the Trellis Peripheral Infusion System (Bacchus Vascular, Santa Clara, Calif) is used to isolate a clot-containing segment and deliver a bolus dose of a thrombolytic drug directly into the thrombus (57,58) (Fig 4). Activation of an oscillating wire is then used to mechanically disperse the drug within the thrombus, after which the drug may be aspirated through a port on the device. Initial reported experiences with these techniques suggest that effective DVT therapy can be accomplished in 80%–90% of patients, of whom perhaps 50% may be treated in a single procedure session. It should be noted that the impact of these techniques on the development of PTS has not been established, to our knowledge. If PTS prevention is achieved with reasonable safety, the efficiency with which these treatments can be delivered seems likely to hasten their widespread adoption.
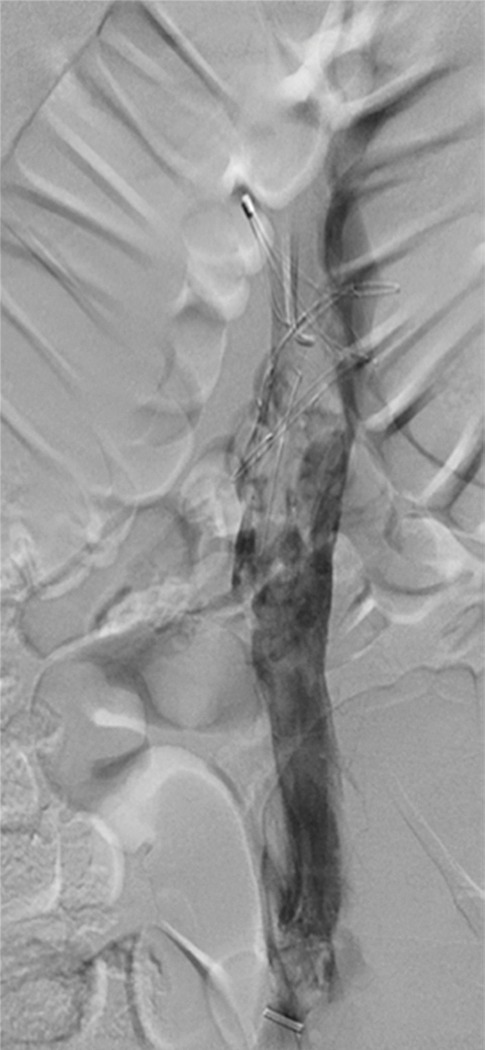
Figure 4a:
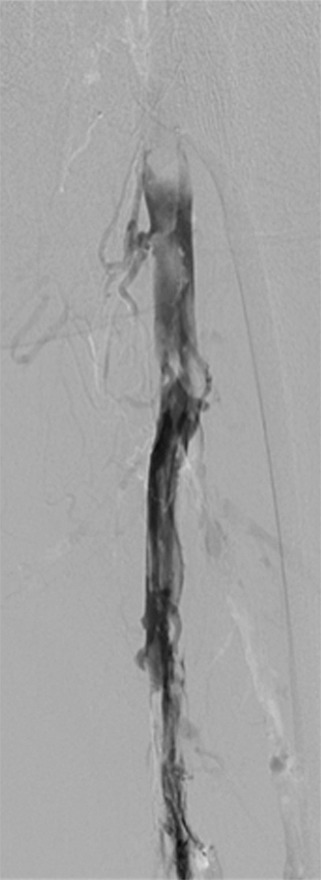
Second-generation PCDT using the Trellis (Bacchus Vascular) device. (a, b) Acute thrombus involving the femoral and iliofemoral deep veins. (c) Fluoroscopic image of the Trellis device deployed along the length of the thrombus. Arrow = the proximal balloon, arrowhead = the macerating wire. Alteplase is being infused along the length of the wire. The distal balloon is not shown. (d, e) Post-PCDT venograms demonstrate successful thrombus removal.
Figure 4b:
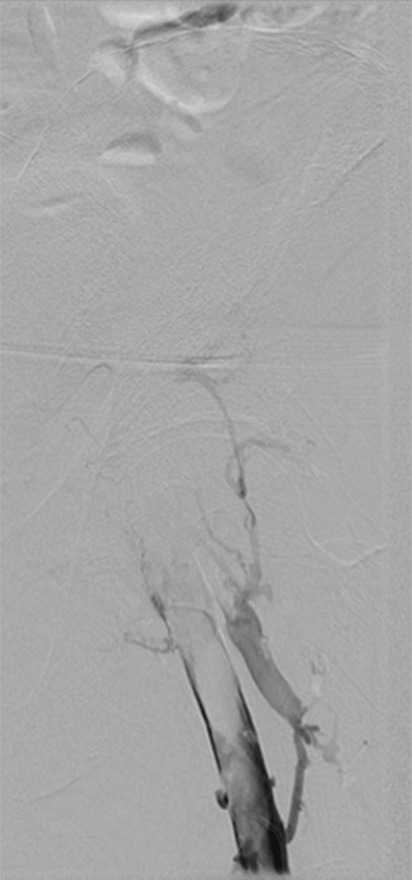
Second-generation PCDT using the Trellis (Bacchus Vascular) device. (a, b) Acute thrombus involving the femoral and iliofemoral deep veins. (c) Fluoroscopic image of the Trellis device deployed along the length of the thrombus. Arrow = the proximal balloon, arrowhead = the macerating wire. Alteplase is being infused along the length of the wire. The distal balloon is not shown. (d, e) Post-PCDT venograms demonstrate successful thrombus removal.
Figure 4c:
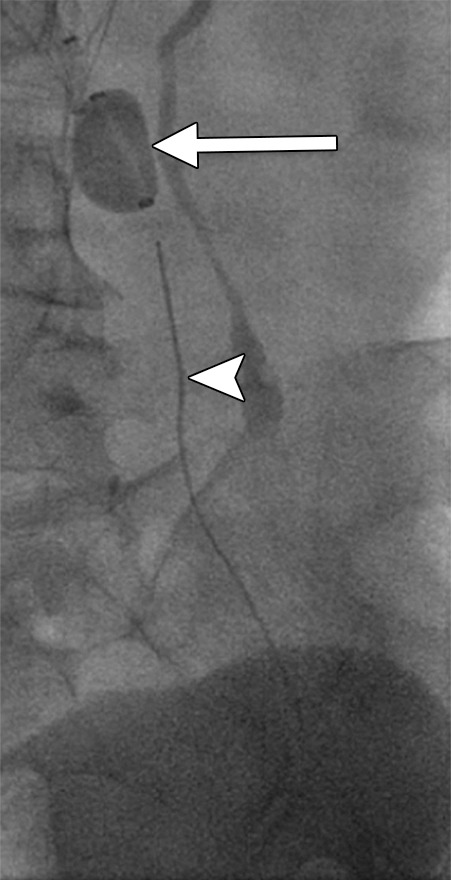
Second-generation PCDT using the Trellis (Bacchus Vascular) device. (a, b) Acute thrombus involving the femoral and iliofemoral deep veins. (c) Fluoroscopic image of the Trellis device deployed along the length of the thrombus. Arrow = the proximal balloon, arrowhead = the macerating wire. Alteplase is being infused along the length of the wire. The distal balloon is not shown. (d, e) Post-PCDT venograms demonstrate successful thrombus removal.
Figure 4d:
Second-generation PCDT using the Trellis (Bacchus Vascular) device. (a, b) Acute thrombus involving the femoral and iliofemoral deep veins. (c) Fluoroscopic image of the Trellis device deployed along the length of the thrombus. Arrow = the proximal balloon, arrowhead = the macerating wire. Alteplase is being infused along the length of the wire. The distal balloon is not shown. (d, e) Post-PCDT venograms demonstrate successful thrombus removal.
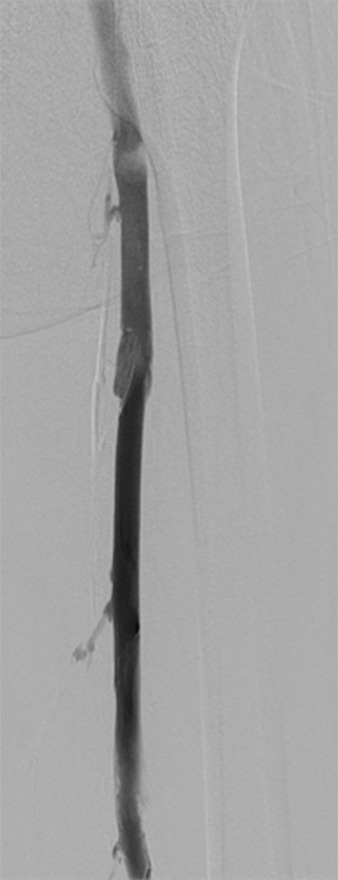
Figure 4e:
Second-generation PCDT using the Trellis (Bacchus Vascular) device. (a, b) Acute thrombus involving the femoral and iliofemoral deep veins. (c) Fluoroscopic image of the Trellis device deployed along the length of the thrombus. Arrow = the proximal balloon, arrowhead = the macerating wire. Alteplase is being infused along the length of the wire. The distal balloon is not shown. (d, e) Post-PCDT venograms demonstrate successful thrombus removal.
Patient Selection for Catheter-directed Therapy: Anatomic and Clinical Considerations
DVT patients require careful evaluation prior to the initiation of CDT therapy (Table 2). Important factors that must be assessed include the following:
Table 2.
Decision Model to Perform CDT Based on Clinical Presentation and Risk of Bleeding
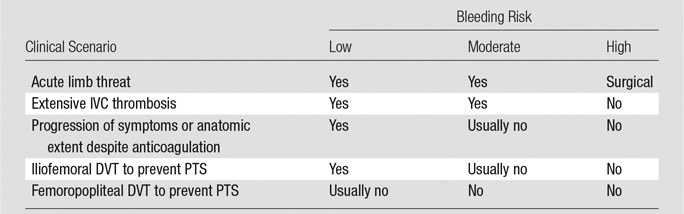
Note.—Adapted, with permission, from reference 109.
Projected risk of bleeding.—All patients in whom CDT is considered must undergo careful evaluation for factors that may increase the risk of major bleeding, including the presence of active bleeding, recent gastrointestinal bleeding (<3 months); recent major surgery, trauma, pregnancy, cardiopulmonary resuscitation, or other invasive procedure; thrombocytopenia or other bleeding diathesis or severe liver dysfunction; the presence of bleeding-prone lesions in critical areas such as the central nervous system; a history of internal eye surgery or hemorrhagic retinopathy within the last 3 months; or a history of stroke or intracranial and/or intraspinal bleeding (47).
Clinical severity of DVT.—The primary intent of aggressive therapy in any patient should be clearly understood and can be grouped into three categories (29,59): Group 1, patients for whom urgent thrombolysis is indicated to prevent life-, limb-, or organ-threatening complications of acute DVT. This would include situations in which limb perfusion is acutely compromised (eg, phlegmasia cerulea dolens) (Fig 5) or when progressive IVC thrombosis (Fig 6b and 6c) despite anticoagulation is believed to increase the risk of fatal pulmonary embolism or acute renal failure to unacceptably high levels. Group 2 includes patients for whom thrombolysis is believed to be reasonable due to a failure of initial anticoagulation to achieve early therapeutic objectives. Such patients are those who have major anatomic DVT progression, a substantial increase in clinical severity, and/or inability to tolerate ongoing major DVT symptoms (ie, pain and swelling that are not relieved or that preclude physical activity) despite the use of initial anticoagulant therapy. In these situations, a low threshold should be applied to exclude patients if there are risk factors for bleeding. Group 3 includes patients with symptomatic DVT for whom thrombolysis is being pursued with the primary purpose being to prevent late PTS. Overall, aggressive therapy for group 1 above should clearly be pursued even when the patient is clinically ill, owing to the absence of other good treatment options, whereas a low threshold for exclusion should be applied to groups 2 and 3 when risk factors for complications exist.
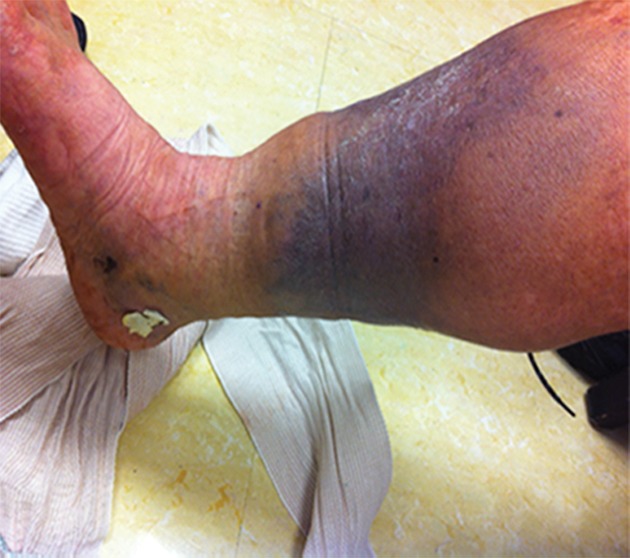
Figure 5a:
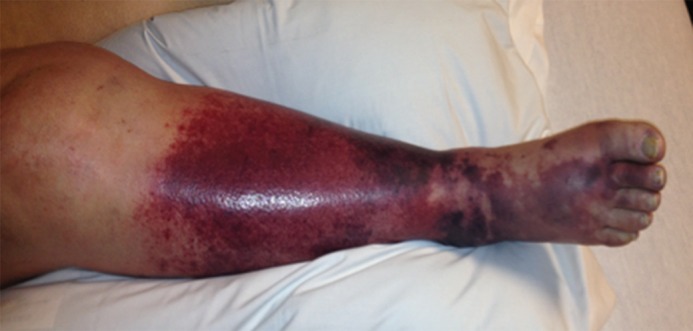
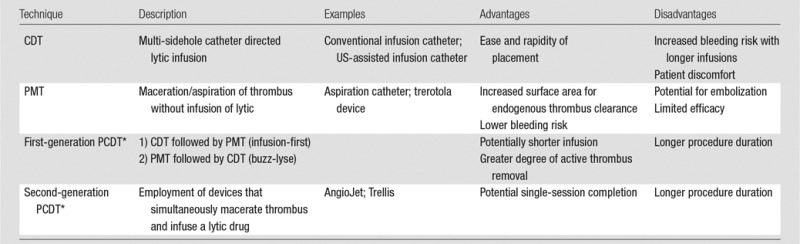
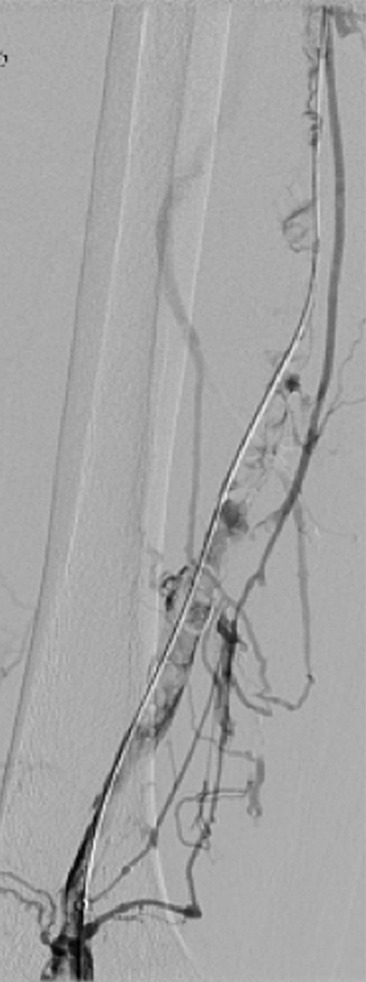
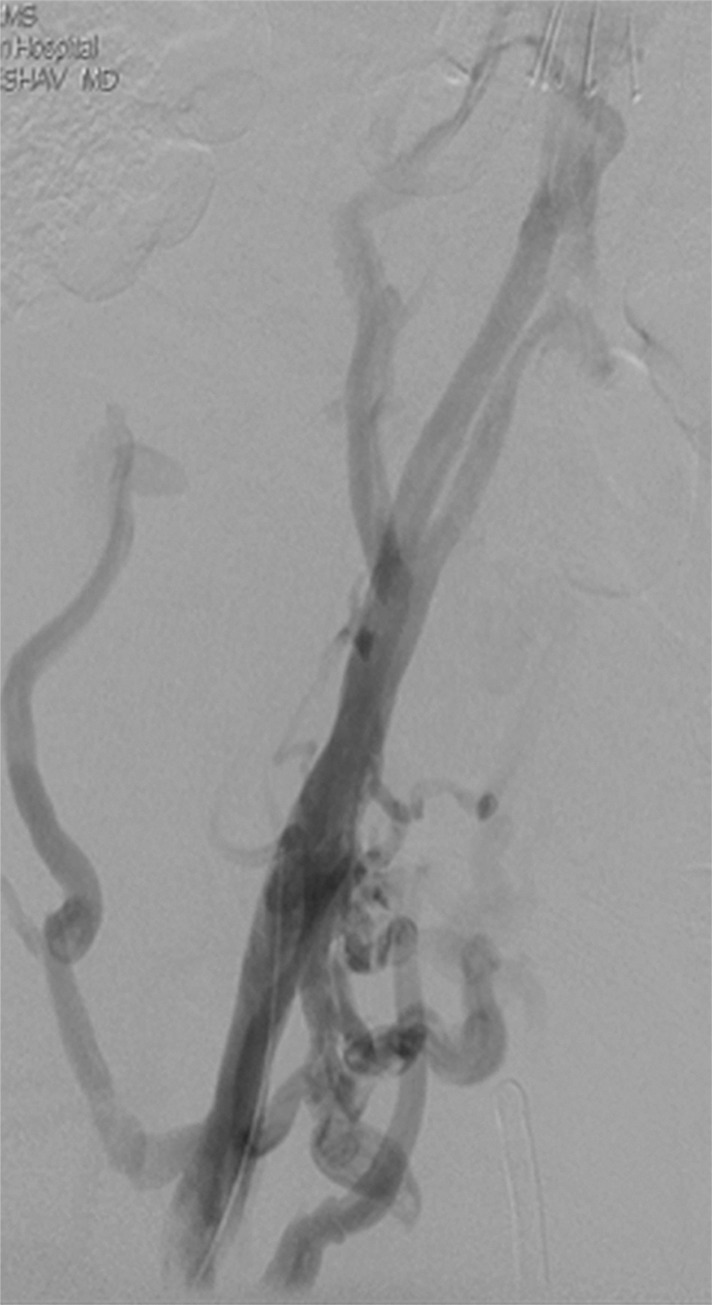
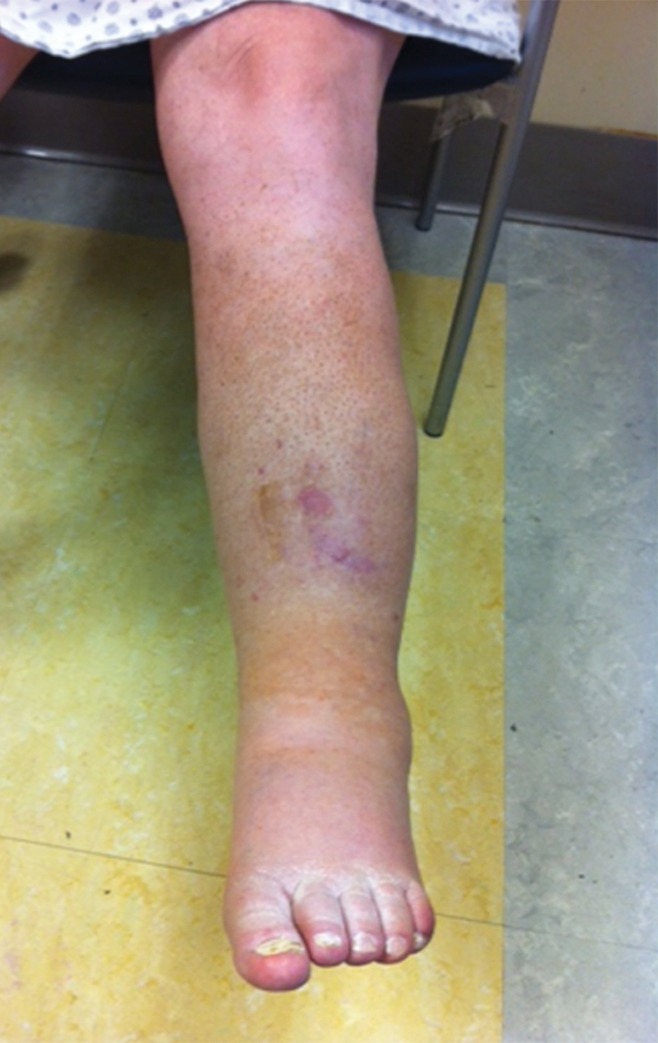
Catheter-directed treatment of phlegmasia. (a) Acutely swollen, mottled, cyanotic leg in the setting of an extensive occlusive DVT. (b, c) Preintervention venograms demonstrate extensive thrombus along the length of the (b) femoral and (c) iliofemoral deep veins. (d, e) After thrombolysis, angioplasty, and stent deployment, the flow through these segments is markedly improved. (f) Appearance of the leg 10 days after treatment. (Case courtesy of Brooke Spencer, MD.)
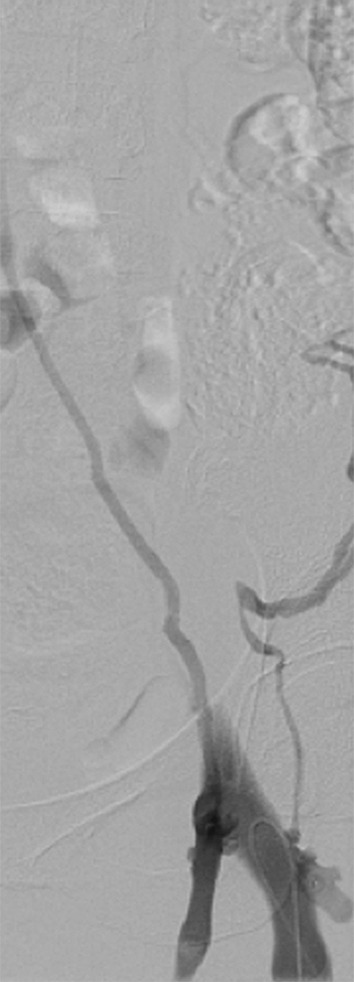
Figure 6b:
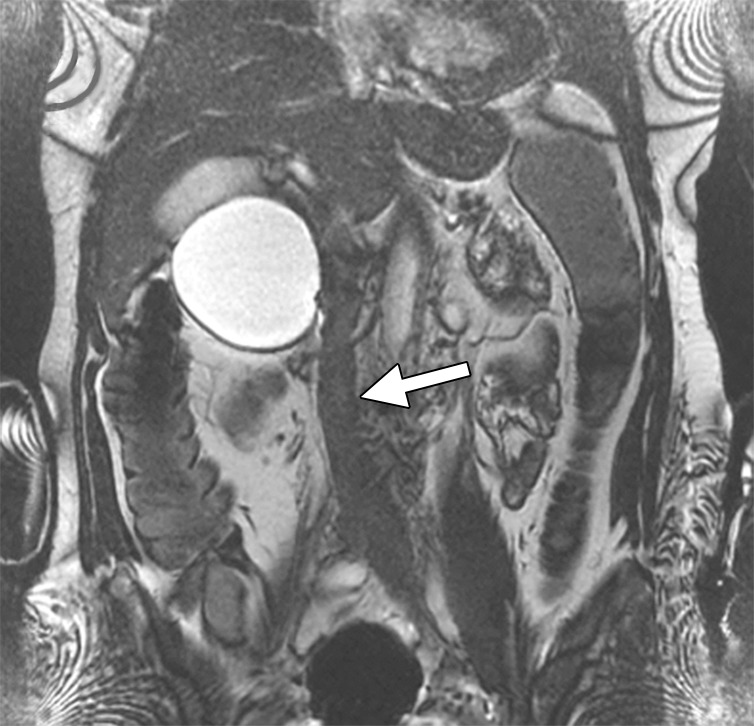
CT and MR appearance of acute iliocaval thrombosis. (a) CT image in a patient with a retrievable infrarenal filter with acute thrombus expanding the IVC and iliac veins (arrowhead). Above the filter, the IVC is patent (arrow). (b, c) Ultrafast T2-weighted coronal MR images in a different patient with acute iliocaval thrombus depict (b) low signal intensity within the IVC (arrow) consistent with thrombus, and (c) a patent intrahepatic IVC (arrow).
Figure 6c:
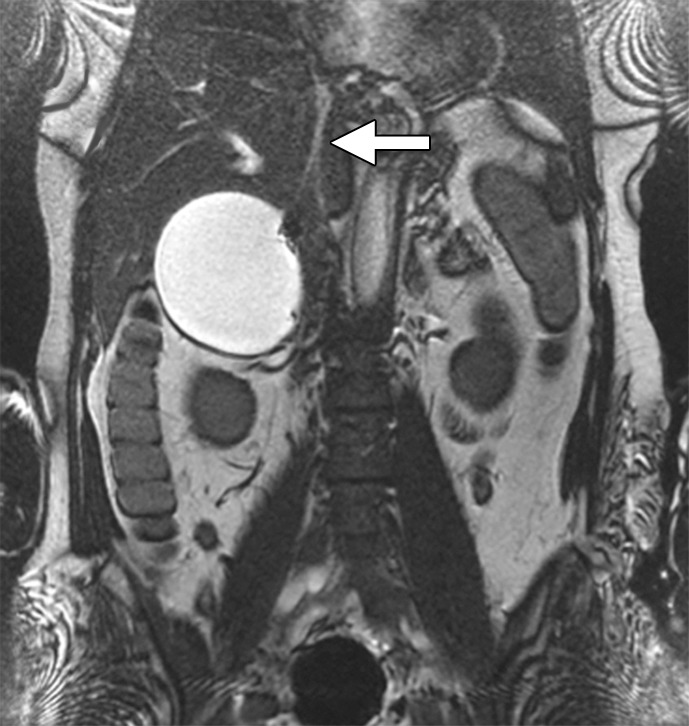
CT and MR appearance of acute iliocaval thrombosis. (a) CT image in a patient with a retrievable infrarenal filter with acute thrombus expanding the IVC and iliac veins (arrowhead). Above the filter, the IVC is patent (arrow). (b, c) Ultrafast T2-weighted coronal MR images in a different patient with acute iliocaval thrombus depict (b) low signal intensity within the IVC (arrow) consistent with thrombus, and (c) a patent intrahepatic IVC (arrow).
Figure 5b:
Catheter-directed treatment of phlegmasia. (a) Acutely swollen, mottled, cyanotic leg in the setting of an extensive occlusive DVT. (b, c) Preintervention venograms demonstrate extensive thrombus along the length of the (b) femoral and (c) iliofemoral deep veins. (d, e) After thrombolysis, angioplasty, and stent deployment, the flow through these segments is markedly improved. (f) Appearance of the leg 10 days after treatment. (Case courtesy of Brooke Spencer, MD.)
Figure 5c:
Catheter-directed treatment of phlegmasia. (a) Acutely swollen, mottled, cyanotic leg in the setting of an extensive occlusive DVT. (b, c) Preintervention venograms demonstrate extensive thrombus along the length of the (b) femoral and (c) iliofemoral deep veins. (d, e) After thrombolysis, angioplasty, and stent deployment, the flow through these segments is markedly improved. (f) Appearance of the leg 10 days after treatment. (Case courtesy of Brooke Spencer, MD.)
Figure 5d:
Catheter-directed treatment of phlegmasia. (a) Acutely swollen, mottled, cyanotic leg in the setting of an extensive occlusive DVT. (b, c) Preintervention venograms demonstrate extensive thrombus along the length of the (b) femoral and (c) iliofemoral deep veins. (d, e) After thrombolysis, angioplasty, and stent deployment, the flow through these segments is markedly improved. (f) Appearance of the leg 10 days after treatment. (Case courtesy of Brooke Spencer, MD.)
Figure 5e:
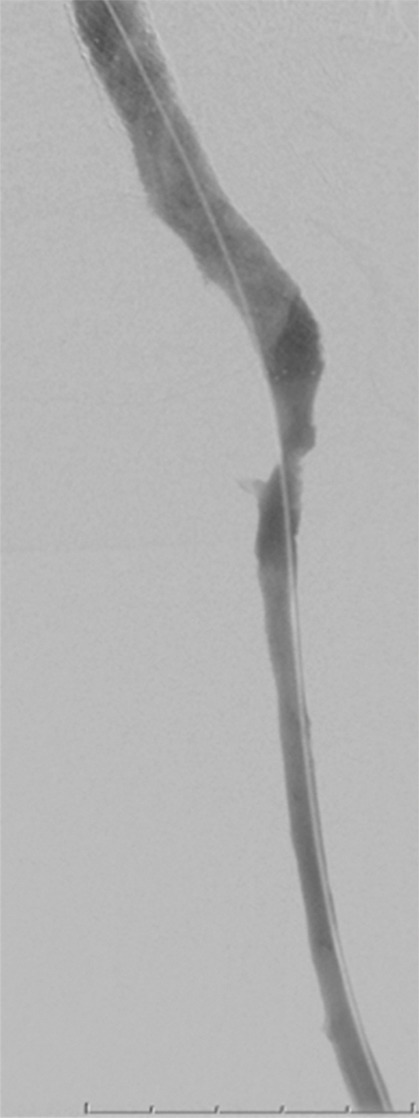
Catheter-directed treatment of phlegmasia. (a) Acutely swollen, mottled, cyanotic leg in the setting of an extensive occlusive DVT. (b, c) Preintervention venograms demonstrate extensive thrombus along the length of the (b) femoral and (c) iliofemoral deep veins. (d, e) After thrombolysis, angioplasty, and stent deployment, the flow through these segments is markedly improved. (f) Appearance of the leg 10 days after treatment. (Case courtesy of Brooke Spencer, MD.)
Figure 5f:
Catheter-directed treatment of phlegmasia. (a) Acutely swollen, mottled, cyanotic leg in the setting of an extensive occlusive DVT. (b, c) Preintervention venograms demonstrate extensive thrombus along the length of the (b) femoral and (c) iliofemoral deep veins. (d, e) After thrombolysis, angioplasty, and stent deployment, the flow through these segments is markedly improved. (f) Appearance of the leg 10 days after treatment. (Case courtesy of Brooke Spencer, MD.)
Anatomic extent of DVT.—It is reasonable to provide patients who have acute iliofemoral DVT and low projected bleeding risk with a balanced discussion of the risks and possible benefits of elective endovascular thrombolysis for the purpose of PTS prevention (29,59). On the contrary, patients with asymptomatic DVT or isolated calf DVT should not be offered CDT since the risk of developing PTS is low (60). For patients with femoropopliteal DVT that does not extend to the level of the common femoral vein, there is little published literature, to our knowledge, to support the added efficacy of thrombolytic therapy, and we suggest therefore that the use of CDT should be limited only to motivated, very symptomatic patients with very low projected risk for bleeding.
Life expectancy, baseline ambulatory capacity, and comorbidities.—Patients who are chronically unable to walk or who have very short life expectancy are less likely to benefit meaningfully from aggressive therapy to prevent PTS, given that PTS affects mobility and functionality and is only truly established 2 years after the acute thrombotic event (7). In addition, patients with substantial respiratory compromise or other acute illness may not be able to tolerate the procedure.
Patients’ personal values and preferences.—For aggressive therapies like DVT thrombolysis for which the benefits have not been conclusively established, it is important for the patient to receive a balanced discussion regarding the rationale, the intended benefits (and possible lack of benefits), the attendant risks and inconveniences, and treatment alternatives. Patients may arrive at different conclusions regarding their own amenability to aggressive therapy (29).
The Role of Imaging in Stratifying Patients and Planning for Potential Intervention
Any patient suspected of having a DVT should be evaluated with duplex US, which has excellent sensitivity and specificity for the detection of infrainguinal DVT (61). Many patients can be evaluated up to the peripheral external iliac vein by using this technique. In patients in whom imaging is difficult, either because of obesity or severe pain with compression US, cross-sectional imaging with computed tomographic (CT) venography or magnetic resonance (MR) venography should be considered. If there is clinical suspicion for IVC involvement (eg, bilateral leg swelling, acute renal failure), cross-sectional imaging can be used to confirm the diagnosis. In the setting of renal failure, non–contrast-enhanced MR venography may be useful (Fig 6b and 6c). If the US examination is negative but there is strong clinical suspicion of a proximal DVT, CT or MR venography can be used to identify a central iliac thrombus. As discussed above, anatomic involvement plays a major role in stratifying patients. Furthermore, it informs the interventionalist of the extent of clot burden and may influence the strategy for endovascular thrombus removal.
Outcomes of Interventional DVT Therapy
The ability of CDT or PCDT to rapidly remove venous thrombus and prevent PTS in proximal DVT patients is supported by a number of comparative studies, although each had substantial methodological limitations. In 2000, Comerota et al (62) analyzed data from 68 CDT-treated acute iliofemoral DVT patients from a multicenter prospective CDT registry and found that they had fewer PTS symptoms (P = .006), better physical functioning (P = .046), less stigma of chronic venous insufficiency (P = .033), and less health distress (P = .022) at a mean follow-up of 16 months than did 30 retrospectively “matched” patients who were treated with anticoagulation alone. However, this comparison was limited by marked age differences in the two cohorts. In 2001, AbuRahma et al (63) described a prospective study in which 51 acute iliofemoral DVT patients were permitted to choose to receive adjunctive CDT (with urokinase or recombinant tissue plasminogen activator) plus anticoagulation or anticoagulation alone. The patients treated with CDT had more frequent venous patency at 6 months (83% versus 24%, P < .0001) and absence of symptoms at 5 years (78% versus 30%, P = .0015). However, this study was limited by nonrandomized design, performance in a single center, and small sample size. In 2002, Elsharawy et al (64) described a single-center Egyptian randomized trial comparing adjunctive CDT (with streptokinase) versus anticoagulation alone in 35 patients with acute iliofemoral DVT. At 6 months, patients treated with CDT had a higher rate of normal venous function (72% versus 12%, P < .001) and less valvular reflux (11% versus 41%, P = .04). However, this study was limited by small sample size and performance in a single center, and it did not evaluate clinically meaningful outcomes such as PTS and quality of life.
The most rigorous currently available data, to our knowledge, on the efficacy of CDT is derived from the multicenter randomized CaVenT Trial, published in 2012. In this study, outcomes were reported in 189 patients with femoral and/or iliac vein DVT in southern Norway who had been randomized to receive either CDT plus anticoagulation or anticoagulation alone (65). At 2-year follow-up, the relative risk of PTS was reduced by 26% with use of CDT (41.1% versus 55.6%, P = .04). A total of 3.3% of CDT-treated patients had major bleeding, of whom one required a blood transfusion and one required surgery to address. There were no CDT-related deaths or intracranial bleeds, and the authors concluded that the bleeding did not affect the patients’ ultimate outcome. This study’s applicability to clinical practice is limited by its modest size and by the fact that an older drug-only infusion CDT technique was used relative to current U.S. practice, which features widespread use of thrombectomy devices (Table 3).
Table 3.
Data from CDT Trials and Registries
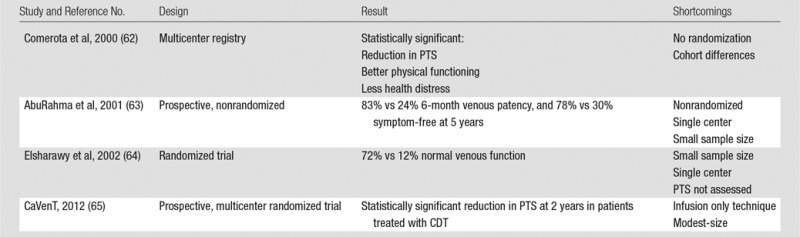
The ATTRACT trial (Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis), is an ongoing, multicenter randomized trial sponsored by the National Heart, Lung, and Blood Institute (www.clinicaltrials.gov, NCT00790335) (66). For this study, patients with symptomatic proximal DVT are being randomized in 50–60 clinical centers to receive either PCDT plus standard DVT therapy (anticoagulant therapy and elastic compression stockings) or standard DVT therapy alone. PTS is assessed at follow-up visits every 6 months during the 2-year follow-up period by using the Villalta PTS scale, a validated measure of PTS that is endorsed by the International Society of Thrombosis and Haemostasis (67,68). Secondary outcomes being assessed include venous disease-specific and generic quality of life; resolution of acute DVT symptoms (pain and swelling); rates of major bleeding, symptomatic pulmonary embolism, recurrent VTE, and death; and cost-effectiveness. ATTRACT, which has enrolled 500 patients and is expected to complete enrollment in 2014, should provide a definitive answer to the question of whether PCDT should be used as first-line therapy for proximal DVT.
Part 2: Interventional Management of Chronic Lower Extremity Deep Venous Disease
Background
As discussed above, chronic lower extremity venous disease is morbid and expensive. Venous ulcers in the United States alone are estimated to cost upward of $3 billion annually and contribute to the loss of 2 million working days, and quality of life is substantially worsened (69). Chronic venous disease can result from prior thrombosis, nonthrombotic obstruction, and/or superficial venous disease. This discussion will focus on the chronic sequelae of thrombotic and nonthrombotic deep venous disease; it is important to note that a significant number of individuals with chronic venous disease have both deep and superficial venous disease, and treating both may be necessary to alleviate symptoms (70).
Greater than 12% of chronic lower extremity venous disease is attributable to PTS (71), with the rest made up by nonthrombotic obstructive deep venous disease, venous reflux, superficial venous disease, or a combination of these. The presence of thrombus in the deep venous system results in a significant inflammatory response (72), mediated by cellular components in the venous wall and circulating leukocytes. The sequence of events is not completely understood, but incomplete fibrinolysis and thrombus fragmentation from this response result in incomplete recanalization of the occluded venous segment with intraluminal endothelial-lined pockets and webs (73,74). While the channels within these webs permit some blood flow, the segment has a higher resistance, leading to venous hypertension, which also affects valvular function if the increased capacitance does not allow valve leaflets to coapt. Collaterals subsequently develop to decompress the affected limb. Although poorly understood or characterized, the degree of collateral formation plays a major role in whether and to what extent an individual develops PTS. The majority of patients who develop the PTS will have mild or moderate forms of the disease. A minority will go on to develop severe PTS and/or venous ulcers (7).
Nonthrombotic causes of chronic lower extremity venous disease include extrinsic compression, trauma, surgery, and congenital abnormalities (49). Lower extremity central venous access, either in the setting of dialysis or acute hospitalization, can result in a deep venous stenosis or occlusion. Extrinsic compression may be secondary to nonneoplastic anatomic factors, such as May-Thurner syndrome and its variants, in which the common iliac vein is compressed between a pulsating adjacent artery, most commonly the right common iliac artery, and a vertebral body (discussed further below). Pelvic or abdominal neoplasms, lymphadenopathy, or lymphoceles may compress or obstruct the deep pelvic veins. Penetrating trauma may cause laceration or complete avulsion of the IVC or pelvic veins (discussed further below). Congenital abnormalities, such as IVC atresia, may manifest in early adolescence or adulthood as chronic venous disease.
Patient Assessment
A thorough clinical history should be obtained for any patient presenting with signs and symptoms of chronic venous disease. Contributory data include a history of VTE, any past surgeries, trauma, a history of lower extremity fistula creation or dialysis catheter insertion, remote central catheter placement, IVC filter placement, and catheterization in the right side of the heart. If the patient has a known malignancy, cross-sectional imaging may reveal obstructing abdominopelvic masses or lymphadenopathy.
Important factors include the duration and severity of symptoms. If the patient describes an acute exacerbation, acute deep venous thrombosis needs to be either diagnosed or excluded, most commonly with lower extremity duplex US evaluation. Other symptoms that are consistent with chronic venous disease include heaviness, pain, paresthesia, and fatigue, especially later in the day. Pertinent physical examination documentation includes the degree of swelling, the presence of dermatitis, and active or healed ulceration. Calf and thigh circumferences, and if possible, a photograph of the affected limb(s), establish a preintervention baseline. Involvement of the calf alone implies femoral or femoropopliteal disease, whereas thigh and calf symptoms together suggest iliofemoral obstruction. Bilateral lower extremity symptoms could indicate a caval lesion, especially with a history of IVC filter placement. Chronicling chronic venous disease with standardized scales, including the “C” or “clinical class” of the CEAP classification (Table 4) or the Villalta scale (Table 5), adds to the baseline data and quantifies disease severity. The clinical class of the CEAP assessment is based on the physical examination findings ranging from swelling to ulceration to categorize the severity of venous insufficiency, while the Villalta scale takes into account both signs and symptoms to determine the presence of mild, moderate, or severe disease.
Table 4.
Clinical Portion of CEAP Classification of Chronic Venous Disease
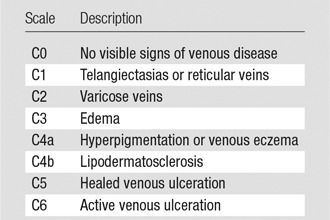
Table 5.
Villalta Scale for PTS
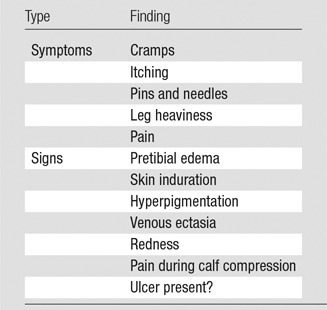
Note.—Each symptom or sign is assigned a grade of none/minimal, mild, moderate, or severe, with 0–3 points assigned for each. The presence of an ulcer automatically confers severe PTS. A score greater than 5 is considered diagnostic of PTS.
Noninterventional Management
Conservative management should be optimized for all patients with chronic venous disease. If the patient has had a DVT within the past 3 months, ensuring that she or he has not improperly terminated or been subtherapeutic on anticoagulation is essential, given that rethrombosis is a major risk factor for PTS. Moreover, many patients with PTS have an ongoing thrombotic risk, either from an obstruction or identified or occult thrombophilia, and require prolonged anticoagulation. Close partnership and consultation with hematologists ensures optimal medical management for these patients.
Compression stockings may be considered for limbs in those with chronic venous disease and may reduce symptoms and swelling in certain individuals (2). As mentioned above, they play an equivocal role in preventing PTS, but may provide symptomatic relief. Automated pneumatic compression devices can be considered for symptomatic relief in some patients (75,76), although their consistent ability to reduce PTS scores has not been documented.
Lifestyle modification, including weight loss, smoking cessation, and exercise, should be encouraged for all patients with chronic venous disease, given that the former two are risk factors for venous thrombosis and can exacerbate existing disease and the last may have some symptomatic benefit (77).
While a full discussion on optimal wound care is beyond the scope of this article, venous ulcers should be aggressively and actively treated through a combination of compression, analgesics and anti-inflammatories, targeted lymphedema therapy, and minor surgical procedures and antibiotics if necessary. In addition to these measures, pentoxifylline and micronized purified flavinoid fraction have demonstrated benefit (78,79).
Imaging Assessment
There are several indications for imaging the deep venous system in chronic venous disease: (a) worsening symptoms, (b) determining etiology, and (c) treatment planning. If the patient is presenting with an acute exacerbation of swelling or pain, lower extremity duplex US can be used to identify whether an acute DVT is the reason. If the duplex study is negative, cross-sectional imaging, either with CT or MR, can be used to uncover acute iliocaval thrombosis (Fig 6).
Figure 6a:
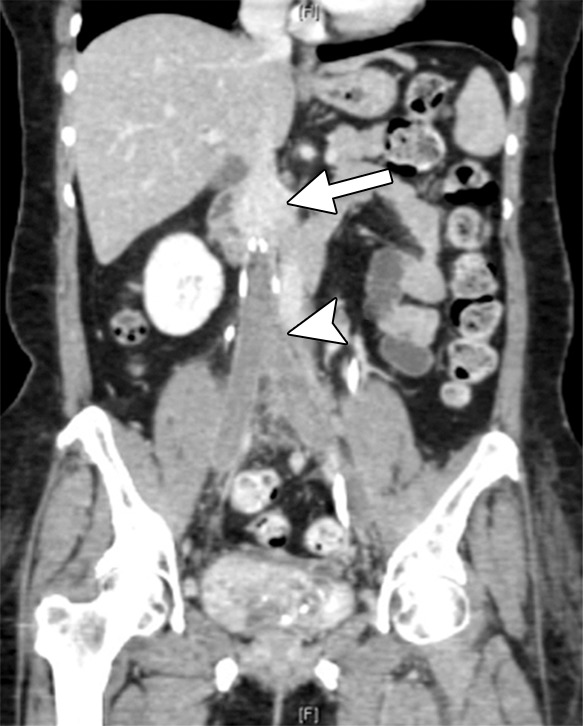
CT and MR appearance of acute iliocaval thrombosis. (a) CT image in a patient with a retrievable infrarenal filter with acute thrombus expanding the IVC and iliac veins (arrowhead). Above the filter, the IVC is patent (arrow). (b, c) Ultrafast T2-weighted coronal MR images in a different patient with acute iliocaval thrombus depict (b) low signal intensity within the IVC (arrow) consistent with thrombus, and (c) a patent intrahepatic IVC (arrow).
If the presenting symptoms are more chronic, imaging can often be used to determine the cause. Duplex US can frequently be used to evaluate up to the external iliac vein, especially in thin patients. It can be used to identify areas of chronic thrombosis, narrowing, or wall thickening, and can be used to assess which venous segments are patent. More central obstructions can be inferred from waveform analysis. Deep and superficial venous reflux can also be documented (80). Cross-sectional imaging, either with CT or MR venography, can be used to detect extrinsic (masses/lymphadenopathy) or intrinsic lesions (81,82). CT is usually more diagnostic than MR if an IVC filter is present, as some filters contain metallic elements that result in considerable MR signal loss. CT assessment in the setting of an IVC filter can be used to confirm the presence or absence of caval narrowing or chronic thrombosis (Fig 7). The caliber of the deep pelvic veins and IVC can also be used to assess noninvasively with MR or CT venography, although the diameter needs to be interpreted with caution, since respiratory variability, position, and hydration status affect this measurement. However, if there is an abrupt caliber change, real stenosis should be entertained (Fig 8). Quality CT venography or high spatial and contrast resolution MR imaging, particularly with venous blood pool agents, may be used to detect intraluminal webs that form from repeated trauma from arterial pulsations and compression.
Figure 7a:
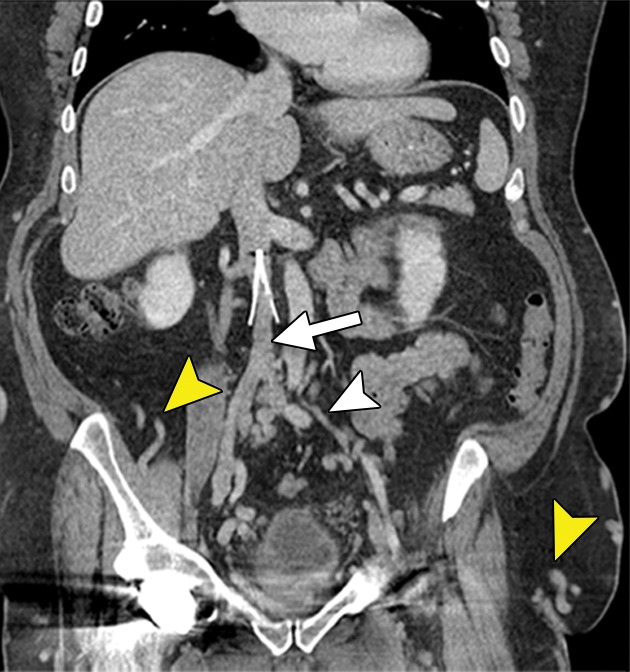
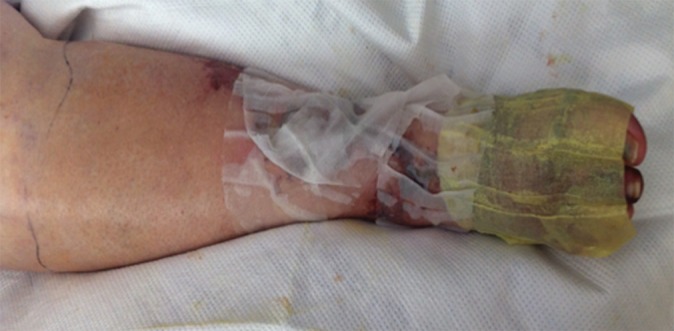
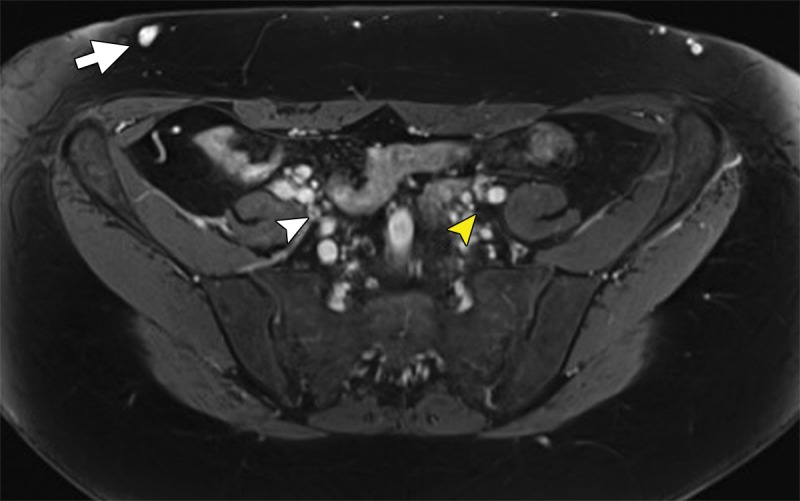
Chronic venous disease from filter-induced caval narrowing. (a) Coronal contrast-enhanced CT reformation demonstrates a chronically indwelling Greenfield infrarenal IVC filter with caval narrowing (arrow) and markedly atretic left common iliac vein (white arrowhead) with intraabdominal and body-wall collaterals (yellow arrowheads). (b) Patient’s leg demonstrates characteristic changes, including redness, swelling, hyperpigmentation, and ulceration treated with a medicated dressing. (c) Right iliac venogram illustrates an atretic common iliac vein and caudal IVC with marked collateralization.
Figure 8a:
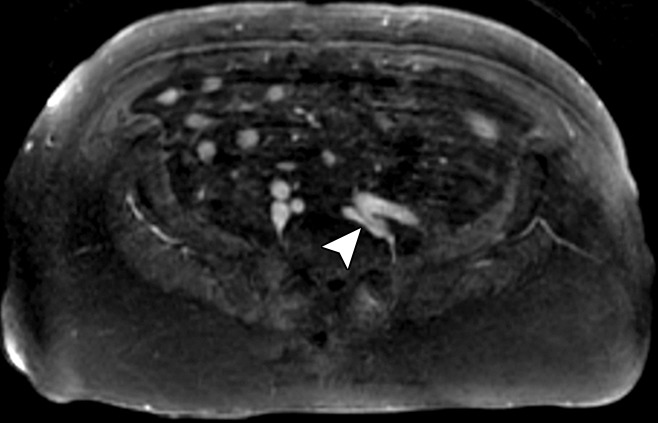
MR appearance of May-Thurner variant. (a) On the high-spatial-resolution blood-pool steady-state axial MR image, the left common iliac vein is compressed (arrowhead) between the left internal iliac artery and vertebral body. (b) The patient’s left leg has altered pigmentation, swelling, and an ulcer overlying the anterior tibia.
Figure 7b:
Chronic venous disease from filter-induced caval narrowing. (a) Coronal contrast-enhanced CT reformation demonstrates a chronically indwelling Greenfield infrarenal IVC filter with caval narrowing (arrow) and markedly atretic left common iliac vein (white arrowhead) with intraabdominal and body-wall collaterals (yellow arrowheads). (b) Patient’s leg demonstrates characteristic changes, including redness, swelling, hyperpigmentation, and ulceration treated with a medicated dressing. (c) Right iliac venogram illustrates an atretic common iliac vein and caudal IVC with marked collateralization.
Figure 7c:
Chronic venous disease from filter-induced caval narrowing. (a) Coronal contrast-enhanced CT reformation demonstrates a chronically indwelling Greenfield infrarenal IVC filter with caval narrowing (arrow) and markedly atretic left common iliac vein (white arrowhead) with intraabdominal and body-wall collaterals (yellow arrowheads). (b) Patient’s leg demonstrates characteristic changes, including redness, swelling, hyperpigmentation, and ulceration treated with a medicated dressing. (c) Right iliac venogram illustrates an atretic common iliac vein and caudal IVC with marked collateralization.
Figure 8b:
MR appearance of May-Thurner variant. (a) On the high-spatial-resolution blood-pool steady-state axial MR image, the left common iliac vein is compressed (arrowhead) between the left internal iliac artery and vertebral body. (b) The patient’s left leg has altered pigmentation, swelling, and an ulcer overlying the anterior tibia.
Imaging can also aid in treatment planning. In the setting of an IVC filter, either contrast-enhanced or unenhanced CT can be used to identify the type of filter, its position in the cava, the direction of the hook (if present), and leg penetration. Such information can be valuable if a complex retrieval is planned (83). US, CT, and MR can all be useful in determining the extent of venous involvement, which in turn informs the interventionalist about the best potential site of entry into the venous system (eg, the internal jugular vein, common femoral vein, or popliteal vein). The level of obstruction can also be inferred by the presence of body wall, cross-pelvic, or thigh collaterals.
Patient Selection and Preparation
After conservative management has been optimized with the strategies described above, many patients may be candidates for endovascular intervention. Before the advent of these techniques, patients underwent surgical bypass (84). Now, surgery is considered in specialized centers for those patients in whom endovascular techniques fail or for whom endovascular techniques are not possible. A full discussion of surgical options for these patients is beyond the scope of this article. Patients should be selected for interventional procedures by balancing the likelihood of improving their symptoms with the risk of procedural complications. Additionally, since many PTS patients require angioplasty and/or stent placement, they should be candidates for anticoagulation to prevent early rethrombosis (70). Patient work-up should include a complete blood count, basic metabolic panel, and coagulation parameters, with particular attention paid to renal function, platelet count, and the international normalized ratio. Many interventionalists will perform these procedures while there is full anticoagulation in the patient, given the propensity toward intraprocedure thrombosis. Procedures can be lengthy, so a patient needs to be able to tolerate moderate sedation. Additionally, prone positioning is required if the popliteal vein is to be accessed. For individuals unable to tolerate a lengthy or positionally challenging procedure, general anesthesia may be required.
Interventional Treatment of Iliocaval Stenoses and Occlusions
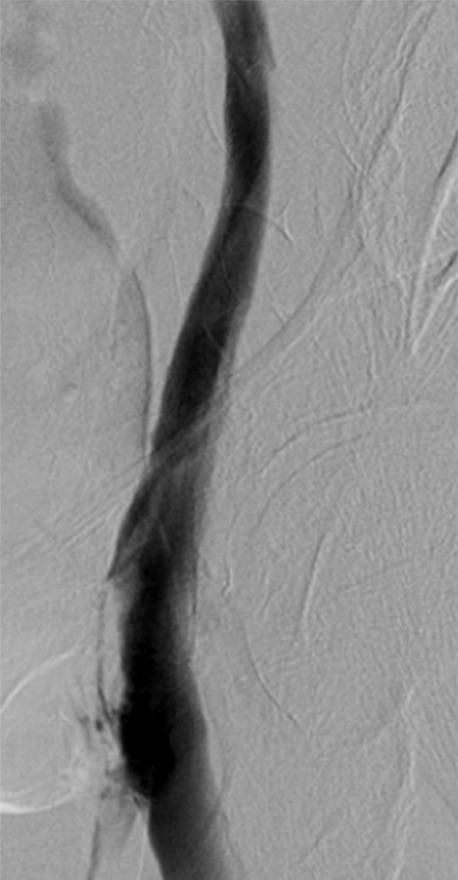
Chronic iliocaval obstructions may be postthrombotic, compressive, or a combination. Depending on the severity and duration of the obstruction, the vein may appear markedly diminished or even nonvisible on cross-sectional images (Fig 9). This finding does not necessarily mean that the lesion cannot undergo recanalization, as frequently there is an infundibulum that leads into the atretic lumen that can be traversed with a wire. Specific scenarios are discussed below.
Figure 9:
MR appearance of postthrombotic iliac veins. Contrast-enhanced blood pool axial image demonstrates no visible left common iliac vein (yellow arrowhead) and an atretic right common iliac vein (white arrowhead), with body wall collaterals (arrow).
IVC obstruction or stenosis caused by a chronically embedded IVC filter.—More scrutiny has been placed on IVC filters in recent years, due to increased awareness of complications including migration, penetration, and fracture. Another known complication is caval stenosis (85). Whether this stenosis is secondary to thrombus formation in the filter that propagates caudally or a primary venous response is unclear. Regardless, a certain number of patients develop symptoms from this stenosis, which can extend into the iliac veins and result in recurrent thrombotic episodes. The PREPIC trial from the late 1990s demonstrated that patients with IVC filters were found to have a higher rate of DVT than those without, although the overall rate of VTE was found to be similar because of a lower incidence of pulmonary embolism (86). To our knowledge, the relationship of IVC filters to the development of PTS is unclear. In a nonrandomized retrospective study from 2007, patients without DVTs who had prophylactic filters placed developed PTS at a similar rate to patients with proximal DVTs without filters. Patients with DVTs that required caval filtration had a higher rate of PTS, with approximately 50% developing the syndrome and approximately 14% developing severe PTS (87). Hence, while there may be a relationship between IVC filters and PTS, no randomized trial has confirmed an association, to our knowledge.
Patients with IVC filters who have symptomatic PTS or penetration-associated morbidity (bowel perforation, pain) should be evaluated for need for the filter (ie, continued high risk of pulmonary embolism and contraindication to anticoagulation), the integrity of the device, and IVC patency. When IVC recanalization is planned, the identity of the filter should be determined with certainty, as retrieval may be considered prior to IVC angioplasty and/or stent placement. CT scans in the venous phase provide excellent information about filter position, penetration, and caval patency. Thin CT reformations and scout images can be used to assess filter integrity and the brand of device implanted. If necessary, the high spatial resolution of abdominal radiographs can aid in these assessments (83). In general, filters with extensive IVC wall contact are more difficult to retrieve after extended indwell times (88).
Well-centered and positioned filters can often be retrieved by using standard snares and sheaths. Devices that are tilted such that the hook is covered by neo-endothelium may be removed by using more advanced techniques such as rigid bronchoscopy forceps (89). When the device elements are firmly adherent to the wall of the IVC, removal can be accomplished using an excimer laser sheath (90). Open-design filters with deeply penetrated legs or arms, including those that penetrated adjacent structures, can be removed safely provided that the filter hook can be engaged (91). Advanced removal techniques should be performed by experienced individuals and after fully considering all of the management options. Major complications are rare but have occurred, including IVC disruption and fragmentation of filters with central embolization to the heart and pulmonary circulation (92). Kuo et al (90) cited a 3% major complication rate and a 7% minor complication rate in their series of 100 consecutive patients undergoing complex filter removal with excimer laser assistance.
Filters associated with IVC stenosis or occlusion that cannot be removed can be managed by placement of stents through the filter followed by angioplasty to collapse and displace the filter elements (93). A stent with sufficient radial force or postangioplasty rigidity should be used. Stents may extend to a suprarenal location; noncovered stents should be deployed to avoid blocking renal veins inflow. This approach increases the luminal diameter of the IVC and traps the filter between the stent and the IVC wall. Subsequent filter removal, if necessary, would require surgical access to the IVC. The long-term patency rates of stents placed through IVC filters is not known, to our knowledge.
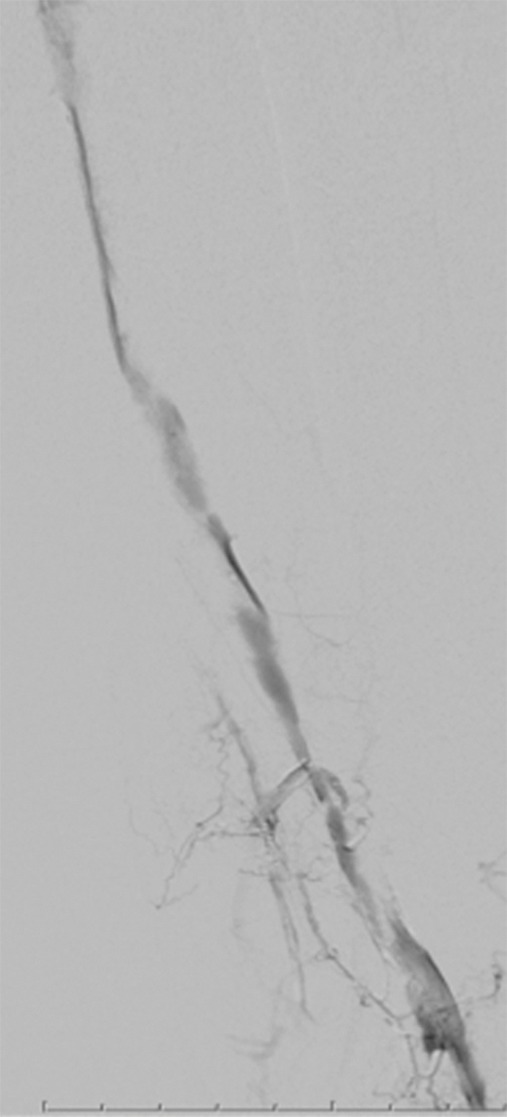
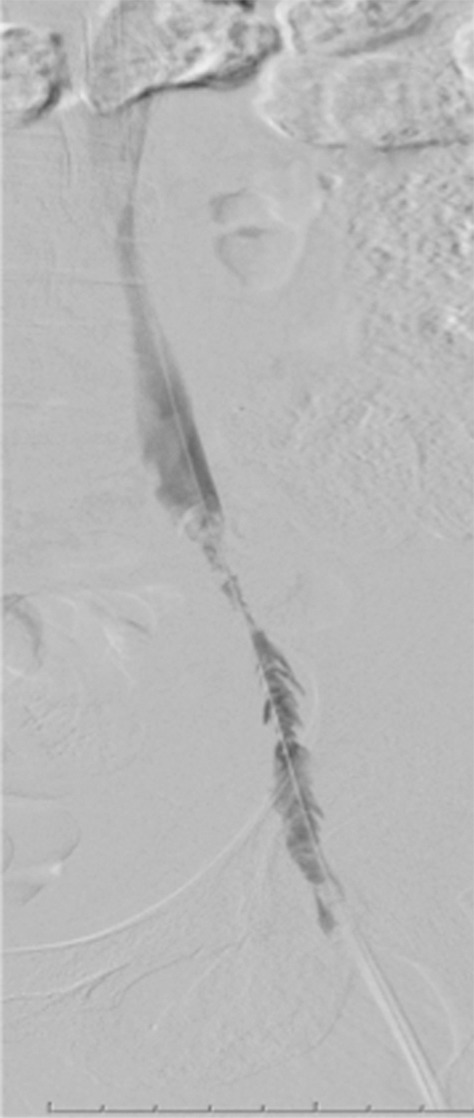
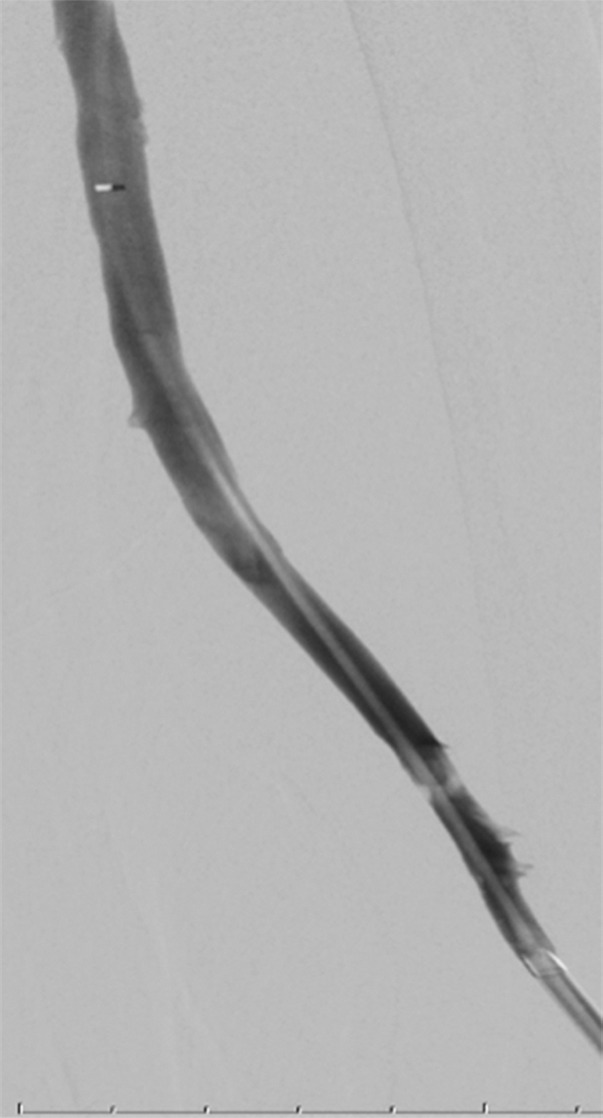
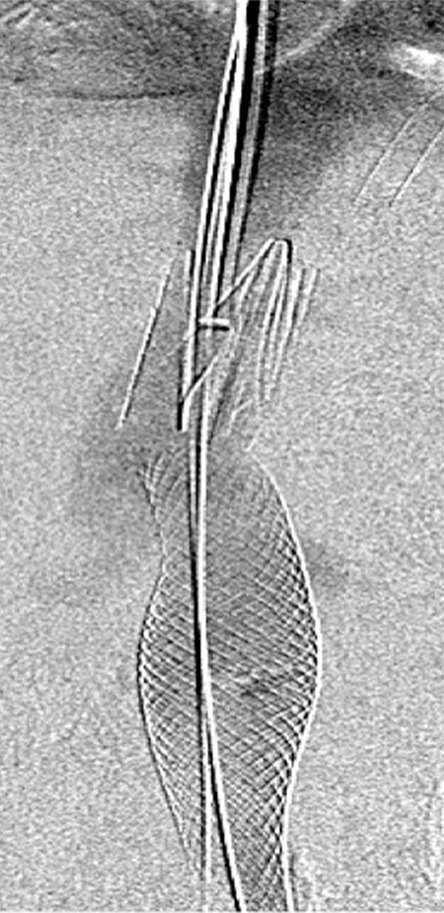
Endovascular treatment of caval stenosis, occlusion, or absence.—Either because of prior surgical ligation, trauma, atresia, or long-standing thrombotic disease, the IVC may be markedly narrowed, stenotic, or completely absent for a portion of its length. Surgical reconstruction can be a morbid and difficult procedure. For this reason, endovascular techniques ranging from wire manipulation to sharp recanalization have been used with success to traverse obstructions or connect discontinuous caval segments (Fig 10) (94). If conventional wire manipulation is not possible due to a recalcitrant obstruction or a completely discontinuous cava, sharp recanalization by means of the back end of a stiff wire, a thin-gauge needle, or powered wires (ie, radiofrequency/laser-assisted) may be used (95). While these latter wires have the ability to penetrate through nearly any obstruction, they are no more directable than other sharp tools and, given the three-dimensional limitations of fluoroscopy, may not offer much more benefit except in very specific circumstances. Moreover, these techniques are new and unproven and require substantial expertise and experience to be used safely. If sharp recanalization is necessary, access is most commonly gained from above and below. Sharp techniques are used on one or both ends until the recanalization instruments are near one another. A snare catheter is used to grasp the wire coming from the other access site. The snared wire is then pulled through the first access site, and through and through access is achieved. After balloon dilatation, a stent is placed that bridges the discontinuous or obstructed segment. The stent is most commonly 20–24 mm in size and can be uncovered and self-expanding. Even in the setting of complete caval discontinuity, the retroperitoneal fat provides a sufficient “wall” for an uncovered stent. Internal balloon angioplasty then expands the stent to its desired diameter, and the gap between IVC segments is effectively bridged (Fig 11).
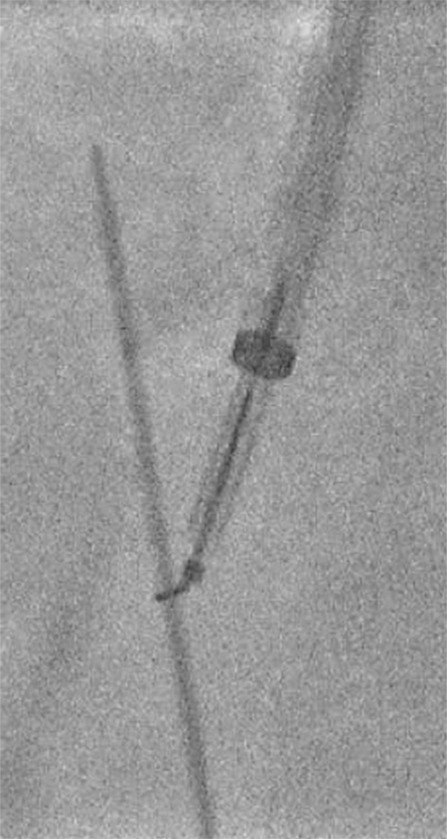
Figure 10a:
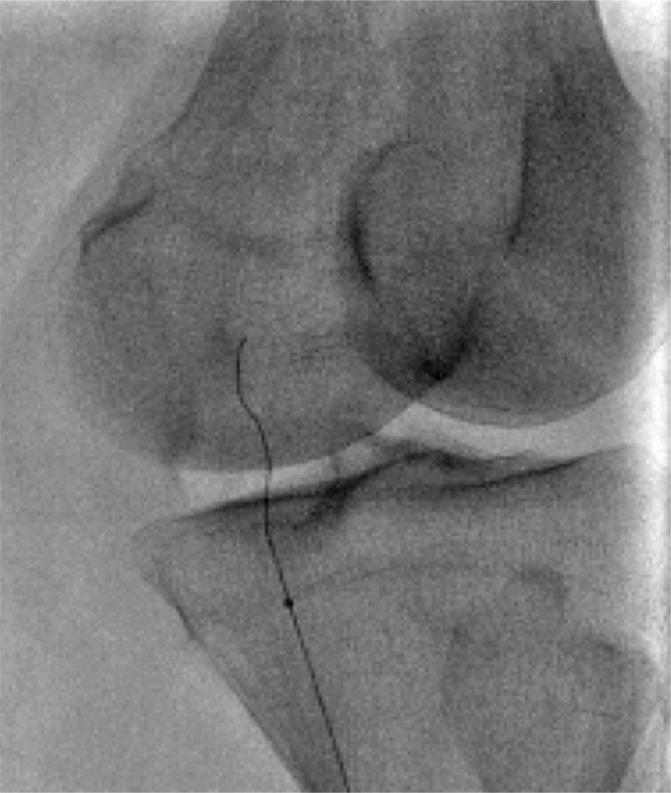
Examples of chronic recanalization techniques. (a) Combination of a stiff-tipped hydrophilic catheter guided by an angled stiff hydrophilic wire. This combination is useful in traversing most chronic venous occlusions. (b) Fluoroscopic image obtained during recanalization of an occluded stent demonstrates the back end of a stiff wire within a metal cannula contained within a sheath. The combination is advanced along the length of the occluded stent until the other side is reached.
Figure 11a:
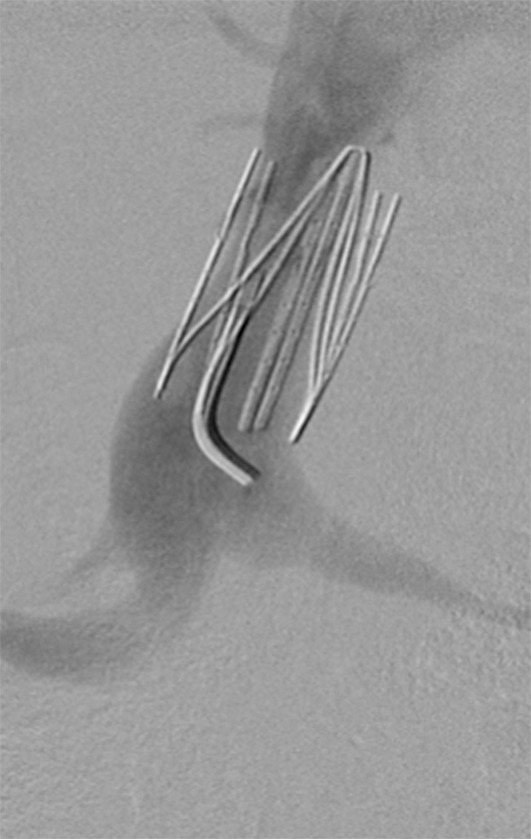
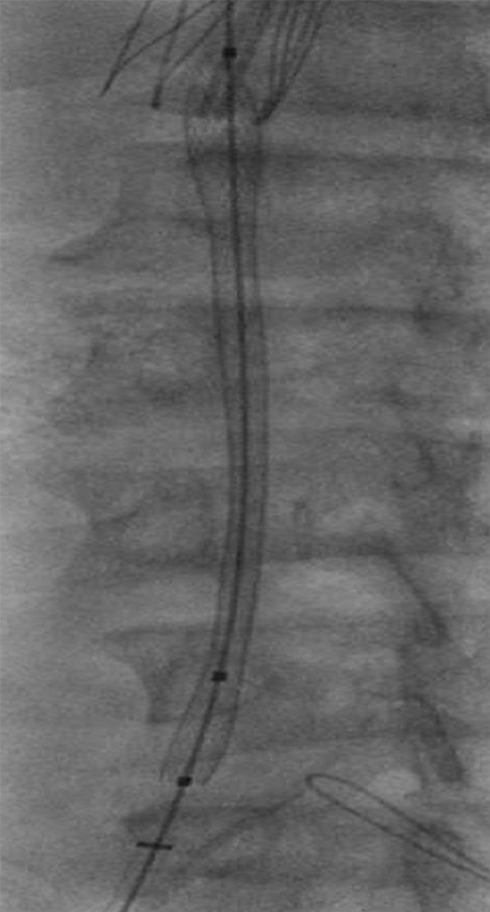
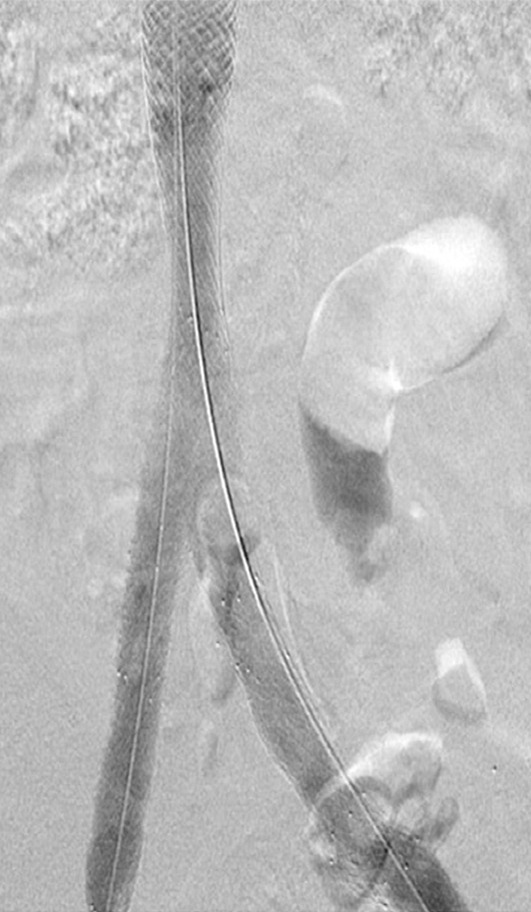
Creation of a neo-IVC 17 years after traumatic laceration. (a) Transjugular IVC contrast material injection demonstrates no detectable infrarenal IVC. (b) Right iliac venogram shows numerous collaterals and diminutive native vein. (c) Spot fluoroscopic image demonstrates a snare within a sheath that has been advanced through sharp recanalization (arrow) and two wires via bilateral common femoral veins (arrowheads). The right iliac wire has entered a lumbar collateral (based on subsequent imaging) and is not in the native IVC. (d) Spot fluoroscopic image of the snare cinching the back end of a stiff wire within the retroperitoneal fat. (e) Fluoroscopic image of undilated self-expanding stainless steel stent spanning the length of the absent IVC. (f, g) Digital subtraction venograms demonstrate brisk flow through the iliac and IVC stents into the suprarenal IVC and right atrium. See also Figs E2a–E2d in this patient (online). (Case courtesy of Thomas Sos, MD, and Akhilesh Sista, MD, Weill Cornell Medical College.)
Figure 10b:
Examples of chronic recanalization techniques. (a) Combination of a stiff-tipped hydrophilic catheter guided by an angled stiff hydrophilic wire. This combination is useful in traversing most chronic venous occlusions. (b) Fluoroscopic image obtained during recanalization of an occluded stent demonstrates the back end of a stiff wire within a metal cannula contained within a sheath. The combination is advanced along the length of the occluded stent until the other side is reached.
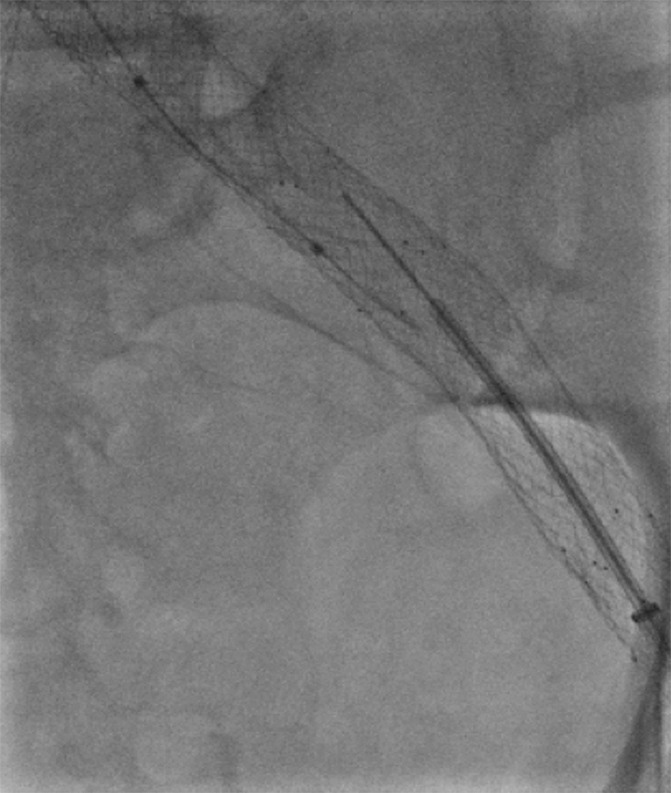
Figure 11b:
Creation of a neo-IVC 17 years after traumatic laceration. (a) Transjugular IVC contrast material injection demonstrates no detectable infrarenal IVC. (b) Right iliac venogram shows numerous collaterals and diminutive native vein. (c) Spot fluoroscopic image demonstrates a snare within a sheath that has been advanced through sharp recanalization (arrow) and two wires via bilateral common femoral veins (arrowheads). The right iliac wire has entered a lumbar collateral (based on subsequent imaging) and is not in the native IVC. (d) Spot fluoroscopic image of the snare cinching the back end of a stiff wire within the retroperitoneal fat. (e) Fluoroscopic image of undilated self-expanding stainless steel stent spanning the length of the absent IVC. (f, g) Digital subtraction venograms demonstrate brisk flow through the iliac and IVC stents into the suprarenal IVC and right atrium. See also Figs E2a–E2d in this patient (online). (Case courtesy of Thomas Sos, MD, and Akhilesh Sista, MD, Weill Cornell Medical College.)
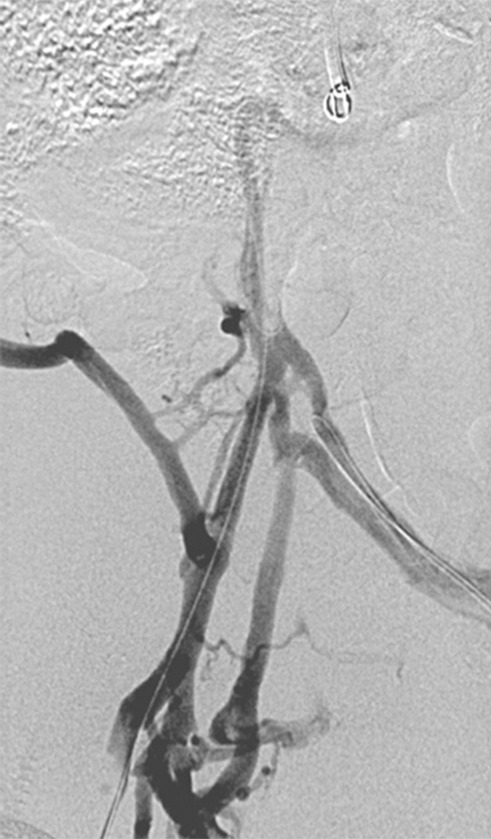
Figure 11c:
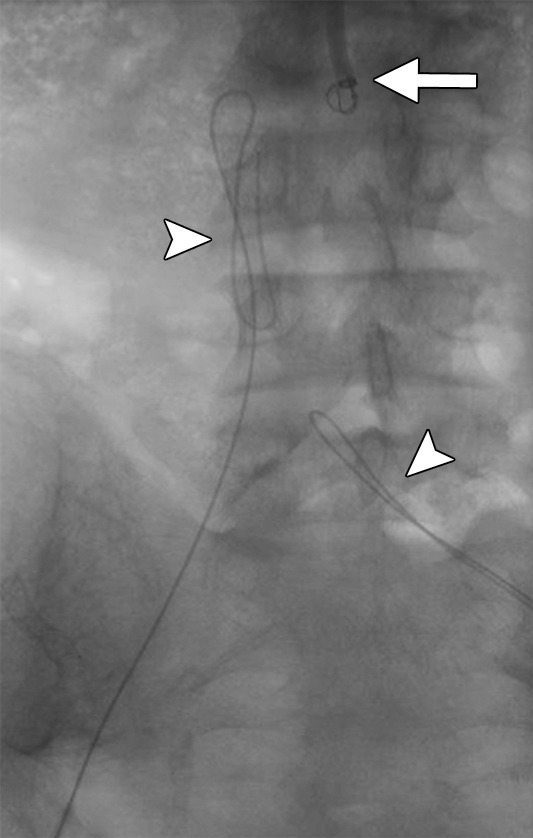
Creation of a neo-IVC 17 years after traumatic laceration. (a) Transjugular IVC contrast material injection demonstrates no detectable infrarenal IVC. (b) Right iliac venogram shows numerous collaterals and diminutive native vein. (c) Spot fluoroscopic image demonstrates a snare within a sheath that has been advanced through sharp recanalization (arrow) and two wires via bilateral common femoral veins (arrowheads). The right iliac wire has entered a lumbar collateral (based on subsequent imaging) and is not in the native IVC. (d) Spot fluoroscopic image of the snare cinching the back end of a stiff wire within the retroperitoneal fat. (e) Fluoroscopic image of undilated self-expanding stainless steel stent spanning the length of the absent IVC. (f, g) Digital subtraction venograms demonstrate brisk flow through the iliac and IVC stents into the suprarenal IVC and right atrium. See also Figs E2a–E2d in this patient (online). (Case courtesy of Thomas Sos, MD, and Akhilesh Sista, MD, Weill Cornell Medical College.)
Figure 11d:
Creation of a neo-IVC 17 years after traumatic laceration. (a) Transjugular IVC contrast material injection demonstrates no detectable infrarenal IVC. (b) Right iliac venogram shows numerous collaterals and diminutive native vein. (c) Spot fluoroscopic image demonstrates a snare within a sheath that has been advanced through sharp recanalization (arrow) and two wires via bilateral common femoral veins (arrowheads). The right iliac wire has entered a lumbar collateral (based on subsequent imaging) and is not in the native IVC. (d) Spot fluoroscopic image of the snare cinching the back end of a stiff wire within the retroperitoneal fat. (e) Fluoroscopic image of undilated self-expanding stainless steel stent spanning the length of the absent IVC. (f, g) Digital subtraction venograms demonstrate brisk flow through the iliac and IVC stents into the suprarenal IVC and right atrium. See also Figs E2a–E2d in this patient (online). (Case courtesy of Thomas Sos, MD, and Akhilesh Sista, MD, Weill Cornell Medical College.)
Figure 11e:
Creation of a neo-IVC 17 years after traumatic laceration. (a) Transjugular IVC contrast material injection demonstrates no detectable infrarenal IVC. (b) Right iliac venogram shows numerous collaterals and diminutive native vein. (c) Spot fluoroscopic image demonstrates a snare within a sheath that has been advanced through sharp recanalization (arrow) and two wires via bilateral common femoral veins (arrowheads). The right iliac wire has entered a lumbar collateral (based on subsequent imaging) and is not in the native IVC. (d) Spot fluoroscopic image of the snare cinching the back end of a stiff wire within the retroperitoneal fat. (e) Fluoroscopic image of undilated self-expanding stainless steel stent spanning the length of the absent IVC. (f, g) Digital subtraction venograms demonstrate brisk flow through the iliac and IVC stents into the suprarenal IVC and right atrium. See also Figs E2a–E2d in this patient (online). (Case courtesy of Thomas Sos, MD, and Akhilesh Sista, MD, Weill Cornell Medical College.)
Figure 11f:
Creation of a neo-IVC 17 years after traumatic laceration. (a) Transjugular IVC contrast material injection demonstrates no detectable infrarenal IVC. (b) Right iliac venogram shows numerous collaterals and diminutive native vein. (c) Spot fluoroscopic image demonstrates a snare within a sheath that has been advanced through sharp recanalization (arrow) and two wires via bilateral common femoral veins (arrowheads). The right iliac wire has entered a lumbar collateral (based on subsequent imaging) and is not in the native IVC. (d) Spot fluoroscopic image of the snare cinching the back end of a stiff wire within the retroperitoneal fat. (e) Fluoroscopic image of undilated self-expanding stainless steel stent spanning the length of the absent IVC. (f, g) Digital subtraction venograms demonstrate brisk flow through the iliac and IVC stents into the suprarenal IVC and right atrium. See also Figs E2a–E2d in this patient (online). (Case courtesy of Thomas Sos, MD, and Akhilesh Sista, MD, Weill Cornell Medical College.)
Figure 11g:
Creation of a neo-IVC 17 years after traumatic laceration. (a) Transjugular IVC contrast material injection demonstrates no detectable infrarenal IVC. (b) Right iliac venogram shows numerous collaterals and diminutive native vein. (c) Spot fluoroscopic image demonstrates a snare within a sheath that has been advanced through sharp recanalization (arrow) and two wires via bilateral common femoral veins (arrowheads). The right iliac wire has entered a lumbar collateral (based on subsequent imaging) and is not in the native IVC. (d) Spot fluoroscopic image of the snare cinching the back end of a stiff wire within the retroperitoneal fat. (e) Fluoroscopic image of undilated self-expanding stainless steel stent spanning the length of the absent IVC. (f, g) Digital subtraction venograms demonstrate brisk flow through the iliac and IVC stents into the suprarenal IVC and right atrium. See also Figs E2a–E2d in this patient (online). (Case courtesy of Thomas Sos, MD, and Akhilesh Sista, MD, Weill Cornell Medical College.)
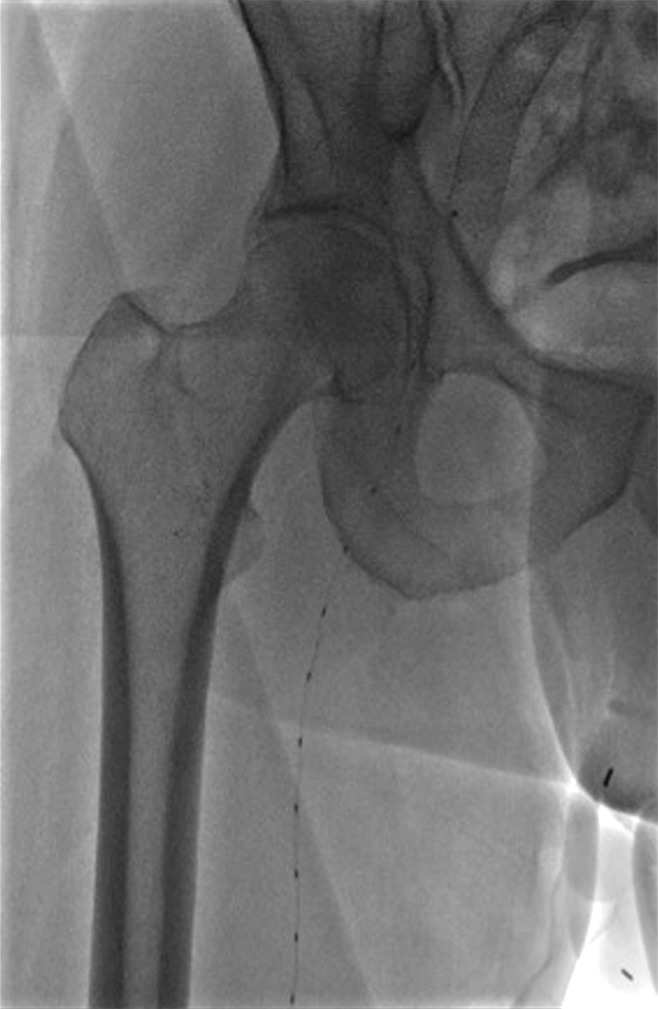
Interventional treatment of May-Thurner syndrome and its variants.—The May-Thurner compression is a controversial topic for radiologists, since most individuals with this anatomic finding are asymptomatic. In one study, the mean compression of the left common iliac vein by the crossing artery in individuals scanned for other reasons was greater than 30% (96). Classic May-Thurner syndrome represents a compression of the left common iliac vein between the crossing right common iliac artery and the vertebral body. The chronic compression is thought to lead to endothelial damage, and a fibrotic response can lead to webs within the venous lumen. Variants can occur anywhere along the length of the iliac vein, including at the bifurcation of the common iliac artery. There are several lines of evidence that indicate that May-Thurner syndrome is significant for some individuals. In nonthrombotic patients with left lower extremity swelling and no other identifiable cause, correcting the obstruction leads to symptomatic relief (97). Moreover, it is considered a risk factor for DVT. In a case-control series, the degree of compression correlated with the likelihood of DVT development (98). Furthermore, when combined with another risk factor, such as oral contraceptive use, the odds of developing a DVT are multiplicative (99). It is important to recognize that nonthrombotic compression is a risk factor for thrombosis. While the mechanism is not clearly understood, any stenotic lesion can result in stasis, endothelial disruption, and turbulent flow, promoting thrombus formation. Many of these individuals develop significant chronic venous disease.
It is important to distinguish thrombotic from nonthrombotic May-Thurner syndrome, as treatment of nonthrombotic disease is usually a simpler procedure and arguably requires less anticoagulation after stent placement. On the other hand, May-Thurner syndrome complicated by thrombosis will likely require more extensive stent procedures, possibly a lower access point if the disease extends infrainguinally, and longer anticoagulation after treatment.
Nonthrombotic May-Thurner syndrome can usually be treated in the supine position. Access is gained via the ipsilateral common femoral vein, and a vascular sheath is placed. After the lesion is traversed with a wire, venography will classically show a flattened common iliac vein just peripheral to the caval bifurcation, with cross-pelvic and lumbar collaterals (Fig 12). Intravascular US can be used to assess the lumen of the iliac vein at an area of stenosis (Fig 13). It can reveal intraluminal webs and be used to measure a comparative cross-sectional diameter. Intravascular US is more sensitive than venography for the detection of luminal narrowing (100); however, it is unclear whether or to what degree this increased sensitivity should be acted on. If the classic venographic findings are present and the patient is symptomatic, most practitioners will place a stent in the lesion. Self-expanding nitinol or stainless steel stents are most commonly used and are typically 14–16 mm in size (101–103). They are extended either to the iliac bifurcation or into the IVC. Appropriate stent sizing is important to prevent migration and edge stenoses. Venography after stent placement typically demonstrates resolution of collateral vessels and brisk flow into the cava.
Figure 12:
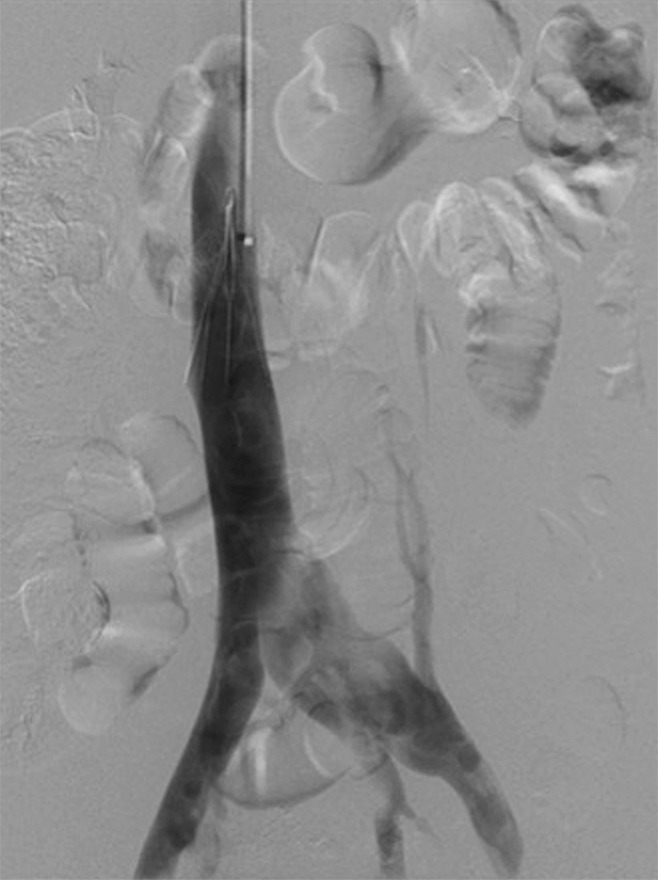
May-Thurner syndrome. Inferior vena cavagram of procedure performed from an internal jugular venous approach demonstrates flattening/effacement of the left common iliac vein with a well-formed lumbar collateral. Also seen is an infrarenal IVC filter.
Figure 13a:
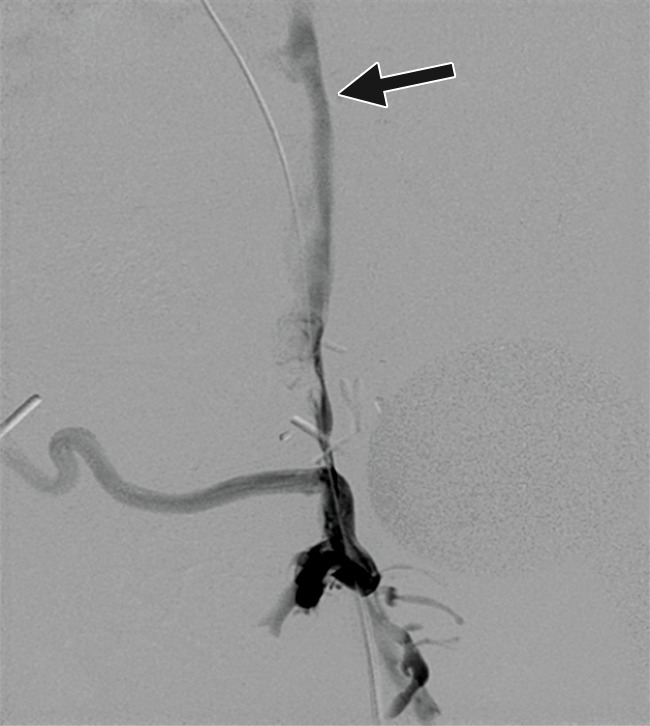
Intravascular US before and after stent placement. (a) Left iliac venogram demonstrates an occlusive lesion in the peripheral external iliac vein (arrow). (b) Intravascular US image at the level of compression. The arrows delineate the estimated borders of the narrowed venous segment. (c) Intravascular US image of the common iliac vein just central to the stenosis in image b. (d) Venogram obtained after stent placement demonstrates markedly improved flow and caliber of the left pelvic deep veins. (e) Intravascular US image obtained after stent placement at the level of the stenosis in image b reveals the improved caliber and the hyperechoic stent in cross section.
Figure 13b:
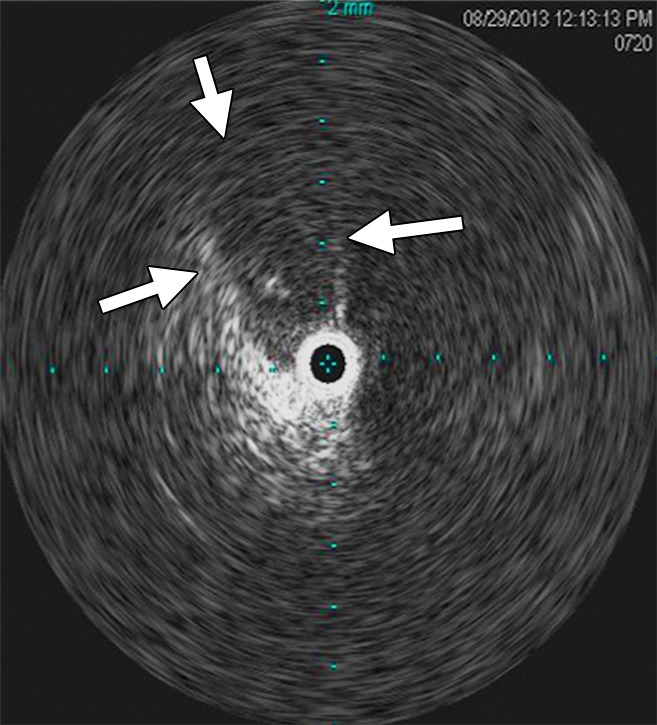
Intravascular US before and after stent placement. (a) Left iliac venogram demonstrates an occlusive lesion in the peripheral external iliac vein (arrow). (b) Intravascular US image at the level of compression. The arrows delineate the estimated borders of the narrowed venous segment. (c) Intravascular US image of the common iliac vein just central to the stenosis in image b. (d) Venogram obtained after stent placement demonstrates markedly improved flow and caliber of the left pelvic deep veins. (e) Intravascular US image obtained after stent placement at the level of the stenosis in image b reveals the improved caliber and the hyperechoic stent in cross section.
Figure 13c:
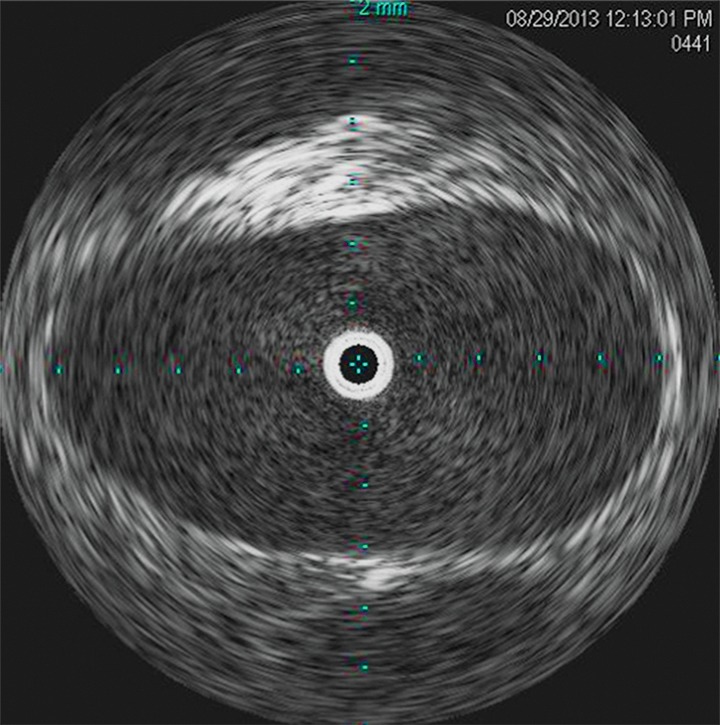
Intravascular US before and after stent placement. (a) Left iliac venogram demonstrates an occlusive lesion in the peripheral external iliac vein (arrow). (b) Intravascular US image at the level of compression. The arrows delineate the estimated borders of the narrowed venous segment. (c) Intravascular US image of the common iliac vein just central to the stenosis in image b. (d) Venogram obtained after stent placement demonstrates markedly improved flow and caliber of the left pelvic deep veins. (e) Intravascular US image obtained after stent placement at the level of the stenosis in image b reveals the improved caliber and the hyperechoic stent in cross section.
Figure 13d:
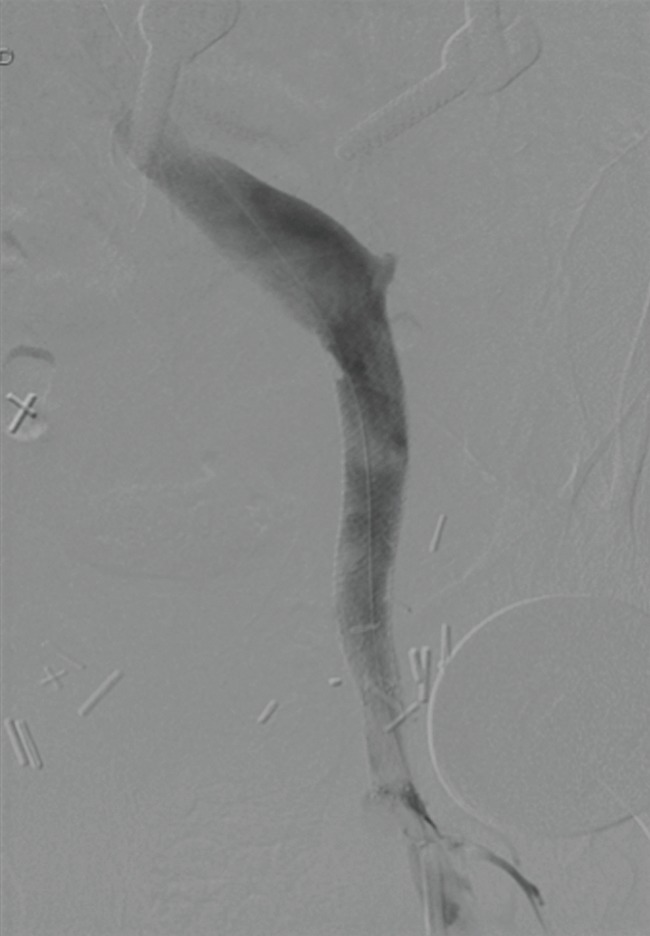
Intravascular US before and after stent placement. (a) Left iliac venogram demonstrates an occlusive lesion in the peripheral external iliac vein (arrow). (b) Intravascular US image at the level of compression. The arrows delineate the estimated borders of the narrowed venous segment. (c) Intravascular US image of the common iliac vein just central to the stenosis in image b. (d) Venogram obtained after stent placement demonstrates markedly improved flow and caliber of the left pelvic deep veins. (e) Intravascular US image obtained after stent placement at the level of the stenosis in image b reveals the improved caliber and the hyperechoic stent in cross section.
Figure 13e:
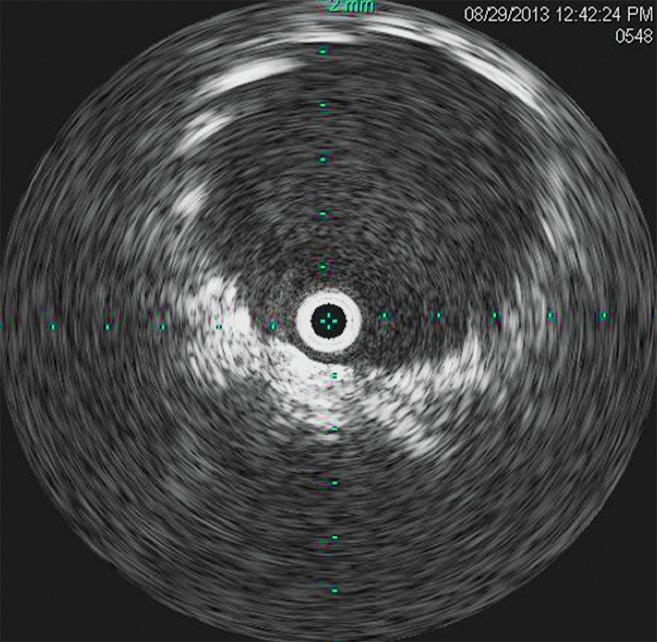
Intravascular US before and after stent placement. (a) Left iliac venogram demonstrates an occlusive lesion in the peripheral external iliac vein (arrow). (b) Intravascular US image at the level of compression. The arrows delineate the estimated borders of the narrowed venous segment. (c) Intravascular US image of the common iliac vein just central to the stenosis in image b. (d) Venogram obtained after stent placement demonstrates markedly improved flow and caliber of the left pelvic deep veins. (e) Intravascular US image obtained after stent placement at the level of the stenosis in image b reveals the improved caliber and the hyperechoic stent in cross section.
Thrombotic May-Thurner syndrome is a more difficult disease to treat, as the postthrombotic vein peripheral to the lesion is frequently scarred and diminutive. Moreover, disease may extend below the inguinal ligament, requiring access from a lower venous segment. While some practitioners will access the femoral vein, most select the popliteal vein for initial access. While the former offers the convenience of keeping the patient supine, it is difficult to compress the femoral vein, which runs deep within the thigh, and a postprocedural hematoma may develop. Popliteal access requires the patient to be positioned prone. Once the obstruction is reached, venography typically shows abrupt tapering of the native channel with marked collateralization around the obstruction. Recanalization of the atretic postthrombotic veins can be challenging, especially in long-standing obstructions (>1 year). Hydrophilic wires and catheters can usually traverse such obstructions; however, occasionally sharp recanalization techniques are needed. Once the thrombotic lesion is crossed, angioplasty is sometimes required before stent placement to allow passage of larger catheters and sheaths. Stents are deployed along the entire length of the obstruction and are frequently extended below the inguinal ligament into the common femoral vein. Doing so has not adversely affected stent patency and, while there is mobility across the inguinal ligament, self-expanding stents in this location perform well (70,101). Extending stents into the femoral vein is not routinely recommended, as stent patency in the femoral vein has been historically poor (45). Some also argue that stents compromise valvular function in the femoral vein and could lead to reflux. However, it should be noted that inflow into the pelvic stents is absolutely essential to maintain patency, which in some cases can only be achieved by stent placement into the femoral or deep femoral vein. Venography performed after stent placement typically demonstrates brisk flow through the treated lesion with resolution of collaterals (Fig 14).
Figure 14a:
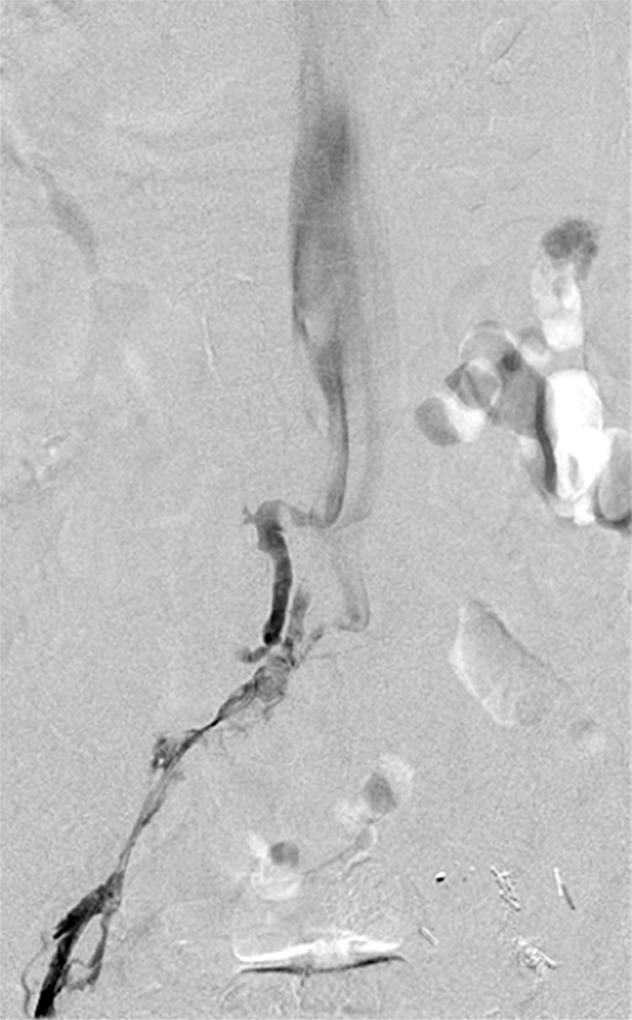
May-Thurner syndrome complicated by chronic thrombosis. (a) Venogram of the left common iliac vein (patient is prone) demonstrates chronic effacement and thrombosis of the proximal left common iliac vein and an atretic and postthrombotic left iliac venous system. (b) Venogram obtained after stent placement demonstrates brisk flow from the left common femoral vein into the IVC. See also Figs E3a and E3b in this patient (online).
Figure 14b:
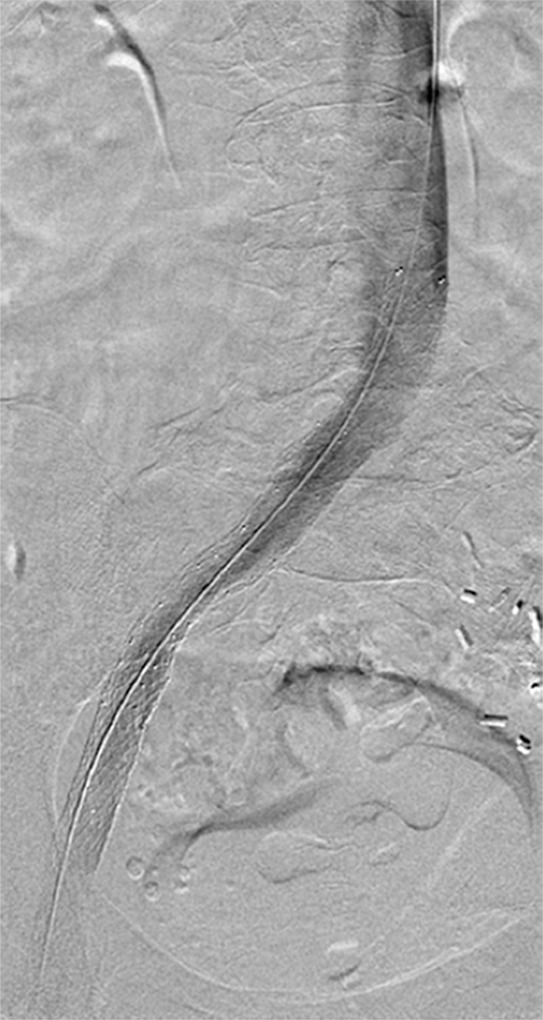
May-Thurner syndrome complicated by chronic thrombosis. (a) Venogram of the left common iliac vein (patient is prone) demonstrates chronic effacement and thrombosis of the proximal left common iliac vein and an atretic and postthrombotic left iliac venous system. (b) Venogram obtained after stent placement demonstrates brisk flow from the left common femoral vein into the IVC. See also Figs E3a and E3b in this patient (online).
Femoropopliteal disease.—Most individuals who have isolated femoropopliteal disease are not affected with severe PTS (7). For those who do develop chronic venous disease in the setting of femoropopliteal thrombosis, endovascular recanalization is controversial. Treating such patients requires a careful risk-benefit analysis. If the patient is motivated because of lifestyle limitations and is at very low risk of complications, he or she may be suitable for such a procedure. It should be noted that data are lacking in the treatment of femoropopliteal chronic disease, and that, at this time, much of the experience is anecdotal. The recanalization procedure often involves access of a calf vein or the posterior tibial vein at the ankle, such that an open vein is accessed. Next, wire access through the native deep venous system is typically achieved with a combination of a stiff hydrophilic wire and a stiff hydrophilic catheter. The recanalization is often performed with angioplasty (Fig 15). The treatment of chronic femoropopliteal thrombotic disease is actively being researched.
Figure 15a:
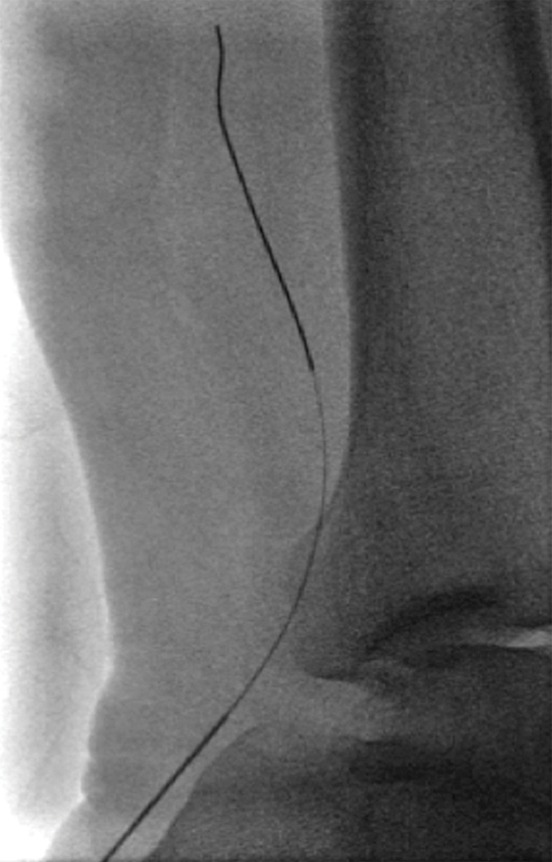
Recanalization of chronically occluded left femoropopliteal veins. (a) Spot fluoroscopic image of wire access into the left posterior tibial vein at the level of the ankle. (b) Popliteal venogram demonstrates heavily diseased popliteal vein, expanded by chronic thrombus, with partially recanalized channels. (c) Femoral venogram shows atretic femoral vein with collateralization. (d) Prolonged balloon angioplasty of the central femoral vein, with a representative waist at areas of stenosis. (e–g) Venograms obtained after angioplasty show improved flow and resolution of collaterals through the (e) popliteal, (f) peripheral femoral, and (g) central femoral veins. Three months later, the patient had no objective PTS per Villalta assessment, and (h) duplex US demonstrated a patent femoral vein. See also Figs E4a and E4b in this patient (online).
Figure 15b:
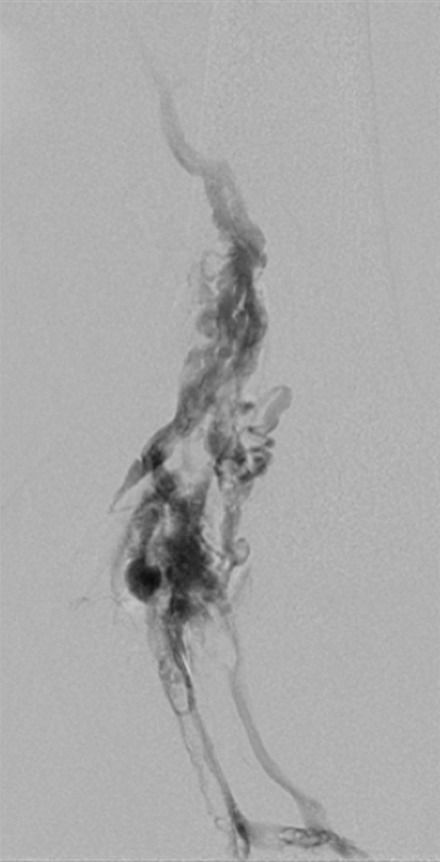
Recanalization of chronically occluded left femoropopliteal veins. (a) Spot fluoroscopic image of wire access into the left posterior tibial vein at the level of the ankle. (b) Popliteal venogram demonstrates heavily diseased popliteal vein, expanded by chronic thrombus, with partially recanalized channels. (c) Femoral venogram shows atretic femoral vein with collateralization. (d) Prolonged balloon angioplasty of the central femoral vein, with a representative waist at areas of stenosis. (e–g) Venograms obtained after angioplasty show improved flow and resolution of collaterals through the (e) popliteal, (f) peripheral femoral, and (g) central femoral veins. Three months later, the patient had no objective PTS per Villalta assessment, and (h) duplex US demonstrated a patent femoral vein. See also Figs E4a and E4b in this patient (online).
Figure 15c:
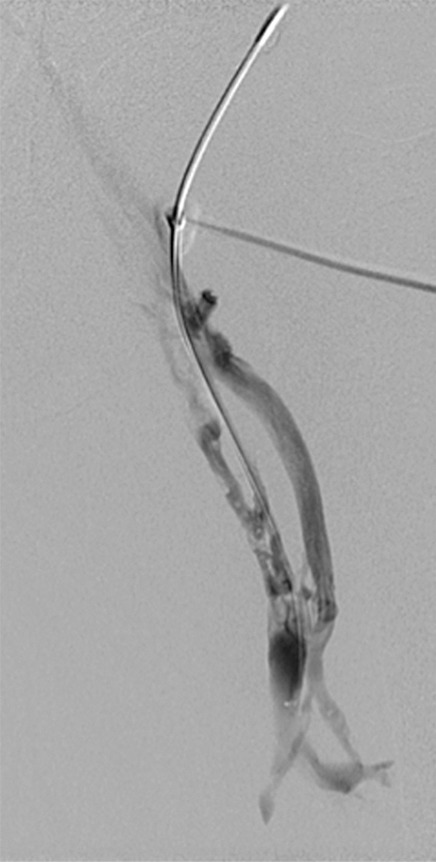
Recanalization of chronically occluded left femoropopliteal veins. (a) Spot fluoroscopic image of wire access into the left posterior tibial vein at the level of the ankle. (b) Popliteal venogram demonstrates heavily diseased popliteal vein, expanded by chronic thrombus, with partially recanalized channels. (c) Femoral venogram shows atretic femoral vein with collateralization. (d) Prolonged balloon angioplasty of the central femoral vein, with a representative waist at areas of stenosis. (e–g) Venograms obtained after angioplasty show improved flow and resolution of collaterals through the (e) popliteal, (f) peripheral femoral, and (g) central femoral veins. Three months later, the patient had no objective PTS per Villalta assessment, and (h) duplex US demonstrated a patent femoral vein. See also Figs E4a and E4b in this patient (online).
Figure 15d:
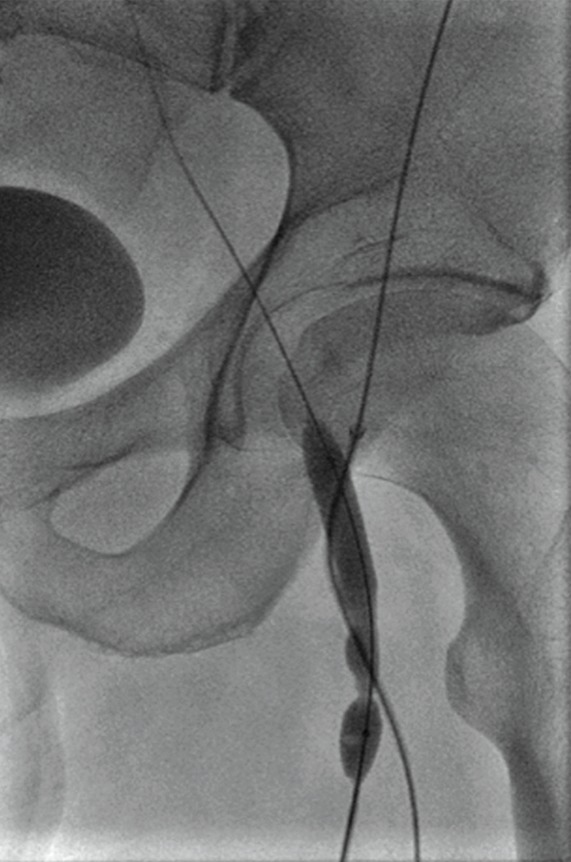
Recanalization of chronically occluded left femoropopliteal veins. (a) Spot fluoroscopic image of wire access into the left posterior tibial vein at the level of the ankle. (b) Popliteal venogram demonstrates heavily diseased popliteal vein, expanded by chronic thrombus, with partially recanalized channels. (c) Femoral venogram shows atretic femoral vein with collateralization. (d) Prolonged balloon angioplasty of the central femoral vein, with a representative waist at areas of stenosis. (e–g) Venograms obtained after angioplasty show improved flow and resolution of collaterals through the (e) popliteal, (f) peripheral femoral, and (g) central femoral veins. Three months later, the patient had no objective PTS per Villalta assessment, and (h) duplex US demonstrated a patent femoral vein. See also Figs E4a and E4b in this patient (online).
Figure 15e:
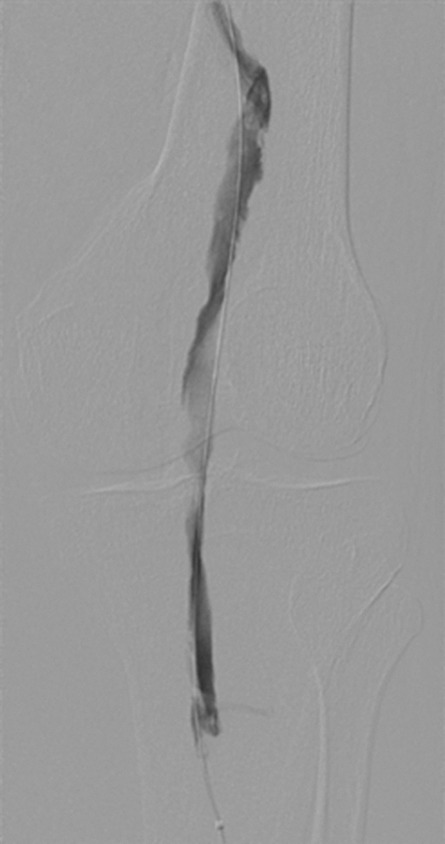
Recanalization of chronically occluded left femoropopliteal veins. (a) Spot fluoroscopic image of wire access into the left posterior tibial vein at the level of the ankle. (b) Popliteal venogram demonstrates heavily diseased popliteal vein, expanded by chronic thrombus, with partially recanalized channels. (c) Femoral venogram shows atretic femoral vein with collateralization. (d) Prolonged balloon angioplasty of the central femoral vein, with a representative waist at areas of stenosis. (e–g) Venograms obtained after angioplasty show improved flow and resolution of collaterals through the (e) popliteal, (f) peripheral femoral, and (g) central femoral veins. Three months later, the patient had no objective PTS per Villalta assessment, and (h) duplex US demonstrated a patent femoral vein. See also Figs E4a and E4b in this patient (online).
Figure 15f:
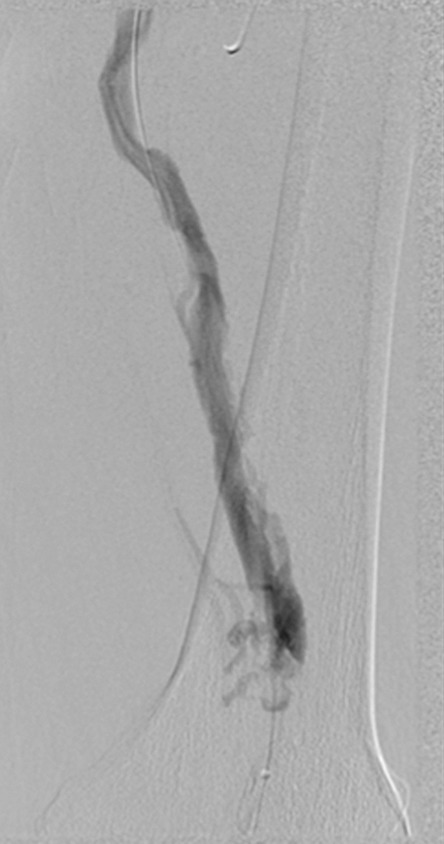
Recanalization of chronically occluded left femoropopliteal veins. (a) Spot fluoroscopic image of wire access into the left posterior tibial vein at the level of the ankle. (b) Popliteal venogram demonstrates heavily diseased popliteal vein, expanded by chronic thrombus, with partially recanalized channels. (c) Femoral venogram shows atretic femoral vein with collateralization. (d) Prolonged balloon angioplasty of the central femoral vein, with a representative waist at areas of stenosis. (e–g) Venograms obtained after angioplasty show improved flow and resolution of collaterals through the (e) popliteal, (f) peripheral femoral, and (g) central femoral veins. Three months later, the patient had no objective PTS per Villalta assessment, and (h) duplex US demonstrated a patent femoral vein. See also Figs E4a and E4b in this patient (online).
Figure 15g:
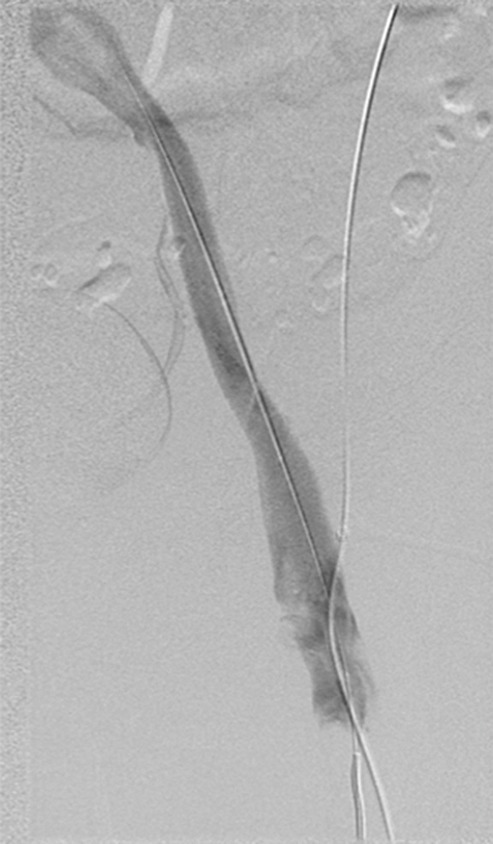
Recanalization of chronically occluded left femoropopliteal veins. (a) Spot fluoroscopic image of wire access into the left posterior tibial vein at the level of the ankle. (b) Popliteal venogram demonstrates heavily diseased popliteal vein, expanded by chronic thrombus, with partially recanalized channels. (c) Femoral venogram shows atretic femoral vein with collateralization. (d) Prolonged balloon angioplasty of the central femoral vein, with a representative waist at areas of stenosis. (e–g) Venograms obtained after angioplasty show improved flow and resolution of collaterals through the (e) popliteal, (f) peripheral femoral, and (g) central femoral veins. Three months later, the patient had no objective PTS per Villalta assessment, and (h) duplex US demonstrated a patent femoral vein. See also Figs E4a and E4b in this patient (online).
Figure 15h:
Recanalization of chronically occluded left femoropopliteal veins. (a) Spot fluoroscopic image of wire access into the left posterior tibial vein at the level of the ankle. (b) Popliteal venogram demonstrates heavily diseased popliteal vein, expanded by chronic thrombus, with partially recanalized channels. (c) Femoral venogram shows atretic femoral vein with collateralization. (d) Prolonged balloon angioplasty of the central femoral vein, with a representative waist at areas of stenosis. (e–g) Venograms obtained after angioplasty show improved flow and resolution of collaterals through the (e) popliteal, (f) peripheral femoral, and (g) central femoral veins. Three months later, the patient had no objective PTS per Villalta assessment, and (h) duplex US demonstrated a patent femoral vein. See also Figs E4a and E4b in this patient (online).
Complications
The procedures described above are generally safe given that most work is being done in the venous system (101–103). The most common complication is access site bleeding, especially in the setting of anticoagulation during the procedure. Typically this minor complication is easily controlled with manual compression, and rarely does anticoagulation need to be stopped for access site bleeding. As part of either blunt or sharp recanalization, wires and catheters may exit the venous system. However, given the low to zero flow through occluded segments, significant bleeding from such transgressions is rare, and the case continues after the interventionalist withdraws to the point of exit and reattempts recanalization through the native channel. Patients may experience considerable pain during angioplasty and stent treatment of chronic occlusions. The pain may last for several days after the procedure, so adequate outpatient pain management is essential. Stent migration occurs rarely, and is usually secondary to undersizing or misplacement (104). Patients should also be cautioned that the procedure might not be technically successful and that there is a reasonably high chance of reintervention at a later date; between 15% and 40% of patients who undergo stent placement require reintervention within 4 years (70,102).
Postprocedural Management
Anticoagulation.—The duration and type of anticoagulation after chronic recanalization procedures is not well studied. A general rule of thumb is that thrombotic venous disease needs to be medically managed more aggressively after a procedure than nonthrombotic disease owing to higher rethrombosis rates in the former group (discussed further below). A typical regimen will include an enoxaparin bridge to warfarin. Some advocate 1 month of enoxaparin before switching to warfarin. More practitioners are considering rivaroxaban as a warfarin alternative; however, data are lacking regarding its efficacy after recanalization and in the setting of stent procedures. The duration of anticoagulation is also variable. For postthrombotic lesions, most recommend long-term therapy, while nonthrombotic disease is typically managed with 1–3 months of anticoagulation. Antiplatelet agents such as aspirin and clopidogrel are used by many after venous stent placement, but this practice is based on arterial data and physiology (105).
Adjunctive supportive care.—Compression stockings, pneumatic compression, and wound care should continue as before the procedure, based on each treatment’s relative efficacy. Patients should be seen at set follow-up points to mark progress and adjust anticoagulation and other supportive measures as necessary.
Outcomes
Technical and clinical outcomes for venous recanalization in chronic venous disease have been documented by several studies (Table 6). In a large series (> 900 limbs) from 2007, accounting for both thrombotic and nonthrombotic lesions, primary patency was found to be 67%, while primary-assisted and secondary patency were found to be 89% and 93%, respectively, with 5-year follow-up (103). Unsurprisingly, primary patency was found to be higher in nonthrombotic limbs. The next largest series in 2012, involving 224 limbs, showed excellent primary and primary-assisted patencies of 98.7% and 100% at 4 years (97). Importantly, these data were derived from nonthrombotic limbs. Early stent thrombosis is relatively rare, between 1% and 7% in published reports (105). Clinical outcomes are promising as well. In a series of 504 patients, stent treatment in the setting of deep venous reflux significantly decreased pain and swelling and had a positive impact on patients with the worst CEAP classifications. In patients with a classification of C4 prior to intervention, venous dermatitis was resolved in 80%. Preintervention C5 patients (healed ulcers) had an 88% likelihood of being ulcer-free after stent placement. In 50% of C6 patients (active ulcers), there was complete resolution of their ulcers following stent procedures (106). In the 2012 study mentioned above, treated patients demonstrated a statistically significant decrease in pain, edema, fatigue, and sleep disturbance. In the same study, 78% of patients had edema prior to treatment, while only 10% had edema after treatment. The ulcer rate dropped from 27% to 6%. A separate single-center retrospective review demonstrated an 80% complete or partial response to endovascular techniques in patients with established PTS (70).
Table 6.
Clinical Outcomes for Endovascular Treatment of Established Chronic Venous Disease
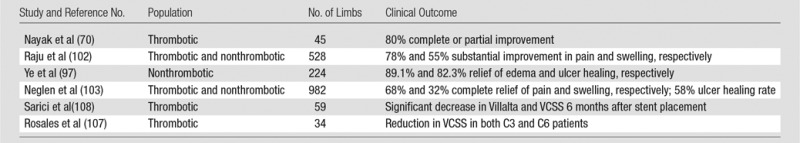
Note.—VCSS = venous clinical severity score.
Conclusion
Endovascular management of lower extremity deep venous disease is complex and dynamic, with new treatments emerging to treat its deleterious acute and chronic manifestations. Advances in knowledge, endovascular techniques, and medical devices have made venous interventions safer and more effective. It is therefore likely that these minimally invasive and often effective techniques will continue to play an important role in the treatment of lower extremity deep venous disease; their exact role will be determined by their continued evolution and the results of prospective clinical trials.
Essentials
■ Lower extremity deep venous disease is the third most common cardiovascular disease and is associated with significant individual morbidity and high societal cost.
■ Postthrombotic syndrome (PTS) is a long-term complication of acute deep venous thrombosis that occurs in a large number of individuals despite optimal anticoagulation.
■ Endovascular interventions such as pharmacomechanical catheter-directed therapy (PCDT) have been developed to prevent PTS; prior studies have shown promise, and the ongoing ATTRACT trial will better define the role of PCDT in the prevention of this syndrome.
■ Established PTS should be managed with optimal noninvasive methods; select patients may benefit from endovascular recanalization.
■ Stent placement in the setting of chronic thrombotic and nonthrombotic deep venous disease has shown good long-term clinical outcomes, with secondary patency greater than 85% in most studies.
SUPPLEMENTAL FIGURES
Received November 17, 2013; revision requested December 20; revision received February 4, 2014; accepted February 17; final version accepted March 21.
Funding: S.V. supported by the National Institutes of Health National Heart, Lung, and Blood Institute (grants U01-HL-088476 and U01-HL-112321).
Disclosures of Conflicts of Interest: A.K.S. disclosed no relevant relationships. S.V. Activities related to the present article: none to disclose. Activities not related to the present article: grants from Covidien, Bayer Healthcare, Genentech; nonfinancial support from Genentech and BSN Medical. Other relationships: none to disclose. J.A.K. Activities related to the present article: none to disclose. Activities not related to the present article: grant from Cook Group for IVC filter registry; personal fees from Crux Medical, for work on DSMB for clinical trial of IVC filter; Other, stock ownership in Veniti, a start-up company focused on venous diseases (no commercial activity at this time), and stock options in Bio2 Medical, a start-up company with venous filter in early clinical trial. Other relationships: none to disclose. D.C.M. disclosed no relevant relationships.
Abbreviations:
- CDT
- catheter-directed thrombolysis
- DVT
- deep vein thrombosis
- IVC
- inferior vena cava
- PCDT
- pharmacomechanical CDT
- PMT
- percutaneous mechanical thrombectomy
- PTS
- postthrombotic syndrome
- VTE
- venous thromboembolism
References
- 1.U.S. Department of Health and Human Services . The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. September 15, 2008. Washington, DC: U.S. Department of Health and Human Services, 2008. [Google Scholar]
- 2.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e419S–e494S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349(2):146–153. [DOI] [PubMed] [Google Scholar]
- 4.Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med 2002;162(15):1729–1735. [DOI] [PubMed] [Google Scholar]
- 5.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361(24):2342–2352. [DOI] [PubMed] [Google Scholar]
- 6.EINSTEIN Investigators , Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363(26):2499–2510. [DOI] [PubMed] [Google Scholar]
- 7.Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008;149(10):698–707. [DOI] [PubMed] [Google Scholar]
- 8.Beyth RJ, Cohen AM, Landefeld CS. Long-term outcomes of deep-vein thrombosis. Arch Intern Med 1995;155(10):1031–1037. [PubMed] [Google Scholar]
- 9.Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost 2008;6(7):1105–1112. [DOI] [PubMed] [Google Scholar]
- 10.Caprini JA, Botteman MF, Stephens JM, et al. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health 2003;6(1):59–74. [DOI] [PubMed] [Google Scholar]
- 11.Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994;31(1):49–53. [DOI] [PubMed] [Google Scholar]
- 12.Bergqvist D, Jendteg S, Johansen L, Persson U, Odegaard K. Cost of long-term complications of deep venous thrombosis of the lower extremities: an analysis of a defined patient population in Sweden. Ann Intern Med 1997;126(6):454–457. [DOI] [PubMed] [Google Scholar]
- 13.Olin JW, Beusterien KM, Childs MB, Seavey C, McHugh L, Griffiths RI. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vasc Med 1999;4(1):1–7. [DOI] [PubMed] [Google Scholar]
- 14.Shbaklo H, Holcroft CA, Kahn SR. Levels of inflammatory markers and the development of the post-thrombotic syndrome. Thromb Haemost 2009;101(3):505–512. [PubMed] [Google Scholar]
- 15.Wakefield TW, Myers DD, Henke PK. Role of selectins and fibrinolysis in VTE. Thromb Res 2009;123(Suppl 4):S35–S40. [DOI] [PubMed] [Google Scholar]
- 16.Roumen-Klappe EM, Janssen MC, Van Rossum J, et al. Inflammation in deep vein thrombosis and the development of post-thrombotic syndrome: a prospective study. J Thromb Haemost 2009;7(4):582–587. [DOI] [PubMed] [Google Scholar]
- 17.Deroo S, Deatrick KB, Henke PK. The vessel wall: A forgotten player in post thrombotic syndrome. Thromb Haemost 2010;104(4):681–692. [DOI] [PubMed] [Google Scholar]
- 18.Markel A, Manzo RA, Bergelin RO, Strandness DE, Jr. Valvular reflux after deep vein thrombosis: incidence and time of occurrence. J Vasc Surg 1992;15(2):377–382; discussion 383–384. [PubMed] [Google Scholar]
- 19.Caps MT, Manzo RA, Bergelin RO, Meissner MH, Strandness DE, Jr. Venous valvular reflux in veins not involved at the time of acute deep vein thrombosis. J Vasc Surg 1995;22(5):524–531. [DOI] [PubMed] [Google Scholar]
- 20.Shull KC, Nicolaides AN, Fernandes é Fernandes J, et al. Significance of popliteal reflux in relation to ambulatory venous pressure and ulceration. Arch Surg 1979;114(11):1304–1306. [DOI] [PubMed] [Google Scholar]
- 21.Prandoni P, Frulla M, Sartor D, Concolato A, Girolami A. Vein abnormalities and the post-thrombotic syndrome. J Thromb Haemost 2005;3(2):401–402. [DOI] [PubMed] [Google Scholar]
- 22.Meissner MH, Manzo RA, Bergelin RO, Markel A, Strandness DE, Jr. Deep venous insufficiency: the relationship between lysis and subsequent reflux. J Vasc Surg 1993;18(4):596–605; discussion 606–608. [PubMed] [Google Scholar]
- 23.Nicolaides AN, Hussein MK, Szendro G, Christopoulos D, Vasdekis S, Clarke H. The relation of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg 1993;17(2):414–419. [DOI] [PubMed] [Google Scholar]
- 24.Welkie JF, Comerota AJ, Katz ML, Aldridge SC, Kerr RP, White JV. Hemodynamic deterioration in chronic venous disease. J Vasc Surg 1992;16(5):733–740. [PubMed] [Google Scholar]
- 25.Araki CT, Back TL, Padberg FT, et al. The significance of calf muscle pump function in venous ulceration. J Vasc Surg 1994;20(6):872–877; discussion 878–879. [DOI] [PubMed] [Google Scholar]
- 26.Johnson BF, Manzo RA, Bergelin RO, Strandness DE, Jr. Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one- to six-year follow-up. J Vasc Surg 1995;21(2):307–312; discussion 313. [DOI] [PubMed] [Google Scholar]
- 27.van Dongen CJJ, Prandoni P, Frulla M, Marchiori A, Prins MH, Hutten BA. Relation between quality of anticoagulant treatment and the development of the postthrombotic syndrome. J Thromb Haemost 2005;3(5):939–942. [DOI] [PubMed] [Google Scholar]
- 28.Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med 2001;110(7):515–519. [DOI] [PubMed] [Google Scholar]
- 29.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123(16):1788–1830. [DOI] [PubMed] [Google Scholar]
- 30.Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg 2004;239(1):118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997;349(9054):759–762. [DOI] [PubMed] [Google Scholar]
- 32.Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004;141(4):249–256. [DOI] [PubMed] [Google Scholar]
- 33.Kahn SR, Shbaklo H, Shapiro S, et al. Effectiveness of compression stockings to prevent the post-thrombotic syndrome (the SOX Trial and Bio-SOX biomarker substudy): a randomized controlled trial. BMC Cardiovasc Disord 2007;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahn SR, Shapiro S, Wells PS, et al. A multicenter randomized placebo controlled trial of compression stockings to prevent the post-thrombotic syndrome after proximal deep venous thrombosis [abstr]. Presented at the 54th Annual Meeting and Exposition of the American Society of Hematology, Atlanta, Georgia, December 9, 2012.
- 35.Hull RD, Marder VJ, Mah AF, Biel RK, Brant RF. Quantitative assessment of thrombus burden predicts the outcome of treatment for venous thrombosis: a systematic review. Am J Med 2005;118(5):456–464. [DOI] [PubMed] [Google Scholar]
- 36.Plate G, Einarsson E, Ohlin P, Jensen R, Qvarfordt P, Eklöf B. Thrombectomy with temporary arteriovenous fistula: the treatment of choice in acute iliofemoral venous thrombosis. J Vasc Surg 1984;1(6):867–876. [DOI] [PubMed] [Google Scholar]
- 37.Plate G, Akesson H, Einarsson E, Ohlin P, Eklöf B. Long-term results of venous thrombectomy combined with a temporary arterio-venous fistula. Eur J Vasc Surg 1990;4(5):483–489. [DOI] [PubMed] [Google Scholar]
- 38.Goldhaber SZ, Buring JE, Lipnick RJ, Hennekens CH. Pooled analyses of randomized trials of streptokinase and heparin in phlebographically documented acute deep venous thrombosis. Am J Med 1984;76(3):393–397. [DOI] [PubMed] [Google Scholar]
- 39.Elliot MS, Immelman EJ, Jeffery P, et al. A comparative randomized trial of heparin versus streptokinase in the treatment of acute proximal venous thrombosis: an interim report of a prospective trial. Br J Surg 1979;66(12):838–843. [DOI] [PubMed] [Google Scholar]
- 40.Arnesen H, Høiseth A, Ly B. Streptokinase of heparin in the treatment of deep vein thrombosis. Follow-up results of a prospective study. Acta Med Scand 1982;211(1-2):65–68. [PubMed] [Google Scholar]
- 41.Turpie AGG, Levine MN, Hirsh J, et al. Tissue plasminogen activator (rt-PA) vs heparin in deep vein thrombosis. Results of a randomized trial. Chest 1990;97(4 Suppl):172S–175S. [PubMed] [Google Scholar]
- 42.Goldhaber SZ, Meyerovitz MF, Green D, et al. Randomized controlled trial of tissue plasminogen activator in proximal deep venous thrombosis. Am J Med 1990;88(3):235–240. [DOI] [PubMed] [Google Scholar]
- 43.Meyerovitz MF, Polak JF, Goldhaber SZ. Short-term response to thrombolytic therapy in deep venous thrombosis: predictive value of venographic appearance. Radiology 1992;184(2):345–348. [DOI] [PubMed] [Google Scholar]
- 44.Schwieder G, Grimm W, Siemens HJ, et al. Intermittent regional therapy with rt-PA is not superior to systemic thrombolysis in deep vein thrombosis (DVT)—a German multicenter trial. Thromb Haemost 1995;74(5):1240–1243. [PubMed] [Google Scholar]
- 45.Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology 1999;211(1):39–49. [DOI] [PubMed] [Google Scholar]
- 46.Semba CP, Dake MD. Iliofemoral deep venous thrombosis: aggressive therapy with catheter-directed thrombolysis. Radiology 1994;191(2):487–494. [DOI] [PubMed] [Google Scholar]
- 47.Vedantham S, Thorpe PE, Cardella JF, et al. Quality improvement guidelines for the treatment of lower extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol 2006;17(3):435–447; quiz 448. [DOI] [PubMed] [Google Scholar]
- 48.Shortell CK, Queiroz R, Johansson M, et al. Safety and efficacy of limited-dose tissue plasminogen activator in acute vascular occlusion. J Vasc Surg 2001;34(5):854–859. [DOI] [PubMed] [Google Scholar]
- 49.Sugimoto K, Hofmann LV, Razavi MK, et al. The safety, efficacy, and pharmacoeconomics of low-dose alteplase compared with urokinase for catheter-directed thrombolysis of arterial and venous occlusions. J Vasc Surg 2003;37(3):512–517. [DOI] [PubMed] [Google Scholar]
- 50.Grunwald MR, Hofmann LV. Comparison of urokinase, alteplase, and reteplase for catheter-directed thrombolysis of deep venous thrombosis. J Vasc Interv Radiol 2004;15(4):347–352. [DOI] [PubMed] [Google Scholar]
- 51.Parikh S, Motarjeme A, McNamara T, et al. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J Vasc Interv Radiol 2008;19(4):521–528. [DOI] [PubMed] [Google Scholar]
- 52.Baker R, Samuels S, Benenati JF, Powell A, Uthoff H. Ultrasound-accelerated vs standard catheter-directed thrombolysis—a comparative study in patients with iliofemoral deep vein thrombosis. J Vasc Interv Radiol 2012;23(11):1460–1466. [DOI] [PubMed] [Google Scholar]
- 53.Delomez M, Beregi JP, Willoteaux S, et al. Mechanical thrombectomy in patients with deep venous thrombosis. Cardiovasc Intervent Radiol 2001;24(1):42–48. [DOI] [PubMed] [Google Scholar]
- 54.Kim HS, Patra A, Paxton BE, Khan J, Streiff MB. Adjunctive percutaneous mechanical thrombectomy for lower-extremity deep vein thrombosis: clinical and economic outcomes. J Vasc Interv Radiol 2006;17(7):1099–1104. [DOI] [PubMed] [Google Scholar]
- 55.Cynamon J, Stein EG, Dym RJ, Jagust MB, Binkert CA, Baum RA. A new method for aggressive management of deep vein thrombosis: retrospective study of the power pulse technique. J Vasc Interv Radiol 2006;17(6):1043–1049. [DOI] [PubMed] [Google Scholar]
- 56.Lin PH, Zhou W, Dardik A, et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg 2006;192(6):782–788. [DOI] [PubMed] [Google Scholar]
- 57.O’Sullivan GJ, Lohan DG, Gough N, Cronin CG, Kee ST. Pharmacomechanical thrombectomy of acute deep vein thrombosis with the Trellis-8 isolated thrombolysis catheter. J Vasc Interv Radiol 2007;18(6):715–724. [DOI] [PubMed] [Google Scholar]
- 58.Hilleman DE, Razavi MK. Clinical and economic evaluation of the Trellis-8 infusion catheter for deep vein thrombosis. J Vasc Interv Radiol 2008;19(3):377–383. [DOI] [PubMed] [Google Scholar]
- 59.Vedantham S, Millward SF, Cardella JF, et al. Society of Interventional Radiology position statement: treatment of acute iliofemoral deep vein thrombosis with use of adjunctive catheter-directed intrathrombus thrombolysis. J Vasc Interv Radiol 2006;17(4):613–616. [DOI] [PubMed] [Google Scholar]
- 60.Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med 2001;161(17):2105–2109. [DOI] [PubMed] [Google Scholar]
- 61.Kearon C, Julian JA, Newman TE, Ginsberg JS. Noninvasive diagnosis of deep venous thrombosis. McMaster Diagnostic Imaging Practice Guidelines Initiative. Ann Intern Med 1998;128(8):663–677. [DOI] [PubMed] [Google Scholar]
- 62.Comerota AJ, Throm RC, Mathias SD, Haughton S, Mewissen M. Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J Vasc Surg 2000;32(1):130–137. [DOI] [PubMed] [Google Scholar]
- 63.AbuRahma AF, Perkins SE, Wulu JT, Ng HK. Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg 2001;233(6):752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg 2002;24(3):209–214. [DOI] [PubMed] [Google Scholar]
- 65.Enden T, Haig Y, Kløw NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012;379(9810):31–38. [DOI] [PubMed] [Google Scholar]
- 66.Vedantham S, Goldhaber SZ, Kahn SR, et al. Rationale and design of the ATTRACT Study: a multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. Am Heart J 2013;165(4):523–553, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost 2009;7(5):879–883. [DOI] [PubMed] [Google Scholar]
- 68.Kahn SR. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J Thromb Haemost 2009;7(5):884–888. [DOI] [PubMed] [Google Scholar]
- 69.Raffetto JD. Inflammation in chronic venous ulcers. Phlebology 2013;28(Suppl 1):61–67. [DOI] [PubMed] [Google Scholar]
- 70.Nayak L, Hildebolt CF, Vedantham S. Postthrombotic syndrome: feasibility of a strategy of imaging-guided endovascular intervention. J Vasc Interv Radiol 2012;23(9):1165–1173. [DOI] [PubMed] [Google Scholar]
- 71.Heit JA, Rooke TW, Silverstein MD, et al. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25-year population-based study. J Vasc Surg 2001;33(5):1022–1027. [DOI] [PubMed] [Google Scholar]
- 72.Roumen-Klappe EM, Janssen MC, Van Rossum J, et al. Inflammation in deep vein thrombosis and the development of post-thrombotic syndrome: a prospective study. J Thromb Haemost 2009;7(4):582–587. [DOI] [PubMed] [Google Scholar]
- 73.Sevitt S. The vascularisation of deep-vein thrombi and their fibrous residue: a post mortem angio-graphic study. J Pathol 1973;111(1):1–11. [DOI] [PubMed] [Google Scholar]
- 74.Sevitt S. The mechanisms of canalisation in deep vein thrombosis. J Pathol 1973;110(2):153–165. [DOI] [PubMed] [Google Scholar]
- 75.Ginsberg JS, Magier D, Mackinnon B, Gent M, Hirsh J. Intermittent compression units for severe post-phlebitic syndrome: a randomized crossover study. CMAJ 1999;160(9):1303–1306. [PMC free article] [PubMed] [Google Scholar]
- 76.O’Donnell MJ, McRae S, Kahn SR, et al. Evaluation of a venous-return assist device to treat severe post-thrombotic syndrome (VENOPTS). A randomized controlled trial. Thromb Haemost 2008;99(3):623–629. [DOI] [PubMed] [Google Scholar]
- 77.Kahn SR, Shrier I, Shapiro S, et al. Six-month exercise training program to treat post-thrombotic syndrome: a randomized controlled two-centre trial. CMAJ 2011;183(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg 2005;30(2):198–208. [DOI] [PubMed] [Google Scholar]
- 79.Jull A, Arroll B, Parag V, Waters J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev 2007 (3):CD001733. [DOI] [PubMed] [Google Scholar]
- 80.Hamper UM, DeJong MR, Scoutt LM. Ultrasound evaluation of the lower extremity veins. Radiol Clin North Am 2007;45(3):525–547, ix. [DOI] [PubMed] [Google Scholar]
- 81.Koizumi J, Horie T, Muro I, et al. Magnetic resonance venography of the lower limb. Int Angiol 2007;26(2):171–182. [PubMed] [Google Scholar]
- 82.Thomas SM, Goodacre SW, Sampson FC, van Beek EJ. Diagnostic value of CT for deep vein thrombosis: results of a systematic review and meta-analysis. Clin Radiol 2008;63(3):299–304. [DOI] [PubMed] [Google Scholar]
- 83.Dinglasan LA, Oh JC, Schmitt JE, Trerotola SO, Shlansky-Goldberg RD, Stavropoulos SW. Complicated inferior vena cava filter retrievals: associated factors identified at preretrieval CT. Radiology 2013;266(1):347–354. [DOI] [PubMed] [Google Scholar]
- 84.Jost CJ, Gloviczki P, Cherry KJ, Jr, et al. Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg 2001;33(2):320–327; discussion 327–328. [DOI] [PubMed] [Google Scholar]
- 85.Angel LF, Tapson V, Galgon RE, Restrepo MI, Kaufman J. Systematic review of the use of retrievable inferior vena cava filters. J Vasc Interv Radiol 2011;22(11):1522–1530, e3. [DOI] [PubMed] [Google Scholar]
- 86.Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med 1998;338(7):409–415. [DOI] [PubMed] [Google Scholar]
- 87.Fox MA, Kahn SR. Postthrombotic syndrome in relation to vena cava filter placement: a systematic review. J Vasc Interv Radiol 2008;19(7):981–985. [DOI] [PubMed] [Google Scholar]
- 88.Rimon U, Bensaid P, Golan G, et al. Optease vena cava filter optimal indwelling time and retrievability. Cardiovasc Intervent Radiol 2011;34(3):532–535. [DOI] [PubMed] [Google Scholar]
- 89.Stavropoulos SW, Dixon RG, Burke CT, et al. Embedded inferior vena cava filter removal: use of endobronchial forceps. J Vasc Interv Radiol 2008;19(9):1297–1301. [DOI] [PubMed] [Google Scholar]
- 90.Kuo WT, Odegaard JI, Rosenberg JK, Hofmann LV. Excimer laser-assisted removal of embedded inferior vena cava filters: a single-center prospective study. Circ Cardiovasc Interv 2013;6(5):560–566. [DOI] [PubMed] [Google Scholar]
- 91.Oh JC, Trerotola SO, Dagli M, et al. Removal of retrievable inferior vena cava filters with computed tomography findings indicating tenting or penetration of the inferior vena cava wall. J Vasc Interv Radiol 2011;22(1):70–74. [DOI] [PubMed] [Google Scholar]
- 92.Hill DA, Goldstein N, Kuo EY. Vena cava filter fracture with migration to the pulmonary artery. Ann Thorac Surg 2013;95(1):342–345. [DOI] [PubMed] [Google Scholar]
- 93.Neglén P, Oglesbee M, Olivier J, Raju S. Stenting of chronically obstructed inferior vena cava filters. J Vasc Surg 2011;54(1):153–161. [DOI] [PubMed] [Google Scholar]
- 94.Razavi MK, Hansch EC, Kee ST, Sze DY, Semba CP, Dake MD. Chronically occluded inferior venae cavae: endovascular treatment. Radiology 2000;214(1):133–138. [DOI] [PubMed] [Google Scholar]
- 95.Guimaraes M, Schonholz C, Hannegan C, Anderson MB, Shi J, Selby B, Jr. Radiofrequency wire for the recanalization of central vein occlusions that have failed conventional endovascular techniques. J Vasc Interv Radiol 2012;23(8):1016–1021. [DOI] [PubMed] [Google Scholar]
- 96.Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg 2004;39(5):937–943. [DOI] [PubMed] [Google Scholar]
- 97.Ye K, Lu X, Li W, et al. Long-term outcomes of stent placement for symptomatic nonthrombotic iliac vein compression lesions in chronic venous disease. J Vasc Interv Radiol 2012;23(4):497–502. [DOI] [PubMed] [Google Scholar]
- 98.Carr S, Chan K, Rosenberg J, et al. Correlation of the diameter of the left common iliac vein with the risk of lower-extremity deep venous thrombosis. J Vasc Interv Radiol 2012;23(11):1467–1472. [DOI] [PubMed] [Google Scholar]
- 99.Chan KT, Tye GA, Popat RA, et al. Common iliac vein stenosis: a risk factor for oral contraceptive-induced deep vein thrombosis. Am J Obstet Gynecol 2011;205(6):e1–e6. [DOI] [PubMed] [Google Scholar]
- 100.Raju S, Martin A, Davis M. The importance of IVUS assessment in venous thrombolytic regimens. J Vasc Surg 2013;1(1):108. [DOI] [PubMed] [Google Scholar]
- 101.Raju S. Long-term outcomes of stent placement for symptomatic nonthrombotic iliac vein compression lesions in chronic venous disease. J Vasc Interv Radiol 2012;23(4):502–503. [DOI] [PubMed] [Google Scholar]
- 102.Raju S, Neglén P. Percutaneous recanalization of total occlusions of the iliac vein. J Vasc Surg 2009;50(2):360–368. [DOI] [PubMed] [Google Scholar]
- 103.Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 2007;46(5):979–990. [DOI] [PubMed] [Google Scholar]
- 104.Brountzos EN, Binkert CA, Panagiotou IE, Petersen BD, Timmermans H, Lakin PC. Clinical outcome after intrahepatic venous stent placement for malignant inferior vena cava syndrome. Cardiovasc Intervent Radiol 2004;27(2):129–136. [DOI] [PubMed] [Google Scholar]
- 105.Meissner MH. Indications for platelet aggregation inhibitors after venous stents. Phlebology 2013;28(Suppl 1):91–98. [DOI] [PubMed] [Google Scholar]
- 106.Raju S, Darcey R, Neglén P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg 2010;51(2):401–408; discussion 408. [DOI] [PubMed] [Google Scholar]
- 107.Rosales A, Sandbaek G, Jørgensen JJ. Stenting for chronic post-thrombotic vena cava and iliofemoral venous occlusions: mid-term patency and clinical outcome. Eur J Vasc Endovasc Surg 2010;40(2):234–240. [DOI] [PubMed] [Google Scholar]
- 108.Sarici IS, Yanar F, Agcaoglu O, et al. Our early experience with iliofemoral vein stenting in patients with post-thrombotic syndrome. Phlebology 2013;29(5):298–303. [DOI] [PubMed] [Google Scholar]
- 109.Vedantham S. Interventional approaches to deep vein thrombosis. Am J Hematol 2012;87(Suppl 1):S113–S118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


