Structured Summary
Background
The recently updated White House National HIV/AIDS Strategy (NHAS) includes specific progress indicators for improving the HIV care continuum, but the economic and epidemiological impact of achieving those indicators remains unclear.
Methods
We constructed a dynamic transmission model of HIV progression and care engagement to project HIV incidence, prevalence, mortality, and costs among adults in the United States over ten years. We specifically considered achievement of the 2020 targets set forth in NHAS progress indicator 1 (90% awareness of serostatus), indicator 4 (85% linkage within one month), and indicator 5 (90% of diagnosed individuals in care).
Finding
At current rates of engagement in the HIV care continuum, we project 524,000 (95% Uncertainty Range 442,000 – 712,000) new HIV infections and 375,000 deaths (364,000 – 578,000) between 2016 and 2025. Achieving NHAS progress indicators 1 and 4 has modest epidemiologic impact (new infections reduced by 2·0% and 3·9%, respectively). By contrast, increasing the proportion of diagnosed individuals in care (indicator 5) averts 52% (47-56%) of new infections. Achieving all NHAS targets resulted in a 58% reduction (52%-61%) in new infections and 128,000 lives saved (106,000-223,000) at an incremental health system cost of $105 billion dollars.
Interpretation
Achievement of NHAS progress indicators for screening, linkage, and particularly improving retention in care, can substantially reduce the burden of HIV in the United States.
Introduction
Since the HIV care continuum in the United States (US) was first described1, stakeholders at the federal, state and local levels have sought to identify and address gaps at each step of the cascade. These steps include HIV diagnosis, linkage to medical care, retention, prescription of antiretroviral therapy [ART], and viral suppression. Broader screening and early ART initiation have been shown to confer both therapeutic and preventive benefits2, 3. However, 2012 figures from the Centers for Disease Control and Prevention (CDC) indicate that of the estimated 1·2 million people living with HIV (PLWH) in the US, approximately one out of eight remained unaware of their infection, and only three out of ten achieved viral suppression4. Given that more than 90% of transmissions have been estimated to be attributable to PLWH who were either undiagnosed or not retained in medical care5, the need for sustained and coordinated efforts along the continuum is tremendous.
In 2013, the HIV Care Continuum Initiative was established to accelerate progress towards specific quantitative goals outlined in the first comprehensive National HIV/AIDS Strategy (NHAS)6. The White House recently updated the NHAS care continuum targets to 90% of PLWH knowing their serostatus (progress indicator 1), 85% of newly diagnosed individuals linking to clinical care within one month of their diagnosis (progress indicator 4), and 90% of diagnosed individuals engaged in HIV medical care (progress indicator 5)7. These align closely with the Joint United Nations Programme on HIV/AIDS (UNAIDS) ambitious 90-90-90 targets seeking to end the epidemic as a major global health threat by 20308.
The current 2015 NHAS additionally contains two epidemiological targets: reduction in the number of new HIV diagnoses of at least 25% by 2020 (progress indicator 2) and a reduction in mortality among persons diagnosed with HIV of 33% (progress indicator 8). However, the extent to which achieving revised NHAS progress indicators for improved screening, linkage, and care engagement would advance progress toward these epidemiological targets, and the magnitude of resources required to achieve desired levels of coverage have not yet been quantified. Therefore, we constructed a dynamic transmission model to project incident HIV infections, prevalence, mortality, and health care costs over the next ten years (2016-2025). For each outcome, we modeled the impact of attaining the three 2020 national progress indicators for serostatus awareness, linkage to care, and retention.
Methods
Model description
The Johns Hopkins HIV economic-epidemic model (JHHEM)9, 10 is a compartmental model of the US HIV epidemic that incorporates transmission, disease progression, and health system engagement. A full description has been published previously9. Briefly, our model partitions the adult population (18 to 78 years) of the US based on sex, age, HIV infection, and transmission category (heterosexuals, men who have sex with men [MSM], and people who inject drugs [PWID]). PLWH are further characterized by CD4 strata and location on the HIV care continuum (e.g. unaware of HIV status, aware but out of care, in care but not on ART, on ART but not virologically suppressed, and virologically suppressed). HIV infection, transition through the care continuum, and demographic changes are modeled dynamically as a system of ordinary differential equations9.
The model parameters (Table 1) 3, 11-18 were updated and calibrated to reflect most recent CDC data on the US HIV epidemic and continuum of care4, 9, 19 (Supplement). The model incorporates HIV transmission through sex (heterosexual or male homosexual) and through needle sharing. We explicitly modeled rates of annual HIV testing, initial linkage to care, discontinuation and reengagement in care, and ART initiation, calibrating those rates to reflect current national estimates of engagement in the continuum of care19, 20 (Supplemental Figure 3). To determine transmission probabilities during model calibration (average yearly partnerships and probability of transmission per partnership), we brought the model to equilibrium in 2006 and then allowed population growth, identifying parameter values in an iterative process for which the model outputs best reflected epidemiologic data on incidence and prevalence between 2007-2012 (model projections and CDC estimates shown in Supplemental Figure 24, 19).
Table 1.
Key model parameters
| Variable | Value | Range for sensitivity analysis | References |
|---|---|---|---|
| HIV Disease Dynamics with ART‡ | |||
| Reduction in rate of transmission | 93% | 80-99·5% | 3 |
| Time to viral suppression on ART | 6 months | 2-12 months | 11 |
| Transmission Dynamics* | |||
| Annual partnerships per year | 1·8 – 6·3 | 1·3-7·8 | Calibrated* |
| Transmission per partnership (male to female) | 2·2-3·1% | 1·7-3·9% | Calibrated* |
| Transmission per partnership (female to male) | 2-2·7% | 1·5-3·4% | Calibrated* |
| Transmission per partnership (MSM) | 2·9% | 2·2-3·7% | Calibrated* |
| Transmission probability per needle sharing partnership (PWID) | 0·0026 | 0·001-0·0075 | Calibrated* |
| Relative risk increase in transmission probability during acute HIV | 12 | 2-24 | 12 |
| Engagement in Care dynamics, base-case† | |||
| % HIV test in past 12 months | 7·5-27·5% | 5-35% | Calibrated† |
| Rate of disengagement from care annually | 0·175 – 0·25 | 0·13-0·32 | 13, Calibrated† |
| Rate of reengagement in care annually | 0·125 per year | 0·09-0·16 | 14, 15, Calibrated† |
| COSTS†† | |||
| HIV test | $33 | $10-50 | 16 |
| HIV viral load | $116 | $50-150 | 17 |
| Outpatient Visit | $129 | $55-274 | 17 |
| CD4 Test | $49 | $20-$90 | 17 |
| Annual ART | $32,000 | $10000-$45000 | 18 |
Transmission in the model is a function of average partnerships per year and average probability of transmission per partnership within risk groups. We began with published estimates and then calibrated these parameters (Supplement for additional details and references) to fit observed HIV incidence and prevalence in the US and varied by gender and risk group4, 19.
Annual HIV screening rates, percent linkage, and disengagement from care were varied by gender and risk group. To obtain parameter estimates, we began with published data and calibrated model to fit published estimates of HIV care engagement in the US4, 19 (Supplement for additional details and references). Individuals without initial linkage to care were eligible to engage in care at later times.
We additionally included annual health care utilization costs for individuals not in care or on ART (e.g. hospitalizations, ED visits). ART regimen costs shown are for first line integrase inhibitor-based regimens21 ( Additional cost details and references are in Supplement).
All populations eligible for ART initiation regardless of CD4 count21.
Statistical Anlaysis
Our primary outcomes were HIV incidence, prevalence, mortality and health care costs over a 10-year time horizon. In the base-case, we assumed implementation of updated guidelines that ART be initiated regardless of CD4 count21, but with continuation of current trends in the HIV care continuum 19, 20. We then estimated the epidemiologic and economic impact of reaching targets set forth in the new NHAS progress indicators (indicators 1, 4, and 5 either individually or in combinations) by 2020 by comparing the following scenarios to the base-case:
NHAS Indicator 1—“Increase the percentage of PLWH who know their serostatus to at least 90%”: We increased annual testing rates for high-risk individuals (MSM, PWID, and heterosexuals aged 18-30 years old), with ongoing screening at current rates in the general population, to a level that would achieve 90% serostatus awareness by 2020. During model calibration of intervention scenarios, this corresponded to an approximate 50% increase compared to current annual testing rates among MSM, PWID, and young heterosexuals (Supplement).
NHAS Indicator 4—“Increase the percentage of newly diagnosed persons linked to HIV medical care within 1 month of their HIV diagnosis to at least 85%”: We increased the proportion of newly diagnosed persons completing an HIV care visit within a month to 85% by 2020.
NHAS Indicator 5—“Increase the percentage of persons with diagnosed HIV infection who are retained in HIV medical care to at least 90%”: We iteratively decreased the annual rate of disengagement in care (from 18-25% per year [depending on risk group] in the base-case to 4-6% per year), and increased the rate of reengagement in care among those out of care (from 12·5% per year to 50% per year), until the point that the proportion of serostatus-aware PLWH engaged in care was 90% in 2020 (Supplement).
Costs were calculated from a health-system perspective with a unit-costing (Table 1) approach that considers the person-time spent in each model compartment (e.g., person-time on ART) and the number of transitions between compartments (e.g. transition from unaware to aware as a result of HIV testing). In the base-case, we considered costs related to HIV testing, linkage referrals, and clinical care (e.g. viral loads, genotypes, ART usage, hospitalizations). We additionally included costs of existing retention in care services, based on staffing for social work, nurse-managers, and case-managers at local HIV clinics (base-case, $300 per patient in care per year [range $50-$1500])9. All costs are reported in 2015 US dollars; future costs were discounted at an annual rate of 3%22. Estimates of disease burden (e.g., infections or deaths) averted are reported without discounting.
In primary analysis we did not consider infrastructural or programmatic costs of interventions to improve the HIV care continuum. However, costs related to increased health care utilization when achieving NHAS targets as a result of increased care-engagement were incorporated (e.g. increased numbers of tests, increased ART usage). We explored a range of intervention costs in secondary analyses . We conducted a probabilistic uncertainty analysis by simultaneously varying all parameter values over beta distributions bounded by their ranges (Table 1). We report 95% uncertainty ranges (URs) as the 2·5th and 97·5th percentile of those simulations.
We performed all analyses using R version 3·0·1(R Foundation for Statistical Computing).
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
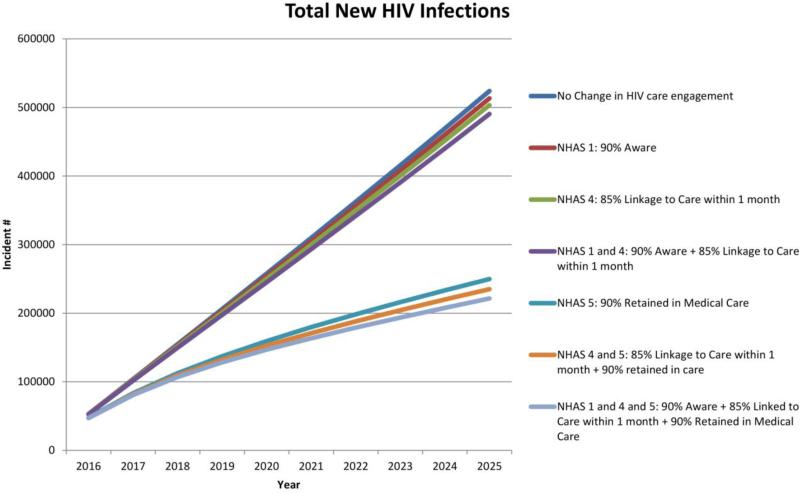
If the current rates of screening, linkage, and retention in care as part of the US HIV care continuum persists, approximately 524,000 (95%UR 442,000 – 712,000) new HIV infections would occur between 2016 and 2025 (Figure 1), with approximately 375,000 deaths (from all causes) among PLWH (95%UR 364,000 – 578,000). This results in a projected prevalence of 1·47 million PLWH in 2025, of whom 39·7% (582,000) would be virally suppressed. HIV care would cost the US health system $254 billion (95%UR $186 billion - $297 billion) with 3% discounting (Table 2) over the next decade ($295 billion without discounting). The epidemiologic and economic impact of achieving the three 2020 NHAS targets for improving HIV awareness (progress indicator 1), linkage to care (progress indicator 4), and retention in care (progress indicator 5) is shown in Table 2 and Figure 1.
Figure 1. Total new HIV infections over the next decade, by achievement of NHAS targets.
Cumulative new HIV infections at varying levels of HIV care engagement. In the base-case scenario, the current rates of HIV care engagement (screening, linkage, retention in care) persist. In the NHAS scenarios, we project improvements in care engagement (through increased yearly screening, improved linkage to care after diagnosis, and reduced care disengagement) that allows achievement of NHAS indicators 1, 4, and 5 by 2020.
Table 2.
Economic and epidemiologic impact from 2016-2025 of achieving NHAS targets for care engagement by 2020
| HIV Incidence | HIV Prevalence* | Deaths | Costs†† | |
|---|---|---|---|---|
| NHAS targets within progress indicators | Total (% Δ from base-case) | Tota(% Δ from base-case) | Total (% Δ from base-case) | Total (% Δ from base-case) |
| Base-case: No change in care | 524,000 (0%) | 1·47 million (0·0%) | 375,000 (0·0%) | $254 billion (0·0%) |
| NHAS 1: 90% awareness by 2020 | 513,000 (−2·0%) | 1-46 million (−0·5%) | 373,000 (−0·4%) | $258 billion (+1·5%) |
| NHAS 4: 85% linkage within 1 month† | 503,000 (−3·9%) | 1·46 million (−0·7%) | 363,000 (−3·1%) | $261 billion (+2·6%) |
| NHAS 5: 90% in care by 2020† | 250,000 (−52·3%) | 1·30 million (−11·1%) | 256,000 (−31·7%) | $350 billion (+37·9%) |
| NHAS 1 and 4: 90% awareness and 85% linkage | 490,000 (−6·4%) | 1·45 million (−1·3%) | 361,000 (−3·5%) | $265 billion (+4·3%) |
| NHAS 4 and 5: 85% linkage, and 90% in care | 235,000 (−55·1%) | 1·30 million (−11·6%) | 249,000 (−33·7%) | $355 billion (+39·7%) |
| NHAS 1 and 4 and 5: 90% awareness, 85% linkage, and 90% in care | 221,000 (−57·7%) | 1·29 million (−12·3%) | 246,000 (−34·3%) | $359 billion (+41·3%) |
Table legend: Abbreviations: SOC, standard of care; NHAS, National HIV/AIDS strategy; PLWH: People Living with HIV. Incremental percent reductions are based on precise model outputs; total values rounded to three significant digits.
We modeled scenarios of achieving the targets set forth in the NHAS progress indicators independently, and in combination. In all scenarios, individuals that do not link to care initially are eligible to engage in care at any later time point. NHAS 4 independent scenarios utilizes current rates of HIV screening, but improves linkage to care within 1 month to 85%. NHAS 5 independent scenario enhances care-retention (reduced rates of yearly loss from care, and improved reengagement rates for those out of care) such that 90% of those aware of their diagnosis are in care in 2020; in this scenario, the yearly rates of HIV screening and linkage to care after HIV diagnosis are the same as the base-case.
Costs represent 3% discounting of future costs. For the NHAS scenarios, costs represent total health system costs accounting for increased HIV tests and health care engagement and ART usage, but do not include costs related to potential interventions needed to achieve improved HIV care continuum engagement.
Projected number of people living with HIV in 2025.Yearly prevalence is shown in Supplemental figure 5.
Increasing screening rates to allow achievement of NHAS indicator 1 (90% serostatus awareness by 2020) would avert 10,600 new HIV infections (95%UR 6,000-18,000), or a 2·0% reduction (95%UR 1·1%-2·8%) over the next 10 years. However, improved screening alone has little impact on overall number of PLWH achieving viral suppression (589,000 of 1·46 million PLWH, 40·3%) in 2025. Consequently, enhanced screening would avert only 0·4% (1400) of deaths among PLWH during the analysis period, if current rates of care retention persist.
If 85% of those newly diagnosed with HIV were linked to care within 1 month (NHAS indicator 4), with current rates of screening and retention in care, the US would avert an estimated 20,600 (95%UR 14,000-35,000) HIV infections (3·9% reduction, 95%UR 2·8-5·4), and would avert 11,600 (95%UR 8,000-25,000) deaths (3·1% reduction; 95%UR 2·0%-4·9). In isolation, improved linkage would increase the percent of total PLWH that are virally suppressed to 41·1% (598,000 of 1·46 million PLWH).
Achieving the retention in care targets in NHAS progress indicator 5 (90% of diagnosed persons in care) was projected to avert a much larger disease burden: a 52·3% reduction (95%UR 47-56%) in HIV infections (274,000 infections averted, 95%UR 220,000 – 372,000) and a 31·7% reduction (95%UR 24%-39%) in deaths (119,000 lives saved, 95%UR 94,700-256,000) over 10 years (with 76·6% [999,000 of 1·30 million] of all PLWH virally suppressed), even at current rates of serostatus awareness and linkage to care.
Simultaneously improving all aspects of the HIV care continuum to reach all three NHAS care targets increased the estimated point prevalence of virological suppression among all PLWH (1.01 million of 1.29million PLWH) in 2025 from the base-case of 39·7% to 78·8% (82% of those diagnosed [1.24million]). Consequently, this scenario resulted in a 57·7% reduction (95%UR 52%-61%) in HIV infections (302,000 HIV infections averted, 95%UR 240,000-421,000) and a 34·3% reduction (95% UR 26%-41%) in deaths (128,000 lives saved, 95%UR 106,000-223,000) (Table 2, Supplemental Figure 5). The increased testing, care-engagement, and ART usage if all NHAS care targets are met is projected to cost $105 billion more over 10 years (with 3% discounting; $124 billion incremental increase without discounting) than if current levels of the HIV care continuum persisted (Table 2, Supplemental Figure 6).
In one-way sensitivity analysis, total costs were most influenced by ART prices (Supplemental Figure 7 and 8). If ART prices could be reduced to the $10,000 per person-year, costs in the current care continuum scenario dropped from $254 billion to $107 billion dollars; alternatively, if ART regimen costs rose to $45,000 per person-year, costs increased to $341 billion over 10 years. The incremental cost to achieve all NHAS care continuum targets likewise primarily reflected the costs related to increased ART usage (range of incremental NHAS achievement cost of $26 billion to $152 billion, for ART costs of $10,000 to $45,000 per person-year, respectively).
In a secondary analysis, we estimated health system costs inclusive of hypothetical intervention costs that would allow increased care engagement to achieve NHAS progress indicators. We explored up to a 20% increase in testing costs, along with an additional $500 per person linking to care, additional $5,000 per person-year to improve retention in care (above current retention costs), and $1,000 per person re-engaged in care. In this scenario, the total costs (with 3% discounting) of achieving all three NHAS care targets (NHAS 1, 4 and 5) was $408 billion (an increase of $49 billion [13·6% increase] compared to our primary NHAS analysis [without intervention costs], and an incremental increase of $154 billion over the scenario of continued current continuum of care).
DISCUSSION
The US NHAS was updated in July 2015 with a vision to reduce new infections and provide high quality care for PLWH. Our model projects that there will be more than half million new HIV infections in the next decade if improvements to diagnosis and care are not realized. By contrast, our results suggest that this number can be cut by almost 60%, resulting in over 125,000 lives saved, if comprehensive steps are taken to achieve 2020 targets for screening, linkage and care-engagement set forth in NHAS progress indicators. The primary gap in the current continuum of care is in care retention, which is needed to realize the prevention and health benefits of ART usage and viral suppression; achieving the NHAS goal of engaging 90% of those diagnosed in care could halve the number of HIV infections over the next 10 years. These results offer credence to the importance of achieving the NHAS progress indicators in order to ‘bend the curve’ of the HIV epidemic.
Currently, CDC estimates that over 85% of PLWH are aware of their serostatus, and there are recommendations to increase HIV screening among high-risk groups19, 23. As such, our modeling efforts found that incrementally increasing HIV diagnoses alone to meet NHAS targets (90% awareness by 2020), has relatively little impact on the overall HIV epidemic. The NHAS additionally outlines the need to increase the percentage of diagnosed PLWH who are virally suppressed to at least 80%. We found that increased screening and linkage alone is insufficient to meet this goal, due to inadequate long-term ART usage. By contrast, the effects of improved HIV screening and linkage could be greatly enhanced if coupled with improved care retention. Under the most intensive scenario, wherein screening, linkage, and proportion of PLWH in care are improved to meet the NHAS goals, nearly 82% of diagnosed PLWH are expected to be virally suppressed, exceeding the NHAS targets for viral suppression.
The NHAS has also set ambitious epidemiological goals, including a 25% reduction in HIV diagnoses (as a surrogate for incident infections) and a 33% reduction in mortality among (diagnosed) PLWH by 2020. Our results suggest that both of these goals are within reach, but only by redoubling efforts to improve care retention. In particular, only when incorporating scenarios with a high proportion of PLWH in care do we observe significant declines in incidence (and mortality), with eventual reductions in the total number of PLWH over time. These results suggest that a change in thinking about the HIV response is necessary if we are to meaningfully bend the curve of HIV transmission. To date, most studies have evaluated the HIV care continuum cross-sectionally. However, continuous retention is required for long-term success in HIV treatment24. Specifically identifying evidence-based interventions to improve long term retention and close this gap in the HIV care continuum25 must be recognized as an urgent priority. Interventions such as housing assistance, case management, or opioid substitution therapy can be helpful, but ultimately we must develop a comprehensive, patient-centered approach to retention and re-engagement in care that considers the diverse needs of PLWH and seeks to meet those needs in holistic fashion. Losing a patient to HIV care should be considered a “sentinel event,” with necessary structures put in place to prevent such events from ever occurring.
Our results also highlight the financial resources that are likely required to achieve these ambitious goals. Currently, the federal domestic HIV budget is $25·3 billion for fiscal year 2016 (21% increase since 2010)26, a funding rate consistent with model projections of total HIV health system costs ($254 billion) over the next decade if the current HIV care continuum persists. In order to achieve levels of care retention necessary to change the trajectory of HIV epidemiology in the US, a similar restructuring in thinking regarding funding of the HIV/AIDS response may be required. Specifically, we estimate that achieving NHAS targets for serostatus awareness, linkage to care, and retention in care would cost an incremental $105 billion in health care costs alone over 10 years, representing an over 40% increase in expenditures – the vast majority of which represent additional costs of ART associated with a greater number of PLWH engaged in care. Thus, if we are to succeed in curbing transmission, we must also reframe our approach to funding the HIV response, seeking innovative and targeted funding mechanisms to facilitate this long term treatment and retention. Reducing ART costs through such mechanisms as 430B pricing, ADAP programs, and generic manufacturing of key drugs could offer marked health system cost savings.. Efforts to both expand funding for HIV care, including ancillary services and interventions to promote long term retention, and lower the costs of ART must both be prioritized.
Our results build on other models of HIV transmission in the US. A study assessing the impact of the original NHAS (2010) linkage goals found a similarly small impact of linkage interventions on transmission27. Compared to other authors28, our results suggest a relatively more modest impact on HIV incidence of expanded screening interventions. The current results also expand on prior efforts by our group to evaluate the impact of the care continuum on HIV transmission in the US5, 9. We now provide updated projections of incidence and prevalence incorporating recently revised estimates of HIV epidemiology and the care continuum4, 19, and quantify the economic and epidemiologic impact of achieving targets for care engagement within NHAS progress indicators.
Our study has several limitations. For all scenarios, we assume that PLWH will have timely access to ART after entry into care, as per current guidelines. Delays or gaps in therapy would lessen the impact of increased care engagement. Our model is also calibrated to currently available national estimates of engagement in the care continuum19. Data from large city and county programs have generally suggested more optimistic estimates of current HIV care engagement and viral suppression29, 30. To the extent that engagement in care and viral suppression in our base-case scenario are underestimated, the incremental impact of achieving NHAS targets may be more modest, and our model may be optimistically biased. Bias in our model estimates may also result from applying nationally representative numbers to any specific city or locale. Nonetheless, our model provides absolute, in addition to relative, projections on incidence and prevalence with uncertainty ranges under various engagement in care scenarios, while reinforcing the importance of accurate national continuum of care estimates. Our model did not evaluate specific interventions (or associated costs) that will likely be needed to achieve NHAS targets. Efforts to improve testing, linkage, and improve adherence or care-engagement will almost certainly require a combination of health system, policy, and clinical innovations. Nonetheless, we conducted secondary analyses to explore costs inclusive of hypothetical HIV care continuum interventions and found modest relative to the health system costs attributable to increased ART usage and clinical care expected to incur with achieving NHAS targets. Our model also found a significant number of new infections despite achievement of comprehensive improvements to screening, linkage, and care engagement, suggesting that additional prevention strategies may be needed to reduce transmissions further. Our model does not address the potential costs and effects of scale up of pre-exposure prophylaxis (PrEP), or other such prevention interventions that fall outside the care continuum. Improved HIV serostatus awareness with consequent consistent PrEP utilization could further decrease transmission among high risk groups, but could also substantially increase prevention costs.
In conclusion, our results offer quantitative evidence of the extent to which achievement of NHAS targets for improving HIV testing, linkage, and care-engagement impacts the parallel NHAS goals of reducing HIV incidence and prevalence. As the US moves to implement the new NHAS, our model suggests that sustained improvements to retention in care must be the highest priority to reach the ambitious epidemiological targets for reduced HIV incidence and prevalence.
Supplementary Material
Acknowledgments
Funding
The authors declare that they have no conflicts of interest or relevant financial interests or activities in relationship to this manuscript. This work was supported by funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under grant # K23AI089259, and T32AI102623, the B. Frank and Kathleen Polk Assistant Professorship in Epidemiology, and the Emory University Center for AIDS Research (P30 AI050409). Funders had no role in the design or conduct of the study, analysis or interpretation of the results, manuscript writing or decision to publish results. We acknowledge support from the CDC/NCHHSTP Epidemiological and Economic Modeling Agreement (5U38PS004646). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Research in Context:
Evidence before this study: We searched PubMed to identify studies related to the National HIV/AIDS strategy (NHAS), and mathematical models of the care continuum in the US. We searched terms (“HIV” or “AIDS”) and (“National HIV/AIDS strategy” or “model” or “continuum”) and included all studies published from 2010 (initial NHAS release) until November 16, 2015 without language restrictions. Our search identified no models or epidemiological studies related to the recently updated US National HIV/AIDS strategy. Our search identified one cost-effectiveness analysis of the prior 2010 NHAS linkage to care target, that showed improved early linkage would cost an extra $62,200 per QALY-gained. Our prior mathematical model of HIV transmission in the US suggested that improving yearly rates of retention in care by 50% could avert 35% of new infections.
Added Value of this Study
Our results demonstrate that achieving the updated NHAS progress indicators of 90% awareness of HIV serostatus, 85% rapid linkage, and 90% of diagnosed individuals in care by 2020 could allow the United States to reach NHAS epidemiological goals and halve new infections over 10 years with eventual reductions in HIV prevalence. The primary driver of this achievement (and also the progress indicator furthest from being met) is retention in care. Meeting NHAS care targets would require an increase of $105 billion of HIV care costs compared to continuation of current practice; the majority of these additional costs represent ART costs for PLWH who are not currently virally suppressed.
Implications of all the available evidence: The evidence suggests that continuous retention in care is critical for long term success in HIV treatment. Meeting the NHAS progress indicators for improved HIV screening, linkage, and care-engagement could alter the trajectory of the US HIV epidemic, but will require continued and increased investment. ART drug prices are the most influential drivers of cost in meeting the goals of improving HIV care engagement.
Contributors: MS, ESR, DWD, and PS conceived and designed the study and led manuscript writing. CDR assisted with data interpretation and manuscript writing. MS, AP, KR and DD executed the study and performed data analysis. SK, AS and JG assisted with manuscript writing, literature search, and data collection.
Declarations of interest: The authors declare they have no conflicts of interest or financial interests in connection to this work.
REFERENCES
- 1.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The Spectrum of Engagement in HIV Care and its Relevance to Test-and-Treat Strategies for Prevention of HIV Infection. Clinical Infectious Diseases. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New England Journal of Medicine. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 3.Cohen M. Final results of the HPTN 052 randomized controlled trial: antiretroviral therapy prevents HIV transmission Program number MOAC0106LB, Track C. International AIDS Society; Vancouver; 2015. 2015. [Google Scholar]
- 4.Centers for Disease Control and Prevention Monitoring selected national HIV prevention and care objectives by using HIV surveillance data-United States and 6 dependent areas--2013. [Sept 29 2015];HIV surveillance Supplemental Report. 2015 2015 http://www.cdc.gov/hiv/library/reports/surveillance/
- 5.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA internal medicine. 2015;175(4):588–96. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 6.White House Office of National AIDS Policy [06/25/2015];National HIV/AIDS Strategy for the United States. 2010 https://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf.
- 7.White House Office of National AIDS Policy [October 19, 2015];National HIV/AIDS Strategy for the United States. 2015 https://www.whitehouse.gov/administration/eop/onap/nhas.
- 8.UNAIDS [06/30/2015];90-90-90: An ambitious treatment target to help end the AIDS epidemic. 2014 http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
- 9.Shah M, Risher K, Berry SA, Dowdy DW. The epidemiologic and economic impact of improving HIV testing, linkage, and retention in care in the United States. Clin Infect Dis. 2015 doi: 10.1093/cid/civ801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddali MV, Dowdy DW, Gupta A, Shah M. Economic and epidemiological impact of early antiretroviral therapy initiation in India. J Int AIDS Soc. 2015;18(1):20217. doi: 10.7448/IAS.18.1.20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Currie S, Rogstad KE, Piyadigamage A, Herman S. Time taken to undetectable viral load, following the initiation of HAART. Int J STD AIDS. 2009;20(4):265–6. doi: 10.1258/ijsa.2008.008268. [DOI] [PubMed] [Google Scholar]
- 12.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198(5):687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 13.Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA. Network HIVR. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60(3):249–59. doi: 10.1097/QAI.0b013e318258c696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udeagu CC, Webster TR, Bocour A, Michel P, Shepard CW. Lost or just not following up: public health effort to re-engage HIV-infected persons lost to follow-up into HIV medical care. Aids. 2013;27(14):2271–9. doi: 10.1097/QAD.0b013e328362fdde. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham CO, Buck J, Shaw FM, Spiegel LS, Heo M, Agins BD. Factors Associated With Returning to HIV Care After a Gap in Care in New York State. J Acquir Immune Defic Syndr. 2014;66(4):419–27. doi: 10.1097/QAI.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Medicaid and Medicare Services Medicaid Fee Schedule. 2014 [Google Scholar]
- 17.Gebo KA, Fleishman JA, Conviser R, et al. Contemporary costs of HIV healthcare in the HAART era. Aids. 2010;24(17):2705–15. doi: 10.1097/QAD.0b013e32833f3c14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AIDSinfo [Sept 29, 2015];Clinical Guidelines Portal--Cost considerations and Antiretroviral Therapy. 2015 https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/459/cost-considerations-and-antiretroviral-therapy.
- 19.Bradley H, Hall HI, Wolitski RJ, et al. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Vital signs: HIV prevention through care and treatment--United States. MMWR Morb Mortal Wkly Rep. 2011;60(47):1618–23. [PubMed] [Google Scholar]
- 21.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents [Sept 29, 2015];Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2015 http://aidsinfo.nih.gov/guidelines.
- 22.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. Jama. 1996;276(15):1253–8. [PubMed] [Google Scholar]
- 23.United States Preventive Services Task Force Human Immunodeficiency Virus (HIV) Infection: Screening. 2013 Apr; 2013. http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/human-immunodeficiency-virus-hiv-infection-screening.
- 24.Colasanti J, Kelly J, Pennisi E, et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis. 2015 doi: 10.1093/cid/civ941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higa DH, Crepaz N, Mullins MM, Prevention Research Synthesis P. Identifying Best Practices for Increasing Linkage to, Retention, and Re-engagement in HIV Medical Care: Findings from a Systematic Review, 1996-2014. AIDS Behav. 2015 doi: 10.1007/s10461-015-1204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser Family Foundation [Oct 2, 2015];US Federal Funding for HIV/AIDS: The president's FY 2016 budget request. 2015 http://kff.org/global-health-policy/fact-sheet/u-s-federal-funding-forhivaids-the-presidents-fy-2016-budget-request/
- 27.Gopalappa C, Farnham PG, Hutchinson AB, Sansom SL. Cost effectiveness of the National HIV/AIDS Strategy goal of increasing linkage to care for HIV-infected persons. J Acquir Immune Defic Syndr. 2012;61(1):99–105. doi: 10.1097/QAI.0b013e31825bd862. [DOI] [PubMed] [Google Scholar]
- 28.Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Annals of internal medicine. 2010;153(12):778–89. doi: 10.1059/0003-4819-153-12-201012210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laffoon BT, Hall HI, Surendera Babu A, et al. HIV Infection and Linkage to HIV-Related Medical Care in Large Urban Areas in the United States, 2009. J Acquir Immune Defic Syndr. 2015;69(4):487–92. doi: 10.1097/QAI.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray KM, Cohen SM, Hu X, Li J, Mermin J, Hall HI. Jurisdiction level differences in HIV diagnosis, retention in care, and viral suppression in the United States. J Acquir Immune Defic Syndr. 2014;65(2):129–32. doi: 10.1097/QAI.0000000000000028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.