Abstract
The aqueous humor (AH) is the fluid that fills the anterior and posterior chambers of the eye. Its main roles are to provide nourishment and metabolic waste removal to active metabolic ocular structures that are avascular and to contribute maintaining a normal intraocular pressure (IOP) without altering the refractive status of the eye. Its composition and the fluid dynamics associated with its flow are voluble and undergo changes associated with age and disease. Of particular importance is that the resistance to the outflow of AH from the anterior chamber is influenced by morphologic, physiologic, and biochemical dynamic factors.1 Beside aqueous nutritional importance, its solutes also participate in establishing the anterior chamber associate immune deviation, and carry and distribute the different proteins and molecules that promote and direct tissue remodeling and changes in the anterior segment that are associated with both age and disease.
Keywords: Aqueous, Formation, Outflow, Trabecular meshwork, Anatomy, Canine
Three major distinct aspects of the aqueous need to be considered:
Aqueous production
Aqueous composition
Aqueous outflow
In this review, the authors focus on aqueous outflow and introduce concepts of aqueous production, mentioning some of the dynamic variations in aqueous composition that may influence the physiologic and pathologic changes seen with aging and glaucoma.
The physiologic range of IOP is maintained through the constant balance between aqueous production and aqueous outflow. The pressure gradient within the eye is comprised within specific values that may vary individually in different daily patterns and with aging. A rule of the thumb indicates normal IOP in dogs to be between 12 and 25 mm Hg; however, most dogs tend to have a normal pressure below the 20s.2–6 IOP values may also vary depending on the time and technique of measurement. The IOP fluctuates during the day with circadian phases that peak and drop at different times of the day, depending on the species. In dogs, like in humans, the highest normal IOP values are measured in the morning, and IOPs are lower in the evening.3,5 In dogs, a decrease in baseline IOP is associated with increased age2; a range of diverse findings has been reported in different human studies.7 There are differences in ethnic groups, and both positive and negative relationships between increased age and IOP have been described.8,9 Although the outflow seems to decrease with age, the production of AH may also decrease.10
Aqueous Production
Two mechanisms—passive and active—are responsible for aqueous production and contribute to its composition.11 Passive diffusion and ultrafiltration of plasma occur in the vascularized ciliary body stroma. The passive mechanisms do not contribute significantly to the formation of AH. Because the ciliary blood vessel endothelia are fenestrated, diffusion of solutes travels according to a concentration gradient, trying to maintain a balance between different tissues/compartments. Substances with high lipid solubility coefficients can easily move across cellular membranes.
Ultrafiltration allows molecules to cross a cell membrane following a hydrostatic force or an osmotic gradient, and it results from differences between the pressure of the ciliary body capillaries and the IOP and solute differences. The hydrostatic pressure of ciliary body capillaries has been estimated to be between 25 and 33 mm Hg,12,13 whereas the oncotic pressure of vascular proteins is about 14 mm Hg.14 Hydrostatic and oncotic forces would both actually favor resorption of AH.15 If we consider the IOP around the value of 15 mm Hg, we can understand how a much higher hydrostatic pressure would be needed to achieve relevant amount of aqueous formation through this passive mechanism.
Passive mechanisms are, however, able to generate a reservoir fluid within the ciliary body.16 Furthermore, because of the lack of a true epithelium on the anterior surface of the iris, leakage and diffusion of diluted plasma occurs from the ciliary vessels into the anterior chamber.17
Basically, AH is secreted across the ciliary epithelium by transferring solutes, mainly NaCl, from the stroma to the posterior chamber of the eye, with a subsequent passive movement of water in the same direction.18 At least 80% to 90% of AH formation occurs with active mechanisms.19 Active secretion requires energy that is provided by the hydrolysis of adenine triphosphate and relies mainly on 2 enzymes: an adenosine triphosphatase Na/K pump and carbonic anhydrase. The structural site for active secretion resides in the inner (facing the posterior chamber), nonpigmented ciliary epithelium of the ciliary processes where these 2 enzymes are highly concentrated. Active formation through an adenosine triphosphatase Na/K pump is responsible for more than 70% of the aqueous production.13,20 Besides the increased concentration of NA+ ions in the posterior chamber, secondary active transport mechanisms increase the concentration of other solutes, including Cl−. Active formation is also catalyzed by the enzyme carbonic anhydrase that is particularly present in the nonpigmented epithelium of the ciliary body.21 The latter accounts for about 40% to 50% of the aqueous production.22 Carbonic anhydrase catalyzes the reaction H2O + CO2 ↔ HCO3− + H+. Bicarbonate moves then in the posterior chamber influencing fluid transport by also affecting Na+, possibly by regulating the pH for optimal active ion transport.15 The 2 active mechanisms share some of the pathways, explaining the mathematics of the rate of production. When active release of sodium (Na+) or bicarbonate (HCO3−) ions into the posterior chamber is mediated by these enzymes, an osmotic gradient is created and the plasma ultrafiltrate can move from the stroma of the ciliary body into the posterior chamber (Fig. 1). This mechanism is sensitive to the level of IOP and decreases with increased IOP. However, this effect is insufficient to serve as a protective mechanism against the development of glaucoma.
Fig. 1.
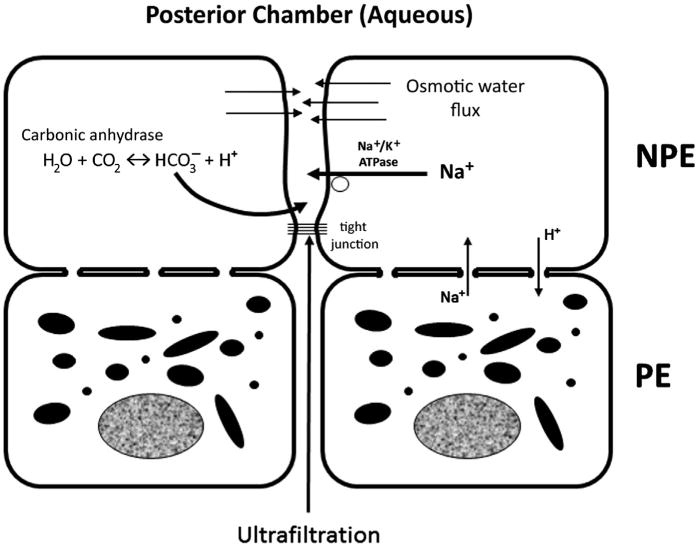
Mechanism for aqueous secretion. An active movement of Na+ and HCO3− increases the solute concentrations in the posterior chamber in proximity of the ciliary processes and creates a positive osmotic gradient that recalls fluids collected in the ciliary tissues because of diffusion and ultrafiltration. ATPase, adenosine triphosphatase; NPE, nonpigmented epithelium; PE, pigmented epithelium.
The amount of AH production is indirectly calculated by measuring the amount of outflow.23 Outflow facility (c) indirectly indicates the amount of aqueous production and it can be expressed as μL/min or μL/min/mm Hg. In normal humans, c is about 2.75 ± 0.63 μL/min (range, 1.8–4.3) or about 0.3 μL/min/mm Hg.24 In dogs, the total value has been manually calculated with a mean ± SD equal to 5.22 ± 1.87 μL/min, whereas when calculated by an automatic software the flow rate was 4.54 ± 2.57 μL/min.25 These values grossly mirror the values of 0.24 to 0.30 μL/m/mm Hg reported by Gum and colleagues.26
Individual variations are following circadian rhythms and are also influenced by age.24,27 In humans, AH formation and outflow both decrease with aging.10,28
Although the site and the mechanisms of aqueous formation seem to be well-established and described, the mechanisms for outflow are still a large field for research, especially when related to the pathophysiology of the different phenotypes of glaucoma.
Pathways of Aqueous Outflow
The outflow facilities are a complex hydraulic system that allows the AH to exit the eye consistently, yet maintaining a physiologic IOP balanced with aqueous secretion. When the regulation of the outflow is impaired, an increase in IOP occurs. No active transport mechanisms is involved in the outflow. AH passes through the trabecular meshwork (TM) as bulk flow driven by the pressure gradient, which is higher in the eye when compared with the distal outflow vessels.29,30 The posterior, uveoscleral outflow (USO) is passive and largely independent from the IOP; it is mostly regulated by osmotic gradients.31
The pathways of canine aqueous outflow include several different anatomic structures whose nomenclature has been variously and differently described, used, and classified.32–38 The understanding of the normal morphology and composition of these structures, and the array of dynamic physiologic changes that occur in different breeds and aging are important considerations when pathologic changes are then analyzed and therapeutic agents selected.
Besides an irrelevant corneal and uveal permeability, 2 main, different outflow pathways are usually considered the most essential to IOP balance:
The anterior/trabecular or conventional outflow
The posterior or unconventional, or the USO
Trabecular Outflow
The anatomic terminology related to the trabecular outflow system (Figs. 2–4) includes the following:
Iridocorneal angle (ICA)
Ciliary cleft (CC)
Pectinate ligament (PL)
- The TM system, which includes
- Uveal TM (UTM)
- Corneoscleral TM (CSTM) and uveoscleral TM (USTM)
- Juxtacanalicular tissue (JCT)
- Angular aqueous plexus (AAP)
- Inner wall (IW)
- Inner collector channels
Radial collector channels
Episcleral veins and intrascleral venous plexus (ISVP) or circle of Hovius
Fig. 2.
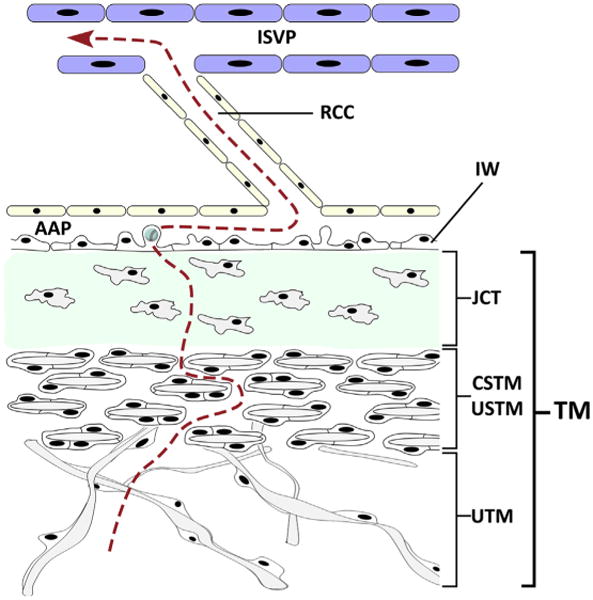
Aqueous (red dotted line) trabecular meshwork (TM) outflow pathway from the ciliary cleft through the trabecular beams of the uveal TM (UTM), the lamellar corneoscleral or uveoscleral trabecula meshwork (CSTM/USTM) and the extracellular matrix of the juxtacanalicular connective tissue (JCT). The fluid moves through the inner wall (IW) endothelial cells by formation of intracellular giant vacuoles that open into the lumen of angular aqueous plexus (AAP). The AAP is connected to larger radial collector channels (RCC) leading to the intrascleral venous plexus (ISVP). (Adapted from Swaminathan SS, Oh DJ, Kang MH, et al. Aqueous outflow: segmental and distal flow. J Cataract Refract Surg 2014;40(8):1264; with permission.)
Fig. 4.
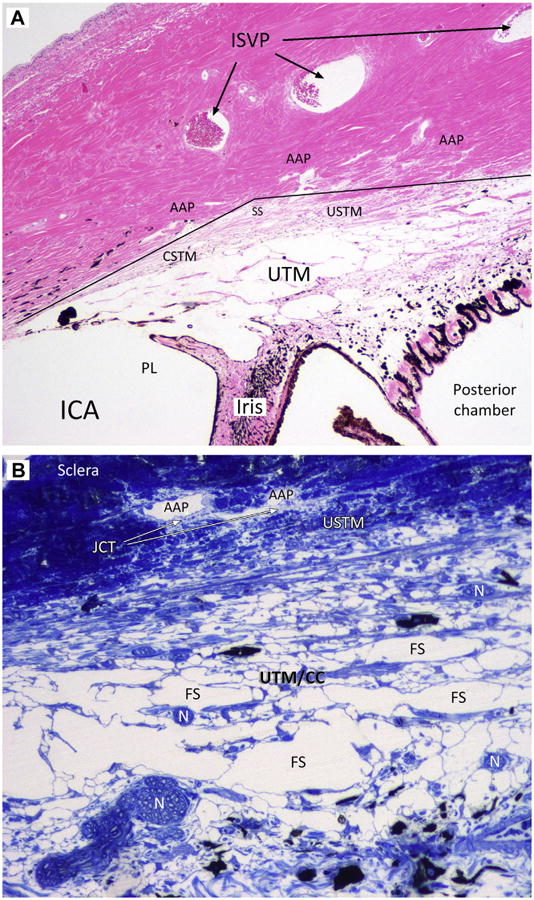
Microphotographs of sagittal sections of canine trabecular outflow tissues. (A) Hematoxylin and eosin staining. A partial section of a pectinate ligament (PL) can be recognized anteriorly and separates the iridocorneal angle (ICA) from the ciliary cleft that is filled with irregularly distributed beams of the uveal trabecular meshwork (UTM). It contains the Fontana spaces. The corneoscleral trabecular meshwork (CSTM) and the uveoscleral TM (USTM) are a transition zone between the sclera and the UTM and lie in the scleral sulcus (SS and continuous line). Note the wide spreading of the angular aqueous plexus (AAP) in the inner sclera. Three large venous vessels can be seen in the midsclera. They constitute the intrascleral venous plexus (ISVP; arrows). (B) Toluidine blue staining at higher magnification. The progressive reduction of the Fontana spaces (FS) can be appreciated. Large spaces converge into smaller intertrabecular spaces in the USTM. With this coloration, the translucency of the juxtacanalicular connective tissue (JCT) are appreciated around the angular aqueous plexus. Several nerve bundles (N) are noted within the UTM.
Iridocorneal Angle
The ICA represents the peripheral, circumferential portion of the anterior chamber, where the cornea, sclera, and base of the iris converge. It can be examined clinically with a gonioscopic lens and the gross appearance of the ICA is characterized by the presence of slender strands of uveal tissue, PLs that connect the peripheral base of the iris to the peripheral cornea, just posterior to the deep scleral pigmented line, which approximately corresponds with where the Descemet membrane ends. The junction between the cornea and PL is labeled Schwalbe's line (see Fig. 3).
Fig. 3.
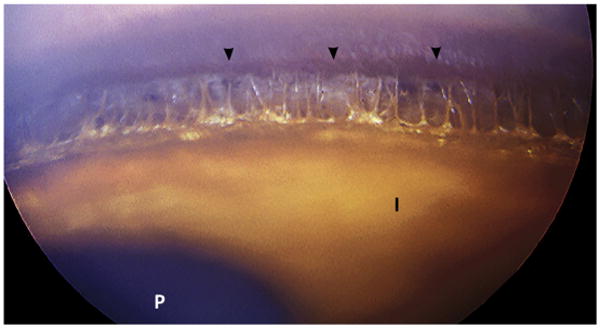
Normal iridocorneal angle in a dog at gonioscopy. Lightly pigmented iris tissue strands can be observed spanning the peripheral angular space and introducing to the posterior space of the ciliary cleft. The corneoscleral pigmented line (Schwalbe's line) is indicated by the arrowheads. I, anterior surface of the iris; P, pupil.
Pectinate Ligaments
The iconic PL is formed by a slender, branching palisade of discrete beams of iris tissue that span the ICA. The number, pattern, length, and thickness of the PLs vary among breeds and individuals.33,36,39,40 The width of the ICA can also vary depending on breed and age.41–44 The PLs are composed of a core of collagen and lined by iridal melanocytes and fibroblasts.33,36 They are usually pigmented unless the dog is subalbinotic. However, with aging, dispersed pigment from microdamage to the posterior epithelium of the iris may accumulate in the ICA even in dogs with a blue iris. The most anterior ligaments are thicker than the beams that form the UTM more posteriorly. PLs branch and anastomose between themselves and with the beams of the UTM. The morphologic transition between the tougher PLs and the thinner trabecular beams can be gradual or abrupt.
Ciliary Cleft
The CC names the peripheral circumferential space posterior to the ICA. This almost virtual space extends posteriorly to the PLs into the posterior ciliary body with a triangular shape with anterior base. Its anatomic boundaries are represented by the PLs anteriorly, the iris root and the anterior pars plicata of the ciliary body internally, the ciliary body matrix and muscle posteriorly, and the sclera externally. Although it is a separate entity with its own boundaries, some authors include the CC in the posterior part of the ICA. The width and depth of the CC varies in different individuals, breeds and with aging.33 The CC is the container for the UTM.
The Trabecular Meshwork
The TM system is a useful nomenclature to group the whole proximal (converging) trabecular outflow system. The TM in dogs consists of 3 main different sections listed from internal to external: (1) The cobweblike UTM, (2) the lamellated CSTM and USTM, and (3) the nonlamellated cribriform region or JCT. The latter is intimately connected to the IW of channels that form the AAP (see Fig. 4).
The Uveal Trabecular Meshwork
The CC contains the UTM, which is a spongiform, cobweblike tissue defined by irregularly anastomosing trabecular beams. The empty spaces between the beams are named Fontana's spaces. Outwards and posteriorly, these spaces reduce progressively because of the increasing interlacing between the beams. The core of the beams are made by connective tissue with collagen and elastin fibers and other extracellular matrix (ECM) proteins.33 The beams are lined by endothelial cells and their basal membrane; these cells are considered to be an extension of the corneal endothelium and play a substantial role in regulating outflow and IOP.35 TM cells respond to mechanical strain increasing ECM turnover, altered gene expression, and release of several cytokines.45 TM cells have phagocytic properties,46,47 and with age they tend to engulf melanin granules released by the posterior surface of the iris (Fig. 5).48,49 If the TM cells become extremely engulfed, they may slough off from the beam, exposing the underlying ECM. Marked trabecular cell loss has been associated to collapse and fusion of the lamellae and loss of intertrabecular spaces.50,51 In primates with primary open-angle glaucoma, there is a marked loss of TM cells when compared with normal age-matched eyes.52,53
Fig. 5.
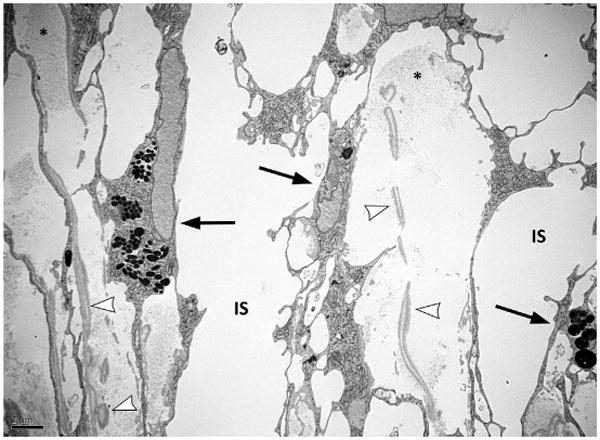
Transmission electron microscopy. Microphotograph of the beams of the canine uveoscleral trabecular meshwork. The beams are interposed to the intertrabecular spaces (IS). The beams are showing collagen (asterisks) and elastin (arrowheads) fibers in their core. Black arrows point to trabecular meshwork endothelial cells that line the outside of the beams and have phagocyted melanin granules.
Anterior tendons of the ciliary muscle insert on the elastic fibers of the UTM and JCT,54 and seemingly the inner scleral tissue, mostly at the outer portion in the posterior half of the CC. The effect of these tendons and their relative muscle on the outflow in dogs has not been well-determined yet, but, considering their anatomic distribution, are likely to help in opening the spaces of the AAP (Fig. 6). Myofibroblast-like cells have been reported in different species to be present in the TM, including the outermost region transitioning into the collector channels/Schlemm's canal.33,54 All this region has high content of the elastic fiber system, which is composed of a core of elastin and surrounding microfibrils (fibrillin-1 and microfibrillar-associated protein-1 and -2). The system provides support, resilience, and allows recoiling to regions under biomechanical stress.55 Elastin deposition and its microfibrillar envelopment, because the thickness of the basal membrane of the endothelial monolayer of the CC increase with age.35 The thickening of the elastin system with aging is in part owing an increased fibers cross-linking.56 These changes are associated with an increased stiffness of the tissues of the TM.
Fig. 6.
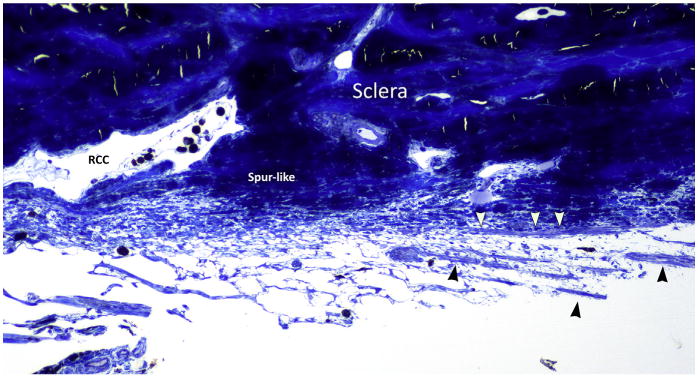
Semithin section of the posterior trabecular meshwork of a canine eye. Toluidine blue staining. Longitudinal ciliary body muscle fibers are indicated by the arrowheads. They direct to the inner sclera, in an area that reminds scleral spur in primates and that is just posterior to a large radial collector channel (RCC).
The capability of this region to undergo constant modifications associated with muscular activity matches with the diffuse presence of nerve endings (see Fig. 4). Both parasympathetic (from the ciliary ganglion) and sympathetic (from the cranial cervical ganglion) systems innervate the ciliary body.26 Sensorial nerves are also provided by branches of the V cranial nerve.
Parasympathetic fibers can be assumed to be the most influential on the longitudinal ciliary muscle in dogs. Other neurotransmitters may play a role in the outflow. Nitrovasodilators in bovine induce relaxation of the myofibroblast-like cells in the TM, increasing the outflow.57,58 Nitrous oxide has also shown interesting effects in reducing the IOP in normotensive and dogs with primary open-angle glaucoma.59
Collagen V and VI are also present. The nonfibrillar collagen type IV is associated with the endothelial basal membranes, together with laminin, fibronectin and perlecan.54,60 Fibronectin and laminin are also found interspersed within the ECM in the beams of TM and in the JCT.61 The beams of the UTM thicken with age and glaucoma in dogs.1,35 Fibronectin is among the major protein components of the ECM of the TM and, although few controversial opinions, increased deposit of fibronectin in trabecular tissues with aging and glaucoma is consistently reported.56,61–63
UTM cells also undergo modulating production of several non-structural proteins that regulate ECM turnover and remodeling, including, but not limited to, transforming growth factor beta-2 (TGFβ2), stromelysin, secreted protein acidic and rich in cysteine (SPARC), trombospondin-1 and -2, bone morphogenic protein-7, tenascin-C, matrix metalloproteinases and their inhibitors.64–68 In addition, TM cells can express several receptors that can be activated by cytokines present in the aqueous (aquecrine/paracrine response) or by growth factors produced by the TM cells themselves (autocrine response).69 These highly convoluted and variably dynamic mechanisms of cellular and matrix communication, which only represent a partial portion, constitute part of the complex mechanisms that regulate tissue homeostasis, remodeling, and changes that occur physiologically and pathologically in the outflow pathways. The boundaries between homeostasis and disease, innate or/and adaptive immune reactions, parainflammation and inflammation are not sharp and often overlapping.70,71 These interactions are not well defined or at least not well understood as a whole yet. In adjunct, polymorphic alleles in coding genes or/and promoters and enhancers and linkage disequilibrium with major histocompatibility complex haplotypes can have an influence on individual diversity in the dynamic cascades involving these factors or can explain breed predisposition to specific multifactorial diseases.
Corneoscleral and Uveoscleral Trabecular Meshwork
The CSTM and the USTM are essentially the same tissue that assumes different names depending on its location and the relationship with surrounding tissues (Fig. 7). The CSTM and the UTMS place in the inner most part of the sclera, occupying the scleral sulcus, a circular, shallow, and wide triangular groove with its outer apex approximately corresponding with the base of the iris (see Fig. 4). Their inclusion within the CC is questionable. In primates, the scleral sulcus is the site that harbors the TM and an equivalent scleral modification can be observed in canine eyes that have been sectioned after fixation with agents that tend to better preserve the ocular anatomy (see Fig.4). The scleral sulcus extends from the peripheral edge of Descemet's membrane to the posterior boundaries of the CC. The morphologic cobweblike network pattern of the UTM differs from the more organized and compact arrangement of the connective lamellae in the CSTM and USTM. The lamellae are disposed in tangentially oriented parallel layers band with evidently and progressively reduced intertrabecular spaces while approaching the AAP. These spaces are still lined by endothelial cells. As mentioned, the CSTM and the USTM share similar anatomy and organization and their division is purely made for anatomic nomenclature dictated by their location.
Fig. 7.
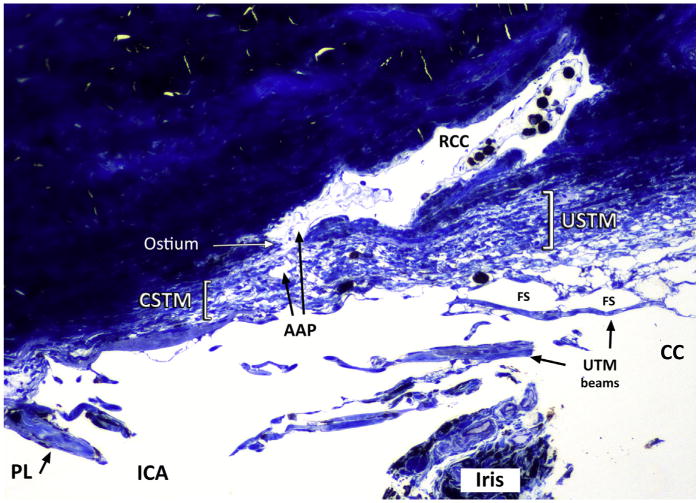
Semithin section and toluidine blue stain of the iridocorneal angle (ICA) and anterior ciliary cleft (CC). The corneoscleral and uveoscleral trabecula meshwork (CSTM and USTM) are the same, lamellar, more compact tissue than the uveal trabecular meshwork (UTM). The name changes depending on anterior (more corneal) or posterior (uveal) position. An ostium between inner collector channels (ICC) and a radial collector channel (RCC) is indicated with the arrow. FS, Fontana spaces; PL, pectinate ligament.
The CSTM is cranial and fades into the cornea approximately around the inner limbal area. The USTM is more posterior and represents the outer boundary of the posterior CC. The CSTM and the USTM represent the transition tissue between the UTM and the JCT (Fig. 8).
Fig. 8.
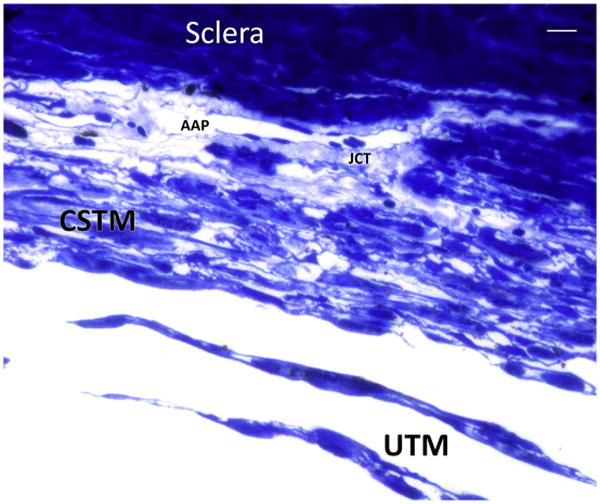
Semithin section with toluidine blue staining shows the transition between the uveal trabecular meshwork (UTM) represented by irregularly distributed beams of connective tissue lined by endothelial cells into a more lamellar and organized tissue represented by the corneoscleral trabecular meshwork (CSTM). Small, interlamellar spaces can be seen. The justacanalicular connective tissue (JCT) is a thin layer rich in extracellular matrix that surround the inner wall of the endothelial cells of angular aqueous plexus (AAP). Bar 5 10 μ.
The Cribriform Region or Juxtacanalicular Tissue and the Inner Wall of Inner Collector Channels
The whole anterior outflow system can be imagined as an hourglass with sand replaced by the aqueous. The proximal, converging spaces are inner to the eye and can be identified with the anterior chamber, the cleft with the UTM, the CSTM and the USTM, whereas the distal, progressively diverging spaces are represented by the lumen of the AAP, radial collector channels, the larger outer collector channels, the episcleral veins, and the ISVP. The scientific consensus is that the site of higher resistance is represented by both the thin layer of JCT around the AAP and their endothelium that represents the IW.54,72–75 This critical area, its structural and functional characteristics, and the changes that occur with aging and disease are the primary region of interest in the normal regulation and dysregulation of IOP. In dogs, in which closed angle glaucoma is the most common phenotype of the disease, other anatomic regions may be of interest as well.
The JCT (also called the endothelial meshwork or the cribriform region) is the outermost region of the TM and lies underneath the IW of the AAP. Despite some morphologic differences between primate and non primate mammals, the JCT has been identified in dogs and bovines.35,36,76,77 The tissue is extremely thin and likely variable in thickness and composition at different sites.78 Its thickness is reported to vary between 5 to 10, 7 to 14, and 2 to 20 μ.74,78–80 The JCT represents a nonlamellar, loose, and not well organized tissue. Fibroblastlike cells are embedded into an almost fluid fibrillary ECM abundant in proteoglycans and glycosaminoglycans (GAGs), and rich in unarranged elastin and collagen fibers.74,75 The cells are kept in mutual contact with each other as well as the IW endothelial cells by extensions of their cytoplasmic membranes.
In dogs, the JCT is not as compact as in humans (Fig. 9).33 Electron-lucent or “empty” spaces have been described in the JCT. These spaces may actually be filled with GAGs35 and seem to be empty owing to sample processing (see Fig. 9). On histologic and transmission electron microscopy sections, the JCT is dogs is more discernible in proximity of the IW of the AAP (see Figs. 8 and 9).
Fig. 9.
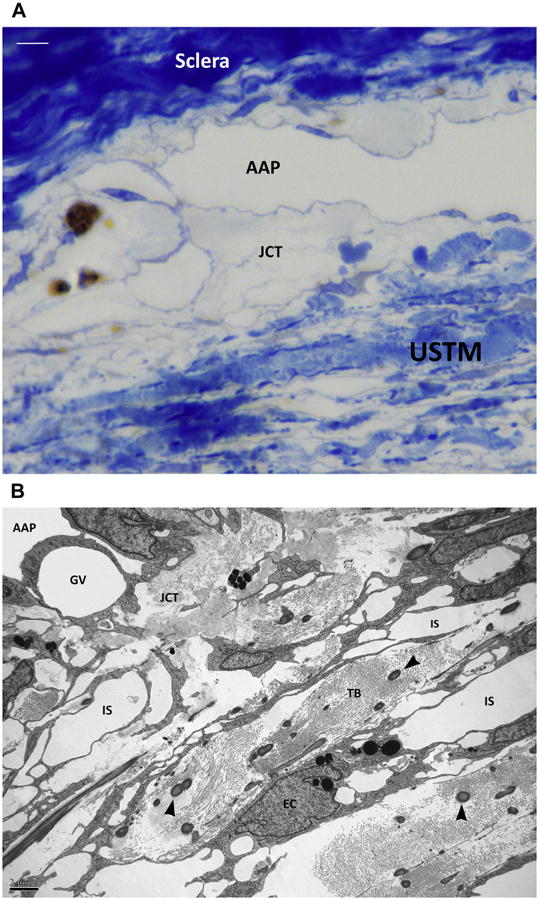
(A) Semithin section with toluidine blue staining of the angular aqueous plexus (AAP) region in a canine eye. The lamellar organization of the uveoscleral trabecular meshwork (USTM) can be recognized at the bottom. Some interlamellar spaces can be seen. The transition region between the USTM and the AAP is represented by a loose translucent tissue, the juxtacanalicular connective tissue (JCT). Bar 5 5 μ. (B) Transmission electron microscopy. A microphotograph of a same corresponding area shows the trabecular beams (TB) of the USTM. The beams are constituted by collagen fibers and larger elastin fibers (arrowheads). Endothelial trabecular meshwork cells line the beams and engulf melanin granules (EC). Intertrabecular spaces (IS) are reducing toward the JCT. A giant vacuole (GV) can be seen within the cytoplasm of an endothelial cell of the inner wall of the angular aqueous plexus (AAP).
The spaces of Fontana and the intertrabecular spaces of the CSTM and USTM are large enough not to offer resistance to the aqueous flow. The ECM of the JCT is then considered to assume a pivotal role in the regulation of IOP in both normal and open angle glaucomatous eyes.81 The elastic fibers allow plasticity of the tissue in response to fluctuations in IOP.79 However, collagen cross-linking occurs with age and it is increased in glaucoma, making the ECM stiffer.82,83 GAGs and their associated proteoglycans, particularly dermatan sulfate, chondroitin sulfate, hyaluronic acid84 and versican79 separate the cells from the fibrillar collagen and have a primary role in modulating resistance.79 Numerous other structural and functional proteins are present, like fibronectin, laminin, collagen types I, III, IV, V, and VI (but not type II), and some of them actively participate in ECM remodeling like myocilin, trombospondin-1, and SPARC.74,79,85,86 Trombospondin-1 is one of the activators of latent transforming growth factor beta-2 abundantly present within the anterior segment,87,88 whereas SPARC is a matricellular protein known to regulate ECM in many tissues and is highly expressed in the TM. SPARC-null mice demonstrated a lower IOP, which is associated with a more uniform outflow pattern and decreased collagen fibril diameter.85
With age, the components of the ECM of this region tend to accumulate plaques of amorphous material supposedly derived from the sheaths of the elastic fibers and collagen VI,72,89 and the outflow resistance increases proportionally.74
The JCT is separated from the lumen of the AAP by a monolayer of endothelial cells. The endothelium of the IW has 2 opposite functions. Although it allows aqueous percolation from the underlying JCT through the formation of giant vacuoles and pores, at the same time it prevents blood products from entering the eye if elevated episcleral venous pressure exceeds IOP because of the presence of tight junctions. For the latter reason, the endothelium should be considered part of the blood–aqueous barrier along with the ciliary epithelium, the iris vascular endothelium, and the posterior iris epithelium.90 The basement membrane of endothelial cells of Schlemm canal in humans and the basement membrane of the AAP in dogs are not continuous.36,80 This feature, associated with the presence of intercellular continuous tight junctions, categorizes the AAP in between lymphatic channels and venules.91 The discontinuity of the basal lamina creates a direct connection between the ECM of the JCT and the IW. The transit of the aqueous from the JCT to the AAP lumen occurs through the formation of endothelial giant vacuoles and intracellular pores or directly through the intercellular pores formed between 2 neighbor cells.80,92
AH filters through the TM as bulk flow that is influenced by, and positively correlates with the pressure gradient; no active transport is involved.74 Several studies have demonstrated a pivotal role of GAGs and their protein complexes, proteoglycans, in regulating the flow through the TM, in particular in the JCT and IW.93–96 All of the normal GAGs have been identified, with hyaluronic acid or hyaluronan and chondroitin sulfate being the most preponderant.81 The hydrophilic properties of their moieties are able to absorb fluids easily. Although initial publications assigned to GAGs a contributing role in flow resistance,1,96–98 more recent works have shown that primary open-angle glaucoma is actually associated with a loss of hyaluronic acid from the TM and that treatment with chondroitinases and hyaluronidase intracamerally in monkey eyes does not change the outflow facility or IOP.99,100
The composition of the JCT and the IW of the Schlemm's canal in primates and of the AAP in dogs has been thoroughly described both ultrastructurally and functionally.33,35,36,54,79 It would be a mistake though to think about this area as a stable, uniform tissue. The JCT must be considered a dynamic tissue undergoing continuous remodeling.79,101 Dynamic changes both structurally and in the ECM occur with age and different conditions.35,74 Nonstructural proteins and molecules are produced and released during tissue remodeling and under the influence of cytokines and growth factors present in the aqueous at different times.70,79 The influence of different cytokines and growth factors (ie, tumor necrosis factor-α, transforming growth factor beta-2) and matricellular proteins on ECM permeability have been extensively studied to understand and differentiate dynamic changes seen in aging and glaucoma.89,101
The most common phenotypes of glaucoma in dogs are those classified as angle closure glaucoma. In these cases, the consistent pathologic finding is the collapse/closure of the CC. Whether these changes are the consequence of a primary obstruction starting in the JCT/AAP or a primary event into the CC itself or if both sites can simultaneously be affected is unknown. Glaucoma is an age-related disease in most of the instances and the histologic changes that occur with aging in normal canine eyes are described mostly with pigment dispersion, increased number of melanin-laden macrophages within the CB, CC, and AAP, and visible collagen deposition in the CB.102 In humans and dogs, an increased permeability of the blood–aqueous barrier with aging has been described,103–106 supporting the idea that its protein content and quality vary and may influence and explain the dynamic remodeling of the outflow tissues.
The intertrabecular spaces, the loose matrix of JCT and the giant vacuoles in the IW endothelium are the expression of how the aqueous permeates through the outer CSTM/USTM and JCT into the AAP. The fine mechanisms by which the fluid passes through the tissue into the lumen are still, however, under investigation. The bulk flow of the aqueous is a passive mechanism that follows pressure gradients. IOP is higher than pressure within the outer draining venous system. Episcleral pressure both in humans and dogs has been measured between 8 and 12 mm Hg.107,108 However, in dogs, an unknown role is also played by the ISVP and vortex veins,36,97 whereas in primates the distal outflow is regulated only through the episcleral and anterior ciliary veins.109 The morphologic presence of giant vacuoles within the endothelial cells of Schlemm's canal (better seen under flow condition or perfusion-fixed eyes) with or without basal opening and micron-sized (0.5–1.5 m) pores on the luminal surface of the endothelial cells have been documented in primates, dogs, rabbits, and bovine.35,36,92,110 Pores of the IW cells can be observed with both transmission electron microscopy and scanning electron microscopy.35,92 The endothelial surface of the AAP is also covered by a thin layer of glycocalix that may play a role in regulating aqueous outflow resistance.111
Endothelial pores are nonuniformly distributed112 and their density proportionally reflects the segmental pattern of higher AH outflow through the TM/JCT.113 IW pore density proportionally increases with increased perfusion in normal eyes.114 However, the density of the pores decreases on the endothelial surface of Schlemm's canal in human glaucomatous eyes.114
Besides the formation of giant vacuoles, pynocytic, intracytoplasmic microvesicles have been observed; however, their role and relevance in aqueous outflow is unknown.35 The giant vacuoles are seen most commonly within the endothelial cells that line the inner side of the AAP, facing the JCT.92 Studies using eyes perfused with a tracer have shown that tracer accumulation corresponds to the location of collector channel ostia109 and with regions of JCT containing less versican.115
The anterior outflow is not uniformly distributed over the circumference of the meshwork, but has a segmental pattern in adult humans,116 bovine,117,118 porcine,115 nonhuman primates, and mice.85 The areas of preferential flow correspond to areas of lower resistance and are located in the proximity of the radial collector channels and vary with their distribution.119 Sectorial flow may occur because some collector channels reside near larger episcleral venules, the final destination of aqueous.109 Although the nasal116 and inferior120 quadrants have been suggested to be more active,109 no consistency in the pattern of segmental outflow is yet accepted.116 The segmental portions of active outflow decrease in glaucomatous patients or with increasing IOP.117,121
Angular Aqueous Plexus
Tripathi37 was the first to use the nomenclature of “angular aqueous plexus (AAP)” to name the complex pattern of the inner-scleral venous aqueous outflow in nonprimate mammals and to recognize, despite morphologic differences, the anatomic and functional similarity of these canalicular structures with Schlemm's canal in primates.36,37
The AAP in dogs is a complex, tangled vascular network that expands from the Schwalbe's line anteriorly to the end of the CC posteriorly, spanning for hundreds of microns. The plexus occupies the inner sclera at the transition zone between the cornea and the outer uvea. The area seems to be similar to a triangular scleral sulcus, with the outer vertex corresponding more or less with the transition area between the ICA and the CC (see Fig. 4; Fig. 10B).
Fig. 10.
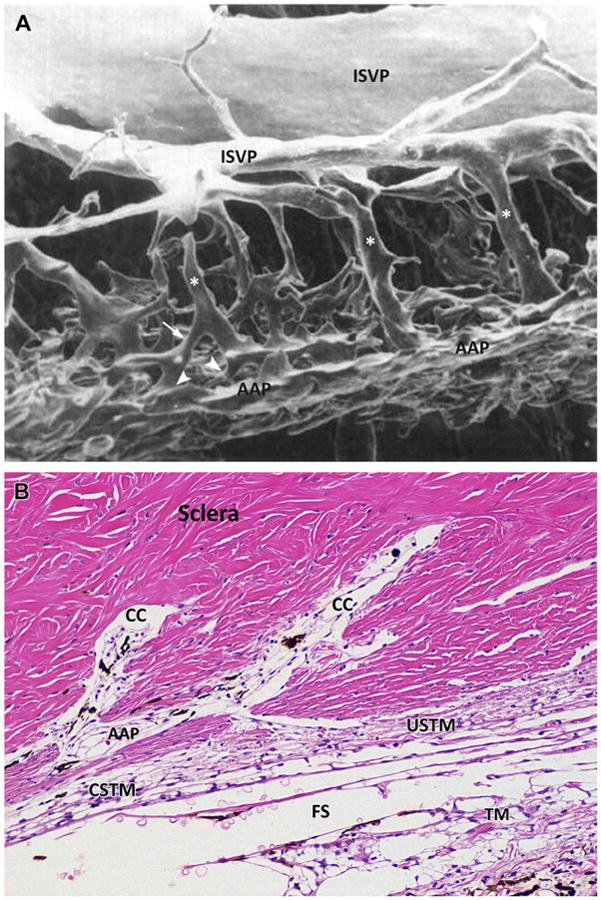
(A) Luminal cast of the canine trabecular outflow pathway. The vessels of the outflow at the level of the angular aqueous plexus (AAP) display a complex and tangled network made by smaller channels oriented tangentially in different planes and directions. Larger radial collector channels (asterisks) connect the network to larger venous vessels in the outer sclera, the intrascleral venous plexus (ISVP). Radial channels are displaced at different anterior and posterior sites. Irregular patterns of anastomoses are present among all the channels and shorter radial channels are seen (arrow). The connections between almost perpendicular inner and radial channels are called ostia (arrowheads and Fig. 7). (B) Microphotograph of a histologic sagittal section of the inner scleral region at the level of the scleral sulcus in a normal canine eye showing the complex anastomotic pattern of the drainage system in dogs. Hematoxylin and eosin staining. Two large, radial collector channels (CC) are visible anteriorly and posteriorly. These collector channels convey the aqueous filtered through the complex system represented by trabecular meshwork (TM), CSTM, uveoscleral TM (USTM), and angular aqueous plexus (AAP). Fontana spaces (FS) are present between the beams of the TM. (Adapted from Van Buskirk EM. The canine eye: the vessels of aqueous drainage. Invest Ophthalmol Vis Sci 1979;18:226; with permission.)
The plexus is made by several channels that have different diameters and directions and lie in different layers at different depths in the inner sclera(see Fig. 10).They represent the first, distal dilation after the JCT and IW. Thin, inner channels are close to the CSTM and USTM and form an intricate network with incomplete circumferential, oblique and meridional tangential directions. These channels are lined by endothelial cells that are the true functional equivalent of the IW of Schlemm's canal in primates and represent the AAP in dogs.36,37 The plasma membranes of the endothelial cells adhere with tight junctions and for this reason are considered part of the blood–aqueous barrier.75
The multiple tangential channels forming the AAP merge then into perpendicular, radial collector channels through openings called “ostia.” Because the tangential channels may be displaced in different planes, short radial collector channels may be present together with longer and larger outer radial channels. As many as 30 to 35 external collector channels have been reported in the human eye.84 Radial channels have different, anterior (corneoscleral) and posterior (uveoscleral) distributions (see Fig. 10). High variability, however, exists and complex patterns of anastomoses can be seen at light microscopy depending not only on the species of animal, but also on the different sections of an individual eye.36,37 The complex patterns of anastomoses can also be clearly observed in luminal casts.38 The histologic sectorial changes in radial channels may reflect the sectorial distribution of the collector channels described in primates, mice and bovines. Preferential outflows may occur in different sectors, which is confirmed by pigment deposition in the near JCT, as described for other species.74,109 Unevenly distributed radial collector channels are present in primates, more being on the nasal than on the temporal side of the eye.84
Larger radial collector channels direct to the outer sclera and anastomose with the anterior ciliary veins or the ISVP (or the venous circle of Hovius); both drain into the orbit via the ophthalmic vein (the former) and via the vortex veins (the latter).38 There are 2 to 4 large venous vessels that compose the ISVP, which is located in the outer third of the sclera. The anterior venules are usually smaller and the caliber of the vessels increases posteriorly (Fig. 11).
Fig. 11.
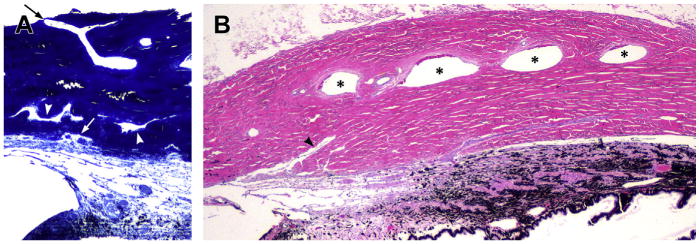
The scleral outflow systems. (A) The complex angular aqueous plexus (AAP) and collector channels are displaced in different layers with different directions and different sizes in the inner sclera. The arrowheads indicate collector channels. The white arrow points to an ostium between AAP and a short radial collector channel. In the outer sclera an aqueous vein can be seen directed to the limbus and to the anterior ciliary veins (black arrow) (toluidine blue). (B) Longitudinal section of the sclera corresponding to the ciliary body area. Four large scleral veins representing the intrascleral venous plexus (ISVP) are indicated with asterisks. The arrowhead points to a large, radial collector channel directing to the ISVP area (Hematoxylin and eosin).
Posterior, Uveoscleral, or Unconventional Outflow
The uveoscleral or unconventional outflow represents the amount of aqueous that once has entered the TM escapes the trabecular outflow and directs posteriorly where it can filter through the porosity of the loose connective tissue and its ECM in the ciliary body. The connective tissue between the longitudinal bundles of the ciliary muscle is loose and permeable. After percolating through the ECM of the ciliary body, the aqueous collects into the uveoscleral space, a virtual space between the posterior ciliary body/anterior choroid and the sclera; from here, the AH can reach the posterior suprachoroidal space. This flow is passive and mostly independent from IOP. It is driven by a positive osmotic gradient between the colloids in the choroidal and scleral blood vessels and the uveal tissue of the posterior ciliary body. Furthermore, the hydraulic conductivity of the scleral tissue is able to absorb and remove 4.3 μL/min in humans.122
In normal dogs, the USO allows full permeability to 1 μ spheres that can easily reach the suprachoroidal space and the connected vascular outflow. In glaucomatous dogs, this ability is reduced significantly.123 The unconventional outflow represents 15% of the total outflow in normal Beagles, whereas it is reduced to 3% in glaucomatous Beagles.124 It is only 3% in normal cats.125 The normal rate of outflow through the unconventional pathway varies in different species and decreases with age.31,56 In nonhuman primates, 40% to 60% of the aqueous leaves the globe through this pathway, whereas in people several rates have been reported, ranging between 12% and 54%.31 The lower values are associated with advanced age.31 In particular the reduction in outflow in normal people has been quantified in about 7% to 10% per decade.31
In younger primates, the connective tissue between the muscular bundles in the ciliary body is sparse. In aging dogs, the loose connective tissue between the muscular bundles of the longitudinal ciliary muscle accumulates large melanin-laden macrophages that progressively obstruct and likely activate or contribute to remodeling the region (see Fig. 12).102
Fig. 12.
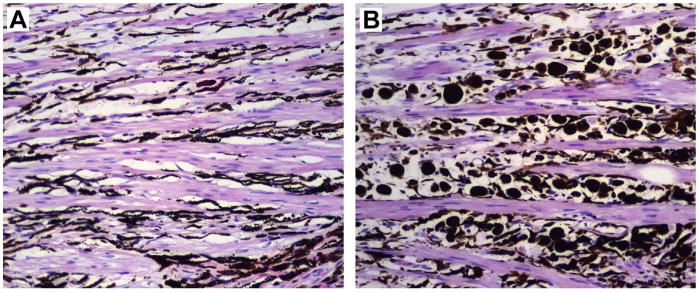
Photomicrographs of ciliary body sections posterior to the trabecular meshwork in 2 normal eyes from different dogs. (A) Ten month old male Labrador. The longitudinal bundles of the ciliary muscle alternate with connective tissue mostly filled with fusiform dendritic melanocytes representing the normal pigmented component of uveal ocular tissue. (B) Fifteen year old spayed female Labrador. The intermuscular spaces present a large number of plump large pigmented cells representing infiltrating macrophages melanin-laden (melanophages).
The most recent calculations of the amount of flow through the USO in humans have provided higher values than early studies, increasing the actual relevance of the unconventional outflow. High variability is determined by age. Healthy humans between 20 and 30 years of age showed rates of USO between 36% and 54%, whereas people of 60 years or older had rates between 12% and 42%.10 This is likely to happen in dogs as well. Unfortunately, the USO has been limitedly investigated only Beagles in the 1980s.123
Along with the decreased outflow that occurs with age, there is also a decrease in aqueous production and the clinical influence of the decreased facility in normal patients is limited by this factor.10
The point of greatest resistance is considered the ECM of the posterior ciliary body. The connective spaces between the longitudinal muscular bundles decrease with age and become less permeable.10 The composition, tightness, and permeability of the ECM of the ciliary body can also differ and change depending on several physiologic, pharmacologic, and inflammatory factors. The contraction or relaxation of the longitudinal ciliary muscle has some influence on the rate of the outflow. Atropine increases the permeability of the ciliary muscle in nonhuman primates, whereas pilocarpine decreases it.126 However, the overall clinical effect of pilocarpine on IOP may be different because of the contraction of the tendons of the ciliary muscles also opens the Fontana spaces, hypothetically shifting the outflow to the anterior pathways. Tendons of the longitudinal ciliary muscle, the only ciliary muscle present in dogs, are directed to the posterior half of the scleral sulcus. According to this morphologic interpretation, ciliary muscle contraction might open the radial collector channels (see Fig. 6).
The USO is the target for powerful medications used to treat glaucomas in dogs. Because the anterior outflow is usually collapsed in advanced glaucoma, decreasing AH production and increasing the posterior outflow are the most rationale pharmacologic goals. Synthetic prostaglandins increase the USO by increasing the permeability of the ECM in the ciliary body. The effect is mediated by the increased expression of matrix metalloproteinases that remodel the ECM of the ciliary body.127
Summary
Knowledge of the anatomic and physiologic background of aqueous dynamics is important for the clinician. Glaucoma is a heterogeneous group of diseases with multiple phenotypes. Accurate evaluation of the clinical conditions is pivotal to understanding the possible therapeutic options and prognosis for an affected patient. It is also important to shift the consideration of anatomy from a steady perspective to a dynamic process in which age is a clear influencing factor and is affecting the structure and the function of ocular outflow.
Moreover, a remarkable benefit of knowing the pathophysiology and relative functional anatomy of the region resides in the possible establishment of predictive models in cases of early disease. The attention to details at the beginning of the disease may allow the clinician to reach the longest control over a pathologic condition that unfortunately is associated with a high occurrence of blindness and patient discomfort.
Key Points.
Understanding the composition and function of the outflow system helps to interpret clinical signs, choose therapies, and formulate the prognosis for patients affected with glaucoma.
Normal intraocular pressure is maintained because there is a balance between aqueous formation and outflow.
The outflow pathways in dogs have clear morphologic and topographic differences from the corresponding structures in primates.
The functional anatomy is a dynamic concept and there are physiologic changes that occur with age and tissue remodeling.
More advanced and extensive changes that are similar to those occurring with aging may occur in glaucoma.
Footnotes
The authors have nothing to disclose.
References
- 1.Gum GG, Samuelson DO, Gelatt KN. Effect of hyaluronidase on aqueous outflow resistance in normotensive and glaucomatous eyes of dogs. Am J Vet Res. 1992;53(5):767–70. [PubMed] [Google Scholar]
- 2.Gelatt KN, MacKay EO. Distribution of intraocular pressure in dogs. Vet Ophthalmol. 1998;1(2–3):109–14. doi: 10.1046/j.1463-5224.1998.00024.x. [DOI] [PubMed] [Google Scholar]
- 3.Giannetto C, Piccione G, Giudice E. Daytime profile of the intraocular pressure and tear production in normal dog. Vet Ophthalmol. 2009;12(5):302–5. doi: 10.1111/j.1463-5224.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 4.Leiva M, Naranjo C, Pena MT. Comparison of the rebound tonometer (ICare) to the applanation tonometer (Tonopen XL) in normotensive dogs. Vet Ophthalmol. 2006;9(1):17–21. doi: 10.1111/j.1463-5224.2005.00429.x. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Suarez E, Molleda C, Tardon R, et al. Diurnal variations of central corneal thickness and intraocular pressure in dogs from 8:00 am to 8:00 pm. Can Vet J. 2014;55(4):361–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade SF, Palozzi RJ, Giuffrida R, et al. Comparison of intraocular pressure measurements between the Tono-Pen XL(R) and Perkins(R) applanation tonometers in dogs and cats. Vet Ophthalmol. 2012;15(Suppl 1):14–20. doi: 10.1111/j.1463-5224.2011.00926.x. [DOI] [PubMed] [Google Scholar]
- 7.David R, Zangwill L, Stone D, et al. Epidemiology of intraocular pressure in a population screened for glaucoma. Br J Ophthalmol. 1987;71(10):766–71. doi: 10.1136/bjo.71.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek SU, Kee C, Suh W. Longitudinal analysis of age-related changes in intraocular pressure in South Korea. Eye (Lond) 2015;29(5):625–9. doi: 10.1038/eye.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong TT, Wong TY, Foster PJ, et al. The relationship of intraocular pressure with age, systolic blood pressure, and central corneal thickness in an Asian population. Invest Ophthalmol Vis Sci. 2009;50(9):4097–102. doi: 10.1167/iovs.08-2822. [DOI] [PubMed] [Google Scholar]
- 10.Toris CB, Yablonski ME, Wang YL, et al. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol. 1999;127(4):407–12. doi: 10.1016/s0002-9394(98)00436-x. [DOI] [PubMed] [Google Scholar]
- 11.Millar C, Kaufman PL. Aqueous humor: secretion and dynamics. In: Tasman W, Jaeger EA, editors. Duane's foundations of clinical ophthalmology. Vol. 2. Philadelphia: Lippincott-Raven; 1995. pp. 1–51. [Google Scholar]
- 12.Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975;55(3):383–417. doi: 10.1152/physrev.1975.55.3.383. [DOI] [PubMed] [Google Scholar]
- 13.Cole DF. Secretion of the aqueous humour. Exp Eye Res. 1977;25(Suppl):161–76. doi: 10.1016/s0014-4835(77)80015-8. [DOI] [PubMed] [Google Scholar]
- Caprioli J. The ciliary epithelia and aqueous humor. In: Hart WM, editor. Adler's physiology of the eye Clinical application. 9th. St Louis (MO): Mosby; 1993. pp. 228–47. [Google Scholar]
- 15.Goel M, Picciani RG, Lee RK, et al. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52–9. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. Aqueous humor hydrodynamics. In: Kaufman PL, Albert A, editors. Adler's physiology of the eye Clinical application. 10th. St Louis (MO): Mosby; 2003. pp. 237–89. [Google Scholar]
- 17.Freddo TF. A contemporary concept of the blood-aqueous barrier. Prog Retin Eye Res. 2013;32:181–95. doi: 10.1016/j.preteyeres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Civan MM, Macknight AD. The ins and outs of aqueous humour secretion. Exp Eye Res. 2004;78(3):625–31. doi: 10.1016/j.exer.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Green K, Pederson JE. Contribution of secretion and filtration to aqueous humor formation. Am J Physiol. 1972;222(5):1218–26. doi: 10.1152/ajplegacy.1972.222.5.1218. [DOI] [PubMed] [Google Scholar]
- 20.Bonting SL, Becker B. Studies on sodium-potassium activated adenosine triphosphatase. Xiv. Inhibition of enzyme activity and aqueous humor flow in the rabbit eye after intravitreal injection of ouabain. Invest Ophthalmol. 1964;3:523–33. [PubMed] [Google Scholar]
- 21.Bhattacherjee P. Distribution of carbonic anhydrase in the rabbit eye as demonstrated histochemically. Exp Eye Res. 1971;12(3):356–9. doi: 10.1016/0014-4835(71)90160-6. [DOI] [PubMed] [Google Scholar]
- 22.Lutjen-Drecoll E, Lonnerholm G, Eichhorn M. Carbonic anhydrase distribution in the human and monkey eye by light and electron microscopy. Graefes Arch Clin Exp Ophthalmol. 1983;220(6):285–91. doi: 10.1007/BF00231357. [DOI] [PubMed] [Google Scholar]
- 23.McLaren JW. Measurement of aqueous humor flow. Exp Eye Res. 2009;88(4):641–7. doi: 10.1016/j.exer.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Schneider TL, Brubaker RF. Effect of chronic epinephrine on aqueous humor flow during the day and during sleep in normal healthy subjects. Invest Ophthalmol Vis Sci. 1991;32(9):2507–10. [PubMed] [Google Scholar]
- 25.Ward DA, Cawrse MA, Hendrix DV. Fluorophotometric determination of aqueous humor flow rate in clinically normal dogs. Am J Vet Res. 2001;62(6):853–8. doi: 10.2460/ajvr.2001.62.853. [DOI] [PubMed] [Google Scholar]
- 26.Gum GG, Gelatt KN, Esson DW. Physiology of the eye. In: Gelatt KN, editor. Veterinary ophthalmology. 4th. Vol. 1. Ames (IA); Wiley-Blackwell: 2007. pp. 149–82. [Google Scholar]
- 27.Naushad M, Alothman ZA, Khan MR. Removal of malathion from aqueous solution using De-Acidite FF-IP resin and determination by UPLC-MS/MS: equilibrium, kinetics and thermodynamics studies. Talanta. 2013;115:15–23. doi: 10.1016/j.talanta.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Gaasterland D, Kupfer C, Milton R, et al. Studies of aqueous humour dynamics in man. VI. Effect of age upon parameters of intraocular pressure in normal human eyes. Exp Eye Res. 1978;26(6):651–6. doi: 10.1016/0014-4835(78)90099-4. [DOI] [PubMed] [Google Scholar]
- 29.Van Buskirk EM, Brett J. The canine eye: in vitro studies of the intraocular pressure and facility of aqueous outflow. Invest Ophthalmol Vis Sci. 1978;17(4):373–7. [PubMed] [Google Scholar]
- 30.VanBuskirk EM, Grant WM. Influence of temperature and the question of involvement of cellular metabolism in aqueous outflow. Am J Ophthalmol. 1974;77(4):565–72. doi: 10.1016/0002-9394(74)90472-3. [DOI] [PubMed] [Google Scholar]
- 31.Alm A, Nilsson SF. Uveoscleral outflow–a review. Exp Eye Res. 2009;88(4):760–8. doi: 10.1016/j.exer.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Samuelson DA. A reevaluation of the comparative anatomy of the eutherian iridocorneal angle and associated ciliary body musculature. Vet Comp Ophthalmol. 1996;6(3):153–72. [Google Scholar]
- 33.Samuelson DA. Ophthalmic anatomy. In: Gelatt KN, Gilger BC, Kern TJ, editors. Veterinary ophthalmology. 5th. Vol. 1. Ames (IA); Wiley-Blackwell: 2013. pp. 39–170. [Google Scholar]
- 34.Samuelson DA, Gelatt KN. Aqueous outflow in the beagle. I. Postnatal morphologic development of the iridocorneal angle: pectinate ligament and uveal trabecular meshwork. Curr Eye Res. 1984;3(6):783–94. doi: 10.3109/02713688409000790. [DOI] [PubMed] [Google Scholar]
- 35.Samuelson DA, Gelatt KN. Aqueous outflow in the Beagle. II. Postnatal morphologic development of the iridocorneal angle: corneoscleral trabecular mesh work and angular aqueous plexus. Curr Eye Res. 1984;3(6):795–807. doi: 10.3109/02713688409000791. [DOI] [PubMed] [Google Scholar]
- 36.Bedford PG, Grierson I. Aqueous drainage in the dog. Res Vet Sci. 1986;41(2):172–86. [PubMed] [Google Scholar]
- 37.Tripathi RC. Ultrastructure of the exit pathway of the aqueous in lower mammals. (A preliminary report on the “angular aqueous plexus”) Exp Eye Res. 1971;12(3):311–4. doi: 10.1016/0014-4835(71)90155-2. [DOI] [PubMed] [Google Scholar]
- 38.Van Buskirk EM. The canine eye: the vessels of aqueous drainage. Invest Ophthalmol Vis Sci. 1979;18(3):223–30. [PubMed] [Google Scholar]
- 39.Read RA, Wood JL, Lakhani KH. Pectinate ligament dysplasia (PLD) and glaucoma in Flat Coated Retrievers. I. Objectives, technique and results of a PLD survey. Vet Ophthalmol. 1998;1(2–3):85–90. doi: 10.1046/j.1463-5224.1998.00019.x. [DOI] [PubMed] [Google Scholar]
- 40.Bedford PG. Gonioscopy in the dog. J Small Anim Pract. 1977;18(10):615–29. doi: 10.1111/j.1748-5827.1977.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 41.Pearl R, Gould D, Spiess B. Progression of pectinate ligament dysplasia over time in two populations of Flat-Coated Retrievers. Vet Ophthalmol. 2015;18(1):6–12. doi: 10.1111/vop.12098. [DOI] [PubMed] [Google Scholar]
- 42.Bjerkas E, Ekesten B, Farstad W. Pectinate ligament dysplasia and narrowing of the iridocorneal angle associated with glaucoma in the English Springer Spaniel. Vet Ophthalmol. 2002;5(1):49–54. doi: 10.1046/j.1463-5224.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 43.Ekesten B, Narfstrom K. Correlation of morphologic features of the iridocorneal angle to intraocular pressure in Samoyeds. Am J Vet Res. 1991;52(11):1875–8. [PubMed] [Google Scholar]
- 44.Crumley W, Gionfriddo JR, Radecki SV. Relationship of the iridocorneal angle, as measured using ultrasound biomicroscopy, with post-operative increases in intraocular pressure post-phacoemulsification in dogs. Vet Ophthalmol. 2009;12(1):22–7. doi: 10.1111/j.1463-5224.2009.00669.x. [DOI] [PubMed] [Google Scholar]
- 45.WuDunn D. Mechanobiology of trabecular meshwork cells. Exp Eye Res. 2009;88(4):718–23. doi: 10.1016/j.exer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Samuelson DA, Gelatt KN, Gum GG. Kinetics of phagocytosis in the normal canine iridocorneal angle. Am J Vet Res. 1984;45(11):2359–66. [PubMed] [Google Scholar]
- 47.Gasiorowski JZ, Russell P. Biological properties of trabecular meshwork cells. Exp Eye Res. 2009;88(4):671–5. doi: 10.1016/j.exer.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pizzirani S, Desai SJ, Pirie CG, et al. Age related changes in the anterior segment of the eye in normal dogs. Vet Ophthalmol. 2010;13(6):421. [Google Scholar]
- 49.Cracknell KP, Grierson I, Hogg P, et al. Melanin in the trabecular meshwork is associated with age, POAG but not Latanoprost treatment. A masked morphometric study. Exp Eye Res. 2006;82(6):986–93. doi: 10.1016/j.exer.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Gottanka J, Johnson DH, Grehn F, et al. Histologic findings in pigment dispersion syndrome and pigmentary glaucoma. J Glaucoma. 2006;15(2):142–51. doi: 10.1097/00061198-200604000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Rohen JW, van der Zypen E. The phagocytic activity of the trabecular meshwork endothelium. An electron-microscopic study of the vervet (Cercopithecus aethiops) Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1968;175(2):143–60. doi: 10.1007/BF02385060. [DOI] [PubMed] [Google Scholar]
- 52.Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91(6):564–79. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- 53.Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye. 1987;1(Pt 2):204–10. doi: 10.1038/eye.1987.38. [DOI] [PubMed] [Google Scholar]
- 54.Lutjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1998;18(1):91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- 55.Hann CR, Fautsch MP. The elastin fiber system between and adjacent to collector channels in the human juxtacanalicular tissue. Invest Ophthalmol Vis Sci. 2011;52(1):45–50. doi: 10.1167/iovs.10-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24(5):612–37. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994;35(5):2515–20. [PubMed] [Google Scholar]
- 58.Stumpff F, Strauss O, Boxberger M, et al. Characterization of maxi-K-channels in bovine trabecular meshwork and their activation by cyclic guanosine monophosphate. Invest Ophthalmol Vis Sci. 1997;38(9):1883–92. [PubMed] [Google Scholar]
- 59.Impagnatiello F, Borghi V, Gale DC, et al. A dual acting compound with latanoprost amide and nitric oxide releasing properties, shows ocular hypotensive effects in rabbits and dogs. Exp Eye Res. 2011;93(3):243–9. doi: 10.1016/j.exer.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Marshall GE, Konstas AG, Lee WR. Immunogold ultrastructural localization of collagens in the aged human outflow system. Ophthalmology. 1991;98(5):692–700. doi: 10.1016/s0161-6420(91)32232-2. [DOI] [PubMed] [Google Scholar]
- 61.Hann CR, Springett MJ, Wang X, et al. Ultrastructural localization of collagen IV, fibronectin, and laminin in the trabecular meshwork of normal and glaucomatous eyes. Ophthalmic Res. 2001;33(6):314–24. doi: 10.1159/000055687. [DOI] [PubMed] [Google Scholar]
- 62.Babizhayev MA, Brodskaya MW. Fibronectin detection in drainage outflow system of human eyes in ageing and progression of open-angle glaucoma. Mech Ageing Dev. 1989;47(2):145–57. doi: 10.1016/0047-6374(89)90017-1. [DOI] [PubMed] [Google Scholar]
- 63.Faralli JA, Schwinn MK, Gonzalez JM, Jr, et al. Functional properties of fibronectin in the trabecular meshwork. Exp Eye Res. 2009;88(4):689–93. doi: 10.1016/j.exer.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tripathi RC, Chan WFA, Li J, et al. Trabecular cells express the TGF-beta2 gene and secrete the cytokine. Exp Eye Res. 1994;58(5):523–8. doi: 10.1006/exer.1994.1046. [DOI] [PubMed] [Google Scholar]
- 65.Fuchshofer R, Yu AH, Welge-Lussen U, et al. Bone Morphogenetic Protein-7 Is an Antagonist of Transforming Growth Factor beta 2 in Human Trabecular Meshwork Cells. Invest Ophthalmol Vis Sci. 2007;48(2):715–26. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- 66.Alexander JP, Samples JR, Van Buskirk EM, et al. Expression of Matrix Metalloproteinases and Inhibitor by Human Trabecular Meshwork. Invest Ophthalmol Vis Sci. 1991;32(1):172–80. [PubMed] [Google Scholar]
- 67.Rhee DJ, Haddadin RI, Kang MH, et al. Matricellular proteins in the trabecular meshwork. Exp Eye Res. 2009;88(4):694–703. doi: 10.1016/j.exer.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 68.Flugel-Koch C, Ohlmann A, Fuchshofer R, et al. Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone in vitro. Exp Eye Res. 2004;79(5):649–63. doi: 10.1016/j.exer.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Wordinger RJ, Clark AF, Agarwal R, et al. Cultured human trabecular meshwork cells express functional growth factor receptors. Invest Ophthalmol Vis Sci. 1998;39(9):1575–89. [PubMed] [Google Scholar]
- 70.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28(5):348–68. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 71.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 72.Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res. 2009;88(4):656–70. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gong H, Freddo TF. The washout phenomenon in aqueous outflow – Why does it matter? Exp Eye Res. 2009;88(4):729–37. doi: 10.1016/j.exer.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009;88(4):648–55. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 75.Johnson M. What controls aqueous humour outflow resistance? Exp Eye Res. 2006;82(4):545–57. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gong H, Yang CY. Morphological and hydrodynamic correlations with increasing outflow facility by rho-kinase inhibitor Y-27632. J Ocul Pharmacol Ther. 2014;30(2–3):143–53. doi: 10.1089/jop.2013.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scott PA, Overby DR, Freddo TF, et al. Comparative studies between species that do and do not exhibit the washout effect. Exp Eye Res. 2007;84(3):435–43. doi: 10.1016/j.exer.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ten Hulzen RD, Johnson DH. Effect of fixation pressure on juxtacanalicular tissue and Schlemm's canal. Invest Ophthalmol Vis Sci. 1996;37(1):114–24. [PubMed] [Google Scholar]
- 79.Keller KE, Acott TS. The juxtacanalicular region of ocular trabecular meshwork: a tissue with a unique extracellular matrix and specialized function. J Ocul Biol. 2013;1(1):3. [PMC free article] [PubMed] [Google Scholar]
- 80.Gong H, Ruberti J, Overby D, et al. A new view of the human trabecular meshwork using quick-freeze, deep-etch electron microscopy. Exp Eye Res. 2002;75(3):347–58. [PubMed] [Google Scholar]
- 81.Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86(4):543–61. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sethi A, Mao W, Wordinger RJ, et al. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52(8):5240–50. doi: 10.1167/iovs.11-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Last JA, Pan T, Ding Y, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52(5):2147–52. doi: 10.1167/iovs.10-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gong H, Tripathi RC, Tripathi BJ. Morphology of the aqueous outflow pathway. Microsc Res Tech. 1996;33(4):336–67. doi: 10.1002/(SICI)1097-0029(19960301)33:4<336::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 85.Swaminathan SS, Oh DJ, Kang MH, et al. Secreted protein acidic and rich in cysteine (SPARC)-null mice exhibit more uniform outflow. Invest Ophthalmol Vis Sci. 2013;54(3):2035–47. doi: 10.1167/iovs.12-10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ueda J, Wentz-Hunter K, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci. 2002;43(4):1068–76. [PubMed] [Google Scholar]
- 87.Daniel C, Wiede J, Krutzsch HC, et al. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65(2):459–68. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 88.Kuchtey J, Kuchtey RW. The microfibril hypothesis of glaucoma: implications for treatment of elevated intraocular pressure. J Ocul Pharmacol Ther. 2014;30(2–3):170–80. doi: 10.1089/jop.2013.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rohen JW, Futa R, Lutjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981;21(4):574–85. [PubMed] [Google Scholar]
- 90.Johnstone MA, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75(3):365–83. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- 91.Kizhatil K, Ryan M, Marchant JK, et al. Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental. process PLoS Biol. 2014;12(7):e1001912. doi: 10.1371/journal.pbio.1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tripathi RC, Tripathi BJ. The mechanism of aqueous outflow in lower mammals. Exp Eye Res. 1972;14(1):73–9. doi: 10.1016/0014-4835(72)90146-7. [DOI] [PubMed] [Google Scholar]
- 93.Grierson I, Lee WR. Acid mucopolysaccharides in the outflow apparatus. Exp Eye Res. 1975;21(5):417–31. doi: 10.1016/0014-4835(75)90124-4. [DOI] [PubMed] [Google Scholar]
- 94.Lerner LE, Polansky JR, Howes EL, et al. Hyaluronan in the human trabecular meshwork. Invest Ophthalmol Vis Sci. 1997;38(6):1222–8. [PubMed] [Google Scholar]
- 95.Usui T, Nakajima F, Ideta R, et al. Hyaluronan synthase in trabecular meshwork cells. Br J Ophthalmol. 2003;87(3):357–60. doi: 10.1136/bjo.87.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knepper PA, Goossens W, Palmberg PF. Glycosaminoglycan stratification of the juxtacanalicular tissue in normal and primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1996;37(12):2414–25. [PubMed] [Google Scholar]
- 97.Van Buskirk EM, Brett J. The canine eye: in vitro dissolution of the barriers to aqueous outflow. Invest Ophthalmol Vis Sci. 1978;17(3):258–71. [PubMed] [Google Scholar]
- 98.Barany EH. The action of different kinds of hyaluronidase on the resistance to flow through the angle of the anterior chamber. Acta Ophthalmol. 1956;34(5):397–403. [PubMed] [Google Scholar]
- 99.Hubbard WC, Johnson M, Gong H, et al. Intraocular pressure and outflow facility are unchanged following acute and chronic intracameral chondroitinase ABC and hyaluronidase in monkeys. Exp Eye Res. 1997;65(2):177–90. doi: 10.1006/exer.1997.0319. [DOI] [PubMed] [Google Scholar]
- 100.Gong H, Freddo TF. Hyaluronic-Acid in the Normal and Glaucomatous Human Outflow Pathway. Invest Ophthalmol Vis Sci. 1994;35(4):2083. [Google Scholar]
- 101.Fuchshofer R, Tamm ER. Modulation of extracellular matrix turnover in the trabecular meshwork. Exp Eye Res. 2009;88(4):683–8. doi: 10.1016/j.exer.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Pizzirani S, Desai SJ, Pirie CG, et al. Age related changes in the anterior segment of the eye in normal dogs; Paper presented at: 41st Annual Meeting of the American College of Veterinary Ophthalmologists; San Diego, CA. October 6–9, 2010. [Google Scholar]
- 103.Oshika T, Kato S, Sawa M, et al. Aqueous flare intensity and age. Jpn J Ophthalmol. 1989;33(2):237–42. [PubMed] [Google Scholar]
- 104.Onodera T, Gimbel HV, DeBroff BM. Aqueous flare and cell number in healthy eyes of Caucasians. Jpn J Ophthalmol. 1993;37(4):445–51. [PubMed] [Google Scholar]
- 105.Satoh K, Takaku Y, Ohtsuki K, et al. Effects of aging on fluorescein leakage in the iris and angle in normal subjects. Jpn J Ophthalmol. 1999;43(3):166–70. doi: 10.1016/s0021-5155(99)00012-x. [DOI] [PubMed] [Google Scholar]
- 106.Pizzirani S, Rankin AJ, Meekins JM, et al. Anterior chamber fluorophotometry in normal dogs of different ages; Paper presented at: 45th Annual Meeting of the American College of Veterinary Ophthalmologists; Fort Worth. October 8–11, 2014. [Google Scholar]
- 107.Talusan ED, Schwartz B. Episcleral venous pressure. Differences between normal, ocular hypertensive, and primary open angle glaucomas. Arch Ophthalmol. 1981;99(5):824–8. doi: 10.1001/archopht.1981.03930010824006. [DOI] [PubMed] [Google Scholar]
- 108.Gelatt KN, Gum GG, Merideth RE, et al. Episcleral venous pressure in normotensive and glaucomatous beagles. Invest Ophthalmol Vis Sci. 1982;23(1):131–5. [PubMed] [Google Scholar]
- 109.Hann CR, Fautsch MP. Preferential fluid flow in the human trabecular meshwork near collector channels. Invest Ophthalmol Vis Sci. 2009;50(4):1692–7. doi: 10.1167/iovs.08-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Inomata H, Bill A, Smelser GK. Aqueous humor pathways through the trabecular meshwork and into Schlemm's canal in the cynomolgus monkey (Macaca irus). An electron microscopic study. Am J Ophthalmol. 1972;73(5):760–89. doi: 10.1016/0002-9394(72)90394-7. [DOI] [PubMed] [Google Scholar]
- 111.Yang CY, Huynh T, Johnson M, et al. Endothelial glycocalyx layer in the aqueous outflow pathway of bovine and human eyes. Exp Eye Res. 2014;128:27–33. doi: 10.1016/j.exer.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allingham RR, de Kater AW, Ethier CR, et al. The relationship between pore density and outflow facility in human eyes. Invest Ophthalmol Vis Sci. 1992;33(5):1661–9. [PubMed] [Google Scholar]
- 113.Braakman ST, Read AT, Chan DW, et al. Colocalization of outflow segmentation and pores along the inner wall of Schlemm's canal. Exp Eye Res. 2015;130:87–96. doi: 10.1016/j.exer.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnson M, Chan D, Read AT, et al. The pore density in the inner wall endothelium of Schlemm's canal of glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002;43(9):2950–5. [PubMed] [Google Scholar]
- 115.Keller KE, Bradley JM, Vranka JA, et al. Segmental versican expression in the trabecular meshwork and involvement in outflow facility. Invest Ophthalmol Vis Sci. 2011;52(8):5049–57. doi: 10.1167/iovs.10-6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chang JY, Folz SJ, Laryea SN, et al. Multi-scale analysis of segmental outflow patterns in human trabecular meshwork with changing intraocular pressure. J Ocul Pharmacol Ther. 2014;30(2–3):213–23. doi: 10.1089/jop.2013.0182. [DOI] [PubMed] [Google Scholar]
- 117.Battista SA, Lu Z, Hofmann S, et al. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49(12):5346–52. doi: 10.1167/iovs.08-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu JY, Ye W, Gong HY. Development of a novel two color tracer perfusion technique for the hydrodynamic study of aqueous outflow in bovine eyes. Chin Med J. 2010;123(5):599–605. [PMC free article] [PubMed] [Google Scholar]
- 119.Parc CE, Johnson DH, Brilakis HS. Giant vacuoles are found preferentially near collector channels. Invest Ophthalmol Vis Sci. 2000;41(10):2984–90. [PubMed] [Google Scholar]
- 120.Dvorak-Theobald G. Further studies on the canal of Schlemm; its anastomoses and anatomic relations. Am J Ophthalmol. 1955;39(4 Pt 2):65–89. doi: 10.1016/0002-9394(55)90154-9. [DOI] [PubMed] [Google Scholar]
- 121.Cha E, Jin R, Gong H. The relationship between morphological changes and reduction of active areas of aqueous outflow in eyes with primary open angle glaucoma; Paper presented at: ARVO 2013 Annual Meeting; Seattle, WA. May 5–9, 2013. [Google Scholar]
- 122.Jackson TL, Hussain A, Hodgetts A, et al. Human scleral hydraulic conductivity: age-related changes, topographical variation, and potential scleral outflow facility. Invest Ophthalmol Vis Sci. 2006;47(11):4942–6. doi: 10.1167/iovs.06-0362. [DOI] [PubMed] [Google Scholar]
- 123.Barrie KP, Gum GG, Samuelson DA, et al. Morphologic studies of uveoscleral outflow in normotensive and glaucomatous beagles with fluorescein-labeled dextran. Am J Vet Res. 1985;46(1):89–97. [PubMed] [Google Scholar]
- 124.Barrie KP, Gum GG, Samuelson DA, et al. Quantitation of uveoscleral outflow in normotensive and glaucomatous Beagles by 3H-labeled dextran. Am J Vet Res. 1985;46(1):84–8. [PubMed] [Google Scholar]
- 125.Bill A. Formation and drainage of aqueous humour in cats. Exp Eye Res. 1966;5:185–90. doi: 10.1016/s0014-4835(66)80020-9. [DOI] [PubMed] [Google Scholar]
- 126.Bill A. Effects of atropine and pilocarpine on aqueous humour dynamics in cynomolgus monkeys (Macaca irus) Exp Eye Res. 1967;6(2):120–5. doi: 10.1016/s0014-4835(67)80062-9. [DOI] [PubMed] [Google Scholar]
- 127.Lim KS, Nau CB, O'Byrne MM, et al. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology. 2008;115(5):790–5.e4. doi: 10.1016/j.ophtha.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


