Abstract
Purpose
Patients receiving intravenous tissue plasminogen activator (tPA) for ischemic stroke are monitored in an intensive care unit (ICU) or a comparable unit capable of ICU interventions due to the high frequency of standardized neurological exams and vital sign checks. The present study evaluates quantitative infarct volume on early post tPA MRI as a predictor of critical care needs, and aims to identify patients who may not require resource intense monitoring.
Methods
We identified 46 patients who underwent MRI within 6 hours of thrombolysis. Infarct volume was measured using semiautomated software. Logistic regression and receiver operating characteristics (ROC) analysis were used to determine factors associated with ICU needs.
Results
Infarct volume was an independent predictor of ICU need in adjusted models (OR 1.027 per cc increase in volume, 95% CI 1.002-1.052). The ROC curve with infarct volume alone achieved an area under the curve (AUC) of 0.766 (95% CI 0.605-0.927), while the AUC was 0.906 (95% CI 0.814-0.998) after adjusting for race, systolic blood pressure, and NIH Stroke Scale. Maximum Youden index calculations identified an optimal infarct volume cut-point of 6.8 cc (sensitivity 75.0%, specificity 76.7%). Infarct volume greater than 3 cc predicted need for critical care interventions with 81.3% sensitivity and 66.7% specificity.
Conclusion
Infarct volume may be used to triage tPA patients to the resource appropriate monitoring environment.
Keywords: infarct volume, ICU needs, critical care needs, IV thrombolysis, IV tPA
Introduction
Intravenous (IV) thrombolysis with recombinant tissue plasminogen activator (tPA) is the only approved therapy for acute ischemic stroke and is currently the cornerstone of therapy for patients presenting within 4.5 hours of symptom onset [1].
It is well established that stroke patients cared for in dedicated stroke units have improved mortality and long-term functional outcomes [2-4]. Stroke units, while providing organized, guideline-driven care, vary significantly in their capacity to provide ICU interventions. Whether all patients undergoing IV thrombolysis require monitoring in an environment capable of ICU interventions (i.e. for invasive monitoring or mechanical ventilation), is not clear. Current guidelines suggest that post tPA patients are monitored in an intensive care unit (ICU) or a comparable environment capable of ICU interventions for frequent vital sign checks and neurological examinations. This monitoring is resource intensive, often requiring one to one nursing care to allow for detection and early intervention of potential complications, such as symptomatic intracranial hemorrhage [5]. However, most patients remain free of complications requiring ICU level interventions. It is currently unclear whether routine ICU-admission or intensive monitoring is medically necessary for all post tPA patients.
ICU resources are scarce and costly. Appropriate utilization of resources in the ICU is of vital importance to provide safe and cost-effective health care. Unnecessary ICU admissions may lead to ICU and Emergency Department (ED) overcrowding, prolonged ED boarding times, and adverse patient outcomes [6]. We have previously identified African American race, systolic blood pressure, and stroke severity by NIH stroke scale (NIHSS) as predictors of critical care needs in post tPA patients [7]; however, the utility of post tPA neuroimaging to predict the need for intense monitoring and ICU care post tPA has not previously been investigated.
In the present study we aimed to evaluate and identify early imaging characteristics on MRI that determine ICU needs post tPA. We tested the hypothesis that quantitative measurement of stroke volume in the immediate post tPA time period may predict need for critical care intervention. To our knowledge, this is the first study to explore associations between quantitative analysis of stroke volume immediately post tPA and need for ICU care.
Methods
IV thrombolysis protocol
At our institution, IV tPA is administered according to the American Heart Association's national guidelines [5]. Any patient presenting within 4.5 hours with a potentially disabling deficit is considered for IV tPA. Post tPA monitoring conforms to the recommendations of the Brain Attack Coalition, which have become the standard of care for most stroke centers. All patients treated with IV tPA are monitored in the neurointensive care unit for at least 24 hours after initiation of thrombolysis, and undergo neuroimaging with either CT or MRI within 24 hours after treatment before being considered for transfer to the floor.
Patients and study design
This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. Patients who were treated with IV tPA for presumed acute ischemic stroke in the ED at Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center between January 2010 and January 2014 were retrospectively identified from our prospectively collected stroke database. Patients were included in the analysis if they additionally had undergone post tPA brain MR imaging within 6 hours of tPA administration. Patients undergoing intra-arterial therapy were excluded from the study, as this therapy is inherently different from IV tPA and therefore may have other characteristics in regards to the need for ICU interventions. In addition, most patients who underwent intra-arterial therapy at our institutions during the study period were routinely sedated and intubated for the procedure, as this had been the prevailing clinical practice until recently, thus requiring post-procedure ICU care solely by virtue of having undergone intervention. Some of the included patients were included in a previously published study[7].
Demographic data including age, sex, and race were collected for all patients. Other variables of interest included stroke risk factors: hypertension, hyperlipidemia, diabetes mellitus, smoking status, coronary artery disease (CAD), history of atrial fibrillation, prior history of stroke, and the pre-hospital use of antiplatelet agents, anticoagulation, and statins. NIHSS and the following physiologic parameters at presentation were recorded: blood pressure, blood glucose, and estimated glomerular filtration rate (eGFR) by Modification of Diet in Renal Disease (MDRD) equation [8]. Data on total length of stay (LOS), length of ICU stay, discharge destination, and in-hospital mortality were collected. The presence of any critical care intervention was recorded. A critical care intervention was considered any therapy or intervention that required ICU resources as defined previously [7]. Specifically, ICU admission criteria included: uncontrolled hypertension requiring titration of IV antihypertensives, use of vasopressors either for symptomatic systemic hypotension or blood pressure augmentation, need for invasive hemodynamic monitoring, uncontrolled hyperglycemia requiring IV Insulin, respiratory compromise resulting in either initiation of bilevel positive airway pressure (BiPAP) or mechanical ventilation, arterial bleeding, management of cerebral edema and increased ICP, neurosurgical intervention such as decompressive craniectomy, or interventional angiography with or without intervention. Our definition of an ICU intervention also included patients with any event or complication that would require monitoring in an ICU setting even if no immediate ICU intervention was performed, such as progressive decrease in mental status with impaired airway protection, increasing oxygen requirement, or detection of potentially life-threatening arrhythmia. Patients who required ICU resources by the end of their tPA infusion or at any time over the next 24 hours were compared with those patients who did not have an ICU intervention during the same time period. Symptomatic intracerebral hemorrhage (sICH) was defined as any ICH with neurological deterioration, as indicated by a change in NIHSS ≥ 4 compared to the baseline as described previously [9].
Imaging Analysis
All patients underwent MR imaging within 6 hours of tPA administration. MRI examinations were performed using a 1.5T MRI system (Siemens, PA). All MR images were reviewed by a vascular neurologist (RF). A subset of images was reviewed by a second investigator (VCU), and an intraclass correlation coefficient for a two-way random effects model was used to assess inter-rater agreement of stroke volume. Interrater reliability for quantitative infarct volume was high (ICC 0.998, 95% CI 0.991-0.999). Acute stroke on post tPA MRI was determined qualitatively by presence of increased signal on diffusion weighted imaging (DWI) with corresponding low signal on the apparent diffusion coefficient (ADC) maps. Large artery signal drop-out of the large artery supplying the area of the stroke was identified by qualitative visual inspection on time-of flight MRA. Quantitative analysis of stroke volume was performed by commercially available FDA-approved semiquantitative software (Olea Sphere, Olea Medical SAS, France). DWI, ADC, and fluid attenuated inversion recovery (FLAIR) images for each patient were coregistered with the Olea software using a 12-degree-of-freedom transformation with a mutual information cost function. This was followed by visual inspection to ensure adequate alignment. The volume of the infarction was calculated by the software using threshold method defined as an apparent diffusion coefficient value less than 600×10-6 mm2/s [10, 11].
Statistical Analysis
Statistical analysis was performed using Stata version 13 (Stata Statistical Software: Release 13. College Station, TX) and SAS 9.2 (SAS Institute Inc., Cary, NC). A two-sided p-value of <0.05 was considered statistically significant. Continuous variables were analyzed using Student's t-tests for normally distributed variables, and Wilcoxon rank-sum tests (Mann-Whitney U test) for non-normally distributed variables. Categorical variables were analyzed using Pearson's Chi2 analysis, and Fisher's exact tests, when appropriate.
Logistic regression models were developed to identify predictors of need for ICU resources. The primary outcome of interest was need for ICU-intervention. Stroke volume on DWI-MRI was the primary predictor of interest. Multivariable logistic regression was performed adjusting for basic demographic variables including age, sex, and race. Other variables felt to be clinically important for predicting critical care needs based on univariate analysis were included, such as NIHSS and systolic blood pressure (SBP). Receiver operating characteristics (ROC) analysis was conducted using DWI data with and without additional predictors. Model selection for ROC curves was done using Akaike information criterion (AIC) values from logistic regression analysis. Maximum Youden index was used to determine the optimal statistical cut-point with equal weight given to sensitivity and specificity.
Results
Patient selection and characteristics
Two hundred and two patients received IV tPA for acute ischemic stroke at our institution between January 2010 and January 2014, of which 148 were confirmed to have an ischemic stroke by MRI or CT. A total of 46 consecutive patients with post tPA MR imaging within the first 6 hours of tPA administration were identified from our database and were included in this study. The remaining patients were excluded because MRI was either not performed (i.e. due to pacemaker, claustrophobia, etc.) or was obtained more than 6 hours after tPA administration. Included and excluded patients did not significantly differ with regards to age, sex, race, NIHSS at presentation, blood pressure, frequency of ICU need, and other clinical or physiological characteristics. Baseline characteristic of patients who were screened but not included in the final analysis can be found as a supplemental table.
The average age for the 46 patients included in this study was 64.4 years (range 32-89); 60.9% were male; and 43.5% were African American. The median NIHSS was 8 (Interquartile range [IQR] 4-16). Thirty-five patients (76.1%) had a history of hypertension, 21 (45.7%) had a history of hyperlipidemia, 10 (21.8%) had a prior history of diabetes mellitus, and 7 (15.2%) had a history of atrial fibrillation. The mean systolic blood pressure (SBP) at presentation was 164 mmHg, and the median serum glucose upon presentation was 119 mg/dL. Two patients (4.4%) developed a symptomatic ICH. The median time to MRI after tPA was 1 hour (IQR 0-2), and the median stroke volume was 2.8 cc (IQR 0.5-17.4). The median duration of ICU stay was 2 days (IQR 2-3). Further baseline patient characteristics are presented in Table 1.
Table 1.
Baseline characteristics of patients with post tPA stroke on MRI by ICU need. P-values are given for the comparison of those with and without need for ICU care. NIHSS: NIHS Stroke Scale; BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; tPA: tissue plasminogen activator; eGFR: estimated glomerular filtration rate; LA: larger artery; vol: volume; ICH: intracerebral hemorrhage; LOS: length of stay; ICU: intensive care unit.
| Characteristics | All patients (n=46) |
With ICU intervention (n=16) |
No ICU intervention (n=30) |
p-value |
|---|---|---|---|---|
| Age – years: mean (SD) | 64.4 (14.4) | 69.5 (10.8) | 61.7 (15.5) | 0.079 |
| range | 32-89 | 54-89 | 32-89 | |
| Race – African American: n (%) | 20 (43.5) | 11 (68.8) | 9 (30.0) | 0.012 |
| Gender – male: n (%) | 28 (60.9) | 9 (56.3) | 19 (63.3) | 0.639 |
| NIHSS – median (IQR) | 8 (4-16) | 14 (4-18.5) | 7 (3-11) | 0.033 |
| BP – mm Hg: mean (SD) | ||||
| SBP | 164 (29) | 179 (27) | 156 (27) | 0.009 |
| DBP | 90 (21) | 97 (27) | 87 (16) | 0.112 |
| tPA window <3hrs – n (%) | 31 (67.4) | 12 (75.0) | 19 (63.3) | 0.421 |
| Glucose – mg/dl: median (IQR) | 119 (101-161) | 142 (106-170) | 111 (100-150) | 0.166 |
| eGFR < 60 ml/min – n (%) | 13 (28.3) | 5 (31.3) | 8 (26.7) | 0.742 |
| Risk factors for stroke – n (%) | ||||
| Hypertension | 35 (76.1) | 14 (87.5) | 21 (70.0) | 0.185 |
| Hyperlipidemia | 21 (45.7) | 8 (50.0) | 13 (43.3) | 0.665 |
| Diabetes mellitus | 10 (21.8) | 6 (37.5) | 4 (13.3) | 0.058 |
| Coronary Artery Disease | 13 (28.3) | 5 (31.3) | 8 (26.7) | 0.744 |
| Atrial fibrillation | 7 (15.2) | 4 (25.0) | 3 (10.0) | 0.216 |
| Prior ischemic stroke | 14 (30.4) | 5 (31.3) | 9 (30.0) | 1.000 |
| Current smoking | 20 (43.5) | 5 (31.3) | 15 (50.0) | 0.222 |
| Medications – n (%) | ||||
| Antiplatelet agent | 19 (41.3) | 7 (43.8) | 12 (40.0) | 0.806 |
| Anticoagulation | 1 (2.2) | 0 (0) | 1 (3.3) | 1.000 |
| Statin | 18 (39.1) | 7 (43.8) | 11 (36.7) | 0.639 |
| Imaging | ||||
| tPA to MRI – hrs: median (IQR) | 1 (0-2) | 1 (0.5-2) | 1 (0-2) | 0.416 |
| Anterior circulation – n (%) | 39 (84.8) | 14 (87.5) | 25 (83.3) | 0.708 |
| Supratentorial location | 43 (93.5) | 14 (87.5) | 29 (96.7) | 0.274 |
| Laterality (supratentorial, n=43) | 0.302 | |||
| Left | 25 (58.1) | 6 (42.8) | 19 (65.5) | |
| Right | 14 (32.6) | 6 (42.8) | 8 (27.6) | |
| Bilateral | 4 (9.3) | 2 (14.4) | 2 (6.9) | |
| LA drop-out on MRA – n (%) | 14 (30.4) | 7 (43.8) | 7 (23.3) | 0.152 |
| Infarct vol. in cc – median (IQR) | 2.8 (0.5-17.8) | 17.0 (5.0-109.7) | 1.2 (0.3-5.6) | 0.003 |
| Symptomatic ICH – n (%) | 2 (4.4) | 2 (12.5) | 0 (0) | 0.116 |
| LOS – days: median (IQR) | 5 (3-8) | 6 (4.5-8.5) | 5 (3-6) | 0.114 |
| ICU stay – days: median (IQR) | 2 (2-3) | 3 (2-4) | 2 (1-2) | 0.006 |
| Discharge to home – n (%) | 21 (45.7) | 3 (18.8) | 18 (60.0) | 0.007 |
| Mortality – n (%) | 6 (13.0) | 6 (37.5) | 0 (0) | 0.001 |
Patients requiring an ICU intervention (16; 34.8%) were compared to those patients who did not have critical care needs (30; 65.2%). The characteristics of these 2 groups including age, gender, race, NIHSS, stroke risk factors, medications for secondary stroke prevention, as well as blood pressure, blood glucose, eGFR at presentation, and relevant imaging characteristics are summarized in Table 1. Patients requiring an ICU intervention were more likely to be African American (p=0.012), presented with higher SBP (p=0.009), and had higher infarct volumes (p=0.003). The need for an ICU interventions was associated with longer ICU stay (p=0.006) and increased mortality (p=0.001).
Of the 16 patients requiring ICU care, the most common critical care interventions were: management of respiratory compromise (10/16, 62.5%), IV drips for uncontrolled hypertension (6/16, 37.5%), management of cerebral edema (4/16, 25%), and management of sICH (2/16, 12.5%). Six of the 16 patients (37.5%) required more than one critical care intervention.
Infarct volume predicts need for ICU care
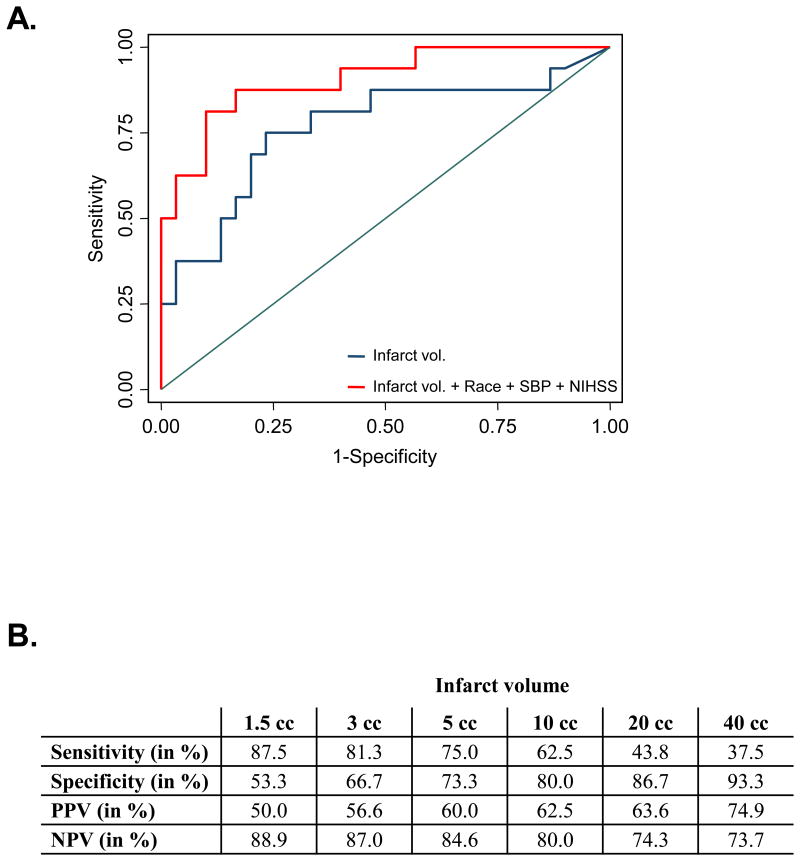
In simple logistic regression analyses infarct volume (odds ratio [OR] 1.024 per cc increase in volume, 95% confidence interval [CI] 1.003-1.044), African American race (OR 5.13 compared to whites, 95% CI 1.38-19.11), NIHSS (OR 1.12 per point increase, 95% CI 1.01-1.23), and SBP (OR 1.03 per 1 mm Hg increase, 95% CI 1.01-1.06) were associated with requiring ICU level of care. In multivariate analysis infarct volume remained an independent predictor of ICU care after adjusting for age, sex, and race (OR 1.026, 95% CI 1.003-1.050, p=0.025). This association remained significant after additionally adjusting for SBP, CAD, and NIHSS (OR 1.031, 95% CI 1.004-1.058, p=0.024). The ROC curves for the logistic regression models of ICU need are shown in Figure 1A. The model including infarct volume achieved an AUC of 0.766 (95% CI 0.605-0.927). There was no statistically significant difference in the AUC after including race (AUC 0.828, p=0.346), SBP (AUC 0.840, p=0.303), or NIHSS (AUC 0.779, p=0.835) when compared with infarct volume alone. The model adjusted for race, SBP, and NIHSS was the best-fitting model and performed better than infarct volume alone (AUC 0.906, 95% CI 0.814-0.998, p=0.037).
Figure 1.
A. The ROC curves for logistic regression models for ICU need are shown. B. Results of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) at a range of infarct volumes in cubic centimeters (cc).
A range of infarct volume cut-offs were tested for sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). An infarct volume cut-off of 3 cubic centimeters (cc) identified patients requiring ICU interventions with a sensitivity of 81.3%, specificity of 66.7%, PPV of 56.6%, and NPV of 87.0%, while an infarct volume cut-off of 10 cc identified need for ICU care with a sensitivity of 62.5%, specificity of 80.0%, PPV of 62.5%, and NPV of 80.0% (Figure 1B). Maximum Youden index calculations identified an optimal infarct volume cut-point of 6.8 cc (Youden index 0.52; sensitivity 75.0%; specificity 76.7%). The predicted values for sensitivity, specificity, PPV, and NPV at various infarct volumes are shown in Figure 1B.
Discussion
All post tPA patients undergo resource intense monitoring for at least 24 hours regardless of patient demographics or other clinical or physiological variables, typically in an ICU or comparable stroke unit, capable of ICU interventions and monitoring. Little data exist as to whether this “one size fits all” approach is necessary, however, since post tPA patients vary with regards to age, stroke severity, and other medical comorbidities, it is conceivable that resource intense monitoring may be necessary for some, but not all patients. In the present study we show that quantitative stroke volume on post tPA MRI is associated with increased need for ICU care, and we define infarct volume cut-offs predicting need for ICU care.
Clinical parameters have previously been used to predict critical care needs in post tPA patients. We have recently shown that NIHSS as a measure of stroke severity is a predictor of ICU care in post tPA patients [7]. NIHSS correlates with infarct volume [12] and large arterial occlusion [13]. NIHSS and infarct volume were positively correlated in our study (Spearman's Rho 0.52, p<0.001); however, infarct volume was a better predictor of critical care needs than NIHSS and remained an independent predictor of requiring ICU care after adjusting for NIHSS in our multivariate model.
In order to utilize MRI as a triaging tool to determine the appropriate monitoring environment for tPA patients, timely imaging is crucial in order to prevent triage delay. Infarct volume at any given ADC threshold is dynamic over time in the setting of ongoing ischemia. Pre-tPA MRI has previously been used to predict outcome in stroke patients [14]; however, routine pre-tPA MRI is currently not feasible at most institutions and may delay initiation of treatment. In the present study we included patients with MR imaging obtained within the first 6 hours of tPA administration, although as many as 75% of patients underwent MR imaging within the first 2 hours. The ADC threshold of 600 was chosen as this is widely considered the threshold associated with irreversible tissue damage [15]. In addition, DWI volume at this threshold has been shown to provide a surrogate of final infarct volume [16].
Although we selected all patients on the basis of a DWI lesion by visual inspection of post tPA MRI, a small subgroup of 4 patients had a quantitative stroke volume of 0 cc at ADC threshold 600. The presence of DWI changes with corresponding ADC values above 600 indicates that irreversible tissue damage may not have taken place at the time of imaging. Since our measured infarct volumes do not account for areas of diffusion restriction at ADC values exceeding 600, our computed cut-off values may underestimate the DWI-volume estimated by mere visual inspection.
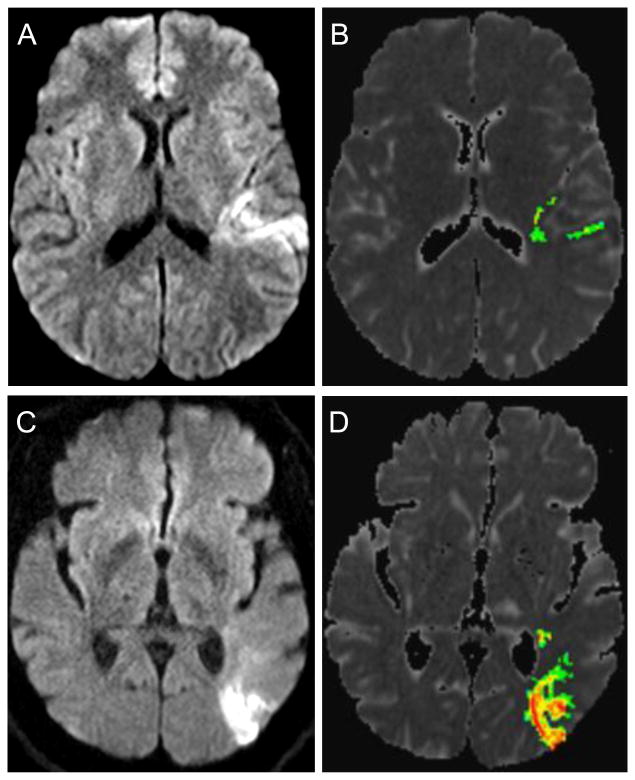
Figure 2 shows the MRIs of 2 representative patients, both with seemingly comparable DWI volumes by visual inspection (Figure 2 A, C). Patient 1 has a DWI lesion with a relatively small core of diffusion restriction below ADC threshold 600, with surrounding tissue restricting diffusion at higher ADC values (Figure 2 A, B). In contrast, the DWI lesion of patient 2 restricts almost entirely below the 600 threshold (Figure 2 C, D). The computed infarct volumes at ADC threshold 600 were 3.9 cc for patient 1, and 9.2 cc for patient 2. Patient 2 required ICU care while patient 1 did not. Both patients underwent MRI within the first hour of completion of tPA. This example emphasizes the point that measured infarct volume at ADC threshold 600 may predict need for ICU care more reliably than the size of the DWI lesion obtained by mere visual inspection.
Figure 2.
MRIs are shown for 2 representative patients, both with similar appearing DWI volumes by visual inspection. The upper panel shows the DWI lesion by visual inspection (A), and by volumetric analysis at ADC threshold 600 (B) for patient 1. The lower panel shows a DWI lesion of seemingly similar volume by visual inspection for patient 2 (C), however, volumetric analysis reveals a significantly higher infarct volume restricting diffusion below the ADC threshold of 600 (D).
The clinically “optimal” infarct volume cut-off for any given test depends on the desired sensitivity and specificity with which prediction is made. The optimal infarct volume cut-off for predicting ICU need was 6.8 cc when accounting for sensitivity and specificity with equal weight (Youden index 0.52). Safety and early recognition of complication is of vital importance in post tPA monitoring, rendering high sensitivity with a low rate of false negatives desirable (patients falsely classified as not requiring ICU care who later end up needing ICU care). Thus, a lower infarct volume cut-point may be considered “optimal” in order to predict need for ICU care with a high level of sensitivity (i.e. 3 cc predicting need for ICU care with a sensitivity of more than 80%).
Our infarct volume cut-offs predicting critical care needs might appear relatively modest at first glance. However, since perfusion volume was not taken into account in the present study, the infarct volume within the first hours after tPA obtained here may underestimate the more commonly published final infarct volume because of ongoing ischemia in hypoperfused brain tissue, and because early infarcted brain tissue may not entirely restrict diffusion at the ADC 600 threshold yet when assessed within 6 hours.
Our study has several limitations. This is a retrospective analysis of a relatively small number of patients. Patients were selected based on the presence of ischemia on DWI imaging obtained within 6 hours of tPA administration. Since there currently is no convincing evidence to support early MR imaging after tPA, most patients in our database screened for this study had undergone MR imaging beyond 6 hours after tPA. The time-point of MR imaging at our institution is mainly driven by MR scanner availability, time of the day, ED workload, and ICU bed availability, rather than clinical criteria, although we cannot exclude that some of the MRIs obtained within 6 hours post tPA were done for a clinical reason. In addition, triaging of post tPA patients is not contingent on MR imaging characteristics at our institutions, and currently all patients undergoing IV thrombolysis are subsequently monitored in the ICU for at least 24 hours. While ICU interventions, procedures, and medication administration were well documented in the vast majority of cases, relying on accuracy of medical records has the potential to result in missing or inaccurate information by virtue of the retrospective nature of this study. Our study population was derived from 2 single stroke centers over the course of 4 years. Therefore, extrapolating our results to community hospitals must be done with caution. In addition, MRI is not universally and readily available at all institutions, potentially limiting generalizability. Finally, indications for ICU admission and interventions may differ among institutions across the United States, and the model described in this study might therefore not be valid in institutions where ICU admission criteria differ significantly from ours.
Triaging decisions in stroke patients are multifactorial and complex. Clinicians have to take a variety of parameters into account when determining the appropriate monitoring environment for each patient, including demographics, physiological parameters, and disease trajectory. Automated imaging systems have been developed to select patients for acute stroke therapy [17]. A similar approach may be taken with regard to monitoring intensity after IV thrombolysis, in which MRI-based selection of a distinct subpopulation of patients with low probability of requiring ICU resources is feasible. Such patients may be safely monitored in a dedicated stroke unit without ICU capabilities. From a practical standpoint, the only MRI sequences required to determine infarct volume are DWI and FLAIR. Both sequences can be obtained in less than 10 minutes, thus presenting only minimal interruption of the recommended post tPA monitoring schedule, if any. In addition, post-processing of MR images with the commercially available software used our study can be accomplished within several minutes and requires relatively little effort and training.
We propose that volumetric analysis on post tPA MRI may aid in the selection of patients who are at low risk for requiring ICU resources. Thus, such patients might be considered for monitoring in a low-intensity monitoring environment.
Supplementary Material
Footnotes
Conflicts of interest: VCU is the PI for the investigator initiated trial SAIL ON (a pilot clinical trial of IV tPA treatment for patients that wake up with stroke). Genentec Inc. has provided funding for this trial. VCU is also PI at Johns Hopkins for the multicenter clinical trial DIAS 4. Sponsor is Lundbeck. There are no patents, products in development or marketed products to declare.
References
- 1.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S, ATLANTIS Trials Investigators, ECASS Trials Investigators, NINDS rt-PA Study Group Investigators Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 2.Ronning OM, Guldvog B. Stroke unit versus general medical wards, II: neurological deficits and activities of daily living: a quasi-randomized controlled trial. Stroke. 1998;29:586–590. doi: 10.1161/01.str.29.3.586. [DOI] [PubMed] [Google Scholar]
- 3.Indredavik B, Bakke F, Solberg R, Rokseth R, Haaheim LL, Holme I. Benefit of a stroke unit: a randomized controlled trial. Stroke. 1991;22:1026–1031. doi: 10.1161/01.str.22.8.1026. [DOI] [PubMed] [Google Scholar]
- 4.Ringelstein EB, Chamorro A, Kaste M, Langhorne P, Leys D, Lyrer P, Thijs V, Thomassen L, Toni D, ESO Stroke Unit Certification Committee European Stroke Organisation recommendations to establish a stroke unit and stroke center. Stroke. 2013;44:828–840. doi: 10.1161/STROKEAHA.112.670430. [DOI] [PubMed] [Google Scholar]
- 5.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H, American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Peripheral Vascular Disease, Council on Clinical Cardiology Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 6.Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP, DELAY-ED study group Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007;35:1477–1483. doi: 10.1097/01.CCM.0000266585.74905.5A. [DOI] [PubMed] [Google Scholar]
- 7.Faigle R, Sharrief A, Marsh EB, Llinas RH, Urrutia VC. Predictors of Critical Care Needs after IV Thrombolysis for Acute Ischemic Stroke. PLoS One. 2014;9:e88652. doi: 10.1371/journal.pone.0088652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 9.Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 10.Dardzinski BJ, Sotak CH, Fisher M, Hasegawa Y, Li L, Minematsu K. Apparent diffusion coefficient mapping of experimental focal cerebral ischemia using diffusion-weighted echo-planar imaging. Magn Reson Med. 1993;30:318–325. doi: 10.1002/mrm.1910300307. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer PW, Hassankhani A, Putman C, Sorensen AG, Schwamm L, Koroshetz W, Gonzalez RG. Characterization and evolution of diffusion MR imaging abnormalities in stroke patients undergoing intra-arterial thrombolysis. AJNR Am J Neuroradiol. 2004;25:951–957. [PMC free article] [PubMed] [Google Scholar]
- 12.Saver JL, Johnston KC, Homer D, Wityk R, Koroshetz W, Truskowski LL, Haley EC. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke. 1999;30:293–298. doi: 10.1161/01.str.30.2.293. [DOI] [PubMed] [Google Scholar]
- 13.Olavarria VV, Delgado I, Hoppe A, Brunser A, Carcamo D, Diaz-Tapia V, Lavados PM. Validity of the NIHSS in predicting arterial occlusion in cerebral infarction is time-dependent. Neurology. 2011;76:62–68. doi: 10.1212/WNL.0b013e318203e977. [DOI] [PubMed] [Google Scholar]
- 14.Kruetzelmann A, Kohrmann M, Sobesky J, Cheng B, Rosenkranz M, Rother J, Schellinger PD, Ringleb P, Gerloff C, Fiehler J, Thomalla G. Pretreatment diffusion-weighted imaging lesion volume predicts favorable outcome after intravenous thrombolysis with tissue-type plasminogen activator in acute ischemic stroke. Stroke. 2011;42:1251–1254. doi: 10.1161/STROKEAHA.110.600148. [DOI] [PubMed] [Google Scholar]
- 15.Purushotham A, Campbell BC, Straka M, Mlynash M, Olivot JM, Bammer R, Kemp SM, Albers GW, Lansberg MG. Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke. 2013 doi: 10.1111/ijs.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler HM, Mlynash M, Inoue M, Tipirneni A, Liggins J, Zaharchuk G, Straka M, Kemp S, Bammer R, Lansberg MG, Albers GW, DEFUSE 2 Investigators Early diffusion-weighted imaging and perfusion-weighted imaging lesion volumes forecast final infarct size in DEFUSE 2. Stroke. 2013;44:681–685. doi: 10.1161/STROKEAHA.111.000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, Campbell BC, Bammer R, Olivot JM, Desmond P, Davis SM, Donnan GA, Albers GW. RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke. 2011;42:1608–1614. doi: 10.1161/STROKEAHA.110.609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.