Abstract
The field of intracellular traffic is celebrating the awarding of the 2013 Nobel Prize in Physiology or Medicine to three of its most distinguished scientists, James Rothman, Randy Schekman and Thomas Südhof, for their discoveries on the molecular mechanisms of vesicular transport. Their outstanding achievements serve as inspiration for the next generation of scientists to continue the task of uncovering new principles of intracellular compartmentalization and dynamics.
The morning of October 7th, 2013, we woke up to the exciting news that James Rothman, Randy Schekman and Thomas Südhof had been awarded the 2013 Nobel Prize in Physiology or Medicine “for their discoveries of machinery regulating vesicle traffic, a major transport system in our cells” [1]. It was a fitting and long-awaited recognition to three scientists who over a period spanning four decades had discovered the fundamental mechanisms by which cargo macromolecules are conveyed between the membrane-enclosed compartments that make up the secretory system of eukaryotic cells. When these scientists started their work, George Palade and colleagues had already established that newly-synthesized secretory proteins are transported in a vectorial fashion from their site of birth in the endoplasmic reticulum (ER) to the Golgi complex and then to secretory granules prior to their release into the extracellular space. Transport was proposed to occur by encapsulation of secretory proteins into small vesicles that budded from a donor compartment and fused with an acceptor compartment at each step of the secretory pathway (Figure 1). Thus the vesicular transport hypothesis was born. It fell to the next generation of scientists to prove this hypothesis and unravel its mechanistic underpinnings.
Figure 1.
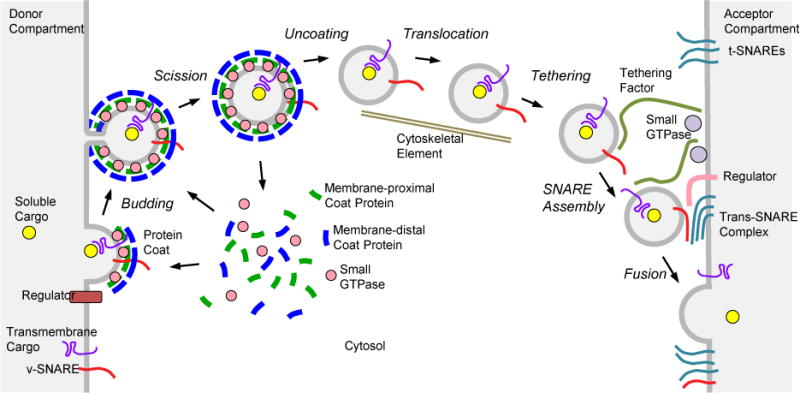
The life cycle of a transport vesicle. The scheme illustrates the steps of vesicle budding and fusion in a generic vesicular transport process. Adapted from reference 16.
Both Schekman and Rothman were inspired by Arthur Kornberg’s earlier biochemical dissection of the enzymatic reactions involved in DNA synthesis. They independently set out to uncover the molecular machinery for vesicular transport using very different experimental systems. Schekman, though a biochemist at heart, initially chose a genetic approach that consisted of isolating yeast mutants defective in invertase secretion (sec mutants) [2] and cloning the mutated genes by complementation. Over the years, this approach allowed the identification of more than 50 proteins involved in transport at different stages of the secretory pathway. Among these proteins were components of the COPII coat (SEC13, SEC23, SEC24, SEC31) that mediates vesicle budding from the ER for transport to the Golgi complex. Ultimate proof of the function of this coat was obtained by reconstitution of vesicle formation and cargo selection from purified components in the test tube [3]. Rothman focused on the biochemical approach right from the start. He developed a cell-free reconstitution assay using mammalian Golgi fractions, which recapitulated both the budding and fusion steps of the transport reaction [4]. This assay also enabled the discovery of many components of the vesicular transport machinery, most notably the SNAREs (SNAP receptors), membrane-bound proteins that come together to promote fusion of transport vesicles with acceptor organelles [5]. In a stunning convergence of findings, some of the proteins identified from the yeast screens (e.g., SEC17 and SEC18) turned out to be orthologous to proteins identified through the use of the mammalian-based cell-free assays (α-SNAP and NSF, respectively). This convergence demonstrated the robustness of the genetic and biochemical approaches, and, most importantly, highlighted the phylogenetic conservation of the protein transport machinery in all eukaryotes from baker’s yeast to humans. Studies performed in model organisms thus acquired critical relevance to human biology, including the elucidation of the pathogenesis of many human diseases.
In yet another coincidence, the SNAREs identified using Rothman’s assay [5] were found to be homologous to VAMPs/synaptobrevins [6] and syntaxins [7], proteins that had been previously proposed to mediate docking and fusion of synaptic vesicles with the plasma membrane in the process of Ca2+-triggered neurotransmitter release. The mechanisms of vesicular transport were therefore also conserved at different steps of the secretory pathway. It was in this context that Südhof identified several proteins that regulate SNARE-mediated neurotransmitter release, including synaptotagmin-1 (which confers regulation by Ca2+) [8], complexin (which keeps fusion in check until induced by synaptotagmin-1) [9], and Munc-18-1 (a homolog of yeast SEC1 and C. elegans UNC-18 that coordinates SNARE complex assembly through interaction with syntaxin-1) [10]. Südhof’s findings added a layer of regulation by extracellular signals and second messengers to the basic process of SNARE-mediated fusion.
The discoveries by Rothman, Schekman, Südhof and their co-workers are far broader than those outlined in the preceding paragraphs. Together, they have contributed to the establishment of a general paradigm for the mechanisms of vesicular transport that applies not only to the secretory pathway but also to other intracellular trafficking processes such as endocytosis, retrograde transport from endosomes to the Golgi complex and from the Golgi complex to the ER, and transport to lysosomes. In this paradigm (Figure 1), a transport vesicle is sculpted from the donor membrane by the assembly of a protein coat. This coat promotes vesicle budding while selecting specific cargos for incorporation into the vesicle. After scission from the donor membrane, the vesicle loses its coat and translocates through the cytoplasm. Tethering factors associated with the acceptor membrane then capture the vesicle and promote the formation of complexes between vesicle SNAREs (v-SNAREs) and target SNAREs (t-SNAREs). “Zippering” of the SNAREs triggers membrane fusion, resulting in the delivery of cargo into the acceptor compartment. An assortment of regulatory factors modulates both the budding and fusion aspects of the vesicular transport process. Specificity is determined by the use of different protein coats, SNAREs and regulatory factors at distinct transport steps.
After such astounding discoveries, one is left to ponder whether more fundamental principles in intracellular traffic remain to be uncovered. I have heard the assertion that intracellular traffic is a “mature field”, like an oil field that is past its peak production and can only deliver diminishing yields. Many young scientists are said to be attracted to more fashionable fields such as “omics” or translational research. Despite the obvious appeal of new technologies or applications, my opinion is that the field of intracellular traffic research is far from waning or démodé. While the vesicular transport paradigm outlined above applies to many intracellular traffic events, there are plenty of other processes that rely on partly or wholly different mechanisms. Consider, for instance, the “inward” budding of the endosomal membrane to generate intraluminal vesicles in the process of multivesicular body formation [11]. This process is topologically opposite to the “outward” budding of transport vesicles, and depends on an entirely different molecular machinery. Likewise, the envelopment of cytoplasmic organelles and particles into autophagic vacuoles, while partly relying on SNAREs, is mediated by a largely distinct mechanism [12]. There are also glycosphingolipid- and cholesterol-based mechanisms of transport carrier formation that do not utilize conventional protein coats [13]. Moreover, the translocation of vesicles between the donor and acceptor compartments and the long range movement of cytoplasmic organelles are known to be mediated by a variety of motor molecules associated with cytoskeletal structures [14], but the determinants of specificity and their role in sorting vesicles to different cellular domains are only now beginning to be understood. All of these are vibrant areas of research in intracellular traffic that have yet to yield some of their most revealing findings. In other emerging topics we have only spotted the proverbial tip of the iceberg. For instance, we are learning that intracellular traffic processes are not merely “constitutive” but are subject to genetic control in response to metabolic needs, as recently found for lysosome and autophagosome biogenesis [15]. How many other trafficking pathways might be similarly regulated at the level of gene expression? And there are yet other processes that to date we can only contemplate with perplexity. What is the mechanistic basis for the bewildering complexity of the tubular endosomal network? And what of trafficking processes that occur only in specialized cells, or in the context of whole tissues or multicellular organisms? These few, arbitrarily chosen examples illustrate that our understanding of the structural and functional compartmentalization of the cell, though growing, is still very limited, posing a challenge for another generation of cell biologists to emulate the outstanding achievements of the 2013 Nobel Prize winners.
References
- 1.Zierath JR, Lendahl U. Machinery regulating vesicle traffic, a major transport system in our cells. The 2013 Nobel Prize in Physiology or Medicine - Advanced Information. 2013 Nobelprize.org. Nobel Media AB 2013.
- 2.Novick P, et al. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 3.Barlowe C, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 4.Balch WE, et al. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- 5.Sollner T, et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 6.Trimble WS, et al. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci U S A. 1988;85:4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett MK, et al. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 8.Perin MS, et al. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 9.McMahon HT, et al. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 10.Hata Y, et al. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 11.Odorizzi G, et al. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- 12.Takeshige K, et al. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romer W, et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–675. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 14.Vale RD, et al. Movement of organelles along filaments dissociated from the axoplasm of the squid giant axon. Cell. 1985;40:449–454. doi: 10.1016/0092-8674(85)90159-x. [DOI] [PubMed] [Google Scholar]
- 15.Sardiello M, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 16.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]


