Abstract
Seminal proteins from the Drosophila male accessory gland induce post-mating responses (PMR) in females. The PMR comprise behavioral and physiological changes that include increased egg laying, decreased receptivity to courting males, and changes in the storage and use of sperm. Many of these changes are induced by a “sex peptide” (SP) and are maintained by SP’s binding to, and slow release from, sperm. The accessory gland contains two secretory cell types with distinct morphological and developmental characteristics. Products of these “main” and “secondary” cells work interdependently to induce and maintain the PMR. To identify individual genes needed for the morphology and function of secondary cells, we studied iab-6cocu males, whose secondary cells have abnormal morphology and fail to provide products to maintain the PMR. By RNA-seq, we identified 77 genes that are downregulated by a factor of >5× in iab-6cocu males. By functional assays and microscopy, we tested 20 candidate genes and found that at least 9 are required for normal storage and release of SP in mated females. Knockdown of each of these 9 genes consequently leads to a reduction in egg laying and an increase in receptivity over time, confirming a role for the secondary cells in maintaining the long-term PMR. Interestingly, only 1 of the 9 genes, CG3349, encodes a previously reported seminal fluid protein (Sfp), suggesting that secondary cells may perform essential functions beyond the production and modification of known Sfps. At least 3 of the 9 genes also regulate the size and/or abundance of secondary cell vacuoles, suggesting that the vacuoles’ contents may be important for the machinery used to maintain the PMR.
Keywords: reproduction, Drosophila, sex peptide, gene regulation, gene expression
SEMINAL fluid proteins (Sfps) play an essential role in reproduction. Sfps interact with the female, locally in the reproductive tract and more globally in the central nervous system, resulting in changes in female gene expression, behavior (in insects), and physiology (reviewed in Sirot et al. 2009). In Drosophila, these changes are collectively referred to as the female post-mating response (PMR). The male accessory gland (AG) is the source of many important Sfps that cause the PMR (Takemori and Yamamoto 2009). Each lobe of the accessory gland consists of a monolayer of secretory cells composed of two morphologically distinct cell types. Flat, polygonal “main cells” make up 96% of each lobe. The remaining 4% of the cells are large, spherical, vacuole-filled “secondary cells” (Bairati 1968; Bertram, et al. 1992) that are located only at the distal tip of each lobe. The multi-cell state of the AG is not unique. Other secretory organs, such as the pancreas (Brissova et al. 2005) and the epididymis (reviewed in Cornwall 2009), are also composed of multiple cell types, the products of which work together to regulate complex processes (Hermo 1995; Cheung et al. 2005; Pietrement et al. 2006; Kujala et al. 2007).
The two cell types of the Drosophila melanogaster male accessory gland make distinct products (Bertram et al. 1992; Gligorov et al. 2013) that act in a network (Ravi Ram and Wolfner 2009; Findlay et al. 2014) that allows for the seminal sex peptide (SP) to bind to sperm, enter the female sperm storage organs, and subsequently be cleaved from the sperm in storage (Liu and Kubli 2003; Peng et al. 2005a). The binding of SP to sperm and its gradual release by proteolytic cleavage allows its effects to persist long after mating has occurred (Chen and Buhler 1970; Chapman et al. 2003; Liu and Kubli 2003; Peng et al. 2005a). Through this mechanism, SP has been implicated in regulating long-term changes in female egg laying, receptivity, and various other post-mating responses (Chapman et al. 2003; Liu and Kubli 2003; Peng et al. 2005a,b; Avila et al. 2010; Apger-McGlaughon and Wolfner 2013). Collectively, the prolonged post-mating response that is mediated by SP is called the long-term response (LTR) (Peng et al. 2005a; Ravi Ram and Wolfner 2007). While main cells produce SP and other proteins necessary to initiate and maintain the LTR (Styger 1992; Liu and Kubli 2003), secondary cell products are essential to maintain the LTR (Leiblich et al. 2012; Minami et al. 2012; Gligorov et al. 2013). A few of these secondary cell products were identified by examining the cell type expression of known accessory gland proteins (Gligorov et al. 2013), but an unbiased search for other secondary cell products that mediate the PMR has not been done. Previously, we generated an enhancer deletion, iab-6cocu, that removes the expression of the Hox gene, Abdominal-B, from the secondary cells of the male accessory gland. iab-6cocu mutants display abnormal secondary cell morphology and are unable to maintain the LTR in their mates (Gligorov et al. 2013). The three secondary cell proteins known to be important for the long-term storage of SP (CG1652, CG1656, and CG17575) were still produced in iab-6cocu males (Ravi Ram and Wolfner 2009; Gligorov et al. 2013) and transferred to females during mating. This suggests that the inability of iab-6cocu mutants to maintain the LTR in their mates is caused by as-yet-unidentified protein(s) and that the mutant may provide a way to identify new secondary cell products necessary for the regulation of the LTR.
To identify secondary cell genes whose function is needed for the LTR, we used RNA-seq to compare transcripts from iab-6cocu and wild-type accessory glands. Using both unbiased screening methods via secondary cell-specific RNA interference (RNAi) and more targeted bioinformatics approaches to narrow down targets, we selected 20 transcripts that were downregulated in iab-6cocu for further study. Of these, 18 were efficiently knocked down by RNAi, and we characterized their effects on the regulation of the PMR. Nine of these genes are necessary for the normal long-term increase in egg laying and long-term reduction in female receptivity. Furthermore, knockdown of several of these genes impacted the number and size of the secondary cell’s characteristic vacuoles, suggesting that these vacuoles may be essential for the regulation of the PMR. Surprisingly, only one of the nine genes encodes a protein that was previously known to be transferred to females, CG3349. One explanation for this finding is that the iab-6cocu mutant may not primarily affect Sfps directly but instead might work through disrupting other, intracellular, functions such as vacuole-associated secretion.
Materials and Methods
Fly stocks and media
Flies were raised at room temperature (23° ± 1°) in bottles on standard yeast–glucose media (8.2% w/w yeast, 8.2% w/w glucose, 1% w/w agar, 1.2% v/w acid mix). Virgin females were aged 3–5 days from eclosion in groups of 5–12 in vials with added yeast. Male flies were aged 3–5 days from eclosion in groups of 10–20 in vials on standard yeast–glucose media. Fly lines containing a UAS-hairpin construct specific to each gene of interest were obtained from the Vienna Drosophila RNAi Center (VDRC) (Dietzl et al. 2007); line numbers for candidate genes discussed in this article are given in Supporting Information, Table S1 and Table S2. To generate knockdown males, each UAS-hairpin line was crossed to the secondary cell driver iab-6D1-Gal4/CyO (Gligorov et al. 2013); control flies were generated by crossing the driver to the appropriate background line [w[1118];P{attP,y[+],w[3′] (Attp60100)] for KK insertion lines and w1118 for GD insertion lines. The level of knockdown of the targeted gene’s expression, relative to controls, was confirmed by RT-PCR as described in Findlay et al. (2014) except that messenger RNA (mRNA) samples were extracted from dissected accessory glands rather than from whole flies (Table S1). In cases where knockdown was not detected with the iab-6D1-Gal4 driver, which might occur for a gene that is normally expressed in both main and secondary cell types, we also used tubulin-Gal4 to check for the function of the RNAi construct (Table S1). If both drivers suggested that knockdown did not occur, the line was excluded from analysis. To generate GFP expression in the secondary cells, the iab-6D1-Gal4 driver was recombined with a UAS-GFP transgene on the second chromosome (generating w; iab-6D1-Gal4, UAS-GFP) and crossed to RNAi lines as previously described.
mRNA-seq
To control for genetic background and for the PhiC31 insertion that was used to make the iab-6cocu mutant (Gligorov et al. 2013) the iab-5,6rescue line, which integrates a wild-type copy of the iab-6 cis-regulatory region instead of the iab-6cocu mutation, was used as the source of control accessory glands. Total RNA was isolated from 100 pairs of accessory glands per genotype from iab5,6rescue and iab-6cocu (iab-5,6∆5) males using the miRNeasy Mini Kit (catalog no. 217004, Qiagen). Approximately 10 μg of total RNA was obtained per genotype and was sent to Fasteris (Fasteris SA, Geneva) for sequencing on an Illumina sequencer and for bioinformatic analysis. For these experiments only a single round of “paired-end” mRNA sequencing was performed per genotype (Table S3). HiSeq was performed on a Hi-Sequation 2000 using 100-bp reads (1 × 100 + 7). Samples were prepared using the TruSeq SBS v5 kit, and the data analysis pipeline was carried out using HiSeq Control Software V. 1.1.37.8, RTA 1.7.48, CASAVA 1.7. Sequences were aligned to the D. melanogaster genome sequence revision 5.30. Counts were normalized as reads per million by dividing by the total number of reads and multiplying by 1 million. Changes in the expression of candidate genes were verified through in situ hybridization to wild-type and mutant glands.
RNAi screening for genes affecting cellular morphology
For the 61 genes of the 77 downregulated genes for which UAS-short hairpin RNA (shRNA) lines were available, male flies carrying the UAS-shRNA constructs were crossed to iab-6DI-Gal4 and iab-6DI-Gal4, UAS-GFP driver line females (all lines tested are shown in Table S4). The GFP marker, which is excluded from the secondary cell vacuoles, was used as an aid to visualize the cellular phenotype. Driver lines with and without a GFP marker were used separately to eliminate the effect, if any, of the UAS-GFP on the phenotype. For each cross, we analyzed the accessory glands of three, 3-day-old males using Nomarski microscopy for male progeny of iab-6D1-Gal4 mothers and a further three, 3-day-old males using fluorescence microscopy for male progeny from iab-6D1-Gal4, UAS-GFP mothers. Based on our examinations under fluorescence and Nomarski microscopy, we did not observe any effect stemming from the UAS-GFP marker.
Vacuole size was measured using ImageJ v1.48 software (Wayne Rasband, National Institutes of Health) using images taken on a Zeiss Axioplan fluorescent microscope. Statistical significance was determined by ANOVA. The average vacuole diameters in the knockdown flies reported all are statistically different from wild-type controls (iab-6DI-Gal4, UAS-GFP) (P < 0.0005) (Figure S1).
Data analysis for candidate selection
We used publicly available database information such as predicted function, the presence of a predicted signal sequence, and expression pattern to select additional candidates for functional analysis that did not impact cellular morphology. For each gene, the presence or absence of a predicted signal sequence was determined using SignalP (Petersen et al. 2011), and expression patterns outside the accessory gland were obtained from data reported by FlyBase (1999), Fly Atlas (Chintapalli et al. 2007), and ModENCODE (Roy et al. 2010; McQuilton et al. 2012; Young et al. 2012). Genes were counted as being expressed in a given location if at least one of these three sources reported expression there. However, preference was given to genes whose expression in the accessory gland was reported by multiple sources. For genes with no listed function in FlyBase, Pfam (Punta et al. 2012) was used to identify conserved domains where possible and function was proposed accordingly. For Gene Ontology (GO) Enrichment Analysis, the 77 genes that were downregulated in iab-6cocu were run through the Database for Visualization and Integrated Discovery (DAVID) at high stringency using the complete list of genes detected by our RNA-seq run as background (Huang da et al. 2007, 2009).
Fertility/fecundity assays
Fertility/fecundity assays were performed as described in Gligorov et al. (2013) and Findlay et al. (2014). Briefly, 3- to 5-day-old virgin knockdown or control males were singly mated with Canton-S virgin females; males were then removed. Females, kept singly in vials, were transferred to fresh vials daily. The number of eggs laid each day was counted (fecundity) through day 10, and the number of progeny from each vial was also counted (fertility). Wilcoxon nonparametric tests were used to compare results for mates of RNAi and control males in total and on individual days. The overall 10-day trends were analyzed by repeated measures ANOVA (rmANOVA). All statistical analysis was performed with the JMP9 software (SAS Institute, 2010). For confirmation with additional RNAi lines, the same protocol was used except that egg counts were carried out for only 5 days since reduced egg laying was readily detectable in all of the initially tested lines for these genes by day 3.
Receptivity assays
Receptivity assays were performed as described in Gligorov et al. (2013) and Findlay et al. (2014). Briefly, 3- to 5-day-old virgin knockdown and control males were singly mated to Canton-S virgin females. Males were then removed and the females were aged individually in vials. Four days after the mating, a virgin Canton S male was introduced into each vial, and the vial was scored for whether or not the female remated within an hour of male introduction. Comparisons of remating frequency between females mated to either control or RNAi males were conducted using a Wilcoxon ranked-sums test using JMP9 software (SAS Institute, 2010).
Western blots
Females were frozen at 30 min after the start of mating (ASM) in liquid nitrogen or held in vials with yeast–glucose food for 4 days prior to being frozen. Virgin flies were flash-frozen at 3–5 days old in groups of 10–20 in liquid nitrogen. After flash freezing, all samples were stored at −80° prior to dissection. Protein sample preparation and Western blot analyses were performed as in Ravi Ram et al. (2005), Ravi Ram and Wolfner (2009), and Gligorov et al. (2013). Primary antibodies for each of the LTR proteins (Liu and Kubli 2003; Ravi Ram and Wolfner 2009, 2007) were used at the following dilutions: SP (1:2000), CG1656 (1:1000), CG1652 (1:500), and CG9997 (1:2000).
Results
RNA-seq reveals 77 candidate genes downregulated by more than fivefold in the accessory glands of iab-6cocu males
Previously, we discovered that the homeotic gene, Abdominal-B, is specifically expressed in the secondary cells of the accessory gland and that removal of its secondary cell-specific enhancer resulted in morphological defects in the secondary cells and a reduction of the LTR in the mated female (Gligorov et al. 2013). To identify additional secondary cell genes needed for the LTR, we attempted to identify mRNAs whose abundance differed in iab-6cocu males relative to controls using RNAseq. Although RNAseq is generally used to characterize transcriptomes, we decided to use it here strictly as a tool to find new genes for functional testing of phenotypes in the accessory gland. Hence, we performed only RNAseq single runs on wild-type or iab-6cocu accessory glands.
Our HiSeq run yielded 66,943,897 reads: 36,740,061 for iab-5,6rescue (a wild-type line generated by the same genetic manipulation techniques that were used to create iab-6cocu) and 30,203,836 for iab-6cocu, mapping to 8764 genes. Fold differences were calculated by dividing the normalized number of reads per gene of each genotype: iab-5,6rescue/iab-6cocu for “downregulated genes” (which may require Abd-B for high expression in the accessory gland) and the inverse for upregulated genes (which might normally be repressed by Abd-B). Using a cutoff value of 5×, we found that 77 genes were downregulated in iab-6cocu relative to controls and 115 genes were upregulated (for a complete list of detected RNAs, see Table S5; for downregulated genes, see Table S6; and for upregulated genes, see Table S7). For this study, we focused primarily on the downregulated genes, as described below.
Eight Sfps had previously been shown to be necessary to bind SP to sperm, allowing for the LTR. Previously, we showed that five of these “SP network” Sfps, and the SP itself, are present at wild-type levels in the accessory glands of iab-6cocu males (Gligorov et al. 2013). Recently, three additional Sfps were found to be part of the network (Findlay et al. 2014). Because antibodies for these proteins (Intrepid, Antares, and Aquarius) are not available, we examined the abundance of their RNAs in our Hiseq database. We detected no differences in expression between iab-6cocu and control accessory glands for any of these new SP network genes or for the five genes previously analyzed by Western blot (Table S8). These results agree with our previous conclusion that the loss of known LTR Sfps does not underlie the PMR phenotypes observed in mates of iab-6cocu males (Gligorov et al. 2013).
We reasoned that since iab-6cocu flies are defective in SP’s ability to bind to sperm and enter into storage, products necessary for this process to occur are probably depleted or missing from the accessory glands of these males. This is consistent with the kinds of phenotypes seen in males knocked down or null for SP network genes upstream of SP storage (Ravi Ram and Wolfner 2009, 2007; Findlay et al. 2014). Therefore, we focused on the 77 genes that we found to be downregulated in iab-6cocu (Table S6), as those genes might be most expected to include ones encoding proteins needed for the LTR. DAVID analysis for enriched GO terms within the 77 downregulated genes did not identify any significant classes or functions for the proteins encoded by these genes, perhaps in part because the most common predicted function of the genes that we detected was “unknown.” Based on archived experimental and prediction data in FlyBase and conserved protein domains (determined using Pfam), the most common predicted functions for these genes, after unknown (32.5% or 25 genes), were serine-type endopeptidase activity (7.7% or 6 genes), transferase activity (7.7% or 6 genes), sodium:iodide symporter activity (7.7% or 6 genes), and transmembrane transporter function (5.2% or 4 genes). These particular classes of protein are interesting for several reasons. Proteases are a common constituent of seminal fluid in all animals (LaFlamme and Wolfner 2013) and are implicated in the regulation of the PMR in D. melanogaster (Findlay et al. 2014). The predicted transferases that we identified are primarily glycosyltransferases, which can catalyze reactions to generate O-linked or N-linked glycosylation. Glycosylation is often essential for the structure, function, and transport of glycoproteins and glycolipids. Furthermore, the glycosylation state of several essential PMR proteins is abnormal in iab-6cocu males (Gligorov et al. 2013). The presence of sodium:iodide symporters might suggest a role for iodine in secondary cell function or secretions. While only 5 of the 77 genes encode proteins previously known to be transferred in seminal fluid (Table S9), 38 of the 77 predicted proteins (49.4%) have a predicted signal sequence, suggesting that these proteins could be secreted and thus may be previously undetected Sfps (Table S6). The even split between proteins with and without predicted signal sequences suggests that both intra- and extracellular functions of the secondary cells are likely impacted by the iab-6cocu mutation.
Interestingly, very few of the 77 genes that are downregulated in iab-6cocu accessory glands are primarily or exclusively expressed in these glands. This suggests that these genes may be important for general cellular functions or that they may have additional functions outside of reproduction. The most common sites of highest expression of these genes were the Malpighian tubules (32.5% or 25 genes), the testis (15.6% or 12 genes), and the midgut (11.7% or 9 genes) with only 2 genes (2.6%) expressed primarily in the AG. These two AG-specific genes are CG11598 and CG3349. They encode two previously identified Sfps (Ravi Ram et al. 2005) that have not yet been characterized. The high level of Malpighian tubule expression was initially surprising; however, other known Sfps also show expression in this organ. For example, the candidate gene CG13309 (Ravi Ram and Wolfner 2007) is most highly expressed in the Malpighian tubules (Chintapalli et al. 2007). However, CG13309 also has male-biased expression (Roy et al. 2010; McQuilton et al. 2012), is expressed at very low (Chintapalli et al. 2007) to moderate (Celniker et al. 2009) levels in the accessory gland, and encodes a protein known to be transferred to females during mating (Ravi Ram et al. 2005). Thus, we believe that the majority of the genes that we identified were detected based on accessory gland expression differences and were not due to contaminating tissues. The large overlap in expression of candidate genes between the accessory glands and the Malphigian tubule may instead suggest that these organs share some functions, the most likely of which is related to their roles as secretory tissues. A detailed account of the molecular characteristics and mRNA-seq results for the 77 genes can be found in Table S6.
Finally, it is interesting to note that a few of the genes cluster at a specific genetic locus. CG12809, CG33783, CG33784, CG33631, CG5361, and CG33630 are all located next to each other within a 13-kb region of chromosome 3. The genomic colocalization of the genes, together with the RNA-seq results, suggests that these genes may be co-regulated as a gene neighborhood.
Prioritizing candidates for functional screening
Genes that regulate secondary cell morphology:
The iab-6cocu mutation was first identified based on its impact on secondary cell morphology. Males homozygous for this mutation have secondary cells that have a hexagonal shape, much like that of main cells, rather than being spherical. These cells also lack the large vacuoles that are characteristic of normal secondary cells (Gligorov et al. 2013). The cause of these morphology differences and whether they are related to the LTR phenotypes observed in mates of iab-6cocu males is unclear. To begin to investigate this, we screened via knockdown all of the available candidates for cellular morphology phenotypes similar to those seen in iab-6cocu mutants. For each gene for which an shRNA line was available (61 of the 77 genes), knockdown was performed using the iab-6D1-Gal4, UAS-GFP driver line (Gligorov et al. 2013). The iab-6D1-Gal4 driver is a secondary cell-specific driver similar to the previously described iab-6D5-Gal4 driver except that the putative enhancer region controlling GAL4 expression in the secondary cells is 1.1 kb rather than the 2.8-kb region used in the iab-6D5 driver.
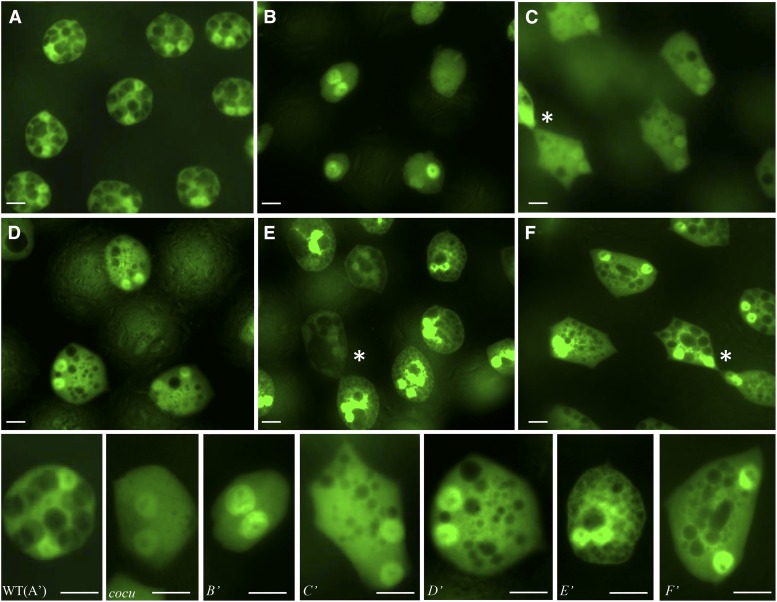
Of the 61 RNAi lines tested, knockdown of five genes—CG7882, CG9509, CG10514, CG14069, and CG31034—resulted in a detectable secondary cell morphology phenotype. For each case, the phenotypes were consistent between individual males but varied slightly between individual cells in a single gland (both more and less severe than the iab-6cocu phenotype) (Figure 1). CG14069 knockdown resulted in the most iab-6cocu-like vacuolar phenotype, where the large vacuoles in the secondary cells were completely absent (Figure 1B). However, CG14069 knockdown also displayed phenotypes not seen in iab6cocu mutants, as the cells within a single gland varied in size and they appeared to partially lose their binucleate character. Knockdown of CG31034 and CG7882 resulted in weaker vacuole phenotypes (Figure 1, C and D) where some large vacuoles were present but were reduced in size [vacuole size: 2.42 ± 1.69 µm (n = 246) (CG31034); 2.40 ± 1.80 µm (n = 178) (CG7882)] and number relative to wild-type AGs [vacuole size: 4.74 ± 1.11 µm (n = 173)]. Knockdown of CG10514 showed an even weaker vacuole phenotype: the number of vacuoles was only slightly reduced, but the general size of the vacuoles was notably smaller [vacuole size 2.87 ± 1.54 µm (n = 243)] (Figure 1F). Finally, knockdown of CG9509 showed a unique secondary cell morphological phenotype characterized by what looked like multiple small vacuoles, which appeared to be interconnected, giving the cells a “grainy” appearance [vacuole size 2.36 ± 2.26 µm (n = 288)] (Figure 1E).
Figure 1.
Knockdown of five genes showed consistent secondary cell morphological phenotypes. (A) GFP fluorescence in an accessory gland expressing UAS-GFP under the control of the iab6DI-Gal4 driver. The GFP signal can be seen exclusively in the secondary cells both in the nuclei and in the cytoplasm. Note that GFP signal is excluded from the vacuoles, which appear as black holes in the secondary cells. (B–F) Accessory glands expressing GFP and RNAi hairpins demonstrating the severity and variability of observed secondary cell morphology phenotypes between lines. The genes knocked down are as follows: (B) CG14069, (C) CG31034, (D) CG7889, (E) CG9509, and (F) CG10514. (A′–F′) Individual cells from A through F that are typical of each knockdown line (C′–F′) have been placed next to each other alongside a wild-type cell (A′) and an example of a secondary cell from the iab-6cocu mutant for ease of comparison. In C, E, and F the asterisk (*) indicates visible cell contacts between secondary cells. Bars, 10 μm for all panels. Average vacuole diameters: control (iab-6DI-Gal4, UAS-GFP) 4.74 ± 1.11 µm; CG31034 2.42 ± 1.69 µm; CG7889 2.40 ± 1.80 µm; CG9509 2.36 ± 2.26 µm; and CG10514 2.87 ± 1.54 µm (all values from these knockdown lines are significantly different from the controls (ANOVA P < 0.0005).
Another cell morphological characteristic of the secondary cells is their round shape; the cells became notably more hexagonal in iab-6cocu mutants (Figure 1). Knockdown of a number of genes affected the cell shape and positioning of the secondary cells within the AG. The clearest examples of this are CG10514 and CG31034: in knockdowns of each of these genes, the secondary cells adopted more angular forms. In other knockdown lines, the cell shape changes were more subtle and might simply be linked to the loss of cell size from the loss of vacuoles. It is interesting to note, however, that many of the knockdown lines also displayed visible secondary cell-to-secondary cell contacts that are not normally seen in wild-type glands, but are seen in iab-6cocu mutants (Figure 1, marked by an asterisk).
Ten more genes—CG5106, CG31198, CG33630, CG9294, CG15406, CG6602, CG12809, CG13538, CG18088, and CG14681—showed less penetrant knockdown phenotypes where morphological defects were seen in only one or two of the three accessory glands dissected (Figure S2). In the glands that had secondary cell phenotypes, most showed a reduction of the size of the vacuoles present compared to wild type. The vacuole sizes within the cells were variable, ranging from barely visible to close to the size of wild-type vacuoles. The remaining candidates screened did not display any secondary cell morphological phenotype upon knockdown, suggesting either insufficient knockdown, functional redundancy, or that the gene is not involved in regulating secondary cell morphology.
To test whether the genes that affected secondary cell morphology also affected the PMR, we performed 5-day egg-laying tests on the 15 genes that displayed some effect on cellular morphology. Four of the knockdown lines tested (CG7882, CG9509, CG14069, and CG15406) showed egg-laying phenotypes (see below). These results suggest that some but not all changes in vacuole number and/or size correlate with LTR defects. Consistent with these conclusions, independent results show that there are different types of vacuoles in secondary cells, with different functions (F. Karch laboratory, unpublished results; Bairati 1968). Based on these initial screens, we selected CG7882, CG9509, CG14069, and CG15406 for further analysis.
Other genes prioritized for functional screening:
The lack of complete congruence between genes affecting secondary cell morphology and ones affecting the PMR led us to hypothesize that some genes needed for the PMR might not have been detected in our cellular morphology screen. Therefore, we also selected several other candidates from the initial 77 most-downregulated genes for functionality testing. We selected these additional candidates based on (1) their predicted function, (2) the presence of a predicted signal sequence in their encoded protein, and (3) confirmed expression in the accessory gland based on FlyAtlas and modENCODE (Chintapalli et al. 2007; Roy et al. 2010; McQuilton et al. 2012). We focused on candidate genes with predicted functions related to post-translational modification of proteins; with predicted roles in sugar transport, binding, or transfer; or with predicted roles in vacuole function (regardless of signal sequence). These functions were selected because our previous study suggested that the accessory glands of iab-6cocu males were abnormal in these processes (Gligorov et al. 2013). We also included a few candidates that did not fall into these categories but encoded proteins with a putative signal sequence that thus might be Sfps, were members of a group of genes that were candidates to be regulated as part of a gene neighborhood, or encoded proteins in functional classes already known to be important for reproduction in flies or other organisms. Based on these selection criteria, we finished with a list of 20 candidate genes for further functional testing via secondary cell-specific RNAi (Table 1). This group included the four genes that were identified in our cellular morphology screen, all of which also met the functional criteria used to select the remaining candidates. Of these 20 candidate genes, we were able to confirm knockdown in the secondary cells via RT-PCR for 18 (Table S1). The remaining 2 candidates were excluded from further analysis.
Table 1. Candidate genes.
| CG no. | Chromosome | ID | Fold decrease | Signal sequence | Function | Highest expression |
|---|---|---|---|---|---|---|
| CG14069 | 2L | CG14069 | — | Yes | Unknown | Testis |
| CG15406 | 2L | CG15406 | — | No | General/sugar transporter | Malpighian tubules |
| CG3285 | 2L | CG3285 | — | No | General/sugar transporter | Malpighian tubules |
| CG33783 | 3R | CG33783 | 1043.24 | No | Unknown | Unknown |
| CG33784 | 3R | CG33784 | 578.00 | Yes | Unknown | Eye |
| CG33631 | 3R | CG33631 | 160.31 | Yes | Unknown | Broad |
| CG11598 | 3R | CG11598 | 66.11 | Yes | Lipase | Accessory gland |
| CG15902 | 3R | Ugt86Dj | 46.04 | Yes | UDP-glucosyl transferase | Midgut |
| CG14376 | 3R | CG14376 | 41.93 | Yes | Solute-binding protein | Broad |
| CG7882 | 2R | CG7882 | 30.01 | No | Transporter, general sugar | Malpighian tubules |
| CG43161 | 3R | Skeletor | 28.02 | Yes | Spindle assembly | — |
| CG9509 | X | CG9509 | 26.31 | No | Glucose-methanol-choline oxidoreductase | Malpighian tubules |
| CG13309 | 3L | CG13309 | 26.31 | Yes | Unknown | Malpighian tubules |
| CG5361 | 3R | CG5361 | 22.20 | No | Alkaline phosphatase | Malpighian tubules |
| CG1112 | 3R | α-Est7 | 21.92 | No | Carboxyl-esterase | Broad |
| CG14292 | 3R | CG14292 | 18.09 | Yes | Unknown | Malpighian tubules |
| CG33630 | 3R | CG33630 | 16.44 | Yes | Unknown | Broad |
| CG14715 | 3R | CG14715 | 7.35 | Yes | cis-transisomerase | Broad |
| CG3349 | 3L | CG3349 | 5.77 | Yes | Unknown | Accessory gland |
| CG18088 | 2L | CG18088 | 5.49 | Yes | Alkaline phosphatase | Salivary gland |
| CG31326 | 3R | CG31326 | 0.38 | Yes | Serine protease | Spermathecae |
We selected candidate genes based on a conglomeration of criteria including fold change, signal sequence, predicted function, expression pattern, and sex-biased expression. Particular preference was given to candidate genes that might be involved with glycosylation, sugar transport, or other post-translational modification to try to determine if these phenotypes in the iab-6cocu mutant relate directly to the LTR. Fold decrease could not be calculated for CG14069, CG15406, and CG3285 since these transcripts were not detectable in iab-6cocu males. Expression data were obtained from Fly Atlas (Chintapalli et al. 2007) or, in the case of male-biased data, from modENCODE (Roy et al. 2010; McQuilton et al. 2012). Tissue-specific expression data were not available for CG43161. The italicized gene, CG31326, is an off-target for the CG15406 RNAi line.
Receptivity suppression is compromised in mates of males knocked down for nine candidate genes
Mates of iab-6cocu males do not exhibit the extended post-mating responses associated with the LTR. In contrast to mates of control males, which remain unreceptive to remating for up to 10 days following an initial mating, mates of iab-6cocu males return to virgin levels of receptivity by 4 days ASM. To test whether any of our candidate genes were needed for the LTR, we crossed virgin Canton-S females to either RNAi or control males and then removed the males. We tested the females individually for re-mating 4 days later, by presenting them with a wild-type male for 1 hr. Nine of the 18 candidate genes tested were needed to maintain decreased female receptivity; mates of males knocked down for CG15902, CG14292, CG3349, CG15406, CG43161, CG3285, CG7882, CG9509, or CG14069 were significantly more receptive to remating at 4 days ASM than mates of control males (Figure 2). Of these positive candidates, only CG3349 was previously known to encode a transferred Sfp (Findlay et al. 2009). These results suggest that the iab-6cocu mutation impacts the expression of multiple genes that encode proteins necessary for males to maintain the suppression of receptivity normally observed in mated females and may play a role in regulating the long-term post-mating response. Alternate lines for eight of the positive candidates confirmed these results; the second available line for CG15902 did not cause knockdown (Figure S3).
Figure 2.
Mates of males knocked down for each of nine secondary-cell-expressed genes have an increased remating rate. The percentage of previously mated females that were willing to remate 4 days ASM to a wild-type male within 1 hr of exposure. At 4 days ASM females initially mated to males knocked down for CG14069, CG14292, CG15406, CG15902, CG3285, CG3349, CG43161, CG7882, and CG9509 are significantly more receptive to courting males compared to mates of control males (Wilcoxon ranked-sums test: *P < 0.05, **P < 0.01, and ***P < 0.0001; control—n = 14–42, average 25; RNAi—n = 14–39, average 25; specific values can be found in Table S2). Knockdown of CG31326, the potential off-target for CG15406, did not show a phenotype, confirming that the phenotype seen with knockdown of CG15406 is not the consequence of off-target effects. These results were confirmed using additional lines for all significant genes where available (Figure S3).
Mates of candidate males lay fewer eggs than mates of control males
To test if the genes uncovered in our receptivity screen also contribute to the ability of males to induce and maintain normal egg laying in their mates, another aspect of the LTR, RNAi or control males were crossed to Canton-S virgin females. Mates of knockdown males for each of the nine candidate genes identified in the receptivity screen showed the normal post-mating increase in egg laying in the first day after mating. However, this increase was significantly lower in mates of CG15406 knockdown males than in controls. Consistent with our receptivity results, mates of knockdowns of each of the candidate genes laid fewer eggs than mates of control males at post-day 1 time points. Thus, the same nine genes that are needed to maintain the reduced receptivity that is characteristic of mated females also play a role in maintaining long term egg laying (Figure 3). The available alternate lines for each of the positive candidates confirmed these results (Figure S3). The necessity of all nine genes in the accessory glands of males for the maintenance of the PMR in their mates suggests that each of these genes is important for the maintenance of long-term post-mating responses in mated females.
Figure 3.
Mates of males knocked down for each of nine secondary-cell-expressed genes also show reduced long-term egg laying. The mean number of eggs laid per female mated to either control males (gray), knockdown males (black), or off-target knockdown males (black dashed line) over a 10-day period. Knockdown of CG15406 in males resulted in a difference in egg laying at 24 hr ASM (Wilcoxon ranked-sums test: P = 0.0042). However, the increase in egg laying observed in mates of CG15406 knockdown males appears to be an off-target effect of CG31326 (dashed line, Wilcoxon ranked-sums test: P = 0.0008) since mates of males knocked down for both genes (CG15406 and the off-target CG31326) or just CG31326 share this effect. There were no other differences at the 24 hr ASM time point across mates of knockdown males compared to controls. Mates of males knocked down for each of the nine candidates lay significantly fewer eggs over 10 days when compared to mates of control males (rmANOVA: *P = <0.05, **P = <0.01, ***P = <0.0001; control: n = 18–27, average 22; knockdown: n = 14–23, average 19; see Table S2 for specific n and P-values). The number of progeny that were produced by each female were also counted so that hatchability (no. of progeny/no. of eggs) could be calculated. No significant differences in hatchability from controls were detected for mates of knockdown males of any of these candidate genes (data not shown). These results were confirmed using additional UAS-RNAi lines and a shorter 5-day egg-laying assay (Figure S4).
Sex peptide storage is abnormal in mates of males knocked down for each of the nine candidate genes
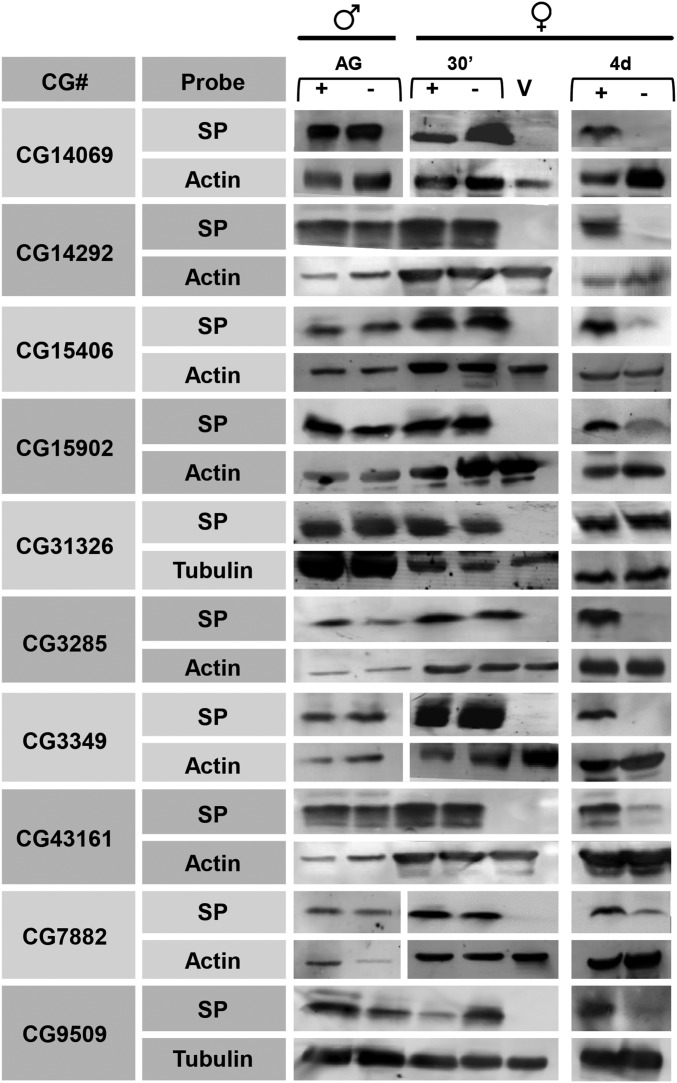
The LTR is due to the binding of SP to, and slow release of SP from, sperm (Peng et al. 2005a). In mates of iab-6cocu males, SP fails to enter storage in the female seminal receptacle and as such is unable to maintain the LTR. Thus, a possible explanation for why knockdown of CG15902, CG14292, CG3349, CG3285, CG14069, CG7882, CG15406, CG9509, and CG43161 in the male impaired the LTR could be impairment of SP storage in mates of knockdown males. To test this hypothesis, we performed Western blots using SP antibodies to detect differences in SP levels in mates of control or knockdown males at 30 min ASM (to detect SP transfer levels) and 4 days ASM, when egg laying and receptivity differences between mates of knockdown and control males are readily apparent and SP levels reflect stored, sperm-bound SP (Ravi Ram and Wolfner 2009; Gligorov et al. 2013; Findlay et al. 2014).
In all cases, equivalent amounts of SP were produced by control and knockdown males and transferred to females. However, mates of males knocked down for any of the nine candidate genes tested show little or no SP retention in storage at the 4-day time point compared to controls (Figure 4). These data indicate that knockdown of any of these nine genes results in a defect in SP retention, thus explaining the defects in the LTR observed in mates of their knockdown males. Protein products of those genes that contain signal sequences could potentially function in the LTR network within the mated female. However, the predicted proteins encoded by CG9509, CG15406, CG7882, and CG3285 either do not contain predicted signal sequences or also contain predicted transmembrane domains. If these proteins are not secreted, they could indirectly influence the LTR by being necessary for proper post-translational modification, secretion, or function of LTR network proteins.
Figure 4.
Sex peptide storage is impaired in mates of males knocked down for each of nine candidate genes. Mates of each of our nine knockdown males have less SP, as detected by antibodies to SP, in their reproductive tract at 4 days ASM. Tubulin or Actin was used as a loading control. Accessory gland extracts from a single male [Control (+) or RNAi (−)] were used as a positive control, and reproductive tract extracts from four virgin females were used as a negative control (V). Reproductive tract (RT) extracts were obtained from females mated to either control (+) or RNAi (−) males at 30 min ASM (2 RTs per) and 4 days ASM (18 RTs per). CG31326, an off-target for the CG15406 VDRC line, was included as a control. All mates of RNAi males have at least as much SP present in their reproductive tracts at 30 min ASM as do mates of control males, indicating normal SP transfer by knockdown males. However, significantly less SP is present in the female reproductive tract at 4 days ASM to any of the RNAi males, except for the off-target control CG31326, as expected, than is present in mates of control males, suggesting that SP storage is impaired.
Discussion
Using RNA-seq we identified 77 transcripts that are downregulated by at least fivefold in the absence of Abd-B expression in the secondary cells of the male accessory gland. We chose a subset of these genes for further study based on expression pattern, predicted function, the presence of a signal sequence, or modifications in cellular morphology upon knockdown. We found that at least nine genes (CG14292, CG3349, CG14069, CG7882, CG15406, CG15902, CG43161, CG9509, and CG3285) are required for the secondary cell’s function in maintaining the post-mating response. Knockdown of each of these nine gene candidates in the secondary cells resulted in a reduction in stored SP, consistent with their effect on the LTR. Four of our candidates were also necessary for the characteristic vacuoles and/or outward cellular morphology of the secondary cells.
Although we concentrated on the verification of the downregulated genes from our RNA-seq screen, we actually found more genes upregulated by >5× in our experiment than downregulated by >5× (115 vs. 73 genes). Among upregulated genes are a few genes previously identified as encoding constituents of the seminal fluid, such as Acp24A4 (Findlay et al. 2008), PEBme (Lung and Wolfner 2001), and PEBII (Bretman et al. 2010). Interestingly, PEBme (also known as Ejaculatory bulb protein of melanogaster or Ebp) and PEBII (also known as Ejaculatory bulb protein II or EbpII) are genes known be expressed in the ejaculatory bulb, but are 301 and 438× overexpressed, respectively, in iab-6cocu mutants. Thus, we hypothesize that the iab-6cocu mutation may cause secondary cell expression of genes whose transcription is normally repressed in those cells. Given that iab-6cocu mutants disrupt Abd-B expression in the secondary cells and that Abd-B is a Hox gene that may contribute to the determination of secondary cell identity, our result may indicate that the iab-6cocu phenotype could due to a failure to completely differentiate from a precursor cell type. Alternatively, it is also possible that secondary cells might influence the expression of genes in other cells through intercellular signaling. This latter hypothesis is supported by our RNAseq data for genes like CG11598. In wild-type accessory glands, CG11598 is expressed at high levels in both main cells and secondary cells (verified by in situ hybridization; data not shown) but is 66× downregulated in the iab-6cocu flies. As secondary cells represent only 4% of the cells in the gland, this extremely large downregulation can be accounted for only by a change in CG11598 expression in the main cells.
Proteins important for multiple processes underlie the LTR defects in mates of iab-6cocu males
Based on our results, it is clear that the iab-6cocu mutation impacts a wide array of systems in the secondary cell that have consequences for the LTR. Of the nine candidates, five encode predicted secreted proteins, and only CG3349 is known to be transferred to females during mating (Findlay et al. 2008). However, the other four proteins may not have been detectable given that the secondary cells make up ∼4% of the accessory gland, and as such these proteins may be present in low abundance. These proteins are potential candidates for being members of the network that binds SP to sperm.
Two of these proteins, encoded by CG3349 and CG14292, have no domains predictive of their function, but the other three predicted proteins do (Drysdale 2008; Punta et al. 2012). CG14069 is predicted to have cytokine activity based on its similarity to the Drosophila growth-blocking peptide, a cytokine that regulates stressor-induced antimicrobial peptide expression in insects (Tsuzuki et al. 2012, 2014). While cytokines have not been previously described as transferred seminal fluid proteins in Drosophila, cytokines are present in the seminal fluid of most mammals (Gruschwitz et al. 1996; Dousset et al. 1997; Vera et al. 2003). In mammals, seminal cytokines, particularly TGF-β in human and mouse (Sharkey et al. 2012a), are activated in the seminal fluid and have been shown to interact with uterine and cervical epithelial cells to initiate pro-inflammatory cytokine synthesis and leukocyte recruitment in the female (Sharkey et al. 2007, 2012a,b). Sfps, including the sex peptide, also elicit an immune response in Drosophila (Peng et al. 2005b), suggesting a possible role for CG14069 in regulating this process. How CG14069 functions in SP storage and dynamics will require future study.
CG43161 (skeletor) encodes four alternative splice products, at least one of which is a component of the intracellular mitotic spindle matrix. The Skeletor-associated spindle is composed of filaments that do not contain actin or tubulin (Bairati 1968; Walker et al. 2000; Zheng 2010). Since there is no apparent difference in accessory gland size or secondary cell number in iab-6cocu males (Gligorov et al. 2013), it seems unlikely that the reduction in CG43161 seen in these males leads to defects in cellular division. Non-tubulin-containing filaments were previously reported inside the vacuoles of the secondary cell as well as in the lumen of the accessory gland (Bairati 1966, 1968; Chen 1984) and appear to be transferred to females during mating (Bairati 1966). It will be intriguing to test whether Skeletor comprises these filaments and thus contributes to the PMR. Alternatively, three secreted splice variants of CG43161 contain DMB and DOMON domains, which are associated with modifying cell-surface proteins (Aravind 2001; Iyer et al. 2007) and could potentially thereby contribute to the function of the LTR network.
CG15902 encodes a protein predicted to have glycosyltransferase activity. At least two Sfps that are part of the SP pathway are known to be glycosylated: the lectins CG1656 and CG1652 (Gligorov et al. 2013; Ravi Ram and Wolfner 2007, 2009). The substrates for these proteins and the importance of their glycosylation state are currently unknown. CG15902 may play a role in regulating the sugars necessary for these seminal lectins to bind their targets or may suggest a regulatory role for glycosylation in the accessory gland. Further work looking into the substrates of CG15902 is needed to determine how it may help regulate the LTR.
The four other new genes needed for the LTR (CG9509, CG15406, CG7882, and CG3285) encode predicted intracellular or transmembrane proteins. Thus their likely function is in the production, secretion, or modification of secreted LTR proteins needed for the storage of SP. CG9509 is a member of the GMC oxidoreductase family of enzymes and has predicted choline dehydrogenase activity (Takeuchi et al. 2005). Other members of this family in mammals are important for reproduction, including the canonical choline dehydrogenase, which is essential for normal sperm motility and male fertility (Johnson et al. 2010, 2012).
The remaining three genes are predicted to encode members of the major facilitator superfamily, the largest known superfamily of secondary transport carriers (reviewed in Pao et al. 1998 and Reddy et al. 2012); CG15406, a predicted fructose transporter; CG7882, a glucose transporter; and CG3285, a general sugar transporter (FlyBase). The role of such sugar transporters in the LTR is unclear. One possibility is that they, potentially along with the glycosyltransferase CG15902, may facilitate the glycosylation of Sfps. Consistent with this idea, iab-6cocu males impact the stability and glycosylation of some LTR network proteins (Gligorov et al. 2013). Whether these differences are directly related to the SP retention defect seen in mates of iab-6cocu males is unclear. Accordingly, we tested mates of males knocked down for all 4 genes, as well as the 14 other candidate genes, by Western blot using antibodies to the proteins known to be abnormally glycosylated (CG1656/CG1652) in iab-6cocu males or processed (CG9997) in their mates. No differences in CG9997 processing or in CG1656/CG1652 apparent molecular weight (Figure S5 and Figure S6) were detected in the mates of males knocked down for any of our candidate genes. Based on these results, we cannot draw any definitive conclusions about the relationship between the glycosylation of CG1656/CG1652 or the processing of CG9997 and SP storage. However, these results suggest that none of our candidate genes are involved in either process. Alternatively, these sugar transport proteins could affect the level of sugars in the ejaculate, promoting sperm motility, as occurs in mammals and honey bees (Poole and Edwards 1970; Caballero Peregrin et al. 1979; Hinton and Howards 1982; Hinton et al. 1983; Gonzales 2001; Gonzales and Villena 2001; Collins et al. 2006; King et al. 2011; Vivas-Acevedo et al. 2011), or possibly affecting the activity of SPR-expressing neurons, which express the fructose-responsive gustatory receptor 43a (Yapici et al. 2008; Miyamoto and Amrein 2014).
When changes in cellular morphology correlate with LTR differences, vacuole number is reduced
The original phenotype that led to the identification of iab-6cocu as a secondary cell mutant was a dramatic change in cellular morphology from large, spherical-shaped cells filled with large vacuoles to the more main-cell-like small, hexagonal, vacuole-less cells. We were able to identify multiple genes whose products are required for the normal overall cell morphology. Knockdown of five genes (CG9509, CG14069, CG7882, CG31034, and CG10514) consistently affects vacuole number/size and alters the external cellular shape. We found that three of these genes, CG9509, CG14069, and CG7882, also affected the male’s ability to induce the LTR. The lack of perfect correlation between these morphological phenotypes and changes in the LTR cannot be explained simply by the severity of the morphological changes. While the knockdown of CG10514 was much milder than the three lines that displayed LTR defects, knockdown of CG31034 gave a quite strong phenotype, affecting both the vacuole number/size and the external cellular shape. One possible way to explain part of this discrepancy could be that there are multiple types of vacuoles. Indeed, electron microscopy work performed in the 1960s (Bairati 1968) showed at least two distinct types of vacuoles in the secondary cells: one containing electron dense “filaments” and the other containing less dense material. Thus it is possible that knockdown of some of these gene products affects a class of vacuole that is important for the LTR, while the others affect a different class of vacuoles that do not play a definable role in the LTR pathway. CG31034 knockdown has a few vacuoles of close-to-normal size, but lacks most of the vacuoles seen in a normal secondary cell. Perhaps the remaining vacuoles are the ones important for the PMR.
How each of these five genes influences vacuole formation is less clear. One probable hypothesis is that the loss of vacuoles is only a readout of a more major change in the biology of the cells. This can perhaps be evidenced by the general changes in cell shape seen with the knockdown of some candidates. Normally, secondary cells are large, “pear-like” cells (Bairati 1968) sticking out of the main cell monolayer into the lumen. Although some of the effect on cell shape can be attributed simply to a loss of cytoplasmic volume from vacuole loss, knockdown of CG31034 and CG10514 creates prominent changes in cell shape that cannot be attributed solely to a reduction in vacuole numbers, which are less affected than in other knockdown lines. In particular, the connections often seen between secondary cells after knockdown of these genes, something that is not observed in wild-type glands, seem to indicate that the adherence properties (and maybe the cellular polarity) of these cells may be different. Given the predicted functions of CG31034 (endopeptidase) and CG10514 (phosphotransferase), cell adhesion or polarity might be modulated by protein modifications predicted to be mediated by these candidates. In the case of the predicted cytokine, CG14069, there is some evidence that cytokines can influence the vacuole formation that leads to endothelial tubes in human endothelial cells in vitro (Bayless 2000; Senger 1996) and also play a role in regulating the formation of autophagic vacuoles in macrophages (Harris 2007). However, given the multifaceted effect of the knockdown of this gene on cell size, vacuole number and nuclei number, a specific effect on vacuole formation seems less likely. It is possible, for example, that CG14069 instead might be important simply to preserve the secondary cell identity. That this effect is not seen in iab-6cocu mutants could be attributed the complexity of the iab-6cocu phenotype, which may somehow compensate for the effects of CG14069 knockdown through the regulation of other genes. More work is needed to determine the specific function of these vacuoles beyond that they have some important role in regulating the PMR.
Conclusion
Previously, we observed that the iab-6cocu mutation influences post-translational modification of some Sfps, cellular morphology, and the maintenance of the LTR. We have demonstrated that at least nine individual genes that are downregulated in the accessory gland of iab-6cocu males encode proteins that are necessary for maintenance of SP in the mated female, and thus for the LTR. Knockdown of three of these genes also severely impacts the number of vacuoles present in the secondary cells and one mildly impacts both the vacuole number and the shape of these cells. Our results suggest that these phenotypes are interrelated but genetically separable and may argue for the importance of some secondary cell vacuoles in the maintenance of the LTR. Furthermore, we conclude that the iab-6cocu mutation impacts the expression of genes required for multiple secondary cell functions, beyond simply the expression and secretion of transferred seminal fluid proteins, that are essential for the maintenance of the long-term response.
Acknowledgments
We thank Eric Alani and Dan Barbash for comments and suggestions during the initial stages of this research and manuscript; Clement Chow for suggestions on figure organization; Frank Avila and anonymous reviewers for helpful comments on the manuscript; the members of the Karch and Wolfner laboratories for helpful suggestions during the course of this research; Yohan El Bali, Jean-Michel Gibert, and Vincent Mayer for their help during the initial steps of the screening performed in Geneva; and Elodie Prince for communicating unpublished observations. We acknowledge the State of Geneva, the Swiss National Fund for Research (grant no. 310003A_149634) and the Fondation Claraz for their support of the Karch laboratory and National Institutes of Health grants R01-HD038921 (to M.F.W.) and R01-HD059060 (to M.F.W.) and Andrew Clark for support of this work.
Footnotes
Communicating editor: B. P. Lazzaro
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.181644/-/DC1.
Literature Cited
- Apger-McGlaughon J., Wolfner M. F., 2013. Post-mating change in excretion by mated Drosophila melanogaster females is a long-term response that depends on sex peptide and sperm. J. Insect Physiol. 59: 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L., 2001. DOMON: an ancient extracellular domain in dopamine beta-monooxygenase and other proteins. Trends Biochem. Sci. 26(9): 524–526. [DOI] [PubMed] [Google Scholar]
- Avila F. W., Ravi Ram K., Bloch Qazi M. C., Wolfner M. F., 2010. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics 186(2): 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairati A., 1966. Filamentous structures in spermatic fluid of Drosophila melanogaster. J. Microsc. (Paris) 5: 265. [Google Scholar]
- Bairati, A. (1968). Structure and ultrastructure of the male reproductive system in Drosophila melanogaster (English trans.) Monitore Zoologico Italiano 2: 105–182.
- Bayless K. J., Salazar R., Davis G. E., 2000. RGD-Dependent Vacuolation and Lumen Formation Observed during Endothelial Cell Morphogenesis in Three-Dimensional Fibrin Matrices Involves the αvβ3 and α5β1 Integrins. The American Journal of Pathology. 156(5): 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram M. J., Akerkar G. A., Ard R. L., Gonzalez C., Wolfner M. F., 1992. Cell type-specific gene expression in the Drosophila melanogaster male accessory gland. Mech. Dev. 38(1): 33–40. [DOI] [PubMed] [Google Scholar]
- Bretman A., Lawniczak M., Boone J., Chapman T., 2010. A mating plug protein reduces early female remating in Drosophila melanogaster. J. Insect Physiol. 56(1): 107–113. [DOI] [PubMed] [Google Scholar]
- Brissova M., Fowler M. J., Nicholson W. E., Chu A., Hirshberg B., et al. , 2005. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 53(9): 1087–1097. [DOI] [PubMed] [Google Scholar]
- Caballero Peregrin B., Gonzalez Guerbolini C., Farinas Fernandez F., 1979. Fructose concentration in sperm liquid and its correspondence with sperm motility: vertical progression of the spermatozoons and the Botella-Casares index. (English trans.) Acta Ginecol. (Madr.) 33(9): 367–372. [PubMed] [Google Scholar]
- Celniker S. E., Dillon L. A., Gerstein M. B., Gunsalus K. C., Henikoff S. et al, 2009. Unlocking the secrets of the genome. Nature 459(7249): 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T., Bangham J., Vinti G., Seifried B., Lung O., et al. , 2003. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl. Acad. Sci. USA 100(17): 9923–9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. S., 1984. The functional morphology and biochemistry of insect male accessory glands and thier secretions. Annu. Rev. Entomol. 29: 233–255. [Google Scholar]
- Chen P. S., Buhler R., 1970. Isolation and function of sex peptide in Drosophila melanogaster. (English trans.) Rev. Suisse Zool. 77(3): 548–554. [PubMed] [Google Scholar]
- Cheung K. H., Leung G. P., Leung M. C., Shum W. W., Zhou W. L., et al. , 2005. Cell-cell interaction underlies formation of fluid in the male reproductive tract of the rat. J. Gen. Physiol. 125(5): 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A., 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39(6): 715–720. [DOI] [PubMed] [Google Scholar]
- Collins A. M., Caperna T. J., Williams V., Garrett W. M., Evans J. D., 2006. Proteomic analyses of male contributions to honey bee sperm storage and mating. Insect Mol. Biol. 15(5): 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall G. A., 2009. New insights into epididymal biology and function. Hum. Reprod. Update 15(2): 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448(7150): 151–156. [DOI] [PubMed] [Google Scholar]
- Dousset B., Hussenet F., Daudin M., Bujan L., Foliguet B., et al. , 1997. Seminal cytokine concentrations (IL-1beta, IL-2, IL-6, sR IL-2, sR IL-6), semen parameters and blood hormonal status in male infertility. Hum. Reprod. 12(7): 1476–1479. [DOI] [PubMed] [Google Scholar]
- Drysdale R., 2008. FlyBase: a database for the Drosophila research community. Methods Mol. Biol. 420: 45–59. [DOI] [PubMed] [Google Scholar]
- Findlay G. D., Yi X. H., MacCoss M. J., Swanson W. J., 2008. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 6(7): 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay G. D., MacCoss M. J., Swanson W. J., 2009. Proteomic discovery of previously unannotated, rapidly evolving seminal fluid genes in Drosophila. Genome Res. 19(5): 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay G. D., Sitnik J. L., Wang W., Aquadro C. F., Clark N. L., et al. , 2014. Evolutionary rate covariation identifies new members of a protein network required for Drosophila melanogaster female post-mating responses. PLoS Genet. 10(1): e1004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FlyBase Consortium , 1999. The FlyBase database of the Drosophila Genome Projects and community literature. Nucleic Acids Res. 27(1): 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gligorov D., Sitnik J. L., Maeda R. K., Wolfner M. F., Karch F., 2013. A novel function for the Hox gene Abd-B in the male accessory gland regulates the long-term female post-mating response in Drosophila. PLoS Genet. 9(3): e1003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales G. F., 2001. Function of seminal vesicles and their role on male fertility. Asian J. Androl. 3(4): 251–258. [PubMed] [Google Scholar]
- Gonzales G. F., Villena A., 2001. True corrected seminal fructose level: a better marker of the function of seminal vesicles in infertile men. Int. J. Androl. 24(5): 255–260. [DOI] [PubMed] [Google Scholar]
- Gruschwitz M. S., Brezinschek R., Brezinschek H. P., 1996. Cytokine levels in the seminal plasma of infertile males. J. Androl. 17(2): 158–163. [PubMed] [Google Scholar]
- Harris J., De Haro S. A., Master S. S., Keane J., Roberts E. A., Delgado M H., et al. , 2007. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 27(3): 505–17. [DOI] [PubMed] [Google Scholar]
- Hermo L., 1995. Structural features and functions of principal cells of the intermediate zone of the epididymis of adult rats. Anat. Rec. 242(4): 515–530. [DOI] [PubMed] [Google Scholar]
- Hinton B. T., Howards S. S., 1982. Rat testis and epididymis can transport [3H] 3-O-methyl-D-glucose, [3H] inositol and [3H] alpha-aminoisobutyric acid across its epithelia in vivo. Biol. Reprod. 27(5): 1181–1189. [DOI] [PubMed] [Google Scholar]
- Hinton B. T., Hernandez H., Howards S. S., 1983. The male antifertility agents alpha chlorohydrin, 5-thio-D-glucose, and 6-chloro-6-deoxy-D-glucose interfere with sugar transport across the epithelium of the rat caput epididymidis. J. Androl. 4(3): 216–221. [DOI] [PubMed] [Google Scholar]
- Huang da, W., B. T. Sherman, Q. Tan, J. Kir, D. Liu et al, 2007 “DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists.” Nucleic Acids Res. 35(Web Server issue): W169–W175. [DOI] [PMC free article] [PubMed]
- Huang da , W., B. T. Sherman, and R. A. Lempicki, 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4(1): 44–57. [DOI] [PubMed] [Google Scholar]
- Iyer L. M., Anantharaman V., Aravind L., 2007. The DOMON domains are involved in heme and sugar recognition. Bioinformatics 23(20): 2660–2664. [DOI] [PubMed] [Google Scholar]
- JMP, Version 9. 2010 SAS Institute Inc., Cary, NC.
- Johnson A. R., Craciunescu C. N., Guo Z., Teng Y. W., Thresher R. J., et al. , 2010. Deletion of murine choline dehydrogenase results in diminished sperm motility. FASEB J. 24(8): 2752–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R., Lao S., Wang T., Galanko J. A., Zeisel S. H., 2012. Choline dehydrogenase polymorphism rs12676 is a functional variation and is associated with changes in human sperm cell function. PLoS One 7(4): e36047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M., Eubel H., Millar A. H., Baer B., 2011. Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. J. Insect Physiol. 57(3): 409–414. [DOI] [PubMed] [Google Scholar]
- Kujala M., Hihnala S., Tienari J., Kaunisto K., Hastbacka J., et al. , 2007. Expression of ion transport-associated proteins in human efferent and epididymal ducts. Reproduction 133(4): 775–784. [DOI] [PubMed] [Google Scholar]
- LaFlamme B. A., Wolfner M. F., 2013. Identification and function of proteolysis regulators in seminal fluid. Mol. Reprod. Dev. 80(2): 80–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiblich A., Marsden L., Gandy C., Corrigan L., Jenkins R., et al. , 2012. Bone morphogenetic protein- and mating-dependent secretory cell growth and migration in the Drosophila accessory gland. Proc. Natl. Acad. Sci. USA 109(47): 19292–19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Kubli E., 2003. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 100(17): 9929–9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung O., Wolfner M. F., 2001. Identification and characterization of the major Drosophila melanogaster maing plug protein. Insect Biochem. Mol. Biol. 31(6): 543–551. [DOI] [PubMed] [Google Scholar]
- McQuilton P., St. Pierre S. E., Thurmond J., 2012. FlyBase 101: the basics of navigating FlyBase. Nucleic Acids Res. 40(Database issue): D706–D714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami R., Wakabayashi M., Sugimori S., Taniguchi K., Kokuryo A., et al. , 2012. The homeodomain protein defective proventriculus is essential for male accessory gland development to enhance fecundity in Drosophila. PLoS One 7(3): e32302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Amrein H., 2014. Diverse roles for the Drosophila fructose sensor Gr43a. Fly (Austin) 8(1): 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao S. S., Paulsen I. T., Saier M. H., Jr, 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62(1): 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Chen S., Busser S., Liu H., Honegger T., et al. , 2005a Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr. Biol. 15(3): 207–213. [DOI] [PubMed] [Google Scholar]
- Peng J., Zipperlen P., Kubli E., 2005b Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr. Biol. 15(18): 1690–1694. [DOI] [PubMed] [Google Scholar]
- Petersen T. N., Brunak S., von Heijne G., Nielsen H., 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8(10): 785–786. [DOI] [PubMed] [Google Scholar]
- Pietrement C., Sun-Wada G. H., Silva N. D., McKee M., Marshansky V., et al. , 2006. Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol. Reprod. 74(1): 185–194. [DOI] [PubMed] [Google Scholar]
- Poole H. K., Edwards J. F., 1970. Induction of motility in honey bee (Apis mellifera L.) spermatozoa by sugars. Experientia 26(8): 859–860. [DOI] [PubMed] [Google Scholar]
- Punta M., Coggill P. C., Eberhardt R. Y., Mistry J., Tate J., et al. , 2012. The Pfam protein families database. Nucleic Acids Res. 40(Database issue): D290–D301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K. R., Wolfner M. F., 2007. Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet. 3(12): e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K. R., Wolfner M. F., 2009. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc. Natl. Acad. Sci. USA 106(36): 15384–15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K., Ji S., Wolfner M. F., 2005. Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem. Mol. Biol. 35(9): 1059–1071. [DOI] [PubMed] [Google Scholar]
- Reddy V. S., Shlykov M. A., Castillo R., Sun E. I., Saier M. H., Jr, 2012. The major facilitator superfamily (MFS) revisited. FEBS J. 279(11): 2022–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Ernst J., Kharchenko P. V., Kheradpour P., Negre N., Eaton M. L., et al. , 2010. modENCODE ConsortiumS. Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science 330(6012): 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger D. R., 1996. Molecular framework for angiogenesis: a complex web of interactions between extravasated plasma proteins and endothelial cell proteins induced by angiogenic cytokines. Amer. J. Path. 149(1): 1–7. [PMC free article] [PubMed] [Google Scholar]
- Sharkey D. J., Macpherson A. M., Tremellen K. P., Robertson S. A., 2007. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol. Hum. Reprod. 13(7): 491–501. [DOI] [PubMed] [Google Scholar]
- Sharkey D. J., Macpherson A. M., Tremellen K. P., Mottershead D. G., Gilchrist R. B., et al. , 2012a TGF-beta mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J. Immunol. 189(2): 1024–1035. [DOI] [PubMed] [Google Scholar]
- Sharkey D. J., Tremellen K. P., Jasper M. J., Gemzell-Danielsson K., Robertson S. A., 2012b Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J. Immunol. 188(5): 2445–2454. [DOI] [PubMed] [Google Scholar]
- Sirot L. K., LaFlamme B. A., Sitnik J. L., Rubinstein C. D., Avila F. W., et al. , 2009. Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Adv. Genet. 68: 23–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styger, D., 1992 Molekulare Analyse des Sexpeptidgens aus Drosophila melanogaster. Ph.D. Thesis, University of Zurich, Zurich, Switzerland. [Google Scholar]
- Takemori N., Yamamoto M. T., 2009. Proteome mapping of the Drosophila melanogaster male reproductive system. Proteomics 9(9): 2484–2493. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Rigden D. J., Ebrahimi B., Turner P. C., Rees H. H., 2005. Regulation of ecdysteroid signalling during Drosophila development: identification, characterization and modelling of ecdysone oxidase, an enzyme involved in control of ligand concentration. Biochem. J. 389(Pt 3): 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki S., Ochiai M., Matsumoto H., Kurata S., Ohnishi A., et al. , 2012. Drosophila growth-blocking peptide-like factor mediates acute immune reactions during infectious and non-infectious stress. Sci. Rep. 2: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki S., Matsumoto H., Furihata S., Ryuda M., Tanaka H., et al. , 2014. Switching between humoral and cellular immune responses in Drosophila is guided by the cytokine GBP. Nat. Commun. 5: 4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera O., Vasqucz L. A., Munoz M. G., 2003. Semen quality and presence of cytokines in seminal fluid of bull ejaculates. Theriogenology 60(3): 553–558. [DOI] [PubMed] [Google Scholar]
- Vivas-Acevedo G., Lozano-Hernandez R., Camejo M. I., 2011. Markers of accessory sex glands function in men with varicocele, relationship with seminal parameters. Can. J. Urol. 18(5): 5884–5889. [PubMed] [Google Scholar]
- Walker D. L., Wang D., Jin Y., Rath U., Wang Y., et al. , 2000. Skeletor, a novel chromosomal protein that redistributes during mitosis provides evidence for the formation of a spindle matrix. J. Cell Biol. 151(7): 1401–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N., Kim Y. J., Ribeiro C., Dickson B. J., 2008. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451(7174): 33–37. [DOI] [PubMed] [Google Scholar]
- Young R. S., Marques A. C., Tibbit C., Haerty W., Bassett A. R., et al. , 2012. Identification and properties of 1,119 candidate lincRNA loci in the Drosophila melanogaster genome. Genome Biol. Evol. 4(4): 427–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., 2010. A membranous spindle matrix orchestrates cell division. Nat. Rev. Mol. Cell Biol. 11(7): 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]