Abstract
Lipids play a pivotal role in embryogenesis as structural components of cellular membranes, as a source of energy, and as signaling molecules. On the basis of a collection of temperature-sensitive embryonic lethal mutants, a systematic database search, and a subsequent microscopic analysis of >300 interference RNA (RNAi)–treated/mutant worms, we identified a couple of evolutionary conserved genes associated with lipid storage in Caenorhabditis elegans embryos. The genes include cpl-1 (cathepsin L–like cysteine protease), ccz-1 (guanine nucleotide exchange factor subunit), and asm-3 (acid sphingomyelinase), which is closely related to the human Niemann-Pick disease–causing gene SMPD1. The respective mutant embryos accumulate enlarged droplets of neutral lipids (cpl-1) and yolk-containing lipid droplets (ccz-1) or have larger genuine lipid droplets (asm-3). The asm-3 mutant embryos additionally showed an enhanced resistance against C band ultraviolet (UV-C) light. Herein we propose that cpl-1, ccz-1, and asm-3 are genes required for the processing of lipid-containing droplets in C. elegans embryos. Owing to the high levels of conservation, the identified genes are also useful in studies of embryonic lipid storage in other organisms.
Keywords: C. elegans, embryonic lipid droplets, embryo development, essential genes, yolk
AFTER fertilization,an embryo undergoes cell cleavage, cellular differentiation, gastrulation, and morphogenesis. These fundamental processes require the proper metabolism of carbohydrates and lipids (Biggers et al. 1967; Harvey and Orbidans 2011; Leese 2012; Minevich et al. 2012). For example, the maturation of oocytes and developing embryos depends on the metabolism of glucose and pyruvate (Gardner et al. 2000; Sutton-McDowall et al. 2010). However, the exact functions of lipids in embryogenesis are not clear (Dunning et al. 2010, 2014; McKeegan and Sturmey 2011). Generally, lipids function as rich sources of energy, signaling molecules, and structural components of plasma and organelle membranes. Given their diverse and important functions, the formation, accumulation, and storage of lipids during oogenesis and embryogenesis have to be precisely regulated. Females of nearly all oviparous species synthesize vitellogenin, a yolk precursor lipoprotein, which is incorporated in oocytes (Byrne et al. 1989; Chen et al. 1997; Sappington and Raikhel 1998; Wallace 1985). In insects, vitellogenins are synthesized primarily in fat cells (Pan et al. 1969; Tufail and Takeda 2009); in vertebrates, the lipoprotein originates from the liver (Deeley et al. 1975; Wangh and Knowland 1975; Wahli et al. 1981; Mosconi et al. 2002). Yolk complexes undergo catabolism in order to provide raw materials, such as amino acids, carbohydrates, and lipids, for the developing embryo (Reimer and Crawford 1995; Jorgensen et al. 2009). In mammals, lipids are also directly provided by cumulus cells surrounding the maturing oocyte (Gilchrist et al. 2008; Prates et al. 2014). Inside the oocyte and embryo, yolk-storing platelets and other lipid-containing droplets have been observed, but their mechanistic roles and significance have yet to be elucidated (Reimer and Crawford 1995; Grant and Hirsh 1999; McEvoy et al. 2000; Ferguson and Leese 2006; Aardema et al. 2011).
The free-living nematode Caenorhabditis elegans serves as an excellent genetic model organism for the study of diverse mechanisms, including embryonic development and lipid metabolism (Byerly et al. 1976; Hodgkin and Barnes 1991; Muschiol et al. 2009; Harvey and Orbidans 2011). Most studies have examined lipid metabolism in adult worms (Ashrafi et al. 2003; Mullaney and Ashrafi 2009; Watts 2009; Klapper et al. 2011;). In the C. elegans embryo, lipid metabolism has been studied primarily with respect to the processing of yolk platelets. C. elegans vitellogenins are synthesized in the intestine of adult hermaphrodites, secreted into the pseudocoelomic space, and transported to the oocytes in the proximal gonadal arm (Kimble and Sharrock 1983; Hall et al. 1999; Kuo et al. 2013). The growing oocyte takes up plenty of yolk through receptor-mediated endocytosis and stores the yolk in yolk platelets (Sharrock et al. 1990; Grant and Hirsh 1999). In C. elegans, yolk seems to be only partially degraded during embryogenesis, as evidenced by the presence of yolk particles in larval intestines (Bossinger and Schierenberg 1992, 1996; Grant and Hirsh 1999). Further, lipid-containing droplets have been partially characterized in the C. elegans embryo (Levitte et al. 2010; Ehmke et al. 2014). In this study, we used C. elegans to identify genes that function in the processing of lipid-containing droplets, especially in the embryo. Our study identified candidate genes, including cpl-1 (cathepsin L–like cysteine protease), ccz-1 (guanine nucleotide exchange factor subunit), and asm-3 (acid sphingomyelinase 3). These genes were further characterized.
Materials and Methods
C. elegans strains and maintenance
Bristol N2 was used as a wild-type reference strain. The mutant strains rrf-3(pk1426), asm-3(ok1744), hjIs67[atgl-1p::atgl-1::GFP], hjls9[pges-1::glo-1::GFP], wels15[pie-1p::GFP::eea-1(FYVEx2)+unc-119(+)], and pwls23[vit-2::GFP] were provided by the C. elegans Genetic Center (CGC), which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). The strains asm-3(tm2384), asm-1(tm5267), asm-2(tm3764), and hosl-1(tm2369) were received from the National BioResource Project (NBRP). Twenty-eight temperature-sensitive mutants (Supporting Information, Table S1) were generated in the laboratory of R. Schnabel (TU Braunschweig) (R. Schnabel et al., unpublished material). All mutant strains were backcrossed to wild-type N2. ccz-1(t3403); atgl-1p::atgl-1::GFP, ccz-1(t3213); pwls23[vit-2::GFP], ccz-1(t3403); pwls23[vit-2::GFP], ccz-1(t3403); hjls9[pges-1::glo-1::GFP], cpl-1(t3423); atgl-1p::atgl-1::GFP, cpl-1(t3423); pwls23[vit-2::GFP], asm-3(ok1744); atgl-1p::atgl-1::GFP, asm-3(ok1744); pwls23[vit-2::GFP], and asm-3(ok1744); wels15[pie-1p::GFP::eea-1(FYVEx2)+unc-119(+)] double mutants were generated by standard genetic techniques, and the presence of the homozygous mutant alleles was confirmed by PCR, embryonic phenotype, and/or GFP expression. Nematodes were maintained at 15º (ts mutants) or 20º on NGM agar plates with Escherichia coli OP50 lawns as food sources using standard methods (Brenner 1974). Experiments with the ts mutants were performed at 15º (permissive temperature) and 25º (restrictive temperature). For all experiments, nematodes were synchronized via the hypochlorite treatment of gravid adults.
Temperature-sensitive mutant screen
We screened a collection of 1669 ts mutants obtained from the laboratory of R. Schnabel (TU Braunschweig) [partially described in Ehmke et al. (2014)]. We selected 28 of these mutants showing either an enlargement or a depletion of droplets as observed in four-dimensional (4D) microscopic analyses for further characterization. The embryos of self-fertilizing hermaphrodites of the selected mutants were scored for enlarged cytoplasmic droplets, droplet depletion, embryonic lethality, and brood size. Eight promising candidates were chosen from this group, and the mutations responsible for the droplet phenotype were identified. For this step, we combined SNP mapping (Davis et al. 2005) with whole-genome sequencing (GATC NextGen Sequencing, GATC Biotech, Konstanz, Germany). The resulting data sets were analyzed on the Galaxy Web platform (http://usegalaxy) using the cloud-based pipeline CloudMap (http://usegalaxy.org/cloudmap) established by the Hobert Laboratory (Minevich et al. 2012). We identified the phenotype-causing mutations for four alleles, two alleles each of cpl-1 and ccz-1. For the remaining four ts alleles, we found relevant mutations in more than one candidate gene (Table S1).
Database search
The WormMart (BioMart v 0.7, http://caprica.caltech.edu:9002/biomart/martview/), PhenomicDB (v 3.9.7, http://phenomicdb.info/), RNAi Database (release 5 http://rnai.org/), WormBase (v WS244, http://www.wormbase.org/), Tissue-specific expression predictions for C. elegans (v 1.0 http://worm-tissue.princeton.edu/search), KEGG Pathway Database (http://www.genome.jp/kegg/pathway.html), Expression Patterns for C. elegans promoter::GFP fusions Database (Hunt-Newbury et al. 2007), DAVID Gene Functional Annotation Tool (resource 6.7, https://david.ncifcrf.gov/), and National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/) GenBank databases were used to identify candidate genes that met one or more of the following criteria: embryonic lethal with unknown or predicted function, embryonic lethal or embryonic expression and known function in lipid metabolism, known phenotypes that are associated with lipid storage (e.g., lipid depleted, lipid content reduced or increased), and orthologs of human genes implicated in lipid diseases.
RNAi screen by feeding
For the interference RNA (RNAi) screen, clones from the Ahringer C. elegans RNAi library, obtained from Gene Services (Source BioScience, Berlin, Germany), were used (Kamath and Ahringer 2003). Bacteria containing the 316 different RNAi clones were cultured in Luria broth medium with 50 µg/ml ampicillin and 5 µg/ml tetracycline at 37º for 16–18 hr. Afterwards, 50 µl of the culture was seeded on NGM plates containing 2.5 mM IPTG and 50 µg/ml ampicillin. The RNAi plates were incubated at 37º overnight. The RNAi-hypersensitive rrf-3 strain was used for the screen. Ten synchronized L1 larvae were placed on each RNAi plate and incubated at 20º. After 48 hr, Nile Red was added on the top of each RNAi plate to a final concentration of 0.05 µg/ml. Embryos of the F1 generation were scored for their overall droplet phenotype and Nile Red incorporation into the droplets after 48 and 72 hr, respectively, using an Axio Zoom.V16 Stereo Microscope (Zeiss, Jena, Germany). Additionally, the phenotypes of the embryos in the F2 generation were determined. In each experiment, an L4440 empty vector and OP50 bacteria were used as negative controls, and the RNAi clones T03E6.7 (coding for cpl-1) and C49F5.1 (coding for sams-1) were used as positive controls, showing the droplet phenotype and embryonic lethality. RNAi clones resulting in a droplet phenotype were rescreened twice.
Microscopy and staining methods
Synchronized adult worms were harvested and washed in M9 buffer. Embryos were obtained by dissecting adult worms at the vulva and subsequently releasing the embryos from the uterus. The embryos were transferred on top of a small 5% agar pad on an objective glass slide. For vital staining, the embryos were directly incubated with BODIPY 493/503 (1:300, 15 min), LipidTOX Red (1:100, 40 min), or LysoTracker (1:50, 20 min) at room temperature. For fixative BODIPY 493/503 and LipidTOX Red staining, nematodes were washed in M9, incubated in 4% paraformaldehyde (PFA) solution for 15 min, followed by three freeze-thaw cycles in liquid nitrogen. After the PFA was removed by three washing steps with M9, the worms were incubated in 500 µl of 1 µg/ml dye in M9 for 1 hr at room temperature. Subsequently, the nematodes were washed three times with M9 buffer. Finally, the worms were mounted for microscopic analysis. Images were taken either by laser-scanning confocal fluorescence microscopy (Zeiss Axio Imager Z2 Upright Microscope equipped with a Zeiss LSM 700 scanning confocal imaging system) or by an Axio Imager Z1 Microscope (Zeiss) equipped with ApoTome.2, Colibri.2, and an AxioCam MRm camera. For fluorescent images, an 81 HE DAPI/FITC/Rhodamine/Cy5 filter (Zeiss) was used. Objects were magnified using a 63×/1.4 oil objective. All experiments were performed three to five times.
Brood size and lethality assays
Brood size and lethality assays were performed on NGM plates at 15 and 25º, respectively. Three synchronized L4 larvae were placed on the NGM plates, grown to adults, and transferred to new NGM plates twice a day during the egg-laying period. The number of eggs laid was counted, and the eggs were allowed to develop for 24 hr. Residual eggs were counted. The overall brood size per worm and the lethality rate were determined. All experiments were performed three to five times.
Temperature-shift assay
Adult worms were synchronized by hypochlorite treatment. The embryos obtained were either shifted directly to the restrictive temperature (25º) or maintained at the permissive temperature (15º) to the L4 stage. Afterwards, larvae were shifted to 25º. The overall larval development into fertile adults and the embryonic lethality rate of subsequent generations were determined.
UV survival assay
The UV survival assay was performed on NGM plates at 20º. Embryos were collected from gravid adults by hypochlorite treatment, and ∼50–60 embryos were transferred to plates seeded with OP50 bacteria. Animals were irradiated directly after hypochlorite treatment for the indicated periods of time (0–180 min). For irradiation, a Philips G30T8 lamp (30 W) emitting a constant C band ultraviolet (UV-C) dose was used. The exact number of irradiated eggs was determined. After 24 hr of development, the remaining eggs were counted, and the lethality rate was calculated. The experiment was performed three to four times.
Analysis of embryonic lipid composition
For analyzing the lipid composition of the wild-type N2 and asm-3(ok1744) embryos, ∼100,000 eggs were obtained by hypochlorite treatment of gravid adults. M9 buffer was removed, and the samples were stored at −80º. Five biological samples were collected per strain, and lipidomic analyses were performed on a high-resolution LC-MS/MS platform according to Fauland et al. (2011). Data processing was accomplished by a Lipid Data Analyzer (Hartler et al. 2011). The following lipid classes were quantified: triacylglycerol (TG), sphingomyelin (SM), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphoinositol (PI), and plasmalogen (aPE). The results were normalized to class amount, protein content, and sample weight. The distribution of lipid species was analyzed using GraphPad Prism (v 4.0) and Microsoft Excel (v 2013).
Protein alignment
We used the SIAS tool (imed.med.ucm.es) with the BLSOSUM62 matrix to calculate the overall protein identity and similarity. The alignment of catalytic domains was performed using the GENtle tool (v 1.9.4). The catalytic regions of human SMPD1 (aa 203–498) and C. elegans ASM-3 (aa 143–439) were aligned using the Clustal-W algorithm. Identical amino acids are indicated with asterisks.
Statistical analyses
Statistical analyses were performed using GraphPad Prism (v 4.0). The data presented represent means ± SEM of results from three or more independent experiments. Significances were calculated using Student’s unpaired t-test or one-way ANOVA. Differences were considered to be significant when *P < 0.05, **P < 0.005, and ***P < 0.001.
Results
Screening of mutant alleles altering the phenotype of putative lipid-containing droplets present in the C. elegans embryo
We searched for C. elegans mutant embryos with altered droplet phenotypes. We assumed that the screening and subsequent analyses of the mutants would lead to identification of genes functioning in the processing of lipid-containing droplets. Our first approach was based on a collection of 1669 ts embryonic lethal mutants and 4D DIC recordings of embryos (R. Schnabel et al., unpublished material). We have already shown that one of these mutants, sams-1(t3210), displayed an expansion of genuine lipid droplets (Ehmke et al. 2014). In this study, the embryos of self-fertilizing hermaphrodites of 28 selected mutants were scored for both embryonic lethality and the appearance and size of droplet structures (Figure 1 and Table S1). By combining SNP mapping with whole-genome sequencing, we identified two new alleles of cpl-1, t3423 and t3438, and of ccz-1, t3403 and t3213 (Figure S1). The pronounced and enlarged droplets in the cpl-1 and ccz-1 mutant embryos (Figure 2) prompted us to further characterize the phenotypes.
Figure 1.
Identification of genes associated with lipid metabolism and/or embryonic lethality. (A) Schematic representation of the approaches performed. The screening of ts alleles resulted in identification of eight possible candidates. The database research identified 1085 candidate genes, of which 316 were further analyzed by functional RNAi testing and/or mutant analysis. (B) Three different approaches were used to identify genes associated with lipid droplets in embryos. First, 28 ts alleles were screened for embryonic lethality and an enlargement or reduction of lipid droplets in embryos (I). Second, 1085 candidate genes of the database screen (II) were grouped in three partly overlapping categories: embryonic lethal, known function, and unknown/predicted function. Third, candidates from the database screen were functionally tested by RNAi (III). See Materials and Methods and Results for more information.
Figure 2.
Identified candidate genes associated with a droplet phenotype in the embryo. (A–I) DIC images of homozygous embryos. (A) Wild-type N2. Homozygous cpl-1(t3423) (B) and cpl-1(t3438) (C) embryos at the restrictive temperature. Homozygous ccz-1(t3403) (D) and ccz-1(t3213) (E) embryos at the restrictive temperature. (F) asm-3(ok1744) embryo. (G) asm-3(tm2384) mutant. (H) hosl-1(tm2369) embryo. (I) Downregulation of E01A2.1 transcript by RNAi in rrf-3(pk1426) embryo. Scale bar, 10 µm.
To identify the additional genes influencing the droplet phenotype of the C. elegans embryo, we collected and combined phenotypic information from several databases (see Materials and Methods). This resulted in a list of 1085 candidate genes, of which 725 were genes with known functions and 360 had unknown or predicted functions (Figure 1 and Table S2). The latter group was of particular interest. From 316 selected genes (Table S3), we identified RNAi-treated and/or mutant embryos showing altered droplet structures (e.g., size, distribution) by staining with dyes to trace the lipids. Of 22 candidate genes (Table S4), the most robust droplet phenotype was observed in embryos lacking asm-3, hosl-1, or E01A2.1 (Figure 2). We focused on the acid sphingomyelinase–encoding gene asm-3 because this gene has not been associated with lipid storage in embryos previously.
Mutant embryos of cpl-1 exhibit large droplets containing neutral lipids
The cpl-1 gene encodes a member of the cathepsin L–like cysteine protease family that seems to be involved in the proteolytic processing of yolk proteins (Hashmi et al. 2002; Britton and Murray 2004). The high embryonic lethality of the identified alleles, cpl-1(t3423) and cpl-1(t3438), at the restrictive temperature (Figure 3A and Table S1) correlated with the reported lethality of the cpl-1(ok360) null mutant (Britton and Murray 2004). In agreement with the strictly maternal function of cpl-1 (Hashmi et al. 2002; Britton and Murray 2004), a temperature shift during gametogenesis drastically enhanced the embryonic lethality of the F1 generation of cpl-1(t3423) (Figure 3B). Both cpl-1(t3423) and cpl-1(t3438) showed a minor increase in brood size compared with wild type at the restrictive temperature (+26.3%, P = 0.2343, and +20.6%, P = 0.361, respectively) (Figure 3C). Based on these results, the cpl-1 mutants exhibited normal or even enhanced fertility, whereas their embryonic development was disturbed.
Figure 3.
cpl-1 mutant embryos exhibit enlarged lipid-laden droplets. (A) Embryonic lethality at permissive temperature (15º, white bar) and restrictive temperature (25º, gray bar). (B) Temperature-shift experiment. cpl-1(t3423) mutants are shifted to restrictive temperature (25º) at the indicated stages. The level of embryonic lethality (emb) in the F1 generation is stated. (C) Brood size at permissive temperature (15º) and restrictive temperature (25º). (**P < 0.005; ***P < 0.001). (D–I) DIC images of cpl-1(t3423) mutant embryos and adults at the restrictive temperature. (D–D″) Intestinal region of adult homozygous cpl-1(t3423) mutant stained with LipidTOX (red, autofluorescence in blue). (E–E″) Gonadal tissue of adult cpl-1 mutant expressing vit-2::GFP and stained with the lipid marker LipidTOX. (F–F″) cpl-1 mutant embryo stained with BODIPY to visualize neutral lipids. (G–G″) Fixative LipidTOX staining of cpl-1 mutant embryo. (H–H″) cpl-1 mutant embryo expressing the lipid droplet marker atgl-1::GFP. (I–I″) Embryo expressing vit-2::GFP in cpl-1 mutant background. Higher-magnification images represent the area outlined in the dotted box. Arrows point to enlarged droplets. Scale bar, 10 µm; scale bar in magnification images, 2 µm.
Regarding the function of cpl-1 during gametogenesis and embryogenesis, the cpl-1(t3423) and cpl-1(t3438) mutant alleles showed normal droplet size in the intestines of adults (Figure 3D) but enlarged droplet sizes in oocytes (Figure 3E) and embryos (Figure 3F). To ascertain the mechanism responsible for the formation of the large droplets, we performed vital and fixative staining experiments with dyes (BODIPY493/503 and LipidTOX Red) tracing neutral lipids. Similar to other vital staining methods (O’Rourke et al. 2009; Wahlby et al. 2014), our short-term (20-min) vital staining approach (Klapper et al. 2011) was capable of visualizing lipid droplets. The fat-staining signals of the dyes did not co-localize with the autofluorescent signals of lysosome-related organelles (LROs) in the intestine (Figure 3D) or with the signals of the yolk marker VIT-2::GFP in oocytes (Figure 3E). Based on our vital fat-staining method, we showed that the expanded droplets present in the oocytes (Figure 3E) and embryos (Figure 3F) of the cpl-1(ts) mutants indeed contained neutral lipids. The fixative fat-staining method confirmed the lipid-containing character of the enlarged droplets of the cpl-1(ts) mutant embryos (Figure 3G). Signals from the ATGL-1::GFP fusion protein, a marker of canonical lipid droplets, were not detectable on the expanded droplets of the cpl-1(t3423) mutant embryos (Figure 3H). However, small embryonic droplets were visualized by ATGL-1::GFP (Figure 3H). According to a previous report on a cpl-1 null mutant (Britton and Murray 2004), the VIT-2::GFP signal was not visible on the large droplets of the cpl-1(ts) mutant embryos (Figure 3I). In conclusion, we found that the cpl-1 mutant embryos accumulated enlarged lipid-containing droplets that did not co-localize with marker proteins for yolk or lipid droplets.
Mutant embryos of ccz-1 accumulate enlarged yolk-containing lipid droplets
The novel alleles of ccz-1, t3213 and t3403, produced mostly dead embryos at the restrictive temperature (Figure 4A and Table S1). At the permissive temperature, the two mutants showed a lethality of approximately 50%. We further observed a high level of embryonic lethality (>95%) independent of whether we shifted the ccz-1(ts) mutants to the restrictive temperature early in development or after reaching the L4 stage (Figure 4B). The two ccz-1(ts) mutants produced considerably fewer embryos at the restrictive temperature than at the permissive temperature (Figure 4C). The high embryonic lethality and impaired fertility of the ccz-1(ts) mutants confirmed the essential role of CCZ-1 in gametogenesis and embryogenesis (Nieto et al. 2010). It has been reported that ccz-1 is involved in the digestion of apoptotic corpses, phagosome maturation, and processing of LROs in the intestine (Poteryaev et al. 2007; Nieto et al. 2010; Delahaye et al. 2014). We found large droplets in the intestine, oocytes, and embryos of the ccz-1(ts) mutants (Figure 4, D–F, and Table S1). Both vital and fixative fat-staining methods revealed that the expanded droplets contained neutral lipids (Figure 4, F and G). The signals of the lipid droplet marker protein ATGL-1::GFP and the yolk marker VIT-2::GFP are clearly visible on the large droplets of the ccz-1(ts) mutant (Figure 4, H and I). In contrast, the LRO marker GLO-1::GFP is not present on the large intestinal droplets of the ccz-1(ts) mutant (Figure 4J). Taken together, the ccz-1 mutant embryos accumulated enlarged lipid-containing droplets, which are characterized by the presence of marker proteins for both yolk and lipid droplets.
Figure 4.
ccz-1 mutant embryos possess enlarged yolk-enriched lipid droplets. (A) Overall embryonic lethality and (B) lethality in temperature-shift assay at permissive temperature (15º, white bar) and restrictive temperature (25º, gray bar). ccz-1(t3403) mutants are shifted to the restrictive temperature at the indicated stages. The level of embryonic lethality (emb) in the F1 generation is stated. (C) Number of progeny per worm (*P < 0.05; **P < 0.005). (D–J) DIC images of ccz-1 mutant embryos or adults at the restrictive temperature. (D–D″) Intestinal region of adult homozygous ccz-1(t3403) mutant stained with LipidTOX (red, autofluorescence in blue). (E–E″) Gonadal tissue of adult ccz-1(tm3403) mutant expressing vit-2::GFP and stained with the lipid marker LipidTOX. (F–F″) ccz-1(t3403) mutant embryo stained with BODIPY to visualize neutral lipids. (G–G″) Fixative BODIPY staining of ccz-1 mutant embryo. (H–H″) Embryo expressing atgl-1::GFP in ccz-1(t3403) mutant background. (I–I″) ccz-1(t3213) mutant embryo expressing vit-2::GFP as yolk marker. (J–J″) Intestinal region of adult ccz-1(t3403) mutant expressing glo-1::GFP and stained with the lipid marker LipidTOX (autofluorescence in blue). Higher-magnification images represent the area outlined in the dotted box. Arrows point to enlarged droplets. Scale bar, 10 µm; scale bar in magnification images, 2 µm.
Mutant embryos of asm-3 are more resistant to UV-C exposure, accumulate enlarged genuine lipid droplets, and show distinct changes in their lipid profiles
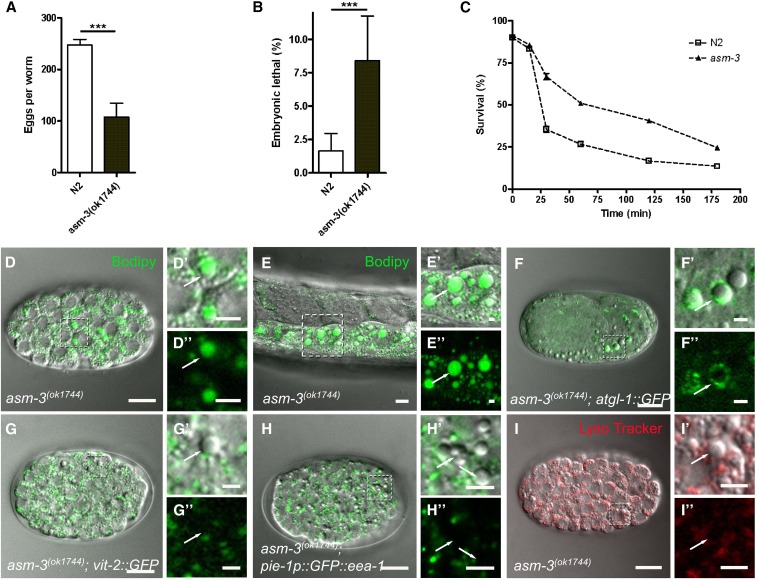
The asm-3 gene is closely related to the C. elegans acid sphingomyelinase genes asm-1 and asm-2 and to the human Niemann-Pick disease gene SMPD1 (Figure S2A). The large-droplet phenotype of the asm-3 mutant embryo (Figure 2, F and G) was not observable in the C. elegans mutants asm-1 and asm-2 (Figure S2, B and C). The asm-3(ok1744) mutant displayed a 56% reduced brood size and a slightly increased embryonic lethality compared with wild-type embryos (Figure 5, A and B). A previous study showed that adult asm-3 mutant worms were characterized by an increased stress resistance (Kim and Sun 2012). We found that the embryos of the asm-3(ok1744) mutant survived for longer times than wild-type embryos under UV-C irradiation (Figure 5C). Thus, the asm-3 mutant embryos were more resistant to UV-C stress.
Figure 5.
Lipid droplets are enlarged in asm-3 mutant embryos. The brood size (A) and embryonic lethality (B) of wild-type N2 and asm-3(ok1744) mutants are shown. (C) Survival rate of wild-type N2 and asm-3(ok1744) embryos after UV-C irradiation (***P < 0.001). (D–I) DIC images of homozygous asm-3(ok1744) mutant embryos and adults. (D–D″) asm-3 mutant embryo stained with BODIPY. (E–E″) Intestinal region of adult asm-3 mutant stained with BODIPY. (F–F″) Embryo expressing the lipid droplet marker atgl-1::GFP in asm-3 mutant background. (G–G″) asm-3 mutant embryo expressing vit-2::GFP. (H–H″) asm-3 mutant embryo expressing the early endosome marker pie-1p::GFP::eea-1. (I–I″) asm-3 mutant embryo stained with the lysosomal marker LysoTracker. Higher-magnification images represent the areas in the dotted box. Arrows point to enlarged droplets. Scale bar, 10 µm; scale bar in magnification images, 2 µm.
The asm-3(ok1744) mutant was characterized by the presence of enlarged droplets in all embryonic stages and in maturing oocytes (Figure S3), indicating a maternal contribution to asm-3 expression. To validate this observation, we crossed homozygous asm-3 mutant hermaphrodites with wild-type N2 males and examined the offspring of the F1 and F2 generations for the incidence of large droplets (Figure S4). The results demonstrated a maternal contribution to asm-3 gene expression. To analyze whether the large droplets of the asm-3(ok1744) mutant embryo contained neutral lipids, we used the vital BODIPY 493/503 staining method. This approach showed that the large droplets of the asm-3(ok1744) mutant embryo were BODIPY 493/503 positive (Figure 5D). In the asm-3(ok1744) adult worms, we also found enlarged BODIPY 493/503–positive droplets (Figure 5E). Moreover, the lipid droplet marker protein ATGL-1::GFP was clearly visible on the surfaces of the enlarged droplets in the asm-3(ok1744) mutant embryos (Figure 5F). In contrast, the expanded droplets were not marked by reporters for yolk (VIT-2::GFP), a subset of early endosomes (pie-1p::GFP::EEA-1), or lysosome (LysoTracker) (Figure 5, G–I). We concluded that the loss of asm-3 resulted in the accumulation of genuine lipid droplets in the embryo.
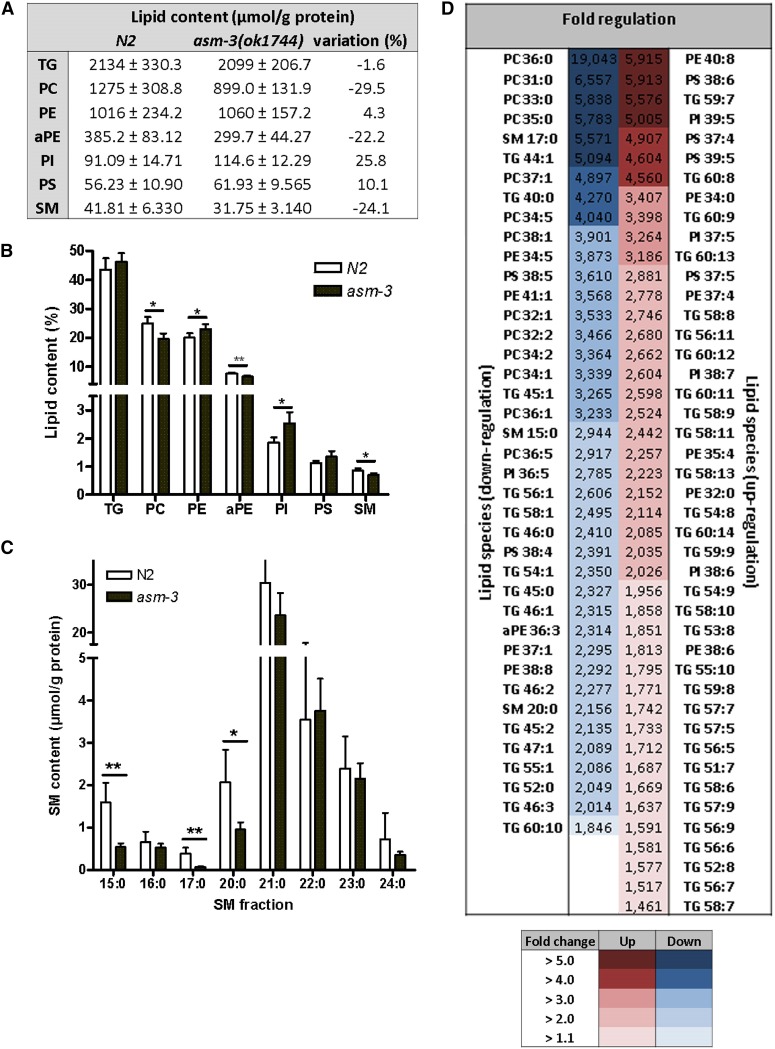
To identify the changes in the lipid profile of asm-3 mutant embryos, we performed a lipodomics analysis and identified 265 relevant individual lipid species. The total lipid amount (N2: 4999 µmol/g protein; asm-3: 4566 µmol/g protein) and the relative distribution of the seven lipid classes were comparable between the asm-3(ok1744) and wild-type embryos (Figure 6, A and B). In the sphingomyelin group, the most prominent effects were observed for SM 15:0 [−2.9-fold lower in asm-3 (ok1744) embryos compared with wild type], SM 17:0 (−5.8-fold), and SM 20:0 (−2.2-fold) (Figure 6C). In the triglyceride species, we observed an overall shift toward long-chain and polyunsaturated fatty acids in the asm-3(ok1744) mutants (Figure S5). When considering the other lipid classes, we found robust differences for PC 36:0 (−19-fold), PC 31:0 (−6.6-fold), PE 40:8 (+5.9-fold), and PS 38:6 (+5.9-fold) (Figure 6D). Overall, 84 lipid species (31.7%) showed significantly higher or lower levels (44 and 40 compounds, respectively) in the asm-3(ok1744) embryos compared with wild-type embryos (Figure 6D). The results of the lipodomics analyses showed that the asm-3 mutant embryo was characterized by certain changes in the lipid profile.
Figure 6.
Lipidomic analysis of sphingomyelin and triglyceride in N2 and asm-3 mutant embryos. (A–D) Results of lipidomic analysis of wild-type N2 and asm-3(ok1744) mutant embryos. (A) Quantification of lipid classes. (B) Distribution of lipids in different classes. (C) Comparison of sphingomyelin fatty acid species. (D) Heat map of significantly regulated lipid species with fold changes. Indicated numbers refer to fatty acids (*P < 0.05; **P < 0.005).
Discussion
Yolk complexes and lipid droplets are distinct lipid storage organelles in C. elegans embryos
Yolk complexes are lipoprotein complexes that serve as energy reserves for the nourishment of developing embryos (Wallace 1985; Byrne et al. 1989; Tufail and Takeda 2009). The precursors of the yolk proteins, vitellogenins, are typically synthesized in organs distant from the gonad and are then endocytosed by the developing oocytes (Mosconi et al. 2002; Tufail and Takeda 2009). Here they are stored in yolk platelets until they are degraded (Reimer and Crawford 1995; Jorgensen et al. 2009). In C. elegans, the cathepsin L–like cysteine protease CPL-1 is involved in the degradation of yolk proteins during embryogenesis (Hashmi et al. 2002; Britton and Murray 2004). In agreement with previous studies (Hashmi et al. 2002; Britton and Murray 2004), we observed enlarged droplets in both oocytes and embryos in the newly identified cpl-1(ts) mutants. Although the yolk marker protein VIT-2::GFP was not detectable on the large droplets, Britton and Murray (2004) showed that the droplets still contained yolk. Similarly, we found that the enlarged droplets of the cpl-1(ts) mutant embryos also contained neutral lipids not marked by the lipid droplet reporter protein ATGL-1::GFP. These findings suggested that the loss of function of cpl-1 causes an enlargement of lipid-containing yolk complexes distinct from genuine lipid droplets.
It has been shown that CCZ-1 acts in complex with SAND-1 as a guanine nucleotide exchange factor for RAB-7 and is involved in the digestion of apoptotic corpses, phagosome maturation, and the processing of lysosome-related organelles (Poteryaev et al. 2007; Nieto et al. 2010; Delahaye et al. 2014). As found in the isolated ccz-1(ts) mutant embryos, sand-1 mutants also accumulate yolk-enriched large droplets in oocytes and embryos (Poteryaev and Spang 2005; Poteryaev et al. 2007). Our further examination of the ccz-1(ts) mutant embryos revealed that the enlarged droplets contained neutral lipids and were marked with reporter proteins for genuine lipid droplets and yolk complexes. Therefore, we proposed that ATGL-positive lipid droplets undergo fusion with yolk complexes to transiently form a mixture of genuine lipid droplets and yolk. These structures might be involved in separating the lipid moiety from vitellogenins and in the subsequent storage of lipids in genuine lipid droplets.
Involvement of the asm-3 gene in altering the size of lipid droplets and in the stress response
Loss of the human sphingomyelinase-encoding gene SMPD1 causes Niemann-Pick disease types A and B, which are lysosomal storage diseases (Schuchman et al. 1992; Jones et al. 2008; Jenkins et al. 2009). In Niemann-Pick patients, lipid-laden foam cells can be found in bone marrow, spleen, and lymph nodes (Viana et al. 1990; Putterman et al. 1992; Thurberg et al. 2012). At a cellular level, loss of SMPD1 results in the accumulation of SM in large droplets within endolysosomal compartments (Schuchman et al. 1992; Jones et al. 2008; Jenkins et al. 2009). C. elegans encodes three acidic sphingomyelinases: asm-1, asm-2, and asm-3 (Lin et al. 1998; Kim and Sun 2012). In this study, we showed that asm-3 mutant oocytes and embryos exclusively accumulated enlarged droplets containing neutral lipids marked by the lipid droplet marker ATGL-1::GFP. Because the droplets are not marked by reporter proteins for lysosomes, yolk, or a subset of early endosomes, we concluded that loss of asm-3 function causes an enlargement of genuine lipid droplets.
The reduction of PC levels in the asm-3(ok1744) mutant embryos was remarkable because a decrease of this lipid class increases the size of the lipid droplets in the intestine of adult worms (Krahmer et al. 2011; Xu et al. 2012; Ehmke et al. 2014). This also could be the reason for lipid droplet enlargement in the asm-3 mutant embryos. Acid sphingomyelinases are crucial in establishing a well-balanced level between SM and ceramide production in the plasma membrane (Goni and Alonso 2002; Jenkins et al. 2009; Young et al. 2013). However, SM has been discovered in lipid droplet fractions in a variety of different cell types (Hood and Patton 1973; Ishii et al. 1995; Tauchi-Sato et al. 2002; McIntosh et al. 2010; Storey et al. 2011). Moreover, SMs, PCs, and PIs are enriched in functionally active lipid droplets (Storey et al. 2011). The conversion of SMs to ceramide causes structural changes in membranes, facilitating the formation of vesicles (Wheeler et al. 2009; Mitsutake et al. 2011). Indeed, the addition of bacterial sphingomyelinase to giant unilamellar vesicles results in the formation of intraluminal vesicles (Trajkovic et al. 2008; Ibarguren et al. 2013), indicating an involvement of sphingomyelinases in vesicle formation. Thus, sphingomyelinase-induced SM hydrolysis also could be involved in determining the size of lipid droplets. We further observed a shift toward long-chain and polyunsaturated fatty acids (PUFAs) in triglyceride species, indicating an upregulation of the desaturase genes (i.e., fat-1, fat-3, and fat-4). This likely increases the fluidity of membranes (Dietrich et al. 2001; Roux et al. 2005) and facilitates the enlargement of lipid droplets.
Acid sphingomyelinases have been implicated in mediating apoptotic and stress responses to Fas ligands and ionizing and UV irradiation (lin et al. 2000; Garcia-Barros et al. 2003; Zeidan et al. 2008; Appelqvist et al. 2013). The C. elegans sphingomyelinase functions as a positive regulator of the DAF-2/insulin signaling pathway, which is involved in the regulation of lifespan, metabolism, reproduction, and development (Kimura et al. 1997; Kim and Sun 2012). Accordingly, we observed a drastically reduced brood size and slightly increased embryonic lethality in asm-3 mutants. Reduced fertility also has been documented in mouse models for Niemann-Pick disease and in other lysosomal storage diseases (Trasler et al. 1998; Adamali et al. 1999; Butler et al. 2007). Kim and Sun (2012) showed that asm-3(ok1744) mutant worms have a prolonged lifespan, their dauer arrest is promoted, and they are more resistant to oxidative or heat stresses. We could show that UV-induced embryonic lethality was lower in the asm-3 null allele ok1744, and thus, stress resistance was already established in embryos. By searching for lipid species that might be important for this phenotype, we found that GPEtn (40:8) was increased in the asm-3 mutant embryo. Assuming that this lipid species is composed of two arachidonic acids (20:4), this fatty acid could contribute to the enhanced stress resistance (O’Rourke et al. 2013) of the asm-3 mutant embryos.
Conclusion
We identified a few evolutionarily conserved and essential genes that seem to be involved in the processing of lipid-containing droplets in C. elegans embryos. Using marker proteins and vital and fixative fat-staining methods, we found that the mutant embryos of the genes cpl-1 (cathepsin L–like cysteine protease), ccz-1 (guanine nucleotide exchange factor subunit), and asm-3 (acid sphingomyelinase 3) showed accumulations of three distinct types of enlarged neutral lipid-containing droplets. These mutant phenotypes indicated that asm-3, ccz-1, and cpl-1 are critical for the distinct steps in the processing of lipid-containing droplets in the C. elegans embryo. The identified genes may be useful for studying embryonic lipid storage in other organisms as well.
Footnotes
Communicating editor: M. P. Colaiácovo
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.179127/-/DC1.
Literature Cited
- Aardema H., Vos P. L., Lolicato F., Roelen B. A., Knijn H. M., et al. , 2011. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biol. Reprod. 85: 62–69. [DOI] [PubMed] [Google Scholar]
- Adamali H. I., Somani I. H., Huang J. Q., Mahuran D., Gravel R. A., et al. , 1999. I. Abnormalities in cells of the testis, efferent ducts, and epididymis in juvenile and adult mice with beta-hexosaminidase A and B deficiency. J. Androl. 20: 779–802. [PubMed] [Google Scholar]
- Appelqvist H., Waster P., Eriksson I., Rosdahl I., Ollinger K., 2013. Lysosomal exocytosis and caspase-8-mediated apoptosis in UVA-irradiated keratinocytes. J. Cell Sci. 126: 5578–5584. [DOI] [PubMed] [Google Scholar]
- Ashrafi K., Chang F. Y., Watts J. L., Fraser A. G., Kamath R. S., et al. , 2003. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421: 268–272. [DOI] [PubMed] [Google Scholar]
- Biggers J. D., Whittingham D. G., Donahue R. P., 1967. The pattern of energy metabolism in the mouse oocyte and zygote. Proc. Natl. Acad. Sci. USA 58: 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossinger O., Schierenberg E., 1992. Cell-cell communication in the embryo of Caenorhabditis elegans. Dev. Biol. 151: 401–409. [DOI] [PubMed] [Google Scholar]
- Bossinger O., Schierenberg E., 1996. The use of fluorescent marker dyes for studying intercellular communication in nematode embryos. Int. J. Dev. Biol. 40: 431–439. [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton C., Murray L., 2004. Cathepsin L protease (CPL-1) is essential for yolk processing during embryogenesis in Caenorhabditis elegans. J. Cell Sci. 117: 5133–5143. [DOI] [PubMed] [Google Scholar]
- Butler A., Gordon R. E., Gatt S., Schuchman E. H., 2007. Sperm abnormalities in heterozygous acid sphingomyelinase knockout mice reveal a novel approach for the prevention of genetic diseases. Am. J. Pathol. 170: 2077–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Cassada R. C., Russell R. L., 1976. The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction. Dev. Biol. 51: 23–33. [DOI] [PubMed] [Google Scholar]
- Byrne B. M., Gruber M., Ab G., 1989. The evolution of egg yolk proteins. Prog. Biophys. Mol. Biol. 53: 33–69. [DOI] [PubMed] [Google Scholar]
- Chen J. S., Sappington T. W., Raikhel A. S., 1997. Extensive sequence conservation among insect, nematode, and vertebrate vitellogenins reveals ancient common ancestry. J. Mol. Evol. 44: 440–451. [DOI] [PubMed] [Google Scholar]
- Davis M. W., Hammarlund M., Harrach T., Hullett P., Olsen S., et al. , 2005. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics 6: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley R. G., Mullinix D. P., Wetekam W., Kronenberg H. M., Meyers M., et al. , 1975. Vitellogenin synthesis in the avian liver: vitellogenin is the precursor of the egg yolk phosphoproteins. J. Biol. Chem. 250: 9060–9066. [PubMed] [Google Scholar]
- Delahaye J. L., Foster O. K., Vine A., Saxton D. S., Curtin T. P., et al. , 2014. Caenorhabditis elegans HOPS and CCZ-1 mediate trafficking to lysosome-related organelles independently of RAB-7 and SAND-1. Mol. Biol. Cell 25: 1073–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C., Bagatolli L. A., Volovyk Z. N., Thompson N. L., Levi M., et al. , 2001. Lipid rafts reconstituted in model membranes. Biophys. J. 80: 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning K. R., Cashman K., Russell D. L., Thompson J. G., Norman R. J., et al. , 2010. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol. Reprod. 83: 909–918. [DOI] [PubMed] [Google Scholar]
- Dunning K. R., Russell D. L., Robker R. L., 2014. Lipids and oocyte developmental competence: the role of fatty acids and beta-oxidation. Reproduction 148: R15–R27. [DOI] [PubMed] [Google Scholar]
- Ehmke M., Luthe K., Schnabel R., Doring F., 2014. S-Adenosyl methionine synthetase 1 limits fat storage in Caenorhabditis elegans. Genes Nutr. 9: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauland A., Kofeler H., Trotzmuller M., Knopf A., Hartler J., et al. , 2011. A comprehensive method for lipid profiling by liquid chromatography-ion cyclotron resonance mass spectrometry. J. Lipid Res. 52: 2314–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E. M., Leese H. J., 2006. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 73: 1195–1201. [DOI] [PubMed] [Google Scholar]
- Garcia-Barros M., Paris F., Cordon-Cardo C., Lyden D., Rafii S., et al. , 2003. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300: 1155–1159. [DOI] [PubMed] [Google Scholar]
- Gardner D. K., Pool T. B., Lane M., 2000. Embryo nutrition and energy metabolism and its relationship to embryo growth, differentiation, and viability. Semin. Reprod. Med. 18: 205–218. [DOI] [PubMed] [Google Scholar]
- Gilchrist R. B., Lane M., Thompson J. G., 2008. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 14: 159–177. [DOI] [PubMed] [Google Scholar]
- Goni F. M., Alonso A., 2002. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 531: 38–46. [DOI] [PubMed] [Google Scholar]
- Grant B., Hirsh D., 1999. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10: 4311–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Winfrey V. P., Blaeuer G., Hoffman L. H., Furuta T., et al. , 1999. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev. Biol. 212: 101–123. [DOI] [PubMed] [Google Scholar]
- Hartler J., Trotzmuller M., Chitraju C., Spener F., Kofeler H. C., et al. , 2011. Lipid Data Analyzer: unattended identification and quantitation of lipids in LC-MS data. Bioinformatics 27: 572–577. [DOI] [PubMed] [Google Scholar]
- Harvey S. C., Orbidans H. E., 2011. All eggs are not equal: the maternal environment affects progeny reproduction and developmental fate in Caenorhabditis elegans. PLoS One 6: e25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi S., Britton C., Liu J., Guiliano D. B., Oksov Y., et al. , 2002. Cathepsin L is essential for embryogenesis and development of Caenorhabditis elegans. J. Biol. Chem. 277: 3477–3486. [DOI] [PubMed] [Google Scholar]
- Hodgkin J., Barnes T. M., 1991. More is not better: brood size and population growth in a self-fertilizing nematode. Proc. Biol. Sci. 246: 19–24. [DOI] [PubMed] [Google Scholar]
- Hood L. F., Patton S., 1973. Isolation and characterization of intracellular lipid droplets from bovine mammary tissue. J. Dairy Sci. 56: 858–863. [DOI] [PubMed] [Google Scholar]
- Hunt-Newbury R., Viveiros R., Johnsen R., Mah A., Anastas D., et al. , 2007. High-throughput in vivo analysis of gene expression in Caenorhabditis elegans. PLoS Biol. 5: e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarguren M., Sot J., Montes L. R., Vasil A. I., Vasil M. L., et al. , 2013. Recruitment of a phospholipase C/sphingomyelinase into non-lamellar lipid droplets during hydrolysis of lipid bilayers. Chem. Phys. Lipids 166: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii I., Onozaki R., Takahashi E., Takahashi S., Fujio N., et al. , 1995. Regulation of neutral cholesterol esterase activity by phospholipids containing negative charges in substrate liposome. J. Lipid Res. 36: 2303–2310. [PubMed] [Google Scholar]
- Jenkins R. W., Canals D., Hannun Y. A., 2009. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell. Signal. 21: 836–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones I., He X., Katouzian F., Darroch P. I., Schuchman E. H., 2008. Characterization of common SMPD1 mutations causing types A and B Niemann-Pick disease and generation of mutation-specific mouse models. Mol. Genet. Metab. 95: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P., Steen J. A., Steen H., Kirschner M. W., 2009. The mechanism and pattern of yolk consumption provide insight into embryonic nutrition in Xenopus. Development 136: 1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Ahringer J., 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30: 313–321. [DOI] [PubMed] [Google Scholar]
- Kim Y., Sun H., 2012. ASM-3 acid sphingomyelinase functions as a positive regulator of the DAF-2/AGE-1 signaling pathway and serves as a novel anti-aging target. PLoS One 7: e45890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J., Sharrock W. J., 1983. Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev. Biol. 96: 189–196. [DOI] [PubMed] [Google Scholar]
- Kimura K. D., Tissenbaum H. A., Liu Y., Ruvkun G., 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946. [DOI] [PubMed] [Google Scholar]
- Klapper M., Ehmke M., Palgunow D., Bohme M., Matthaus C., et al. , 2011. Fluorescence-based fixative and vital staining of lipid droplets in Caenorhabditis elegans reveal fat stores using microscopy and flow cytometry approaches. J. Lipid Res. 52: 1281–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahmer N., Guo Y., Wilfling F., Hilger M., Lingrell S., et al. , 2011. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 14: 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y., Hsu T. Y., Wu Y. C., Chang H. C., 2013. Fluorescent nanodiamond as a probe for the intercellular transport of proteins in vivo. Biomaterials 34: 8352–8360. [DOI] [PubMed] [Google Scholar]
- Leese H. J., 2012. Metabolism of the preimplantation embryo: 40 years on. Reproduction 143: 417–427. [DOI] [PubMed] [Google Scholar]
- Levitte S., Salesky R., King B., Coe Smith S., Depper M., et al. , 2010. A Caenorhabditis elegans model of orotic aciduria reveals enlarged lysosome-related organelles in embryos lacking umps-1 function. FEBS J. 277: 1420–1439. [DOI] [PubMed] [Google Scholar]
- Lin T., Genestier L., Pinkoski M. J., Castro A., Nicholas S., et al. , 2000. Role of acidic sphingomyelinase in Fas/CD95-mediated cell death. J. Biol. Chem. 275: 8657–8663. [DOI] [PubMed] [Google Scholar]
- Lin X., Hengartner M. O., Kolesnick R., 1998. Caenorhabditis elegans contains two distinct acid sphingomyelinases. J. Biol. Chem. 273: 14374–14379. [DOI] [PubMed] [Google Scholar]
- McEvoy T. G., Coull G. D., Broadbent P. J., Hutchinson J. S., Speake B. K., 2000. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J. Reprod. Fertil. 118: 163–170. [PubMed] [Google Scholar]
- McIntosh A. L., Storey S. M., Atshaves B. P., 2010. Intracellular lipid droplets contain dynamic pools of sphingomyelin: ADRP binds phospholipids with high affinity. Lipids 45: 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeegan P. J., Sturmey R. G., 2011. The role of fatty acids in oocyte and early embryo development. Reprod. Fertil. Dev. 24: 59–67. [DOI] [PubMed] [Google Scholar]
- Minevich G., Park D. S., Blankenberg D., Poole R. J., Hobert O., 2012. CloudMap: a cloud-based pipeline for analysis of mutant genome sequences. Genetics 192: 1249–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsutake S., Zama K., Yokota H., Yoshida T., Tanaka M., et al. , 2011. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J. Biol. Chem. 286: 28544–28555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi G., Carnevali O., Habibi H. R., Sanyal R., Polzonetti-Magni A. M., 2002. Hormonal mechanisms regulating hepatic vitellogenin synthesis in the gilthead sea bream, Sparus aurata. Am. J. Physiol. Cell Physiol. 283: C673–C678. [DOI] [PubMed] [Google Scholar]
- Mullaney B. C., Ashrafi K., 2009. C. elegans fat storage and metabolic regulation. Biochim. Biophys. Acta 1791: 474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschiol D., Schroeder F., Traunspurger W., 2009. Life cycle and population growth rate of Caenorhabditis elegans studied by a new method. BMC Ecol. 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto C., Almendinger J., Gysi S., Gomez-Orte E., Kaech A., et al. , 2010. ccz-1 mediates the digestion of apoptotic corpses in C. elegans. J. Cell Sci. 123: 2001–2007. [DOI] [PubMed] [Google Scholar]
- O’Rourke E. J., Soukas A. A., Carr C. E., Ruvkun G., 2009. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 10: 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke E. J., Kuballa P., Xavier R., Ruvkun G., 2013. ω-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 27: 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M. L., Bell W. J., Telfer W. H., 1969. Vitellogenic blood protein synthesis by insect fat body. Science 165: 393–394. [DOI] [PubMed] [Google Scholar]
- Poteryaev D., Spang A., 2005. A role of SAND-family proteins in endocytosis. Biochem. Soc. Trans. 33: 606–608. [DOI] [PubMed] [Google Scholar]
- Poteryaev D., Fares H., Bowerman B., Spang A., 2007. Caenorhabditis elegans SAND-1 is essential for RAB-7 function in endosomal traffic. EMBO J. 26: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prates E. G., Nunes J. T., Pereira R. M., 2014. A role of lipid metabolism during cumulus-oocyte complex maturation: impact of lipid modulators to improve embryo production. Mediators Inflamm. 2014: 692067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterman C., Zelingher J., Shouval D., 1992. Liver failure and the sea-blue histiocyte/adult Niemann-Pick disease: case report and review of the literature. J. Clin. Gastroenterol. 15: 146–149. [DOI] [PubMed] [Google Scholar]
- Reimer C. L., Crawford B. J., 1995. Identification and partial characterization of yolk and cortical granule proteins in eggs and embryos of the starfish, Pisaster ochraceus. Dev. Biol. 167: 439–457. [DOI] [PubMed] [Google Scholar]
- Roux A., Cuvelier D., Nassoy P., Prost J., Bassereau P., et al. , 2005. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J. 24: 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington T. W., Raikhel A. S., 1998. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 28: 277–300. [DOI] [PubMed] [Google Scholar]
- Schuchman E. H., Levran O., Pereira L. V., Desnick R. J., 1992. Structural organization and complete nucleotide sequence of the gene encoding human acid sphingomyelinase (SMPD1). Genomics 12: 197–205. [DOI] [PubMed] [Google Scholar]
- Sharrock W. J., Sutherlin M. E., Leske K., Cheng T. K., Kim T. Y., 1990. Two distinct yolk lipoprotein complexes from Caenorhabditis elegans. J. Biol. Chem. 265: 14422–14431. [PubMed] [Google Scholar]
- Storey S. M., McIntosh A. L., Senthivinayagam S., Moon K. C., Atshaves B. P., 2011. The phospholipid monolayer associated with perilipin-enriched lipid droplets is a highly organized rigid membrane structure. Am. J. Physiol. Endocrinol. Metab. 301: E991–E1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton-McDowall M. L., Gilchrist R. B., Thompson J. G., 2010. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 139: 685–695. [DOI] [PubMed] [Google Scholar]
- Tauchi-Sato K., Ozeki S., Houjou T., Taguchi R., Fujimoto T., 2002. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 277: 44507–44512. [DOI] [PubMed] [Google Scholar]
- Thurberg B. L., Wasserstein M. P., Schiano T., O’Brien F., Richards S., et al. , 2012. Liver and skin histopathology in adults with acid sphingomyelinase deficiency (Niemann-Pick disease type B). Am. J. Surg. Pathol. 36: 1234–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., et al. , 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247. [DOI] [PubMed] [Google Scholar]
- Trasler J., Saberi F., Somani I. H., Adamali H. I., Huang J. Q., et al. , 1998. Characterization of the testis and epididymis in mouse models of human Tay Sachs and Sandhoff diseases and partial determination of accumulated gangliosides. Endocrinology 139: 3280–3288. [DOI] [PubMed] [Google Scholar]
- Tufail M., Takeda M., 2009. Insect vitellogenin/lipophorin receptors: molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 55: 87–103. [DOI] [PubMed] [Google Scholar]
- Viana M. B., Giugliani R., Leite V. H., Barth M. L., Lekhwani C., et al. , 1990. Very low levels of high density lipoprotein cholesterol in four sibs of a family with non-neuropathic Niemann-Pick disease and sea-blue histiocytosis. J. Med. Genet. 27: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlby C., Conery A. L., Bray M. A., Kamentsky L., Larkins-Ford J., et al. , 2014. High- and low-throughput scoring of fat mass and body fat distribution in C. elegans. Methods 68: 492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Ryffel G. U., Weber R., 1981. Vitellogenesis and the vitellogenin gene family. Science 212: 298–304. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., 1985. Vitellogenesis and oocyte growth in nonmammalian vertebrates. Dev. Biol. 1: 127–177. [DOI] [PubMed] [Google Scholar]
- Wangh L. J., Knowland J., 1975. Synthesis of vitellogenin in cultures of male and female frog liver regulated by estradiol treatment in vitro. Proc. Natl. Acad. Sci. USA 72: 3172–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. L., 2009. Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrinol. Metab. 20: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D., Knapp E., Bandaru V. V., Wang Y., Knorr D., et al. , 2009. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J. Neurochem. 109: 1237–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Zhang S. O., Cole R. A., McKinney S. A., Guo F., et al. , 2012. The FATP1-DGAT2 complex facilitates lipid droplet expansion at the ER-lipid droplet interface. J. Cell Biol. 198: 895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. M., Kester M., Wang H. G., 2013. Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 54: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan Y. H., Wu B. X., Jenkins R. W., Obeid L. M., Hannun Y. A., 2008. A novel role for protein kinase Cδ-mediated phosphorylation of acid sphingomyelinase in UV light-induced mitochondrial injury. FASEB J. 22: 183–193. [DOI] [PubMed] [Google Scholar]