Abstract
Cdk1 activity drives both mitotic entry and the metaphase-to-anaphase transition in all eukaryotes. The kinase Wee1 and the phosphatase Cdc25 regulate the mitotic activity of Cdk1 by the reversible phosphorylation of a conserved tyrosine residue. Mutation of cdc25 in Schizosaccharomyces pombe blocks Cdk1 dephosphorylation and causes cell cycle arrest. In contrast, deletion of MIH1, the cdc25 homolog in Saccharomyces cerevisiae, is viable. Although Cdk1-Y19 phosphorylation is elevated during mitosis in mih1∆ cells, Cdk1 is dephosphorylated as cells progress into G1, suggesting that additional phosphatases regulate Cdk1 dephosphorylation. Here we show that the phosphatase Ptp1 also regulates Cdk1 dephosphorylation in vivo and can directly dephosphorylate Cdk1 in vitro. Using a novel in vivo phosphatase assay, we also show that PP2A bound to Rts1, the budding yeast B56-regulatory subunit, regulates dephosphorylation of Cdk1 independently of a function regulating Swe1, Mih1, or Ptp1, suggesting that PP2ARts1 either directly dephosphorylates Cdk1-Y19 or regulates an unidentified phosphatase.
Keywords: Cdc25/Mih1, Cdk1, PP2A, Wee1/Swe1, mitosis
MITOTIC onset is regulated in all eukaryotes by an increase in Cdk1 activity caused by the dephosphorylation of Cdk1 on a conserved inhibitory tyrosine (tyrosine 19 in budding yeast) (Nurse 1990). The Wee1 kinase phosphorylates and inhibits Cdk1 (Gould and Nurse 1989; Parker et al. 1992), and the Cdc25 phosphatase acts as a mitotic inducer by dephosphorylating and activating Cdk1 (Dunphy and Kumagai 1991; Gautier et al. 1991). wee1 mutants in fission yeast shorten G2 by prematurely activating Cdk1 (Nurse 1975; Russell and Nurse 1987), whereas cdc25 mutants cannot accumulate sufficient Cdk1 activity to enter mitosis and arrest (Russell and Nurse 1986). Both Wee1 and Cdc25 are the targets of numerous cell-cycle checkpoints, all of which delay mitotic entry by activating Wee1 or inhibiting Cdc25 (Kellogg 2003). In budding yeast, Swe1 (the Wee1 homolog) and Mih1 (the Cdc25 homolog) also function prior to mitosis (Russell et al. 1989; Booher et al. 1993; Harvey and Kellogg 2003; Pal et al. 2008), but our recent work revealed that overexpression of Swe1 or activation of a Swe1-dependent checkpoint arrests cells in metaphase (Lianga et al. 2013).
Deletion of cdc25 in fission yeast is lethal and arrests cells in G2, indicating the essential role of Cdk1-Y15 dephosphorylation in fission yeast (Russell and Nurse 1986). In contrast, although deletion of MIH1 exhibits high Cdk1-Y19 phosphorylation during mitosis, these cells have only mild delays in mitotic entry and anaphase onset and initiate Cdk1-Y19 dephosphorylation at anaphase onset (Russell et al. 1989; Rudner et al. 2000; Pal et al. 2008; Lianga et al. 2013). This behavior argues that at least one additional phosphatase functions redundantly with Mih1.
Russell and colleagues (Millar et al. 1992) identified the fission yeast Pyp3 as a phosphatase that functions redundantly with Cdc25. Increased expression of pyp3 or the budding yeast and mammalian homologs PTP1, PTP1B and TC-PTP1, respectively, suppresses the temperature sensitivity of cdc25-22, and the pyp3∆ mutant exacerbates the defects of cdc25-22 (Gould et al. 1990; Millar et al. 1992; Hannig et al. 1993), suggesting that Ptp1 has a conserved function dephosphorylating Cdk1.
Protein phosphatase 2A (PP2A) also may act redundantly with Mih1. PP2A is a heterotrimeric complex composed of a catalytic subunit, a scaffolding subunit, and one of two B-regulatory subunits, Cdc55 (homologous to B55/B) or Rts1 (homologous to B56/B′) (Healy et al. 1991; Stark 1996; Shu et al. 1997; Zhao et al. 1997). Deletion of CDC55 or RTS1 elevates Cdk1-Y19 phosphorylation (Minshull et al. 1996; Zapata et al. 2014), and both mutants display synthetic interactions when combined with mih1∆ (Pal et al. 2008; Costanzo et al. 2010), suggesting that PP2A may function with Mih1. However, these synthetic interactions instead may be explained by negative regulation of Swe1 by PP2ACdc55 and PP2ARts1 (Harvey et al. 2011). In addition, although PP2A is capable of tyrosine dephosphorylation in vitro, it is believed to function solely as a serine/threonine phosphatase in vivo (Foulkes et al. 1983; Janssens and Goris 2001; Shi 2009; Tonks 2013).
Here we show that Ptp1, the budding yeast homolog of fission yeast Pyp3, also regulates Cdk1 dephosphorylation in vivo. However, despite delayed Cdk1-Y19 dephosphorylation in anaphase, mih1∆ ptp1∆ cells are as healthy as mih1∆ cells, suggesting the existence of an additional redundant phosphatase. Using an in vivo Cdk1-Y19 phosphatase assay, we show that PP2ARts1, but not PP2ACdc55, regulates dephosphorylation of Cdk1-Y19 independently of its role regulating Swe1. Although we are not able to confirm whether PP2ARts1 directly dephosphorylates Cdk1-Y19 or regulates an unidentified phosphatase, our results suggest that Mih1, Ptp1, and PP2ARts1 function redundantly to regulate the spatial and temporal activation of Cdk1, providing a mechanism for the observed stepwise activation of Cdk1 prior to anaphase onset.
Materials and Methods
A complete description of our methods can be found in Supporting Information, File S1.
Results
Ptp1 regulates Cdk1 tyrosine dephosphorylation
In fission yeast, the Pyp3 phosphatase acts redundantly with Cdc25 in vivo and in vitro (Millar et al. 1992), so we examined the role of the budding yeast homolog Ptp1 in regulating tyrosine phosphorylation on Cdk1. Asynchronously growing ptp1∆ cells have elevated Cdk1-Y19 phosphorylation (Figure 1A), but this increase is not as dramatic as what is observed in mih1∆ cells. (See Table S1 and Table S2 for a complete list of all the strains used in this study.)
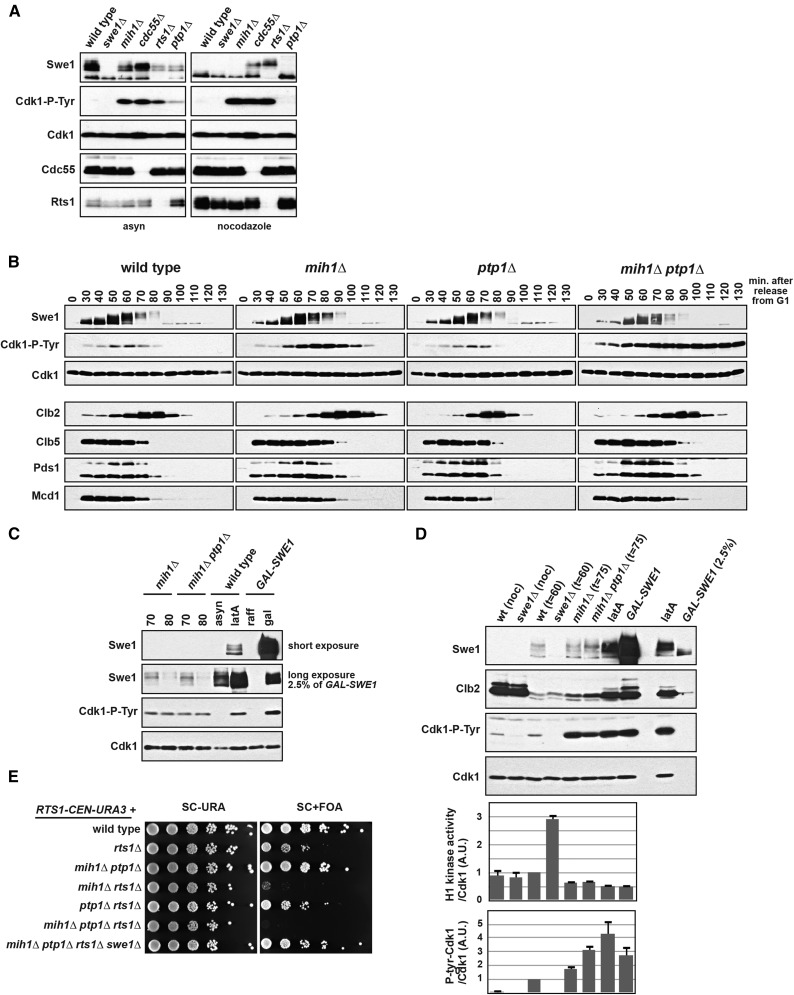
Figure 1.
PTP1 regulates Cdk1-Y19 phosphorylation in vivo. (A) mih1∆, cdc55∆, rts1∆, and ptp1∆ cells have increased Cdk1-Y19 phosphorylation. Wild-type, swe1∆, mih1∆, cdc55∆, rts1∆, and ptp1∆ cells were grown at 25° asynchronously (asyn) or arrested in mitosis with nocodazole, harvested, lysed, and blotted with the indicated antibodies. (B) Wild-type, mih1∆, ptp1∆, and mih1∆ ptp1∆ cells were grown at 25°, arrested with mating pheromone, and released into the cell cycle at t = 0. Cells were harvested, lysed, and blotted with the indicated antibodies. (C) A comparison of Cdk1-Y19 phosphorylation in mih1∆ and mih1∆ ptp1∆ cells in samples from B at t = 70 min and t = 80 min, wild-type cells treated with latA (2.5 µM), and cells overexpressing Swe1 from a GAL-SWE1 gene that replaces the endogenous SWE1. Cells arrested by latA or Swe1 overexpression have significantly more Swe1 than mih1∆ and mih1∆ ptp1∆ cells and have slightly higher levels of phosphorylated Cdk1-Y19. (D) Differences in Cdk1-Y19 phosphorylation correlate with changes in Cdk1/Clb2 histone 1H-kinase activity. Wild-type, swe1∆, mih1∆, and mih1∆ ptp1∆ cells were grown in nocodazole (noc) or arrested with mating pheromone (αf; 100 ng/ml) and released into the cell cycle at t = 0 and either harvested at the time of peak Cdk1-Y19 phosphorylation (t = 60 or 75 min post-release) or incubated with latA for 2 hr. GAL-SWE1 cells were grown in YEP + 2% raffinose, arrested in nocodazole, and then grown for 90 min after the addition of 2% galactose. Cells were harvested, lysed, and blotted with the indicated antibodies (top panels), or Cdk1/Clb2 complexes were immunoprecipitated with anti-Clb2 antibody and blotted with anti-Cdk1 and anti-Cdk1-P-tyr antibodies, or the Clb2-associated histone H1 kinase activity was assayed. The indicated ratios [average (±SEM) of three independent experiments] were calculated and normalized so that the ratio of synchronous mitotic wild-type cells (t = 60 min) is set to 1. (E) mih1∆ rts1∆ and mih1∆ ptp1∆ rts1∆ cells are inviable, and this inviablity is rescued by swe1∆. Tenfold serial dilutions of the indicated strains were spotted on SC-URA or SC + FOA medium and grown at 25°. All strains harbor a RTS1-CEN-URA3 plasmid, and their dependence on RTS1 was examined by selecting for loss of the plasmid using 5-FOA, which counterselects for URA3 cells.
mih1∆ ptp1∆ double mutants are viable and healthy, so we examined the effect of mutating both phosphatases on entry into and progression through mitosis. Deletion of MIH1 causes an increase in Cdk1-Y19 phosphorylation during mitosis, delays mitotic entry, and the length of mitosis increases (Lianga et al. 2013) (Figure 1B). ptp1∆ mutant cells behave similarly to wild-type cells, but in mih1∆ ptp1∆ double-mutant cells, Cdk1-Y19 levels remain high as the cells progress through mitosis and reenter G1. Surprisingly, despite this persistent phosphorylation, we observed no change in the timing of degradation of the mitotic cyclins Clb2 and Clb5, the anaphase inhibitor Pds1/securin, and the cohesin subunit Mcd1/Scc1 in mih1∆ ptp1∆ cells compared to mih1∆ cells. Although Cdk1-Y19 phosphorylation does not appear to drop as mih1∆ ptp1∆ cells exit mitosis, the lower levels present after G1 arrest caused by the mating pheromone α-factor (αf; t = 0) suggest that an additional phosphatase may function redundantly with Mih1 and Ptp1.
Although the level of Cdk1-Y19 phosphorylation in mih1∆ and mih1∆ ptp1∆ cells is high relative to wild-type cells, it is lower than the extent of phosphorylation in wild-type cells arrested by the actin-depolymerizing drug latrunculin A (latA), which activates a Swe1-dependent checkpoint, or in cells overexpressing Swe1 (GAL-SWE1) (Figure 1C). These two treatments arrest the cell cycle and depend on Y19 phosphorylation on Cdk1 (Booher et al. 1993; Lew and Reed 1995), suggesting that small differences in Cdk1-Y19 phosphorylation have large phenotypic effects.
We assessed the extent of inhibition of Cdk1 activity caused by Y19 phosphorylation in vivo by measuring the Clb2-associated histone H1 kinase activity in synchronous mitotic cells (Figure 1D). By normalizing the activity to the total Cdk1 present in each kinase reaction, we were able to compare the relative activity and Y19 phosphorylation of each Cdk1/Clb2 complex present in a cell. Cdk1 precipitated from synchronous mitotic mih1∆ and mih1∆ ptp1∆ cells (t = 75 min after release from G1 arrest) has approximately 65% of wild-type activity, while arrest caused by latA or GAL-SWE1 further reduces Cdk1 activity to 50% of wild type. The reduction in Cdk1 activity in these cells is mirrored by concomitant increases in Cdk1-Y19 phosphorylation. These measurements also allow us to infer the relative stoichiometry of Cdk1-Y19 phosphorylation and suggest that small decreases in Cdk1 activity (and increased Y19 phosphorylation) in latA- or GAL-SWE1-arrested cells halts cell-cycle progression. These data are consistent with our prior observation that ∼30% of the Cdk1 remains active during an arrest caused by GAL-SWE1 (Lianga et al. 2013).
Cdk1 precipitated from mitotic swe1∆ cells (t = 60 min) has threefold higher activity than Cdk1 from synchronous mitotic wild-type cells (t = 60 min) (Figure 1D), suggesting that two-thirds of the Cdk1 in mitotic wild-type cells is phosphorylated on Y19. We were surprised, however, that mitotic Cdk1 activity in wild-type cells does not differ from the activity of Cdk1 precipitated from wild-type or swe1∆ cells arrested in mitosis by nocodazole despite the lack of Cdk1-Y19 phosphorylation. We hypothesize that an unidentified mechanism reduces Cdk1 activity in nocodazole-arrested cells and may be used to control the increase in total Cdk1 activity as Clb2 protein rises during the arrest.
PP2ARts1 acts redundantly with Mih1 and Ptp1
Mutation of the B-regulatory subunits of PP2A, CDC55 and RTS1, also causes an increase in Cdk1-Y19 phosphorylation (Minshull et al. 1996; Zapata et al. 2014) (Figure 1A). cdc55∆ and rts1∆ are also synthetically lethal or result in sickness, respectively, in combination with mih1∆, and these interactions are completely suppressed by deletion of SWE1 (Pal et al. 2008; Costanzo et al. 2010) (Figure 1E). In addition, the temperature sensitivity of rts1∆ is partially suppressed by swe1∆ (Figure S1A). The increase in Cdk1-Y19 phosphorylation in these mutants and their interaction with mih1∆ can at least be partially attributed to upregulation of Swe1 protein in both cdc55∆ and rts1∆ cells (Minshull et al. 1996; Harvey et al. 2011; Zapata et al. 2014) and downregulation of Mih1 in cdc55∆ cells (Pal et al. 2008), but it also may be caused by other functions of PP2A.
To test whether PP2A regulates Cdk1-Y19 phosphorylation independently of its function regulating Swe1 and Mih1, we developed an in vivo phosphatase assay in which we monitor Cdk1-Y19 dephosphorylation in mitotically arrested cells that first induce high levels of Swe1 and Cdk1-Y19 phosphorylation using galactose induction of a GAL-SWE1-AID gene, then rapidly repress SWE1 transcription with glucose, and degrade the Swe1-AID protein with the addition of auxin (Figure 2). This experimental design allows us to assess the role of PP2A independently of its function regulating Swe1 and in the absence of Swe1-dependent rephosphorylation of Cdk1. GAL-SWE1-AID replaces the endogenous SWE1, so when grown on glucose, these strains behave effectively as swe1∆ cells, suppressing the lethality of several of the mutant combinations tested.
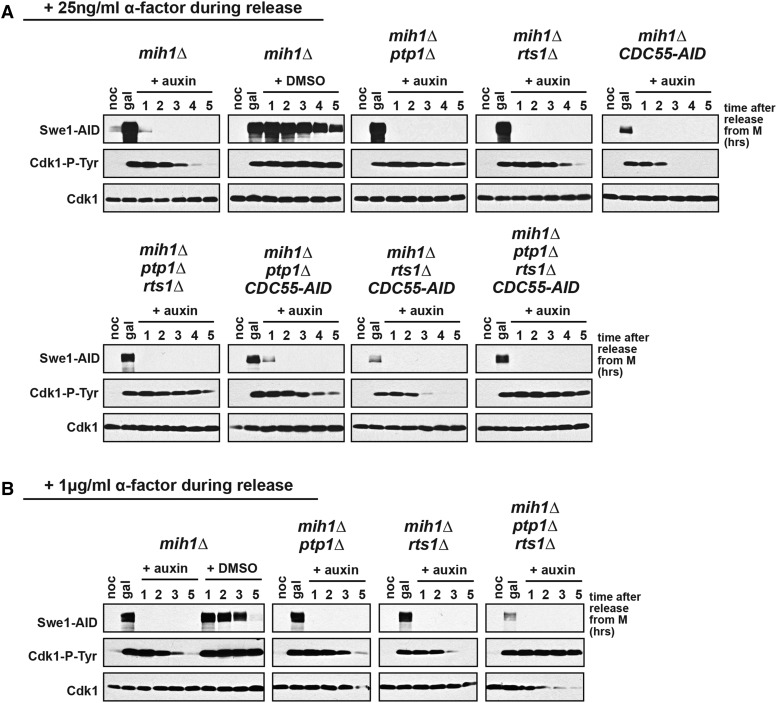
Figure 2.
MIH1, PTP1, and RTS1 redundantly regulate Cdk1-Y19 dephosphorylation. The indicated strains were grown overnight in YEP + raffinose at 25° and arrested in mitosis with nocodazole (noc), and then galactose was added to induce high levels of Swe1-AID and Cdk1-Y19 phosphorylation. After 1 hr of induction (gal), cells were released from the nocodazole arrest, dextrose was added to repress transcription of GAL-SWE1-AID, and auxin was added to induce Tir1-dependent degradation of Swe1-AID and Cdc55-AID, if present in the strain. Control mih1∆ cells also were released into medium containing dextrose and vehicle (DMSO). ⍺-factor was added 90 min after release at (A) 25 ng/ml or (B) 1 µg/ml in order to rearrest the cells in the subsequent G1. Cells were harvested at the indicated times for immunoblotting with the indicated antibodies.
In mih1∆ cells, Cdk1 is dephosphorylated between 2 and 4 hr after the addition of glucose and auxin (Figure 2, A and B). If only glucose, but not auxin, is added, Swe1-AID is degraded slowly, and Cdk1-Y19 phosphorylation persists throughout the experiment. Cdk1 is dephosphorylated slowly in mih1∆ ptp1∆ cells (Figure 2A), consistent with our observation that these cells are defective in the dephosphorylation of Cdk1-Y19 in anaphase (Figure 1B). To simplify our analysis of these experiments, we added αf during the release from nocodazole, arresting cells in the subsequent G1. We noticed that although adding either 25 ng/ml or 1 µg/ml of αf will arrest cells in G1 (Figure S2, A and B), Cdk1-Y19 dephosphorylation in mih1∆ ptp1∆ cells is similar to that in mih1∆ cells when treated with a higher concentration of αf (Figure 2B). However, the triple-mutant combination mih1∆ ptp1∆ rts1∆ completely blocks dephosphorylation of Cdk1 at both concentrations of pheromone (Figure 2, A and B), suggesting that PP2ARts1 may directly target Cdk1-Y19 or may negatively regulate an unidentified fourth phosphatase that targets Cdk1. Consistent with redundancy among these three phosphatases, the deletion of PTP1 in mih1∆ rts1∆ cells causes lethality that is fully suppressed by deletion of SWE1 (Figure 1E). Although mih1∆ ptp1∆ rts1∆ cells block Cdk1-Y19 dephosphorylation, they do not remain arrested in mitosis, as judged by the degradation of Clb2, Clb5, Pds1, and Mcd1 (Figure S2, A and B).
To investigate whether PP2ACdc55 also functions independently of Swe1 to regulate Cdk1-Y19 dephosphorylation, we used strains containing CDC55-AID rather than a deletion of CDC55 because cdc55∆ cells grow poorly in medium containing raffinose or galactose, both of which are used in this experiment (Figure S1B). In the presence of auxin, CDC55-AID is lethal in combination with mih1∆, and strains containing CDC55-AID grow poorly on raffinose and galactose medium (Figure S1, B and C), suggesting that CDC55-AID behaves as a null mutant when such cells are grown on auxin. In the in vivo phosphatase assay, mih1∆ CDC55-AID and mih1∆ ptp1∆ CDC55-AID cells induce lower levels of Swe1-AID, but enough is produced to allow the accumulation of high levels of Cdk1-Y19 phosphorylation (Figure 2A). As in mih1∆ cells, Cdk1 is dephosphorylated between 2 and 4 hr after the addition of glucose and auxin, suggesting that PP2ACdc55 regulates Cdk1-Y19 phosphorylation indirectly through Swe1 and Mih1. We were surprised that mih1∆ ptp1∆ CDC55-AID cells dephosphorylate Cdk1-Y19 more rapidly than mih1∆ ptp1∆ cells, suggesting that PP2ACdc55 may have an additional function negatively regulating Cdk1-Y19 dephosphorylation.
Ptp1 dephosphorylates Cdk1 in vitro
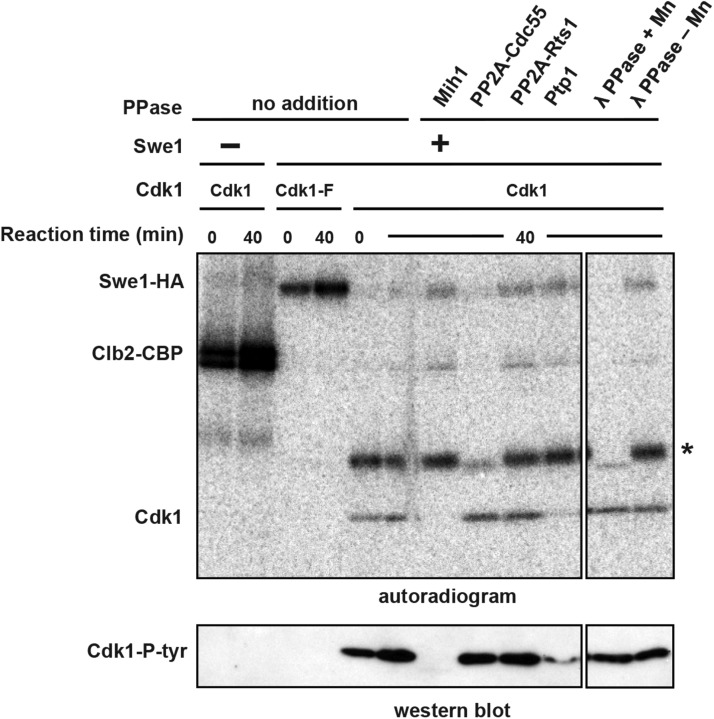
To validate our genetic results, we tested whether purified phosphatases can dephosphorylate Cdk1 in vitro (Figure S3). Immunopurified Swe1-phosphorylated Cdk1/Clb2 complexes were incubated with Mih1, Ptp1, PP2ACdc55, or PP2ARts1 purified from yeast, or with lambda phosphatase. Both Mih1 and Ptp1 can dephosphorylate Cdk1 in vitro, but both forms of PP2A and lambda phosphatase cannot (Figure 3). The dephosphorylation of a fortuitous background protein (indicated by an asterisk) confirms that purified PP2ACdc55 and lambda phosphatase are active in this experiment and that Mih1 and Ptp1, which do not dephosphorylate this protein, display Cdk1-Y19 specificity. PP2ARts1, which our prior experiments suggested may directly dephosphorylate Cdk1, had no activity in this experiment, but we have no evidence that the purified enzyme is active.
Figure 3.
Mih1 and Ptp1 dephosphorylate Cdk1-Y19 in vitro. Purified Cdk1/Clb2-CBP was phosphorylated by bead-bound Swe1-HA in the presence of γ-[32P]ATP. Following phosphorylation, Cdk1/Clb2-CBP was removed from the beads and mixed with purified phosphatases and an excess of unlabeled ATP to prevent rephosphorylation of Cdk1 by γ-[32P]ATP and Swe1-HA that was washed off the beads. Reactions were run on a polyacrylamide gel and either dried and exposed to a phosphorimager screen or transferred to nitrocellulose and immunoblotted with anti-Cdk1-P-Y19 antibody. In the absence of Swe1-HA, Clb2 is heavily phosphorylated by Cdk1. In the presence of Swe1-HA, Clb2 is not phosphorylated, showing that Cdk1 is completely inhibited by Swe1-dependent phosphorylation of Y19. Swe1 does not phosphorylate Cdk1-F, but Cdk1-F phosphorylates Swe1 (Harvey et al. 2005). After incubation for 40 min at 25°, purified Mih1 and Ptp1 (see Figure S3) can dephosphorylate Cdk1-Y19 in vitro, but PP2ACdc55, PP2ARts1, and lambda phosphatase (λ-PPase) cannot. PP2ACdc55 and λ-PPase can effectively dephosphorylate a fortuitous phosphorylated background protein (indicated with an asterisk), providing a control indicating that both PP2ACdc55 and λ-PPase are active.
Discussion
We have identified two phosphatases, Ptp1 and PP2ARts1, that work redundantly with Mih1 to regulate Cdk1-Y19 dephosphorylation. Past work had suggested that Ptp1 may regulate Cdk1-Y19 dephosphorylation in budding yeast (Hannig et al. 1993), and we have shown that combining deletions in PTP1 and MIH1 severely impairs Cdk1-Y19 dephosphorylation during anaphase. mih1∆ ptp1∆ cells still dephosphorylate Cdk1-Y19 during a prolonged G1 arrest, suggesting that at least one additional phosphatase may regulate Cdk1-Y19 dephosphorylation.
Cdk1-Y19 dephosphorylation in mih1∆ ptp1∆ cells could be caused by turnover of Cdk1 because newly synthesized Cdk1 would not be rephosphorylated by Swe1, which is absent in G1 (Figure 1B). Although we did not directly measure the half-life of Cdk1 in budding yeast, we think this mechanism is unlikely or plays only a minor function because the half-life of Cdk1 in cultured mammalian cells in G1 is ∼18 hr (Welch and Wang 1992; Gannon et al. 1998).
To test whether PP2A functions redundantly with Mih1 and Ptp1, we developed a novel in vivo phosphatase assay that allowed us to examine whether PP2A regulates Cdk1-Y19 phosphorylation independently of its known role regulating Swe1 and Mih1. We discovered that PP2ARts1 either directly dephosphorylates Cdk1-Y19 or inhibits a fourth phosphatase that does (Figure 2). In contrast, we have shown that PP2ACdc55 does not regulate Cdk1-Y19 dephosphorylation independently of its function inhibiting Swe1 and activating Mih1 and cannot dephosphorylate Cdk1-Y19 in vitro. Acute loss of Cdc55 using an auxin-inducible degron, in fact, appears to cause more rapid dephosphorylation of Cdk1-Y19, suggesting that PP2ACdc55 may negatively regulate Cdk1-Y19 dephosphorylation (Figure 2A). This function of PP2ACdc55 may work via PP2ARts1 because deletion of RTS1 is epistatic to this behavior of CDC55-AID.
Although we have demonstrated that purified Mih1 and Ptp1 dephosphorylate Cdk1-Y19 in vitro (Figure 3), we were unable to rigorously test PP2ARts1 function in vitro. PP2ARts1 substrates are thought to be phosphorylated only on serine and threonine residues (Janssens and Goris 2001; Shi 2009), but past work has shown that PP2A can acquire activity against tyrosine residues in vitro in specific reaction conditions or in the presence of PTPA (encoded by Rrd1 and Rrd2 in budding yeast), whose in vivo role is poorly understood (Cayla et al. 1990; Van Hoof et al. 1994; Van Hoof 2001).
We found it surprising that mih1∆ ptp1∆ cells initiate anaphase and enter G1 with little change in the level of Cdk1-Y19 phosphorylation. Our measurements of Cdk1-Y19 phosphorylation and Cdk1 activity in these cells suggest that an additional one-third of the active Cdk1/Clb2 complexes are inhibited in these cells compared to wild-type cells, which increases to half when cells are arrested by overexpression of Swe1 or activation of a SWE1-dependent checkpoint (Figure 1D). This difference suggests that there is sufficient Cdk1 activity in mih1∆ ptp1∆ cells to drive entry into mitosis and the metaphase-to-anaphase transition and that the difference between cell-cycle arrest and progression may reflect a small change in the extent of inhibition of Cdk1 or the targeted inhibition of a pool of Cdk1. Our data lead to the hypothesis that PP2ARts1 acts with Mih1 and Ptp1 to activate this pool of Cdk1 activity.
One aim of this study was to assess whether preventing Cdk1-Y19 dephosphorylation would block anaphase onset in a similar way as we have described in cells overexpressing Swe1 or arrested by latA treatment (Lianga et al. 2013). Although mih1∆ ptp1∆ cells block Cdk1-Y19 dephosphorylation during anaphase in an unperturbed cell cycle (Figure 1B) and mih1∆ ptp1∆ rts1∆ cells block dephosphorylation in the in vivo phosphatase assay (Figure 2), both mutant combinations do not block anaphase onset, as judged by activation of the anaphase-promoting complex (APC) and loss of the cohesin subunit Mcd1 (Figure S2). These results may indicate that Cdk1-Y19 dephosphorylation is not needed to trigger anaphase, but we think that it is more likely that Cdk1-Y19 phosphorylation does not reach the level needed for cell-cycle arrest in these experiments. rts1∆ cells, like CDC55-AID cells, induce GAL-SWE1-AID poorly, and lower levels of Cdk1-Y19 phosphorylation accumulate in these cells prior to the addition of auxin and dextrose (Figure 2).
Numerous studies have suggested that inhibitory phosphorylation regulates Cdk1 activation in a stepwise manner in both vertebrates and yeast (Stern and Nurse 1996; Pomerening et al. 2003, 2005; Deibler and Kirschner 2010; Harvey et al. 2011). Lower levels of Cdk1 activity are needed to induce the entry into mitosis than the metaphase-to-anaphase transition, and Cdk1-Y19 phosphorylation functions at both these thresholds (Rahal and Amon 2008; Lianga et al. 2013). Our results suggest that at least three phosphatases regulate Cdk1 dephosphorylation and that the regulation of their activity may provide a mechanism for cells to activate pools of Cdk1 at different times and in different cellular locations. PP2ARts1 is localized to the pericentromere (Gentry and Hallberg 2002; Kitajima et al. 2006; Riedel et al. 2006), and its activity there may allow kinetochore activation of Cdk1 and assist in triggering anaphase onset.
Cdc25 inhibitors have been pursued as promising chemotherapeutic agents that could block cell-cycle progression in cancerous cells, but these compounds have had only limited clinical success (Lavecchia et al. 2012). If similar redundancy exists in human cells as we have uncovered in budding yeast, then several different phosphatase inhibitors may be needed to effectively block Cdk1 activation and arrest the cell cycle.
Acknowledgments
We thank Thomas Eng and Doug Koshland for plasmids; Doug Kellogg, Mark Hall, Kathy Gould, Mike Downey, Hilary Phenix, Dara Spatz Friedman, and current and former members of the Rudner laboratory for invaluable discussions, technical advice, and unwavering support. Immunization of rabbits for antibody production was performed at the Ottawa University Animal Care Facility. This work was supported by grants from the Canadian Institutes of Health Research (177774), the Canada Foundation for Innovation (13119), and the University of Ottawa Research Development Program–Bridge Funding Opportunity and CIHR New Investigator and Ontario Early Researcher awards. E.K.K. was supported by an Ontario Graduate Scholarship; M.D. was supported by a Natural Sciences and Engineering Research Council Undergraduate Student Research Award; and N.L. and E.C.W. were supported by CIHR Canada Graduate Fellowships.
Footnotes
Communicating editor: S. Biggins
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.182469/-/DC1
Literature Cited
- Booher R. N., Deshaies R. J., Kirschner M. W., 1993. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 12: 3417–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayla X., Goris J., Hermann J., Hendrix P., Ozon R., et al. , 1990. Isolation and characterization of a tyrosyl phosphatase activator from rabbit skeletal muscle and Xenopus laevis oocytes. Biochemistry 29: 658–667. [DOI] [PubMed] [Google Scholar]
- Costanzo M., Baryshnikova A., Bellay J., Kim Y., Spear E. D., et al. , 2010. The genetic landscape of a cell. Science 327: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibler R. W., Kirschner M. W., 2010. Quantitative reconstitution of mitotic CDK1 activation in somatic cell extracts. Mol. Cell 37: 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Kumagai A., 1991. The cdc25 protein contains an intrinsic phosphatase activity. Cell 67: 189–196. [DOI] [PubMed] [Google Scholar]
- Foulkes J. G., Erikson E., Erikson R. L., 1983. Separation of multiple phosphotyrosyl- and phosphoseryl-protein phosphatases from chicken brain. J. Biol. Chem. 258: 431–438. [PubMed] [Google Scholar]
- Gannon J. V., Nebreda A., Goodger N. M., Morgan P. R., Hunt T., 1998. A measure of the mitotic index: studies of the abundance and half-life of p34cdc2 in cultured cells and normal and neoplastic tissues. Genes Cells 3: 17–27. [DOI] [PubMed] [Google Scholar]
- Gautier J., Solomon M. J., Booher R. N., Bazan J. F., Kirschner M. W., 1991. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell 67: 197–211. [DOI] [PubMed] [Google Scholar]
- Gentry M. S., Hallberg R. L., 2002. Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol. Biol. Cell 13: 3477–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Nurse P., 1989. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature 342: 39–45. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Moreno S., Tonks N. K., Nurse P., 1990. Complementation of the mitotic activator, p80cdc25, by a human protein-tyrosine phosphatase. Science 250: 1573–1576. [DOI] [PubMed] [Google Scholar]
- Hannig G., Ottilie S., Schievella A. R., Erikson R. L., 1993. Comparison of the biochemical and biological functions of tyrosine phosphatases from fission yeast, budding yeast and animal cells. Yeast 9: 1039–1052. [DOI] [PubMed] [Google Scholar]
- Harvey S. L., Kellogg D. R., 2003. Conservation of mechanisms controlling entry into mitosis: budding yeast wee1 delays entry into mitosis and is required for cell size control. Curr. Biol. 13: 264–275. [DOI] [PubMed] [Google Scholar]
- Harvey S. L., Charlet A., Haas W., Gygi S. P., Kellogg D. R., 2005. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell 122: 407–420. [DOI] [PubMed] [Google Scholar]
- Harvey S. L., Enciso G., Dephoure N., Gygi S. P., Gunawardena J., et al. , 2011. A phosphatase threshold sets the level of Cdk1 activity in early mitosis in budding yeast. Mol. Biol. Cell 22: 3595–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy A. M., Zolnierowicz S., Stapleton A. E., Goebl M., DePaoli-Roach A. A., et al. , 1991. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol. Cell. Biol. 11: 5767–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V., Goris J., 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353: 417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. R., 2003. Wee1-dependent mechanisms required for coordination of cell growth and cell division. J. Cell Sci. 116: 4883–4890. [DOI] [PubMed] [Google Scholar]
- Kitajima T. S., Sakuno T., Ishiguro K.-I., Iemura S.-I., Natsume T., et al. , 2006. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441: 46–52. [DOI] [PubMed] [Google Scholar]
- Lavecchia A., Di Giovanni C., Novellino E., 2012. CDC25 phosphatase inhibitors: an update. Mini Rev. Med. Chem. 12: 62–73. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Reed S. I., 1995. A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J. Cell Biol. 129: 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianga N., Williams E. C., Kennedy E. K., Doré C., Pilon S., et al. , 2013. A Wee1 checkpoint inhibits anaphase onset. J. Cell Biol. 201: 843–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. B., Lenaers G., Russell P., 1992. Pyp3 PTPase acts as a mitotic inducer in fission yeast. EMBO J. 11: 4933–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Straight A., Rudner A. D., Dernburg A. F., Belmont A., et al. , 1996. Protein phosphatase 2A regulates MPF activity and sister chromatid cohesion in budding yeast. Curr. Biol. 6: 1609–1620. [DOI] [PubMed] [Google Scholar]
- Nurse P., 1975. Genetic control of cell size at cell division in yeast. Nature 256: 547–551. [DOI] [PubMed] [Google Scholar]
- Nurse P., 1990. Universal control mechanism regulating onset of M-phase. Nature 344: 503–508. [DOI] [PubMed] [Google Scholar]
- Pal G., Paraz M. T. Z., Kellogg D. R., 2008. Regulation of Mih1/Cdc25 by protein phosphatase 2A and casein kinase 1. J. Cell Biol. 180: 931–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L. L., Atherton-Fessler S., Piwnica-Worms H., 1992. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc. Natl. Acad. Sci. USA 89: 2917–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerening J. R., Sontag E. D., Ferrell J. E., 2003. Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat. Cell Biol. 5: 346–351. [DOI] [PubMed] [Google Scholar]
- Pomerening J. R., Kim S. Y., Ferrell J. E., 2005. Systems-level dissection of the cell-cycle oscillator: bypassing positive feedback produces damped oscillations. Cell 122: 565–578. [DOI] [PubMed] [Google Scholar]
- Rahal R., Amon A., 2008. Mitotic CDKs control the metaphase-anaphase transition and trigger spindle elongation. Genes Dev. 22: 1534–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel C. G., Katis V. L., Katou Y., Mori S., Itoh T., et al. , 2006. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441: 53–61. [DOI] [PubMed] [Google Scholar]
- Rudner A. D., Hardwick K. G., Murray A. W., 2000. Cdc28 activates exit from mitosis in budding yeast. J. Cell Biol. 149: 1361–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P., 1986. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell 45: 145–153. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P., 1987. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell 49: 559–567. [DOI] [PubMed] [Google Scholar]
- Russell P., Moreno S., Reed S. I., 1989. Conservation of mitotic controls in fission and budding yeasts. Cell 57: 295–303. [DOI] [PubMed] [Google Scholar]
- Shi Y., 2009. Serine/threonine phosphatases: mechanism through structure. Cell 139: 468–484. [DOI] [PubMed] [Google Scholar]
- Shu Y., Yang H., Hallberg E., Hallberg R., 1997. Molecular genetic analysis of Rts1p, a B′ regulatory subunit of Saccharomyces cerevisiae protein phosphatase 2A. Mol. Cell. Biol. 17: 3242–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., 1996. Yeast protein serine/threonine phosphatases: multiple roles and diverse regulation. Yeast 12: 1647–1675. [DOI] [PubMed] [Google Scholar]
- Stern B., Nurse P., 1996. A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet. 12: 345–350. [PubMed] [Google Scholar]
- Tonks N. K., 2013. Protein tyrosine phosphatases—from housekeeping enzymes to master regulators of signal transduction. FEBS J. 280: 346–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof C., 2001. The Saccharomyces cerevisiae phosphotyrosyl phosphatase activator proteins are required for a subset of the functions disrupted by protein phosphatase 2A mutations. Exp. Cell Res. 264: 372–387. [DOI] [PubMed] [Google Scholar]
- Van Hoof C., Cayla X., Bosch M., Merlevede W., Goris J., 1994. The phosphotyrosyl phosphatase activator of protein phosphatase 2A: a novel purification method, immunological and enzymic characterization. Eur. J. Biochem. 226: 899–907. [DOI] [PubMed] [Google Scholar]
- Welch P. J., Wang J. Y., 1992. Coordinated synthesis and degradation of cdc2 in the mammalian cell cycle. Proc. Natl. Acad. Sci. USA 89: 3093–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata J., Dephoure N., Macdonough T., Yu Y., Parnell E. J., et al. , 2014. PP2ARts1 is a master regulator of pathways that control cell size. J. Cell Biol. 204: 359–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Boguslawski G., Zitomer R. S., DePaoli-Roach A. A., 1997. Saccharomyces cerevisiae homologs of mammalian B and B’ subunits of protein phosphatase 2A direct the enzyme to distinct cellular functions. J. Biol. Chem. 272: 8256–8262. [DOI] [PubMed] [Google Scholar]