Abstract
SWI/SNF ATP-dependent chromatin-remodeling complexes have been related to several cellular processes such as transcription, regulation of chromosomal stability, and DNA repair. The Caenorhabditis elegans gene ham-3 (also known as swsn-2.1) and its paralog swsn-2.2 encode accessory subunits of SWI/SNF complexes. Using RNA interference (RNAi) assays and diverse alleles we investigated whether ham-3 and swsn-2.2 have different functions during C. elegans development since they encode proteins that are probably mutually exclusive in a given SWI/SNF complex. We found that ham-3 and swsn-2.2 display similar functions in vulva specification, germline development, and intestinal cell proliferation, but have distinct roles in embryonic development. Accordingly, we detected functional redundancy in some developmental processes and demonstrated by RNA sequencing of RNAi-treated L4 animals that ham-3 and swsn-2.2 regulate the expression of a common subset of genes but also have specific targets. Cell lineage analyses in the embryo revealed hyper-proliferation of intestinal cells in ham-3 null mutants whereas swsn-2.2 is required for proper cell divisions. Using a proteomic approach, we identified SWSN-2.2-interacting proteins needed for early cell divisions, such as SAO-1 and ATX-2, and also nuclear envelope proteins such as MEL-28. swsn-2.2 mutants phenocopy mel-28 loss-of-function, and we observed that SWSN-2.2 and MEL-28 colocalize in mitotic and meiotic chromosomes. Moreover, we demonstrated that SWSN-2.2 is required for correct chromosome segregation and nuclear reassembly after mitosis including recruitment of MEL-28 to the nuclear periphery.
Keywords: SWI/SNF, Caenorhabditis elegans, chromatin, development, nuclear envelope
CHROMATIN is a dynamic structure required not only for the packaging of large amounts of DNA in the limited space of eukaryotic nuclei, but also for the regulation of gene expression (Ho and Crabtree 2010; Narlikar et al. 2013). SWI/SNF complexes, which are conserved from yeast to mammals, modify the state of the chromatin in an ATP-dependent manner and, therefore, the accessibility of distinct proteins to a given DNA region (Hargreaves and Crabtree 2011; Euskirchen et al. 2012). Such activity in the DNA regulates various cellular aspects like proliferation, differentiation, chromosomal stability, and DNA repair (Reisman et al. 2009; Lans et al. 2010; Euskirchen et al. 2011). SWI/SNF complexes are involved in gene-specific regulation since only a low percentage of gene expression (6% in budding yeast, 7.5% in Caenorhabditis elegans) is regulated by these complexes (Holstege et al. 1998; Riedel et al. 2013).
A canonical SWI/SNF complex consists of a central ATPase subunit, two or three core components, and several (five to eight) accessory subunits (Hargreaves and Crabtree 2011). While all SWI/SNF complexes include core subunits that are in charge of remodeling nucleosomes (Phelan et al. 1999), the accessory proteins confer specificity to a given complex and their presence varies depending on the tissue and/or cellular state (Weissman and Knudsen 2009; Euskirchen et al. 2012). Traditionally, SWI/SNF complexes have been classified into two subclasses, named BAF/BAP or PBAF/PBAP, depending on their signature subunits (Figure S1A). The human accessory subunits BAF60a/SMARCD1, BAF60b/SMARCD2, and BAF60c/SMARCD3 and their worm homologs HAM-3 and SWSN-2.2 derive from the same evolutionary ancestor and are expected to belong to both subclasses of complexes (Shibata et al. 2012; Weinberg et al. 2013) (Figure S1 and Figure S2). The three human BAF60 proteins, which present ∼60% of similarity in their amino acid sequences, are mutually exclusive in a given SWI/SNF complex displaying distinct expression patterns and functions in humans (Oh et al. 2008; Puri and Mercola 2012; Jordan et al. 2013; Watanabe et al. 2014). BAF60c for example, is specifically required for the transcription of myogenic-specific genes and, consequently, muscle differentiation (Forcales et al. 2012). Importantly, alterations in these three BAF proteins have been associated with the progression of diverse types of cancer such as neuroblastoma, breast cancer, and lung cancer (Weissman and Knudsen 2009). Beyond cancer, mutations in SWI/SNF components contribute to the pathogenesis of other disorders, including viral infections, intellectual disability, and muscular dystrophy (Santen et al. 2012; Berdasco and Esteller 2013; Masliah-Planchon et al. 2015).
ham-3 and swsn-2.2 are paralog genes with 67% similarity at the amino acid sequence level (Figure S3). These two genes have previously been related to several developmental processes and pathways in C. elegans. ham-3 and swsn-2.2 exhibit RNA interference (RNAi) phenotypes in vulva development and fertility, present a synthetic genetic interaction with lin-35/Rb (Cui et al. 2004; Ceron et al. 2007), and are implicated in somatic gonad development (Large and Mathies 2014). In addition to these common functions, ham-3 has been described to be involved in neuronal specification and in the transcriptional regulation of specific microRNAs (Hayes et al. 2011; Weinberg et al. 2013). Differently from ham-3, loss of swsn-2.2 produces Emb (embryonic lethality) and Psa (phasmid socket absent; specific cells acquiring hypodermal fate instead of the neuronal fate) phenotypes at high penetrance (Sawa et al. 2000; Large and Mathies 2014).
Although ham-3 and swsn-2.2 have been associated with various developmental mechanisms, the functional interplay of the two proteins in different stages and tissues has not been formally studied. We have compiled mutant alleles for the two genes and isolated he159, a new allele for ham-3. In addition, we used RNAi to uncover functional redundancies masked by the strong phenotypes caused by null alleles. We also performed embryonic lineage analyses, RNA sequencing, and proteomics to provide a comprehensive study of these two paralogs during development. We further investigated one of our findings to uncover a functional link between SWSN-2.2 and the nuclear envelope structure.
Materials and Methods
Strains and maintenance
Standard methods were used to culture and manipulate worms (Stiernagle 2006). Wild-type strain Bristol N2 and the following mutant and reporter strains were used: BN311 bqIs311 [gfp::mel-28] II, CER30 ham-3(he159) III/hT2 (I;III); rtIs18[Pelt-2::gfp] I, CER31 swsn-2.2(ok3161) I/hT2 (I;III); rtIs18[Pelt-2::gfp] I, CER123 ham-3(he159) III/hT2 (I;III), HA661 rtIs18[Pelt-2::gfp] I, MT2124 let-60(n1046) IV, MT3971 ham-3(n1654) III, RA440 swsn-2.2(tm3395) I/hT2 (I;III), RA459 ham-3(tm3309) III/hT2 (I;III), VC2789 swsn-2.2(ok3161) I/hT2 (I;III), MT12839 lin-61(n3809) I; lin-8(n2731) II, and PS3972 unc-119(ed4) syIs90 [egl-17::yfp + unc-119(+)] III.
RNA interference
RNAi by feeding was performed on NGM plates supplemented with 50 µg/ml ampicillin, 12.5 µg/ml tetracycline, and 3 mM IPTG. RNAi clones were validated by PCR and/or sequencing. Synchronized L1 populations were seeded on the RNAi clones and incubated at 20° or 25°. The RNAi clone for ham-3 was taken from the ORFeome library. In the case of swsn-2.2, no RNAi clone was available. To generate one, complementary DNA (cDNA) of N2 animals was used as template, and PCR using specific primers for swsn-2.2 was performed. Using the MultiSite Gateway Vector Construction Kit, the insert was cloned into the pGC49 vector and transformed into the bacterial strain DH5α. Once amplified and purified, the plasmid was transformed into the definitive bacterial host strain HT115.
Video recording and lineage analysis
Embryos used for the lineage analyses described in this work were prepared and mounted as described by Sulston et al. (1983). Gravid hermaphrodites were dissected, and two- to four-cell-stage embryos were mounted on 4% agar pads in water and sealed with Vaseline. Four-dimensional microscopy (3D of the embryo + time) was carried out using a multifocal plane and a time-lapse recording system. The device was based on a Leica DM 6000 microscope fitted with Nomarski microscopy. Recordings were made using a 100×/1.4 PL APO objective, and the temperature was kept constant at 25°. The microscope was controlled with the open-source software Micro-manager. Pictures from 30 focal planes (1 μm/section) were taken every 30 sec for up to 12 hr. The SIMI Biocell software allowed tracing of the cell lineage of the embryos in time and space as well as tracing of the mitoses and apoptosis. Lineages of apoptotic cells were followed until the onset of the dead cell, and then the corpse was followed until it was engulfed and disappeared, as described in Nieto et al. (2010).
Immunofluorescence
Gonads were dissected and fixed in a glass multi-well plate (Pyrex Plate, catalog no. 71563-01) as described in Fontrodona et al. (2013). For blocking, the fixed gonads were incubated with PBS–Tween + 0.1% BSA for 1 hr at room temperature (RT). Incubation with primary antibodies was performed at 4° overnight, and incubation with secondary antibodies for 2 hr at RT. Gonads were counterstained with DAPI included in mounting media. Immunostaining of embryos was performed following the Freeze-Crack protocol (Askjaer et al. 2014). Q5536 is a polyclonal antibody generated upon our request against the first 89 amino acids of the N terminus of SWSN-2.2. Antibodies were used at the following dilutions: anti-SWSN-2.2 (SDIX, Q5536; 1:500), anti-GFP (Molecular Probes, A11120, 1:200), anti-MEL-28 (BUD3; 1:250), mAb414 (Covance MMS-120R; 1:250), anti-rabbit (Abcam, Alexa Fluor 568; 1:500), and anti-mouse (Abcam, Alexa Fluor 488; 1:500).
RNA sequencing
RNA from gfp(RNAi), ham-3(RNAi), and swsn-2.2(RNAi) L4 animals was isolated with TRIReagent and purified by using the Purelink RNA Mini kit (Ambion) and DNAse I (Ambion). Quality of RNA samples was analyzed on the Agilent 2010 Bioanalyzer. Samples were multiplexed in libraries for RNA sequencing on an Illumina HiSeq 2000 platform through the Centre Nacional d’Anàlisi Genòmica sequencing facility at the Barcelona Parc Científic. More than 50 million reads for the sample were mapped against the C. elegans worm version WS236 following the GEMTools pipeline (http://gemtools.github.io/). The resulting BAM files were analyzed using SeqSolve software, which uses Cufflinks/Cuffdiff for differential gene/transcript expression analyses (Trapnell et al. 2012). The sequence data for the three transcriptomes analyzed in this study are available at the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus website (http://www.ncbi.nlm.nih.gov/geo/) under accession no. GSE75703.
Co-immunoprecipitation and mass spectrometry
For immunoprecipitation, the Dynabeads Co-immunopreciptation Kit was used. The included immunoprecipitation (IP) buffer was modified with 100 mM NaCl and protease and phosphatase inhibitors. Mixed populations or synchronized young adult N2 animals were harvested, washed with the modified IP buffer, and added dropwise into liquid nitrogen to produce worm pearls. These pearls were grounded to a fine powder in liquid nitrogen using a mortar and pestle and allowed to thaw on ice. By centrifugation, solid particles were separated from the protein extract. Antibody-conjugated beads were prepared using 2 mg of Dynabeads and 10 μg of antibody per reaction. The antibodies used were anti-SWSN-2.2 (Q5536) and unspecific anti-rabbit (TEBU-BIO, 036SC-2027). For immunoprecipitation, 3 mg of total protein was brought to a volume of 1 ml with modified IP buffer. A total of 1.5 mg of antibody-conjugated beads was added to the protein sample and incubated for 20 min at 4° under rotation. Subsequent washing steps were performed according to the manufacturer’s instructions.
For mass spectrometry, complexes were eluted by resuspending the beads for 30 sec in 50 μl glycin, pH 2.5, followed by neutralization with 5 μl Tris–HCl, pH 10.4. Complexes were precipitated with ice-cold acetone overnight. Pellets were resuspended with 6 M urea/200 mM NH4HCO3 and digested with trypsin. Then 45% of the co-immunoprecipitation (ChIP) samples and 0.5% of the Input were injected in an Orbitrap XL. BSA controls were included both in the digestion and the liquid chromatography-tandem mass spectrometry analyses for quality control. The data were analyzed using an internal version of the search algorithm Mascot (http://www.matrixscience.com) against an NCBI C. elegans (February 2014) database. Peptides were filtered based on peptide score. The protein/peptide identification information was obtained from Proteome Discoverer software (v1.4.1.14).
RT-PCR
Synchronized worm populations were harvested and frozen in TRI Reagent, and total RNA was isolated using standard methods. To eliminate DNA contamination, the DNAse I Amplification Grade system (Invitrogen) was used. cDNA was synthesized with oligo(dT) primers using the RevertAid H Minus First Strand cDNA synthesis kit (Thermo Scientific) following the manufacturer’s instructions. Semiquantitative PCR was performed using the BIOTAQ PCR kit. For the quantification of gene expression levels, the Roche LightCycler 480 Instrument I and SYBR green I Master kit were used. Gene expression was normalized to transcript levels of the housekeeping gene act-1.
Results
Mutant alleles for ham-3 and swsn-2.2
ham-3(he159) is a novel deletion allele with phenotypes similar to other ham-3 mutants:
Recently, two ham-3 alleles, n1654 and tm3309, have been described (Weinberg et al. 2013; Large and Mathies 2014). While n1654 is a point mutation, tm3309 produces a 243-bp deletion and a 4-bp insertion. Both alleles result in premature termination codons and presumably in the degradation of their transcripts by the nonsense-mediated decay pathway (Figure 1). We tested this assumption by RT-PCR with cDNA from both mutant strains, and none of the ham-3 transcripts was detected (Figure S4). Therefore, we conclude that n1654 and tm3309 are null alleles.
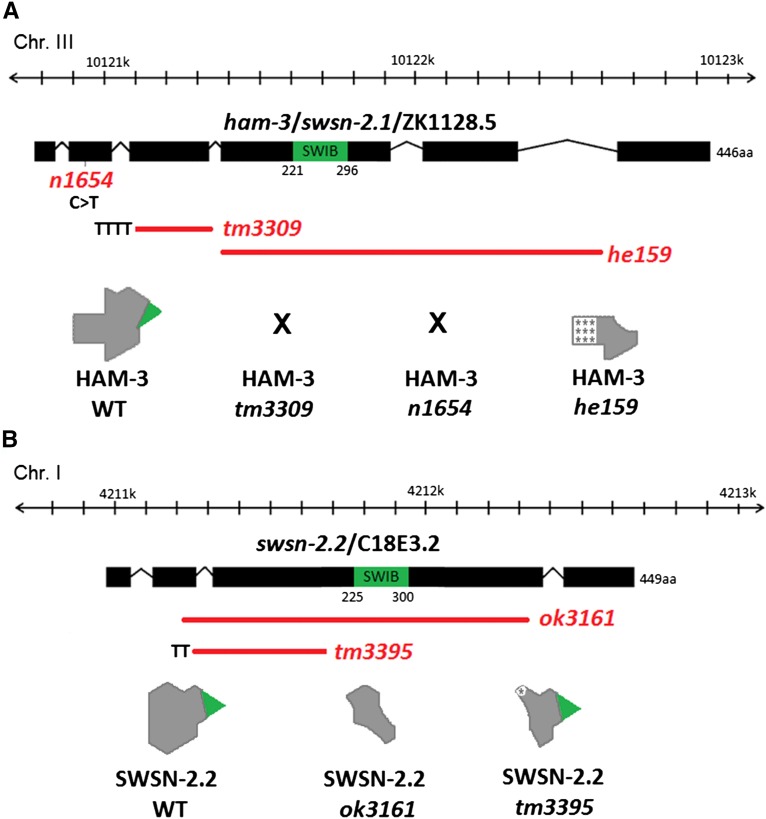
Figure 1.
Scheme of ham-3 and swsn-2.2 mutant alleles and their expected gene products. The SWIB domain is labeled in green. Red bars represent deletions. Letters indicate insertions or transitions. In the drawing for the expected protein products, asterisks in the gray background indicate amino acids that are different from the original sequence. (A) ham-3(n1654) and ham-3(tm3309) are putative null alleles. ham-3(he159) encodes a truncated protein lacking the central SWIB domain. (B) Both swsn-2.2 mutant alleles produce truncated proteins. While the product encoded by swsn-2.2(ok3161) lacks the central domain, swsn-2.2(tm3395) gives a chimeric protein containing the SWIB motif.
From a deletion library we isolated the novel ham-3 allele he159, which consists of a 1211-bp deletion that causes a frameshift and encodes a truncated protein [189 vs. 446 amino acids (aa)] (Figure 1). The predicted protein lacks a region of 257 aa including the central SWIB domain and is identical only to the wild-type protein in the first 154 aa (Figure S5). RT-PCR confirmed that the he159 allele produces a shorter transcript (Figure S4).
All three ham-3 alleles (he159, n1654, and tm3309) present similar phenotypes such as short body length (Sma), egg-laying defects (Egl), adult lethality (Adl), and protruding vulva (Pvl) (Table 1 and Figure S6). Due to these phenotypic similarities and the absence of the SWIB domain in the product encoded by he159, we conclude that he159 most likely also represents a null mutation.
Table 1. Temperature dependence of some ham-3 and swsn-2.2 mutant phenotypes.
| Phenotype: | Pvl (%) | Egl (%) | Adl (%) | |||
|---|---|---|---|---|---|---|
| Temperature: | 15° | 25° | 15° | 25° | 15° | 25° |
| ham-3(tm3309) [m+,z−] | 100 | 72.5 | 100 | ND (Ste) | 87.2 | 52 |
| ham-3(n1654) [m−,z−] | 62.2 | 80.7 | 100 | 100 | 91.7 | 100 |
| ham-3(he159) [m−,z−] | 59.9 | 100 | 100 | 100 | 80 | 90 |
| swsn-2.2(ok3161) [m+,z−] | 89.3 | 91.6 | 37.5 | 45.8 | 11.1 | 50 |
| swsn-2.2(tm3395) [m−,z−] | 58.7 | 95 | 0 | 6.25 | 35 | 35 |
To score the Protruding vulva (Pvl) phenotype, homozygote animals were grown at 15°, gravid mothers were dissected, and the progeny were incubated at the respective temperatures until reaching adult stage (n ≥ 122). To investigate Egg-laying defects (Egl), homozygote animals were grown at 15° until reaching L4 stage, singled out, and incubated at the indicated temperatures. Mothers that accumulated embryos and/or died due to the Bag of worms (Bag) or Rupturing vulva (Rup) phenotypes were regarded as Egl (n ≥ 30). Animals that died within 96 or 72 hr (at 15° or 25°, respectively) were scored as adult lethal (Adl). [m+ or m−] indicates maternal; [z+ or z−] indicates zygotic contribution of the respective wild-type protein.
Two swsn-2.2 alleles, ok3161 and tm3395, produce truncated proteins:
The swsn-2.2 in-frame deletion allele ok3161 lacks 1122 bp and encodes a truncated form of SWSN-2.2 (91 aa instead of 449 aa) in which the SWIB domain is absent (Figure 1 and Figure S7). RT-PCR of swsn-2.2(ok3161) animals confirmed the presence of a truncated transcript (Figure S4). Although this mutation had previously been described as inviable due to a larval arrest phenotype (Lva) (Weinberg et al. 2013), others and we have observed homozygous swsn-2.2(ok3161) adult worms (Large and Mathies 2014). swsn-2.2(ok3161) adults present dramatic levels of embryonic lethality (Figure 4A), and the F1 escaper larvae do not reach adulthood (Figure S8). Therefore, in contrast to ham-3 alleles, swsn-2.2(ok3161) cannot be maintained in an unbalanced form. Homozygote swsn-2.2(ok3161) worms deriving from heterozygous mothers display some phenotypes that are also observed in ham-3 alleles, such as Sma, Adl, Pvl, and Egl (Table 1 and Figure S6).
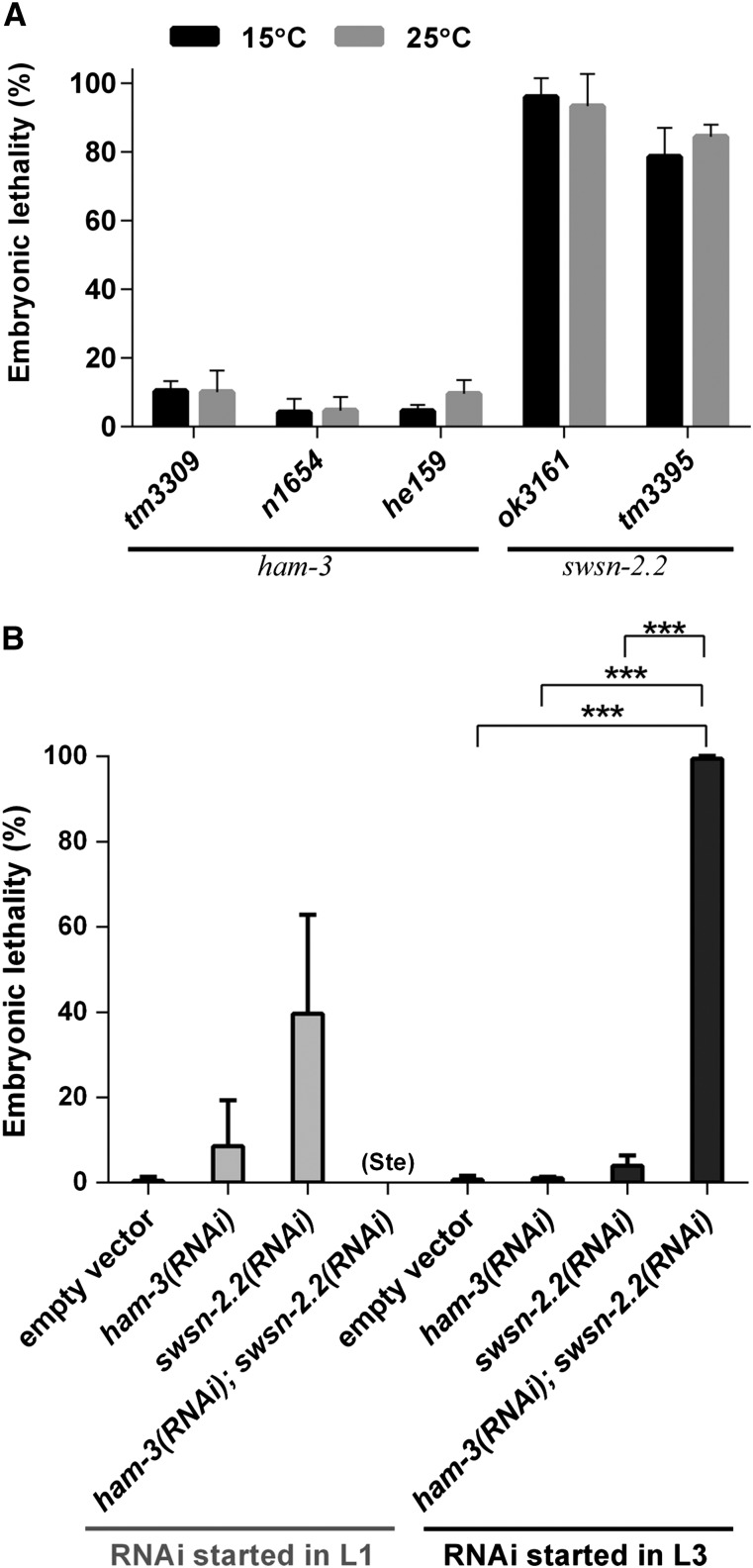
Figure 4.
Embryonic lethality caused by RNAi and mutations of ham-3 and swsn-2.2. (A) swsn-2.2 mutants display higher embryonic lethality than ham-3 mutants. Homozygote mutants (F1) were grown at 15° until reaching the adult stage and were then dissected, and the embryos (F2) were incubated at the indicated temperatures. Twenty-four hours after dissection, the number of eggs unable to hatch was determined (n ≥ 371). Apart from swsn-2.2(ok3161) and ham-3(tm3309), all dissected animals derived from homozygote mothers (P0). (B) RNAi assays uncover a functional redundancy between ham-3 and swsn-2.2 in embryonic development. Starting the RNAi at the L3 stage, the animals fed with both RNAi clones bypassed the sterile phenotype, observed when RNAi starts at L1, and a synthetic embryonic phenotype was observed. Error bars indicate the standard deviation between two independent experiments. The statistical significance was calculated based on a two-tailed student’s t-test. ***P-value ≤ 0.001.
The second mutant allele for swsn-2.2, tm3395, has recently been characterized (Large and Mathies 2014). swsn-2.2(tm3395) is an in-frame deletion–insertion (deletion of 421 bp and insertion of 4 bp) mutation that encodes a truncated product (Figure S4 and Figure S7). The putative truncated protein lacks 127 aa that are replaced by 3 aa but, differently from the rest of the alleles described here, the central SWIB domain is retained (Figure 1). Like swsn-2.2(ok3161) mutants, swsn-2.2(tm3395) worms display embryonic lethality and exhibit several of the phenotypes observed in ham-3 alleles (Table 1). However, the phenotypes are milder in swsn-2.2(tm3395) than in swsn-2.2(ok3161) animals and, as a consequence, swsn-2.2(tm3395) can be maintained as a homozygous strain.
In summary, mutant alleles for ham-3 and swsn-2.2 share some phenotypes, suggesting a partial functional overlap between the two paralogs. We next investigated to what extent their functions are redundant, shared, or unique during development.
ham-3 and swsn-2.2 have redundant functions in germline and vulva development
ham-3 and swsn-2.2 function in gonad development:
Both ham-3 and swsn-2.2 mutants display reduced brood size. We quantified the progeny of these mutants and observed temperature-dependent fertility (Figure S8). To some extent, the reduced number of progeny laid by ham-3 and swsn-2.2 mutants was caused by egg-laying defects (Egl) and adult lethality (Adl) (Table 1). Still, we observed that ham-3 and swsn-2.2 mutants have smaller germ lines (Figure S9), and, as previously reported, some mutant animals presented gonadogenesis defects including the lack of one gonad arm (Large and Mathies 2014).
Since the double mutant for ham-3 and swsn-2.2 dies at early larval stages (Large and Mathies 2014), we used RNAi to investigate whether the two genes have redundant functions in germline development. First, we constructed an RNAi clone for swsn-2.2 since this gene was not represented in any of the two RNAi libraries available in C. elegans (Kamath et al. 2003; Rual et al. 2004). Then, we validated the efficiency of the ham-3 or swsn-2.2 RNAi feeding clones by RT-PCR (Figure S10).
Started at L1 stage, double RNAi of ham-3 and swsn-2.2 caused a synthetic sterile phenotype due to the incapacity to produce embryos (Figure 2A and Figure S11). These sterile animals developed smaller germ lines that were able to produce oocytes and sperm, but accumulated unfertilized endomitotic oocytes (Emo) (Figure S11).
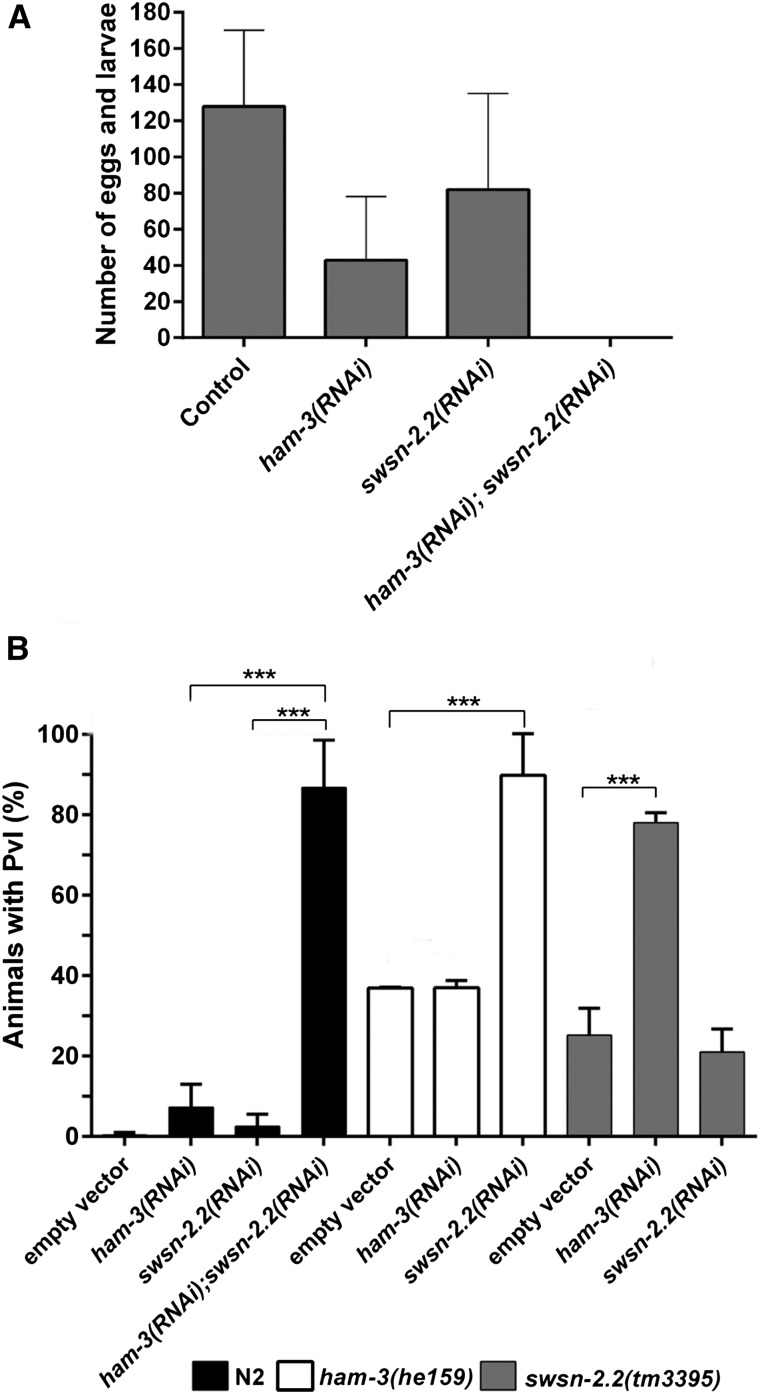
Figure 2.
ham-3 and swsn-2.2 act redundantly in fertility and vulva development. (A) RNAi of ham-3 or swsn-2.2 causes reduced brood size, but double RNAi results in sterility. Synchronized L1 larvae (n ≥ 24) were grown on the indicated RNAi plate at 25° and their progeny were counted. Error bars indicate the standard deviations in the brood sizes determined in two independent experiments. (B) ham-3 and swsn-2.2 act redundantly in vulval induction. Wild type (N2) (n ≥ 86) or mutants (n ≥ 58) were seeded on the indicated RNAi plates at L1 stage. Experiments with N2 were performed at 25°, and the experiments with ham-3 or swsn-2.2 mutants were performed at 20°. The number of animals showing Pvl was determined at the young adult stage. Error bars indicate the standard deviation between two independent experiments. The statistical significance was calculated with a two-tailed student’s t-test. ***P-value ≤ 0.001.
ham-3 and swsn-2.2 act in vulval induction through the let-60/Ras pathway:
The Pvl phenotype observed in ham-3 and swsn-2.2 mutants could be the consequence of alterations in diverse signaling pathways (Wnt, EGF, and Notch) acting in Vulval Precursor Cells (VPCs) and/or uterine tissues during postembryonic development (Sternberg 2005; Gupta et al. 2012). RNAi of ham-3 and swsn-2.2 in wild-type and mutant animals produced a genetic synthetic interaction in vulva development as the percentage of Pvl animals was synergistically higher when both genes were simultaneously inactivated (Figure 2B).
To further explore the function of ham-3 and swsn-2.2 in vulval induction, we performed RNAi assays in the lin-61(n3809); lin-8(n2731) synMuv mutant and in a let-60/Ras gain-of-function background (n1046 allele). Both strains display a multivulva (Muv) phenotype due to excessive induction of VPCs (Sternberg and Han 1998; Ceol et al. 2006; Andersen et al. 2008), and ham-3(RNAi) and swsn-2.2(RNAi) enhanced the Muv phenotype in both mutant backgrounds (Figure S12).
While it cannot be dismissed that the two proteins are involved in more than one aspect of the regulation of vulva development, all our data indicate that ham-3 and swsn-2.2 act redundantly to negatively regulate the induction of VPCs. To further validate their involvement in vulva induction, we used a reporter for the expression of egl-17 (Inoue et al. 2002), which is a target of the Ras-signaling pathway (Yoo et al. 2004). We observed that ham-3(RNAi); swsn-2.2(RNAi) animals show ectopic expression of egl-17 in cells derived from the vulval precursor cells P5.p and P7.p (Figure S13). Therefore, our results indicate that the regulation of the vulval development by ham-3 and swsn-2.2 happens, at least to some extent, through the let-60/Ras pathway.
Number of postembryonic intestinal nuclei is controlled by ham-3 and swsn-2.2
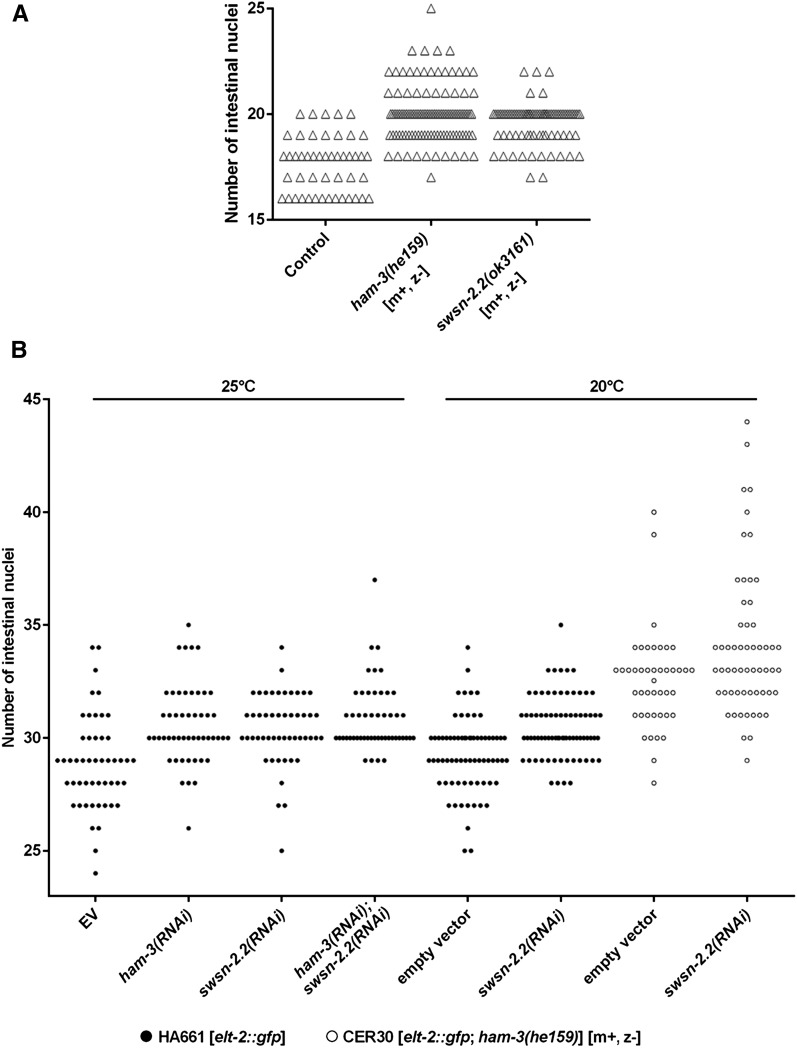
Since SWI/SNF complexes functionally interact with cell-cycle regulators in C. elegans (Cui et al. 2004; Ruijtenberg and van den Heuvel 2015), we investigated the role of ham-3 and swsn-2.2 in the control of the intestinal cell cycle. We took advantage of the Pelt-2::GFP reporter to score the number of intestinal nuclei at L1 stage in ham-3 and swsn-2.2 mutants and observed an increase of the number of intestinal nuclei (Figure 3A). Similarly to the mutant alleles, RNAi of ham-3 and swsn-2.2 produced additional postembryonic intestinal nuclei (Figure 3B). This hyper-proliferative effect seems to be additive when both genes are inactivated using the ham-3(he159) mutation in combination with swsn-2.2(RNAi) (Figure 3B).
Figure 3.
ham-3 and swsn-2.2 regulate the number of intestinal nuclei. (A) The number of intestinal nuclei of homozygous ham-3(he159) and swsn-2.2(ok3161) mutants bearing a Pelt-2::gfp reporter was determined at the L1 stage (n ≥ 48). In both cases mutants came from heterozygous mothers [m+, z−] and were grown at 20°. [m+ or m−] indicates maternal, and [z+ or z−] indicates zygotic contribution of the respective protein. (B) The number of GFP-positive intestinal nuclei was determined at the L4 stage in Pelt-2::gfp animals (n ≥ 44). Due to the sickness of ham-3(he159) mutants at high temperatures, experiments with this allele were performed at 20°.
Distinct and overlapping functions of ham-3 and swsn-2.2 in embryonic development
ham-3 and swsn-2.2 cooperate in embryonic development, but only swsn-2.2 is essential in the early embryo:
The scoring of the embryonic lethality of ham-3 and swsn-2.2 mutants revealed a dramatic difference: while the three ham-3 alleles produced a low percentage of dead embryos, the embryonic lethality in swsn-2.2 mutants ranged from 80 to 100% in swsn-2.2(ok3161) animals (Figure 4A).
Through RNAi of ham-3 and swsn-2.2 we investigated whether these two genes cooperate in any embryonic developmental process. The simultaneous inactivation of these genes by RNAi starting at the L1 stage abolished the production of embryos and therefore produced sterility (Figure 4B). Thus, to study the effect of the loss of both proteins on embryonic lethality, we started the RNAi treatment at later developmental stages. Interestingly, starting at L2/L3, double RNAi of swsn-2.2 and ham-3 uncovered a synthetic embryonic phenotype (Figure 4B) in which embryos die at later stages than swsn-2.2(ok3161) embryos.
ham-3 regulates the number of embryonic intestinal cells and other Wnt-regulated embryonic processes:
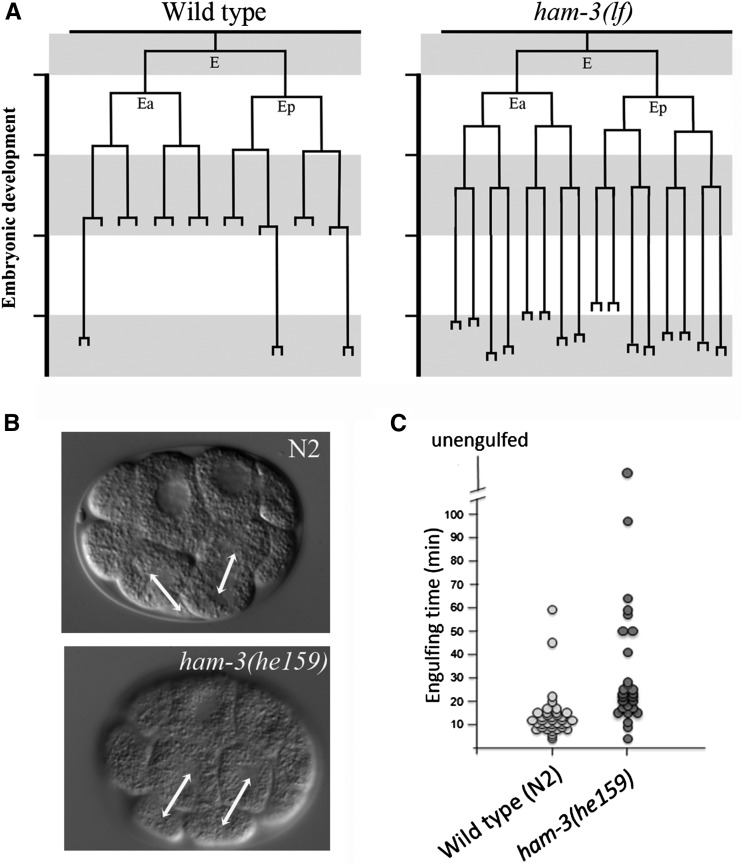
We analyzed the embryonic lineages of ham-3(he159) animals by performing 4D video recording and lineage analyses. Interestingly, we observed that all the ham-3(he159) embryos analyzed (n = 12) had extra cell divisions in the intestinal lineage only (Figure 5A and Figure S14). We confirmed the intestinal hyperplasia of ham-3 mutant embryos by lineage analysis in embryos bearing the ham-3(n1654) allele (Figure 5A and Figure S14).
Figure 5.
Embryonic phenotypes of ham-3 mutants. (A) ham-3(he159) and ham-3(n1654) mutants [represented as ham-3(lf)] exhibit additional cell divisions in the E lineage. (B) ham-3(he159) embryo displaying an abnormal orientation of the mitotic spindle of the embryonic ABar cell. (C) ham-3(he159) embryos show a delay in the engulfment of apoptotic bodies.
We also detected two ham-3(he159) phenotypes that are characteristic for mutants with defects in the Wnt-signaling pathway or its effectors (Cabello et al. 2010; Gómez-Orte et al. 2013). For example, in 1 of 12 analyzed embryos we observed a defect in the orientation of the mitotic spindle of the ABar cell at the eight-cell stage (Figure 5B) (Rocheleau et al. 1997; Herman 2002). The slow engulfment of apoptotic corpses was another phenotype that is shared between ham-3(he159) and mutants affecting the Wnt signaling (Figure 5C).
Thus, we conclude that, in the embryo, ham-3 is involved in the control of the intestinal nuclei number and in the regulation of developmental processes driven by the Wnt pathway.
swsn-2.2 is required for early embryonic cell divisions:
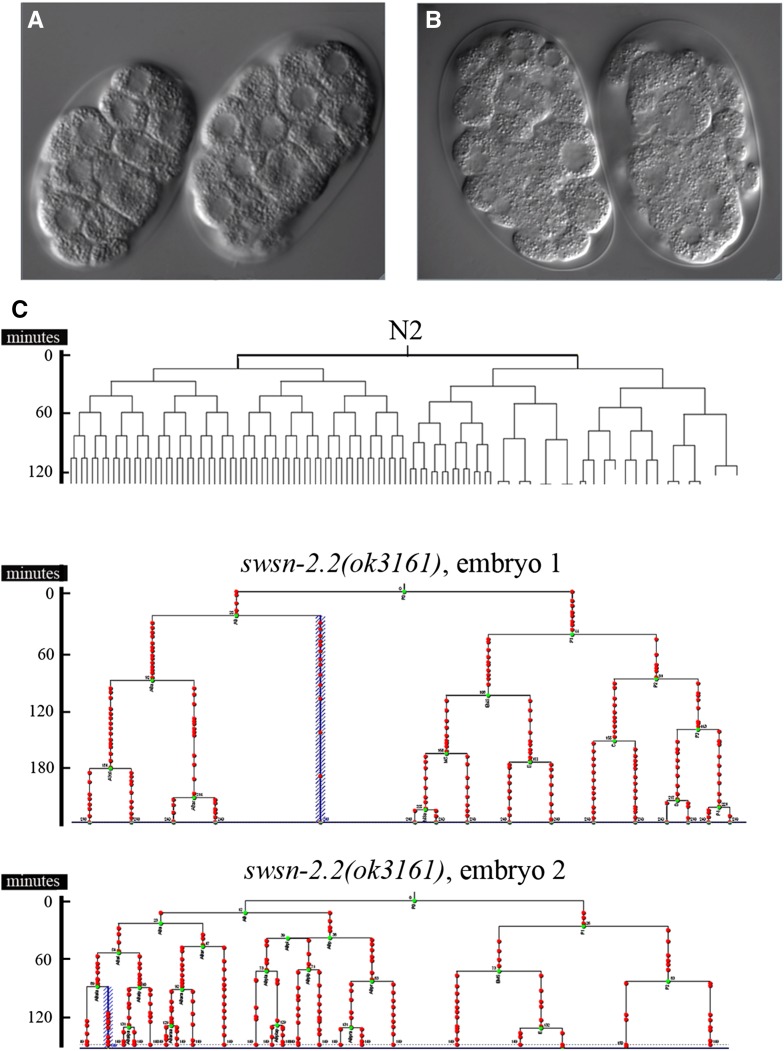
As mentioned above, the loss-of-function alleles swsn-2.2(ok3161) and swsn-2.2(tm3395) cause high rates of embryonic lethality (Figure 4A). 4-D video recording and lineage analysis of swsn-2.2(ok3161) embryos showed that the failure in embryogenesis occurs early in development (File S1 and File S2). The swsn-2.2(ok3161) embryos exhibited severe defects in early cell divisions that resulted in embryos with nuclei of different sizes (Figure 6, A and B). Such abnormal cell division does not allow the lineage analysis later in development and causes the death of the embryo before the comma stage (Figure 6C; File S3; File S4).
Figure 6.
swsn-2.2 mutants present cell division defects in the early embryo. (A) N2 wild-type embryos after few rounds of cell division. The size of nuclei in each embryo is uniform. (B) Early swsn-2.2(ok3161) embryos show nuclei of different sizes, which are indicative of defective cell divisions. (C) Wild-type embryonic cell lineage for the first cell divisions and cell lineages of two swsn-2.2(ok3161) embryos produced by homozygote mothers. Green and red spots indicate cell divisions and points of lineage scoring, respectively. The aberrant cell division pattern in swsn-2.2(ok3161) embryos hampers the lineage analysis after few divisions and causes the death of the embryos before the comma stage.
In summary, null activity of ham-3 and swsn-2.2 produces different embryonic phenotypes indicating specific functions. However, partial depletion of both genes by RNAi unmasks a functional cooperation of these paralogs during embryonic development.
Identification of SWSN-2.2 interactors uncovers a functional link with the nuclear envelope
To identify potential SWSN-2.2 interactors, endogenous SWSN-2.2 was pulled down from two biological replicates of extracts from mixed-stage worm populations. A third replicate of the experiment was performed with a synchronized population of young adult animals. As validation of our approach, mass spectrometry of the Co-IPed complexes identified several SWI/SNF subunits (Table 2). The absence of HAM-3 from any of the three replicates reflects that these two paralogs, like their human counterparts, are mutually exclusive in a given SWI/SNF complex.
Table 2. Some of the proteins identified as interactors of SWSN-2.2 by co-immunoprecipitation and mass spectrometry.
| Mixed stage (replicate 1) | Mixed stage (replicate 2) | Young adults | ||
|---|---|---|---|---|
| Protein | Function | No. of peptides | No. of peptides | No. of peptides |
| SWSN-1 | SWI/SNF subunit | 11 | 16 | 35 |
| SWSN-2.2 | SWI/SNF subunit | 6 | 10 | 12 |
| SWSN-3 | SWI/SNF subunit | 1 | 5 | 13 |
| SWSN-4 | SWI/SNF subunit | 0 | 4 | 13 |
| SWSN-5 (SNFC-5) | SWI/SNF subunit | 0 | 2 | 7 |
| SWSN-6 | SWI/SNF subunit | 0 | 2 | 11 |
| SWSN-7 | SWI/SNF subunit | 0 | 1 | 0 |
| SWSN-8 (LET-526) | SWI/SNF subunit | 0 | 0 | 15 |
| SWSN-9 | SWI/SNF subunit | 0 | 0 | 4 |
| PBRM-1 | SWI/SNF subunit | 0 | 0 | 3 |
| PHF-10 | SWI/SNF subunit | 0 | 0 | 3 |
| SAO-1 | Early embryo | 1 | 4 | 4 |
| ATX-2 | Early embryo | 0 | 3 | 6 |
| NPP-2 | Nuclear envelope | 0 | 1 | 0 |
| NPP-9, isoform a | Nuclear envelope | 2 | 1 | 0 |
| MEL-28 | Nuclear envelope | 1 | 0 | 0 |
| HUM-5 | Myosin-related | 10 | 0 | 0 |
| HUM-2 | Myosin-related | 2 | 0 | 0 |
| MLC-6 | Myosin-related | 3 | 1 | 0 |
| MLC-7 | Myosin-related | 4 | 0 | 0 |
| TNT-4 | Myosin-related | 4 | 0 | 0 |
| C30H6.7 | Acetyltransferase | 7 | 4 | 3 |
| TAF-9 | Transcription | 1 | 1 | 1 |
| ZK973.9 | Arp2/3 complex | 0 | 2 | 2 |
This table lists some of the SWSN-2.2 interactors identified. The full list is available in Table S1. While the first two replicates were performed with mixed developmental stages, a synchronized population of young adults was used for the third experiment. The total number of interactors identified was 64 (mixed stage, replicate 1), 57 (mixed stage, replicate 2), and 99 (young adult stage).
In addition to several SWI/SNF subunits, we found interacting proteins required for early embryogenesis such as SAO-1 and ATX-2 (Kiehl et al. 2000; Hale et al. 2012) (Table 2 and Table S1). Interestingly, we also identified nuclear envelope components, such as the nucleoporins NPP-2, NPP-9, and MEL-28 (Galy et al. 2003, 2006) (Table 2).
The study of the biological interactions between SWSN-2.2 and some of the potential partners identified in this work may expand the catalog of SWSN-2.2 functions. We find the interaction of SWSN-2.2 with a set of myosin-related proteins particularly interesting, since some unconventional myosins have been related to the mitotic spindle dynamics and the regulation of gene expression (Woolner and Bement 2009; Sarshad et al. 2013).
SWSN-2.2 colocalizes with MEL-28 and is required for nuclear reassembly after mitosis and correct chromosome inheritance in the early embryo:
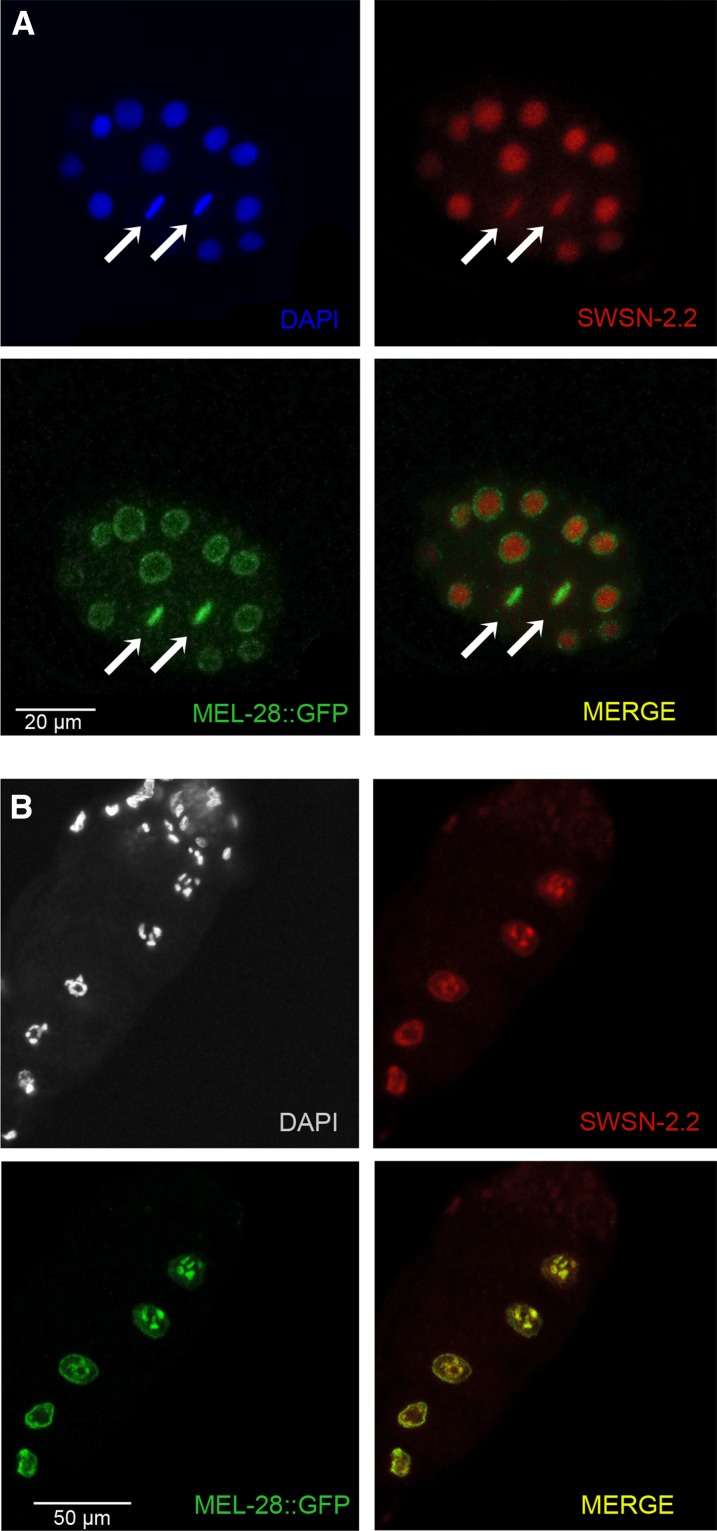
Since mel-28(t1684) and swsn-2.2(ok3161) mutants present similar phenotypes (adults that produce dead embryos at an early stage displaying chromosomal segregation defects) (Galy et al. 2006), chromatin and the nuclear envelope are mechanistically coupled (Mattout et al. 2015), and SWI/SNF proteins copurify with nuclear pore proteins in mouse embryonic stem cells (Ho et al. 2009), we decided to further investigate the functional relationship between these two genes. Taking advantage of a strain expressing GFP::MEL-28, we performed immunofluorescence to test if SWSN-2.2 and MEL-28 colocalize. In interphasic embryonic cells, SWSN-2.2 is diffusely localized in the nucleoplasm, whereas MEL-28 is present at the nuclear envelope and in the nuclear interior (Figure 7A). During mitosis, both proteins associate to chromosomes (Figure 7A). In oocytes, the two proteins localize to meiotic chromosomes and to a variable degree to the nuclear envelope, depending on the oocyte maturation stage (Figure 7B).
Figure 7.
SWSN-2.2 and GFP::MEL-28 colocalize in early embryos and in oocytes. Immunofluorescence with anti-SWSN-2.2 and anti-GFP antibodies in transgenic animals expressing GFP::MEL-28. All images are the projection of three confocal sections of 1 µm. (A) Arrows indicate mitotic chromosomes of early embryonic cells. (B) SWSN-2.2 and GFP::MEL-28 colocalize in the nuclear membrane and in meiotic chromosomes of developing oocytes.
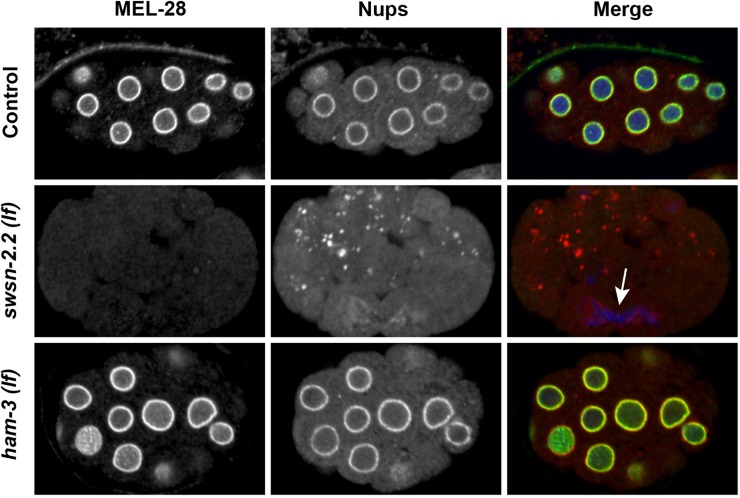
Finally, using the mutant swsn-2.2(ok3161), we observed that SWSN-2.2 is required for nuclear reassembly after mitosis and for the recruitment of MEL-28 to the nuclear periphery in the early embryo and in the adult germline (Figure 8 and Figure S15). On the contrary, ham-3(he159) animals did not show any of these embryonic defects (Figure 8). This further supports that ham-3 and swsn-2.2 have distinct functions in the early embryo.
Figure 8.
SWSN-2.2 is required for correct chromosome inheritance and postmitotic nuclear reassembly in early embryos. Immunostaining with specific antibodies against MEL-28 and mAb414 against several nuclear pore proteins (Nups) in early swsn-2.2(ok3161) and ham-3(he159) embryos shows distinct functions of HAM-3 and SWSN-2.2 in cell division. While absence of functional swsn-2.2 impairs nuclear reassembly and correct chromosome segregation, these processes are not affected by ham-3(lf). In swsn-2.2 mutants, the MEL-28 signal is strongly reduced, whereas Nups accumulate in cytoplasmic aggregates. The arrow indicates chromatin trapped in a cleavage furrow.
Transcriptomic analyses identify common and specific targets of HAM-3 and SWSN-2.2
Finally, to further explore the common and distinct functions of ham-3 and swsn-2.2, the transcriptomes of L4 animals fed with ham-3(RNAi) or swsn-2.2(RNAi) for 36 hr at 25° were compared with that of a control population fed with gfp(RNAi). The reason to choose the L4 stage for this experiment was that somatic and germline genes could be targeted. Beyond that stage, some animals die during the reproductive phase in response to ham-3(RNAi) and swsn-2.2(RNAi). At the stage at which the animals were harvested they begin to display the Protruding Vulva phenotype.
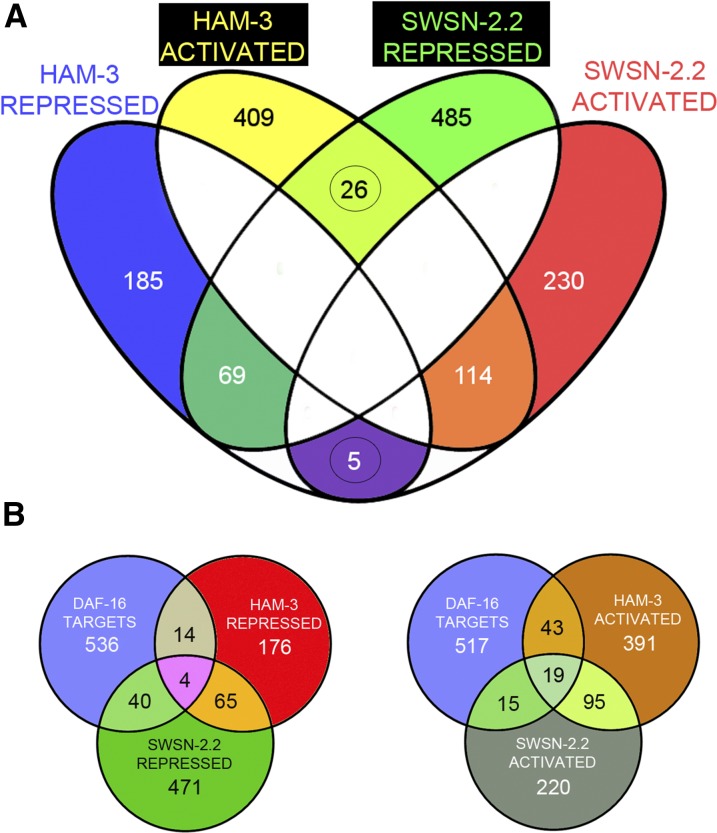
We detected a significant overlap (P ≤ 0,0001, χ2 test) between genes up- or downregulated in ham-3(RNAi) and swsn-2.2(RNAi) animals. Thus, we identified 183 genes that may be coregulated (69 repressed and 114 activated) by both HAM-3 and SWSN-2.2 (Figure 9A and Table S2), suggesting that either paralog could be present in the complexes that control the expression of those genes. In addition, we found genes the expression of which seems to be preferentially affected by one of the two paralogs. The number of genes activated or repressed in ham-3(RNAi) and in swsn-2.2(RNAi) worms suggests that HAM-3 has a major role in gene activation whereas SWSN-2.2 is preferentially involved in gene repression (Figure 9A). We performed Gene Ontology term analyses and found that HAM-3 and SWSN-2.2 function on diverse general biological processes (Figure S16). The influence of both genes in the immune system process is particularly interesting since it has been demonstrated that SWI/SNF proteins physically interact with DAF-16 to regulate DAF-16 targets (Riedel et al. 2013), and DAF-16 itself is the effector of the Insulin/IGF-1 Signaling (IIS) pathway that is involved in the innate immune response (Jensen et al. 2010).
Figure 9.
Transcriptomic analysis of ham-3(RNAi) and swsn-2.2(RNAi) animals. (A) Genes whose expression is activated or repressed by ham-3 or swsn-2.2. L1 animals were fed for 36 hr at 25° with ham-3(RNAi), swsn-2.2(RNAi) or gfp(RNAi) as a control. The 31 genes that seem to be antagonistically regulated by ham-3 and swsn-2.2 are not statistically significant (P ≤ 0.96). The overlaps between genes activated and repressed by both genes were significant with P-values ≤ 0.0001. Expression of five of these genes was analyzed by quantitative PCR, and we detected a differential rather than an antagonistic regulation (Figure S18). (B) HAM-3 and SWSN-2.2 targets significantly overlap with DAF-16 targets (P ≤ 0.0001). A list of 594 genes regulated by DAF-16 was retrieved from Pinkston-Gosse and Kenyon (2007) and compared with genes activated or repressed by HAM-3 or SWSN-2.2. Overlap significance was calculated with χ2 tests with Yates’ correction.
We investigated whether there is an overlap between HAM-3- and SWSN-2.2-regulated genes and a list of DAF-16 targets (Pinkston-Gosse and Kenyon 2007), and we found a significant number of DAF-16 targets among the genes activated or repressed by ham-3 and swsn-2.2 (Figure 9B). We further analyzed our RNA-sequencing data searching for genes deregulated in daf-16 and swsn-1 young adult mutants (Riedel et al. 2013). Although our transcriptomic study was performed at L4, we found that ∼20% of the HAM-3 and SWSN-2.2 targets are also deregulated in daf-16 and/or swsn-1 mutants (Figure S17).
In summary, HAM-3 and SWSN-2.2 influence the transcriptional activity of common genes but also have specific targets. This is in agreement with the overlapping and distinct roles that we describe during development. The differential occupancy of promoter regions of DAF-16 targets suggested by our study, however, needs further investigation by ChIP sequencing.
Discussion
In cancer, as in other diseases, SWI/SNF genes are found inactivated in multiple manners. Mutations in these genes can be somatic or originated in the germ line and produce loss-of-function to different extents. In addition to deletions and point mutations, DNA rearrangements and epigenetic marks can modify SWI/SNF activities (Shain and Pollack 2013; Romero and Sanchez-Cespedes 2014). In our study we analyzed the consequences of the inactivation of two SWI/SNF paralogs by diverse mutations and RNAi. ham-3 and swsn-2.2 double mutants are not viable, but RNAi allows the modulation of loss-of-function effects and thereby the uncovering of new functional relations. Thus, only by RNAi was it possible to identify a functional redundancy between ham-3 and swsn-2.2 in fertility, vulval induction, and embryonic development. The synthetic phenotypes between SWI/SNF subunits help to understand the function of these chromatin remodelers in different processes, but also present an opportunity for therapies (Helming et al. 2014). Today, the emerging CRISPR/Cas9 approaches to editing the genome might fuel the investigation of functional interactions between specific loss-of-function mutations of SWI/SNF genes (Frøkjær-Jensen 2013).
Redundant, shared, and unique functions
Functions of SWI/SNF subunits have been cataloged as redundant, shared, or unique (Bezhani et al. 2007). Others and we have found these three modes of interaction between ham-3 and swsn-2.2. While swsn-2.2 has a unique function in early embryonic cell divisions, only ham-3 affects the development of the hermaphrodite specific neurons (HSNs) (Desai et al. 1988; Weinberg et al. 2013). Since the HSNs stimulate the vulva muscle cells to extrude embryos, the high penetrance of the Egl phenotype in animals where ham-3 was inactivated can be explained by this neuronal defect.
We found that ham-3 and swsn-2.2 act redundantly in developmental processes such as germline or vulva development. This redundancy implies that either HAM-3 or SWSN-2.2 can be part of the SWI/SNF complexes regulating these processes.
Finally, a shared function is assigned when the simultaneous inactivation of both genes does not cause a clear synthetic phenotype, suggesting regulation by more than one SWI/SNF complex. Our data suggest that the control of the intestinal cell number could be a function shared by HAM-3 and SWSN-2.2.
Role in germline development
Double inactivation of ham-3 and swsn-2.2 by RNAi produces endomitotic oocytes (Emo phenotype) resulting in sterility. The Emo phenotype can be caused by defects of the somatic gonad that block the maturation signals to oocytes (McCarter et al. 1997). Since BAF and PBAF complexes have previously been related to C. elegans somatic gonad development (Cui et al. 2004; Shibata et al. 2012; Large and Mathies 2014), HAM-3 and SWSN-2.2 could be involved in the onset of somatic gonad precursors (SGPs) as well as in the differentiation of the cells derived from these SGPs. In fact, ham-3 and swsn-2.2 have been identified as enhancers of the ehn-3(rd2) mutation, which is a mild allele of a gene specifically expressed in SGPs and required for the correct development of the somatic gonad (Large and Mathies 2014). The somatic gonad influences germ-cell proliferation, which may explain the reduced germ-cell number observed when ham-3 and swsn-2.2 activities are inhibited (Kimble and Crittenden 2005; Korta and Hubbard 2010). Given the functional relationship between SWI/SNF complexes and DAF-16, another way for HAM-3 and SWSN-2.2 to play a role in fertility is through the control of germline proliferation orchestrated by the insulin-signaling pathway in somatic tissues (Michaelson et al. 2010; Qi et al. 2012). Still, a direct function of ham-3 and swsn-2.2 in germ cell proliferation cannot be dismissed.
Role in vulva development
We have demonstrated that during postembryonic development ham-3 and swsn-2.2 inhibit the induction of the vulva by acting, at least partially, through the let-60/Ras pathway. The zinc-finger protein SOMI-1 binds the promoter of let-60 to repress its gene expression and therefore inhibit the induction of the VPCs (Hayes et al. 2011). Hayes and coworkers described that HAM-3 cooperates with SOMI-1 in the differentiation of hypodermal cells (Hayes et al. 2011). Therefore, a direct inhibitory action of HAM-3, and maybe of SWSN-2.2, on the promoter of let-60 could explain their role in repressing vulva induction. Moreover, considering that ham-3 genetically interacts with lin-35/Rb (Ceron et al. 2007), which is a synthetic Multivulva class B gene (synMuv B genes produce a multivulva phenotype when a synMuv A or C gene is inactivated in parallel), we cannot discard a role for ham-3 and swsn-2.2 in the synMuv pathway that also represses the induction of the vulva (Fay and Yochem 2007).
Cell-cycle control
A recent study in C. elegans has shown that cell-cycle inhibitors and SWI/SNF subunits regulate the cell-cycle exit (Ruijtenberg and van den Heuvel 2015). Our lineage analyses of ham-3 loss-of-function alleles showed an increase of the intestinal cell number due to extra cell divisions during embryogenesis. This additional round of intestinal cell division indicates a misregulation of the cell-cycle exit and mimics the embryonic phenotypes caused by cki-1 loss-of-function mutations rather than those of cdc-25.1 gain-of-function mutants where cells divide much faster (Clucas et al. 2002; Kostić and Roy 2002). This fact, and the somatic gonad phenotypes caused by the inactivation of cki-1 (Kostić et al. 2003; Fujita et al. 2007), suggest that ham-3, swsn-2.2, and the G1/S inhibitor cki-1 may be functionally related in more than one developmental process.
SWSN-2.2 and the nuclear envelope in the early embryo
Concurrently with previous publications, we observed that inactivation of swsn-2.2 results in embryonic lethality (Sawa et al. 2000; Large and Mathies 2014). In addition, we found that SWSN-2.2 localizes to mitotic chromosomes of early embryonic cells, which is consistent with the aberrant pattern of cell divisions observed in swsn-2.2(ok3161) mutants. Consistently, other SWI/SNF subunits have been found to be associated with chromosomes of diverse postembryonic cell types in C. elegans (Shibata et al. 2012).
We found protein–protein interactions between SWSN-2.2 and nuclear pore proteins. Nuclear pore proteins also copurify with SWI/SNF factors in mouse embryonic stem cells (Ho et al. 2009). However, although it is accepted that chromatin and the nuclear envelope are mechanistically coupled (Mattout et al. 2015), it is quite speculative how components of both structures coordinate their functions. The small interfering RNA knockdown of the SWI/SNF core component BRG1 produces nuclear shape changes (Imbalzano et al. 2013). Thus, the interaction between SWSN-2.2 and nuclear envelope proteins helps in understanding the nuclei of different sizes observed in swsn-2.2(ok3161) embryos.
We observed that SWSN-2.2 and MEL-28 colocalize on mitotic chromosomes in the early embryo. The nuclear envelope breaks down and re-establishes during every round of the cell cycle, and MEL-28 is required for this process in the C. elegans embryo. During interphase MEL-28 is associated with nuclear pore complexes, but it is present at the kinetochores at the onset of mitosis and on chromatin in late mitosis, where it is required for the correct segregation of the chromosomes and for nuclear pore complex assembly (Fernandez and Piano 2006; Galy et al. 2006). Similarly to swsn-2.2(ok3161) mutants, inactivation of mel-28 causes changes in nuclear morphology and abnormal distribution of nuclear pore complexes. We have shown that SWSN-2.2 is not only involved in chromosome segregation but also necessary for the recruitment of MEL-28 to chromatin to induce nuclear reassembly after mitosis.
Therefore, the interaction between SWSN-2.2 and MEL-28 is physical and functional. swsn-2.2 was not found as one of the mel-28 interactors during postembryonic development in C. elegans (Fernandez et al. 2014), suggesting that their functional interaction may occur only at specific developmental stages.
In this study we show how during embryonic development SWSN-2.2, but not its paralog HAM-3, is required for the proper formation of the nuclear envelope. A recent RNAi-based study of chromatin regulators during C. elegans embryogenesis suggests the existence of at least two functionally distinct SWI/SNF complexes in the early embryo (Krüger et al. 2015), supporting the different implications of HAM-3 and SWSN-2.2 in embryonic development demonstrated in our work.
SWI/SNF proteins and the IIS pathway
SWI/SNF proteins physically interact with the transcription factor DAF-16/FOXO to regulate the expression of DAF-16 targets (Riedel et al. 2013). Stress conditions diminish the insulin signaling and increase the amount of nuclear DAF-16. In agreement with a functional correlation between SWI/SNF proteins, DAF-16, and stress, BAF SWI/SNF complexes have been shown to be involved in the stress response through the IIS pathway (Kuzmanov et al. 2014). However, in the absence of stress, we also found that many genes regulated by ham-3 and swsn-2.2 were DAF-16 known targets, which might reflect primarily the presence of HAM-3 and SWSN-2.2 in the promoter region of such DAF-16-regulated genes. Therefore, while SWI/SNF complexes were described to bind predominantly genes activated by DAF-16 under stress conditions (Riedel et al. 2013), we found that HAM-3 and SWSN-2.2 may also have a role in activation and in repression of DAF-16 targets in the absence of stress. ChIP experiments should not only determine to what extent these accessory subunits overlap with DAF-16-binding sites, but also identify genes specifically and differently regulated by HAM-3 or SWSN-2.2.
We have demonstrated that HAM-3 and SWSN-2.2 could have a common and specific impact on diverse cancer-related processes such as the control of the cell cycle or cell division. Moreover, we have related these two SWI/SNF subunits with cancer-related pathways such as Ras or Wnt. The tissue specificity and the synthetic phenotypes reported for these SWI/SNF accessory subunits should encourage deeper research due to their potential for future therapies.
Acknowledgments
Some strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). I.E. was supported by an IDIBELL (Bellvitge Biomedical Research Institute) Ph.D. fellowship. J.C. is a Miguel Servet Researcher (ISCIII). This study was supported by a grant from the Instituto de Salud Carlos III (ISCIII) (PI12/01554), which is cofunded by FEDER funds/European Regional Development Fund—a way to build Europe. The P.A. laboratory was supported by funding from the Spanish Ministry of Economy and Competitiveness (BFU2013-42709-P).
Footnotes
Communicating editor: D. I. Greenstein
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.183533/-/DC1.
Literature Cited
- Andersen E. C., Saffer A. M., Horvitz H. R., 2008. Multiple levels of redundant processes inhibit Caenorhabditis elegans vulval cell fates. Genetics 179: 2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askjaer P., Galy V., Meister P., 2014. Modern tools to study nuclear pore complexes and nucleocytoplasmic transport in Caenorhabditis elegans. Methods Cell Biol. 122: 277–310. [DOI] [PubMed] [Google Scholar]
- Berdasco M., Esteller M., 2013. Genetic syndromes caused by mutations in epigenetic genes. Hum. Genet. 132: 359–383. [DOI] [PubMed] [Google Scholar]
- Bezhani S., Winter C., Hershman S., Wagner J. D., Kennedy J. F., et al. , 2007. Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED. Plant Cell 19: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello J., Neukomm L. J., Günesdogan U., Burkart K., Charette S. J., et al. , 2010. The Wnt pathway controls cell death engulfment, spindle orientation, and migration through CED-10/Rac. PLoS Biol. 8: e1000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceol C. J., Stegmeier F., Harrison M. M., Horvitz H. R., 2006. Identification and classification of genes that act antagonistically to let-60 Ras signaling in Caenorhabditis elegans vulval development. Genetics 173: 709–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceron J., Rual J.-F., Chandra A., Dupuy D., Vidal M., et al. , 2007. Large-scale RNAi screens identify novel genes that interact with the C. elegans retinoblastoma pathway as well as splicing-related components with synMuv B activity. BMC Dev. Biol. 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clucas C., Cabello J., Büssing I., Schnabel R., Johnstone I. L., 2002. Oncogenic potential of a C.elegans cdc25 gene is demonstrated by a gain-of-function allele. EMBO J. 21: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M., Fay D. S., Han M., 2004. lin-35/Rb cooperates with the SWI/SNF complex to control Caenorhabditis elegans larval development. Genetics 167: 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai C., Garriga G., McIntire S. L., Horvitz H. R., 1988. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature 336: 638–646. [DOI] [PubMed] [Google Scholar]
- Euskirchen G. M., Auerbach R. K., Davidov E., Gianoulis T. A., Zhong G., et al. , 2011. Diverse roles and interactions of the SWI/SNF chromatin remodeling complex revealed using global approaches. PLoS Genet. 7: e1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euskirchen G., Auerbach R. K., Snyder M., 2012. SWI/SNF chromatin-remodeling factors: multiscale analyses and diverse functions. J. Biol. Chem. 287: 30897–30905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay D. S., Yochem J., 2007. The SynMuv genes of Caenorhabditis elegans in vulval development and beyond. Dev. Biol. 306: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A. G., Piano F., 2006. MEL-28 is downstream of the Ran cycle and is required for nuclear-envelope function and chromatin maintenance. Curr. Biol. 16: 1757–1763. [DOI] [PubMed] [Google Scholar]
- Fernandez A. G., Mis E. K., Lai A., Mauro M., Quental A., et al. , 2014. Uncovering buffered pleiotropy: a genome-scale screen for mel-28 genetic interactors in Caenorhabditis elegans. G3 (Bethesda) 4: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontrodona, L., M. Porta-de-la-Riva, T. Morán, W. Niu, M. Díaz et al., 2013 RSR-2, the Caenorhabditis elegans ortholog of human spliceosomal component SRm300/SRRM2, regulates development by influencing the transcriptional machinery. PLoS Genet. 9: e1003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcales S. V., Albini S., Giordani L., Malecova B., Cignolo L., et al. , 2012. Signal-dependent incorporation of MyoD-BAF60c into Brg1-based SWI/SNF chromatin-remodelling complex. EMBO J. 31: 301–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C., 2013. Exciting prospects for precise engineering of Caenorhabditis elegans genomes with CRISPR/Cas9. Genetics 195: 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Takeshita H., Sawa H., 2007. Cyclin E and CDK2 repress the terminal differentiation of quiescent cells after asymmetric division in C. elegans. PLoS One 2: e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V., Mattaj I. W., Askjaer P., 2003. Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol. Biol. Cell 14: 5104–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V., Askjaer P., Franz C., López-Iglesias C., Mattaj I. W., 2006. MEL-28, a novel nuclear-envelope and kinetochore protein essential for zygotic nuclear-envelope assembly in C. elegans. Curr. Biol. 16: 1748–1756. [DOI] [PubMed] [Google Scholar]
- Gómez-Orte E., Sáenz-Narciso B., Moreno S., Cabello J., 2013. Multiple functions of the noncanonical Wnt pathway. Trends Genet. 29: 545–553. [DOI] [PubMed] [Google Scholar]
- Gupta B. P., Hanna-Rose W., Sternberg P. W., 2012. Morphogenesis of the vulva and the vulval-uterine connection. WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.152.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale V. A., Guiney E. L., Goldberg L. Y., Haduong J. H., Kwartler C. S., et al. , 2012. Notch signaling is antagonized by SAO-1, a novel GYF-domain protein that interacts with the E3 ubiquitin ligase SEL-10 in Caenorhabditis elegans. Genetics 190: 1043–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves D. C., Crabtree G. R., 2011. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 21: 396–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes G. D., Riedel C. G., Ruvkun G., 2011. The Caenorhabditis elegans SOMI-1 zinc finger protein and SWI/SNF promote regulation of development by the mir-84 microRNA. Genes Dev. 25: 2079–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helming K. C., Wang X., Roberts C. W. M., 2014. Vulnerabilities of mutant SWI/SNF complexes in cancer. Cancer Cell 26: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M. A., 2002. Control of cell polarity by noncanonical Wnt signaling in C. elegans. Semin. Cell Dev. Biol. 13: 233–241. [DOI] [PubMed] [Google Scholar]
- Ho L., Crabtree G. R., 2010. Chromatin remodelling during development. Nature 463: 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L., Ronan J. L., Wu J., Staahl B. T., Chen L., et al. , 2009. An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc. Natl. Acad. Sci. USA 106: 5181–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege F. C., Jennings E. G., Wyrick J. J., Lee T. I., Hengartner C. J., et al. , 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95: 717–728. [DOI] [PubMed] [Google Scholar]
- Imbalzano A. N., Imbalzano K. M., Nickerson J. A., 2013. BRG1, a SWI/SNF chromatin remodeling enzyme ATPase, is required for maintenance of nuclear shape and integrity. Commun. Integr. Biol. 6: e25153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Sherwood D. R., Aspöck G., Butler J. A., Gupta B. P., et al. , 2002. Gene expression markers for Caenorhabditis elegans vulval cells. Mech. Dev. 119(Suppl): S203–S209. [DOI] [PubMed] [Google Scholar]
- Jensen V. L., Simonsen K. T., Lee Y.-H., Park D., Riddle D. L., 2010. RNAi screen of DAF-16/FOXO target genes in C. elegans links pathogenesis and dauer formation. PLoS One 5: e15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan N. V., Prat A., Abell A. N., Zawistowski J. S., Sciaky N., et al. , 2013. SWI/SNF chromatin-remodeling factor Smarcd3/Baf60c controls epithelial-mesenchymal transition by inducing Wnt5a signaling. Mol. Cell. Biol. 33: 3011–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., et al. , 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. [DOI] [PubMed] [Google Scholar]
- Kiehl T.-R., Shibata H., Pulst S.-M., 2000. The ortholog of human ataxin-2 is essential for early embryonic patterning in C. elegans. J. Mol. Neurosci. 15: 231–242. [DOI] [PubMed] [Google Scholar]
- Kimble J., Crittenden S. L., 2005. Germline proliferation and its control. WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.13.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korta D. Z., Hubbard E. J. A., 2010. Soma-germline interactions that influence germline proliferation in Caenorhabditis elegans. Dev. Dyn. 239: 1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostić I., Roy R., 2002. Organ-specific cell division abnormalities caused by mutation in a general cell cycle regulator in C. elegans. Development 129: 2155–2165. [DOI] [PubMed] [Google Scholar]
- Kostić I., Li S., Roy R., 2003. cki-1 links cell division and cell fate acquisition in the C. elegans somatic gonad. Dev. Biol. 263: 242–252. [DOI] [PubMed] [Google Scholar]
- Krüger A. V., Jelier R., Dzyubachyk O., Zimmerman T., Meijering E., et al. , 2015. Comprehensive single cell-resolution analysis of the role of chromatin regulators in early C. elegans embryogenesis. Dev. Biol. 398: 153–162. [DOI] [PubMed] [Google Scholar]
- Kuzmanov A., Karina E. I., Kirienko N. V., Fay D. S., 2014. The conserved PBAF nucleosome-remodeling complex mediates the response to stress in Caenorhabditis elegans. Mol. Cell. Biol. 34: 1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lans H., J. AMarteijn, B. Schumacher, J. H. Hoeijmakers, G. Jansen et al, 2010. Involvement of global genome repair, transcription coupled repair, and chromatin remodeling in UV DNA damage response changes during development. PLoS Genet. 6: e1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large E. E., Mathies L. D., 2014. Caenorhabditis elegans SWI/SNF subunits control sequential developmental stages in the somatic gonad. G3 (Bethesda) 4: 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah-Planchon J., Bièche I., Guinebretière J.-M., Bourdeaut F., Delattre O., 2015. SWI/SNF chromatin remodeling and human malignancies. Annu. Rev. Pathol. 10: 145–171. [DOI] [PubMed] [Google Scholar]
- Mattout A., Cabianca D. S., Gasser S. M., 2015. Chromatin states and nuclear organization in development: a view from the nuclear lamina. Genome Biol. 16: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J., Bartlett B., Dang T., Schedl T., 1997. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev. Biol. 181: 121–143. [DOI] [PubMed] [Google Scholar]
- Michaelson D., Korta D. Z., Capua Y., Hubbard E. J. A., 2010. Insulin signaling promotes germline proliferation in C. elegans. Development 137: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narlikar G. J., Sundaramoorthy R., Owen-Hughes T., 2013. Mechanisms and functions of ATP-dependent chromatin-remodeling enzymes. Cell 154: 490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto C., Almendinger J., Gysi S., Gómez-Orte E., Kaech A., et al. , 2010. ccz-1 mediates the digestion of apoptotic corpses in C. elegans. J. Cell Sci. 123: 2001–2007. [DOI] [PubMed] [Google Scholar]
- Oh J., Sohn D. H., Ko M., Chung H., Jeon S. H., et al. , 2008. BAF60a interacts with p53 to recruit the SWI/SNF complex. J. Biol. Chem. 283: 11924–11934. [DOI] [PubMed] [Google Scholar]
- Phelan M. L., Sif S., Narlikar G. J., Kingston R. E., 1999. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol. Cell 3: 247–253. [DOI] [PubMed] [Google Scholar]
- Pinkston-Gosse J., Kenyon C., 2007. DAF-16/FOXO targets genes that regulate tumor growth in Caenorhabditis elegans. Nat. Genet. 39: 1403–1409. [DOI] [PubMed] [Google Scholar]
- Puri P. L., Mercola M., 2012. BAF60 A, B, and Cs of muscle determination and renewal. Genes Dev. 26: 2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W., Huang X., Neumann-Haefelin E., Schulze E., Baumeister R., 2012. Cell-nonautonomous signaling of FOXO/DAF-16 to the stem cells of Caenorhabditis elegans. PLoS Genet. 8: e1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D., Glaros S., Thompson E. A., 2009. The SWI/SNF complex and cancer. Oncogene 28: 1653–1668. [DOI] [PubMed] [Google Scholar]
- Riedel C. G., Dowen R. H., Lourenco G. F., Kirienko N. V., Heimbucher T., et al. , 2013. DAF-16 employs the chromatin remodeller SWI/SNF to promote stress resistance and longevity. Nat. Cell Biol. 15: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau C. E., Downs W. D., Lin R., Wittmann C., Bei Y., et al. , 1997. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell 90: 707–716. [DOI] [PubMed] [Google Scholar]
- Romero O. A., Sanchez-Cespedes M., 2014. The SWI/SNF genetic blockade: effects in cell differentiation, cancer and developmental diseases. Oncogene 33: 2681–2689. [DOI] [PubMed] [Google Scholar]
- Rual J.-F., Ceron J., Koreth J., Hao T., Nicot A.-S., et al. , 2004. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14: 2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijtenberg S., van den Heuvel S., 2015. G1/S inhibitors and the SWI/SNF complex control cell-cycle exit during muscle differentiation. Cell 162: 300–313. [DOI] [PubMed] [Google Scholar]
- Santen G. W. E., Kriek M., van Attikum H., 2012. SWI/SNF complex in disorder: SWItching from malignancies to intellectual disability. Epigenetics 7: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarshad A., Sadeghifar F., Louvet E., Mori R., Böhm S., et al. , 2013. Nuclear myosin 1c facilitates the chromatin modifications required to activate rRNA gene transcription and cell cycle progression. PLoS Genet. 9: e1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa H., Kouike H., Okano H., 2000. Components of the SWI/SNF complex are required for asymmetric cell division in C. elegans. Mol. Cell 6: 617–624. [DOI] [PubMed] [Google Scholar]
- Shain, A. H., and J. R. Pollack, 2013 The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PLoS One 8: e55119. [DOI] [PMC free article] [PubMed]
- Shibata Y., Uchida M., Takeshita H., Nishiwaki K., Sawa H., 2012. Multiple functions of PBRM-1/Polybromo- and LET-526/Osa-containing chromatin remodeling complexes in C. elegans development. Dev. Biol. 361: 349–357. [DOI] [PubMed] [Google Scholar]
- Stiernagle T., 2006. Maintenance of C. elegans. WormBook: The Online Review of C. elegans. Biology: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg P. W., 2005. Vulval development. WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.6.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg P. W., Han M., 1998. Genetics of RAS signaling in C. elegans. Trends Genet. 14: 466–472. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N., 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64–119. [DOI] [PubMed] [Google Scholar]
- Trapnell C., Roberts A., Goff L., Pertea G., Kim D., et al. , 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7: 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Ui A., Kanno S.-I., Ogiwara H., Nagase T., et al. , 2014. SWI/SNF factors required for cellular resistance to DNA damage include ARID1A and ARID1B and show interdependent protein stability. Cancer Res. 74: 2465–2475. [DOI] [PubMed] [Google Scholar]
- Weinberg P., Flames N., Sawa H., Garriga G., Hobert O., 2013. The SWI/SNF chromatin remodeling complex selectively affects multiple aspects of serotonergic neuron differentiation. Genetics 194: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman B., Knudsen K. E., 2009. Hijacking the chromatin remodeling machinery: impact of SWI/SNF perturbations in cancer. Cancer Res. 69: 8223–8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolner S., Bement W. M., 2009. Unconventional myosins acting unconventionally. Trends Cell Biol. 19: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo A. S., Bais C., Greenwald I., 2004. Crosstalk between the EGFR and LIN-12/Notch pathways in C. elegans vulval development. Science 303: 663–666. [DOI] [PubMed] [Google Scholar]