Abstract
Applied Genetic Technologies Corporation is developing rAAV2tYF-CB-hRS1, a recombinant adeno-associated virus (rAAV) vector for treatment of X-linked retinoschisis (XLRS), an inherited retinal disease characterized by splitting (schisis) of retinal layers causing poor vision. We report here results of a study evaluating the safety and biodistribution of rAAV2tYF-CB-hRS1 in normal cynomolgus macaques. Three groups of male animals (n = 6 per group) received an intravitreal injection in one eye of either vehicle, or rAAV2tYF-CB-hRS1 at one of two dose levels (4 × 1010 or 4 × 1011 vg/eye). Half the animals were sacrificed after 14 days and the others after 91 or 115 days. The intravitreal injection procedure was well tolerated in all groups. Serial ophthalmic examinations demonstrated a dose-related anterior and posterior segment inflammatory response that improved over time. There were no test article-related effects on intraocular pressure, electroretinography, visual evoked potential, hematology, coagulation, clinical chemistry, or gross necropsy observations. Histopathological examination demonstrated minimal or moderate mononuclear infiltrates in 6 of 12 vector-injected eyes. Immunohistochemical staining showed RS1 labeling of the ganglion cell layer at the foveal slope in vector-injected eyes at both dose levels. Serum anti-AAV antibodies were detected in 4 of 6 vector-injected animals at the day 15 sacrifice and all vector-injected animals at later time points. No animals developed antibodies to RS1. Biodistribution studies demonstrated high levels of vector DNA in the injected eye but minimal or no vector DNA in any other tissue. These results support the use of rAAV2tYF-CB-hRS1 in clinical studies in patients with XLRS.
Introduction
X-linked retinoschisis (XLRS) is an early onset retinal disease that affects 1:5,000 to 1:20,000 males worldwide and is the leading cause of vision loss from macular degeneration in young men.1 Characteristic features of XLRS include mild to severe loss of central vision, radial streaks arising from foveal schisis, splitting of inner retinal layers in the peripheral retina, and a negative electroretinogram (ERG) arising from a marked reduction in b-wave amplitude.1,2 Best corrected visual acuity is reduced to 20/100 or worse in most patients although it may vary greatly.3 Disease progression and severity is highly variable even within families. Secondary complications such as retinal detachment and vitreous hemorrhage that occur during the course of the disease can lead to a poor outcome. Female carriers are asymptomatic although detailed clinical examination can reveal minor retinal abnormalities.
XLRS is caused by mutations in the gene encoding a protein called retinoschisin (RS1), a cell-surface adhesion protein expressed by photoreceptor and bipolar cells of the retina.3 The 24 kDa protein has two conserved sequence motifs, an initial signal sequence that targets the protein for secretion and a large discoidin domain that is implicated in cell adhesion. RS1 helps to maintain the structural organization of the retinal cell layers and promotes visual signal transduction.
There is no specific treatment for XLRS. Anecdotal reports suggest that topical carbonic anhydrase inhibitors may provide some reduction in the degree of schisis detected by OCT and improvement in visual acuity in some but not all patients,4–8 but the absence of controlled clinical trials makes interpretation of these reports difficult.
Knockout mice have been used to obtain insight into the role of RS1 in retinal structure, function, and pathology. Studies in these murine models of XLRS have shown that recombinant adeno-associated virus (rAAV) vectors expressing RS1 can provide significant restoration of retinal structure and function, including preservation of photoreceptors, reduction in the number and size of schisis cavities, and restoration of ERG b-wave response.9–15
We are developing an rAAV vector expressing RS1 as a potential product for treatment of XLRS. The product, rAAV2tYF-CB-hRS1, contains a CMV enhancer/chicken beta actin promoter,16 a human RS1 cDNA, and an SV40 polyadenylation sequence and is packed in an AAV2 capsid containing three tyrosine to phenylalanine mutations. As part of our efforts to develop this product, we conducted a toxicology and biodistribution study in normal cynomolgus macaques.
Research Design and Methods
Vector description
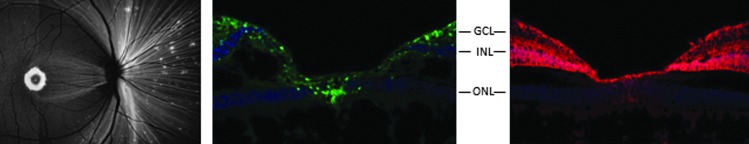
Capsids with mutations in surface-exposed tyrosine residues have been shown to enhance the efficiency of transduction in mouse retinas after intravitreal injection. When administered by subretinal or intravitreal injection in mice, AAV vectors expressing green fluorescent protein (GFP) and packaged in capsids with single tyrosine to phenylalanine (YF) mutations achieved stronger and more widespread transgene expression in many retinal cells compared with their wild-type capsid counterparts.17 Additionally, intravitreal injection of AAV2-GFP vectors packaged in capsids with multiple YF mutations achieved GFP expression in the ganglion, Müller, and inner retinal cells.18 Based on these observations, in a preliminary study we evaluated intravitreal injection in rhesus macaques of AAV vectors expressing either GFP or RS1 that were packaged in an AAV2 capsid containing three YF mutations (AAV2tYF). The amino acid sequences of human and rhesus RS1 are identical. To distinguish the vector-expressed human RS1 protein from the endogenously expressed rhesus RS1 protein, a sequence encoding a 10 amino acid “myc” tag was added to the human RS1 cDNA sequence, and immunohistochemistry using an anti-myc monoclonal antibody was used to visualize the human RS1 expression. This study demonstrated that the intravitreally injected rAAV2tYF-CB-GFP and rAAV2tYF-CB-RS1myc vectors were able to achieve robust transgene expression in the retinal ganglion cell ring and foveal cone photoreceptors (Fig. 1), which are the areas of the retina primarily affected in patients with XLRS.19
Figure 1.
Expression of GFP and RS1myc in nonhuman primate retina. A rhesus macaque received an intravitreal injection of rAAV2tYF-CB-GFP in one eye and rAAV2tYF-CB-RS1myc in the other eye at a dose of 1 × 1011 vg/eye. An in-life fundus fluorescence photograph of an eye injected with the GFP vector (left panel) showed expression of GFP (white) in the macula (donut-shaped area) and fovea (dot in the center of the macula), retinal nerve fibers, and scattered foci in the peripheral retina. Immunohistochemical examination of the same eye (middle panel) showed expression of GFP (green), which is an intracellular protein, in retinal ganglion cells across the macular region of the retina and in foveal cones. Immunohistochemical examination of the other eye (right panel) showed expression of RS1myc (red), demonstrating that the protein was secreted into the extracellular matrix and spread horizontally and vertically through the inner layers of the retina. Slides in the middle and right panels were also stained with 4′,6-diamidino-2-phenylindole to identify nuclei (blue). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
Vector production
The rAAV2tYF-CB-hRS1 vector was produced using a recombinant herpes simplex virus (rHSV) complementation system in suspension-cultured baby hamster kidney (sBHK) cells.20 Two rHSV helper viruses, one containing the AAV2 rep and AAV2tYF cap genes and the other containing the hRS1 expression cassette, were used to coinfect sBHK cells grown in serum-free medium. One day later the cells were lysed with Triton X-100 detergent and treated with Benzonase. Cell lysate containing the AAV vector was clarified by filtration and purified by AVB Sepharose (GE Life Sciences) affinity chromatography followed by CIM SO3− (BIA Separations) cation-exchange chromatography, and eluted in concentrated balanced salt solution containing 0.014% (v/v) Tween-20 (BSST). The purified bulk was concentrated and buffer exchanged to 1× BSST (drug substance) and sterile (0.2 μm) filtered to generate drug product. The drug product was further concentrated, as needed, using a 100 kDa MWCO Ultra centrifugal filter unit (EMD Millipore), and re-filtered (0.2 μm) to generate drug product sublots of specific concentrations, which were stored at ≤65°C.
Vector characterization
Vector concentration (vector genomes [vg] per ml), vector infectivity (tissue culture 50% infectious dose [TCID50]), purity (silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE] analysis), and concentrations of endotoxin and HSV protein were measured as previously described.21 RS1 expression was quantified by co-infection of HEK 293 cells with rAAV2tYF-CB-hRS1 (1 × 105 vg/cell) and human adenovirus type 5 (10 IU/cell). After culture for 2 days, RS1 expressed by the cells and secreted into culture medium was quantified by RS1-specific ELISA. Concentrations of the BHK protein, bovine serum albumin, Benzonase, and AVB ligand were measured by ELISA using commercially available kits. Concentrations of HSV and BHK DNA were measured by quantitative PCR (qPCR). Testing for mycoplasma, bacteria, and fungi was performed using standard microbiological methods. Testing for infectious HSV was by serial passage in V27 cells.
Device compatibility testing
Product stability before and after exposure to the syringe and needle used to administer the vector by intravitreal injection was determined by measuring the vector concentration (vg/ml) and hRS1 protein expression (μg/ml) by ELISA. Vials of vector at a concentration of 1.6 × 1012 vg/ml were thawed and used either undiluted or after dilution with 3 volumes of BSST to achieve a concentration of 4 × 1011 vg/ml. Samples were tested immediately or after 4 hr without exposure to the device, or after exposure to the device by withdrawal into a 1 ml syringe that was kept on ice for 4 hr.
Toxicology study design
Three groups of male cynomolgus macaques (n = 6 per group), 2 years of age and weighing between 2.1 and 2.7 kg, each received an intravitreal injection of 110 μl containing rAAV2tYF-CB-hRS1 at a concentration of 3.6 × 1011 vg/ml (4 × 1010 vg/eye) or 3.6 × 1012 vg/ml (4 × 1011 vg/eye) or 110 μl of vehicle control in the right eye (Table 1). With allometric scaling based on the smaller volume of a primate eye compared to a human eye, these doses bracket the doses planned for a phase 1/2 clinical trial (1 × 1011 to 6 × 1011 vg/eye). The left eyes were untreated. Residual dosing formulations were frozen for later testing by qPCR to confirm the concentration of vector administered.
Table 1.
Study design
| Dosage level | ||||
|---|---|---|---|---|
| Group | Number | Vector concentration | Injection volume | Total dose |
| 1 | 6 males | 0 (control) | 110 μl | 0 |
| 2 | 6 males | 3.6 × 1011 vg/ml | 110 μl | 4 × 1010 vg |
| 3 | 6 males | 3.6 × 1012 vg/ml | 110 μl | 4 × 1011 vg |
Half of the animals were sacrificed 14 days after vector administration (study day 15) and the remaining animals were sacrificed 91 or 115 days after vector administration (study day 92 or 116). The dosing phase was extended to study day 116 for two animals in the high-dose group to assess reversibility of ocular inflammation that was persisting at study day 92. At each sacrifice time point, samples were collected for evaluation of safety and biodistribution.
Animals were observed twice daily for mortality, clinical abnormalities, and signs of pain or distress. Detailed observations were made at least once during the predose phase, before dosing on study day 1, weekly thereafter, and on the day of scheduled sacrifice. Body weights were obtained during the predose phase, on the day of dosing, weekly thereafter, and on the day of sacrifice. An ophthalmic examination (slit lamp biomicroscopy, indirect ophthalmoscopy, and measurement of intraocular pressure [IOP]) was conducted during the predose phase, on study days 3, 8, and 15, and during weeks 4, 5, 9, 13, and 17 for all surviving animals. Aqueous cells and flare and vitreous cells were scored as previously described.22 Aqueous and vitreous cell scores were assigned using the same estimate of cells per single 0.2 mm field of the focused slit lamp beam as 0 (no cells), trace (1–5 cells), 1+ (5–25 cells), 2+ (25–50 cells), 3+ (50–100 cells), or 4+ (>100 cells). Aqueous flare was scored based on presence of protein in the anterior chamber as 0 (no visible protein), trace (visible only to an experienced observer using a small, bright focal light source and magnification), 1+ (mild), 2+ (moderate), 3+ (moderate but more than 2+), or 4+ (severe). Vitreous haze was scored according to the Standardization of Uveitis Nomenclature (SUN) method.23
Scotopic and photopic ERG and visual evoked potentials (VEP) were obtained during the predose phase and during weeks 7 and 13. Blood for hematology, coagulation, and clinical chemistry analysis was obtained during the predose phase and during the week before sacrifice. Blood for qPCR was obtained on study days 3 and 8 and at sacrifice. Samples of tears, nasal secretions, saliva, urine, and feces were obtained on study days 3 and 8 for qPCR analysis to evaluate vector shedding.
Sera for measurement of antibodies to AAV (neutralizing antibody assay) and RS1 (ELISA) were obtained during the predose phase, during study weeks 5 and 9, and at necropsy. Blood for measurement of cell-mediated immune responses to RS1 and AAV capsid peptides by enzyme-linked immunospot (ELISPOT) analysis of peripheral blood mononuclear cells (PBMC) was obtained during the predose phase, on study day 43, and at necropsy.
At necropsy, a complete external and internal examination was performed, including body orifices and cranial, thoracic, and abdominal organs and tissues. Brain, heart, liver, kidneys (pair), spleen, thymus, lungs, adrenals (pair), and testes (pair) were weighed.
The eyes were processed for histology and biodistribution using the approach described by Jacobson et al.24 At the interim sacrifice, both eyes of each animal were enucleated and the anterior segment was removed and processed for qPCR and histopathology evaluation. A section of the eye cup to include the fovea and optic disc was collected for histopathology. From the remaining calotte, a 6-mm-diameter punch that included the macula, and samples of lens, vitreous and optic nerve, were collected and frozen for qPCR analysis. The remaining eyecup and intraorbital optic nerve were processed for histopathology. At the terminal sacrifice, the eyes were enucleated, the optic nerve was removed at the point at which it exits the globe, and the globe was fixed intact for histopathology. The optic nerve most proximal to the globe was collected and frozen for qPCR analysis and the remaining intraorbital optic nerve was processed for histopathology.
At both sacrifice time points, the following tissues were examined histologically: adrenal glands, brain, optic chiasm, optic tract (left and right), lateral geniculate nucleus (left and right), occipital cortex (left and right), cecum, colon, diaphragm, duodenum, epididymides, esophagus, femur with bone marrow (articular surface of the distal end), gall bladder (drained), heart, ileum, jejunum, kidneys, lesions, liver, lung with large bronchi, lymph nodes (mesenteric, deep cervical, preauricular, inguinal and mandibular), mammary gland (females), pancreas, pituitary gland, sciatic nerve, prostate, skin/subcutis, spleen, stomach, skeletal muscle (gastrocnemius), testes, thymus, thyroid with parathyroid, and urinary bladder.
At both sacrifice time points, the following regions of the brain were dissected for qPCR analysis: optic chiasm, optic tract (left and right), lateral geniculate nucleus (LGN; left and right), occipital cortex (left and right), and cerebellum. Samples of heart, lung, liver, spleen, kidneys, testes, and left and right deep cervical, parotid, and mandibular lymph nodes were collected for both qPCR and histopathology. Tissue sections for histopathology were stained with hematoxylin and eosin. Sections of each eye were evaluated for immunohistochemistry using an antibody specific for RS1. At the interim sacrifice, brain tissue was processed only for qPCR and not for histopathology. At the terminal sacrifice, brain tissue was processed for histopathology and qPCR was performed on DNA extracted from formalin-fixed tissue.
Detection of antibodies to AAV and RS1
Antibodies to AAV2tYF capsid were measured using a neutralization assay as previously described.25 Antibodies to RS1 were determined by ELISA. Briefly, microtiter plates were coated with RS1 (generated from a thioredoxin-His6 tagged recombinant fusion protein after removal of the thioredoxin-His6 tag by TEV protease; GeneScript) or monkey IgG (Rockland Immunochemicals) and incubated overnight. Plates were washed and blocked, and diluted monkey test serum, or rabbit anti-RS1 (Sigma-Aldrich) as a positive control, was added. A rabbit anti-hRS1 antibody-positive control was used in the assay since a cynomolgus monkey antibody was not available. After sample incubation, a cocktail of HRP-conjugated anti-monkey IgG and anti-rabbit IgG (Sigma-Aldrich) were added to detect antibodies bound to hRS1. Tetramethyl benzidine substrate was then added and absorbance measured spectophotometically.
Quantitation of vector in blood and tissues
DNA was extracted from blood by automated extraction (AutoGen, Inc.), from urine, feces, buccal swabs, tears, nasal secretions and fresh frozen tissue using QIAamp kits (Qiagen Sciences, Inc.), and from formalin-fixed brain using a LabCorp proprietary method. DNA concentration was determined using Pico Green® (Molecular Probes, Inc.). A 0.2 μg sample was subjected to TaqMan® qPCR analysis (Roche Molecular Systems, Inc.) using the 7900HT Real Time PCR System (Life Technologies) using primers and probe that target the SV40 polyadenylation sequence in the vector or using primers and probe that target human and nonhuman primate glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The cycle threshold (Ct) value was determined and compared to a standard curve based on serial dilutions of a plasmid containing known amounts of SV40 or GAPDH sequences. During assay validation the limit of detection (LOD) was determined to be 1 copy per reaction and the lower limit of quantification (LLOQ) was determined to be 10 copies per reaction. When the assay was performed on samples from this study, all no-template control samples had Ct values >38 and each standard curve had a coefficient of variation <15%, R2 values of >0.98, and a linear range of 101–107 copies per reaction. For samples with low DNA concentrations (lens, vitreous, tears, nasal secretions, saliva, and urine), additional samples were extracted and combined to achieve sufficient DNA mass to allow 0.2 μg per reaction. For samples with <0.2 μg per reaction after multiple extractions, the DNA mass per sample was recorded. Samples with DNA mass <0.03 μg per reaction were spiked with 1 × 103 copies of the GAPDH plasmid per reaction to confirm the absence of inhibitory factors in the sample. GAPDH was amplified from all such samples.
Statistical analyses
Hematology, coagulation, and clinical chemistry data were analyzed using analysis of variance and Dunnett's test. Intraocular pressure and ERG/VEP data were analyzed using repeated measures analysis of covariance. Results were reported as significant at the 5% or 1% level, as applicable.
Results
Characterization of rAAV2tYF-CB-hRS1
Results of release testing for drug product are shown in Table 2. Concentrations of residual process reagents in the drug substance used to produce this drug product were 72 ng/ml for HSV DNA, 1.9 ng/ml for BHK DNA, 120 ng/ml for HSV protein, 49 ng/ml for bovine serum albumin, and <LLOQ for BHK protein, Benzonase, AVB ligand, and endotoxin. Tests for mycoplasma, bacteria, fungi and infections HSV were all negative.
Table 2.
Characterization of rAAV2tYF-CB-hRS1 drug product
| Test | Method | Result |
|---|---|---|
| Vector concentration | Quantitative PCR | 3.6 × 1012 vg/ml |
| Vector infectivity | TCID50 | 3.6 × 1010 IU/ml |
| RS1 expression | Infection of 293 cells | 5.1 μg/ml |
| Endotoxin | LAL | <LLOQ |
| Microbial enumeration | Direct inoculation | <LLOQ |
LAL, limulus amebocyte lysis; LLOQ, lower limit of quantification.
The LLOQ is 0.3 endotoxin units per sample for the LAL assay and 1 colony forming unit per sample for microbial enumeration.
Results of device compatibility testing demonstrated no significant change in vector concentration or vector potency (RS1 expression) after exposure to the administration syringe and needle for 4 hr.
Toxicology study results
The intravitreal injection procedure was well tolerated in all groups. Results of dosing analysis confirmed that the measured concentrations of study agent in the residual dosing formulations were consistent with the concentrations specified in the protocol.
Administration of rAAV2tYF-CB-hRS1 had no effect on qualitative food consumption, body weights, or body weight change. There was no apparent effect of vehicle control or rAAV2tYF-CB-hRS1 at either dose level on IOP. ERG and VEP results were within expected limits in all animals at the predose baseline test and in the two dosage groups tested at 7 and 13 weeks after vector administration. No instances of subnormal (amplitude decrease ≥50%) or extinguished (flat) ERG responses were noted in any of the animals tested. There were no clinically significant intergroup differences in scotopic or photopic ERGs or VEPs. There were no intergroup differences in hematology, coagulation, or clinical chemistry parameters and no test article-related gross necropsy observations. All animals survived to the scheduled sacrifice.
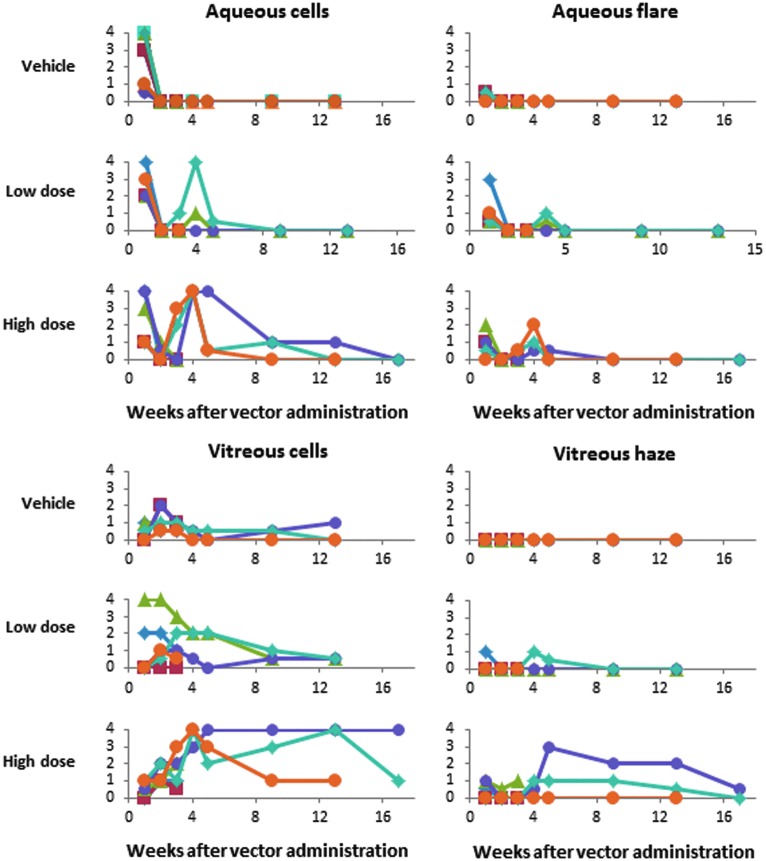
Serial ophthalmic examinations demonstrated a dose-related anterior and posterior segment inflammatory response to intravitreal administration of test articles that improved over time, as depicted in Fig. 2. One animal that received the 4 × 1011 vg dose had squinting of the injected eye on day 24 and was given intramuscular nonsteroidal anti-inflammatory therapy (flunixin meglumine, 2 mg/kg) for 3 days.
Figure 2.
Ocular inflammation findings. Intensity of parameters in individual animals was scored in a standardized fashion as 0, trace (0.5), 1+, 2+, 3+, or 4+ as described in the Research Design and Methods section. Each symbol represents an individual animal, with 6 animals per group through week 2, 3 animals per group during week 3 to week 12, and 2 animals in the high-dose group at week 16. For the animal in the low-dose vector group with the highest grade of vitreous cells, the cells were red in color, indicating vitreous hemorrhage, probably related to the intravitreal injection procedure rather than intraocular inflammation.
Administration of vehicle control was associated with transient and typically mild conjunctival hyperemia, aqueous flare/cell, vitreous cell, and white vitreous floaters near the injection site. Aqueous cells ranged from trace to 4+ at 1 week after injection and resolved by week 2. Vitreous cells ranged from trace to 2+ at 2–3 weeks after injection, resolved by week 4 in four animals, and in the other two animals were resolved or resolving at week 12. None of the animals in this group had vitreous haze.
Ocular inflammation was greater in the vector-treated animals and was more pronounced at the higher dose. Based on findings at the week 4 ocular examination, topical atropine (1% atropine sulfate ointment once daily) was administered for 1 or 5 weeks to 2 animals in the low-dose group and for 5, 13, or 13 weeks to 3 animals on the high-dose group.
Aqueous cells ranged from 1+ to 4+ at week 1 in all animals, including those given vehicle, and resolved or decreased significantly by week 2. In vector-treated animals, aqueous cells recurred at week 2–4 in 2 animals in the low-dose and 3 animals in the high-dose group, and then resolved over the next several weeks. Vitreous cells developed in 5 of 6 animals in the low-dose group and all animals in the high-dose group, and persisted at 2+ or greater for more than 4 weeks in 2 animals in the low-dose and 3 animals in the high-dose group. Moderate vitreous cell (up to 2+) also developed in 3 control animals. Vitreous haze developed at week 4 in 1 animal in the low-dose and 3 animals in the high-dose group and improved or resolved over the next several weeks.
Additional manifestations of ocular inflammation included varying degrees of conjunctival hyperemia, keratic precipitates, white vitreous floaters near the injection site, and white perivascular sheathing around retinal blood vessels. These additional findings are summarized in Table 3.
Table 3.
Additional signs of ocular inflammation after intravitreal injection of rAAV2tYF-CB-hRS1 in cynomolgus macaques
| Finding | Day 3 | Day 8 | Day 15 | Week 4 | Week 5 | Week 9 | Week 13 | Week 17 |
|---|---|---|---|---|---|---|---|---|
| Group 1 (vehicle control) | · | |||||||
| Conjunctival hyperemia | · | |||||||
| 1+ | 5/6 | – | – | – | – | – | – | · |
| Retinal hemorrhage | 1/6 | – | – | – | – | – | – | · |
| Vitreous floaters—white | 1/6 | 3/6 | 1/6 | – | 1/3 | – | – | · |
| Group 2 (4 × 1010 vg/eye) | · | |||||||
| Keratic precipitates | – | – | – | 1/3 | – | – | – | · |
| Conjunctival hyperemia | · | |||||||
| 1+ | 4/6 | – | – | – | – | – | – | · |
| 2+ | 1/6 | – | – | 1/3 | – | – | – | · |
| Incomplete pupil dilation | 1/6 | – | – | – | – | – | – | · |
| Vitreous hemorrhage | 1/6 | – | – | – | – | – | – | · |
| Vitreous floaters—white | 5/6 | 1/6 | 1/6 | 3/3 | 2/3 | 2/3 | 2/3 | · |
| Perivascular sheathing | – | – | – | 1/3 | 1/3 | – | – | · |
| Group 3 (4 × 1011 vg/eye) | ||||||||
| Keratic precipitates | – | – | – | 1/3 | – | – | – | – |
| Conjunctival hyperemia | ||||||||
| 1+ | 6/6 | – | – | 1/3 | – | – | – | – |
| Vitreous floaters—white | 6/6 | 6/6 | 6/6 | 3/3 | 3/3 | 3/3 | 3/3 | 2/2 |
| Perivascular sheathing | – | – | – | 2/3 | 3/3 | 2/3 | 2/3 | 1/2 |
Dash (–), findings not present at this interval; dot (·), not applicable to groups 1 and 2.
Histopathological and immunohistochemical findings
At the interim sacrifice, histopathology demonstrated moderate mononuclear infiltrates at the optic disc of the injected eye in 1 of 3 animals in the high-dose group. At the terminal sacrifice, mononuclear infiltrates at the optic disc were seen in 2 of 3 animals in the low-dose group (both minimal) and 3 of 3 animals in the high-dose group (2 minimal and 1 moderate). All other tissues collected for histopathological examination showed no abnormalities.
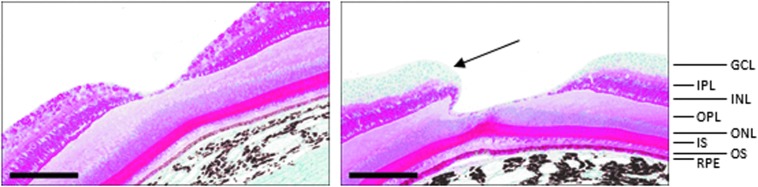
Immunohistochemical staining showed the expected RS1 staining of photoreceptors and inner nuclear and inner plexiform layers in treated and untreated eyes, and also showed RS1 labeling of the ganglion cell layer at the foveal slope in the injected eye in 6 of 6 animals in the high-dose group and 5 of the 5 animals in low-dose group for whom the fovea was present in the slides stained for immunohistochemical analysis. An example is shown in Fig. 3.
Figure 3.
Immunohistochemical staining for RS1. The eye from an animal injected with vehicle control (right) or rAAV2tYF-CB-hRS1 (left) was stained with an antibody specific for human RS1 (red staining). The amino acid sequence of human and cynomolgus RS1 is identical, and the antibody detects RS1 expression in all retinal layers except in the ganglion cell layer in the control eye (blue hematoxylin-stained area indicated by an arrow on the right image) and also detects RS1 expression in the retinal ganglion cell layer of the vector-injected eye on the left image. The size marker is 200 μm. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; IS, inner segment of photoreceptors; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, outer segment of photoreceptors; RPE, retinal pigment epithelium.
Antibody and T-cell responses to AAV and RS1
Serum neutralizing antibodies to AAV2tYF were detected in serum from 4 of 6 vector-injected animals at the day 15 sacrifice and all vector-injected animals at weeks 5 and 9 and the terminal sacrifice (Table 4). No animals developed antibodies to RS1 (data not shown).
Table 4.
Serum anti-AAV2tYF antibody titers in cynomolgus macaques
| Group | Animal | Predose | Day 15 | Week 5 | Week 9 | Day 92 | Day 116 |
|---|---|---|---|---|---|---|---|
| 1 | I03225 | <5 | <5 | ||||
| 1 | I03226 | <5 | <5 | ||||
| 1 | I03227 | <5 | <5 | ||||
| 1 | I03228 | <5 | <5 | <5 | <5 | ||
| 1 | I03229 | <5 | <5 | <5 | <5 | ||
| 1 | I03230 | <5 | <5 | 5 | <5 | ||
| 2 | I03231 | <5 | <5 | ||||
| 2 | I03233 | <5 | <5 | ||||
| 2 | I03243 | <5 | 10 | ||||
| 2 | I03234 | <5 | 40 | 40 | 20 | ||
| 2 | I03235 | <5 | 80 | 80 | 20 | ||
| 2 | I03236 | <5 | 80 | 160 | 80 | ||
| 3 | I03237 | <5 | 20 | ||||
| 3 | I03238 | <5 | 10 | ||||
| 3 | I03239 | <5 | 20 | ||||
| 3 | I03242 | <5 | 80 | 160 | 80 | ||
| 3 | I03240 | 5 | 160 | 640 | 320 | ||
| 3 | I03241 | <5 | 80 | 160 | 160 |
ELISPOT testing with pools of peptides from AAV2tYF capsid demonstrated a positive response (SFU per million PBMC >65 and >3 times the medium control) to 1 AAV2tYF peptide pool at day 43 in 2 samples (100 SFU/106 PBMC in 1 low-dose animal and 63 SFU/106 PBMC in 1 high-dose animal). Results at day 92 were negative for both animals, and all other ELISPOT responses to AAV2tYF capsid peptides from all animals were negative at each time point at which the assay was performed. No animals had a positive ELISPOT response to RS1 peptides.
Biodistribution
For biodistribution analysis, DNA was extracted from tissues and 0.2 μg of DNA was tested by qPCR, and results with reported as vector DNA copies per μg of host DNA. For samples containing very little DNA, the entire extracted sample was dried and used for qPCR analysis, with results reported as copies per sample (and as copies per μg of DNA if the measured mass of DNA recovered was greater than 0).
At the interim sacrifice on day 15, high levels of vector DNA were present in the anterior segment and retina of the treated right eye of both the lower and higher dose animals. Very low levels of vector DNA were detected in the optic chiasm and optic nerves, with even lower levels of vector DNA in the lateral geniculate nucleus of vector-treated animals. Only one animal had a low level of vector DNA detected in the left occipital cortex. One control animal had a very low level of vector DNA (57 copies per μg DNA, with an LLOQ of 50 copies per μg DNA) in the retina (Table 5).
Table 5.
Vector DNA in ocular tissue, visual pathways, and cerebellum at the interim sacrifice
| Group | 1 (vehicle control) | 2 (4 × 1010 vg/eye) | 3 (4 × 1011 vg/eye) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal ID number | 3225 | 3226 | 3227 | 3231 | 3233 | 3243 | 3237 | 3238 | 3239 |
| Ocular tissue | |||||||||
| Anterior segment (left) | – | – | – | – | – | – | – | – | – |
| Retina (left) | – | – | 57 | – | – | – | – | – | – |
| Optic nerve (left) | – | – | – | – | – | – | – | – | – |
| Anterior segment (right) | – | – | – | 655,161 | 170,905 | 87,462 | 1,042,311 | 1,668,346 | 2,462,150 |
| Retina (right) | – | – | – | 426,370 | 3,135,758 | 2,551,060 | 31,639,615 | 88,946,710 | 7,815,759 |
| Optic nerve (right) | – | – | – | 679 | 695 | 508 | 549 | 1,813 | 2,505 |
| Optic chiasm | – | – | – | 302 | 158 | 114 | 155 | 787 | 509 |
| Visual pathways | |||||||||
| Optic tract (left) | – | – | – | 580 | 354 | 137 | 647 | 615 | 772 |
| LGN (left) | – | – | – | 158 | 381 | 275 | 88 | 337 | 835 |
| Occipital cortex (left) | – | – | – | 173 | – | – | – | – | – |
| Optic tract (right) | – | – | – | 62 | 249 | 99 | 117 | 541 | 284 |
| LGN (right) | – | – | – | – | 245 | 271 | 105 | 158 | 166 |
| Occipital cortex (right) | – | – | – | – | – | – | – | – | – |
| Cerebellum | – | – | – | – | – | – | – | – | – |
Dash (–), less than lower limit of quantification; LGN, lateral geniculate nucleus.
Data expressed as vector copies per μg DNA.
High levels of vector DNA were detected in the lens and vitreous in the vector-treated eyes (Table 6), but the amount per μg of DNA was usually not quantifiable because the measured mass of DNA recovered was 0. In the untreated left eye, low levels of vector DNA, ranging from 11 to 361 copies per sample, were detected in 4 of 9 lens samples and 3 of 9 vitreous samples.
Table 6.
Vector DNA in lens and vitreous at the interim sacrifice
| Copies per sample | Mass per sample (μg) | Copies per μg | Copies per sample | Mass per sample (μg) | Copies per μg | ||
|---|---|---|---|---|---|---|---|
| Group | Animal number | Lens (left) | Lens (right) | ||||
| 1 | 3225 | – | 0.016 | – | – | 0.014 | – |
| 1 | 3226 | 361 | 0.014 | 25,901 | – | 0.015 | – |
| 1 | 3227 | – | 0.000 | NA | – | 0.000 | NA |
| 2 | 3231 | 18 | 0.000 | NA | 28,232 | 0.019 | 1,485,891 |
| 2 | 3233 | – | 0.015 | – | 584,266 | 0.014 | 41,932,945 |
| 2 | 3243 | – | 0.000 | NA | 224,008 | 0.024 | 9,307,825 |
| 3 | 3237 | 47 | 0.027 | 1,753 | 396,530 | 0.016 | 24,080,769 |
| 3 | 3238 | – | 0.020 | – | 373,827 | 0.000 | NA |
| 3 | 3239 | 12 | 0.019 | 627 | 17,208 | 0.015 | 1,132,136 |
| Vitreous (left) | Vitreous (right) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 3225 | – | 0.000 | NA | – | 0.000 | NA |
| 1 | 3226 | – | 0.000 | NA | – | 0.000 | NA |
| 1 | 3227 | 11 | 0.000 | NA | – | 0.000 | NA |
| 2 | 3231 | 16 | 0.000 | NA | 20,634 | 0.000 | NA |
| 2 | 3233 | – | 0.000 | NA | 714,913 | 0.000 | NA |
| 2 | 3243 | – | 0.000 | NA | 45,827 | 0.000 | NA |
| 3 | 3237 | 126 | 0.000 | NA | 863,494 | 0.000 | NA |
| 3 | 3238 | – | 0.000 | NA | 1,980,176 | 0.000 | NA |
| 3 | 3239 | – | 0.000 | NA | 18,416,140 | 0.000 | NA |
Dash (–), less than lower limit of quantification.
NA, not applicable, because the mass of DNA extracted from the tissue was 0.
At the terminal sacrifice on day 92 or day 116, vector DNA was not detected in any of the ocular, visual pathway or brain tissues tested.
At both the interim and terminal sacrifice time points, low levels of vector DNA were detected in the spleen of most vector-treated animals, and in a few lymph nodes from some of the vector-treated animals. A very low level of vector DNA was also detected in a few other organs at one of the sacrifice time points in a small number of vector-treated animals and in the spleen of one control animal. There was no vector DNA detected in the heart or testes of any animal at either time point (Table 7).
Table 7.
Vector DNA in nonocular tissues
| Group | 1 (vehicle control) | 2 (4 × 1010 vg/eye) | 3 (4 × 1011 vg/eye) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal number | 3225 | 3226 | 3227 | 3231 | 3233 | 3243 | 3237 | 3238 | 3239 |
| Interim sacrifice | |||||||||
| Heart | – | – | – | – | – | – | – | – | – |
| Lung | – | – | – | – | – | – | – | – | – |
| Liver | – | – | – | – | – | – | – | 161 | 90 |
| Spleen | – | – | 2,780 | 66 | – | – | 455 | 1,688 | 339 |
| Kidney | – | – | – | – | – | – | – | – | – |
| Testes | – | – | – | – | – | – | – | – | – |
| Lymph nodes | |||||||||
| Cervical (left) | – | – | – | 66 | – | – | – | 56 | – |
| Cervical (right) | – | – | – | – | – | – | – | – | – |
| Mandibular (left) | – | – | – | – | – | – | 58 | – | 109 |
| Mandibular (right) | – | – | – | – | – | – | – | – | – |
| Parotid (left) | – | – | – | – | – | – | – | – | – |
| Parotid (right) | – | – | – | 165 | – | 2,908 | – | – | 6,800 |
| Animal number | 3228 | 3229 | 3230 | 3234 | 3235 | 3246 | 3242 | 3240 | 3241 |
|---|---|---|---|---|---|---|---|---|---|
| Terminal sacrifice | |||||||||
| Heart | – | – | – | – | – | – | – | – | – |
| Lung | – | – | – | – | – | 68 | – | – | – |
| Liver | – | – | – | – | – | – | – | – | – |
| Spleen | – | – | – | 125 | 498 | 68 | 110 | 195 | 262 |
| Kidney | – | – | – | – | – | 308 | – | – | – |
| Testes | – | – | – | – | – | – | – | – | – |
| Lymph nodes | |||||||||
| Cervical (left) | – | – | – | – | – | – | – | – | – |
| Cervical (right) | – | – | – | – | – | – | – | – | – |
| Mandibular (left) | – | – | – | – | – | – | – | 64 | – |
| Mandibular (right) | – | – | – | – | – | – | – | 10,646 | – |
| Parotid (left) | – | – | – | – | – | – | – | – | – |
| Parotid (right) | – | – | – | – | 98 | – | – | – | – |
Dash (–), less than lower limit of quantification.
Data expressed as vector copies per μg DNA.
Blood samples were collected from all animals at day 3 and day 8, and all were negative for vector DNA. One animal in the high-dose vector group had 108,846 copies per μg DNA in blood collected at the day 15 sacrifice, and one animal in the low-dose vector group had a low level of vector DNA (151 copies per μg of DNA) in blood collected at the day 92 sacrifice, but all other blood samples collected at the interim or terminal sacrifice were negative.
The overall conclusion from the biodistribution studies is that after intravitreal injection there were high levels of vector DNA in the injected eye but minimal or no vector DNA in any other tissue.
Vector shedding
Vector shedding was evaluated by measuring vector DNA in samples of tears, saliva, nasal secretions, urine, and feces collected on study days 3 and 8. Only for feces was the amount of DNA recovered sufficient to reliably quantify the vector copies per μg of DNA. Seven animals had vector DNA detected in feces on day 3 (127 or 867 copies per μg of DNA in 2 low-dose animals and 253, 409, 730, 1178, or 1596 copies per μg of DNA in 5 high-dose animals). All samples collected on day 8 were negative.
Very small amounts or no measurable amounts of vector DNA were obtained from the other bodily fluids tested. Some of these samples had very small amounts of vector DNA detected, but in only 10 instances among the 180 samples tested was the amount more than 100 copies per sample, including tears from 1 low-dose and 1 high-dose animal on day 3 (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/humc), nasal secretions from 1 low-dose and 4 high-dose animals on day 3 (Supplementary Table S2), and urine from 3 high-dose animals on day 3 (Supplementary Table S3).
Values of 11–100 copies per sample were detected in tear samples from 5 control animals, 9 low-dose animals, and 9 high-dose animals. Values of 11–100 copies per sample were also detected in nasal secretion samples from 3 low-dose animals and 3 high-dose animals, from saliva in 1 high-dose animal, and from urine in 2 control animals, 2 low-dose animals, and 3 high-dose animals.
The overall conclusion from the vector shedding analysis is that only small amounts of vector DNA were detected in bodily fluids from a few animals on day 3 (2 days after vector administration) and rarely on day 8 (7 days after vector administration). Very small amounts of vector DNA, between 11 and 100 copies per sample, were reported in tear and urine samples from control animals as well as vector-treated animals.
Discussion
An AAV vector expressing human RS1 driven by a CB promoter was previously shown to rescue the abnormal phenotype when administered by subretinal injection in RS1-deficient mice,10,12 and subretinal injections of rAAV vectors expressing RPE65 have been used to treat patients with Leber congenital amaurosis type 2.26–28 However, the retinas of patients with XLRS are more fragile and prone to disease complications such as vitreous hemorrhage and retinal detachment, which may lead to severe visual impairment,29 and a subretinal injection in this patient population may pose a significant risk to visual function.14 Intravitreal injection is therefore considered the preferred route for administration of gene therapy vectors to patients with XLRS.
The key toxicology finding in the current study was that intravitreal administration of rAAV2tYF-CB-RS1 resulted in a dose-related anterior and posterior segment inflammatory response that improved over time. There appeared to be two phases of this inflammatory response. The initial phase, which was seen in vehicle-injected as well as vector-injected eyes, was likely related to the intravitreal injection procedure. The recurrence of ocular inflammation in vector-injected eyes several weeks after intravitreal injections suggests that the inflammation might have been related to immune responses to the vector. The intensity and duration of abnormalities on ophthalmic examination, the frequency and intensity of ocular histologic findings, and the maximum titer of antibodies to AAV2tYF capsids were all higher in the high-dose group, but it is not known if any of these observations are causally related or are independent responses to the higher dose administered. A transient, borderline-positive ELISPOT response to AAV capsid peptides occured in only 1 of 3 animals in each vector dose group, suggesting that T-cells were not mediating the ocular inflammation.
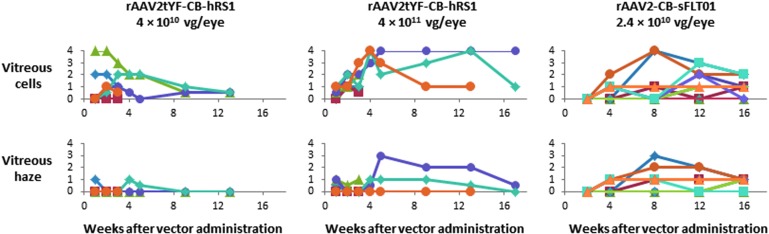
The ocular inflammation observations after intravitreal injection of rAAV2tYF-CB-RS1 are similar to those reported in a study in which rAAV2-CB-sFLT01, an AAV vector expressing a vascular endothelial growth factor inhibitor driven by the CB promoter and packaged in wild-type AAV2 capsids, was administered by intravitreal injection in cynomolgus macaques.30 The vitreous cells and vitreous haze scores after intravitreal injection of rAAV2-CB-sFLT01 at a dose of 2.4 × 1010 vg per eye were similar to the vitreous cells and vitreous haze scores after intravitreal injection of rAAV2tYF-CB-RS1 at a dose of 4 × 1011 vg per eye (Fig. 4). When the rAAV2-CB-sFLT01 vector was administered in a clinical trial to patients with age-related macular degeneration at a dose of 2.4 × 1010 vg per eye, only 1 of 11 subjects developed ocular inflammation, which resolved after 5 weeks of topical anti-inflammatory treatment.31 It will be important to determine the frequency and intensity of inflammation after intravitreal injections of rAAV2tYF-CB-RS1 in patients with XLRS. In the initial clinical trial of rAAV2tYF-CB-RS1, subjects will have ocular examinations scheduled at 1, 7, and 14 days and 1, 2, and 3 months after vector administration, and a plan will be implemented to manage ocular inflammation if it occurs.
Figure 4.
Comparison of ocular inflammation in cynomolgus macaques after intravitreal injection of rAAV2tYF-CB-hSR1 or rAAV2-CB-sFLT01. Each symbol represents an individual animal. Intensity of vitreous cells and vitreous haze in individual animals was scored in a standardized fashion as 0, trace (0.5), 1+, 2+, 3+, or 4+. Each symbol represents an individual animal. In the rAAV2tYF-CB-hRS1 study there were 6 animals per group through week 2, 3 animals per group during week 3 to week 12, and 2 animals in the high-dose group at week 16. There were 12 animals in the rAAV2-CB-sFLT01 study.
Other parameters tested provided no evidence of local or systemic toxicity related to the vector. There was no apparent effect at either dose level on intraocular pressure, ERG or VEP responses, and no evidence of clinical toxicity or changes in hematology, coagulation, or clinical chemistry parameters.
Results of biodistribution studies demonstrated that the vector does not spread widely or consistently outside the injected eye. High levels of vector DNA were found in the injected eye but minimal or no vector DNA was found in any other tissue. Vector shedding studies showed that only small amounts of vector DNA were detected in bodily fluids from a few animals on day 3 (2 days after vector administration). The presence of very low amounts of vector DNA in bodily fluids from both control and treated animals suggests that many of these represent false-positive results when using a qPCR assay designed to have sensitivity of 50 copies per μg DNA (10 copies per sample for samples containing 0.2 μg DNA). When shedding did occur it was almost always short-lived; three animals had positive nasal secretions on day 8, in each instance with a lower value on day 8 than on day 3, and only one animal had a positive tear sample on day 8. Vector DNA was not detected in any samples of saliva, urine, or feces on day 8.
We have previously used sBHK cells grown in medium containing fetal bovine serum to produce an rAAV1-CB-hAAT vector that was evaluated in patients with alpha-1 antitrypsin (AAT) deficiency.21,32,33 The process used for purification of that vector employed a CIM Q column followed by an AVB column. In contrast, the use of serum-free sBHK cells enables AVB Sepharose affinity resin capture of rAAV2tYF-CB-hRS1 directly from clarified lysate without the need for precolumn concentration or buffer exchange. The AVB Sepharose eluate is then subjected to CIM SO3− monolith chromatography, which permits elution of rAAV2tYF-CB-hRS1 into a buffer matrix similar to that of the final formulation buffer.
The process used to produce and purify rAAV2tYF-CB-hRS1 resulted in a product with purity >90%; low levels of residual BHK, HSV, and BSA protein; low levels of BHK and HSV DNA; and undetectable levels of Benzonase and AVB ligand. These results are similar to the low levels of process residuals seen in toxicology and clinical batches of the rAAV1-CB-hAAT product that had a favorable safety profile when administered in a clinical trial at doses up to 6 × 1012 vg/kg (4.2 × 1014 total vg in a 70 kg person) in subjects with alpha-1 antitrypsin deficiency.32,33
In conclusion, intravitreal injection of rAAV2tYF-CB-hRS1 at doses of 4 × 1010 or 4 × 1011 vg per eye was well tolerated but was associated with a dose-related anterior and posterior segment inflammatory response that improved over time. Histopathological examination demonstrated minimal or moderate mononuclear infiltrates in 6 of 12 vector-injected eyes, and immunohistochemical staining showed RS1 labeling of the ganglion cell layer at the foveal slope in vector-injected eyes at both dose levels. Biodistribution studies demonstrated high levels of vector DNA in the injected eye but minimal or no vector DNA in any other tissue. These results support the use of rAAV2tYF-CB-hRS1 in clinical studies in patients with XLRS. A phase 1/2 clinical trial evaluating rAAV2tYF-CB-hRS1 at dose levels ranging from 1 × 1011 to 6 × 1011 vg per eye administered by intravitreal injection in patients with XLRS was initiated in July 2015.34
Supplementary Material
Acknowledgments
This study was supported in part by grant TA-GT-0911-0559-AGTC-WG from the Foundation Fighting Blindness. We thank T. Michael Nork for performing the intravitreal injections; Roberto Calcedo for AAV antibody testing; and Nadezhda Kulagina for RS1 antibody testing.
Author Disclosure
G.Y., D.R.K., and J.D.C. are employees and shareholders of Applied Genetic Technologies Corporation and have a conflict of interest to the extent that this work potentially increases their financial interests. None of the other authors have a competing financial interest.
References
- 1.George ND, Yates JR, Moore AT. X linked retinoschisis. Br J Ophthalmol 1995;79:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tantri A, Vrabec TR, Cu-Unjieng A, et al. X-linked retinoschisis: A clinical and molecular genetic review. Surv Ophthalmol 2004;49:214–230 [DOI] [PubMed] [Google Scholar]
- 3.Molday RS, Kellner U, Weber BH. X-linked juvenile retinoschisis: Clinical diagnosis, genetic analysis, and molecular mechanisms. Prog Retin Eye Res 2012;31:195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apushkin MA, Fishman GA. Use of dorzolamide for patients with X-linked retinoschisis. Retina 2006;26:741–745 [DOI] [PubMed] [Google Scholar]
- 5.′Genead MA, Fishman GA, Walia S. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with X-linked retinoschisis. Arch Ophthalmol 2010;128:190–197 [DOI] [PubMed] [Google Scholar]
- 6.Ghajarnia M, Gorin MB. Acetazolamide in the treatment of X-linked retinoschisis maculopathy. Arch Ophthalmol 2007;125:571–573 [DOI] [PubMed] [Google Scholar]
- 7.Khandhadia S, Trump D, Menon G, Lotery AJ. X-linked retinoschisis maculopathy treated with topical dorzolamide, and relationship to genotype. Eye (Lond) 2011;25:922–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walia S, Fishman GA, Molday RS, et al. Relation of response to treatment with dorzolamide in X-linked retinoschisis to the mechanism of functional loss in retinoschisin. Am J Ophthalmol 2009;147:111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng Y, Takada Y, Kjellstrom S, et al. RS-1 gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-linked retinoschisis. Invest Ophthalmol Vis Sci 2004;45:3279–3285 [DOI] [PubMed] [Google Scholar]
- 10.Min SH, Molday LL, Seeliger MW, et al. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h-deficient mouse model of x-linked juvenile retinoschisis. Mol Ther 2005;12:644–651 [DOI] [PubMed] [Google Scholar]
- 11.Kjellstrom S, Bush RA, Zeng Y, et al. Retinoschisin gene therapy and natural history in the Rs1h-KO mouse: Long-term rescue from retinal degeneration. Invest Ophthalmol Vis Sci 2007;48:3837–3845 [DOI] [PubMed] [Google Scholar]
- 12.Janssen A, Min SH, Molday LL, et al. Effect of late-stage therapy on disease progression in AAV-mediated rescue of photoreceptor cells in the retinoschisin-deficient mouse. Mol Ther 2008;16:1010–1017 [DOI] [PubMed] [Google Scholar]
- 13.Takada Y, Vijayasarathy C, Zeng Y, et al. Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery. Invest Ophthalmol Vis Sci 2008;49:3677–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park TK, Wu Z, Kjellstrom S, et al. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther 2009;16:916–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne LC, Ozturk BE, Lee T, et al. Retinoschisin gene therapy in photoreceptors, Muller glia or all retinal cells in the Rs1h-/- mouse. Gene Ther 2014;21:585–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song S, Embury J, Laipis PJ, et al. Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (rAAV) vectors. Gene Ther 2001;8:1299–1306 [DOI] [PubMed] [Google Scholar]
- 17.Petrs-Silva H, Dinculescu A, Li Q, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther 2009;17:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrs-Silva H, Yasumura D, Matthes MT, et al. Suppression of rds expression by siRNA and gene replacement strategies for gene therapy using rAAV vector. Adv Exp Med Biol 2012;723:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apushkin MA, Fishman GA, Janowicz MJ. Correlation of optical coherence tomography findings with visual acuity and macular lesions in patients with X-linked retinoschisis. Ophthalmology 2005;112:495–501 [DOI] [PubMed] [Google Scholar]
- 20.Thomas DL, Wang L, Niamke J, et al. Scalable recombinant adeno-associated virus production using recombinant herpes simplex virus type 1 coinfection of suspension-adapted mammalian cells. Hum Gene Ther 2009;20:861–870 [DOI] [PubMed] [Google Scholar]
- 21.Chulay JD, Ye GJ, Thomas DL, et al. Preclinical evaluation of a recombinant adeno-associated virus vector expressing human alpha-1 antitrypsin made using a recombinant herpes simplex virus production method. Hum Gene Ther 2011;22:155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin PL, Miller PE, Mata M, Christian BJ. Ocular inflammation in cynomolgus macaques following intravenous administration of a human monoclonal antibody. Int J Toxicol. 2009;28:5–16 [DOI] [PubMed] [Google Scholar]
- 23.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson SG, Boye SL, Aleman TS, et al. Safety in nonhuman primates of ocular AAV2-RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum Gene Ther 2006;17:845–858 [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Calcedo R, Vandenberghe LH, et al. Impact of preexisting vector immunity on the efficacy of adeno-associated virus-based HIV-1 Gag vaccines. Hum Gene Ther 2008;19:663–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: A phase 1 dose-escalation trial. Lancet 2009;374:1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson SG, Cideciyan AV, Ratnakaram R, et al. Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: Safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol 2012;130:9–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bainbridge JW, Mehat MS, Sundaram V, et al. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med 2015;372:1887–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sikkink SK, Biswas S, Parry NR, et al. X-linked retinoschisis: An update. J Med Genet 2007;44:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maclachlan TK, Lukason M, Collins M, et al. Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol Ther 2011;19:326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heier J, Campochiaro P, Dugel P, et al. Preliminary results of phase I study with AAV 2-sFLT01 as gene therapy for treatment of exudative age-related macular degeneration. Paper presented at Retina Society Annual Meeting, September12, 2014, Philadelphia, PA [Google Scholar]
- 32.Flotte TR, Trapnell BC, Humphries M, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha-1 antitrypsin: Interim results. Hum Gene Ther 2011;22:1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller C, Chulay JD, Trapnell BC, et al. Human Treg responses allow sustained recombinant adeno-associated virus-mediated transgene expression. J Clin Invest 2013;123:5310–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AGTC. Safety and efficacy of rAAV-hRS1 in patients with X-linked retinoschisis (XLRS). www.clinicaltrials.gov/ct2/show/NCT02416622 (accessed September2, 2015)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.